Abstract
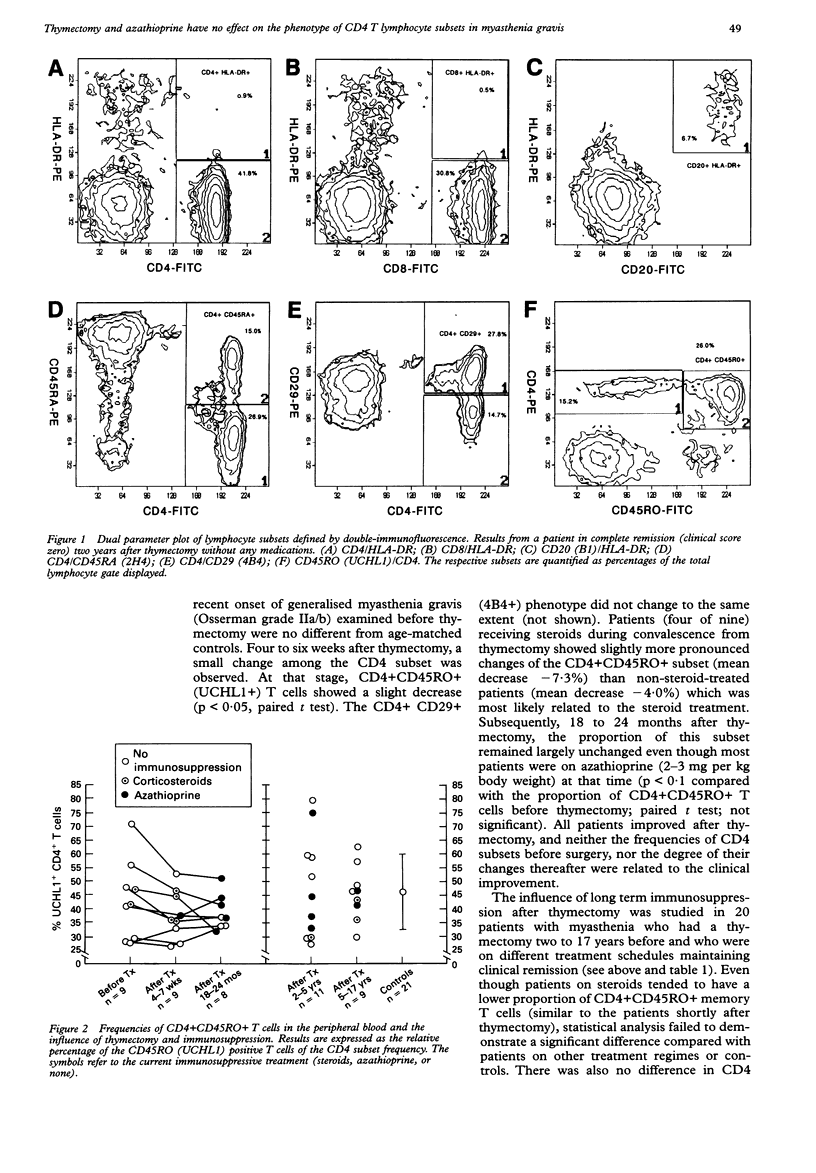
The influence of thymectomy and long term immunosuppression on the phenotype of CD4 T lymphocyte subsets, which were defined by the restricted expression of CD45RA and CD45RO markers, was studied by double immunofluorescence in 29 patients in different clinical stages of generalised myasthenia gravis. In the acute stage of myasthenia, before thymectomy and immunosuppression, no differences in CD4 subsets were observed in the peripheral blood from nine patients and 21 matched controls. Four to seven weeks after thymectomy, there was a slightly decreased proportion of CD4+CD45RO+ (UCHL1+) memory cells (p < 0.05, paired t test). Patients on steroids showed a more pronounced decrease of CD4+CD45RO+ cells suggesting, in addition, a drug-related effect. CD4 subsets (CD45RA, CD45RO, and CD29 positive) in the peripheral blood compartment remained largely stable over 18 to 24 months thereafter. In addition, CD4 subsets were examined in 20 patients with myasthenia gravis who had had a thymectomy between two and 17 years before. With the exception of patients on steroids, there were no differences in CD4 subsets in patients on or off azathioprine. These data did not show any relation of CD4 T cell subsets to the clinical course of myasthenia, or significant changes due to thymectomy, or immunosuppression with azathioprine. These results also complement the authors' clinical experience that thymectomy in adults does not leave a deficit in cell-mediated immunity. The slight change associated with steroid treatment might deserve further attention.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Besinger U. A., Toyka K. V., Hömberg M., Heininger K., Hohlfeld R., Fateh-Moghadam A. Myasthenia gravis: long-term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology. 1983 Oct;33(10):1316–1321. doi: 10.1212/wnl.33.10.1316. [DOI] [PubMed] [Google Scholar]
- Beverley P. C. Human T cell subsets. Immunol Lett. 1987 Apr;14(4):263–267. doi: 10.1016/0165-2478(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Chofflon M., Weiner H. L., Morimoto C., Hafler D. A. Loss of functional suppression is linked to decreases in circulating suppressor inducer (CD4+ 2H4+) T cells in multiple sclerosis. Ann Neurol. 1988 Aug;24(2):185–191. doi: 10.1002/ana.410240203. [DOI] [PubMed] [Google Scholar]
- Drachman D. B. Myasthenia gravis (first of two parts). N Engl J Med. 1978 Jan 19;298(3):136–142. doi: 10.1056/NEJM197801192980305. [DOI] [PubMed] [Google Scholar]
- Ginsberg A. H., Gale M. J., Jr, Rose L. M., Clark E. A. T-cell alterations in late postpoliomyelitis. Arch Neurol. 1989 May;46(5):497–501. doi: 10.1001/archneur.1989.00520410031018. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Harden E. A., Olanow C. W., Eisenbarth G. S., Wechsler A. S., Hensley L. L., Roses A. D. Effect of thymectomy on peripheral lymphocyte subsets in myasthenia gravis: selective effect on T-cells in patients with thymic atrophy. J Immunol. 1983 Aug;131(2):773–777. [PubMed] [Google Scholar]
- Hohlfeld R., Kalies I., Kohleisen B., Heininger K., Conti-Tronconi B., Toyka K. V. Myasthenia gravis: stimulation of antireceptor autoantibodies by autoreactive T cell lines. Neurology. 1986 May;36(5):618–621. doi: 10.1212/wnl.36.5.618. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Rowe D., Muir J., Beverley P. C. The tissue distribution of T lymphocytes expressing different CD45 polypeptides. Immunology. 1989 Apr;66(4):517–525. [PMC free article] [PubMed] [Google Scholar]
- Kirchner T., Tzartos S., Hoppe F., Schalke B., Wekerle H., Müller-Hermelink H. K. Pathogenesis of myasthenia gravis. Acetylcholine receptor-related antigenic determinants in tumor-free thymuses and thymic epithelial tumors. Am J Pathol. 1988 Feb;130(2):268–280. [PMC free article] [PubMed] [Google Scholar]
- Melms A., Schalke B. C., Kirchner T., Müller-Hermelink H. K., Albert E., Wekerle H. Thymus in myasthenia gravis. Isolation of T-lymphocyte lines specific for the nicotinic acetylcholine receptor from thymuses of myasthenic patients. J Clin Invest. 1988 Mar;81(3):902–908. doi: 10.1172/JCI113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Hafler D. A., Weiner H. L., Letvin N. L., Hagan M., Daley J., Schlossman S. F. Selective loss of the suppressor-inducer T-cell subset in progressive multiple sclerosis. Analysis with anti-2H4 monoclonal antibody. N Engl J Med. 1987 Jan 8;316(2):67–72. doi: 10.1056/NEJM198701083160202. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Pawelec G. P., Bühring H. J., Busch F. W., Kalthoff F., Wernet P. Different signals for stimulation of proliferation and lymphokine secretion by a CD3+ WT31- cloned cytotoxic lymphocyte. Cell Immunol. 1988 May;113(2):329–340. doi: 10.1016/0008-8749(88)90031-7. [DOI] [PubMed] [Google Scholar]
- Pilarski L. M., Gillitzer R., Zola H., Shortman K., Scollay R. Definition of the thymic generative lineage by selective expression of high molecular weight isoforms of CD45 (T200). Eur J Immunol. 1989 Apr;19(4):589–597. doi: 10.1002/eji.1830190403. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G. H., Covelli M., Meliconi R., Markey A., Panayi G. S. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991 Feb;21(2):369–376. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Reichert R. A., Weissman I. L., Butcher E. C. Dual immunofluorescence studies of cortisone-induced thymic involution: evidence for a major cortical component to cortisone-resistant thymocytes. J Immunol. 1986 May 15;136(10):3529–3534. [PubMed] [Google Scholar]
- Rose L. M., Ginsberg A. H., Rothstein T. L., Ledbetter J. A., Clark E. A. Fluctuations of CD4+ T-cell subsets in remitting-relapsing multiple sclerosis. Ann Neurol. 1988 Aug;24(2):192–199. doi: 10.1002/ana.410240204. [DOI] [PubMed] [Google Scholar]
- Rose L. M., Ginsberg A. H., Rothstein T. L., Ledbetter J. A., Clark E. A. Selective loss of a subset of T helper cells in active multiple sclerosis. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7389–7393. doi: 10.1073/pnas.82.21.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L. P. Therapy in myasthenia gravis: introduction. Ann N Y Acad Sci. 1987;505:566–567. doi: 10.1111/j.1749-6632.1987.tb51324.x. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Sommer N., Willcox N., Harcourt G. C., Newsom-Davis J. Myasthenic thymus and thymoma are selectively enriched in acetylcholine receptor-reactive T cells. Ann Neurol. 1990 Sep;28(3):312–319. doi: 10.1002/ana.410280303. [DOI] [PubMed] [Google Scholar]
- Van de Griend R. J., Carreno M., Van Doorn R., Leupers C. J., Van den Ende A., Wijermans P., Oosterhuis H. J., Astaldi A. Changes in human T lymphocytes after thymectomy and during senescence. J Clin Immunol. 1982 Oct;2(4):289–295. doi: 10.1007/BF00915069. [DOI] [PubMed] [Google Scholar]
- Vincent A. Immunology of acetylcholine receptors in relation to myasthenia gravis. Physiol Rev. 1980 Jul;60(3):756–824. doi: 10.1152/physrev.1980.60.3.756. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet. 1977 Mar 26;1(8013):678–680. doi: 10.1016/s0140-6736(77)92118-3. [DOI] [PubMed] [Google Scholar]


