Summary
Intramuscular fatty infiltration in muscle injuries and diseases, caused by aberrant adipogenesis of fibro-adipogenic progenitors, negatively impacts function. Intramuscular delivery of wingless-type MMTV integration site family 7a (WNT7A) offers a promising strategy to stimulate muscle regeneration, but its effects on adipogenic conversion of fibro-adipogenic progenitors remain unknown. Here, we show that WNT7A decreases adipogenesis of fibro-adipogenic progenitors (FAPs) by inducing nuclear localization of Yes-associated protein (YAP) through Rho in a β-CATENIN-independent manner and by promoting nuclear retention of YAP and transcriptional co-activator with PDZ-binding motif (TAZ) in differentiating FAPs. Furthermore, intramuscular injection of WNT7A in vivo effectively suppresses fatty infiltration in mice following glycerol-induced injury. Our results collectively suggest WNT7A as a potential protein-based therapeutic for diminishing adipogenesis of FAPs and intramuscular fatty infiltration in pathological muscle injuries or diseases.
Keywords: WNT7a, skeletal muscle, fatty infiltration, adipogenesis, YAP/TAZ, fibro-adipogenic progenitors, mesenchymal stem cells
Highlights
-
•
WNT7A decreases the adipogenic potential of fibro-adipogenic progenitors
-
•
WNT7A does not promote nuclear localization of β-CATENIN
-
•
WNT7A promotes nuclear activation of YAP/TAZ in fibro-adipogenic progenitors
-
•
Delivery of WNT7A decreases intramuscular fatty infiltration in vivo
In this article, Fu and colleagues show that WNT7A decreases adipogenesis of skeletal muscle-derived fibro-adipogenic progenitors. WNT7A promotes nuclear localization and retention of YAP/TAZ in a β-CATENIN-independent manner in differentiating fibro-adipogenic progenitors. When administered intramuscularly in vivo following glycerol-induced injury, WNT7A significantly suppressed fatty infiltration.
Introduction
Persistent fatty infiltration, known as myosteatosis, is a common hallmark of chronic skeletal muscle injuries and diseases that negatively impacts function and poses significant health and socioeconomic burden (Minagawa et al., 2013; Yamamoto et al., 2010). For example, in rotator cuff tendon injuries, irreversible fatty infiltration is prevalent in the associated muscles, which directly increases muscle dysfunction and retear rates following surgical repair (Fu et al., 2021; Gladstone et al., 2007; Park et al., 2015b). In muscular dystrophies, pervasive intramuscular fatty infiltration positively correlates with the disease severity (Li et al., 2015). Fatty infiltration is also common in the paraspinal and neck muscles of astronauts following spaceflights (Burkhart et al., 2019; McNamara et al., 2019). Recent evidence suggests that fibro-adipogenic progenitors (FAPs), a population of muscle-resident mesenchymal stem/stromal cells, are the primary cellular culprit that generates intramuscular fatty infiltration (Joe et al., 2010; Liu et al., 2016; Uezumi et al., 2010; Wosczyna et al., 2012). Although this link between FAPs and fatty infiltration is established, therapies that limit such pathologic fatty infiltration without compromising myogenesis currently do not exist.
FAPs play a critical role in muscle regeneration by secreting paracrine factors that promote activation and expansion of muscle stem/satellite cells (Joe et al., 2010; Uezumi et al., 2010; Wosczyna et al., 2012, 2019). Furthermore, skeletal muscles with depleted FAPs not only exhibit significantly impaired muscle regeneration but also lead to muscle atrophy under homeostatic conditions, highlighting the importance of FAPs in muscle maintenance (Wosczyna et al., 2019). Upon muscle injury, immune cells initially infiltrate the injured space to remove debris and activate both FAPs and satellite cells (Butterfield et al., 2006; Heredia et al., 2013; Tidball and Villalta, 2010). As the inflammation resolves, tumor necrosis factor α (TNF-α) released by macrophages induces apoptotic clearances of FAPs, while activated satellite cells continue to undergo myogenesis (Lemos et al., 2015). In chronic muscle pathology, however, FAPs undergo unchecked proliferation and give rise to adipocytes and myofibroblasts (Lemos et al., 2015). The resulting fatty infiltration and fibrosis perturb the highly aligned and organized muscle structure and consequently reduce the ability of muscles to contract and regenerate. Thus, identifying molecular mechanisms that regulate the adipogenic conversion of FAPs is critical for establishing strategies to combat pathologic fatty infiltration.
Intramuscular delivery of wingless-type MMTV integration site family 7a (WNT7A) offers a promising strategy to both stimulate muscle regeneration and prevent muscle degeneration (Han et al., 2019; von Maltzahn et al., 2012, 2013; Schmidt et al., 2020). WNT7A promotes myofiber hypertrophy through the non-canonical AKT/mTOR pathway and increases the symmetric expansion of muscle satellite cells through the non-canonical planar cell polarity pathway (Le Grand et al., 2009; von Maltzahn et al., 2011). In preclinical models of Duchenne muscular dystrophy, WNT7A administration significantly increases satellite cell quantity, myofiber hypertrophy, and muscle strength (von Maltzahn et al., 2012). Controlled delivery of WNT7A using a bioengineered hydrogel also increases satellite cell quantity and myofiber hypertrophy, presenting WNT7A as an effective therapeutic candidate for treating various acute and degenerative muscle conditions (Han et al., 2019). While the past findings collectively corroborate that WNT7A can be used as a potential pro-myogenic therapeutic, the effect of WNT7A on FAPs, and specifically whether it limits adipogenic conversion of FAPs, remains unknown.
The objective of this study was to determine the mechanistic effect of WNT7A on FAPs adipogenic conversion. By using freshly isolated primary murine FAPs, we demonstrate that WNT7A effectively decreases the adipogenic potential of FAPs. We show that while WNT7A does not directly increase nuclear localization of β-CATENIN, it does induce nuclear localization of Yes-associated protein (YAP) through Rho and promotes nuclear retention of YAP and transcriptional co-activator with PDZ-binding motif (TAZ) in differentiating FAPs. We additionally show that WNT7A suppresses intramuscular fatty infiltration following glycerol injury without negatively impacting myogenesis or causing fibrosis in vivo. Collectively, we provide mechanistic evidence that WNT7A effectively reduces adipogenesis of FAPs and intramuscular fatty infiltration.
Results
WNT7A decreases adipogenesis of FAPs
To evaluate the effect of WNT7A on lineage specification of differentiating FAPs, we carried out a series of in vitro experiments using primary murine FAPs. Primary FAPs (CD31−, CD45−, ITGA7−, SCA1+) were isolated from the hindlimb muscles of C57Bl6/J mice using previously reported methods (Figure S1A) (Marinkovic et al., 2019). Freshly isolated FAPs express PDGFRα (80.4% ± 4.8%; Figure S1B), a defining marker of murine FAPs (Joe et al., 2010; Uezumi et al., 2010). Primary FAPs differentiated into myofibroblasts characterized by stress fibers expressing α-smooth muscle actin (αSMA) when maintained in fibrogenic differentiation medium (FM) for 4 days (Figures S1C and S1D; p < 0.001), but when maintained in adipogenic differentiation medium (ADM) for 4 days, FAPs differentiated into oil red O (ORO)+/PLIN1+ adipocytes (Figures S1C–S1F; p < 0.001). Collectively, these results confirm the functional identity of the isolated primary FAPs to be used in the subsequent experiments.
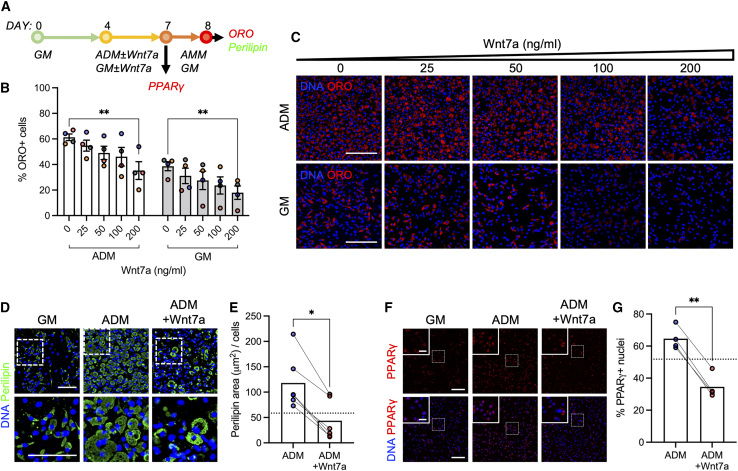
To determine the dose-dependent effect of WNT7A on the adipogenic potential of FAPs, we seeded freshly isolated FAPs in a dish and let the cells proliferate to near confluency for 4 days in growth media. Proliferating FAPs were further maintained in growth medium (GM ± WNT7A) or ADM (ADM ± WNT7A) for an additional 3–4 days (Figure 1A). Note, in GM, the FAPs begin to spontaneously differentiate into both myofibroblasts and adipocytes (Joe et al., 2010). In both GM and ADM, WNT7A decreased adipogenesis in a dose-dependent manner (Figures 1B and 1C). The dose of 200 ng/mL significantly reduced the formation of ORO+ adipocytes compared with the control (0 ng/mL; Figures 1B and 1C; p < 0.01). Based on this, we chose to use 200 ng/mL for the subsequent in vitro experiments.
Figure 1.
WNT7A decreases FAP adipogenesis
(A) Experimental timeline. GM, growth media; ADM, adipogenic differentiation media; AMM, adipogenic maintenance media.
(B) Percentage of ORO+ cells treated with varying doses of WNT7A. Two-way ANOVA with Bonferroni post-hoc analyses. Mean ± SEM. Dose effect p = 0.0021. Media effect p < 0.0001. ∗∗p < 0.01. n = 4. Colors represent biological replicates.
(C) Representative images of ORO-labeled cells treated with varying doses of WNT7A. Scale bar: 100 μm.
(D) Representative immunofluorescence images of perilipin-labeled FAPs cultured in GM and ADM ± WNT7A (200 ng/mL). Scale bar: 100 μm.
(E) Perilipin area normalized by cell quantity. Two-tailed unpaired t test. ∗p < 0.05. n = 6. Dotted line: mean of GM.
(F) Representative immunofluorescence images of PPARγ-labeled FAPs cultured in GM and ADM ± WNT7A (200 ng/mL). Scale bar: 100 μm. Inset scale bar: 25 μm.
(G) Percentage of PPARγ+ nuclei. Two-tailed unpaired t test. ∗∗p < 0.01. n = 4. Dotted line: mean of GM.
To further validate the effect of WNT7A on the suppression of FAPs adipogenesis, we cultured freshly isolated FAPs to near confluency and subsequently maintained the cells in either GM or ADM, with or without 200 ng/mL WNT7A (Figure 1A). Note, the dH2O vehicle for WNT7A (0.2% v/v in media) does not affect FAP adipogenesis (Figure S2). WNT7A significantly reduced the formation of PLIN1+ (perilipin; lipid droplet-associated protein) adipocytes (Figures 1D and 1E; p < 0.0001) as well as nuclear activation of PPARγ, a master regulator of adipogenesis, compared with the control (Figures 1F and 1G; p < 0.01). In the adipogenic condition, WNT7A also reduced PLIN1 and PPARγ expressions to below the mean of spontaneously differentiating condition (GM; Figures 1D–1G). These results demonstrate that WNT7A effectively decreases the adipogenic potential of FAPs.
We next quantified the number of cells expressing αSMA+ stress fibers, a hallmark of activated myofibroblasts (Goffin et al., 2006; Hinz, 2007), to determine if WNT7A increases fibrogenesis in the adipogenic condition. We observed that PLIN1− FAPs in ADM with or without WNT7A treatment exhibited a base level of diffuse αSMA, but only in the fibrogenic (FM) and spontaneously differentiating (GM) conditions did the FAPs differentiate into myofibroblasts, exhibiting structurally apparent αSMA+ stress fibers (Figures S3A and S3B). In ADM with or without WNT7A treatment, significantly fewer αSMA stress fibers-expressing myofibroblasts formed (Figures S3A and S3B; p < 0.0001). This suggests that WNT7A does not promote fibrogenesis in adipogenic culture conditions in vitro. WNT7A treatment also did not affect cell viability and proliferation (Figures S3C, S3D, and S4). Altogether, these results demonstrate that WNT7A dampens adipogenesis of FAPs without promoting fibrogenesis in adipogenic conditions in vitro.
WNT7A reduces adipogenesis of FAPs in a β-CATENIN-independent manner
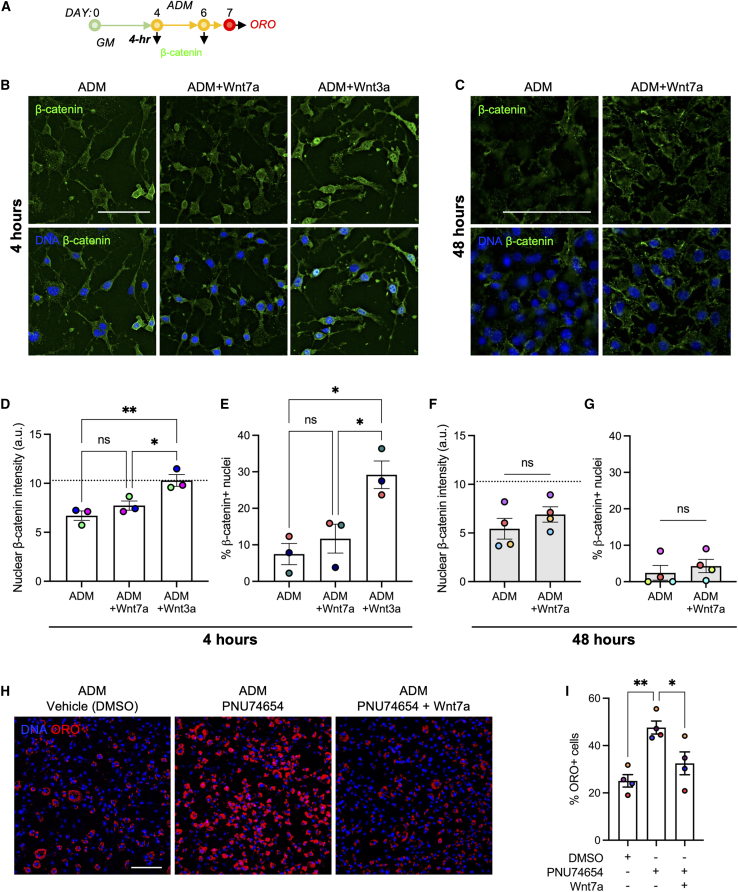
The canonical Wnt signaling inhibits adipogenesis through suppression of the adipogenic transcription factor PPARγ in preadipocytes, marrow-derived mesenchymal stromal cells, and muscle FAPs (Bennett et al., 2002; Kang et al., 2007; Longo et al., 2004; Moldes et al., 2003; Reggio et al., 2020; Ross et al., 2000). To determine if WNT7A inhibits adipogenesis of FAPs through the canonical Wnt pathway, we cultured freshly isolated FAPs in the GM for 4 days and then subsequently treated the cells in the ADM containing WNT7A (200 ng/mL) or the prototypically canonical activator WNT3A (200 ng/mL) for 4 or 48 h (Figures 2A–2C). As expected, brief 4-h treatment with WNT3A exhibited significantly increased nuclear intensity of β-CATENIN compared with both vehicle control and WNT7A conditions (Figures 2B and 2D; p < 0.05 vs. WNT7A, p < 0.01 vs. control). Similarly, WNT3A treatment significantly increased the percentage of β-CATENIN+ nuclei compared with both the vehicle control and WNT7A (Figure 2E; p < 0.05 vs. WNT7A and control). However, FAPs treated with WNT7A did not exhibit an increased nuclear intensity of β-CATENIN after 4- and 48-h treatment compared with the vehicle control (Figures 2B–2F). WNT7A also had no effects on the percentage of β-CATENIN+ nuclei after 4- and 48-h treatment (Figures 2E and 2G). Gene expression analyses of Wnt-related genes of FAPs after 2-day WNT7A treatment revealed that genes related to the canonical WNT signaling (e.g., Lrp5, Lrp6, Axin1, Axin2, Gsk2b, and Ctnnb1) were insignificantly altered (Figures S5A and S5B). These data collectively suggest that WNT7A suppresses adipogenesis of FAPs in a β-CATENIN-independent manner.
Figure 2.
WNT7A reduces adipogenesis of FAPs in a β-CATENIN-independent manner
(A) Experimental timeline of β-CATENIN expression and adipogenesis study. GM, growth media; ADM, adipogenic differentiation media.
(B) Representative immunofluorescence images of β-CATENIN-labeled FAPs treated with vehicle, WNT3A (200 ng/mL), and WNT7A (200 ng/mL) for 4 h. Scale bar: 100 μm.
(C) Representative immunofluorescence images of β-CATENIN-labeled FAPs treated with vehicle and WNT7A (200 ng/mL) for 48 h. Scale bar: 100 μm.
(D) Nuclear β-CATENIN intensity analyzed at the 4-h time point. >1,000 cells analyzed per condition in an automated manner from n = 3 biological replicates. One-way ANOVA with Tukey’s post-hoc analyses applied on the medians of biological donors. Mean ± SEM. ∗p < 0.05; ∗∗p < 0.01. n = 3. Colors represent biological replicates. Dotted line (at 10 a.u.) indicates the mean of WNT3A condition.
(E) Percentage of β-CATENIN+ nuclei analyzed at the 4-h time point. One-way ANOVA with Tukey’s post-hoc analyses. Mean ± SEM. ∗p < 0.05. n = 3. Colors represent biological replicates.
(F) Nuclear β-CATENIN intensity analyzed at the 48-h time point. >440 cells analyzed per condition from n = 4 biological replicates. Two-tailed unpaired t test applied on the medians of biological donors. Mean ± SEM. n = 4. Colors represent biological replicates. Dotted line (at 10 a.u.) indicates the mean of WNT3A condition from (D).
(G) Percentage of β-CATENIN+ nuclei analyzed at the 48-h time point. Two-tailed unpaired t test. Mean ± SEM. n = 4. Colors represent biological replicates.
(H) Representative images of ORO-labeled cells treated with DMSO, PNU74654 (50 μM), and PNU74654 (50 μM) + WNT7A (200 ng/mL). Scale bar: 100 μm.
(I) Percentage of ORO+ cells with DMSO-, PNU74654-, and PNU74654 + Wnt7a-treated conditions. One-way ANOVA with Tukey’s post-hoc analyses. Mean ± SEM. ∗p < 0.05; ∗∗p < 0.01. n = 4. Colors represent biological replicates.
To further corroborate this finding, we next sought to inhibit the activity of β-CATENIN using a small-molecule inhibitor, PNU-74654. This inhibitor specifically prevents the binding of β-CATENIN and the T cell factor (TCF) in the nucleus. In this assay, we found that concentrations beyond 50 μM diminish FAP proliferation in vitro, and thus we chose to use 50 μM for the inhibition study (Figure S5C). As expected, inhibiting β-CATENIN/TCF binding with PNU-74654 significantly increased FAP adipogenesis compared with the DMSO vehicle control (Figures 2H and 2I; p < 0.01). However, treating FAPs with both WNT7A and PNU-74654 resulted in a marked reduction in adipogenesis compared with the PNU-74654 condition (Figures 2H and 2I; p < 0.05). This serves as additional evidence that WNT7A suppresses adipogenesis of FAPs in a β-CATENIN-independent manner, as WNT7A effectively reduces adipogenesis, while β-CATENIN activity remains inhibited.
WNT7A induces nuclear localization and retention of YAP
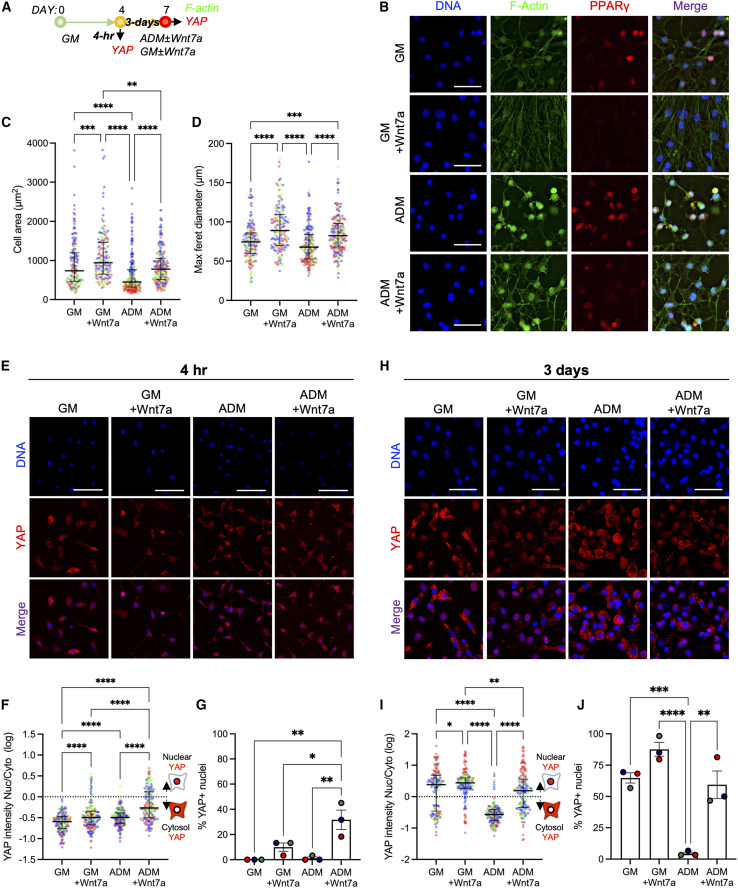
Actin cytoskeleton disassembly through Rho-ROCK signaling and subsequent cell area changes drive adipogenic differentiation of the mouse 3T3-L1 preadipocyte cell line and human stromal stem cells (Chen et al., 2018; Nobusue et al., 2014). To determine whether WNT7A reduces FAP adipogenesis through modulation of cell area and morphology, we expanded freshly isolated FAPs for 4 days and then cultured the cells in either growth and adipogenic differentiation, with or without WNT7A (Figure 3A). FAPs in the adipogenic condition (ADM) exhibited significantly reduced cell area compared with the growth condition (GM; Figures 3B and 3C; p < 0.0001). Note that this decrease in cell area is also accompanied by elevated levels of nuclear PPARγ (Figure 3B). In ADM, WNT7A significantly increased cell area and maximum (max) Feret diameter compared with its WNT7A-free control (Figures 3B–3D; p < 0.0001). In the spontaneously differentiating growth condition (GM), WNT7A also significantly increased both cell area and max Feret diameter compared with its WNT7A-free control (Figures 3B–3D; p < 0.001). WNT7A-treated FAPs in ADM also exhibited cell area and morphology comparable to FAPs maintained in WNT7A-free GM (Figures 3B and 3C). WNT7A treatment does not alter cell density and proliferation (Figure S4), ruling out the possibility of cell density affecting these measurements. Therefore, these results suggest that WNT7A in the adipogenic condition prevents the shrinking of cell area and maintains morphology.
Figure 3.
WNT7A induces nuclear localization and retention of YAP
(A) Experimental timeline of cell morphology and YAP quantification. GM, growth media; ADM, adipogenic differentiation media.
(B) Representative images of F-actin- and PPARγ-labeled FAPs. Scale bar: 50 μm.
(C) Cell area quantification. Kruskal-Wallis test with Dunn’s multiple comparisons. Median ± interquartile range (IQR). ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. n = 179–219 cells analyzed from 3 biological replicates.
(D) Max Feret diameter quantification. Kruskal-Wallis test with Dunn’s multiple comparisons. Median ± IQR. ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. n = 179–219 cells analyzed from 3 biological replicates. Colors represent biological replicates (C and D).
(E) Representative immunofluorescence images of YAP-labeled cells after 4-h treatment in GM ± Wnt7a (200 ng/mL) and ADM ± WNT7A (200 ng/mL). Scale bar: 100 μm.
(F) Quantification of YAP nuclear:cytosol intensity ratio at the 4-h time point. Values were log transformed. Kruskal-Wallis with Dunn’s post-hoc analyses. Median ± IQR. ∗∗∗∗p < 0.0001. n = 180 cells analyzed from 3 biological replicates. Colors represent biological replicates.
(G) Percentage of YAP+ nuclei. One-way ANOVA with Tukey’s post-hoc analyses. Mean ± SEM. ∗p < 0.05; ∗∗p < 0.01. n = 3. Colors represent biological replicates.
(H) Representative immunofluorescence images of YAP-labeled cells after 3-day treatment in GM ± WNT7A (200 ng/mL) and ADM ± WNT7A (200 ng/mL). Scale bars: 25 μm.
(I) Quantification of YAP nuclear:cytosol intensity ratio of the 3-day time point. Values were log transformed. Kruskal-Wallis with Dunn’s post-hoc analyses. Median ± IQR. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001. n = 179 cells analyzed from 3 biological replicates. Colors represent biological replicates.
(J) Percentage of YAP+ nuclei. One-way ANOVA with Tukey’s post-hoc analyses. Mean ± SEM. ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. n = 3. Colors represent biological replicates.
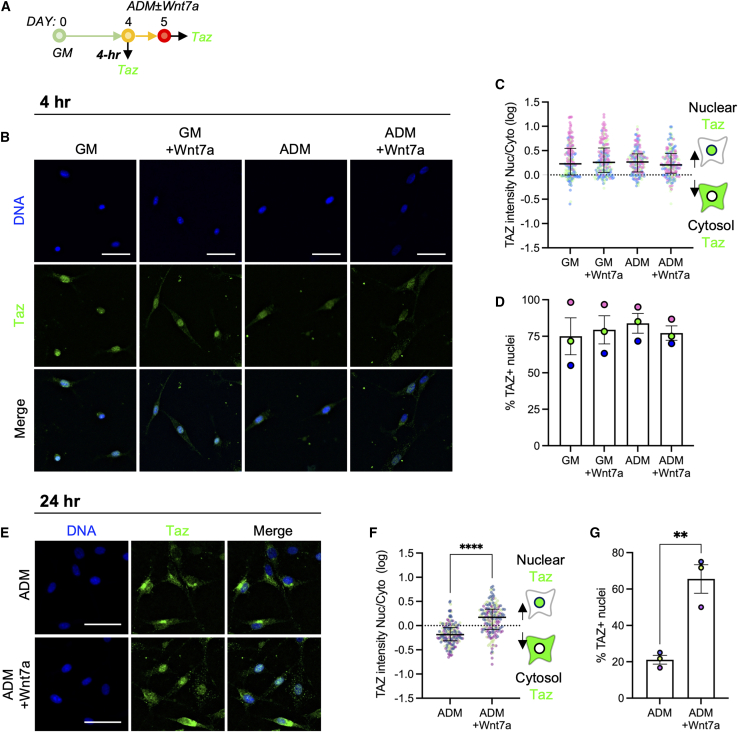
YAP-1 and its paralog TAZ act as biochemical mechanotransducers that convert mechanical cues and resulting cellular changes (e.g., cell contractility and shape) into cell-specific transcriptional activities (Dupont et al., 2011). Recent evidence also suggests that YAP/TAZ also act as downstream modulators of Wnt pathways (Azzolin et al., 2012, 2014; Park et al., 2015a). Based on such evidence and our observation that WNT7A reduces FAP adipogenesis by maintaining cellular shape (Figures 3B–3D), we next questioned whether non-canonical WNT7A signaling promotes nuclear localization of YAP (Figure 3A). Nearly all freshly isolated and proliferating FAPs exhibited higher cytosolic YAP in vitro (Figures 3E–3G). Culturing the proliferating FAPs in WNT7A-containing GM and ADM for 4 h significantly increased the YAP nuclear-to-cytosolic ratio compared with their respective controls (Figures 3E and 3F; p < 0.0001). YAP nuclear localization was also significantly higher in ADM containing WNT7A compared with GM containing WNT7A (Figures 3E and 3F; p < 0.0001), suggesting context-dependent responsivity. The percentage of YAP+ nuclei also significantly increased when FAPs were treated in ADM containing WNT7A compared with all other groups, further corroborating this finding (Figures 3E and 3G; p < 0.05). These results indicate that WNT7A promotes nuclear localization of YAP in proliferating FAPs in vitro.
To further determine if prolonged exposure of FAPs to WNT7A promotes nuclear retention of YAP, we cultured FAPs in growth (GM) and adipogenic (ADM) conditions with or without WNT7A supplementation for 3 days (Figure 3A). In GM, we observed a bimodal distribution of cells expressing nuclear and cytosolic YAP (Figures 3H and 3I), likely indicating the bifurcating lineage commitment of FAPs, but WNT7A significantly increased nuclear retention of YAP (Figures 3H and 3I; p < 0.05). By day 3, FAPs undergoing adipogenesis in ADM exhibited cytosolic YAP (Figures 3H–3J). In ADM, 3-day treatment with WNT7A significantly increased the nuclear retention of YAP and the percentage of YAP+ nuclei compared with its control (Figures 3H–3J; p < 0.01). In addition, this WNT7A treatment also resulted in a comparable distribution of cells exhibiting nuclear and cytosolic YAP (i.e., bimodal) with GM without WNT7A (Figures 3H and 3I; p > 0.05). Finally, we conducted an additional experiment to determine if WNT7A-induced nuclear retention of YAP correlates with non-adipogenic FAPs. To do this, we cultured the FAPs in adipogenic conditions (ADM) with WNT7A for 3 days and co-immunolabeled the cells for both YAP and PLIN1 (Figure S6A). Approximately 85% of cells expressing nuclear YAP were PLIN1− (Figures S6B and S6C), while approximately 35% of the cells expressing nuclear YAP were PLIN1+ (Figures S6B and S6C), indicating that WNT7A-induced nuclear retention of YAP negatively correlates with the adipogenic FAPs. Collectively, these data suggest that WNT7A treatment promotes nuclear localization and retention of YAP, and this likely decreases the adipogenic potential of FAPs.
WNT7A promotes YAP nuclear localization through Rho
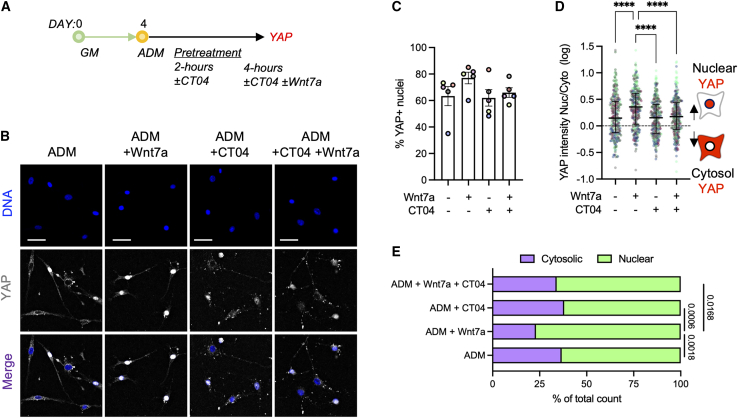
We next asked whether WNT7A promotes nuclear localization of YAP through the alternative Wnt signaling axis involving Wnt-FZD/ROR-Rho GTPases-Lats1/2 (Park et al., 2015a; Thorup et al., 2020). In the alternative Wnt signaling, inhibition of Rho prevents Wnt-induced YAP activation. To mechanistically test this hypothesis, we pretreated FAPs in adipogenic media (ADM) containing a Rho GTPase inhibitor (purified C3 transferase; CT04) or vehicle (dH2O) for 2 h (Figure 4A). The cells were further maintained in ADM with or without CT04 and WNT7A for an additional 4 h (Figure 4A). Here, we note that 6-h culture in ADM begins to increase the fraction of FAPs exhibiting YAP nuclear localization, contributing to an insignificant increase in the percentage of YAP+ nuclei following WNT7A treatment (Figures 4B and 4C). Even so, WNT7A significantly increased the nuclear intensity ratio of YAP compared with the ADM control (Figure 4D; p < 0.0001). Rho inhibition alone did not significantly affect the nuclear intensity ratio of YAP compared with the control (Figure 4D). However, when Rho was inhibited, WNT7A failed to increase the nuclear intensity ratio of YAP (Figure 4D). The overall fraction of FAPs with nuclear or cytosolic YAP also remained unaffected when treated with WNT7A with Rho inhibited (Figure 4E). In sum, the results suggest that Rho is required for WNT7A-induced activation of YAP in FAPs.
Figure 4.
WNT7A activates YAP through Rho
(A) Experimental timeline of Rho inhibition study. GM, growth media; ADM, adipogenic differentiation media.
(B) Representative immunofluorescence images of YAP-labeled cells. Scale bar: 50 μm.
(C) Percentage of YAP+ nuclei. One-way ANOVA with Tukey’s post-hoc analyses. n = 5. Colors represent biological replicates.
(D) Quantification of YAP nuclear:cytosol intensity ratio. Values were log transformed. Kruskal-Wallis with Dunn’s post-hoc analyses. Median ± IQR. ∗∗∗∗p < 0.0001. n = 300 cells analyzed from 5 biological replicates. Colors represent biological replicates.
(E) Quantification of the cell proportions with nuclear and cytoplasmic YAP localization. Chi-squared tests. Adjusted p values for Bonferroni correction.
WNT7A promotes nuclear retention of TAZ
TAZ, a paralog of YAP-1, regulates the differentiation potential of mesenchymal stem cells by directly repressing PPARγ while activating Runx2 genes (Hong et al., 2005). To test if WNT7A promotes TAZ nuclear localization in a similar manner to YAP, we maintained proliferating FAPs in the GM or ADM containing WNT7A for 4 h (Figure 5A). In contrast to YAP (Figures 3E–3G), most FAPs exhibited higher nuclear TAZ in vitro (Figures 5B–5D). WNT7A treatment in either the growth or adipogenic condition did not result in further increases in nuclear TAZ intensity (Figures 5B–5D), suggesting that WNT7A does not promote nuclear localization of TAZ in proliferating FAPs in vitro.
Figure 5.
WNT7A promotes nuclear retention of TAZ
(A) Experimental timeline of TAZ quantification. GM, growth media; ADM, adipogenic differentiation media.
(B) Representative immunofluorescence images of TAZ-labeled cells after 4-h treatment in GM ± WNT7A (200 ng/mL) and ADM ± WNT7A (200 ng/mL). Scale bar: 50 μm.
(C) Quantification of TAZ nuclear:cytosol intensity ratio at the 4-h time point. Values were log transformed. Median ± IQR. n = 180 cells analyzed from 3 biological replicates. Colors represent biological replicates.
(D) Percentage of TAZ+ nuclei at the 4-h time point. Mean ± SEM. n = 3. Colors represent biological replicates.
(E) Representative immunofluorescence images of TAZ-labeled cells after 24-h treatment in ADM ± WNT7A (200 ng/mL). Scale bar: 50 μm.
(F) Quantification of TAZ nuclear:cytosol intensity ratio at the 24-h time point. Values were log transformed. Two-tailed unpaired t test. Median ± IQR. ∗∗∗∗p < 0.0001. n = 180 cells analyzed from 3 biological replicates. Colors represent biological replicates.
(G) Percentage of TAZ+ nuclei at the 24-h time point. Two-tailed unpaired t test. Mean ± SEM. ∗∗p < 0.01. n = 3. Colors represent biological replicates.
To determine if WNT7A promotes nuclear retention of TAZ in differentiating FAPs, we maintained FAPs in the ADM containing WNT7A for 24 h (Figure 5A). Nearly 80% of FAPs maintained in the ADM without WNT7A exhibited cytosolic TAZ (Figures 5E–5G), suggesting that adipogenic FAPs displace TAZ from their nucleus to cytosol. However, 24-h WNT7A treatment significantly increased nuclear retention of TAZ, quantified by both the nuclear intensity ratio of TAZ and the percentage of TAZ+ nuclei (Figures 5E–5G; p < 0.01). In GM, FAPs homogeneously expressed nuclear TAZ at this time point, and WNT7A treatment did not further alter the TAZ nuclear localization (Figures S6D–S6F). Altogether, the results suggest that while WNT7A does not stimulate nuclear localization of TAZ, it effectively promotes nuclear retention of TAZ in differentiating FAPs in the adipogenic condition.
WNT7A suppresses fatty infiltration in skeletal muscle
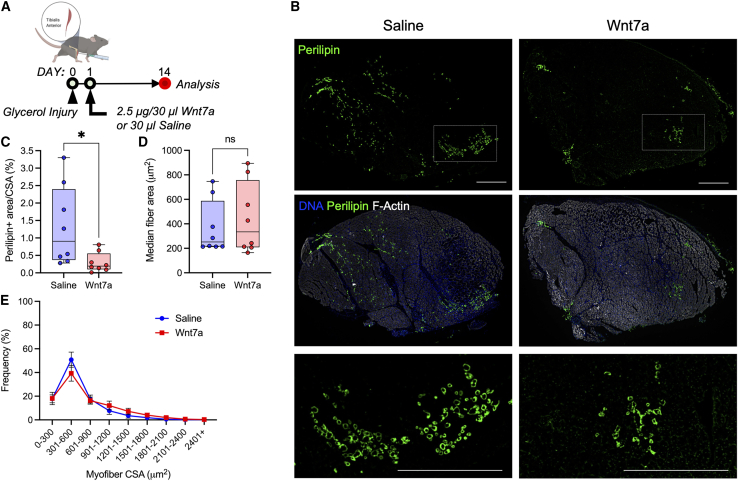
We next sought to evaluate the efficacy of WNT7A in suppressing intramuscular fatty infiltration in vivo using the glycerol injury model. Intramuscular injection of glycerol stimulates a robust and reproducible fatty infiltration without affecting muscle regeneration, thus serving as an excellent proof-of-concept in vivo model to evaluate therapeutics targeted for reducing intramuscular adipogenesis (Pisani et al., 2010). To induce injury, tibialis anterior (TA) muscles were injected with glycerol (50% v/v). WNT7A or saline was injected into the belly of the injured TA muscles 1 day post-injury (Figure 6A). The muscles were then harvested 14 days post-injury for analyses (Figure 6A). Glycerol-injured TAs with saline treatment exhibited a severe fatty infiltration, marked by PLIN1 expression within the interstitial space (Figures 6B and S7A). However, glycerol-injured TAs with WNT7A treatment exhibited a significantly reduced PLIN1 expression (Figures 6B, 6C, and S9A; p < 0.05). We observed no statistically significant differences in the myofiber area distribution (Figures 6D and 6E). We also observed no qualitative and quantitative differences in fibrosis assessed by trichrome staining and polarized light imaging (Figures S7B–S7D). These data collectively show that WNT7A effectively suppresses intramuscular fatty infiltration in vivo without negatively impacting myogenesis or inducing fibrosis.
Figure 6.
WNT7A suppresses fatty infiltration in skeletal muscle
(A) Experimental timeline outlining in vivo glycerol injection, WNT7A administration, and analyses. Created with BioRender.
(B) Representative cross-sections of glycerol-injured TA muscles treated with WNT7A or saline. Scale bars: 500 μm.
(C) Perilipin area normalized by the TA cross-sectional area. Two-tailed unpaired t test. ∗p = 0.027.
(D) Median fiber cross-sectional area. Two-tailed unpaired t test.
(E) Histogram of fiber cross-sectional area. Mean ± SEM.
Discussion
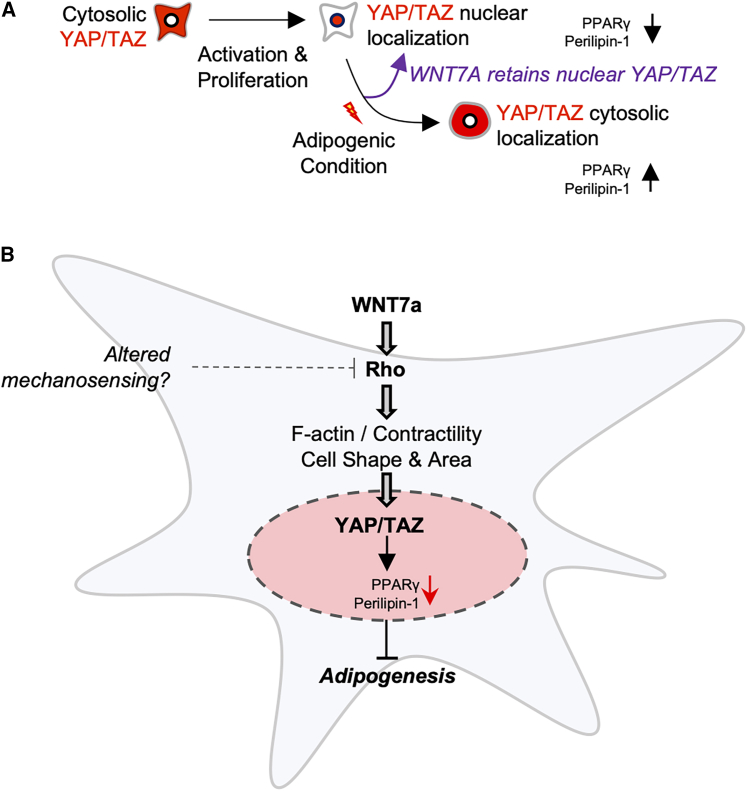
Persistent fatty infiltration is a chronic hallmark of skeletal muscle injuries and diseases, such as rotator cuff injuries and Duchenne muscular dystrophy (Fu et al., 2021; Li et al., 2015). WNT7A has been emerging as a potential therapeutic for muscle diseases and injuries due to its pro-regenerative effects on muscle satellite cells and myofibers (Han et al., 2019; Le Grand et al., 2009; von Maltzahn et al., 2011), but its effects on FAPs remain unknown. Thus, addressing the effects of WNT7A in modulating fatty infiltration and FAP function is critical for translating WNT7A as a potential therapeutic. In this study, we determined the mechanistic effect of WNT7A on adipogenesis of FAPs, which are the precursors to fatty infiltration in skeletal muscle pathology (Joe et al., 2010; Liu et al., 2016; Uezumi et al., 2010; Wosczyna et al., 2012). Our data reveal that WNT7A suppresses adipogenesis by inducing nuclear localization of YAP through Rho and, subsequently, by promoting nuclear retention of YAP and TAZ.
The mechanistic role of canonical Wnt signaling and β-CATENIN in suppressing adipogenesis is well established in other cell types (Bennett et al., 2002; Kang et al., 2007; Longo et al., 2004; Moldes et al., 2003; Reggio et al., 2020; Ross et al., 2000). For instance, WNT5A reduces adipogenesis of FAPs through canonical Wnt signaling (Reggio et al., 2020). Thus, we first sought to determine if WNT7A increased nuclear β-CATENIN through the canonical pathway in FAPs. WNT7A did not increase nuclear β-CATENIN in FAPs (Figures 2B–2G). Furthermore, WNT7A remained effective in reducing adipogenesis even when β-CATENIN activity was inhibited using PNU-74654 (Figures 2H and 2I), suggesting that WNT7A acts through a non-canonical pathway. We also observed that WNT7A retained a larger cell area of FAPs in adipogenic culture conditions while preventing adipogenesis (Figures 3B–3D). Based on these findings, we then asked if WNT7A acts through alternative Wnt signaling, where YAP is activated through the Wnt-FZD/Ror-Rho GTPases-Lats1/2 signaling axis independent of β-CATENIN (Park et al., 2015a). In support of our hypothesis, a brief 4-h WNT7A treatment induced nuclear localization of YAP in FAPs (Figures 3E–3G). We found that WNT7A failed to induce nuclear localization of YAP when Rho was inhibited using CT04 (Figure 4). These data suggest that WNT7A activates YAP through Rho-dependent alternative Wnt signaling in FAPs (Figure S10). These findings also raise an interesting hypothesis in which fatty infiltration that arises from skeletal muscle unloading (Kaneshige et al., 2022) may potentially be compensated through exogenous WNT7A that acts through the mechanosignaling pathway involving Rho and YAP (Figure 7). In contrast to YAP, however, we found that WNT7A does not promote TAZ nuclear localization (Figures 5B–5D). This is likely because most proliferating FAPs express nuclear TAZ (Figures 5B–5D), while YAP is localized in the cytosol (Figures 3E–3G). Nonetheless, FAPs differentiating into adipogenic lineage begin to displace both YAP and TAZ from their nuclei, and WNT7A promotes nuclear retention of YAP and TAZ. (Figures 3H–3J, 5E–5G, and 7).
Figure 7.
WNT7A retains nuclear YAP/TAZ and decreases FAP adipogenesis
(A) During adipogenesis, YAP/TAZ translocate to cytosol. WNT7A promotes nuclear retention of YAP/TAZ within the nucleus, thereby preventing FAP adipogenesis.
(B) WNT7A may rescue mechanical unloading-induced FAP adipogenesis by reinforcing YAP/TAZ activity through cell contractility-mediated mechanisms.
How does WNT7A-induced YAP/TAZ nuclear retention inhibit PPARγ and adipogenesis of FAPs? First, published evidence suggests that the nuclear activity of YAP/TAZ inhibits adipogenesis by repressing PPARγ in multiple cell types (Deng et al., 2019; El Ouarrat et al., 2020; Hong et al., 2005; Lorthongpanich et al., 2019; Pan et al., 2018). Specifically, TAZ directly represses PPARγ while activating Runx2 genes in mesenchymal stem cells (Hong et al., 2005). The same mechanism may also be reducing the adipogenesis of FAPs when treated with WNT7A because WNT7A promotes nuclear retention of YAP/TAZ in differentiating FAPs and suppresses adipogenesis. Second, YAP/TAZ activity and TEAD-induced transcription may trigger the secretion of canonical Wnt modulators (Park et al., 2015a). As noted above, Wnt/β-CATENIN signaling is a crucial mediator of adipogenesis, where its downregulation results in the differentiation of preadipocytes into mature adipocytes (Bennett et al., 2002; Longo et al., 2004; Ross et al., 2000). In the current study, we observed that inhibiting β-CATENIN activity using PNU-74654 alone increased adipogenesis of FAPs (Figures 2H and 2I), suggesting that β-CATENIN indeed plays a role in modulating adipogenesis. However, WNT7A did not promote nuclear localization of β-CATENIN in FAPs after 4- and 48-h treatment (Figures 2B–2G), nor did it significantly increase expressions of genes related to the canonical Wnt signaling (Figures S5A and S5B), suggesting that WNT7A-induced YAP/TAZ activity does not significantly upregulate the canonical Wnt signaling. Interestingly, Wisp1, which directly binds and represses PPARγ to inhibit adipogenesis (Ferrand et al., 2017), was significantly upregulated with WNT7A treatment.
WNT7A promotes nuclear localization of YAP through Rho, increases nuclear retention of YAP/TAZ in differentiating FAPs, and suppresses adipogenesis, but it is currently unclear which receptors expressed by FAPs are binding to WNT7A to elicit the observed downstream effects. WNT7A binds to FZD7 to promote satellite cell expansion and myotube hypertrophy (Le Grand et al., 2009; von Maltzahn et al., 2011), and because FZD7 is also expressed by the FAPs (Reggio et al., 2020), it is also likely that WNT7A promotes nuclear localization and retention through FZD7. Furthermore, based on the published prior studies that describe the mechanistic link between the Wnt and Hippo signaling pathways (Park et al., 2015a; Thorup et al., 2020), we speculate that WNT7A acts by binding to the Frizzled and tyrosine kinase-like orphan receptor-1/2 (ROR1/2) complexes. In this non-canonical pathway, the binding of Wnt ligands to Frizzled and ROR1/2 co-receptors increases Rho activity that subsequently inhibits Lats1/2 (Park et al., 2015a; Thorup et al., 2020). Interestingly, querying the publicly available single-nucleus skeletal muscle gene expression database (https://research.cchmc.org/myoatlas/) (Petrany et al., 2020) revealed that ROR1 is highly expressed by murine FAPs at all ages (postnatal day 3 through 30 months of age). Whether WNT7A binds the ROR1 co-receptor to promote YAP/TAZ nuclear localization and retention remains to be addressed in future studies.
While in vivo administration of WNT7A to the glycerol-injured TA significantly suppressed fatty infiltration, WNT7A did not improve muscle regeneration. This is in contrast to previous findings that demonstrated that WNT7A enhances muscle regeneration (Han et al., 2019; Le Grand et al., 2009; von Maltzahn et al., 2011). The difference between the current and prior findings on the effect of WNT7A on muscle regeneration is likely due to different modes of injury used: cardiotoxin vs. glycerol. While cardiotoxin and glycerol injections induce comparable levels of muscle damage, the regeneration rate following glycerol-induced injury is dampened compared with cardiotoxin-induced injury (Lukjanenko et al., 2013). Furthermore, ectopic fatty infiltration formed following glycerol-induced injury is significantly higher compared with cardiotoxin injection, which may further reduce WNT7A-induced regeneration and hypertrophy (Lukjanenko et al., 2013). Finally, glycerol-induced injury also elicits increased gene expression of anti-inflammatory cytokines compared with cardiotoxin (Lukjanenko et al., 2013). These differences, along with the single time point assessed in the current study, likely contribute to an insignificant improvement in regeneration.
The current study has its limitations. Our current in vitro experiments applied a narrow time frame in which these FAPs are either spontaneously differentiating or induced to differentiate in cell culture settings. Future investigations will validate the observed mechanisms using clinically relevant in vivo injury and disease models. The cellular identity of the WNT7A-treated FAPs in vivo is also an important consideration. To further develop WNT7A as therapeutic, potential cross-talk between FAPs and other cell populations should be considered in vivo in a context-dependent manner as well. Functional measures, including muscle contractile force, mouse gait, and fibrosis, should also be considered to comprehensively evaluate WNT7A as a potential therapeutic. Ultimately, an effective delivery method using biomaterials such as engineered hydrogels should be tested to show the effectiveness and efficiency of WNT7A release on therapeutic models.
In conclusion, we identified that WNT7A suppresses adipogenesis of FAPs by inducing nuclear localization of YAP through Rho in a β-CATENIN-independent manner and by promoting nuclear retention of YAP and TAZ. Our data provide insight into applying WNT7A as a potential therapeutic for mitigating intramuscular fatty infiltration in various skeletal muscle pathologies.
Experimental procedures
Resource availability
Corresponding author
Request for resources, reagents, and protocols should be addressed to the corresponding author, Woojin M. Han, PhD (woojin.han@mssm.edu).
Materials availability
This study did not generate unique materials.
Mice
All animal procedures were conducted under the approved protocol by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. Mice were housed and maintained in the Center for Comparative Medicine and Surgery Facility of the Icahn School of Medicine at Mount Sinai. C57BL/6J mice were acquired from the Jackson Laboratory (stock #000664). Both male and female mice were used in a randomized manner.
Glycerol injuries and WNT7A administration
2-month-old mice were anesthetized with isoflurane. 50 μL 50% glycerol in saline was intramuscularly injected into both TA muscles. After 24 h, 2.5 μg/30 μL recombinant human WNT7A (PeproTech) and 30 μL saline were injected into the TA muscles in a randomized manner. Buprenorphine shots were subcutaneously administered every 12 h at the onset of the procedure for 3 days. Mice were sacrificed on day 14 for histological analyses.
Isolation of FAPs
Primary FAPs were isolated from 4- to 6-week-old mice by magnetic-activated cell sorting (MACS) as described previously (Marinkovic et al., 2019). Mouse hindlimb muscles were dissected and incubated in digestion media (2.5 U/mL Dispase II, Thermo Fisher Scientific, and 0.2% w/v collagenase type II, Worthington, in DMEM) on a shaking incubator at 37°C for 1.5 h. Deactivation media (20% FBS in Ham’s F-10; Gibco) was added to inactivate the reaction. The muscle digest was filtered through a 70-μm cell strainer and then centrifuged (300 × g, 5 min, 4°C). Cell pellets were resuspended in Staining Buffer (0.5% bovine serum albumin and 2 mM EDTA in PBS) and filtered through a 35-μm cell strainer. Cells were incubated with biotin anti-mouse CD31 (BioLegend, cat. no. 102503; 1:150), Biotin anti-mouse CD45 (BioLegend, cat. no. 103103; 1:150), and biotin anti-integrin α7 (Miltenyi, cat. no. 130-101-979; 1:10) antibodies at 4°C for 45 min. Cells were pelleted through centrifugation and incubated with 10 μL streptavidin beads (1:30) at 4°C for 15 min. Labeled cells were passed through an LD column (Miltenyi) for negative selection. The remaining cells were incubated with biotin anti-mouse Ly-6A/E (SCA-1) antibody (BioLegend, cat. no. 122504; 1:75) at 4°C for 20 min and then 10 μL streptavidin beads (1:30) at 4°C for 10 min. Cells were then passed through an LS column (Miltenyi) and enriched for SCA-1+ cells. Cells were filtered through a 35-μm cell strainer once more before cell seeding.
FAP culture and differentiation
Isolated FAPs were seeded at ∼10,000 cells per 1 cm2 well in GM (10% FBS and 1× penicillin-streptomycin in DMEM) containing 2.5 ng/mL bFGF (Peprotech) on laminin- (Gibco; 10 μg/mL) and collagen I-coated (Thermo Fisher Scientific; 5 μg/mL) plates. Cultures were maintained at 37°C and 5% CO2 levels. Adipogenic differentiation was performed by incubating the FAPs in ADM: 0.5 mM 3-isobutyl-1-methylxanthine (Millipore Sigma), 0.25 μM dexamethasone (Millipore Sigma), and 1 μg/mL insulin (Millipore Sigma) in GM and adipogenic maintenance medium: 1 μg/mL insulin in GM. Fibrogenic differentiation was performed by incubating the FAPs in fibrogenic medium: 10 ng/mL TGF-β1 (PeproTech) in GM.
In vitro assays and reagents
Unless otherwise noted, recombinant human WNT7A (PeproTech; 200 ng/mL; vehicle: dH2O) was added to the culture media. To inhibit β-CATENIN binding to TCF4, PNU-74654 (Cayman Chemicals; 50 μM; vehicle: DMSO) was added to the media for 3 days in ADM. To inhibit Rho, CT04 (cytoskeleton; 2 μg/mL; vehicle: dH2O) was added to the media for 2 h as pretreatment plus an additional 4 h with or without WNT7A. The live/dead staining assay was performed using the LIVE/DEAD Cell Imaging Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. Cell-permeant Calcein AM was used as the live cell indicator, and cell-impermeant BOBO-3 iodide nucleic acid dye was used as the dead cell indicator.
Real-time quantitative PCR
mRNA was extracted from cells using the RNeasy Plus Microkit (Qiagen, cat. no. 74034), and cDNA was prepared using the RT2 First Strand Kit (Qiagen, cat. no. 330401). RT2 SYBR Green qPCR Master Mix (Qiagen, cat. no. 330504) and RT2 Profiler PCR Array Mouse WNT Signaling Pathway ABI 7900HT Standard Block plates (Qiagen, cat. No. PAMM-043ZA) were used for qPCR.
Immunocytochemistry staining
Cells were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature. Samples were washed three times with 1× PBS and incubated in blocking/permeabilization buffer (5.0% goat serum, 2.0% bovine serum albumin, 0.5% Triton X-100 in PBS) overnight at 4°C. For PDGFRα staining, blocking/permeabilization buffer without the goat serum (2.0% bovine serum albumin, 0.5% Triton X-100 in PBS) was used. The following primary and secondary antibodies were used for immunocytochemistry in this study: anti-PLIN (Abcam; ab3526; 1:200); anti- αSMA (Abcam; ab7817; 1:200); anti-YAP (Santa Cruz Biotechnology; sc101199; 1:200); anti-TAZ (Cell Signaling Technology; 83669S; 1:100); anti-PPARγ (Santa Cruz Biotechnology; sc7273; 1:200); anti-PDGFRα (R&D Systems; AF1062; 1:200); anti-β-CATENIN (Cell Signaling Technology; 8480S; 1:100); goat anti-rabbit Alexa 488 (Thermo Fisher Scientific; A11008; 1:500); goat anti-mouse Alexa Fluor 546 (Thermo Fisher Scientific; A11003; 1:500); goat anti-mouse Alexa 488 (Thermo Fisher Scientific; PIA32723; 1:500); and goat anti-rabbit Alexa 647 (Thermo Fisher Scientific; PIA32733; 1:500). Hoechst 33342 (Thermo Fisher Scientific; 1:1,000) and Phalloidin-iFluor 488 (Cayman Chemicals; 1:1,000) were used to stain nuclei and F-actin, respectively.
Oil Red O (ORO) staining
Cells were fixed with 4% PFA for 20 min at room temperature. Samples were washed three times with 1× PBS and incubated in blocking/permeabilization buffer (5% goat serum, 2% bovine serum albumin, 0.5% Triton X-100 in PBS) overnight at 4°C. Cells were incubated in isopropanol (60%) for 5 min and then incubated in ORO for 20 min. Cells were washed five times with 1× PBS and then counterstained with Hoechst 33342 (Thermo Fisher Scientific; 1:1,000).
Histology and immunohistochemistry
Detailed protocol is provided in the supplemental experimental procedures.
Imaging and image analysis
Images were taken on a Leica Microsystems THUNDER DMi8 microscope using LAS-X software for processing. Detailed image analysis protocol is provided in the supplemental experimental procedures.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. Normality was determined using the Shapiro-Wilk test and Q-Q plot. To test statistical significance, two-tailed t test, one-way analysis of variance (ANOVA) with Tukey’s post-hoc analysis, two-way ANOVA with Bonferroni post-hoc analysis, and Kruskal-Wallis test with Dunn’s multiple comparisons were performed depending on data normality and the number of comparisons. p <0.05 was considered statistically significant. All experiments and studies had at least three biological replicates.
Author contributions
C.F., B.C.-Y., and W.M.H. conceived and designed the studies. C.F., B.C.-Y., G.P., M.G.-S., D.L., and W.M.H. conducted experiments and analyzed data. C.F., B.C.-Y., G.P., and W.M.H. wrote and revised this manuscript.
Acknowledgments
We thank Nada Marjanovic (Sinai qPCR Core) for her work on the qPCR and gene expression analysis. This study was supported by the Department of Orthopedics at the Icahn School of Medicine at Mount Sinai to W.M.H. and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR080616. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interests
The authors declare no competing interests.
Published: March 30, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.03.001.
Supplemental information
Data and code availability
All data needed to evaluate the conclusion of the paper are present in the paper or the supplemental information.
References
- Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., Cordenonsi M., Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Bennett C.N., Ross S.E., Longo K.A., Bajnok L., Hemati N., Johnson K.W., Harrison S.D., MacDougald O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- Burkhart K., Allaire B., Bouxsein M.L. Negative effects of long-duration spaceflight on paraspinal muscle morphology. Spine. 2019;44:879–886. doi: 10.1097/BRS.0000000000002959. [DOI] [PubMed] [Google Scholar]
- Butterfield T.A., Best T.M., Merrick M.A. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J. Athl. Train. 2006;41:457–465. [PMC free article] [PubMed] [Google Scholar]
- Chen L., Hu H., Qiu W., Shi K., Kassem M. Actin depolymerization enhances adipogenic differentiation in human stromal stem cells. Stem Cell Res. 2018;29:76–83. doi: 10.1016/j.scr.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Deng K., Ren C., Fan Y., Pang J., Zhang G., Zhang Y., You P., Wang F. YAP1 regulates PPARG and RXR alpha expression to affect the proliferation and differentiation of ovine preadipocyte. J. Cell. Biochem. 2019;120:19578–19589. doi: 10.1002/jcb.29265. [DOI] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- El Ouarrat D., Isaac R., Lee Y.S., Oh D.Y., Wollam J., Lackey D., Riopel M., Bandyopadhyay G., Seo J.B., Sampath-Kumar R., Olefsky J.M. TAZ is a negative regulator of PPARγ activity in adipocytes and TAZ deletion improves insulin sensitivity and glucose tolerance. Cell Metabol. 2020;31:162–173.e5. doi: 10.1016/j.cmet.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand N., Béreziat V., Moldes M., Zaoui M., Larsen A.K., Sabbah M. WISP1/CCN4 inhibits adipocyte differentiation through repression of PPARγ activity. Sci. Rep. 2017;7:1749. doi: 10.1038/s41598-017-01866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C., Huang A.H., Galatz L.M., Han W.M. Cellular and molecular modulation of rotator cuff muscle pathophysiology. J. Orthop. Res. 2021;39:2310–2322. doi: 10.1002/jor.25179. [DOI] [PubMed] [Google Scholar]
- Gladstone J.N., Bishop J.Y., Lo I.K.Y., Flatow E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am. J. Sports Med. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- Goffin J.M., Pittet P., Csucs G., Lussi J.W., Meister J.-J., Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J. Cell Biol. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W.M., Mohiuddin M., Anderson S.E., García A.J., Jang Y.C. Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment. Acta Biomater. 2019;94:243–252. doi: 10.1016/j.actbio.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia J.E., Mukundan L., Chen F.M., Mueller A.A., Deo R.C., Locksley R.M., Rando T.A., Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- Hong J.-H., Hwang E.S., McManus M.T., Amsterdam A., Tian Y., Kalmukova R., Mueller E., Benjamin T., Spiegelman B.M., Sharp P.A., et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- Joe A.W.B., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige A., Kaji T., Zhang L., Saito H., Nakamura A., Kurosawa T., Ikemoto-Uezumi M., Tsujikawa K., Seno S., Hori M., et al. Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell. 2022;29:265–280.e6. doi: 10.1016/j.stem.2021.11.003. [DOI] [PubMed] [Google Scholar]
- Kang S., Bennett C.N., Gerin I., Rapp L.A., Hankenson K.D., Macdougald O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- Le Grand F., Jones A.E., Seale V., Scimè A., Rudnicki M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos D.R., Babaeijandaghi F., Low M., Chang C.-K., Lee S.T., Fiore D., Zhang R.-H., Natarajan A., Nedospasov S.A., Rossi F.M.V. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- Li W., Zheng Y., Zhang W., Wang Z., Xiao J., Yuan Y. Progression and variation of fatty infiltration of the thigh muscles in Duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscul. Disord. 2015;25:375–380. doi: 10.1016/j.nmd.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Liu X., Ning A.Y., Chang N.C., Kim H., Nissenson R., Wang L., Feeley B.T. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 2016;6:6–15. doi: 10.11138/mltj/2016.6.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo K.A., Wright W.S., Kang S., Gerin I., Chiang S.-H., Lucas P.C., Opp M.R., MacDougald O.A. Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- Lorthongpanich C., Thumanu K., Tangkiettrakul K., Jiamvoraphong N., Laowtammathron C., Damkham N., U-Pratya Y., Issaragrisil S. YAP as a key regulator of adipo-osteogenic differentiation in human MSCs. Stem Cell Res. Ther. 2019;10:402. doi: 10.1186/s13287-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukjanenko L., Brachat S., Pierrel E., Lach-Trifilieff E., Feige J.N. Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS One. 2013;8:e71084. doi: 10.1371/journal.pone.0071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J., Bentzinger C.F., Rudnicki M.A. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat. Cell Biol. 2011;14:186–191. doi: 10.1038/ncb2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J., Renaud J.-M., Parise G., Rudnicki M.A. Wnt7a treatment ameliorates muscular dystrophy. Proc. Natl. Acad. Sci. USA. 2012;109:20614–20619. doi: 10.1073/pnas.1215765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J., Zinoviev R., Chang N.C., Bentzinger C.F., Rudnicki M.A. A truncated Wnt7a retains full biological activity in skeletal muscle. Nat. Commun. 2013;4:2869. doi: 10.1038/ncomms3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic M., Fuoco C., Sacco F., Cerquone Perpetuini A., Giuliani G., Micarelli E., Pavlidou T., Petrilli L.L., Reggio A., Riccio F., et al. Fibro-adipogenic progenitors of dystrophic mice are insensitive to NOTCH regulation of adipogenesis. Life Sci. Alliance. 2019;2:e201900437. doi: 10.26508/lsa.201900437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara K.P., Greene K.A., Tooze J.A., Dang J., Khattab K., Lenchik L., Weaver A.A. Neck muscle changes following long-duration spaceflight. Front. Physiol. 2019;10:1115. doi: 10.3389/fphys.2019.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa H., Yamamoto N., Abe H., Fukuda M., Seki N., Kikuchi K., Kijima H., Itoi E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass-screening in one village. J. Orthop. 2013;10:8–12. doi: 10.1016/j.jor.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldes M., Zuo Y., Morrison R.F., Silva D., Park B.-H., Liu J., Farmer S.R. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem. J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobusue H., Onishi N., Shimizu T., Sugihara E., Oki Y., Sumikawa Y., Chiyoda T., Akashi K., Saya H., Kano K. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 2014;5:3368. doi: 10.1038/ncomms4368. [DOI] [PubMed] [Google Scholar]
- Pan J.-X., Xiong L., Zhao K., Zeng P., Wang B., Tang F.-L., Sun D., Guo H.-H., Yang X., Cui S., et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Res. 2018;6:18. doi: 10.1038/s41413-018-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.W., Kim Y.C., Yu B., Moroishi T., Mo J.-S., Plouffe S.W., Meng Z., Lin K.C., Yu F.-X., Alexander C.M., et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S., Park H.J., Kim S.H., Oh J.H. Prognostic factors affecting rotator cuff healing after arthroscopic repair in small to medium-sized tears. Am. J. Sports Med. 2015;43:2386–2392. doi: 10.1177/0363546515594449. [DOI] [PubMed] [Google Scholar]
- Petrany M.J., Swoboda C.O., Sun C., Chetal K., Chen X., Weirauch M.T., Salomonis N., Millay D.P. Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers. Nat. Commun. 2020;11:6374. doi: 10.1038/s41467-020-20063-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani D.F., Bottema C.D.K., Butori C., Dani C., Dechesne C.A. Mouse model of skeletal muscle adiposity: a glycerol treatment approach. Biochem. Biophys. Res. Commun. 2010;396:767–773. doi: 10.1016/j.bbrc.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Reggio A., Rosina M., Palma A., Cerquone Perpetuini A., Petrilli L.L., Gargioli C., Fuoco C., Micarelli E., Giuliani G., Cerretani M., et al. Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/β-catenin axis. Cell Death Differ. 2020;27:2921–2941. doi: 10.1038/s41418-020-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L., MacDougald O.A. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Poser C., von Maltzahn J. Wnt7a counteracts cancer cachexia. Mol. Ther. Oncolytics. 2020;16:134–146. doi: 10.1016/j.omto.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup A.-S., Strachan D., Caxaria S., Poulet B., Thomas B.L., Eldridge S.E., Nalesso G., Whiteford J.R., Pitzalis C., Aigner T., et al. ROR2 blockade as a therapy for osteoarthritis. Sci. Transl. Med. 2020;12:eaax3063. doi: 10.1126/scitranslmed.aax3063. [DOI] [PubMed] [Google Scholar]
- Tidball J.G., Villalta S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A., Fukada S.i., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Wosczyna M.N., Biswas A.A., Cogswell C.A., Goldhamer D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner. Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna M.N., Konishi C.T., Perez Carbajal E.E., Wang T.T., Walsh R.A., Gan Q., Wagner M.W., Rando T.A. Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Rep. 2019;27:2029–2035.e5. doi: 10.1016/j.celrep.2019.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Takagishi K., Osawa T., Yanagawa T., Nakajima D., Shitara H., Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusion of the paper are present in the paper or the supplemental information.