Graphical abstract
Keywords: Aortic stenosis, Transcatheter aortic valve implantation, Aortic leaflet avulsion, Extracorporeal membrane oxygenation, Watershed
Highlights
-
•
Aortic valve leaflet avulsion is a rare complication during TAVI.
-
•
Avulsed leaflet traveled to the LA with ECMO flow and migrated to the aorta.
-
•
TEE aided in timely management and helped complete the transcatheter intervention.
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as an alternative procedure to surgical aortic valve replacement for patients with aortic stenosis at high surgical risk,1 and its indications are expanding to include those at low surgical risk.2, 3, 4
Although short-term outcomes following TAVI have improved in the last decade along with advances in equipment, increases in the number of procedures, and accumulation of experience by the heart team, unanticipated complications still occur during the procedure. A few cases of native or prosthetic valve leaflet avulsion flowing to the aorta during TAVI have been previously reported.5, 6, 7, 8 However, no cases of valve leaflet avulsion presenting as a free-floating mass in the left atrium (LA) during TAVI have been reported.
Herein, we report a case of aortic valve leaflet avulsion that traveled upstream to the LA along with the flow of extracorporeal membrane oxygenation (ECMO) and migrated to the descending aorta (DA) immediately after prosthetic valve deployment. The present case demonstrates the importance of transesophageal echocardiography (TEE) in detecting, evaluating, and addressing procedural complications in a timely manner during TAVI.
Case Presentation
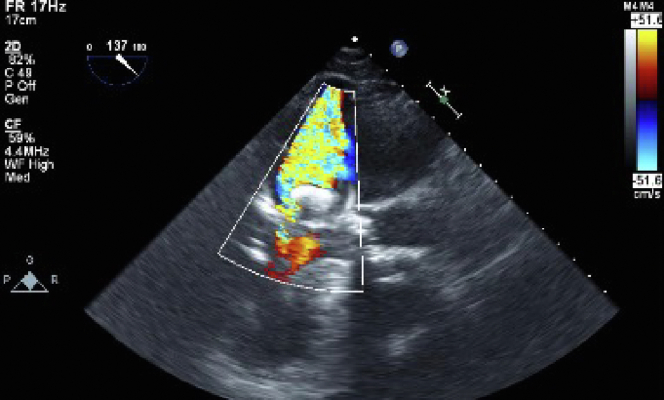
Written informed consent was obtained from the patient for publication of this case. A 92-year-old woman with a history of severe symptomatic aortic stenosis and paroxysmal atrial fibrillation presented with a chief complaint of dyspnea. The patient was scheduled for TAVI using a 23-mm Edwards Sapien 3 valve (Edwards Lifesciences) without predilatation. Transthoracic echocardiography (TTE) showed a severely calcified trileaflet aortic valve (aortic valve area = 0.67 cm2; mean pressure gradient = 44 mm Hg; aortic valve area index = 0.49 cm2/m2) and normal left ventricular (LV) systolic function (ejection fraction = 65%). Color Doppler evaluation at the mitral valve showed mild mitral regurgitation (MR) occupying 9% of the LA with a jet area of less than 3 cm2. Color Doppler evaluation at the aortic valve showed mild aortic regurgitation (AR) with a vena contracta width of 2.8 mm and a ratio of jet width to LV outflow tract width of 20%. Color Doppler evaluation at the tricuspid valve showed that the mild tricuspid regurgitation area was less than 5 cm2 and the inferior vena cava diameter was 16 mm with respiratory variability. Dexmedetomidine and propofol were administered continuously to maintain moderate sedation. A pacemaker wire for rapid ventricular pacing was inserted via the right jugular vein. Vascular sheaths were inserted into the bilateral femoral arteries and the right femoral vein under fluoroscopic and echocardiographic guidance. The right femoral arterial sheath was inserted for the transcatheter heart valve. The valve was advanced over the stiff wire. Before valve deployment, the patient had sudden hypotension that did not improve even after extensive volume resuscitation and pressor support. We decided to use venoarterial ECMO to improve the hemodynamic instability. The patient was administered general anesthesia with endotracheal intubation, and TEE was performed. Fluoroscopic imaging showed that the stiff wire extended into the LA. Transesophageal echocardiography showed that the acute MR was probably caused by entanglement of the stiff wire and the chordae tendineae in the LV cavity. The guidewire protruded into the LA, making it difficult to coapt (Figure 1, Video 1). Once the stiff wire was repositioned, MR was relieved under ECMO with flow at 2.4 L/min. The bioprosthetic valve was then introduced over the wire.
Figure 1.
2D TEE with color flow Doppler, mid esophageal long axis display (137°) demonstrates acute significant MR and the guidewire protruding into the LA.
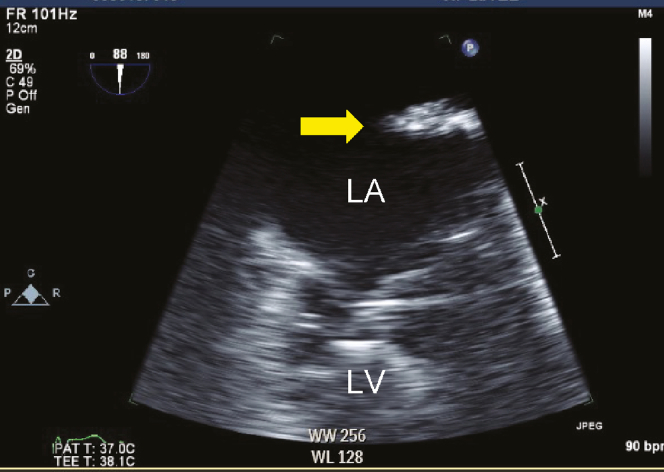
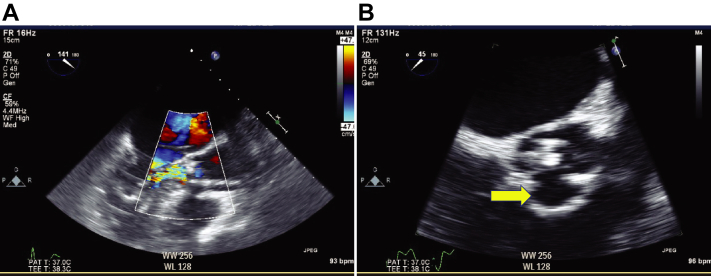
When we tried to advance the bioprosthetic valve across the native aortic valve, it went deeper into the LV side beyond the expected position. We attempted to withdraw the bioprosthetic valve back into the ascending aorta but were unsuccessful due to severe leaflet calcification. We tried to pull the valve to the aortic side against strong resistance and adjust the bioprosthetic valve to the exact position. However, a free-floating mass in the LA was seen on fluoroscopic imaging and TEE (Figure 2, Videos 2-4). In addition, TEE also showed AR due to the disappearance of the right leaflet and central MR with noncoapted mitral leaflets (Figure 3A and B, Videos 5 and 6).
Figure 2.
2D TEE, mid esophageal zoomed LAA view (88°) demonstrates a 3.0 cm × 0.5 cm free-floating mass (yellow arrow).
Figure 3.
(A) 2D TEE with color flow Doppler, mid esophageal long-axis view (141°) demonstrates the acute AR and MR. (B) 2D TEE, mid esophageal zoomed short axis aortic valve view (45°) demonstrates the absence of the right aortic leaflet (yellow arrow).
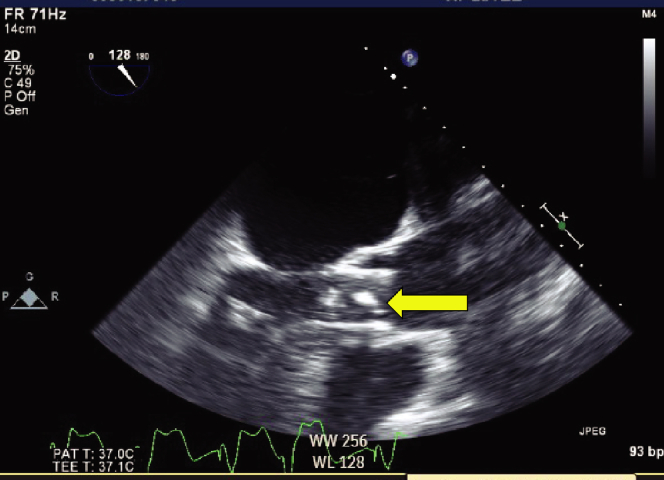
A multidisciplinary heart team discussed the risks and benefits of transcatheter and surgical interventions. Mass removal under cardiopulmonary bypass (CPB) and surgical aortic valve replacement were considered. However, given the patient’s advanced age and general frailty, mass removal under CPB and TAVI was thought to be less invasive. Therefore, we proceeded with TAVI while preparing for emergent CPB. To reduce the risk of ventricular migration during valve deployment, the ECMO flow was stopped and the cannulas were clamped until the valve was released. Rapid pacing was initiated at 180 bpm, with appropriate drops in blood pressure and pulse pressure, and TAVI was performed. The ECMO flow was temporarily increased from 2.4 to 3.5 L/min immediately after deployment so that the floating mass would stay in the LA. However, upon discontinuation of rapid pacing, the mass unexpectedly passed through the prosthetic valve to the DA (Figure 4, Videos 7 and 8).
Figure 4.
2D TEE, mid esophageal zoomed long axis view (128 degrees) demonstrates a mass that was seen to pass through the prosthetic valve (yellow arrow).
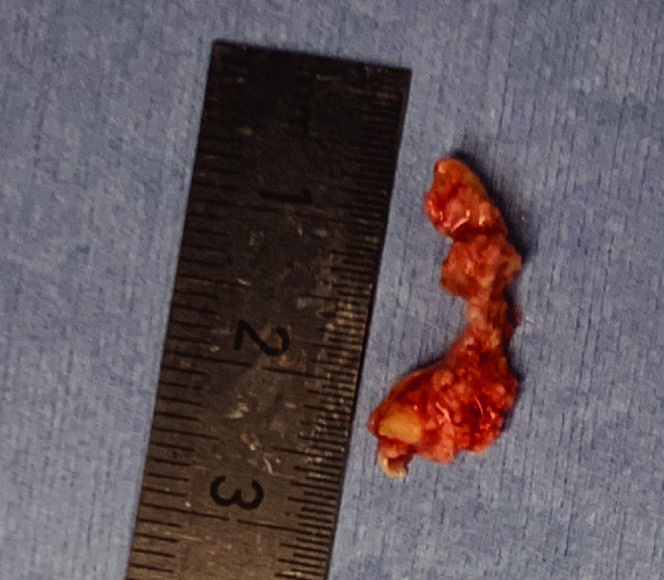
After valve deployment, a mild paravalvular leak remained with no pericardial effusion. After removal of the delivery systems from the LV, TEE showed that MR was improved to the preoperative baseline level, leading to fair coaptation of the mitral leaflets. An endovascular snare system was inserted through the right femoral sheath. The floating mass was successfully removed using the snare system without neurological complications (Figure 5). The ECMO flow was weaned slowly and removed in the operating room. The patient was transferred to the intensive care unit and extubated the next day.
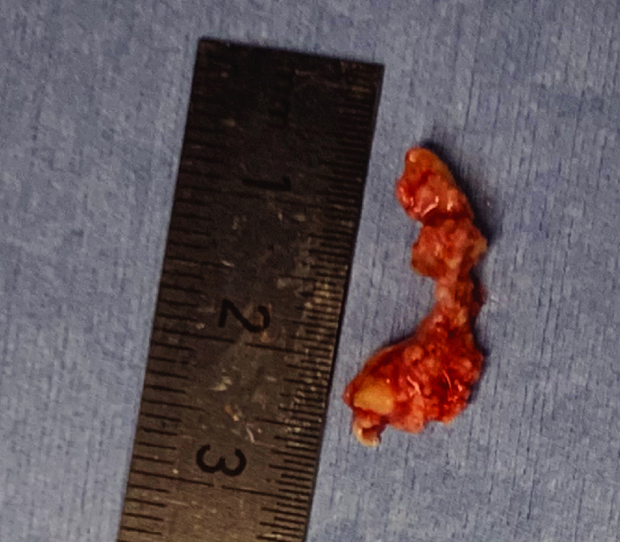
Figure 5.
A 2.0 cm × 0.5 cm leaflet of the native aortic valve was retrieved using a transcatheter snare device.
Transthoracic echocardiography in the intensive care unit showed a normal functioning bioprosthetic valve (peak velocity = 1.46 m/sec; mean gradient = 4.26 mm Hg; circumferential extent of prosthetic valve paravalvular regurgitation = 9%).
Pathologically, the mass was a portion of the aortic valve with degenerative changes.
Discussion
Two uncommon events were shown in this case. First, an aortic valve leaflet avulsed and traveled upstream to the LA due to retrograde ECMO flow. Second, the avulsed aortic valve leaflet migrated to the DA after deployment. Transesophageal echocardiography was proven to be useful for managing both events.
First, the aortic valve leaflet avulsion traveled to the LA, which was induced by retrograde ECMO flow. The current case can be considered unique in that the leaflet was detached from the native aortic valve and traveled upstream to the LA because of the retrograde ECMO flow. Extracorporeal membrane oxygenation can be used successfully in patients undergoing TAVI as a rescue maneuver.9 Extracorporeal membrane oxygenation has unique hemodynamic effects compared with other cardiac support devices, such as intra-aortic balloon pumping and percutaneous transaortic ventricular assist devices. Extracorporeal membrane oxygenation supports circulation to maintain vital organ function. However, blood flow can be antegrade or retrograde depending on the native cardiac output (CO) relative to ECMO flow.10 One explanation for the leaflet having ended up in the LA is that it is easier for the left ventricle (LV) to push the leaflet avulsion backward into the low-pressure LA with the MR flow than to eject it forward against ECMO flow. The presence of MR could be explained by the fact that repositioning of the bioprosthetic valve led to restriction of the chordae tendineae after interaction with the stiff wire.11 Notably, aortic valve leaflet avulsion due to mechanical trauma can be found in the LV, LA, and even the pulmonary vein, especially during ECMO. The heart team should share information about this pitfall and discuss emergency management while the CPB is prepared for immediate use, because surgical removal of an intracardiac free-floating mass is the typical treatment to prevent systemic emboli.
Second, the aortic valve leaflet avulsion migrated to the DA after deployment. Previous case reports have shown that fragments detached from the aortic valve can cause systemic embolism after deployment.7,8 However, our case was different from previous cases, as the aortic valve leaflet was retained in the DA without embolization and was retrieved by endovascular surgery. The migratory aortic valve leaflet eventually remained in the DA due to the retrograde ECMO flow competing with the post-TAVI native CO. In other words, migration of the mass into the DA visually showed the shift of the so-called watershed. We could not perform mass removal under CPB because we underestimated the post-TAVI native CO. Another report showed that rapid improvement in cardiac function triggered stroke in patients with intracardiac thrombosis.12 In this case, migratory leaflet avulsion in the systemic circulation also occurred immediately after improvement in CO with TAVI, suggesting that changes in CO may pose an increased risk of developing embolism, including stroke. An ultra-short-acting β-1 selective antagonist exerting a suppressive effect on native CO could be an option to lock the torn leaflet in the LA and remove it under CPB. An attempt to control the watershed by increasing ECMO flow was not sufficient for an emergent management approach. For patients with a potential risk of embolism resulting from TAVI, a cerebral protection device is also recommended, although it is used off-label in Japan. When sources of emboli, such as leaflet tissue, thrombus, or atheroma, are found prior to valve replacement during ECMO, the heart team should discuss how to address the embolus in addition to the method of valve replacement (i.e., surgical, transcatheter, or both). Although the optimal measures have not been defined, the order of interventions is also important.
Transesophageal echocardiography played a crucial role in both events in this case. The prompt identification of the free-floating mass, which is likely to embolize, helped to determine an emergent management approach for the unanticipated complications. Additionally, real-time tracking of the migration of the aortic valve leaflet avulsion modified the emergent management approach. Although TAVI and mass removal under CPB was the original plan, the rapid assessment obtained with TEE helped us make a prompt decision to remove CPB and led to alternative transcatheter intervention. Therefore, clinicians should track mobile leaflets with caution, especially after deployment. Contrary to our expectation, TAVI and transcatheter mass removal without the need for cardiac arrest and CPB was probably the best alternative for this patient.
Although TTE is typically used as an adjunct to fluoroscopy for simple TAVI,13,14 our case demonstrated that TEE is superior to fluoroscopy in detecting, evaluating, and addressing procedural complications in a timely manner. Transesophageal echocardiography has 2 different strengths in this case compared with angiography. It is important to learn the strengths of echocardiography and choose the optimal imaging method depending on the situation.
First, TEE can better assess the location of a mobile aortic valve leaflet than angiography. Angiography can visualize a large area of the heart and greater vessels at once and projects the image of a three-dimensional structure on a two-dimensional screen. Therefore, multiple projections are necessary to accurately locate the mobile aortic valve leaflet. With TEE, however, detailed information about the location of the mobile aortic leaflet was obtained continuously without contrast. In addition, angiography could not clearly differentiate the calcified, avulsed aortic leaflet from the blood vessels and heart due to the low contrast. With TEE, however, it was easier to detect it because it was shown as a free-floating high echoic mass on the screen. In fact, none of the 4 cardiologists noticed that the mass had migrated to the DA until the echocardiologist mentioned it. This was because they were concentrating on checking the location of the bioprosthetic valve and postdeployment complications.
Second, TEE is superior to angiography in that it allows rapid, repetitive, and constant assessment of valve regurgitation. Transesophageal echocardiography can visualize intracardiac structures and valvular regurgitation rapidly, continuously, and repeatedly without contrast and radiation. Deterioration of AR and absence of the right aortic leaflet were indicative of valve avulsion. If echocardiography identifies a free-floating mass in the left side of the heart during TAVI along with acute AR, clinicians should suspect valve avulsion that can cause adverse complications.
Whether TEE or TTE is the better option to guide TAVI when combined with fluoroscopy has remained controversial.13,14 Transthoracic echocardiography and TEE are useful during TAVI, and both can show almost the same structures. However, TEE remains of great help in complicated TAVI procedures because of its ability to provide high-quality imaging repeatedly, rapidly, and constantly compared with TTE.13 In addition, TEE can provide more effective images, especially of the posterior cardiovascular structures, including the LA and DA. Transthoracic echocardiography could not have tracked the mass that had migrated from the LA to the DA because TTE is not effective at viewing these structures. Assessing the location of the mobile aortic valve leaflet was more effective with TEE, as the TEE probe and posterior structures are sufficiently close to provide a higher-resolution image. As intraoperative complications can be detected more quickly and accurately with TEE than with TTE, TEE is considered an essential monitor for complicated TAVI.
Anesthetic management has increasingly switched from general anesthesia with TEE to moderate sedation with TTE, and the use of TEE has decreased.15 However, clinicians should make a rapid transition from TTE to TEE emergently when the patient develops unexplained life-threatening hemodynamic instability or when procedural complications related to posterior cardiovascular structures, including the LA and DA, occur.
Conclusion
An avulsed aortic leaflet traveled upstream to the LA with retrograde ECMO flow and migrated to the DA after deployment. Transesophageal echocardiography is an essential tool for complicated TAVI and may potentially minimize the damage of procedural complications.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2022.12.008.
Supplementary Data
2D TEE midesophageal long axis (137°) view demonstrates acute significant MR and the stiff wire in the LV cavity interacting with the chordae tendineae as the guidewire is extended into the LA.
X-ray fluoroscopy demonstrates a free-floating mass in the LA, AR, transcatheter aortic valve implantation over the stiff wire, temporary pacer wire, and ECMO venous cannula.
2D TEE midesophageal long axis (88°) LA-focused view demonstrates an echo-bright free-floating mass.
3D TEE midesophageal volume-rendered image from the perspective of the LA demonstrates a free-floating mass.
2D TEE with color flow Doppler, midesophageal long-axis (141°) aortic valve-focused view prior to valve deployment demonstrates the acute AR during diastole and MR during systole.
2D TEE midesophageal short axis (45°) aortic valve–focused view demonstrates the absence of the right aortic leaflet.
2D TEE midesophageal long axis (128°) view demonstrates the mass that passed through the prosthetic valve to the DA at that moment.
X-ray fluoroscopy after deploying the valve demonstrates stagnation of the mass in the DA.
References
- 1.Smith C.R., Leon M.B., Mack M.J., Miller D.C., Moses J.W., Svensson L.G., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 2.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Fleisher L.A., et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner H., Falk V., Bax J.J., De Bonis M., Hamm C., Holm P.J., et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 5.Sherev D., Azizi K., Azimi N.A., Van Nordheim S., Moreno-Cabral R. Delayed management of partial aortic valve avulsion after transcatheter aortic valve replacement. Ann Thorac Surg. 2017;104:e17–e18. doi: 10.1016/j.athoracsur.2017.01.100. [DOI] [PubMed] [Google Scholar]
- 6.Zimmet J., Kaiser E., Tseng E., Shunk K. Successful percutaneous management of partial avulsion of the native aortic valve complex complicating transcatheter aortic valve replacement. J Invasive Cardiol. 2014;26:E137–E140. [PubMed] [Google Scholar]
- 7.Kinthala S., Saththasivam P., Ankam A., Sattur S. Embolization of aortic valve leaflet during valve-in-valve transcatheter aortic valve implantation: a case report. Eur Heart J Case Rep. 2020;4:1–5. doi: 10.1093/ehjcr/ytaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masumoto A., Kitai T., Ota M., Kim K., Ehara N., Furukawa Y. Real-time observation of a high-echoic mass in the left ventricle during transcatheter aortic valve implantation: a case report. Eur Heart J Case Rep. 2020;4:1–4. doi: 10.1093/ehjcr/ytaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husser O., Holzamer A., Philipp A., Nunez J., Bodi V., Müller T., et al. Emergency and prophylactic use of miniaturized veno-arterial extracorporeal membrane oxygenation in transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2013;82:E542–E551. doi: 10.1002/ccd.24806. [DOI] [PubMed] [Google Scholar]
- 10.Rao P., Khalpey Z., Smith R., Burkhoff D., Kociol R.D. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11:e004905. doi: 10.1161/CIRCHEARTFAILURE.118.004905. [DOI] [PubMed] [Google Scholar]
- 11.López-Aguilera J., Mesa-Rubio D., Ruiz-Ortiz M., Delgado-Ortega M., Villanueva-Fernández E., Romo-Peña E., et al. Mitral regurgitation during transcatheter aortic valve implantation: the same complication with a different mechanism. J Invasive Cardiol. 2014;26:603–608. [PubMed] [Google Scholar]
- 12.Kikuchi S., Hibi K., Tamura K., Kimura K. Free-floating left ventricular thrombus after rapid improvement of cardiac function related to mechanical hemodynamic support. J Cardiol Cases. 2020;21:231–233. doi: 10.1016/j.jccase.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronzon I., Jelnin V., Ruiz C.E., Saric M., Williams M.R., Kasel A.M., et al. Optimal imaging for guiding TAVR: transesophageal or transthoracic echocardiography, or just fluoroscopy? JACC Cardiovasc Imaging. 2015;8:361–370. doi: 10.1016/j.jcmg.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Stewart W.J. Imaging the future of transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2008;1:25–28. doi: 10.1016/j.jcmg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Husser O., Fujita B., Hengstenberg C., Frerker C., Beckmann A., Möllmann H., et al. Conscious sedation versus general anesthesia in transcatheter aortic valve replacement: the German Aortic Valve Registry. JACC Cardiovasc Interv. 2018;11:567–578. doi: 10.1016/j.jcin.2017.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2D TEE midesophageal long axis (137°) view demonstrates acute significant MR and the stiff wire in the LV cavity interacting with the chordae tendineae as the guidewire is extended into the LA.
X-ray fluoroscopy demonstrates a free-floating mass in the LA, AR, transcatheter aortic valve implantation over the stiff wire, temporary pacer wire, and ECMO venous cannula.
2D TEE midesophageal long axis (88°) LA-focused view demonstrates an echo-bright free-floating mass.
3D TEE midesophageal volume-rendered image from the perspective of the LA demonstrates a free-floating mass.
2D TEE with color flow Doppler, midesophageal long-axis (141°) aortic valve-focused view prior to valve deployment demonstrates the acute AR during diastole and MR during systole.
2D TEE midesophageal short axis (45°) aortic valve–focused view demonstrates the absence of the right aortic leaflet.
2D TEE midesophageal long axis (128°) view demonstrates the mass that passed through the prosthetic valve to the DA at that moment.
X-ray fluoroscopy after deploying the valve demonstrates stagnation of the mass in the DA.