Abstract
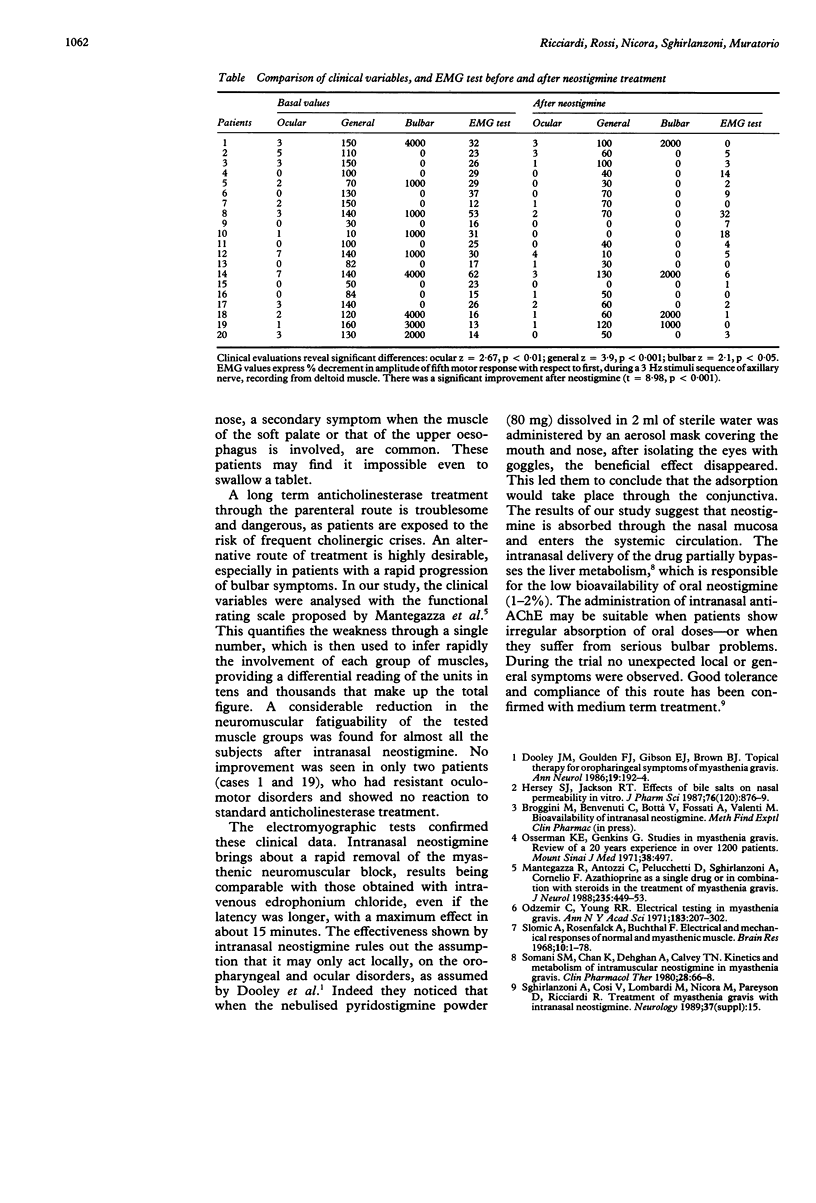
The effectiveness of intranasal neostigmine (9.3-13.8 mg) was tested in 20 subjects with myasthenia gravis, classified as Osserman grades 2A and 2B. In all cases the drug produced significant clinical and electromyographic improvement. No side effects were reported during the treatment.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dooley J. M., Goulden K. J., Gatien J. G., Gibson E. J., Brown B. S. Topical therapy for oropharyngeal symptoms of myasthenia gravis. Ann Neurol. 1986 Feb;19(2):192–194. doi: 10.1002/ana.410190214. [DOI] [PubMed] [Google Scholar]
- Hersey S. J., Jackson R. T. Effect of bile salts on nasal permeability in vitro. J Pharm Sci. 1987 Dec;76(12):876–879. doi: 10.1002/jps.2600761206. [DOI] [PubMed] [Google Scholar]
- Mantegazza R., Antozzi C., Peluchetti D., Sghirlanzoni A., Cornelio F. Azathioprine as a single drug or in combination with steroids in the treatment of myasthenia gravis. J Neurol. 1988 Nov;235(8):449–453. doi: 10.1007/BF00314245. [DOI] [PubMed] [Google Scholar]
- Ozdemir C., Young R. R. Electrical testing in myasthenia gravis. Ann N Y Acad Sci. 1971 Sep 15;183:287–302. doi: 10.1111/j.1749-6632.1971.tb30759.x. [DOI] [PubMed] [Google Scholar]
- Slomić A., Rosenfalck A., Buchthal F. Electrical and mechanical responses of normal and myasthenic muscle. Brain Res. 1968 Aug 5;10(1):1–78. doi: 10.1016/0006-8993(68)90227-8. [DOI] [PubMed] [Google Scholar]


