Abstract
The ability of lactococcal strains to lyse (and release intracellular enzymes) during cheese manufacture can be a very desirable trait and has been associated with improvement in flavor and acceleration of cheese ripening. Using a laboratory-scale cheese manufacturing assay, the autolytic behavior of 31 strains of Lactococcus lactis was assessed. In general, marked variation was observed between strains with a 20-fold difference between the best and worst lysing strains based on the release of the intracellular enzyme lactate dehydrogenase. In a parallel experiment, the genomes of these strains were examined for the presence of prophage integrase (int) sequences by using conserved primer sequences from known lysogenic phage. Results demonstrated that the lytic behavior of lactococcal starter strains significantly correlates with the presence of prophage sequences. These results highlight not only the contribution of prophage to starter cell lysis but also the potential of PCR as a useful initial screen to assess strains for this important industrial trait.
Strains of the lactic acid starter bacterium Lactococcus lactis have been used for millennia in the manufacture of a variety of fermented foods, including Cheddar cheese (19). In general, the level of lactococci in cheese declines during manufacturing and ripening, due to lysis with concomitant release of the lactococcal intracellular enzymes, including peptidases, into the cheese matrix (7, 31, 38). It is recognized that after autolysis, these intracellular peptidases act by degrading casein-derived peptides in cheese, contributing to the desirable flavor characteristics in the final cheese product (22, 35). Indeed, various studies demonstrate the association of starter cell lysis with increased proteolysis and/or flavor development (8, 23, 39). As a result, a number of surveys comparing autolysis of lactococcal strains have been conducted (8, 16, 26, 34) and the autolytic systems of several strains have been characterized (5, 27, 29). It is noteworthy that alternative methods to accelerate ripening are available, and these include the addition of exogenous proteinase or peptidase preparations to the curd at the salting stage (17, 37) or the exploitation of bacteriocin-producing starter strains to mediate targeted lysis of individual starter strains (24).
Various factors, such as pH, temperature, carbon source, and salt concentration, appear to be important for the autolytic process (29). The degree of autolysis is strain dependent, and the structure and components of the cell wall are important contributory factors (25). Importantly, the possession of one or several peptidoglycan hydrolases which are associated with cell wall peptidoglycan cleavage plays a major role (3, 5, 19, 28). These cell wall hydrolases, also known as autolysins, play an important role in cellular processes such as cell wall turnover, cell division, and, in some genera, competence for genetic transformation (30). L. lactis expresses one major autolysin, AcmA, which is responsible for cell division and autolysis during the stationary phase of growth (3, 4). A related group of cell wall hydrolytic enzymes, known as lysins (amidases or muramidases), are encoded by lactococcal bacteriophages and are synthesized during the lytic stage of the bacteriophage growth cycle (9). Located immediately upstream of the lysin gene on the characterized lactococcal temperate bacteriophage genomes is the holin gene, which is responsible for production of the membrane-disruptive holin protein (9, 14). This protein works in tandem with the lysin protein by generating holes in the cytoplasmic membrane of cells, allowing lysin to reach the cell wall peptidoglycan.
The involvement of temperate bacteriophage in cell lysis was first proposed by Feirtag and McKay (11), who demonstrated that lysis could be induced either by UV radiation, by exposure to mitomycin C, or by a temperature shift from 30 to 40°C. These authors proposed that the cooking temperatures used in Cheddar cheese manufacture (38 to 40°C) could therefore act as an environmental stimulus to induce temperate phage excision, causing cell lysis in the cheese curd. Based on this study, it was suggested that lysogenic lactococcal strains be used as starter cultures to enhance lysis of starter cells during cheese ripening. Additionally, investigations performed by Wiederholt and Steele (36) on the thermoinducibility of prophage from L. lactis SK11 and US3 describe the isolation of a prophage from both strains as a result of mitomycin C induction. However, the respective prophage did not appear to be responsible for the thermolytic response, suggesting the presence of a second prophage. Recent studies of the strain L. lactis AM2 support the theory of prophage involvement in starter cell lysis (18, 19). Following mitomycin C induction, Lepeuple et al. (18) successfully isolated a prophage which similarly was found not to be responsible for temperature sensitivity of the strain. They suggested that the induction of prophage during cheese making probably results from the effect of a technological factor other than temperature. They later associated a lytic enzyme encoded by prophage DNA with the early lysis of bacteria in cheese (19). It is important to point out the distinction between this beneficial role of temperate bacteriophages as mediators of desirable enzyme release and the undesirable role that lytic bacteriophages may play in the inhibition of starter cultures, a topic which has been extensively researched in these and other laboratories during the past 2 decades (1, 12, 28).
While autolysis of starter cultures may undoubtedly be affected by a number of factors including temperature, AcmA activity, bacteriocins, etc., in some cases it is evident that prophage can contribute to starter cell lysis. For this reason, the present study explores the relationship between lysogeny and cell lysis in dairy starter cultures. We investigated the efficacy of using the int gene, which is highly conserved among temperate lactococcal bacteriophage genomes (10), to detect lysogenic lactococcal strains. Using this approach, we assessed the genomes of a bank of 31 cheese-making strains of L. lactis for the presence of prophage integrase gene sequences by PCR. In parallel, the lytic behavior of these strains was studied by monitoring the optical densities of broth cultures subjected to temperature shift and also by assaying intracellular lactate dehydrogenase (LDH) enzyme levels in cheese curds. The results obtained demonstrate a significant correlation between the presence of int genes and cell lysis.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used in this study are held in the DPC culture collection and are listed in Table 1. Lactococcal strains were routinely propagated at 30°C in M17 medium (Difco Laboratories, Detroit, Mich.), in M17 medium supplemented with 0.5% lactose (LM17), or in 10% reconstituted skim milk. A 1.5% inoculum of all lactococcal strains used was incubated at 21°C overnight in pasteurized whole milk for mini-cheese trials. Solid media contained 1.0% agar (Oxoid, Basingstokes, Hampshire, United Kingdom). All strains were stocked in LM17 containing 40% glycerol and stored at −80°C. Working cultures were stored at 4°C and transferred periodically.
TABLE 1.
Bacterial strains used in this studya
| L. lactis strain | PCR detection of prophage int | LDH in cheese curd | ODD in broth |
|---|---|---|---|
| AM1 | + | + | + |
| AM2 | + | + | + |
| R1 | + | + | + |
| Z8 | + | + | + |
| US3 | + | + | + |
| KHNZ | + | + | − |
| DPC4270 | + | + | − |
| DPC4269 | + | + | + |
| DPC4271 | + | + | − |
| SK11G | + | + | + |
| HP | + | + | + |
| 077 | + | + | + |
| C13B | + | + | + |
| 290P | − | − | − |
| 310 | + | − | + |
| UC317 | − | − | − |
| 938 | − | + | + |
| SK1 | − | − | − |
| BA1 | − | + | − |
| BA2 | − | − | − |
| 057 | + | + | + |
| DPC4987 | − | + | − |
| 320 | − | − | − |
| 047 | + | + | + |
| DRC3 | − | − | − |
| DPC4268 | + | − | − |
| ML8 | + | + | − |
| 712 | − | − | − |
| DPC452 | − | − | − |
| E8 | − | + | − |
| 007 | + | + | + |
The following criteria were used to assess these strains for lysis: the presence of a prophage integrase (int) gene using PCR primers specific for the lactococcal int gene (+ designates the successful amplification of a 1.0-kb PCR fragment; − indicates no amplification); LDH activity in cheese curd (+ designates LDH scores in excess of the threshold level of 0.05 arbitrary units/ml; − indicates values below the threshold); and ODD of broth cultures (+ indicates a decline in optical density following temperature increase to 39°C; − indicates no decline).
Integrase sequence acquisition and primer design.
All previously identified (available) sequences for temperate lactococcal bacteriophage integrase (int) genes were retrieved from GenBank. These sequences included int genes from the following bacteriophage (numbers in parentheses are reference numbers and database accession numbers, respectively): Tuc2009 (33) (AAA32608), Lc3 (21) (A47085), r1T (34) (AAB18676), and BK5-T (3) (AAA98586). ClustalW alignments of these sequences were performed with DNAStar software (DNAStar Inc., Madison, Wisc.). Degenerate oligonucleotide primers for PCR were designed on the basis of conserved regions within all four integrase determinants. The primers 5′ AAGGACTTCCTCGTCTAAC 3′ and 5′TGCTGTGTGATAGCCACAC 3′ were selected so as to achieve an amplified intergenic product of approximately 1.0 kb.
Chromosomal DNA isolation.
Genomic DNA was isolated from 1.5-ml aliquots of overnight LM17 broth cultures by using a modification of the method of Hoffman and Winston (15). This procedure involves shearing with glass beads to lyse the cells and was modified as outlined by Coakley et al. (6). Following DNA isolation, its concentration was measured spectrophotometrically and was standardized to ensure that all samples had similar template concentrations.
PCR analysis.
Lactococcal strains were assessed for prophage integrase sequences (int) by PCR using int-specific primers. PCR amplifications were performed in a total volume of 50 μl in a Hybaid PCR Express Unit (Hybaid Ltd., Middlesex, United Kingdom). The method was employed essentially as described by Coakley et al. (6) and used 1 μl of each of the int-specific primers (50 pmol/μl) and 1 μl of template DNA (0.5 μg/ml) isolated and standardized by the procedure described above. To determine the reproducibility of the method, the DNA extraction and PCR analyses were performed in triplicate.
Mini-cheese manufacture.
Laboratory-scale cheese trials were performed using 2 liters of pasteurized whole milk, pre-incubated at 32°C for 30 min. A 1.5% inoculum (30 ml) was added and stirred for 35 min until a pH of <6.6 had been achieved. Renneting was performed at 32°C using Chymax rennet (Hansens, Little Island, Cork, Ireland) added at a concentration of 0.077 ml/liter. This was allowed to set for approximately 40 min, after which the curd was cut using a sharp knife to a curd particle size of approximately 1 cm3. Following this, the curd was cooked at 39°C. The whey was drained when the pH had reached 6.1. The pH of the curd was monitored until it reached 5.2, and thereafter it was placed in 500-ml plastic cylindrical cheese molds with draining pores 3 mm in diameter (catalog no. 3404m; Smallholding Supplies, Pikes Farmhouse, Somerset, United Kingdom) for overnight pressing at 4°C. Triplicate curd samples were then centrifuged at 50,000 rpm for 30 min at 4°C using a Sorvall OTD65B Ultracentrifuge. Curd juice was then assayed for LDH activity, and the average LDH measurement of the triplicate samples was determined.
Measurement of intracellular enzyme release in cheese curd.
Levels of LDH, an intracellular marker enzyme used to measure cell lysis, were determined using a modified version of the method outlined by Wittenberger and Angelo (40), which measures the decrease in absorbance at 340 nm resulting from the pyruvate-dependent oxidation of NADH. Activity is expressed as units per milliliter of juice, where 1 U is the amount of enzyme that catalyzes the oxidation of 1 μmol of NADH per min per ml.
Optical density measurements.
Growth characteristics of the lactococcal strains in 12 ml of LM17 broth were determined spectrophotometrically by measuring the absorbance at 600 nm at 30°C over an 8-h period. The effect of temperature on the induction of prophage was examined by shifting the growth temperature of strains in the mid-exponential phase of growth (optical density at 600 nm [OD600] = 0.35) to 39°C for up to 4 h.
CSLM.
In situ confocal scanning laser microscopy (CSLM) observations and viability staining of bacterial cells in Cheddar cheese curds were performed by the method of Auty et al. (unpublished data), using a Zeiss LSM310 confocal scanning laser microscope. Sections of cheese 5 by 5 by 2 mm were cut with a fresh scalpel blade and placed on a microscope slide. Seventy-five microliters of LIVE/DEAD Baclight stain was added to the freshly cut cheese surface, and a coverslip was placed on top. CSLM images were obtained ∼10 μm below the level of the coverslip after 20 min of incubation in the dark at room temperature.
Statistical analysis.
Mann-Whitney U tests were conducted to investigate if LDH values differ between strains positive and negative for the int gene, as assessed by PCR, and between strains that exhibited and did not exhibit optical density decline (ODD+ and ODD− strains, respectively). This test is based on ranks, and thus all descriptives provided are in terms of ranks, not in terms of the original values. That is, the LDH values were ranked (1 representing lowest value) ignoring group membership, and all analyses were performed using these ranks (the ranked LDH values). The average rank associated with each group is presented. Box plots and 95% confidence intervals for each group (positive and negative) for int sequences were obtained. Similar plots for ODD were obtained. A chi-square test was conducted to compare the proportion of ODD+ strains between groups with and without int.
RESULTS AND DISCUSSION
The overall objective of this work was to assess a bank of 31 cheese-making lactococcal strains for autolytic behavior. These included strains for which some evidence of autolysis exists, some strains that have been traditionally used in the Irish dairy industry, and a number of commercially used cheese starter strains. To estimate the lytic potential of these lactococcal strains, separate approaches were adopted in parallel. These included monitoring the ODD associated with temperature shift, measurement of intracellular enzyme release during simulated cheese manufacture, and the use of PCR to detect the presence of prophage-related integrase sequences.
Optical density measurements.
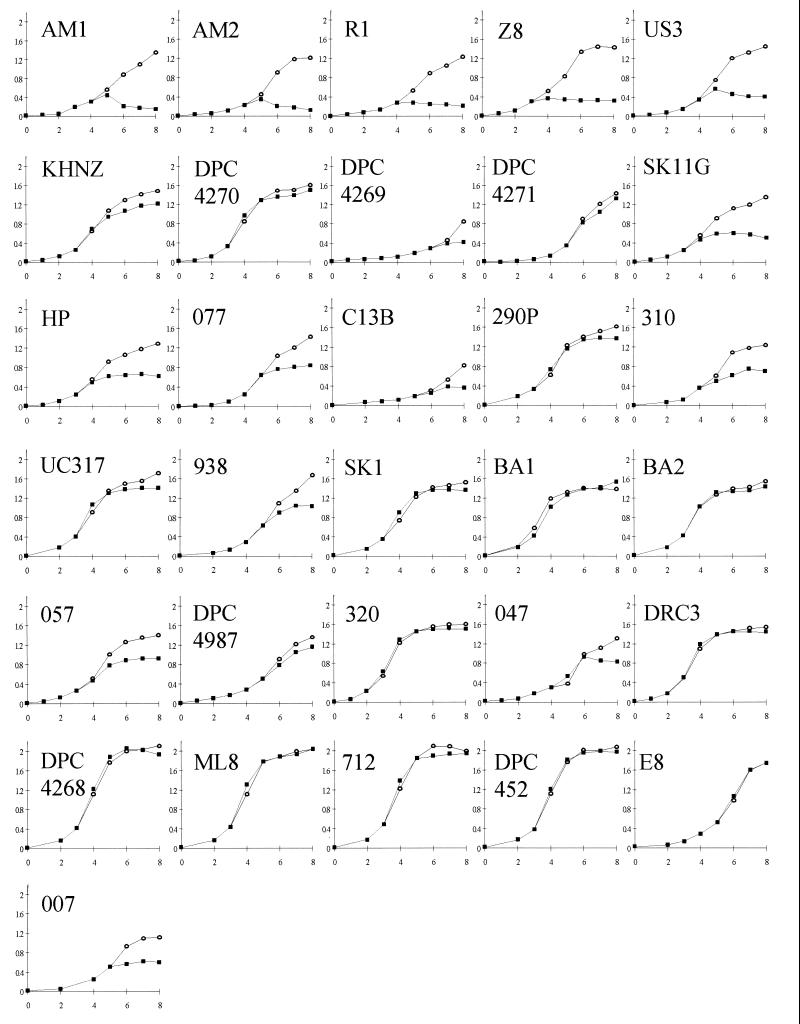
Initially, lysis among the strains was investigated by monitoring the optical densities of broth cultures, in an approach similar to that used in a number of previous studies (11, 29, 34). However, in contrast to previous investigations, this study employed a modified cheese temperature profile, whereby the strains were all allowed to achieve equal biomass (OD600 = 0.35) before being subjected to a temperature shift to 39°C (mimicking the cooking stage in industrial Cheddar cheese manufacture) for up to 4 h. Growth characteristics of the 31 lactococcal strains in LM17 broth were monitored by measuring the absorbance at 600 nm at 30°C over an 8-h period (Fig. 1). The observation of a significant reduction in the optical density for an individual strain subjected to 39°C heat treatment, in comparison to the optical density of its control (which remained incubated at 30°C) was used to group these strains either as thermoinducible or not thermoinducible. Using this approach, 15 of the 31 (48%) lactococcal strains produced an ODD when exposed to 39°C heat treatment and were assigned to the thermoinducible lysis grouping. In some instances it is easy to visualize the distinct lytic effect (e.g., in L. lactis AM1, AM2, R1, US3, and SK11G). However, a number of strains showed less evidence of lysis (e.g., L. lactis 310 and 938), and it is possible in these cases that temperature sensitivity rather than prophage induction was the direct cause for cessation of growth. The remaining 16 (52%) of lactococcal strains showed little or no reduction in biomass when subjected to 39°C heat treatment, indicating that these strains did not encode a thermoinducible lytic system.
FIG. 1.
OD600 values of broth cultures of 31 cheese-making lactococcal strains over a modified cheese temperature profile. Strain designations are indicated on each graph. Symbols: ○, 8 h at 30°C; ■, strain incubated at 30°C until OD600 reached 0.35 and subsequently incubated at 39°C.
Mini-cheese manufacture and measurement of LDH release in cheese curd.
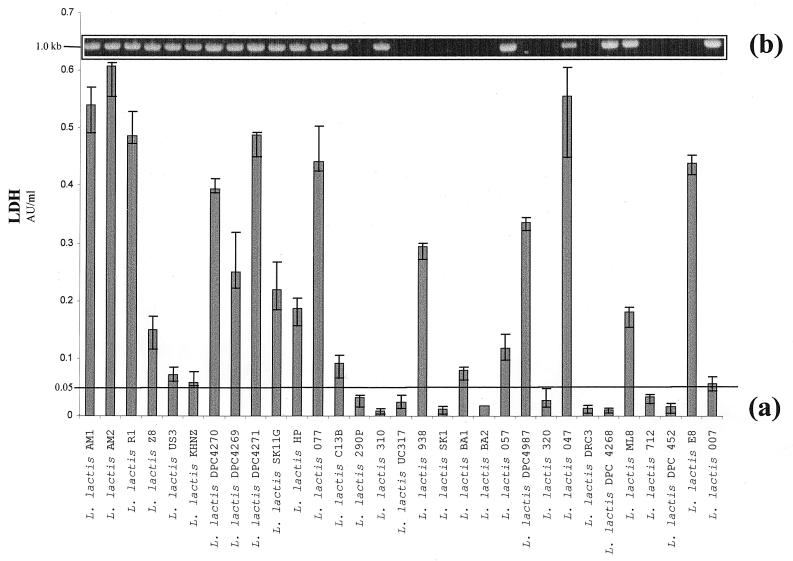
Since it is well established that autolysis of L. lactis strains makes such an important contribution to cheese flavor (7, 39), it was pertinent to evaluate the lytic potential of individual strains in the cheese environment. It was more relevant to assess LDH release in cheese curd, rather than in synthetic medium, since the former is the environment where cell lysis is industrially relevant. A mini-cheese manufacture method for the production of laboratory-scale Cheddar cheese curds was designed, and accordingly, 31 individual laboratory-scale cheeses were made. Yields varied from 150 to 250 g (final curd weight). LDH activity, which is known to be very stable in a cheese environment (13, 39), was determined for the 31 cheese juice samples. Overall, strains exhibited a marked variability with regard to LDH release during cheese manufacture (Fig. 2a). For example, the strain L. lactis AM2 yielded up to 20-fold more enzyme release than strain L. lactis 320 during cheese manufacture. In general, those strains which exhibited significant lysis during temperature shift in broth, such as L. lactis AM1, R1, and SK11G, also gave significantly high LDH release. One of the exceptions to this is L. lactis 310. For this strain the optical density decreased after 39°C heat treatment, indicating thermoinducible lysis. Surprisingly, however, this strain exhibited a low level of LDH following cooking in the cheese. It was considered that this could be due to a low level of LDH production by this strain, although previous studies indicated that little variation exists in the specific activity and kinetics of intracellular LDH within lactococcal strains (13, 39). An LDH activity of 0.05 U/ml was selected as the threshold level for a lytic strain, on the basis of L. lactis US3, which achieved a lower-order LDH score but is cited in the literature as an autolytic strain (11, 36). Using this criterion, 21 of the 31 (68%) cheese juice samples assessed fell into the high lysis category, leaving 10 (32%) of the cheese juice samples falling beneath the threshold mark and assigned to the nonlytic grouping. In addition, a representative strain from each category (L. lactis AM1 from the high lysis group and L. lactis 320 from the nonlytic group) was assessed for LDH release at defined time points (2, 4, and 6 h) during the cheese manufacturing process (data not shown). These experiments provided information on the viability of the starter and the degree of lysis and concomitant LDH release at different stages during cheese manufacture. The representative lytic strain L. lactis AM1 began to lyse and release LDH into the curd approximately 4 h into the cheese manufacturing schedule. In contrast, no significant LDH activity was detected at any stage during the manufacture of cheese with the nonlytic starter strain L. lactis 320. Given that a number of researchers have reported higher levels of free amino acids and different peptide profiles in extracts of cheese made with highly lytic starters (39), the inclusion of strains such as L. lactis AM1 in starter strain systems may prove beneficial in terms of improved cheese flavor and accelerated cheese ripening.
FIG. 2.
(a) Bar chart indicating LDH concentrations in cheese curd following Cheddar cheese manufacture with 31 strains of L. lactis. Strain designations are indicated. AU, arbitrary units. (b) Agarose gel electrophoresis of PCR products from the same 31 cheese-making lactococci with int-specific oligonucleotide primers. Primers used for the detection of prophage integrase genes are based on consensus sequences within the integrase determinants. A 1.0-kb PCR product indicates the presence of prophage integrase determinants.
Primer design and PCR detection of lactococcal prophage integrase sequences.
A number of studies have linked cell lysis to lysogenic phage. In this report we explored the prevalence of prophage DNA among a range of lactococcal cheese-making strains. Since the int gene is highly conserved among bacteriophage genomes (10), oligonucleotide primers for PCR were designed on the basis of conserved regions within four integrase determinants. Using these int-specific primers, a sharp and distinct 1.0-kb amplification product was achieved for 19 of the 31 (61%) strains assessed (Fig. 2b), indicating the presence of a homologous int gene. It is interesting that most of the strains which yielded a positive result by the first two approaches also generated a fragment consistent in size with that of an internal fragment of the int gene. The advantages of the PCR approach are the high throughput of samples (over 30 strains tested in a single assessment), relative speed of assessment, and ease of use. An obvious limitation is that only one factor contributing to cell lysis in cheese, namely, lysogeny, is assessed by this approach. Furthermore, the PCR primers employed in this assessment will only detect a bacteriophage integrase with a specific conserved sequence. It is conceivable that additional primers designed to detect other conserved regions of temperate lactococcal bacteriophage could further extend the accuracy of this method for assessing the capacity for prophage-associated lysis. As more-complete temperate bacteriophage sequences become available, it may be possible to design more-expansive primers, permitting more-comprehensive characterization of lactococcal strains.
CSLM to assess autolysis.
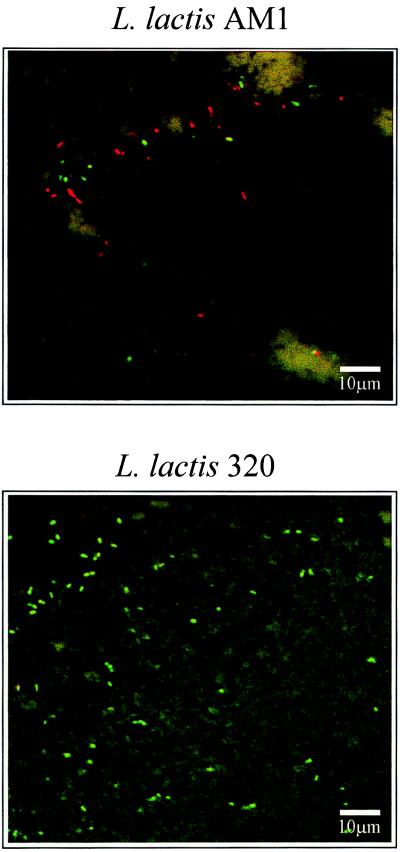
The potential of CSLM as a method to study autolysis was also examined. Selected Cheddar cheese curds were pressed overnight at 7°C in plastic molds and sent for in situ CSLM observation. The viability of starter cultures was assessed using a LIVE/DEAD Baclight stain, which was devised specifically to differentiate between live and dead bacterial cells based on plasma membrane permeability (Auty et al., unpublished data). A classical lytic and nonlytic situation is presented in Fig. 3, with results for the L. lactis strain AM1, a known autolytic strain examined by many groups previously (11, 21, 35), and the nonlytic strain L. lactis 320. A CSLM image taken from a section of curd manufactured with L. lactis AM1 after overnight pressing shows that a high proportion of cells stain red (indicating the majority of cells are nonviable). In agreement with this, it was found that L. lactis AM1 undergoes thermoinducible lysis with a concomitant high-level release of intracellular LDH when subjected to cooking temperature conditions. LDH release from L. lactis AM1 cells monitored during the cheese manufacturing process indicates that increased LDH activity correlates with a decline in cell viability. PCR analysis also indicated that this strain possessed an integrase gene. In contrast, the CSLM image taken from a section of curd manufactured with L. lactis 320 illustrates high numbers of viable (i.e., the membrane is intact) cells that stain green as shown by CSLM observation. This result was supported by PCR analysis of L. lactis 320, which indicated the absence of integrase determinants in the genome of this strain. Furthermore, when subjected to cooking temperature conditions, this strain showed a negligible reduction in biomass, while low-level intracellular enzyme activity was detected in the cheese curd during the manufacturing process. These results highlight the potential use of CSLM as an indicator of bacterial viability in the cheese curd, which indirectly allows a measure of the autolytic potential of starter cultures.
FIG. 3.
CLSM analysis of cheese curd for the lytic strain L. lactis AM1 (top) and nonlytic L. lactis 320 (bottom).
Statistical analysis.
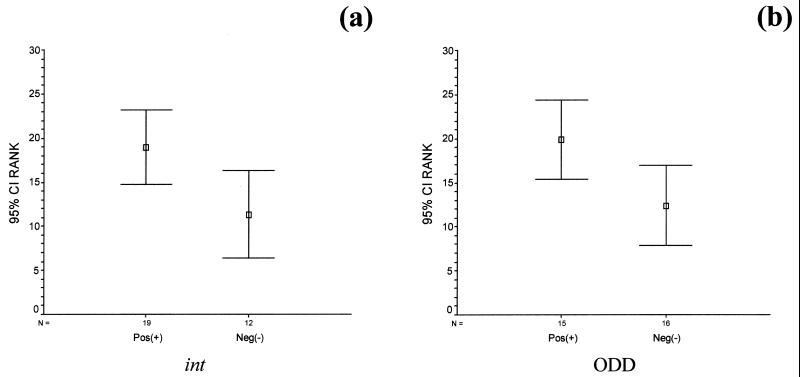
The lytic behavior of the 31 lactococcal strains assessed according to three different criteria is summarized in Table 1. Each criterion was compared to the others and analyzed statistically. It was found that the int results and LDH results were in agreement for 81% of the strains analyzed. The LDH levels in cheese juice were found to be significantly greater for strains possessing int compared to those lacking this gene (Fig. 4a). This is confirmed by the Mann-Whitney U test, which revealed significant differences in LDH values between int-possessing and -lacking groups (U = 58; P < 0.05). The average ranks (ranked LDH values) for int-possessing and -lacking strains are 19.0 and 11.3, respectively. From a statistical viewpoint, it is highly significant that the strains found positive for possession of integrase sequences by PCR tend to give higher-order LDH scores (LDH+).
FIG. 4.
Statistical plots illustrating the 95% confidence interval (CI) for the average rank (ranked LDH values) for each group of int (a) and ODD (b).
A similar profile emerges when LDH values are used to discriminate on the basis of ODD results (Fig. 4b). A Mann-Whitney U test revealed significant differences in LDH values between ODD+ and ODD− groups (U = 62; P < 0.05), with ODD+ strains have significantly higher LDH values than ODD− strains. The average ranks for ODD+ and ODD− strains are 19.9 and 12.4, respectively.
ODD results and int results correlated for 81% of the strains, and the proportion of ODD+ strains was found to be significantly different for int-possessing and int-lacking strains (χ2 = 10.1; df = 12; P = 0.001). int-possessing strains had significantly more ODD+ (74% versus 8% for int-lacking strains). Agreement across all three fields was achieved in 68% of the cases. A selection of strains analyzed deviated from the trend. These included DPC4268, which had the int gene but showed no lytic responses otherwise. A possible explanation for this may be the presence of integrase determinants in remnants of temperate phage genomes which have lost their biological activity during evolution. Alternatively, the prophage may be viable but does not mediate lysis under cheese-making conditions. The strains L. lactis KHNZ, DPC4270, DPC4271, and ML8 all possessed int and were all LDH+ but show no evidence for phage-induced lysis following temperature shift from 30 to 39°C in broth. It is possible with these four strains that temperate phage are not induced by temperature and that some other factor, most probably autolysin activation, caused the lysis during cheese curd manufacture. Indeed, autolysin activation is the most-plausible explanation for the behavior of strains L. lactis E8, DPC4987, and BA1, which lack int and are ODD− yet give higher-order LDH release. However, the possibility that these three lactococcal strains possess a phage with a different integrase gene cannot be overlooked. Again, the development and application of new PCR primers designed to identify other conserved regions of temperate lactococcal bacteriophage would be of considerable benefit for the characterization of these deviant lactococcal strains.
Conclusions.
There are a number of contributory factors for lytic behavior in lactococcal bacteria. Results from this study indicate that an overriding feature in lactococcal strains is the apparent presence of prophage DNA in lytic strains. The PCR strategy devised for the detection of prophage integrase sequences may prove to be a very useful tool for the initial screening of lactococcal cheese starter strains for lytic potential. Over the course of this study we utilized the monitoring of optical density readings of broth cultures, which is a method traditionally employed to assess the level of lysis among lactococcal strains. We also developed the mini-cheese manufacture method specifically for this study, and this represents a new screening approach which negates the need to make pilot-scale cheeses for each test starter strain. The potential of CSLM as an indicator of bacterial viability in the cheese curd was examined, and this may prove to be a valuable technique for indirect measurement of the autolytic capability of starter cultures. The advantages of the PCR approach are the high throughput of samples, relative speed of assessment, clarity of results, and ease of use. The detection of statistically significant, but not absolute, correlation between this approach and the traditional mechanisms for gauging lytic activity is an important finding, but perhaps more interesting are the outliers that do not fit the model. Nevertheless, the PCR approach provides a useful tool to be used in combination with the other more traditional methods for assessing cell lysis.
ACKNOWLEDGMENTS
This research has been partly funded by grant aid under the Food Sub-Programme of the Operational Programme for Industrial Development which is administered by the Department of Agriculture, Food and Forestry, and supported by national and European Union funds. D.O.S was supported by a Teagasc Walsh fellowship.
We thank Mark Auty for CSLM analysis, Kathleen O'Sullivan (University College, Cork) for advice on statistical analysis, and Denis Twomey and Martin Wilkinson for helpful discussions.
REFERENCES
- 1.Allison G E, Klaenhammer T R. Phage resistance mechanisms in lactic acid bacteria. Int Dairy J. 1998;8:207–226. [Google Scholar]
- 2.Boyce J D, Davidson B E, Hillier A J. Sequence analysis of the Lactococcus lactis bacteriophage BK5-T and demonstration that phage DNA has cohesive ends. Appl Environ Microbiol. 1995;61:4089–4098. doi: 10.1128/aem.61.11.4089-4098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buist G, Kok J, Leenhouts K J, Dabrowska M, Venema G, Haandrikman A J. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;6:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buist G, Karsens H, Nauta A, Van Sinderen D, Venema G, Kok J. Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl Environ Microbiol. 1997;63:2722–2728. doi: 10.1128/aem.63.7.2722-2728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapot-Chartier M P, Deniel C, Rousseau M, Vassel L, Gripon J C. Autolysis of two strains of Lactococcus lactis during cheese ripening. Int Dairy J. 1994;4:251–269. [Google Scholar]
- 6.Coakley M, Ross R P, Donnelly D. Application of the polymerase chain reaction to the rapid analysis of brewery yeast strains. J Inst Brew. 1996;102:349–354. [Google Scholar]
- 7.Crow V L, Coolbear T, Gopal P K, Martley F G, McKay L L, Riepe H R. The role of autolysis of lactic acid bacteria in the ripening of cheese. Int Dairy J. 1995;5:855–875. [Google Scholar]
- 8.Crow V L, Martley F G, Coolbear T, Roundhill S J. The influence of phage-assisted lysis of Lactococcus lactis subsp. lactis ML8 on Cheddar cheese ripening. Int Dairy J. 1995;5:451–472. [Google Scholar]
- 9.De Ruyter P G G A, Kuipers O P, Meijer W C, de Vos W M. Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat Biotechnol. 1997;15:976–979. doi: 10.1038/nbt1097-976. [DOI] [PubMed] [Google Scholar]
- 10.Esposito D, Scocca J J. The integrase family of tyrosine recombinases: evolution of a conserved active site domain. Nucleic Acids Res. 1997;25:3605–3614. doi: 10.1093/nar/25.18.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feirtag J M, McKay L L. Thermoinducible lysis of temperature-sensitive Streptococcus cremoris strains. J Dairy Sci. 1987;70:1779–1784. [Google Scholar]
- 12.Forde A, Fitzgerald G F. Bacteriophage defence systems in lactic acid bacteria. Antonie Leeuwenhoek. 1999;76:89–113. [PubMed] [Google Scholar]
- 13.Garvie E L. Bacterial lactate dehydrogenases. Microbiol Rev. 1980;44:106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasson M J. Lytic systems in lactic acid bacteria and their bacteriophages. Antonie Leeuwenhoek. 1996;70:147–159. doi: 10.1007/BF00395931. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman C S, Winston F. A ten minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 16.Law B A, Castanon M J, Sharpe M E. The contribution of starter streptococci to flavour development in Cheddar cheese. J Dairy Res. 1975;8:667–674. [Google Scholar]
- 17.Law B A. Annual Report to the Food Research Institute, Reading, United Kingdom. Reading, United Kingdom: Food Research Council; 1986. The accelerated ripening of cheese; pp. 111–132. [Google Scholar]
- 18.Lepeuple A-S, Vassal L, Cesselin B, Delacroix-Buchet A, Gripon J-C, Chapot-Chartier M-P. Involvement of a prophage in the lysis of Lactococcus lactis subsp. cremoris AM2 during cheese ripening. Int Dairy J. 1998;43:301–311. [Google Scholar]
- 19.Lepeuple A-S, Van Gemert E, Chapot-Chartier M-P. Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identification of a prophage-encoded enzyme. Appl Environ Microbiol. 1998;64:4142–4148. doi: 10.1128/aem.64.11.4142-4148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lillehaug D, Birkeland N K. Characterization of genetic elements required for site-specific integration of the temperate lactococcal bacteriophage φ LC3 and construction of the integration-negative φ LC3 mutants. J Bacteriol. 1993;175:1745–1755. doi: 10.1128/jb.175.6.1745-1755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martley F G, Lawrence R C. Cheddar cheese flavour. II. Characteristics of single strain starters associated with good or poor flavour developement. N Z J Dairy Res. 1972;7:38–53. [Google Scholar]
- 22.Meijer W, Van De Bunt B, Twigt M, De Jonge B, Smit G, Hugenholtz J. Lysis of Lactococcus lactis subsp. cremoris SK110 and its nisin-immune transconjugant in relation to flavor development in cheese. Appl Environ Microbiol. 1998;64:1950–1953. doi: 10.1128/aem.64.5.1950-1953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan S, Ross R P, Hill C. Bacteriolytic activity caused by the presence of a novel lactococcal plasmid encoding lactococcins A, B, and M. Appl Environ Microbiol. 1995;61:2995–3001. doi: 10.1128/aem.61.8.2995-3001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan S, Ross R P, Hill C. Increasing starter cell lysis in Cheddar cheese using a bacteriocin-producing adjunct. J Dairy Res. 1996;80:1–10. [Google Scholar]
- 25.Mou L, Sullivan J J, Fox G R. Autolysis of Streptococcus cremoris. J Dairy Res. 1976;43:275–282. doi: 10.1017/s0022029900015831. [DOI] [PubMed] [Google Scholar]
- 26.O'Donovan C M, Wilkinson M G, Guinee T P, Fox P F. An investigation of the autolytic proporties of three lactococcal strains during cheese ripening. Int Dairy J. 1996;6:1149–1165. [Google Scholar]
- 27.Ostlie H M, Vegraud G, Langsrud T. Autolysis of lactococci: detection of lytic enzymes by polyacrylamide gel electrophoresis and characterization in buffer systems. Appl Environ Microbiol. 1995;61:3598–3603. doi: 10.1128/aem.61.10.3598-3603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Sullivan D, Coffey A C, Fitzgerald G F, Hill C, Ross R P. Design of a phage-insensitive lactococcal dairy starter via sequential transfer of naturally occurring conjugative plasmids. Appl Environ Microbiol. 1998;64:4618–4622. doi: 10.1128/aem.64.11.4618-4622.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riepe H R, Pillidge C J, Gopal P K, McKay L L. Characterization of the highly autolytic Lactococcus lactis subsp. cremoris strains CO and 2250. Appl Environ Microbiol. 1997;63:3757–3763. doi: 10.1128/aem.63.10.3757-3763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shockman G D, Holtje J-V. Microbial peptidoglycan (murein) hydrolases. In: Ghuysen J-M, Hackenbeck R, editors. New comprehensive biochemistry. 27. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1994. pp. 131–167. [Google Scholar]
- 31.Tan P S T, Chapot-Chartier M-P, Pos K M, Rousseau M, Boquein C Y, Gripon J-C, Konings W N. Localization of peptidases in lactococci. Appl Environ Microbiol. 1992;58:285–290. doi: 10.1128/aem.58.1.285-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van de Guchte M, Daly C, Fitzgerald G F, Arendt E K. Identification of int and attP on the genome of lactococcal bacteriophage Tuc2009 and their use for site-specific plasmid integration in the chromosome of Tuc2009-resistant Lactococcus lactis MG1363. Appl Environ Microbiol. 1994;60:2324–2329. doi: 10.1128/aem.60.7.2324-2329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Sinderen D, Karsens H, Kok J, Terpstra P, Ruiters M H J, Venema G, Nauta A. Sequence analysis and molecular characterisation of the temperate lactococcal bacteriophage r1T. Mol Microbiol. 1996;19:1343–1355. doi: 10.1111/j.1365-2958.1996.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 34.Vegarud G, Castberg H B, Langsrud T. Autolysis of group N streptococci, effects of media composition modifications and temperature. J Dairy Sci. 1983;66:2294–2302. [Google Scholar]
- 35.Visser S. Proteolytic enzymes and their relation to cheese ripening. J Dairy Sci. 1993;76:329–350. [Google Scholar]
- 36.Wiederholt K M, Steele J L. Prophage curing and characterisation of temperate bacteriophages from thermolytic strains of Lactococcus lactis subsp. cremoris. J Dairy Sci. 1993;76:921–930. [Google Scholar]
- 37.Wilkinson M G. Acceleration of cheese ripening. In: Fox P F, editor. Cheese: chemistry, physics and microbiology. 2nd ed. Vol. 1. London, United Kingdom: Chapman and Hall; 1993. pp. 523–556. [Google Scholar]
- 38.Wilkinson M G, Guinee T P, Fox P F. Factors which may influence the determination of autolysis of starter bacteria during cheddar cheese ripening. Int Dairy J. 1994;4:141–160. [Google Scholar]
- 39.Wilkinson M G, Guinee T P, O'Callaghan D M, Fox P F. Autolysis and proteolysis in different strains of starter bacteria during cheese ripening. J Dairy Res. 1994;61:249–262. [Google Scholar]
- 40.Wittenberger C L, Angelo N. Purification and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970;101:717–734. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]