Abstract
Background
Melanoma is an aggressive type of cancer that can metastasize to numerous other organs. TGFβ is one of the key signaling pathways in melanoma progression. Previous studies on various types of cancer have shown that both: polyphenols and a static magnetic field (SMF) can be potential chemopreventive/therapeutic agents. Therefore, the aim of the study was to evaluate the effect of a SMF and selected polyphenols on the transcriptional activity of TGFβ genes in melanoma cells.
Methods and results
Experiments were performed on the C32 cell line treated with caffeic or chlorogenic acids, and with simultaneous exposure to a moderate-strength SMF. The RT-qPCR method was used to determine the mRNA level of genes encoding the TGFβ isoforms and their receptors. The concentration of the TGFβ1 and TGFβ2 proteins were also measured in the cell culture supernates. The first response of C32 melanoma cells to both factors is the reduction of TGFβ levels. Then, mRNA level of these molecules returned to values close to pre-treatment level by the end of experiment.
Conclusion
Our study results demonstrate the potential of polyphenols and a moderate-strength SMF to support cancer therapy by altering TGFβ expression, which is a very promising topic for the diagnosis and treatment of melanoma.
Keywords: Static magnetic field, cancer, Chlorogenic acid, Caffeic acid, Melanoma cells, Transforming growth factor β
Introduction
Although cutaneous melanoma (CM) constitute a small proportion of skin cancers, it is the most common cause of cancer death in the young population [1]. According to studies, the incidence of this cancer has increased sharply among people in developed countries [2, 3]. It is a fairly aggressive type of cancer that, despite having the tumor removed, can metastasize to numerous other organs [4]. The etiopathogenesis of the disease is quite complex, however, it has not been fully documented yet. Changes at the molecular level are quite important [5, 6]. Multiple signaling pathways are involved in the development of CM. Mention is made of pathways: Jak/STAT (Janus kinase/signal transducer and activator of transcription), Wnt, NF-κB (nuclear factor kappa B), and also TGFβ (transforming growth factor β) [3, 7, 8]. Impact character of TGFβ is complex, depending on the type of tissue and the stage of the disease [9]. Although TGFβ is a strong inhibitor of epithelial cell proliferation, which is related to the inhibitory effect on the cell cycle [2], the final mechanism of action is quite controversial. It is mainly indicated that TGFβ is an ideal microenvironment for tumor development, promotes angiogenesis and the development of the immunosuppressive mechanism. This confirms previous reports on the secretion of TGFβ by most tumors, including melanomas [10]. It should also be taken into account that the mRNA level of individual TGFβ isoforms depends on the type and nature of cells. As reported in the literature, the mRNA transcript for TGFβ-2 is characteristic only for melanoma cells, while the transcripts for TGFβ-1 and TGFβ-3 - for normal and neoplastic cells. The protein products of TGFβ-2 and TGFβ-3 are expressed only by neoplastic cells and participate in melanoma progression [10].
In recent years, a lot of attention has been paid to substances that can help in the fight against this cancer. Many of them are already known, but the effects of substances of natural origin, commonly found in food products, are still being researched. There are more and more studies proving that more and more compounds of plant origin may constitute a new and important agent with pharmacological properties [11]. The latest literature data indicate a group of those that may show anti-inflammatory properties in terms of preventing the development of neoplastic diseases [12]. Such compounds include chlorogenic acid and caffeic acid.
The first is a derivative of a cinnamic acid, a compound structurally composed of caffeic acid and quinic acid. It is a polyphenol that is commonly found in the diet: apples, carrots, artichokes, etc. [13, 14]. It shows mainly anti-inflammatory, antioxidant, anti-neurodegenerative, but also anti-diabetic and anti-lipidemic effects [13, 15, 16]. However, the anti-cancer effect has been widely reported recently [17, 18]. It has been shown that this compound suppresses the proliferation of melanoma cells and reduces reactive oxygen species (ROS), which may reduce photocarcinogenesis [15].
The second compound is caffeic acid, i.e. 3,4-hydroccinamonic acid. It is found in many plants, including apples and coffee. It has antioxidant, antibacterial and anticancer properties [19]. Numerous studies show that caffeic acid inhibits the proliferation of neoplastic cells. Moreover, it has been shown that this compound has the potential to reduce metastases in the neoplastic process [20]. It should be noted that the exact mechanism of these properties has not been fully explained.
Melanocytes present in the skin, but also in the hair and eyes, protect against reactive oxygen species and UV radiation. Consequently, chlorogenic acid and caffeic acid are commonly used in cosmetic products [21].
In recent years, additional attention has been paid to other factors that may support the action of active substances. Among them, there is a static magnetic field (SMF), which is being borne in mind by numerous scientific studies [22, 23]. Research is underway to draw attention to its potential use in medicine. The action of this factor is discussed in terms of its impact on cellular processes important in the pathogenesis of a given disease entity. It should be noted that in some types of cancer it was possible to confirm its potential therapeutic importance in vitro [24] and in vivo [25]. Moreover, there are indications that it may act synergistically with chemotherapeutic agents [25]. Currently, there are no described studies on the direct effect of a SMF with natural substances on various cancer cells, while those focusing on the use of magnetic nanoparticles activated by a magnetic field (MNP) in the treatment of cancer seem to be promising [26, 27]. Therefore, it seems important to analyse the effect of a SMF in the aspect of the application of a bioactive substance derived from food in order to demonstrate their potential anticancer activity.
Materials and methods
Experiments were performed on the C32 cell line (amelanotic melanoma, ATCC, CRL-1585; Manassas, VA, USA) treated with caffeic (CA) or chlorogenic (CGA) acids, and with simultaneous exposure to a moderate-strength static magnetic field.
Cell culture conditions
The cell cultures were performed in Dulbecco’s Modified Eagle Medium (DMEM, Lonza, Basel, Switzerland) enriched with 10% FBS (fetal bovine serum, Sigma-Aldrich, St. Louis, MO, USA), amphotericin B (0.25 mg/ml), and penicillin (10,000 U/ml) in an incubator (Heraeus, Hanau, Germany) with the atmosphere of 5% CO2 at 37 °C. The Countess™ Automated Cell Counter (Invitrogen, Carlsbad, CA, USA) was used to assess the number and viability of cells. Cells between passages four and eight and at approximately 80% confluency for these experiments were used.
The experiment was conducted under the conditions described previously [28]. The C32 cells were seeded in 25 cm2 cell culture flasks (Sarstedt, Nümbrecht, Germany) and after 24 h of incubation, the cells were treated with chlorogenic acid (CGA, Sigma-Aldrich, St. Louis, MO, USA) or caffeic acid (CA, Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 1 mmol/L with simultaneous exposure to a moderate-intensity static magnetic field (0.7 T) and cultured in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37 °C. Control cells and cells that had been treated with phenolic acids and/or SMF were cultured simultaneously under the same conditions. Following next 24 h of incubation, cells were washed in PBS (Phosphate Buffered Saline, Lonza, Basel, Switzerland) and pelleted to further molecular analysis. At the same time, the culture medium was also harvested. Samples were stored at -80 °C until further analysis. Separate exposures (in triplicate) for analyses were performed.
In our experiments, a static magnetic field was generated in a special magnetic chambers consisting of a ferromagnetic yoke and permanent magnet, and fully fitted to the culture flasks (Fig. 1). In control chambers that were used to culture the unexposed cells, the steel (0.0 T) was used instead of permanent magnet [29, 30]. The design of these magnetic chambers allow for uniform distribution of magnetic flux density over the measurement space of the flask. The flux densities in the chambers were measured using a gauss meter.
Fig. 1.
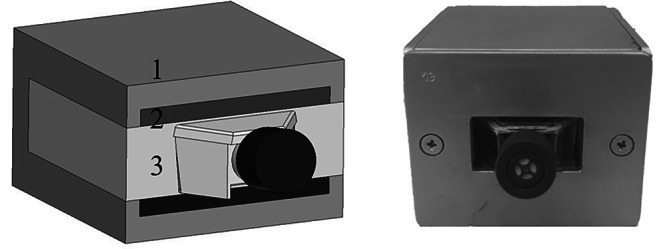
Magnetic test chamber for in vitro culture of cells exposed to a static magnetic field (1 – ferromagnetic yoke; 2 – permanent magnets; 3 – non-magnetic material)
The intensity of field was selected on the based on the previous results that showed the possibility of its use in a cancer treatment [28, 31]. Also the concentration of the phenolic acids was selected on the based on the previous research that showed their cytotoxic properties and the ability to induce apoptosis of melanoma cells while normal cells were not affected [28, 32, 33].
RNA extraction
The RNA extraction was performed using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA) that is specifically formulated to isolate total RNA from many sample types. The procedure was performed according to the manufacturer’s instructions and consisted of the following stages: cell lysis, phase separation, RNA precipitation, RNA wash and RNA dissolving. In the next step, agarose gel electrophoresis and MaestroNano MN-913 (MaestroGen Inc., Las Vegas, NV, USA) spectrophotometer was used for the qualitative and quantitative assessment of RNA extracts.
Real time RT-qPCR assay
The RT-qPCR method (reverse transcription-quantitative polymerase chain reaction) was used to determine the mRNA level of genes encoding the TGFβ isoforms (TGFβ-1, TGFβ-2 and TGFβ-3) and their receptors (TGFβ-R1, TGFβ-R2 and TGFβ-R3). The gene expression were evaluated with SYBR Green I chemistry (SensiFAST SYBR No-ROX One-Step, Bioline, London, UK) and a LightCycler® 480 Instrument II (Roche Life Science, Basel, Switzerland). The oligonucleotide specific primers were commercially available (Sigma-Aldrich, St. Louis, MO, USA). Each reaction was repeated three times. The thermal conditions of the reaction were as follows: reverse transcription, 45 °C − 10 min; activation, 95 °C − 2 min; 45 cycles: denaturation, 95 °C − 5 s; annealing, 60 °C − 10 s and extension, 72 °C − 5 s. At the end melting curve analysis was also performed.
2−(ΔCt) method with β-actin as a reference gene (where ΔCt = Ct of our gene of interest - Ct of β-actin) was used to determine the relative gene expression [34].
ELISA assay
The concentration of the TGFβ1 and TGFβ2 proteins were measured in the acid-activated cell culture supernates using the Quantikine Human TGFβ1 Immunoassay and the Quantikine Human TGFβ2 Immunoassay kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. The absorbance at a wavelength of 450 nm was read on a Wallac 1420 VICTOR microplate reader (PerkinElmer, Waltham, MA, USA). Simultaneously, the TGFβ concentration was measured in the control medium in order to assess the background level of TGFβ. All assays were performed in duplicate.
Statistical analyses
The statistical analyses were performed using Statistica 13.3 software (StatSoft, Tulsa, OK, USA) and the level of significance was set at p < 0.05. In the statistical analyses, the one-way ANOVA and Tukey post hoc tests were used due to the normal distribution of the data.
Results
In our experiment the changes in TGFβ gene expression and TGFβ protein concentration in C32 melanoma cells treated with caffeic or chlorogenic acid and simultaneously exposed to a static magnetic field were determined.
Expression of TGFβ genes and their receptors in C32 melanoma cells
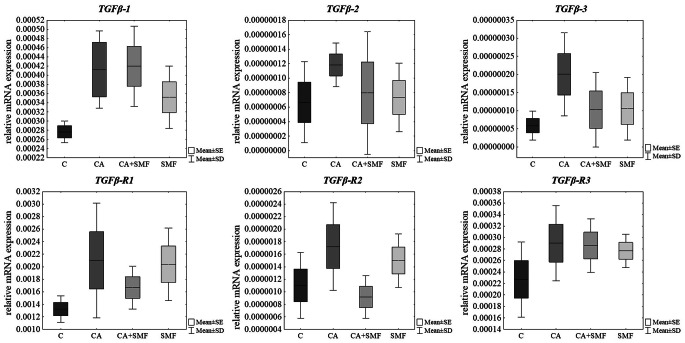
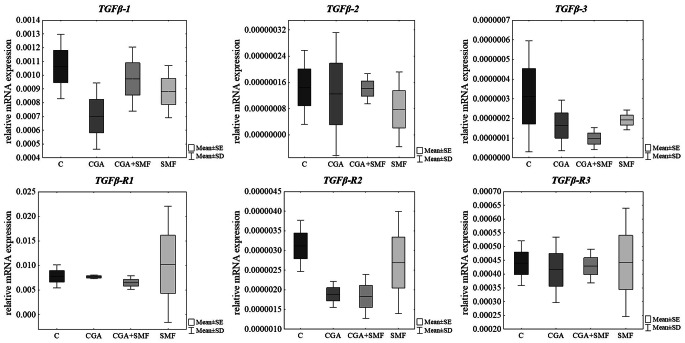
After 24 h of treatment of melanoma cells with a caffeic acid, with a 0.7 T-intensity static magnetic field and both factors no statistically significant alterations in expression of TGFβ isoforms and their receptors in comparison to control cells were observed (Table 1; Fig. 2). Similar results were found after treatment of C32 cells with chlorogenic acid and with simultaneous exposure to a static magnetic field (Table 1; Fig. 3).
Table 1.
Relative mRNA expression of TGFβ isoforms and their receptors in C32 melanoma cells that have been treated with caffeic or chlorogenic acids and that have been exposed to a static magnetic field
| Groups | TGFβ-1 | TGFβ-2 | TGFβ-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FC | Expression | *p | FC | Expression | *p | FC | Expression | *p | |
| Caffeic acid | |||||||||
| CA | 1.49 | ↑ | NS | 1.77 | ↑ | NS | 3.41 | ↑ | NS |
| CA + SMF | 1.52 | ↑ | 1.19 | ↑ | 1.75 | ↑ | |||
| SMF | 1.27 | ↑ | 1.10 | ↑ | 1.80 | ↑ | |||
| Chlorogenic acid | |||||||||
| CGA | -1.51 | ↓ | NS | -1.16 | ↓ | NS | -1.90 | ↓ | NS |
| CGA + SMF | -1.09 | ↓ | -1.03 | ↓ | -3.22 | ↓ | |||
| SMF | -1.20 | ↓ | -1.85 | ↓ | -1.63 | ↓ | |||
| TGFβ-R1 | TGFβ-R2 | TGFβ-R3 | |||||||
| FC | Expression | *p | FC | Expression | *p | FC | Expression | *p | |
| Caffeic acid | |||||||||
| CA | 1.58 | ↑ | NS | 1.56 | ↑ | NS | 1.28 | ↑ | NS |
| CA + SMF | 1.26 | ↑ | -1.20 | ↓ | 1.26 | ↑ | |||
| SMF | 1.54 | ↑ | 1.36 | ↑ | 1.22 | ↑ | |||
| Chlorogenic acid | |||||||||
| CGA | -1.01 | ↓ | NS | -1.65 | ↓ | NS | -1.06 | ↓ | NS |
| CGA + SMF | -1.20 | ↓ | -1.70 | ↓ | -1.02 | ↓ | |||
| SMF | 1.31 | ↑ | -1.16 | ↓ | 1.01 | ↑ | |||
FC – fold change; ↑ - increased expression vs. control; ↓ - decreased expression vs. control;
NS- not significant, one-way ANOVA test;
CA or CGA – cells that have been treated with caffeic or chlorogenic acid;
SMF – cells that have been exposed to a static magnetic field;
CA or CGA + SMF – cells that have simultaneously been exposed to caffeic or chlorogenic acid and a static magnetic field
Fig. 2.
Relative mRNA expression of TGFβ isoforms and their receptors in cells that have been treated with caffeic acid and cells that have been exposed to a static magnetic field. The results are presented as a mean ± SD; C – control cells; CA – cells that have been treated with caffeic acid; SMF – cells that have been exposed to a static magnetic field; CA + SMF – cells that have simultaneously been exposed to caffeic acid and a static magnetic field
Fig. 3.
Relative mRNA expression of TGFβ isoforms and their receptors in cells that have been treated with chlorogenic acid and cells that have been exposed to a static magnetic field. The results are presented as a mean ± SD; C – control cells; CGA – cells that have been treated with chlorogenic acid; SMF – cells that have been exposed to a static magnetic field; CGA + SMF – cells that have simultaneously been exposed to chlorogenic acid and a static magnetic field
Concentration of TGFβ proteins in C32 cell supernates
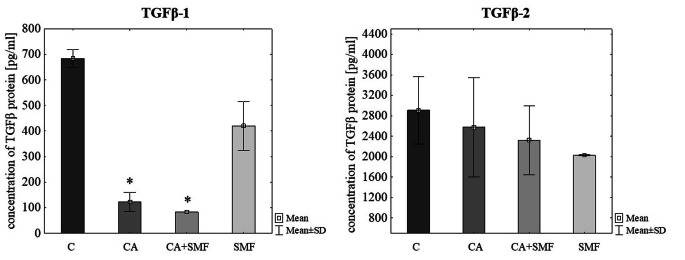
At protein level it was found that caffeic acid and caffeic acid with simultaneous exposure to a moderate-strength static magnetic field cause a statistically significant reduction of TGFβ-1 molecule concentration in comparison to control cells (p = 0.009, p = 0.013, respectively, Tukey post hoc test). However, both factors did not have a significant effect on the concentration of TGFβ-2 protein (Table 2; Fig. 4).
Table 2.
The TGFβ-1 and − 2 protein concentration in supernates of C32 melanoma cell cultures
| Groups | TGFβ-1 | TGFβ-2 | |||
|---|---|---|---|---|---|
| FC | *p | FC | *p | ||
| Caffeic acid | |||||
| CA | -5.57 | 0.008 | -1.13 | NS | |
| CA + SMF | -8.21 | -1.25 | |||
| SMF | -1.63 | -1.44 | |||
| Chlorogenic acid | |||||
| CGA | -1.34 | 0.013 | -1.24 | 0.007 | |
| CGA + SMF | -1.60 | -1.35 | |||
| SMF | -3.45 | -1.52 | |||
FC – fold change;
*p < 0.05, NS- not significant, one-way ANOVA test
CA or CGA – cells that have been treated with caffeic or chlorogenic acid;
SMF – cells that have been exposed to a static magnetic field;
CA or CGA + SMF – cells that have simultaneously been exposed to caffeic or chlorogenic acid and a static magnetic field
Fig. 4.
The TGFβ-1 and TGFβ-1 protein concentration in the C32 cells after exposure to the caffeic acid and a static magnetic field. The results are presented as a mean ± SD; C – control cells; CA – cells that have been treated with caffeic acid; SMF – cells that have been exposed to a static magnetic field; CA + SMF – cells that have simultaneously been exposed to caffeic acid and a static magnetic field; *p < 0.05 vs. C
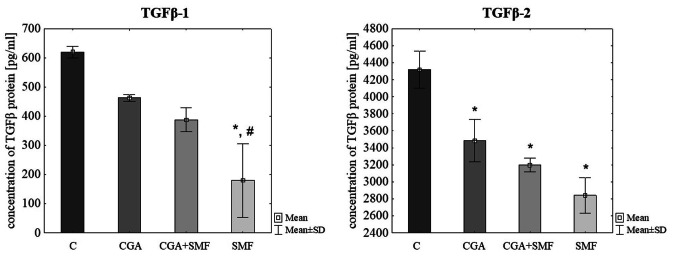
In case of chlorogenic acid there was indicated a significant reduction of TGFβ-1 protein concentration in cells that have been exposed to a SMF in comparison to control (p = 0.010, Tukey post hoc test). Statistically significant differences in the concentration of this protein were also observed between cells that have been treated with a SMF and cells that have been treated with a CGA (p = 0.045, Tukey post hoc test). There was also a statistically significant decrease in the concentration of TGFβ-2 molecule in CGA-treated cells, in SMF-exposed cells, and in cells that have been simultaneously treated with both factors (p = 0.045, p = 0.006, p = 0.017, respectively, Tukey post hoc test) (Table 2; Fig. 5).
Fig. 5.
The TGFβ-1 and TGFβ-1 protein concentration in the C32 cells after exposure to the chlorogenic acid and a static magnetic field. The results are presented as a mean ± SD; C – control cells; CGA – cells that have been treated with chlorogenic acid; SMF – cells that have been exposed to a static magnetic field; CGA + SMF – cells that have simultaneously been exposed to chlorogenic acid and a static magnetic field; *p < 0.05 vs. C; #p < 0.05 vs. CGA
Summarizing the obtained results, the first response of C32 melanoma cells to both factors (phenolic acids and a SMF) is the reduction of TGFβ levels, which is visible in 24 h of experiment at the protein level. Then, mRNA level of these molecules returned to values close to pre-treatment level by the end of experiment.
Discussion
Considering the increasing number of patients diagnosed for melanoma and the still not fully known etiopathogenesis of this disease, newer and newer diagnostic markers of this cancer are being searched for. Our focus was the TGFβ pathway as a therapeutic target in the treatment of melanoma. Previous studies have shown the role of TGFβ in the development and progression of melanoma. Cesaro et al. [9] indicated the participation of TGFβ in tumor development. The research was carried out in terms of the role of the zinc finger protein ZNF224 in signaling the TGFβ pathway as a stimulator of the pro-oncogenic function of TGFβ favourable to epithelial-mesenchymal transition [9]. Moreover, Gipsyianti et al. [35] noted the participation of both TGFβ and cyclooxygenase- 2 (COX-2) in the development of acral melanoma (AM), as well as the depth and invasion of the lesion. The involvement of the TGFβ pathway in the development of melanoma has also been noted by Ren et al. [36]. In our research, we focused on the aspect of changes in TGFβ expression due to the use of plant compounds and the physical factor. The aim of our study was to find out whether these chemical and physical factors could potentially be used to support cancer therapy and limit the tumor development through the suppression of pro-tumorigenic TGFβ. Plant compounds are widely used in the treatment of various diseases, including cancer. In our previous research, we have already proved the positive effect of chlorogenic and caffeic acid and a static magnetic field on melanoma cells in the aspect of oxidative stress, cell redox homeostasis and apoptosis [28, 33]. In turn, Li et al. [37] demonstrated the effectiveness of treatment consisting of a combination of sialic acid modified chlorogenic acid liposome therapy (CA-SAL) in combination with anti-PD1 (anti-programmed death protein 1) antibody treatment. Moreover, the research by Choi et al. [38] also showed the positive effect of Phyllostachys nigra active compounds on melanoma cells. It is worth noting that the active ingredients of this plant include those we have studied: caffeic acid and chlorogenic acid. These acids are also the active ingredients of the tincture of European mistletoe (Viscum album L.). The authors showed that these compounds reduced the growth of tumor cells in a dose-dependent manner in murine melanoma cells [39].
In our study, we decided to check the effect of a SMF in combination with plant compounds on the reduction of tumor development as a result of modification of TGFβ expression. The topic is quite innovative due to the small amount of research on a SMF. It would seem that a SMF is applicable because of its documented performance. A SMF has been shown to influence tumor blood flow by reducing it and thus impair angiogenesis and growth of solid tumors in vivo. There are studies showing that exposure of mice treated with cis-diaminodichloroplatinum (cis-platinum) and (2-chloroethyl) tetrahydro-2 H-1,3,2-oxazaphosphite-2-N, N-bis 2-oxide -amines (cyclophosphamide) to a SMF contributed to the desired therapeutic effect [40]. But molecular mechanism its action on cells is not fully explained. On the other hand, there are recent reports showing that cell response cells to a magnetic field can be related to the radical pair mechanism [41, 42] and ion cyclotron resonance [43].
Polyphenols, including phenolic acids, as bioactive substances, can influence the expression of many genes and thus may regulate a number of signaling pathways in cells, including those related to TGFβ [44, 45]. Also, a SMF can affect signaling pathways by modifying the concentration of secondary messengers such as calcium ions, reactive oxygen species, and changing the biophysical properties of cell membranes [46]. In the study by Bekhite et al. [47], the researchers showed that a static magnetic field enhanced cardiomyocyte differentiation of mouse embryonic stem cells. The proposed mechanism of a SMF action is related with Ca2+ influx that is involved in reactive oxygen species production and induction of cardiomyogenesis. In turn, Novikov et al. [48] observed that combined magnetic field (CMF) caused a decrease of the respiratory burst in neutrophils probably through the effect of a CMF at the resonance frequency of Fe3+. Also Gurhan et al. [49] concluded that a static magnetic field with different intensities affects many biological parameters such as cell growth, membrane potential, mitochondrial calcium concentration and oxidative stress in HT-1080 fibrosarcoma cells. Hence, a both polyphenols and a SMF can act synergistically together. Furthermore, we previously demonstrated their synergistic effect in inducing apoptosis in C32 cells [28] and also that a static magnetic field may be use in the design of new therapeutic strategies for diseases in the pathogenesis of which are played by disturbance of the signaling cascades that are associated with the activity of TGFβ [50]. Their common effect on cells is rather not due to the influence of a SMF on the structure of molecules [51], but it results from their similar mechanisms of action. Moreover, it was found that a high magnetic field influences the regulation of cell signaling pathways by impact on the diffusion of di- and paramagnetic molecules in cytoplasm of cells [52].
In our study, we showed that a SMF together with the tested acids may support the melanoma treatment, and thus reduces the development of cancer, through modification of TGFβ expression. We have shown that in cells that have been exposed to both factors: phenolic acids and a SMF, expression of TGFβ-1 and − 2 was decreased, which is best illustrated by changes in the protein level after 24 h. In turn, mRNA level of these molecules and their receptors was normalized at the end of experiment. Therefore, no statistical differences at mRNA level were noted [53]. A similar trend in the direction and dynamics of changes in mRNA and protein expression was observed by Cheng et al. [54]. They indicated that mRNAs and proteins are modulated with different temporal patterns and they observed the restoration of mRNA levels close to pre-treatment levels. On the other hand, the direction of changes in the level of protein expression was unidirectional [54]. The authors of the review by Liu et al. [55] reached similar conclusions, who considered many possibilities of the observed situation.
It should also be emphasized that in research on gene expression using various techniques, including RT-qPCR, the number of biological replicates is very important. However, the majority of gene expression studies is limited to three replicates which may affect the statistical power [56]. On the other hand, Udvardi et al. [57] and Rieu and Powers [58] found that experiments based on RT-qPCR technique should include at least three biological replicate to facilitate statistical analysis of data. Another important factor affecting gene expression profile and cell metabolism is cell confluency. Hence, the time of experiment and time of cell treatment with tested factors is very essential. Often in in vitro experiments the treatment time is 24 h.
Taken together, our study results demonstrate the potential of polyphenols and a moderate-strength static magnetic field to support cancer therapy by altering TGFβ expression, which is a very promising topic for the diagnosis and treatment of melanoma. It should be noted that although our studies show the potential use of phenolic acids and a SMF in melanoma, they have limitations. The use of a positive control would increase the value of the results, which should be taken into account when planning further experiments. Moreover, further research should be extended to the effects of phenolic acids and a SMF with various magnetic induction on other signaling molecules and also other cancer cell lines.
Abbreviations
- AM
acral melanoma
- C32 cell line
amelanotic melanoma
- CA
caffeic acid
- CGA
chlorogenic acid
- CM
cutaneous melanoma
- CMF
combined magnetic field
- COX-2
cyclooxygenase
- Jak/STAT
Janus kinase/signal transducer and activator of transcription
- MNP
magnetic nanoparticles
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
- SMF
static magnetic field
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- TGFβ
transforming growth factor β
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Magdalena Kimsa-Dudek and Agnieszka Synowiec-Wojtarowicz. The first draft of the manuscript was written by Magdalena Kimsa-Dudek and Agata Krawczyk. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by grant PCN-2-017/N/1/I from the Medical University of Silesia, Katowice, Poland.
Declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dzwierzynski WW. Melanoma Risk factors and Prevention. Clin Plast Surg. 2021;48(4):543–550. doi: 10.1016/j.cps.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera R, Recule F. Unusual clinical presentations of malignant melanoma: a review of clinical and histologic features with special emphasis on dermatoscopic findings. Am J Clin Dermatol. 2018;19(Suppl 1):15–23. doi: 10.1007/s40257-018-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert L, Lebrun JJ. TGF-beta inhibits human cutaneous melanoma cell migration and invasion through regulation of the plasminogen activator system. Cell Signal. 2013;25(2):490–500. doi: 10.1016/j.cellsig.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Curti BD, Faries MB. Recent advances in the treatment of Melanoma. N Engl J Med. 2021;384(23):2229–2240. doi: 10.1056/NEJMra2034861. [DOI] [PubMed] [Google Scholar]
- 5.Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28(6):1005–1011. [PubMed] [Google Scholar]
- 6.Ribero S, Glass D, Bataille V. Genetic epidemiology of melanoma. Eur J Dermatol. 2016;26(4):335–339. doi: 10.1684/ejd.2016.2787. [DOI] [PubMed] [Google Scholar]
- 7.Gajos-Michniewicz A, Czyz M. WNT signaling in Melanoma. Int J Mol Sci. 2020;21(14):4852. doi: 10.3390/ijms21144852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X, Yuan L, Ma T. Mechanisms of JAK-STAT signaling pathway mediated by CXCL8 gene silencing on epithelial-mesenchymal transition of human cutaneous melanoma cells. Oncol Lett. 2020;20(2):1973–1981. doi: 10.3892/ol.2020.11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesaro E, Pastore A, Polverino A, Manna L, Divisato G, Quintavalle C, Sanzo MD, Faniello MC, Grosso M, Costanzo P. ZNF224 is a mediator of TGF-β pro-oncogenic function in melanoma. Hum Mol Genet. 2021;30(22):2100–2109. doi: 10.1093/hmg/ddab173. [DOI] [PubMed] [Google Scholar]
- 10.Busse A, Keilholz U. Role of TGF-β in melanoma. Curr Pharm Biotechnol. 2011;12(12):2165–2175. doi: 10.2174/138920111798808437. [DOI] [PubMed] [Google Scholar]
- 11.Jin S, Kim KC, Kim JS, Jang KI, Hyun TK. Anti-Melanoma Activities and Phytochemical Compositions of Sorbus commixta fruit extracts. Plants (Basel) 2020;9(9):1076. doi: 10.3390/plants9091076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortes C. Are anti-inflammatory foods associated with a protective effect for cutaneous melanoma? Eur J Cancer Prev. 2020;29(5):466–469. doi: 10.1097/CEJ.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 13.Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: recent advances on its dual role as a Food Additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22(3):358. doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santana-Gálvez J, Pérez-Carrillo E, Velázquez-Reyes HH, Cisneros-Zevallos L, Jacobo-Velázquez DA. Application of wounding stress to produce a nutraceutical-rich carrot powder ingredient and its incorporation to nixtamalized corn flour tortillas. J Funct Foods. 2016;27:655–666. doi: 10.1016/j.jff.2016.10.020. [DOI] [Google Scholar]
- 15.Li HR, Habasi M, Xie LZ, Aisa HA. Effect of chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules. 2014;19(9):12940–12948. doi: 10.3390/molecules190912940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang N, Kitts DD. Role of chlorogenic acids in Controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8(1):16. doi: 10.3390/nu8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Changizi Z, Moslehi A, Rohani AH, Eidi A. Chlorogenic acid induces 4T1 breast cancer tumor’s apoptosis via p53, bax, Bcl-2, and caspase-3 signaling pathways in BALB/c mice. J Biochem Mol Toxicol. 2021;35(2):e22642. doi: 10.1002/jbt.22642. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Li X, Cuiping C, Pu R, Weihua Y (2020) Study on the Anticancer Effect of an Astragaloside- and Chlorogenic Acid-Containing Herbal Medicine (RLT-03) in Breast Cancer. Evid Based Complement Alternat Med. 2020:1515081. 10.1155/2020/1515081 [DOI] [PMC free article] [PubMed]
- 19.Maruyama H, Kawakami F, Lwin TT, Imai M, Shamsa F. Biochemical characterization of Ferulic Acid and Caffeic Acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol Pharm Bull. 2018;41(5):806–810. doi: 10.1248/bpb.b17-00892. [DOI] [PubMed] [Google Scholar]
- 20.Pelinson LP, Assmann CE, Palma TV, da Cruz IBM, Pillat MM, Mânica A, Stefanello N, Weis GCC, de Oliveira Alves A, de Andrade CM, Ulrich H, Morsch VMM, Schetinger MRC, Bagatini MD. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol Biol Rep. 2019;46(2):2085–2092. doi: 10.1007/s11033-019-04658-1. [DOI] [PubMed] [Google Scholar]
- 21.Wianowska D, Typek R, Dawidowicz AL. Chlorogenic acid stability in pressurized liquid extraction conditions. J AOAC Int. 2015;98(2):415–421. doi: 10.5740/jaoacint.14-200. [DOI] [PubMed] [Google Scholar]
- 22.Satari M, Javani Jouni F, Abolmaleki P, Soleimani H. Influence of static magnetic field on HeLa and Huo2 cells in the Presence of Aloe vera Extract. Asian Pac J Cancer Prev. 2021;22(S1):9–15. doi: 10.31557/APJCP.2021.22.S1.9. [DOI] [PubMed] [Google Scholar]
- 23.Song C, Yu B, Wang J, Ji X, Zhang L, Tian X, Yu X, Feng C, Wang X, Zhang X (2021) Moderate Static Magnet Fields Suppress Ovarian Cancer Metastasis via ROS-Mediated Oxidative Stress. Oxid Med Cell Longev 2021:7103345. 10.1155/2021/7103345 [DOI] [PMC free article] [PubMed]
- 24.Zhang L, Wang J, Wang H, Wang W, Li Z, Liu J, Yang X, Ji X, Luo Y, Hu C, Hou Y, He Q, Fang J, Wang J, Liu Q, Li G, Lu Q, Zhang X. Moderate and strong static magnetic fields directly affect EGFR kinase domain orientation to inhibit cancer cell proliferation. Oncotarget. 2016;7(27):41527–41539. doi: 10.18632/oncotarget.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gellrich D, Schmidtmayer U, Eckrich J, Hagemann J, Becker S, Strieth S. Modulation of exposure to static magnetic field affects targeted therapy of solid tumors in vivo. Anticancer Res. 2018;38(8):4549–4555. doi: 10.21873/anticanres.12759. [DOI] [PubMed] [Google Scholar]
- 26.Darroudi M, Gholami M, Rezayi M, Khazaei M. An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery. J Nanobiotechnol. 2021;19(1):399. doi: 10.1186/s12951-021-01150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spyridopoulou K, Makridis A, Maniotis N, Karypidou N, Myrovali E, Samaras T, Angelakeris M, Chlichlia K, Kalogirou O. Effect of low frequency magnetic fields on the growth of MNP-treated HT29 colon cancer cells. Nanotechnology. 2018;29(17):175101. doi: 10.1088/1361-6528/aaaea9. [DOI] [PubMed] [Google Scholar]
- 28.Kimsa-Dudek M, Synowiec-Wojtarowicz A, Krawczyk A, Kosowska A, Kimsa-Furdzik M, Francuz T. The apoptotic effect of caffeic or chlorogenic acid on the C32 cells that have simultaneously been exposed to a static magnetic field. Int J Mol Sci. 2022;23(7):3859. doi: 10.3390/ijms23073859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gawron S, Glinka M, Wolnik T. Magnetyczna komora badawcza dedykowana do hodowli komórek. Zesz Probl Masz Elektr. 2012;4:11–16. [Google Scholar]
- 30.Glinka M, Gawron S, Sieroń A, Pawłowska-Góral K, Cieślar G, Sieroń-Stołtny K. Test chambers for cell culture in static magnetic field. J Magn Mater. 2013;331:208–215. doi: 10.1016/j.jmmm.2012.11.042. [DOI] [Google Scholar]
- 31.Dini L, Abbro L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron. 2005;36(3):195–217. doi: 10.1016/j.micron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Kimsa-Dudek M, Krawczyk A, Synowiec-Wojtarowicz A. Redox homeostasis in melanoma cells treated with caffeic acid. In: Biały Ł, Młynarczuk-Biały I, editors. Advances in Biomedical Research—from COVID to Medical Humanities. Lublin: Wydawnictwo Naukowe Tygiel Sp. z o.o; 2020. pp. 88–96. [Google Scholar]
- 33.Kimsa-Dudek M, Krawczyk A, Synowiec-Wojtarowicz A, Dudek S, Pawłowska-Góral K. The impact of the co-exposure of melanoma cells to chlorogenic acid and a moderate-strength static magnetic field. J Food Biochem. 2020;44(12):e13512. doi: 10.1111/jfbc.13512. [DOI] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 35.Gipsyianti N, Aziz A, Hernowo BS, Usman HA. High expression of COX-2 Associated with the depth of Invasion on Acral Melanoma by increasing TGF-β1. Clin Cosmet Investig Dermatol. 2021;14:209–216. doi: 10.2147/CCID.S285564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren YQ, Li QH, Liu LB. USF1 prompt melanoma through upregulating TGF-β signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20(17):3592–3598. [PubMed] [Google Scholar]
- 37.Li X, Zhu S, Yin P, Zhang S, Xu J, Zhang Q, Shi S, Zhang T. Combination immunotherapy of chlorogenic acid liposomes modified with sialic acid and PD-1 blockers effectively enhances the anti-tumor immune response and therapeutic effects. Drug Deliv. 2021;28(1):1849–1860. doi: 10.1080/10717544.2021.1971797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi MH, Jo HG, Yang JH, Ki SH, Shin HJ. Antioxidative and anti-melanogenic activities of bamboo stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF downregulation in B16F10 melanoma cells. Int J Mol Sci. 2018;19(2):409. doi: 10.3390/ijms19020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melo MNO, Oliveira AP, Wiecikowski AF, Carvalho RS, Castro JL, de Oliveira FAG, Pereira HMG, da Veiga VF, Capella MMA, Rocha L, Holandino C. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm J. 2018;26(3):311–322. doi: 10.1016/j.jsps.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tofani S, Barone D, Berardelli M, Berno E, Cintorino M, Foglia L, Ossola P, Ronchetto F, Toso E, Eandi M. Static and ELF magnetic fields enhance the in vivo anti-tumor efficacy of cis-platin against lewis lung carcinoma, but not of cyclophosphamide against B16 melanotic melanoma. Pharmacol Res. 2003;48(1):83–90. [PubMed] [Google Scholar]
- 41.Pooam M, Jourdan N, El Esawi M, Sherrard RM, Ahmad M. HEK293 cell response to static magnetic fields via the radical pair mechanism may explain therapeutic effects of pulsed electromagnetic fields. PLoS ONE. 2020;15(12):e0243038. doi: 10.1371/journal.pone.0243038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zadeh-Haghighi H, Simon C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J R Soc Interface. 2022;19(193):20220325. doi: 10.1098/rsif.2022.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pazur A. Calcium ion cyclotron resonance in dissipative water structures. Electromagn Biol Med. 2018;37(2):100–113. doi: 10.1080/15368378.2018.1434789. [DOI] [PubMed] [Google Scholar]
- 44.Ding S, Xu S, Fang J, Jiang H. The Protective Effect of Polyphenols for Colorectal Cancer. Front Immunol. 2020;11:1407. doi: 10.3389/fimmu.2020.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun LR, Zhou W, Zhang HM, Guo QS, Yang W, Li BJ, Sun ZH, Gao SH, Cui R. Modulation of multiple signaling pathways of the Plant-Derived Natural Products in Cancer. Front Oncol. 2019;9:1153. doi: 10.3389/fonc.2019.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Sarje A, Che PL, Yarema KJ. Moderate strength (0.23–0.28 T) static magnetic fields (SMF) modulate signaling and differentiation in human embryonic cells. BMC Genomics. 2009;10:356. doi: 10.1186/1471-2164-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bekhite MM, Figulla HR, Sauer H, Wartenberg M. Static magnetic fields increase cardiomyocyte differentiation of Flk-1 + cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int J Cardiol. 2013;167(3):798–808. doi: 10.1016/j.ijcard.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Novikov VV, Yablokova EV, Fesenko EE. A decrease of the respiratory burst in neutrophils after exposure to weak combined magnetic Fields of a certain duration. Biophysics. 2020;65:82–87. doi: 10.1134/S0006350920010157. [DOI] [Google Scholar]
- 49.Gurhan H, Bruzon R, Kandala S, Greenebaum B, Barnes F. Effects Induced by a weak static magnetic field of different intensities on HT-1080 Fibrosarcoma cells. Bioelectromagnetics. 2021;42(3):212–223. doi: 10.1002/bem.22332. [DOI] [PubMed] [Google Scholar]
- 50.Kimsa-Dudek M, Synowiec-Wojtarowicz A, Krawczyk A, Kruszniewska-Rajs C, Gola J. A static magnetic field changes the expression profile of the transforming growth factor β family genes in human cells that have been treated with fluoride ions. Cytokine. 2021;143:155537. doi: 10.1016/j.cyto.2021.155537. [DOI] [PubMed] [Google Scholar]
- 51.Panczyk T, Camp PJ. Lorentz forces induced by a static magnetic field have negligible effects on results from classical molecular dynamics simulations of aqueous solutions. J Mol Liq. 2021;330:115701. doi: 10.1016/j.molliq.2021.115701. [DOI] [Google Scholar]
- 52.Zablotskii V, Polyakova T, Dejneka A. Effects of high magnetic Fields on the diffusion of biologically active molecules. Cells. 2021;11(1):81. doi: 10.3390/cells11010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4(9):117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng Z, Teo G, Krueger S, Rock TM, Koh HW, Choi H, Vogel C. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol Syst Biol. 2016;12(1):855. doi: 10.15252/msb.20156423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Beyer A, Aebersold R. On the Dependency of Cellular protein levels on mRNA abundance. Cell. 2016;165(3):535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Kokou F, Adamidou S, Karacostas I, Sarropoulou E. Sample size matters in dietary gene expression studies—A case study in the gilthead sea bream (Sparus aurata L.) Aquaculture Rep. 2016;3:82–87. doi: 10.1016/j.aqrep.2015.12.004. [DOI] [Google Scholar]
- 57.Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20(7):1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rieu I, Powers SJ. Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell. 2009;21(4):1031–1033. doi: 10.1105/tpc.109.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]