Abstract
Nirmatrelvir–ritonavir (Paxlovid) is recommended to reduce the risk of hospitalization from COVID-19 in pregnancy. Data on use in pregnancy, including prescribing patterns and patient experience (adverse effects, incidence of rebound), are limited. We performed a cross-sectional study in which we surveyed a cohort of vaccinated pregnant or lactating individuals with breakthrough COVID-19. Of 35 pregnant respondents, 51.4% were prescribed and 34.3% took nirmatrelvir–ritonavir; of these, 91.7% experienced dysgeusia and 50.0% had rebound (50.0% positive test, 33.3% return of symptoms). Three of five lactating respondents were prescribed and two took nirmatrelvir–ritonavir. There were no significant adverse outcomes. Unknown risk was the most common reason for declining nirmatrelvir–ritonavir. More research is needed to establish the safety of nirmatrelvir–ritonavir in pregnancy and lactation, improve public health messaging, and increase uptake of this treatment.
Precis:
Nirmatrelvir–ritonavir is well-tolerated in pregnancy and lactation, though mild adverse effects and rebound may be common.
Introduction
Nirmatrelvir–ritonavir (Paxlovid) reduces the risk of hospitalization and death from COVID-19 in high-risk populations1, but data in pregnancy and lactation are lacking. Leading professional societies support its use in pregnancy.2,3 Patient experience, such as adverse effects and incidence of rebound symptoms, has not been reported in these groups.
We surveyed a vaccinated cohort of pregnant or lactating individuals about their experience with nirmatrelvir–ritonavir for COVID-19. We aimed to assess the patient clinical experience after treatment, including the rate of rebound symptoms.
Methods
This is a cross-sectional study of individuals who received their initial COVID-19 vaccination series during pregnancy or lactation, the COVID-19 Vaccination in Pregnancy and Lactation (COVIPAL) study. Enrollees receive periodic questionnaires to assess for breakthrough infections and other changes in their health. SARS-CoV-2 infection (confirmed by medical record review) between January – December 2022 received an additional survey about their experience with nirmatrelvir–ritonavir.
The study was approved by the Institutional Review Board of the University of California, San Francisco (IRB#20–32077). All participants provided written informed consent prior to enrollment. Enrollees with COVID-19 received a REDCap-based survey with questions about their experience with nirmatrelvir–ritonavir. See Appendix 1, available online at http://links.lww.com/xxx. Medical records were reviewed to assess the following variables: gestational age at SARS-CoV-2 infection, gestational age at COVID-19 vaccination and booster dose(s), maternal age, gravidity, parity, self-reported race/ethnicity, co-morbidities (hypertensive disorder, diabetes mellitus, pulmonary disease), obstetric outcomes, and hospitalizations. We analyzed descriptive data using medians (range) for continuous variables and frequencies by n (%). Adverse effect severity was graded according to DAIDS criteria.4 We collected information about patient race and ethnicity because racial and ethnic disparities exist in outpatient COVID-19 treatment in the United States.5
Results
Of 371 patients in the parent study, 64 had COVID-19 during the survey period. All cases were mild. Forty participants responded including 35 pregnant and 5 lactating individuals (Figure 1). Surveys were returned a median of 16 days after completing nirmatrelvir–ritonavir.
Figure 1:
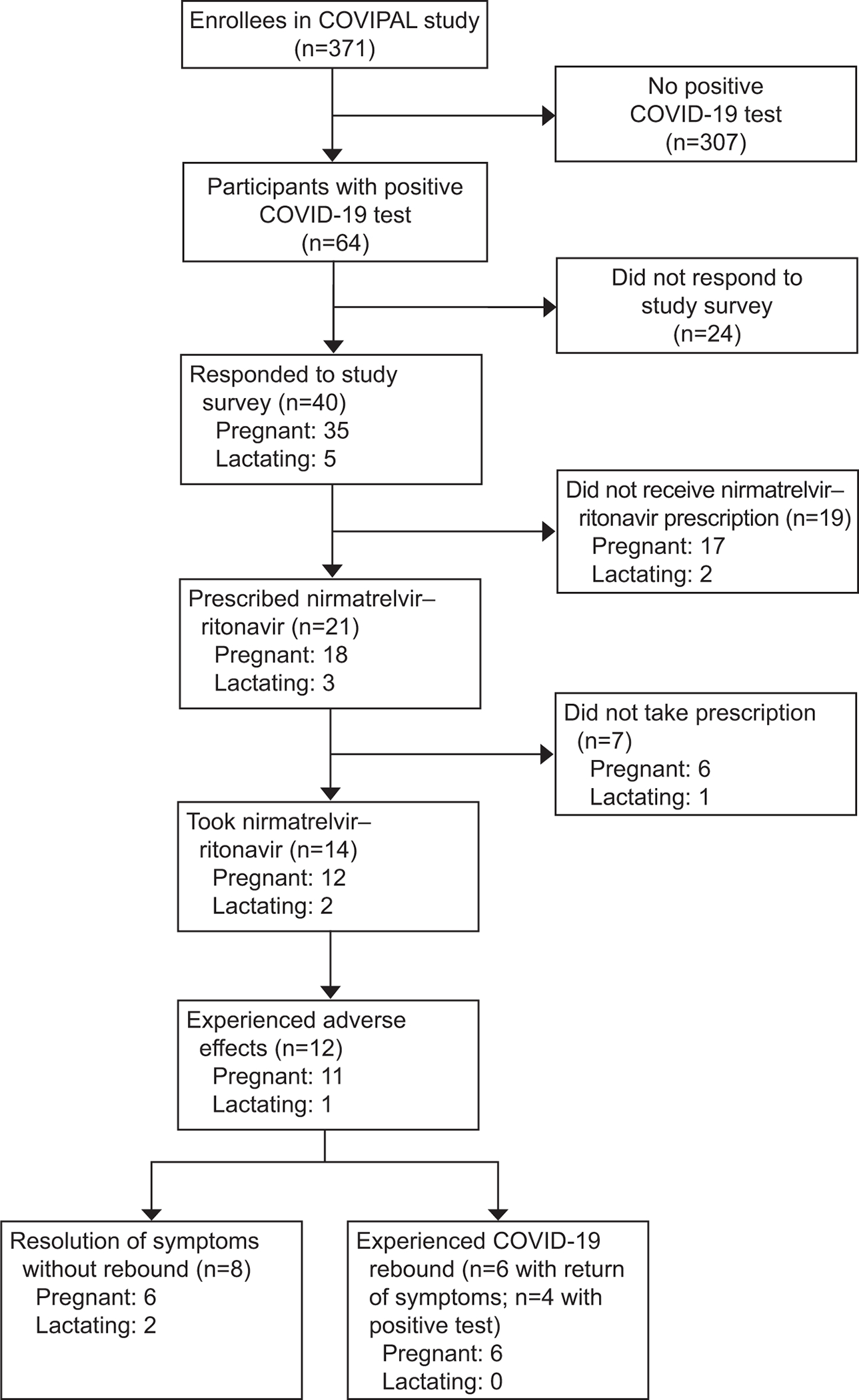
Patient inclusion cohort flow. COVIPAL, COVID-19 Vaccination in Pregnancy and Lactation.
Respondent characteristics are described in Table 1. Of pregnant respondents, 18 (51.4%) received a nirmatrelvir–ritonavir prescription, of whom 66.7% opted to take the full 5-day course (n=12, 34.3% of pregnant respondents). Adverse effects were reported by 91.7%, most commonly dysgeusia (n=11, 91.7%), diarrhea (n=2, 16.7%), and mild abdominal pain (n=1, 8.3%)(Figure 2A). Adverse effects lasted from 72 hours to 1 week. Six respondents (50.0%) reported a positive COVID-19 test after completion of nirmatrelvir–ritonavir; four (33.3%) reported a return of COVID-19 symptoms, most commonly fatigue (100.0%) and nasal congestion (100.0%), cough (50.0%) and headache (50.0%) (Figure 2B).
Table 1:
Characteristics of pregnant or lactating patients vaccinated against COVID-19 prescribed nirmatrelvir–ritonavir
| Patients, Number (%) | ||
|---|---|---|
| Characteristic | Pregnant | Lactating |
| Prescribed medication | N=18 | N=3 |
| Age, median (range), years | 35 (31–44) | 41 (38–44) |
| Race | ||
| Asian | 5 (27.8%) | 1(33.3%) |
| Black/African American | 0 | 0 |
| White | 10 (55.6%) | 1 (33.3%) |
| None of the above | 3 (16.7%) | 1 (33.3%) |
| Ethnicity | ||
| Hispanic/Latine | 3 (16.7%) | 0 |
| Not Hispanic/Latine | 9 (50.0%) | 2 (66.7%) |
| Unknown | 6 (33.3%) | 0 |
| Gestational age at treatment (weeks) (range) | 22 (9.3–39.4) | 64 weeks postpartum (4–114) |
| Trimester of pregnancy at time of infection/treatment | ||
| First | 3 (16.7%) | |
| Second | 9 (50.0%) | |
| Third | 6 (33.3%) | |
| Gravidity (range) | 1 (1–2) | 4 (2–4) |
| Parity (range) | 0 (0–2) | 2 (2–3) |
| Took prescribed medication | N=12 (66.0%) | N=2 |
| Experienced adverse effects* | 11 (91.7%) | 1 |
| Altered sense of taste | 11 (91.7%) | 1 |
| Diarrhea | 2 (16.7%) | 0 |
| Other | 1 (8.3%) | 1 |
| Return of COVID-19 symptoms after completed course † | 4 (33.3%) | 0 |
| Cough | 2 (16.7%) | 0 |
| Shortness of breath/difficulty breathing | 1 (8.3%) | 0 |
| Muscle/body aches | 1 (8.3%) | 0 |
| Headache | 2 (16.7%) | 0 |
| Sore throat | 1 (8.3%) | 0 |
| Congestion/runny nose | 4 (33.3%) | 0 |
| Positive COVID-19 test after completed course | 6 (50.0%) | 0 |
| Time from completion of course to positive test (range) | 7 (2–10) days | N/A |
No patients reported high blood pressure or muscle aches
No patients reported experiencing fever/chills, new loss of taste or smell, nausea/vomiting, diarrhea, abdominal pain, or mastitis
Figure 2:
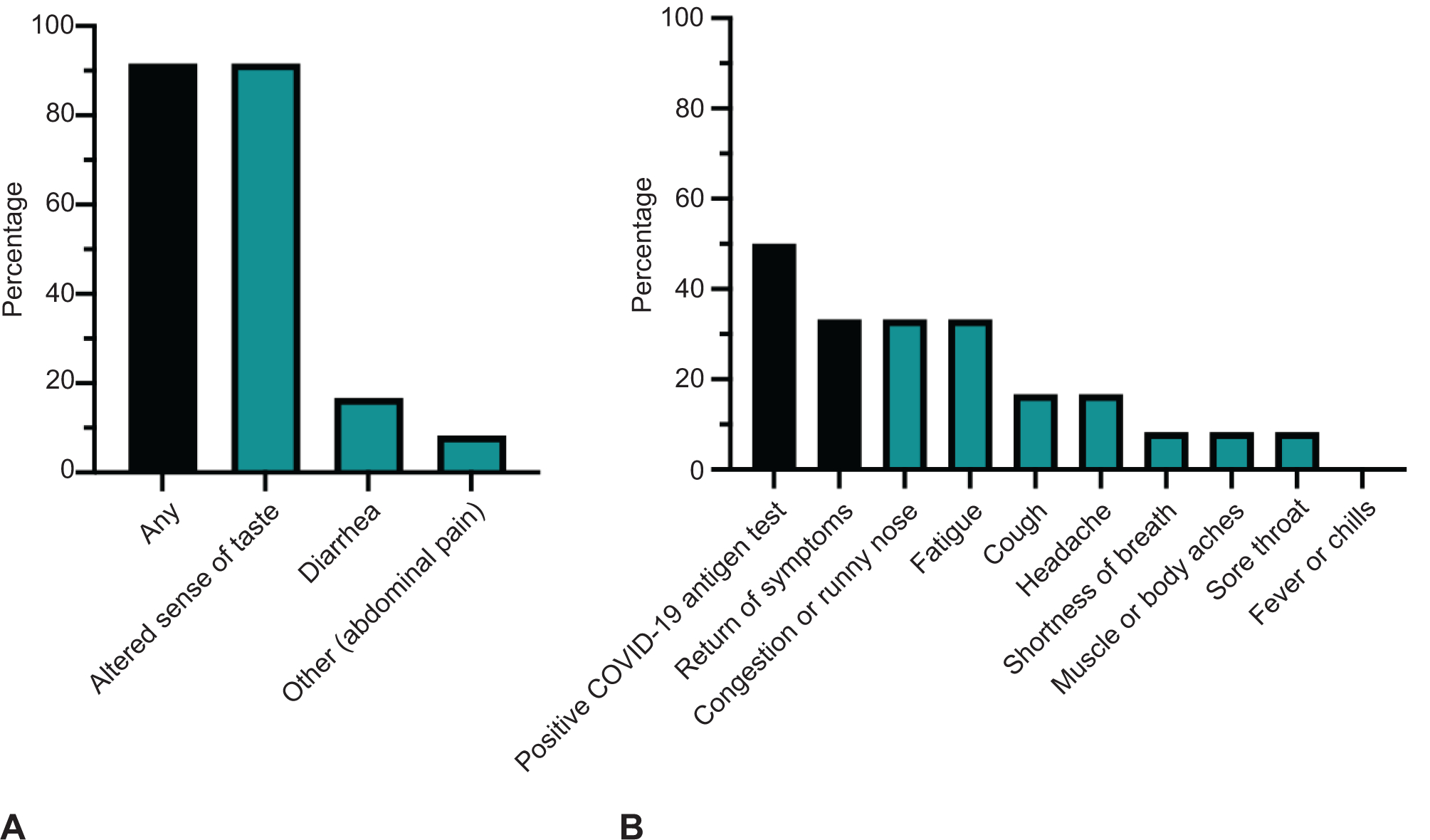
A. Eleven (91.7%) had any medication adverse effects. B. Six (50.0%) had positive test and four (33.0%) had a return of mild symptoms after nirmatrelvir–ritonavir course. COVID-19, coronavirus disease 2019.
Three of five lactating respondents received a nirmatrelvir–ritonavir prescription; two opted to take the medication. One reported altered sense of taste. None reported subsequent COVID-19 rebound (Table 1).
The most common reported reason for avoidance of nirmatrelvir–ritonavir in both groups was lack of data and unknown risks. One participant expressed concern about rebound COVID-19 symptoms. Two participants declined nirmatrelvir–ritonavir due to mildness of symptoms (Appendix 2, available online at http://links.lww.com/xxx).
There were no adverse events, no obstetric complications, and no hospitalizations for COVID-19. Of 12 pregnant individuals who completed the nirmatrelvir–ritonavir course, ten had healthy term pregnancies without complications; one had a preterm twin delivery, and one remains pregnant.
Discussion
In this cohort of vaccinated pregnant individuals, half were prescribed nirmatrelvir–ritonavir. Of those, 66.0% took the medication; most declined nirmatrelvir–ritonavir due to concerns about unknown risks in pregnancy. Nearly all who took nirmatrelvir–ritonavir had mild adverse effects, and half experienced mild COVID-19 rebound. There were no serious adverse events and the medication was overall well-tolerated, consistent with available data.6
Though pregnant status is a high-risk condition, we report low rates of nirmatrelvir–ritonavir prescription receipt. Patient uncertainty about this new medication is a barrier to treatment uptake, even after it’s prescribed by a health care professional. The rate of medication adverse effects and COVID-19 rebound were higher than cited in nirmatrelvir–ritonavir clinical trials (5.6% and 1–2%, respectively) in non-pregnant or lactating individuals.1 There may be bias in survey reporting in this small sample size; larger trials are needed.
Ritonavir is well-studied and widely used in pregnancy and lactation, but nirmatrelvir’s safety is largely based on animal models7, and one series of 46 pregnancies.6 More research is needed to understand if nirmatrelvir–ritonavir is protective against adverse COVID-19 outcomes in this population, including those who are vaccinated. Studies of nirmatrelvir–ritonavir efficacy are mostly in older individuals1,8, and exclude pregnant or lactating individuals. While our study size was too small to infer true medication safety, until clinical trials are completed, this study provides early data on nirmatrelvir–ritonavir experiences in this population.
Supplementary Material
Funding:
This study was supported by the National Institutes of Health (NIAID K08AI141728 (SLG) and K23AI127886 (MKP), the Marino Family Foundation (MKP), UCSF REAC Award (SLG and MKP), Weizmann Institute of Science (YG) and the Human Frontiers in Science Program (YG).
Financial Disclosure
Stephanie L. Gaw reports received funding from California Health Care Foundation, Centers for Disease Control and Prevention Foundation, Bill and Melinda Gates Foundation, Robert Wood Johnson Foundation, and the Yellow Chair Foundation, for work not reported here. The other authors did not report any potential conflicts of interest.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
PEER REVIEW HISTORY
Received January 19, 2023. Received in revised form February 14, 2023. Accepted February 16, 2023. Peer reviews and author correspondence are available at http://links.lww.com/xxx.
References
- 1.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. Accessed December 27, 2022. https://www.acog.org/en/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- 3.World Health Organization. Nirmatrelvir-Ritonavir for COVID-19. World Health Organization; 2022. Accessed December 27, 2022. https://apps.who.int/iris/handle/10665/359751 [Google Scholar]
- 4.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1. [July 2017]. [Google Scholar]
- 5.Boehmer TK. Racial and Ethnic Disparities in Outpatient Treatment of COVID-19 United States, January–July 2022. MMWR Morb Mortal Wkly Rep. 2022;71. doi: 10.15585/mmwr.mm7143a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garneau WM, Jones-Beatty K, Ufua MO, et al. Analysis of Clinical Outcomes of Pregnant Patients Treated With Nirmatrelvir and Ritonavir for Acute SARS-CoV-2 Infection. JAMA Network Open. 2022;5(11):e2244141. doi: 10.1001/jamanetworkopen.2022.44141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catlin NR, Bowman CJ, Campion SN, et al. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 Mpro inhibitor in animal models. Reprod Toxicol. 2022;108:56–61. doi: 10.1016/j.reprotox.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. N Engl J Med. 2022;387(9):790–798. doi: 10.1056/NEJMoa2204919 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


