Abstract
The effect of a mutation affecting flocculation, differentiation into cyst-like forms, and root colonization on nitrogenase expression by Azospirillum brasilense is described. The gene flcA of strain Sp7 restored these phenotypes in spontaneous mutants of both strains Sp7 and Sp245. Employing both constitutive pLA-lacZ and nifH-lacZ reporter fusions expressed in situ, the colony morphology, colonization pattern, and potential for nitrogenase activity of spontaneous mutants and flcA Tn5-induced mutants were established. The results of this study show that the ability of Sp7 and Sp245 mutant strains to remain in a vegetative form improved their ability to express nitrogenase activity in association with wheat in a hydroponic system. Restoring the cyst formation and colonization pattern to the spontaneous mutant Sp7-S reduced nitrogenase activity rates in association with plants to that of the wild-type Sp7. Although Tn5-induced flcA mutants showed higher potentials for nitrogenase expression than Sp7, their potentials were lower than that of Sp7-S, indicating that other factors in this strain contribute to its exceptional nitrogenase activity rates on plants. The lack of lateral flagella is not one of these factors, as Sp7-PM23, a spontaneous mutant impaired in swarming and lateral-flagellum production but not in flocculation, showed wild-type nitrogenase activity and expression. The results also suggest factors of importance in evolving an effective symbiosis between Azospirillum and wheat, such as increasing the availability of microaerobic niches along the root, increased supply of carbon sources by the plant, and the retention of the bacterial cells in vegetative form for faster metabolism.
Observations of nitrogen fixation in the roots of grasses and nonleguminous crops in the 1970s and observations of plant growth-promoting capabilities of Azospirillum initiated a wave of field experiments to study growth stimulation (for reviews, see references 4 and 25). However, a lack of consistency in the field results with respect to positive contributions from biological nitrogen fixation has been reported (5). The challenge of obtaining consistency suggests that further fundamental research is required to understand both rhizosphere interaction and colonization by Azospirillum species before strains can be selected that will perform well using field inoculation.
Azospirillum brasilense strains are known to be highly pleomorphic and to change their metabolic activities swiftly in response to changes in environmental conditions (7, 14, 28, 29). Under low oxygen tension, bacteria of the genus Azospirillum are highly motile, half-curved or vibrioid, gram-negative rods with a long polar flagellum in liquid medium and additional peritrichous flagella on solid medium (35). Under aerobic conditions, particularly in aged cultures, vibrioid cells undergo a transition to round, nonmotile, encapsulated forms (7, 14) that are considered to be cysts (21, 28, 29, 30). Heavy capsulation gives the cells a particular adhesive nature so that they aggregate in a matrix of polysaccharide material, forming large macroscopic clumps that flocculate in liquid cultures (28). Fructose induces flocculation in A. brasilense to a greater extent than other carbon sources (28). During flocculation, the vegetative cells lose motility, assume an enlarged spherical form, and accumulate abundant poly-β-hydroxybutyrate granules, developing an outer layer or coat of polysaccharides (28).
Mutants of A. brasilense unable to undergo transition from vegetative vibrioids into encapsulated forms formed white colonies on Congo red plates in the background of wild-type red colonies (6). Several polysaccharides are known to interact in solution with the dye Congo red (diphenyldiazo-bis-α-naphthylaminesulfonate) (38), suggesting a change in or lack of the external polysaccharides in these mutants. The abilities to form cysts and to flocculate have been correlated in several publications (6, 8, 13, 18, 24, 28). Both features were also correlated with the ability of Azospirillum to colonize the surfaces of wheat roots, where the wild-type strain A. brasilense Sp7 forms a sheath of bacteria on the surface under the conditions of growth in hydroponic solution without competition from other microbial strains (18, 27). The attachment of Azospirillum to wheat roots is mainly dependent on two factors: the existence of a polar flagellum, allowing the bacteria to adsorb to the roots, and the production of exopolysaccharides (EPS), allowing the bacteria to firmly attach to the root surface (11, 23). EPS production is regulated by the flcA gene, although the mechanism of regulation is not known yet (27). The existence of both polar and lateral flagella is essential for normal mobility, the first for swimming in liquid media and the second for swarming in semisolid media (16). Several of these phenotypes are affected in the strain Sp7-S, a spontaneous mutant of A. brasilense Sp7: it does not form cyst-like cells on wheat roots, possibly due to the lack of an extracellular polysaccharide layer (18), and it does not swarm on semisolid media, suggesting that it is affected in its lateral-flagellum production or function (27). These differences in phenotypes may explain the differences in colonization patterns between Sp7-S and the wild-type Sp7. Although strain Sp7-S colonizes the root surface to a lesser extent than the wild type, it reduces acetylene at a higher rate, suggesting that cyst formation and colonization pattern play roles in regulating nitrogenase activity on plants (18).
As a complement to recent publications (18, 27), this article deals with the relationship between cyst formation, colonization patterns, and the potential for nitrogen fixation in two different strains of A. brasilense with different modes of colonization: Sp7 and Sp245. A simple assay to isolate spontaneous mutants impaired in flocculation, based on selection of pale colonies on Congo red agar plates, is described, as well as other features of these representative spontaneous mutants impaired in flocculation. The mutants are then compared with Tn5-induced flcA mutant strains of Sp7, which have been genetically characterized (27), to estimate the effect of encapsulation on nitrogenase activity. The potentials for nitrogenase activity in the different mutants are estimated at the gene regulation level, making use of a nifH-lacZ fusion (22).
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this work are listed in Table 1. Complete medium was nutrient broth (NB; Difco) for A. brasilense and Luria-Bertani medium for Escherichia coli. Otherwise, Azospirillum strains were grown on minimal lactate medium (15) or nitrogen-free malate medium (NFB) (34), which were supplemented with 40 μg of Congo red. The medium for examination of the ability of the strains to swarm was semisolid NB or minimal lactate containing 0.4% agar. The following antibiotic concentrations were used for Azospirillum: tetracycline, 5 μg/ml, and kanamycin, 20 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Genotype or phenotypea | Reference |
|---|---|---|
| E. coli S17-1 | pro thi hsdR recA Tra+ IncP | 33 |
| A. brasilense | ||
| Sp7 | Wild type | 35 |
| Sp7-S | Spontaneous mutant of Sp7; CR− Floc− | 18 |
| Sp245 | Wild type | 3 |
| Sp7-PM23 | Spontaneous mutant of Sp7; CR+ Floc+ Swarm− | This work |
| Sp7-PM35 | Spontaneous mutant of Sp7; CR± Floc− Swarm+ | This work |
| Sp245-M4, -M5, -M6 | Spontaneous mutants of Sp245; CR− Floc− Swarm+ | This work |
| Sp72001, -4 | Tn5-induced flcA mutants of Sp7; Kmr CR− Floc− | 27 |
| Plasmids | ||
| pAB1220-9c | Derivative of pVK100b containing the flcA gene on a 9-kb HindIII fragment; Tcr | 18 |
| pAB2051c | pLA29.17 derivativeb containing flcA on a 1.1-kb PstI fragment constitutively expressed; Tcr | 27 |
| pAB2053c | pLA29.17 derivative containing flcA on a 4.6-kb BamHI-BglII fragment; Tcr | 27 |
| pSP7115d | Plasmid that restored swarming in strain Sp7-S; isolated from a gene bank of A. brasilense Sp7; Tcr | 27 |
| pAB2053Z | lacZ cartridge cloned into pAB2053; Tcr Kmrlac+ | 27 |
| pLA-lacZ | pLA29.17 derivativeb; lacZ constitutive fusion; Tcr Kmr Lac+ | 1 |
| pAB358 | pVK100 derivative; nifH-lacZ fusion; Tcr | 22 |
Ampr, Cmr, Kmr, and Tcr indicate resistance to ampicillin, chloramphenicol, kanamycin, and tetracycline, respectively; CR, Congo red binding; Floc, flocculation; Swarm, swarming ability; lac, lacZ.
pVK100 and pLA29.17 are low-copy-number, broad-host-range cloning-vector derivatives of RK2 that are stable in Azospirillum strains.
In pAB2051, a truncate form of flcA is cloned under a constitutive promoter, while in pAB2053 and pAB1220-9, flcA is cloned under its own promoter.
Isolated by C. Elmerich at the Institute Pasteur, Paris, France.
Examination of flocculation.
Flocculation in minimal medium in the presence of 8 mM fructose and 0.5 mM KNO3 (flocculation medium) was examined using a modification of the procedure outlined by Sadasivan and Neyra (30). The inoculum was harvested from a log-phase culture (2 ml) grown in NB by centrifugation at 5,000 × g for 10 min at room temperature using a minicentrifuge. The pellet was washed with minimal medium and inoculated into the flocculation medium to an absorbance of 0.3 to 0.4 at 600 nm. Experiments were conducted in 50-ml flasks containing 10 ml of flocculation medium, which were incubated at 30°C on a shaker at 200 rpm for approximately 10 h.
Selective mutagenesis of Azospirillum strains.
Spontaneous mutant strains of A. brasilense Sp7 and Sp245 impaired in flocculation were isolated from the supernatants of flocculated cultures. The number of cells per milliliter of supernatant was estimated using a counting slide with an Olympus BHA light microscope. The supernatant was diluted to a final concentration of 2,000 to 3,000 bacteria per ml. Since in previous cases the ability to flocculate was positively correlated with the ability to bind Congo red, nonflocculating mutants were selected on minimal lactate agar plates containing 40 μg of Congo red/ml. Fractions (100 μl) of the diluted supernatant were spread on each of the selective medium plates, which were then incubated over 2 to 3 nights at 37°C. Colonies that failed to bind Congo red and appeared white or light pink were tested further for flocculation. The frequency of the mutation was calculated, and the stability of the mutation was estimated following several reisolations.
Complementation analysis.
Several mutants were transformed with selected plasmids (Table 1): (i) pAB2051 and pAB2053, containing the flcA gene of strain Sp7, which complements flocculation and Congo red binding in strain Sp7-S (27), and (ii) pSP7115, complementing swarming ability in strain Sp7-S (27). Transfer of the plasmids into an Azospirillum recipient was performed by conjugation with E. coli S17-1 as a donor. Transconjugants were selected on minimal lactate medium containing 20 mM ammonium chloride, 5 μg of tetracycline/ml, and, in some cases, 20 μg of kanamycin/ml (15).
Plant assays.
Wheat seedlings of cultivar Miskle were grown in hydroponic growth solution under sterile conditions as described by Zeman et al. (39). Seeds were inoculated with bacteria containing a lacZ fusion as described by Arsène et al. (1). Several plants were treated with 0.7 ppm 2,4-dichlorophenoxyacetic acid (2,4-D). The 2,4-D was added to the hydroponic solution at the time of inoculation. Ten days after inoculation, five plants (unless otherwise mentioned) were assayed for nitrogenase activity by acetylene reduction assays (ARA) and for β-galactosidase activity and in situ X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining of bacteria or were examined under a scanning electron microscope (SEM). Experiments were repeated two or three times.
ARA.
Natural production of ethylene is insignificant compared to the rates of acetylene reduction observed in hydroponic Azospirillum-wheat systems, and 2,4-D does not enhance ethylene production without added acetylene (34). ARA with Azospirillum strains associated with plant roots as well as ARA in free living cultures were performed as described by Katupitiya et al. (18). Ethylene formation was measured with a Shimadzu GC 8F gas chromatograph equipped with a flame ionization detector and a 1-m Porapak T column.
SEM examination of wheat roots inoculated with Azospirillum.
Root segments from inoculated plants were placed on a metal plate, freeze-dried in liquid nitrogen for 40 to 50 s, and immediately observed with a Philips 505 SEM operating at 15 to 20 kV (this novel method was developed by Tony Romeo of the Electron Microscopy Unit [EMU] at the University of Sydney).
Visualization of flagella by TEM.
The broth medium for the examination of polar flagella was minimal medium supplemented with 20 mM ammonium chloride. For the examination of both polar and lateral flagella, the same medium was supplemented with 1.5% agar. Broth cultures were grown to an absorbance of 0.4 to 0.5 at 600 nm, and colonies grown on solid medium were resuspended in saline solution (sterile 0.85% NaCl). Negative staining was performed with 2% phosphotungstic acid, a high-electron-density negative stain, and the samples were observed by transmission electron microscopy (TEM) with a Philips 902 transmission electron microscope operated at 80 kV.
Examination of EPS production by TEM.
For the examination of EPS production, cultures of the mutant strain Sp7-S and the complemented strain Sp7-S pAB1220-9 were grown overnight in nitrogen-free medium supplemented with 0.5% yeast extract and were treated and examined by TEM as described by Katupitiya et al. (18).
Detection of β-galactosidase activity in wheat roots inoculated with Azospirillum.
The colonization pattern was investigated by in situ staining of inoculated roots with X-Gal. Detection of bacteria on the roots by light microscopy, although very accurate quantitatively, is limited to observations of bacterial cells on the root surface only, since bacterial cells in the internal root tissues cannot be brought into focus. Quantitative measurements by β-galactosidase activity of strains carrying lacZ fusions in association with wheat were established as described by Arsène et al. (1). Several lacZ fusions were used with different strains of Azospirillum. (i) pAB358, a nifH-lacZ transcriptional fusion (22), was used for estimating the potential for nitrogenase activity. (ii) pLA-lacZ, containing a constitutive lacZ fusion (1), was used for quantification and detection of bacteria. (iii) pAB2053Z, containing a constitutive lacZ fusion and the flcA gene of strain Sp7 (27), was used for complementation analysis.
Early stages of colonization.
In a separate hydroponic experiment, colonization of wheat roots by Sp7 and Sp72004 was examined 4, 8, 12, and 24 h after inoculation with vegetative-phase bacterial cultures. Colonization of Sp7 was also examined 4 days after inoculation. In this experiment, plant roots were briefly submerged in sterile distilled H2O before determination of β-galactosidase activity to prevent quantitative errors caused by bacteria in the hydroponic growth solution trapped in the root tip hairs.
RESULTS
Effect of EPS and cyst formation on nitrogenase activity rates.
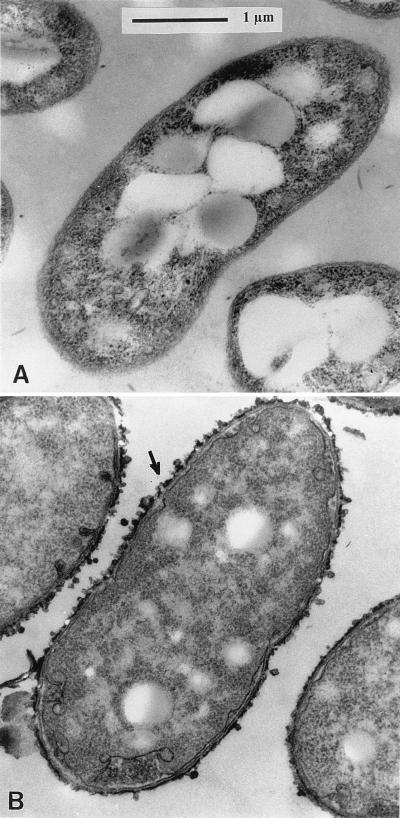
Transverse sections examined by TEM revealed that the introduction of the plasmid pAB1220-9, containing the flcA gene (Table 1), restored the capsular material, which is absent in strain Sp7-S (Fig. 1), and the formation of cyst-like cells on wheat roots (data not shown).
FIG. 1.
Transmission electron micrographs of transverse section of Sp7-S in free-living state, in which the outer layer of EPS is not present (A), and transverse section of Sp7-S complemented with the plasmid pAB1220-9, which shows restoration of the EPS layer (B). The arrow is pointing at the EPS layer around the cell.
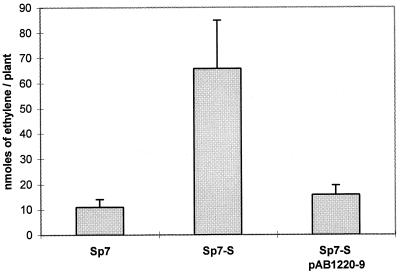
Nitrogenase activity rates for Sp7-S pAB1220-9 in association with 2,4-D-treated wheat were reduced (fivefold) to that of the wild type (Fig. 2). Previously, controls without 2,4-D were shown to have significantly lower acetylene reduction rates for both Sp7 and Sp7-S (18). Still, Sp7-S showed higher rates of ethylene production (18).
FIG. 2.
Acetylene reduction activity associated with 2,4-D-treated plants inoculated with Sp7, Sp7-S, or Sp7-S complemented for flocculation with plasmid clone pAB1220-9. The assays were carried out in a modified atmosphere containing 2.5% oxygen and lasted for 10 h from the injection of acetylene. The data shown are averages of five plants, grown for 10 days after inoculation. No ethylene was detected in noninoculated plants or in controls without acetylene. The error bars indicate standard errors.
Frequency and stability of the spontaneous mutation.
Since the mutant Sp7-S is incapable of flocculation, similar mutants were expected to be found in the supernatant of a well-flocculated culture. It was found that 0.2% of the total number of colonies of A. brasilense Sp7 which originated from the supernatant of a flocculated culture (total, 41,000 colonies) failed to bind Congo red and appeared white or pink (the colonies were labeled Sp7-PM1 to -PM84). Following at least five reisolations on minimal agar containing Congo red, 15 cultures of the 84 mutants of Sp7 regained the ability to bind Congo red (Sp7-PM2, -4, -9, -10, -13, -17, -20, -21, -22, -23, -29, -36, -50, -56, and -77), while 69 of the 84 original mutants retained the mutation and remained pink or white. Therefore, the stability of the mutation, or the percentage of the isolated mutants of Sp7 that preserved the mutation, was 82%.
The effect of the inability to flocculate on the colonization pattern of roots was examined in another strain of A. brasilense, Sp245, which was reported to be a good colonizer (17). Sp245 appeared drier than Sp7 on NFB plates and also showed a higher degree of flocculation, leaving the supernatant visually very clear. This probably explains the higher frequency of the mutation found with Sp245, in which 1.36% of the colonies (60 out of 4,400; named Sp245-M1 to -M60) did not bind Congo red. Only five of the cultures retained a red color (Sp245-M36, -40, -41, -42, and -50); therefore, the stability of the mutation from Sp245 was estimated at 92%.
Characterization of selected mutants of A. brasilense Sp7 and Sp245.
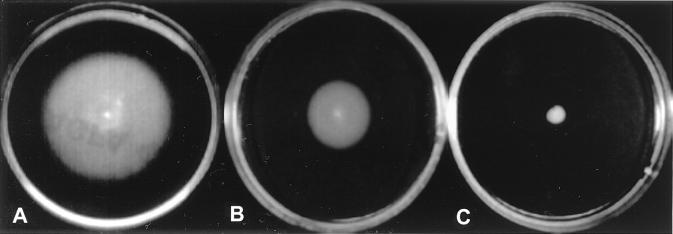
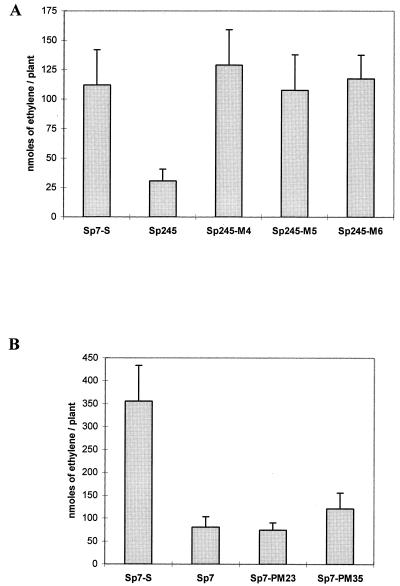
The mutant strains of interest were further investigated and characterized: Sp7-PM23, which is impaired in swarming in semisolid medium (Fig. 3); Sp7-PM35, which partially binds Congo red but does not flocculate; and Sp245-M4, -M5, and -M6, which do not bind Congo red and show higher rates of nitrogenase activity than the wild-type Sp245 in association with plants (Fig. 4).
FIG. 3.
Swarming ability of A. brasilense strains Sp7 3 days (A) and 1 day (B) after inoculation and Sp7-PM23 3 days after inoculation (C). The assay was performed on semisolid NB containing 0.4% agar. Inoculum of freshly grown culture was placed in the center of each plate and allowed to grow over 2 to 3 days at 37°C. All strains examined in this work, except Sp7-S and Sp7-PM23, were able to swarm (i.e., grow in diameter). Swarming tests on semisolid minimal lactate medium (0.4% agar) showed similar results.
FIG. 4.
Acetylene reduction activity of A. brasilense Sp7 and Sp245 strains associated with 2,4-D-treated plants. The plants were inoculated with the wild type and spontaneous mutants of strain Sp245 (Sp245-M4, -M5, and -M6) (A) or with the wild type and spontaneous mutants of strain Sp7 (Sp7-PM23 and -PM35) (B). Strain Sp7-S, in which increased rates of acetylene reduction were reported (18), was used as a control. The assays were carried out in a modified atmosphere containing 2.5% oxygen and lasted for 20 to 24 h (a day) after the injection of acetylene. The data shown are averages of results for 10 plants, assayed 10 days after inoculation. No ethylene was detected in noninoculated plants or in controls without acetylene. The error bars indicate standard errors.
Similar to the mutant Sp7-S, the mutant strains Sp7-PM35 and Sp245-M4, -M5, and -M6 did not flocculate. However, in contrast to Sp7-S, they were able to swarm on semisolid media. All of the strains tested in this work, including Sp7-S, were motile in liquid medium (Table 2).
TABLE 2.
Phenotypes of wild types and mutants of A. brasilense strains Sp7 and Sp245
| Phenotype | Presencea
|
||||||
|---|---|---|---|---|---|---|---|
| Sp7 | Sp7- S | Sp7- PM23 | Sp7- PM35 | Sp245 | Sp245-M4, -M5, -M6 | Sp72001/4b | |
| Congo red binding | + | − | + | P | + | − | − |
| Flocculation | + | − | + | − | + | − | − |
| Growth on nitrate | + | + | + | + | + | + | + |
| Growth on ammonia | + | + | + | + | + | + | + |
| Nitrogen fixation | + | + | + | + | + | + | + |
| Motility in liquid medium | + | + | + | + | + | + | + |
| Polar flagellum | + | + | + | + | + | + | + |
| Swarming (semi-solid media) | + | − | − | + | + | + | + |
| Lateral flagella | + | − | − | NT | NT | NT | NT |
| Growth on dicarboxylic acids | + | + | + | + | + | + | + |
P, partial; +, positive; −, negative; NT, not tested.
These strains are described by Pereg-Gerk and colleagues (27) and are included here as a reference for Tn5-induced flcA mutants.
The wild-type and the mutant strains had the same mucoid appearance in the first 1 to 2 days (at 30°C) on solid agar plates (NB or minimal lactate). However, longer incubation resulted in dry, red (when Congo red was used) colonies of the wild types, while the mutants remained in the mucoid form, suggesting a defect in differentiation into cysts. Strains Sp245-M4, -M5, and -M6 never regained the red color, while in every reisolation of a white colony, approximately 20% of Sp7-PM35 colonies regained the red color (Table 2).
Similar to their wild types, the mutant strains Sp7-PM35 and Sp245-M4, -M5, and -M6 were able to fix nitrogen (as shown by ARA) in pure cultures (the rates were similar to those of the wild type [data not shown]) and were able to utilize both nitrate and ammonium for growth (Table 2).
Both strains Sp7 and Sp7-PM35 could utilize malate, lactate, gluconate, or β-hydroxybutyrate as a sole carbon source and grew poorly on glucose, galactose, or fructose. Strains Sp245 and Sp245-M4, -M5, and -M6 could also efficiently utilize fructose as a sole carbon source (Table 2).
The mutant Sp7-PM23 was similar to the wild type in all of the characteristics mentioned above, except in its inability to swarm in semisolid medium (0.4% agar) (Fig. 3). TEM observations of both strains Sp7-S and Sp7-PM23, grown on solid NFB medium, showed cells lacking lateral flagella (Fig. 5).
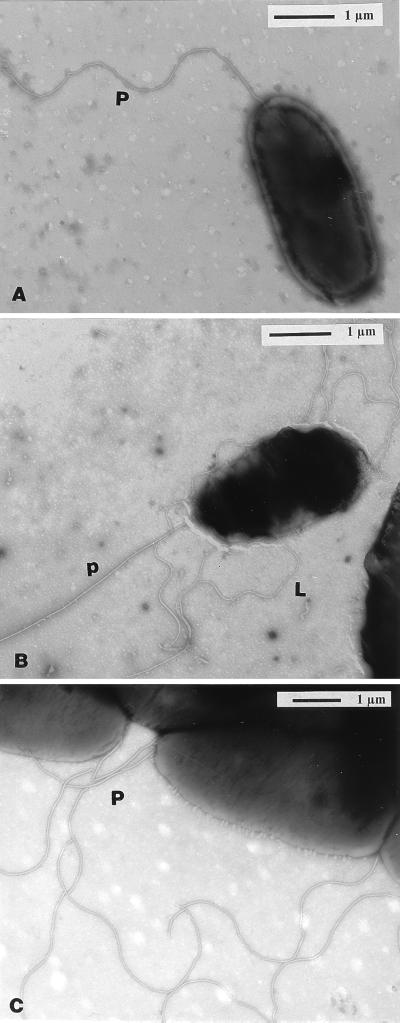
FIG. 5.
Transmission electron micrographs displaying the polar flagellum and lateral flagella of several A. brasilense strains. Negative staining with 2% phosphotungstic acid was used. Strain Sp7 displayed a polar flagellum (P) when grown in liquid medium (minimal lactate) (A) and both lateral (L) and polar (p) flagella when grown on solid or semisolid medium (B). No lateral flagella were observed for the mutant strain Sp7-S (C) or Sp7-PM23 (not shown).
Nitrogenase activity (acetylene reduction) measured with wheat roots inoculated with the mutants Sp245-M4, -M5, and -M6 was significantly higher (between four- and fivefold) than that with roots inoculated with the wild-type Sp245 (Fig. 4A). The acetylene reduction rates in these mutant strains were as high as in the mutant Sp7-S on 2,4-D-treated plants.
There was no difference in acetylene reduction rates between plants inoculated with the wild-type Sp7 and plants inoculated with the nonswarming mutant Sp7-PM23 (Fig. 4B). Plants inoculated with the mutant Sp7-PM35 showed slightly higher (1.5-fold) rates than the wild type, but not as high as the mutant strain Sp7-S (Fig. 4B).
Surface versus protected colonization of wheat roots.
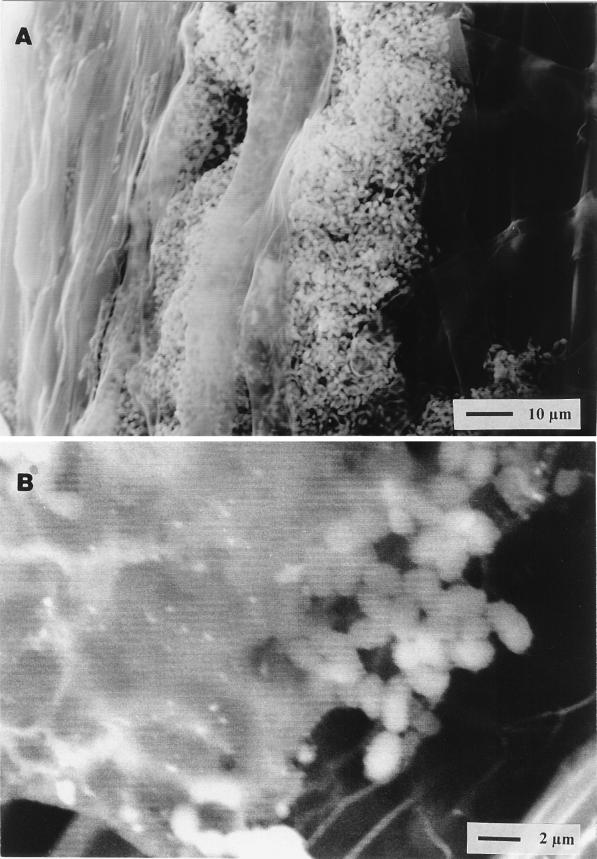
SEM observations confirmed earlier X-Gal staining data (18, 27) showing that the spontaneous mutant Sp7-S and the Tn5-induced flcA mutants Sp72001, -2, and -4 do not colonize the root surface as efficiently as the wild-type strain, Sp7. In addition, they showed that the cell surface of the wild type not only appears as a rough layer (in contrast to the smooth surface of the mutant) but also is, in many cases, covered with a layer of mucus that seems to be of plant origin (Fig. 6). The direct method of fixing the root samples (see Materials and Methods) avoids the disturbance of fragile layers, such as the mucus, which is observed also in the areas of root crevices (Fig. 6).
FIG. 6.
Scanning electron micrographs of wheat root colonization by A. brasilense Sp7, which heavily colonized the surfaces of the roots (A). Note the mucus covering part of the cells of the wild-type strain Sp7 (B), which also appears on the root surface (not shown).
The potential for nitrogenase activity by nonflocculating mutants in association with the roots was studied in this work using both 2,4-D-treated and nontreated plants. However, the pattern of colonization of plant roots by several of the mutants studied here has not been reported previously. The extent of colonization, which was measured with a constitutive lacZ fusion (pLA-lacZ [Table 1]), was summarized from several independent experiments, each of which included a wild-type control. Therefore, the results are presented as percentages of the wild-type colonization efficiency (Table 3). When the fusion pLA-lacZ was used, lower β-galactosidase activity rates were detected for plants inoculated with the mutants than for those inoculated with the wild type (Table 3), indicating a lower extent of overall colonization (1). However, using the mutants Sp7-S, Sp72001 and -4, and Sp7-PM35, a strong X-Gal coloration was detected in the para-nodules of 2,4-D-treated plants compared with very little on the root surface, suggesting a bias towards intercellular or endophytic colonization within the roots.
TABLE 3.
β-Galactosidase activity of wheat roots inoculated with A. brasilense strains Sp245 and Sp7 and flocculation mutants carrying a plasmid containing a lacZ fusion
| Strainb | β-Galactosidase activitya
|
|
|---|---|---|
| 2,4-D-treated plantsc | Plants without 2,4-D | |
| Sp245 derivatives | ||
| Sp245 wild-type pLA-lacZ | 100 ± 8 | 100 ± 25 |
| Sp245-M4 pLA-lacZ | 16 ± 1.9 | 13 ± 3.1 |
| Sp245-M4 pAB2053Z | 110 ± 16 | 73 ± 24 |
| Sp245-M5 pLA-lacZ | 18 ± 3.7 | 12 ± 2.8 |
| Sp245-M5 pAB2053Z | 64 ± 17 | 79 ± 10.5 |
| Sp245-M6 pLA-lacZ | 21 ± 4.3 | 12 ± 2.9 |
| Sp245-M6 pAB2053Z | 56 ± 7 | 76 ± 3.5 |
| Sp7 derivatives | ||
| Sp7 pLA-lacZ | 100 ± 15 | 100 ± 7.7 |
| Sp7-S pLA-lacZ | 30 ± 4.1 | —d |
| Sp72001 pLA-lacZ | 20 ± 2.3 | — |
| Sp72001 pAB2053Z | 100 ± 14.8 | — |
| Sp72004 pLA-lacZ | 25 ± 3.2 | — |
| Sp72004 pAB2053Z | 98 ± 16.9 | — |
| Sp7-PM35 pLA-lacZ | 44 ± 4.3 | 70 ± 10.5 |
| Sp7-PM23 pLA-lacZ | 82 ± 20.2 | 74 ± 14.2 |
| Sp7-PM35 pAB2053Z | NT | 112 ± 13.6 |
| Noninoculated plants | 2 ± 0.2 | 1 ± 0.3 |
Data are presented as percentages of the wild-type activity (Miller units per minute per milligram of plant protein, estimated 10 days after inoculation).
A. brasilense was grown overnight in nitrogen-free medium containing 10 mM ammonia. Plants were inoculated with 0.1 ml of culture (about 5 × 106 bacteria per ml of hydroponic solution). In some cases, 2,4-D (0.7 ppm) was added at the time of inoculation. The data are averages of results for five plants. Standard errors are indicated.
2,4-D-treated plants produced para-nodules in the sites of lateral root emergence.
−, data for plants without 2,4-D are presented in detail by Pereg Gerk and colleagues (27).
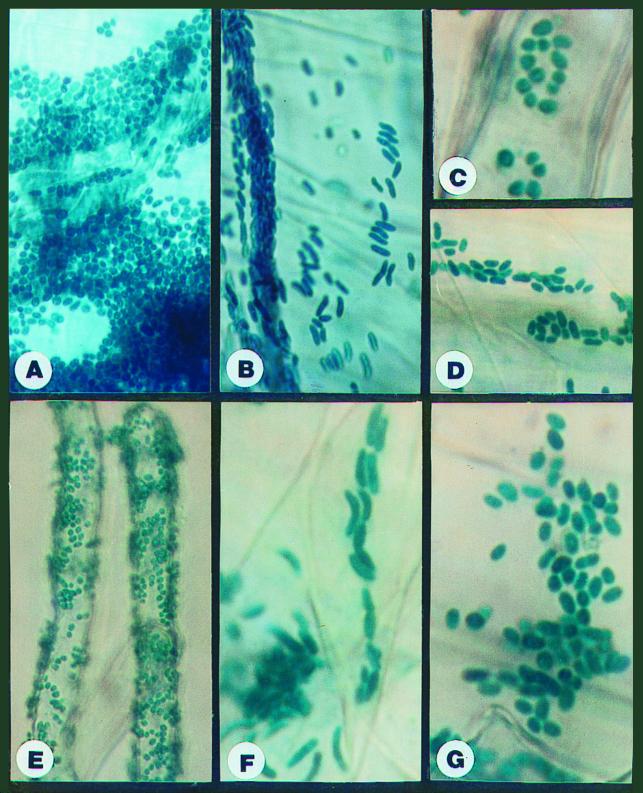
The spontaneous mutants Sp7-PM35 (nonflocculating) and Sp7-PM23 (nonswarming) showed higher surface colonization than Sp7-S (Table 3), which is defective in both flocculation and swarming (Table 2). However, they showed lower surface colonization than the wild type (Table 3). Sp7-PM23 appeared as ovoid cells (Fig. 7C), similar to the wild type (Fig. 7A). Sp7-PM35 appeared on the roots as a mixture of ovoid and vegetative cells (Fig. 7D).
FIG. 7.
Colonization of wheat root surface by several strains of A. brasilense Sp245 and Sp7. (A) Sp7 pLA-lacZ; magnification, ×40. (B) Sp7-S pLA-lacZ; magnification, ×40. (C) Sp7-PM23 pLA-lacZ, magnification, ×100. (D) Sp7-PM35 pLA-lacZ; magnification, ×60. (E) Sp245 pLA-lacZ; magnification, ×40. (F) Sp245-M5 pLA-lacZ; magnification, ×100. (G) Sp245-M5 pAB2053Z; magnification, ×100. β-Galactosidase activity is revealed in situ by the blue color obtained from X-Gal staining.
In agreement with β-galactosidase activity rates obtained with pLA-lacZ (Table 3), heavy root surface colonization by ovoid, cyst-like cells was observed on plants (both treated and not treated with 2,4-D) inoculated with the wild-type strain Sp245 (Fig. 7E). However, similar to Sp7-S (Fig. 7B), plants treated with the mutants Sp245-M4, -M5, and -M6 showed very little or no surface colonization. Bacterial cells, which were observed around lateral root emergence sites, retained the normal curved-rod shape of vegetatively grown azospirilla (Fig. 7F). The mutant strains of both strains Sp7 and Sp245 that were impaired in flocculation and root surface colonization (Sp7-PM35 and Sp245-M4, -M5, and -M6) were fully complemented by the plasmid pAB2053Z (Table 3 and Fig. 7G). Thus, the mutations involved in all of the cases are connected to the flcA gene (27).
All of the strains examined in this work colonized both the crevices surrounding the sites of lateral root emergence and those crevices associated with the para-nodules appearing in 2,4-D-treated roots. However, the colonization of para-nodules was more extensive than that of lateral root emergence sites in plants that were not treated with 2,4-D. No coloration was detected in controls of noninoculated root samples or root samples inoculated with bacteria which did not carry a lacZ fusion.
Time course of colonization.
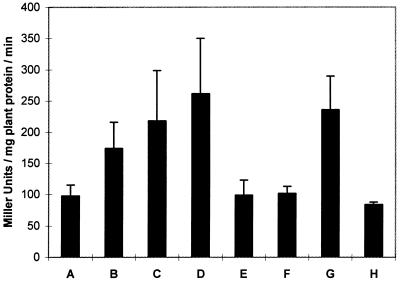
How fast the colonization process is following inoculation in laboratory assays and when the vegetative cells on roots differentiate into cyst-like forms are two key questions in testing colonization. Examination of root segments by both X-Gal staining and β-galactosidase activity showed clear evidence of initiation of root surface colonization 8 h after inoculation with Sp7 (Fig. 8). At this stage, A. brasilense strains Sp7 and Sp72004 were still in their vegetative states and appeared mostly among the root tip hairs. The colonization by Sp7 increased with time: 12 h after inoculation there was already a mixed population of ovoid and curved, rod-like bacteria, but mainly curved; 24 h after inoculation there were a significant number of bacteria on the root surface (Fig. 8) which appeared to be mostly ovoid in shape, indicating differentiation into the cyst-like form (data not shown). Four days after inoculation with Sp7 (Fig. 8), the extent of X-Gal coloration and β-galactosidase activity was similar to that in plants examined 10 days after inoculation (Table 3). Therefore, sufficient time was allowed for the bacteria to differentiate completely into cyst forms during the 10 days following inoculation, when tests for acetylene reduction and colonization were carried out, even for a growing population. Plants (not treated with 2,4-D) inoculated with the flcA mutant strain Sp72004 containing the plasmid pLA-lacZ showed no detectable β-galactosidase activity 24 h after inoculation, indicating that these cells lack the capacity to adhere strongly to the root surface. However, normal activity was restored with the introduction of the plasmid pAB2053Z (Fig. 8).
FIG. 8.
Early stages of colonization of wheat root by A. brasilense Sp7. One-week-old plants were inoculated with 5 × 106 bacteria per ml of hydroponic solution. The β-galactosidase activities of plant extracts inoculated with wild-type strain Sp7 and the mutant strain Sp72004, carrying either pAB2053Z or pLA-lacZ, were measured 4, 8, 12, and 24 h after inoculation. β-Galactosidase activity is expressed as Miller units per minute per milligram of plant protein. The data are averages of five determinations. (A to D) Sp7 pLA-lacZ 4, 8, 12, and 24 h after inoculation, respectively; (E and F) Sp72004 pLA-lacZ 12 and 24 h after inoculation, respectively; (G) Sp72004 pAB2053Z 12 h after inoculation; (H) control noninoculated plants. The error bars indicate standard errors. One-way analysis of variance showed nonsignificant differences between the activities of strain Sp7 during that time (P > 0.05).
Expression of a nifH-lacZ fusion and β-galactosidase activity in association with wheat.
The potential for nitrogen fixation was previously examined by measuring the extent of nifH expression (37), which indicates the levels of environmental factors, such as oxygen and fixed nitrogen, affecting both the synthesis and the activity of the nitrogenase. Moreover, acetylene reduction rates showed high correlation with the extent of nifH expression (nifH-lacZ) in axenic cultures of Sp7 and Sp7-S (12). The potential for nitrogenase activity of nonflocculating mutant strains of Sp7 and Sp245 was tested in this work using the extents of nifH expression.
Although nonflocculating mutants of A. brasilense were defective in root surface colonization (Table 3) (27), there was no significant difference in the expression of nifH between the wild-type strain Sp7 and any of its mutants (Table 4) in associations with nontreated wheat. No strain expressed nifH as highly as the mutant strain Sp7-S in association with 2,4-D-treated plants (Table 4).
TABLE 4.
β-Galactosidase activity of plants inoculated with the wild type and flocculation mutants of A. brasilense containing a nifH-lacZ fusiona
| Strain | β-Galactosidase activityb
|
|
|---|---|---|
| 2,4-D-treated plants | Plants without 2,4-D | |
| Sp245 derivatives | ||
| Sp245 wild-type | 100 ± 22 | 100 ± 17 |
| Sp245-M4 | 67 ± 19 | 15 ± 6 |
| Sp245-M5 | 83 ± 34 | 15 ± 6 |
| Sp245-M6 | 98 ± 40 | 11 ± 2 |
| Noninoculated plants | 24 ± 13 | 1 ± 1 |
| Sp7 derivatives | ||
| Sp7 wild type | 100 ± 33 | 100 ± 35 |
| Sp7-S | 385 ± 150 | 110 ± 35 |
| Sp72001 | 64 ± 5 | 65 ± 34 |
| Sp72004 | 76 ± 18 | 54 ± 27 |
| Sp7-PM35 | 108 ± 45 | 106 ± 53 |
| Sp7-PM23 | 47 ± 15 | 68 ± 58 |
| Noninoculated plants | 17 ± 10 | 1.5 ± 1 |
Plants were inoculated with bacterial cells containing pAB358, a plasmid carrying a nifH-lacZ fusion. The data are averages of results for 10 plants. Standard errors are indicated.
Results obtained using plants treated or not treated with 2,4-D (Miller units per minute per milligram of plant protein) are presented as percentages of the wild-type activity (Sp7 or Sp245, respectively).
On the other hand, wheat roots inoculated with the wild-type Sp245 revealed higher expression of nifH than those inoculated with the flocculation mutants Sp245-M4, -M5, and -M6 (Table 4), in agreement with the colonization pattern (Fig. 7 and Table 3). However, para-nodulated roots inoculated with the wild-type Sp245 displayed an expression of nifH similar to that of those inoculated with the flocculation mutants (Table 4), although the extent of colonization of the entire root obtained with the wild-type was much higher (Table 3).
DISCUSSION
The data reported here show a connection between flocculation, Congo red binding, cyst formation, and root surface colonization in A. brasilense Sp245 similar to that of Sp7 (6, 8, 13, 18, 23, 24, 27, 28). Defects in these phenotypes are complemented with plasmid clones containing the flcA gene of A. brasilense Sp7, as previously shown with several other mutants and wild-type strains of Azospirillum (reference 27 and this work).
It is now clear that nonencapsulated mutants are defective in root surface colonization. But does such a defect affect the nitrogen fixation rates or the potential for nitrogen fixation? We show here that the mutants Sp72001 and -4 (flcA mutants), which colonize only the crevices and the sites of lateral root emergence, have nifH expression rates similar to that of the wild-type Sp7, which colonizes the root surface extensively and thus has larger numbers of its cells present. This may indicate a higher potential for nitrogenase activity per cell by the mutants than by the wild type. However, it is more likely that only those wild-type cells that colonize the crevices and not those that colonize the root surface express nifH strongly and that presenting the nifH activity per cell would most probably be misleading in this case. The observation that the wild-type strain Sp245 expressed nifH more strongly than its nonencysted mutants is not surprising, as this strain is known to colonize wheat roots more endophytically (2, 31); hence, the wild type enjoys a preferred environment for nifH expression. However, the reason for the low nifH expression by both Sp245 and its mutants on the shorter, modified roots following 2,4-D treatment is unclear. Could it be that the treatment with the synthetic auxin disturbed internal colonization by Sp245 along the roots? After all, 2,4-D significantly modifies the root surface tissues, where Sp245 was previously detected (31).
The exceptionally high expression of nifH by Sp7-S in association with 2,4-D-treated roots suggests genetic and phenotypic changes in this strain in addition to a mutation in flcA. The extra crevices in the para-nodulated roots possibly allow this smooth-surfaced strain to colonize the roots predominantly internally, where it is better protected from atmospheric oxygen. It has already been proven that this spontaneous mutant has at least one additional mutation, in swarming ability (27), although it is clearly shown here that this defect alone does not affect the nitrogenase activity of either A. brasilense Sp7 or Sp245 on wheat roots. More studies of the genetic bases for other possible mutations of Sp7-S, such as its phenotype of smaller colony size on NFB or minimal lactate agar (unpublished data), may clarify its unusual performance in nitrogen fixation on para-nodulated roots. The potential for nifH expression, although indicating the suitability of oxygen and available nitrogen levels for nitrogenase activity, should be clearly distinguished from the actual activity of the enzyme in the root-Azospirillum association. The nitrogenase enzyme requires adequate reductant and ATP for its activity (32). The greater nitrogenase activities (as shown by ARA) of the nonencysted mutants of Sp245 compared to that of the wild type are most probably due to the high supply of energy by the plant, which can increase the metabolism rates of the vegetative cells of the mutants (whereas the wild-type, cyst-like cells are metabolically dormant). Indeed, 2,4-D-treated plants contain higher levels of sugars in their roots, particularly glucose and fructose (L. Feng, L. Copeland, and I. R. Kennedy, unpublished data), as well as supporting enhanced nitrogenase activity by azospirilla (9, 36, 39) than do nontreated plants.
Wild-type Sp7 starts colonizing the roots only a few hours after inoculation (under laboratory conditions) and quickly forms cyst-like cells on the root surface. Although cyst-like forms of Azospirillum contain a high ribosome content at the early stages of differentiation and thus may be physiologically active (2), it was shown that they have increased resistance to environmental stress and exhibit very low nitrogenase activity (26). Remaining in vegetative phase may account for increased nitrogenase activity (acetylene reduction) by nonflocculating mutant strains on plants. Indeed, nitrogenase activity by Sp7-S carrying pAB1220-9, which restored colonization by cyst-like cells on the root surface (18, 27), is reduced to wild-type levels, suggesting that flcA is also indirectly influencing the rate of nitrogenase activity.
Azospirillum is well adapted to changes in its environment, as confirmed by the results of this study. When grown in liquid medium, it develops a single polar flagellum, allowing it to swim and exhibit chemotaxis, whereas on solid medium and in mucus (or semisolid medium) it develops additional lateral flagella for swarming motility. Possibly in this way it could conserve the energy involved in the production and operation of the lateral flagella when they are not needed. When conditions are not suitable for growth, Azospirillum differentiates into cyst-like forms lacking flagella and lowers its metabolism. It presumably remains in this relatively dormant form until conditions are suitable for growth. No doubt both features of the wild-type strain are important for its survival.
Nevertheless, nonencysted mutants of A. brasilense can still colonize crevices and points of emergence of lateral roots, as well as the basal zone of the modified lateral roots known as para-nodules and internal channels between cortical cells (20). These conditions are associated with increased nitrogenase activity in hydroponic systems (9, 10, 19, 36, 39). Remaining in vegetative phase, with lowered EPS, may also enhance the tendency towards endophytic colonization. This could arise as a result of a combination of several factors: (i) reduced stickiness of the cell surface, (ii) retention of cell motility by means of a polar flagellum, and (iii) continued cell division. The ease of producing spontaneous nonflocculating mutants otherwise similar to the wild type and the instability of this genetic trait in Azospirillum (reference 27 and this work) may reflect an adaptation of the bacteria to selective root colonization but with the capacity to revert to the cyst-forming genotype.
The fact that nonflocculating, nonencysted strains of Azospirillum are not efficient in root surface colonization but nevertheless show increased efficiency in nitrogenase activity calls into question the importance of having good plant root surface colonizers in the selection of strains for enhanced nitrogen-fixing ability. Indeed, this trait may even be detrimental to the capacity to establish colonies of azospirilla capable of nitrogen fixation with access to carbon substrates. The survival of nonencysted mutants in the soil could be affected by the inability to form stress-resistant cells. However, if seeds or seedlings can be effectively inoculated in growing field crops such as wheat, it may not be important for the bacteria to survive in the soil during the prolonged periods between crops. In this case, the development of inoculation techniques that allow sufficient exposure of the roots to Azospirillum strains would be necessary. Only testing these mutants in soil and under field conditions can verify this hypothesis.
ACKNOWLEDGMENTS
We thank Claudine Elmerich of the Institute Pasteur for the gift of the nifH-lacZ fusion and for providing helpful advice and Tony Romeo of the EMU at the University of Sydney for help with electron microscopy.
This project was supported by Australian GRDC and ARC research funds.
REFERENCES
- 1.Arsène F, Katupitiya S, Kennedy I R, Elmerich C. Use of lacZ fusions to study the expression of nif genes of Azospirillum brasilense in association with plants. Mol Plant-Microbe Interact. 1994;7:748–757. [Google Scholar]
- 2.Assmus B, Hutzler P, Kirchhof G, Amann R, Lawrence J R, Hartmann A. In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labeled, rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy. Appl Environ Microbiol. 1995;61:1013–1019. doi: 10.1128/aem.61.3.1013-1019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldani V L D, Baldani J I, Döbereiner J. Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat. Can J Microbiol. 1983;29:924–929. [Google Scholar]
- 4.Bashan Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv. 1998;16:729–770. [Google Scholar]
- 5.Bashan Y, Levanony H. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can J Microbiol. 1990;36:591–608. [Google Scholar]
- 6.Bastarrachea F, Zamudio M, Rivas R. Non-encapsulated mutants of Azospirillum brasilense and Azospirillum lipoferum. Can J Microbiol. 1988;34:24–29. [Google Scholar]
- 7.Berg R H, Tyler M E, Novick N J, Vasil V, Vasil I K. Biology of Azospirillum-sugarcane association: enhancement of nitrogenase activity. Appl Environ Microbiol. 1980;39:642–649. doi: 10.1128/aem.39.3.642-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleakley B H, Gaskins M H, Hubbell D H, Zam S G. Floc formation by Azospirillum lipoferum grown on poly-β-hydroxybutyrate. Appl Environ Microbiol. 1988;54:2986–2995. doi: 10.1128/aem.54.12.2986-2995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen-Weniger C. N2 fixation by ammonium-excreting Azospirillum brasilense in auxin-induced root tumours of wheat (Triticum aestivum L.) Biol Fert Soils. 1992;13:165–172. [Google Scholar]
- 10.Christiansen-Weniger C, Vanderleyden J. Ammonium-excreting Azospirillum sp. become intracellularly established in maize (Zea mays) para-nodules. Biol Fert Soils. 1994;17:1–8. [Google Scholar]
- 11.Croes C, Moens S, van Bastelaere E, Vanderleyden J, Michiels K. The polar flagellum mediates Azospirillum brasilense adsorption to wheat roots. J Gen Microbiol. 1993;139:2261–2269. [Google Scholar]
- 12.Deaker R, Kennedy I R. Proceedings of the 11th Australian Nitrogen Fixation Conference. Perth, Australia: University of Western Australia; 1996. The use of nifH-lacZ fusions in the detection of nitrogen fixation in associations between Azospirillum spp. and wheat; pp. 34–35. [Google Scholar]
- 13.Del Gallo M M, Negi M, Neyra C A. Calcofluor- and lectin-binding exocellular polysaccharides of Azospirillum brasilense and Azospirillum lipoferum. J Bacteriol. 1989;171:3504–3510. doi: 10.1128/jb.171.6.3504-3510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskew D L, Focht D D, Ting I P. Nitrogen fixation, denitrification and pleomorphic growth in a highly pigmented Spirillum lipoferum. Appl Environ Microbiol. 1977;34:582–585. doi: 10.1128/aem.34.5.582-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galimand M, Perroud B, Delorme F, Paquelin A, Vieille C, Bozouklian H, Elmerich C. Identification of DNA regions homologous to nitrogen fixation genes nifE, nifUS and fixABC in Azospirillum brasilense Sp7. J Gen Microbiol. 1989;135:1047–1059. doi: 10.1099/00221287-135-5-1047. [DOI] [PubMed] [Google Scholar]
- 16.Hall P G, Krieg N R. Swarming of Azospirillum brasilense on solid media. Can J Microbiol. 1983;29:1592–1594. [Google Scholar]
- 17.Jain D K, Patriquin D G. Root hair deformation, bacterial attachment, and plant growth in wheat-Azospirillum associations. Appl Environ Microbiol. 1984;48:1208–1213. doi: 10.1128/aem.48.6.1208-1213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katupitiya S, Millet J, Vesk M, Viccars L, Zeman A, Lidong Z, Elmerich C, Kennedy I R. A mutant of Azospirillum brasilense Sp7 impaired in flocculation with a modified colonization pattern and superior nitrogen fixation in association with wheat. Appl Environ Microbiol. 1995;61:1987–1995. doi: 10.1128/aem.61.5.1987-1995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy I R, Tchan Y T. Biological nitrogen fixation in non-leguminous field crops: recent advances. Plant Soil. 1992;141:93–118. [Google Scholar]
- 20.Kennedy I R, Pereg-Gerk L L, Wood C, Deaker R, Gilchrist K, Katupitiya S. Biological nitrogen fixation in non-leguminous field crops: facilitating the evolution of an effective association between Azospirillum and wheat. Plant Soil. 1997;194:65–79. [Google Scholar]
- 21.Lamm R B, Neyra C A. Characterization and cyst production of azospirilla isolated from selected grass growing in New Jersey and New York. Can J Microbiol. 1981;27:1320–1325. [Google Scholar]
- 22.Liang Y Y, Kaminski P A, Elmerich C. Identification of a nifA-like regulatory gene of Azospirillum brasilense Sp7 expressed under conditions of nitrogen fixation and in the presence of air and ammonia. Mol Microbiol. 1991;5:2735–2744. doi: 10.1111/j.1365-2958.1991.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 23.Michiels K, Croes C L, Vanderleyden J. Two different modes of attachment of Azospirillum brasilense Sp7 to wheat roots. J Gen Microbiol. 1991;137:2241–2246. [Google Scholar]
- 24.Michiels K, Verreth C, Vanderleyden J. Azospirillum lipoferum and Azospirillum brasilense surface polysaccharide mutants that are affected in flocculation. J Appl Bacteriol. 1990;69:705–711. [Google Scholar]
- 25.Okon Y, Labandera-Gonzalez C A. Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol Biochem. 1994;26:1591–1601. [Google Scholar]
- 26.Papen H, Werner D. Organic acid utilization, succinate excretion, encystation and oscillating nitrogenase activity in Azospirillum brasilense under microaerobic conditions. Arch Microbiol. 1982;132:57–61. [Google Scholar]
- 27.Pereg Gerk L, Paquelin A, Gounon P, Kennedy I R, Elmerich C. A transcriptional regulator of the LuxR-UhpA family, FlcA, controls flocculation and wheat root surface colonization by A. brasilense Sp7. Mol Plant-Microbe Interact. 1998;11:177–187. doi: 10.1094/MPMI.1998.11.3.177. [DOI] [PubMed] [Google Scholar]
- 28.Sadasivan L, Neyra C A. Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J Bacteriol. 1985;163:716–723. doi: 10.1128/jb.163.2.716-723.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadasivan L, Neyra C A. Cysts of Azospirilla under various cultural conditions. In: Klingmüller W, editor. Azospirillum. 1985. pp. 230–242. III. Genetics, physiology, ecology. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 30.Sadasivan L, Neyra C A. Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145. J Bacteriol. 1987;169:1670–1677. doi: 10.1128/jb.169.4.1670-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schloter M, Kirchhof G, Hienzmann U, Doebereiner J, Hartmann A. Proceedings of the 6th International Symposium on Nitrogen Fixation with Non-Legumes. Cairo, Egypt: The American University in Cairo Press; 1994. Immunological studies of the wheat-root-colonization by the Azospirillum brasilense strains Sp7 and Sp245 using strain-specific monoclonal antibodies; pp. 291–297. [Google Scholar]
- 32.Schubert K R. Products of biological nitrogen fixation in higher plants: synthesis, transport, and metabolism. Annu Rev Plant Physiol. 1986;37:539–574. [Google Scholar]
- 33.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 34.Sriskandarajah S, Kennedy I R, Yu D, Tchan Y T. Effects of plant growth regulators on acetylene-reducing associations between Azospirillum brasilense and wheat. Plant Soil. 1993;153:165–178. [Google Scholar]
- 35.Tarrand J J, Krieg N R, Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov., and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978;24:967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- 36.Tchan Y T, Zeman A M M, Kennedy I R. Nitrogen fixation in para-nodules of wheat roots by introduced free-living diazotrophs. Plant Soil. 1991;137:43–47. [Google Scholar]
- 37.Vande Broek A, Michiels J, Van Gool A, Vanderleyden J. Spatial-temporal colonization patterns of Azospirillum brasilense on the wheat root surface and expression of the bacterial nifH gene during association. Mol Plant-Microbe Interact. 1993;6:592–600. [Google Scholar]
- 38.Wood P J. Specificity in the interaction of direct dyes with polysaccharides. Carbohydr Res. 1980;85:271–287. [Google Scholar]
- 39.Zeman A M M, Tchan Y T, Elmerich C, Kennedy I R. Nitrogenase activity in wheat seedlings bearing para-nodules induced by 2,4-dichlorophenoxyacetic acid (2,4-D) and inoculated with Azospirillum. Res Microbiol. 1992;143:847–855. doi: 10.1016/0923-2508(92)90072-v. [DOI] [PubMed] [Google Scholar]