Abstract
Introduction
Infective endocarditis (IE) has undergone important changes in its epidemiology worldwide.
Methods
The study aimed to compare IE epidemiological features and outcomes according to predefined European regions and between two different time periods in the twenty-first century.
Results
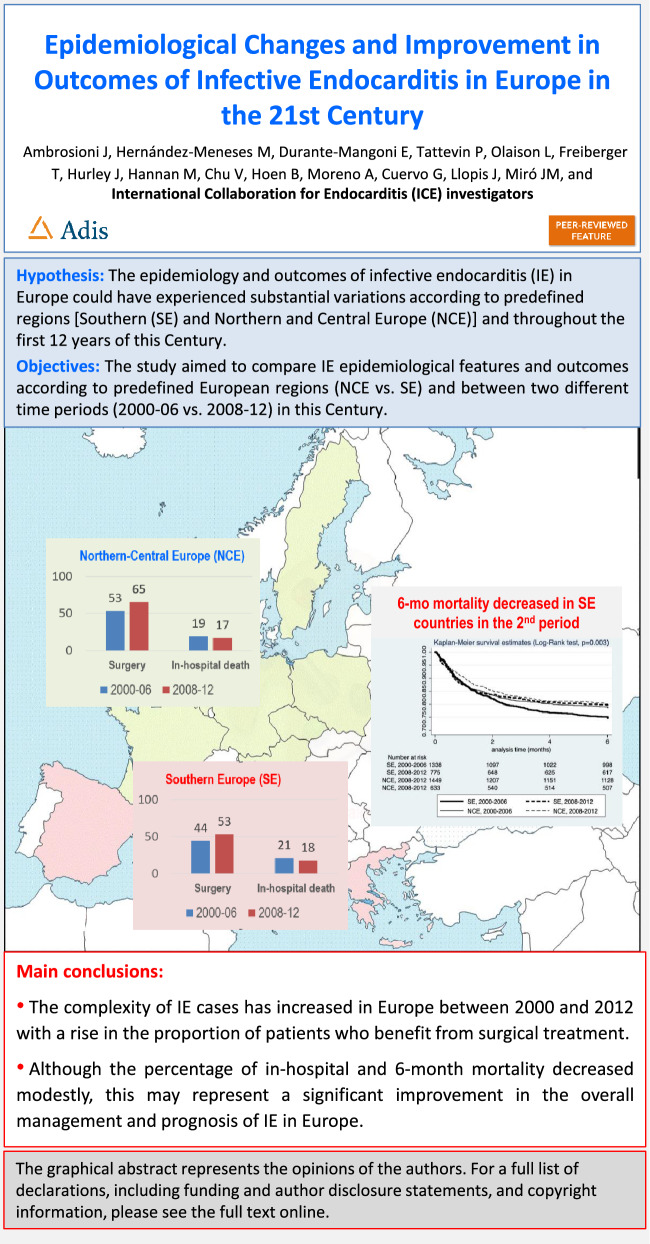
IE cases from 13 European countries were included. Two periods were considered: 2000–2006 and 2008–2012. Two European regions were considered, according to the United Nations geoscheme for Europe: Southern (SE) and Northern–Central Europe (NCE). Comparisons were performed between regions and periods. A total of 4195 episodes of IE were included, 2113 from SE and 2082 from NCE; 2787 cases were included between 2000 and 2006 and 1408 between 2008 and 2012. Median (IQR) age was 63.7 (49–74) years and 69.4% were males. Native valve IE (NVE), prosthetic valve IE (PVE), and device-related IE were diagnosed in 68.3%, 23.9%, and 7.8% of cases, respectively; 52% underwent surgery and 19.3% died during hospitalization. NVE was more prevalent in NCE, whereas device-related IE was more frequent in SE. Higher age, acute presentation, hemodialysis, cancer, and diabetes mellitus all were more prevalent in the second period. NVE decreased and PVE and device-related IE both increased in the second period. Surgical treatment also increased from 48.7% to 58.4% (p < 0.01). In-hospital and 6-month mortality rates were comparable between regions and significantly decreased in the second period.
Conclusions
Despite an increased complexity of IE cases, prognosis improved in recent years with a significant decrease in 6-month mortality. Outcome did not differ according to the European region (SE versus NCE).
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00763-8.
Keywords: Infective endocarditis, Europe, Epidemiology, Cardiac surgery, Mortality
Key Summary Points
| In recent decades, the epidemiology and outcomes of infective endocarditis (IE) have undergone important changes worldwide, but these changes are poorly characterized in the European regions. |
| The study aimed to identify the epidemiological and clinical features of 4,195 episodes of IE in the 13 European countries through the International Collaboration on Endocarditis (ICE) registry, comparing two regions (Southern [SE] vs. Northen-Central Europe [NCE] and two periods of time (2000–2006 vs. 2008–2021) in the twenty-first century. |
| The study revealed an increase in the complexity of the IE profile over time in both of these European regions, including a significant rise in the proportion of patients benefitting from surgical treatment (from 49% to 58%; p < 0.01). In-hospital (19%) and six-month (22%) mortality rates were similar between the regions and significantly decreased in recent years, mainly in the SE countries (from 21% to 18%, p < 0.01). |
| We have learned that, despite the increase in patients´ comorbidities and a more complex endocarditis profile, modestly decreasing in-hospital and six-month mortality may reflect a significant improvement in the overall management and prognosis of IE in Europe. |
Digital Features
This article is published with a digital feature (a graphical abstract). To view digital features for this article go to https://figshare.com/s/e98d8be0814c0d2b240e.
Introduction
Infective endocarditis (IE) has undergone important changes in its epidemiology worldwide. In high-income countries, the proportion of IE related to prior rheumatic disease has decreased significantly and has been replaced proportionally by cases related to degenerative valvulopathies, prosthetic valves, and cardiovascular implantable electronic devices [1]. IE incidence seems to be on the rise in high-income countries [2, 3]. Indeed, community-acquired, nosocomial, and [4] healthcare-related cases have risen in recent years. The proportion of IE caused by staphylococci and the median age of patients have also augmented, which may be partially justified by better reporting of cases. In low-income countries, in contrast, IE remains related to classic risk factors, such as rheumatic disease [5], and streptococci remain the most frequent causative agents [1].
A changing profile of IE has been described in several European countries [6–8]. European regions have large disparities in terms of access to care [3]. Moreover, in regions with comparable access to care, practices vary considerably in different countries (and even within the same country). In the early twenty-first century, IE has been described to be more often an acute disease, characterized by a high rate of Staphylococcus aureus infection, and to affect patients with more comorbid conditions [9]. In parallel, significant improvements in the management of IE, such as larger availability of cardiac surgery when it is indicated [10, 11] or the creation of multidisciplinary IE teams [12], have expanded in recent years. The paradox of a mortality rate that has remained relatively stable may be explained by this parallel increase in the complexity of cases and progress in care. The Euro-Endo registry is a recent prospective registry of IE cases, mainly from Europe, but also from abroad [13]. In the Euro-Endo registry initial report, in-hospital and overall 1-year mortality were 17.1% and 23.1%, respectively [14]. However, there are no reliable reports of previous years to put Euro-Endo registry information in context [15].
It is also unknown whether the epidemiological factors, complications, and outcome associated with IE differ across European regions with different healthcare systems and medical practices. The aim of this study was to compare IE epidemiological variables and outcomes in Europe according to predefined regions and across two different time periods in the twenty-first century, using data from the International Collaboration on Endocarditis (ICE) prospective cohort study (2000–2012).
Methods
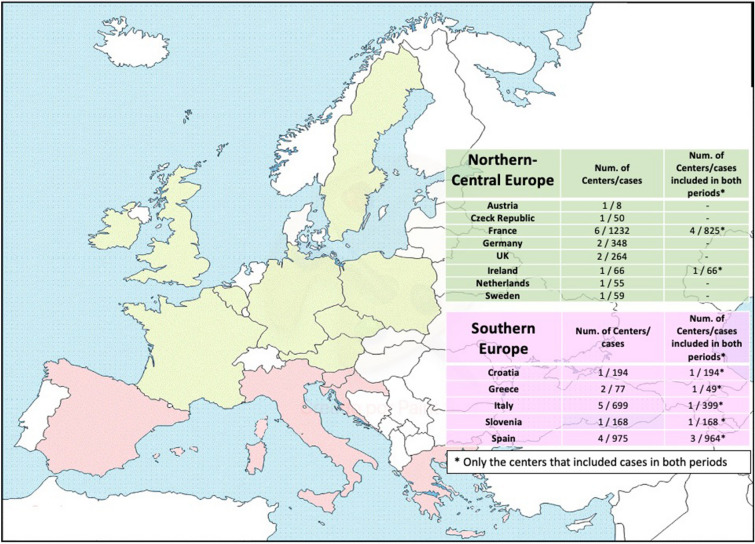
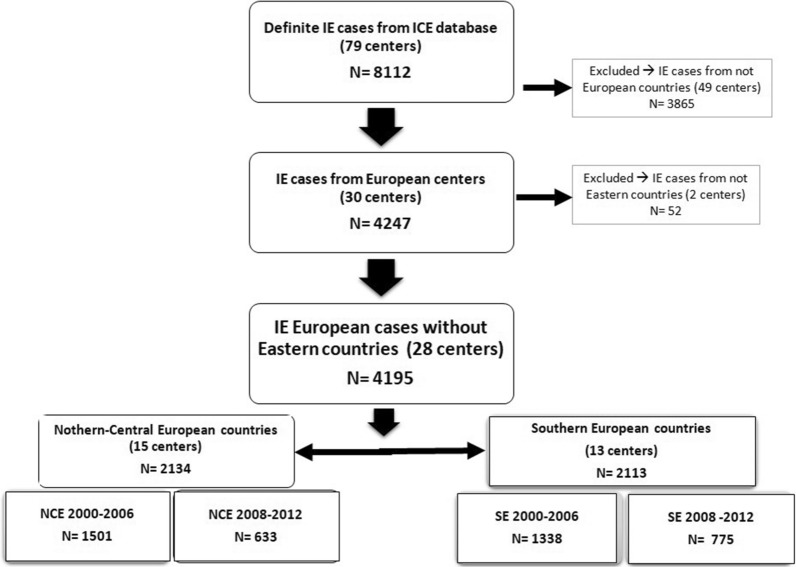
This observational study was based on data within the ICE Prospective Cohort Study and the ICE-Plus databases. The ICE Prospective Cohort Study (ICE-PCS) database contains prospective data on 5591 patients with definite and possible IE from 64 sites in 28 countries collected between January 1 2000 and December 31 2006. The ICE-Plus database contains prospective data on 2124 patients with IE from 34 sites in 18 countries collected between September 1 2008 and December 31 2012 [16]. For the purpose of this study, cases from the 28 European centers were included in the main study, and to overcome the issue of differences in practices between centers, a specific subanalysis was performed only with the 12 European centers reporting cases in both periods of time (see Fig. 1 and Supplementary Fig. 1).
Fig. 1.
Countries, centers, and cases from ICE cohort included in the study. Green: Northern–Central European countries included in the study. Red: Southern European countries included in the study
Briefly, sites of the ICE cohort had a minimum enrollment of 12 cases per year in a center with access to cardiac surgery, patient identification procedures in place to ensure consecutive enrollment and to minimize ascertainment bias, high-quality data, and an institutional review board and/or ethics committee approval.
The ICE registry was funded for two periods: 2000–2006 and 2008–2012, and these two periods were arbitrarily chosen for comparing epidemiological changes and outcomes of early and late IE in the first two decades of the 21st century.
For analyses purposes, two periods were considered: 2000–2006 (early) and 2008–2012 (late), according to data collection in the ICE cohorts. Centers were grouped according to the United Nations geoscheme for Europe [14]. Two European regions were considered for analysis: Southern (SE) and Northern and Central Europe (NCE). Due to very limited data (only two ICE centers, one from Romania and one from Russia, with less than 30 IE cases reported in total), Eastern Europe could not be considered for analysis. Comparisons were performed between both periods (2000–2006 versus 2008–2012) and the two regions (SE versus NCE), including epidemiological factors, microbiology, clinical aspects, echocardiographic findings, and outcome.
The variables included in the study are presented in Tables 1, 2, and 3, and were collected using an standardized case report form. Definitions have been previously described [4]. Definitions for the place of infection were as follows: (a) Cases were considered community-acquired if they were diagnosed within 48 h of admission, and if signs or symptoms consistent with IE developed in a patient without extensive out-of-hospital contact with healthcare interventions or systems; (b) cases were considered nosocomial healthcare associated if they occurred in a patient hospitalized for more than 48 h prior to the onset of signs or symptoms consistent with IE; and (c) cases were considered non-nosocomial healthcare associated if they were diagnosed within 48 h of admission, and if signs or symptoms consistent with IE developed prior to hospitalization in patients with extensive out-of-hospital contact with healthcare interventions or systems, defined as: (1) receipt of intravenous therapy, wound care, or specialized nursing care at home within the 30 days prior to the onset of native valve endocarditis; (2) receipt of hemodialysis or intravenous chemotherapy in the 30 days before the onset of native valve endocarditis; (3) hospitalization for 2 or more days in the 90 days before the onset of native valve endocarditis; or (4) residence in a nursing home or long-term care facility [4].
Table 1.
Baseline characteristics and predisposing conditions of overall cohort
| Total N = 4195 | NCE N = 2082 | SE N = 2113 | p-Value | Early period (2000–2006) N = 2787 | Later period (2008–2012) N = 1408 | p | |
|---|---|---|---|---|---|---|---|
| Baseline characteristics | |||||||
| Age (years, median, IQR) (N = 4195) | 63.7 (49–74) | 62.8 (48–74) | 64.1 (48–74) | < 0.01 | 63.2 (47–73) | 64.8 (51–75) | < 0.01 |
| Male gender (N = 4186) | 2904 (69.4%) | 1441 (69.4%) | 1463 (69.3%) | 0.92 | 1922 (69.1%) | 982 (69.8%) | 0.64 |
| First sign to admission < 1 month (N = 3848) | 3180 (83%) | 1533 (82.2%) | 1647 (83.1%) | 0.48 | 1995 (79.4%) | 1185 (88.8%) | < 0.01 |
| Hemodialysis (N = 3287) | 194 (5.9%) | 93 (5.7%) | 101 (6.1%) | 0.56 | 136 (4.9%) | 58 (11%) | < 0.01 |
| Diabetes (N = 4098) | 776 (18.9%) | 357 (17.9%) | 419 (20%) | 0.08 | 464 (17.1%) | 312 (22.6%) | < 0.01 |
| Cancer (N = 4099) | 469 (11.4%) | 242 (12.1%) | 227 (10.8%) | 0.22 | 278 (10.3%) | 191 (13.8%) | < 0.01 |
| HIV positive (N = 4127) | 88 (2.1%) | 13 (0.6%) | 75 (3.6%) | < 0.01 | 66 (2.4%) | 22 (1.6%) | 0.09 |
| Predisposing conditions | |||||||
| Previous IE (N = 4172) | 343 (8.2%) | 159 (7.7%) | 184 (8.7%) | 0.22 | 235 (8.5%) | 108 (7.8%) | 0.44 |
| Native valve predispositiona (N = 4034) | 1137 (28.2%) | 605 (29.7%) | 532 (26.6%) | 0.03 | 799 (29.1%) | 338 (26.2%) | 0.05 |
| Congenital heart disease (N = 4078) | 373 (9.4%) | 208 (10.3%) | 165 (8%) | 0.01 | 229 (8.5%) | 144 (10.5%) | 0.04 |
| CIED (N = 4160) | 517 (12.4%) | 226 (11%) | 291 (13.8%) | < 0.01 | 352 (12.7%) | 165 (12%) | 0.51 |
| Intravenous drug use (N = 4160) | 324 (7.8%) | 138 (6.7%) | 186 (8.8%) | 0.01 | 245 (8.9%) | 79 (5.7%) | < 0.01 |
| CA-IE (N = 3879) | 2910 (75%) | 1450 (79%) | 1460 (71.5%) | < 0.01 | 1965 (75.7%) | 945 (73.7%) | 0.18 |
| N-IE (N = 3879) | 726 (18.7%) | 272 (14.8%) | 454 (22.2%) | < 0.01 | 470 (18.1%) | 256 (20%) | 0.17 |
| HA-IE (N = 3879) | 243 (6.3%) | 114 (6.2%) | 129 (6.3%) | 0.89 | 162 (6.2%) | 81 (6.3%) | 0.95 |
| IE type | |||||||
| Native (N = 4123) | 2816 (68.3%) | 1458 (70.9%) | 1358 (65.7%) | < 0.01 | 1909 (70%) | 907 (64.9%) | < 0.01 |
| Prosthetic (N = 4123) | 985 (23.9%) | 482 (23.4%) | 503 (24.3%) | 0.49 | 619 (22.7%) | 366 (26.2%) | 0.01 |
| CIED endocarditis (N = 4123) | 322 (7.8%) | 117 (5.7%) | 205 (9.9%) | < 0.01 | 198 (7.3%) | 124 (8.9%) | 0.08 |
NCE Northern and Central European countries; SE Southern European countries; HIV human immunodeficiency virus; IE infective endocarditis; CA community acquired; N nosocomial, HA healthcare-associated; CIED cardiovascular implantable electronic devices
aIncluding aortic regurgitation, aortic stenosis, mitral regurgitation, and mitral stenosis
Table 2.
Microbiologic etiology comparative analyses between the two predefined regions and two periods of overall cohort
| Total N = 4195 | NCE N = 2082 | SE N = 2113 | p-Value | Early period (2000–2006) N = 2787 | Later period (2008–2012) N = 1408 | p-Value | |
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus (N = 3882) | 1039 (26.8%) | 506 (26.2%) | 533 (27.2%) | 0.59 | 711 (27.2%) | 328 (26.3%) | 0.61 |
| Viridans group Streptoccoci (N = 3882) | 655 (17.4%) | 295 (15.9%) | 360 (18.2%) | 0.16 | 443 (18%) | 212 (16.7%) | 0.39 |
| Coagulase negative Staphylococcus (N = 3882) | 510 (13.6%) | 219 (10.8%) | 291 (14.9%) | < 0.01 | 327 (12.5%) | 183 (14.8%) | 0.09 |
| Enterococcus spp. (N = 3882) | 427 (10.5%) | 202 (10.1%) | 225 (10.8%) | 0.60 | 269 (8.9%) | 158 (12.5%) | < 0.01 |
| Streptococcus gallolyticus (N = 3882) | 330 (9%) | 173 (12.3%) | 157 (7.3%) | < 0.01 | 221 (8.8%) | 109 (9%) | 0.90 |
| Other streptococcia (N = 3882) | 257 (7.2%) | 143 (10.1%) | 144 (5.8%) | < 0.01 | 159 (6.4%) | 98 (8.1%) | 0.11 |
| Gram negative (not HACEKb) (N = 3882) | 132 (4%) | 59 (3.1%) | 73 (3.7%) | 0.37 | 83 (3.2%) | 49 (3.9%) | 0.25 |
| Polymicrobial (N = 3882) | 85 (1.6%) | 59 (2.6%) | 26 (1.2%) | < 0.01 | 44 (0.5%) | 41 (3%) | < 0.01 |
| HACEKb (N = 3882) | 50 (1.2%) | 28 (1.5%) | 22 (1.1%) | 0.46 | 35 (1.3%) | 15 (1.1%) | 0.68 |
| Fungi (N = 3882) | 53 (1.2%) | 22 (1.2%) | 31 (1.2%) | 0.99 | 36 (1.3%) | 17 (1.2%) | 0.96 |
| Negative culture (N = 3882) | 235 (4.9%) | 124 (2.6%) | 111 (5.9%) | < 0.01 | 219 (8%) | 16 (1.1%) | < 0.01 |
| Other (N = 3882) | 109 (2.6%) | 53 (2.5%) | 56 (2.7%) | 0.73 | 78 (3%) | 31 (2.2%) | 0.20 |
aOther Streptococci including Streptococcus pneumoniae, beta hemolytic group Streptococci, etc.
bHACEK group includes Haemophilus species, Aggregatibacter actinomycetemcomitans, Aggregatibacter aphrophilus (formerly Haemophilus aphrophilus and Haemophilus paraphrophilus), Cardiobacterium hominis, Eikenella corrodens, and Kingella species.
Table 3.
Echocardiographic findings, complications, treatment, and outcome of overall cohort
| Total N = 4195 | NCE N = 2082 | SE N = 2113 | p-Value | Early period (2000–2006) N = 2787 | Later period (2008–2012) N = 1408 | p-Value | |
|---|---|---|---|---|---|---|---|
| Vegetation present | |||||||
| Intracardiac vegetation (N = 4146) | 3450 (83.2%) | 1650 (80.3%) | 1800 (86.1%) | < 0.01 | 2301 (83.5%) | 1149 (82.7%) | 0.50 |
| Aortic valve (N = 4135) | 1590 (38.5%) | 776 (37.7%) | 814 (39.2%) | 0.35 | 1035 (37.7%) | 555 (39.9%) | 0.18 |
| Mitral valve (N = 4135) | 1546 (37.4%) | 781 (38%) | 765 (36.8%) | 0.42 | 1030 (37.6%) | 516 (37.1%) | 0.76 |
| Tricuspid valve (N = 4131) | 384 (9.3%) | 176 (8.6%) | 208 (10%) | 0.11 | 280 (10.2%) | 104 (7.5%) | < 0.01 |
| Pulmonary valve (N = 4124) | 43 (1%) | 21 (1%) | 22 (1.1%) | 0.91 | 26 (1%) | 17 (1.2%) | 0.43 |
| Complications | |||||||
| Stroke (N = 4107) | 719 (17.5%) | 373 (18.6%) | 346 (16.5%) | 0.08 | 455 (16.8%) | 264 (19%) | 0.08 |
| Embolization non-stroke (N = 4096) | 1136 (27.7%) | 530 (26.4%) | 606 (29%) | 0.07 | 660 (24.4%) | 476 (34.2) | < 0.01 |
| CHF (N = 4093) | 1305 (31.9%) | 578 (28.9%) | 727 (34.7%) | < 0.01 | 793 (29.3%) | 512 (37%) | < 0.01 |
| Persistent positive blood culturea (N = 4027) | 293 (7.3%) | 130 (6.5%) | 163 (8%) | 0.06 | 171 (6.3%) | 122 (9.2%) | < 0.01 |
| Intracardiac abscess (N = 4122) | 698 (16.9%) | 386 (19%) | 312 (14.9%) | < 0.01 | 392 (14.3%) | 306 (22.1%) | < 0.01 |
| Treatment/outcome | |||||||
| Surgical therapyb (N = 4162) | 2163 (52%) | 1177 (56.9%) | 986 (47.1%) | < 0.01 | 1349 (48.7%) | 814 (58.4%) | < 0.01 |
| Time elapsed between admission and surgery (N = 2163) (days) | 1899 (87.8%) | 15 (6–30) | 18 (9–32) | < 0.01 | 17 (8–32) | 15 (6–29) | < 0.01 |
| In-hospital deathc (N = 4169) | 806 (19.3%) | 387 (18.7%) | 419 (19.9%) | 0.34 | 558 (20.1%) | 248 (17.8%) | 0.06 |
| Six-month mortality (N = 4187) | 939 (22.4%) | 439 (21.2%) | 500 (23.7%) | 0.05 | 649 (23.4%) | 290 (20.6%) | 0.04 |
| Relapses (N = 1973) | 109 (5.5%) | 45 (4.6%) | 64 (6.4%) | 0.08 | 70 (5.6%) | 39 (5.5%9 | 0.18 |
NCE Northern and Central European countries; SE Southern European countries; CHF congestive heart failure
aPersistent blood cultures were defined as blood cultures that remained positive after 7 days of effective therapy
bIn the NCE region, the rates of cardiac surgery in the periods 2000–2006 and 2008–2012 were 53.05% (N = 766) and 63.34% (N = 411), respectively. In the SE region they were 43.83% (N = 583) and 52.75% (N = 403) in the same time periods
cIn the NCE region, the in-hospital mortality rates in the periods 2000–2006 and 2008–2012 were 19.25% (N = 279) and 17.06% (N = 411), respectively. In the SE region they were 20.85% (N = 279) and 18.06% (N = 140) in the same time periods
The American and European IE guidelines for the indications of surgery were followed, as was the classification of emergent, urgent, and elective surgery [17, 18].
Compliance with ethics guidelines
The Institutional Review Board (IRB) of the Hospital Clinic of Barcelona approved the implementation of this study (ERB number HCB/2004/4629). The study’s retrospective nature waived the requirement for informed written consent. Patient identification was encoded, complying with the needs of the Organic Law on Data Protection 15/1999.
Statistical Analysis
Continuous variables are presented as medians with 25th and 75th percentiles. Categorical variables are presented as frequencies and percentages of the specified group. The chi-square test was used to compare categorical variables, and the Mann–Whitney or Kruskal–Wallis tests were used, as appropriate, to compare continuous variables. Multivariable analysis was performed to identify variables independently associated with in-hospital and 6-month mortality. We did select the variables using a bivariate analysis. Those with a p-value < 0.20 were considered as candidates for the multivariable analyses. In addition, those variables with an important clinical relevance (e.g., age and gender) were also included in the model. We used both forward stepwise and backward elimination subset selection methods to identify variables independently associated with mortality. The significance level for entering effects was < 0.1, and the significance level for removing effects was < 0.05. Multicollinearity was calculated using the Belsley, Kuh, and Welsch test and principal component analysis [19]. Interaction tests between the Charlson score and the period of time or the European regions were also performed. Kaplan–Meier curves were built to compare 6-month mortality between periods and region. Prognostic factors for in-hospital and 6-month mortality were analysed using a logistic regression model, with comparisons reported with odds ratios (ORs) with 95% confidence intervals (CIs). For all tests, a p-value < 0.05 was considered significant. Statistical analyses were performed using Stata statistical package v.14 (Stata Corporation LLC).
Results
The distribution of countries according to the predefined regions, and the relative proportion of cases and centers provided by each country are shown in Fig. 1. There were 2782 cases in the early period (2000–2006) and 1408 cases in the later period (2008–2012), see Fig. 2. Most cases from the SE region were provided by Spanish (975 cases from four centers) and Italian (699 cases from five centers) centers, whereas most cases from the NCE region were provided by French sites (1232 cases from six centers). In all, 4195 episodes of IE were included in the final analysis, 2113 from SE and 2082 from NCE. Overall, median (IQR) age was 63.7 (49–74) years and 69.4% were males. Native valve IE (NVE), prosthetic valve IE (PVE), and cardiac implantable electronic device-related IE were diagnosed in 68.3%, 23.9%, and 7.8% of cases, respectively; 52% underwent surgery and 19.3% died during hospitalization.
Fig. 2.
Flow chart of cases included in the study. NCE Northern and Central European countries (according to the UN geoscheme), SE Southern European countries (according to the UN geoscheme)
Baseline Characteristics and Predisposing Conditions
Baseline characteristics and predisposing conditions of patients are presented in Table 1. Human immunodeficiency virus (HIV) infection was more prevalent in SE centers. Native valve involvement was more prevalent in NCE, whereas device-related IE was more frequent in SE (p < 0.01 for all comparisons). When comparing time periods, patient age increased (p < 0.01) and acute presentation, hemodialysis, cancer, and diabetes mellitus was all more prevalent in the second period (p < 0.01 for all comparisons). Intravenous drug use became less prevalent (p < 0.01). Native valve IE decreased (p < 0.01) and prosthetic (p = 0.01) and device-related IE both increased, although the latter not significantly (p = 0.08).
Microbiological Findings
Microbiological features are presented in Table 2. Overall, S. aureus was the most prevalent microbial etiology, in 26.8% of cases and it was equally distributed in NCE and SE countries. Coagulase-negative staphylococci (CoNS) IE was more frequent in SE, viridans group Streptococcus (VGS) and Enterococcus spp. were equally distributed, and S. gallolyticus (formerly S. bovis) was more frequent in NCE countries. When comparing time periods, Enterococcus spp. increased with no other relevant difference among microorganisms. Notably, the proportion of culture negative cases was extremely low in the second period, accounting for only 1.1% of cases.
Echocardiographic Findings, Treatment, and Outcome
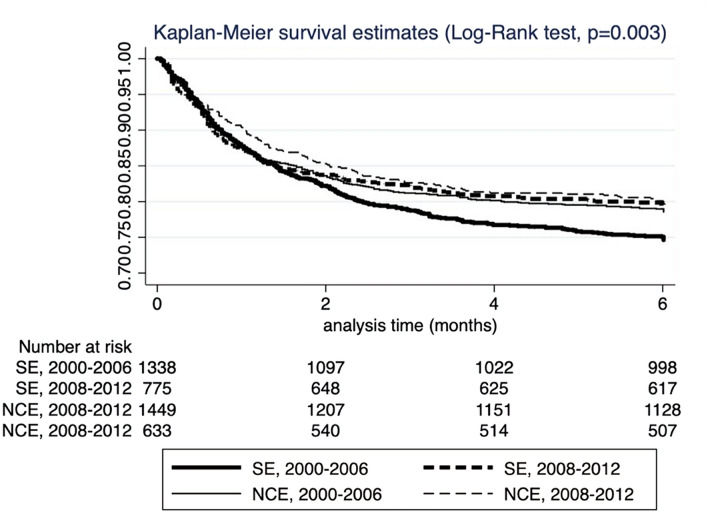
Echocardiographic findings, complications, treatment, and outcome are presented in Table 3. Valve involvement was not different between regions. Stroke and intracardiac abscesses were more prevalent in NCE, while systemic embolization was more prevalent in SE, although not statistically significant. Stroke (p = 0.08), congestive heart failure, systemic embolization, persistently positive blood cultures, and intracardiac abscesses increased in the second period (p < 0.01 for all comparisons). Surgical treatment was applied significantly more often in NCE countries, and regarding time periods, increased from 48.7% to 58.4% (p < 0.001). Stratified by group, surgery recourse remained stable in NCE countries in both periods (61.7% versus 64.6%, p = 0.39), but significantly increased in the SE countries (44.8% versus 50.1%, p = 0.03). In-hospital and 6-month mortality were comparable between regions and significantly decreased at 6 months in the second period from 23.4% to 20.6% (p = 0.04). When analyzed by period and by region, a more pronounced decrease in mortality was observed in SE countries (Fig. 3).
Fig. 3.
Kaplan–Meir curves of 6-month mortality for 4195 IE cases included in the study, according to pre-established regions and periods. SE Southern-European Countries; NCE Northern–Central European Countries
Multivariable analysis of factors associated with in-hospital mortality is presented in Table 4. The multicollinearity index was weak (maximum of 3.22). Classic IE prognostic factors (such as Charlson score, PVE, Staphylococcus aureus etiology, congestive heart failure, stroke, persistently positive blood cultures, or paravalvular complications), were independently related to increased mortality. VGS etiology and surgery were protective factors. Surgery was also protective when we excluded the patients who died before 2 weeks without surgery, with an OR (95%CI) of 0.67 (0.57, 0.80). The region (NCE versus SE) was unrelated to both in-hospital and 6-month mortality, but being diagnosed in the second period was a protective factor (OR of 0.54 and 0.53, respectively, for in-hospital and 6-month mortality).
Table 4.
Multivariable analysis of factors associated with in-hospital mortality and 6-month mortality of overall cohort
| In-hospital mortality | Six-month mortality | |||||
|---|---|---|---|---|---|---|
| Multivariate OR |
CI 95% | p-Value | Multivariate OR |
CI 95% | p-Value | |
| Charlson score | 1.36 | (1.24, 1.50) | < 0.01 | 1.34 | (1.01, 1.66) | 0.04 |
| Prosthetic valve IE | 1.62 | (1.26, 2.10) | < 0.01 | 1.68 | (1.30, 2.17) | < 0.01 |
| Staphylococcus aureusa | 1.82 | (1.43, 2.34) | < 0.01 | 1.72 | (1.32, 2.24) | < 0.01 |
| ConSa | 1.59 | (1.17, 2.21) | < 0.01 | 1.48 | (1.07, 2.09) | < 0.01 |
| Viridans group Streptoccocia | 0.38 | (0.21, 0.72) | < 0.01 | 0.64 | (0.44, 0,92) | 0.02 |
| Intracardiac vegetation | 1.57 | (1.14, 2.19) | < 0.01 | 1.59 | (1.15, 2.19) | < 0.01 |
| Stroke | 2.47 | (1.91, 3.18) | < 0.01 | 2.31 | (1.79, 3.01) | < 0.01 |
| CHF | 2.79 | (2.24, 3.49) | < 0.01 | 2.77 | (2.21, 3.46) | < 0.01 |
| Persistent positive blood culture | 2.69 | (1.91, 3.78) | < 0.01 | 2.65 | (1.84, 3.81) | < 0.01 |
| Paravalvular complications | 1.83 | (1.43, 2.32) | < 0.01 | 1.81 | (1.42, 2.32) | < 0.01 |
| N IE and HA IE versus CA IE | 1.89 | (1.56, 2.27) | < 0.01 | 1.30 | (1.01, 1.66) | 0.04 |
| In-hospital surgery | 0.69 | (0.55, 0.87) | < 0.01 | 0.68 | (0.54, 0.86) | < 0.01 |
| European region (SE versus NCE) | 1.33 | (0.88, 1.45) | 0.18 | 1.33 | (0.91, 1.41) | 0.27 |
| Period (2008–2012 versus 2000–2006) | 0.54 | (0.40, 0.76) | < 0.01 | 0.53 | (0.39, 0.73) | < 0.01 |
This analysis was adjusted by age and gender
NCE Northern and Central European countries; SE Southern European countries; IE infective endocarditis; CHF congestive heart failure; N nosocomial; HA healthcare associated; CA community acquired; IE infective endocarditis
aThe reference group is the rest of the etiological microorganisms
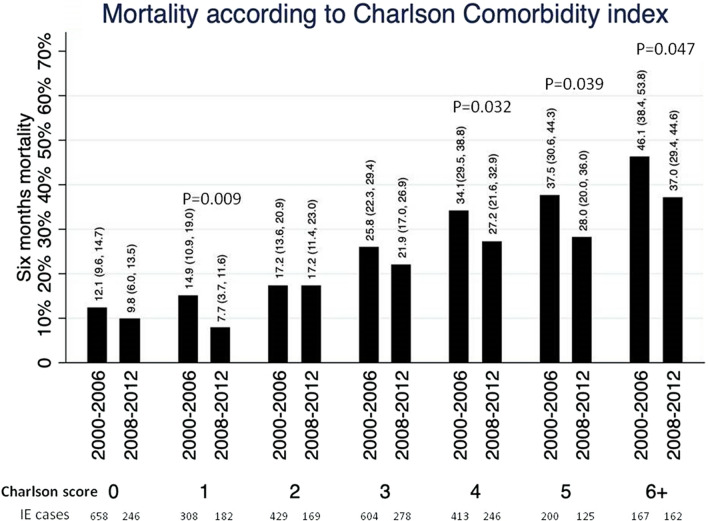
A subanalysis was performed with Charlson co-morbidity index (Fig. 4), showing that 6-month mortality was consistently lower in the second period for a given Charlson score, without interaction between variables (interaction test p = 0.08).
Fig. 4.
Analysis of mortality rate according to Charlson score, stratified by period (2000–2006 and 2008–2012)
The specific subanalysis performed only with centers reporting cases in both periods (N = 2665 cases, from 12 centers) showed no major differences in results, compared with the entire cohort (Supplementary Fig. 1 and Supplementary Tables 1–4).
Discussion
In recent decades, the epidemiology of IE in developed countries has shown a trend toward a higher comorbidity index, and increased complexity of cases, and therefore, a shift in the microbiological causes, favoring staphylococcal etiology [1]. Despite these recognized changes of IE in recent years, no study has compared different European regions or has evaluated prognosis trends over time. Our study shows an overall improvement in outcomes in Europe, despite the increased complexity of cases (shown by the higher rates of comorbidities such as hemodialysis or diabetes mellitus). Moreover, as shown in Fig. 4, for a given Charlson score, mortality was always lower in the second period.
The reasons for this overall better prognosis in the later period are not completely understood, although they may reflect several factors such as the increased proportion of early surgery (although not performed faster after admission in the second period); the better management of IE complications such as CNS emboli, or the utilization of more effective, better tolerated, and active antimicrobial agents; and the multidisciplinary approach by IE teams, among other factors. Although these data were not analyzed in our article, the role of newer antimicrobials or newer combinations of drugs in Europe may have had an impact. In fact, although recommended treatment regimens have remained almost unchanged for decades [15, 20], antimicrobial management of IE in reference centers frequently differ from the recommendations, even in centers whose clinical specialists have co-written the international management guidelines [21] The creation of specific teams dedicated to the management of these increasingly complex cases (so-called IE teams) [12], might have also impacted the observed global improvement. These teams, an integration of ID specialists, clinical microbiologists, cardiologists, cardiac surgeons, nuclear medicine, and radiology specialists, among others, have been shown to significantly reduce the mortality of IE cases [22], and have become more frequent in many centers recently.
Regarding geographical trends among regions, during the early period, prognosis was slightly better in NCE countries, and was associated with higher rates of cardiac surgery. As shown in Fig. 3, in the most recent chronological period these differences in mortality were not observed among regions, and overall mortality has decreased in association with an increase in the proportion of IE patients treated by cardiac surgery in SE countries. Unfortunately, we were unable to analyze the situation of IE in Eastern Europe, where access to care may be more limited and where intravenous drug use has importantly risen recently, with HIV and IE related to intravenous drug use becoming a major concern [23]. In this context, we would expect a quite different epidemiological and microbiological profile, with a higher proportion of right-sided IE and higher prevalence of co-morbidities such as HIV and HCV co-infection [24].
We did not identify major microbiological relevant differences between regions or periods, apart from a significant increase in the proportion of enterococcal IE, which may be related to the progressive aging of patients with IE [25] and the increasing prevalence of colorectal pathology in the general population [26]. Enterococcal IE is expected to rise even more, considering that Enterococcus spp. is the main cause of IE in transcatheter aortic valve replacement (TAVR) cases, and the number of TAVR cases is also expected to increase in the future, due to expanding indications [25–28]. S. aureus and CoNS IE, on the rise during in recent decades, remained stable during the two periods of our study.
However, the reduction in mortality shown in our study represents a modest but positive trend in the field of IE. Putting our data in context, with respect to the Euro-Endo registry, in hospital mortality in our study was 20.1% for the first period and 17.8% for the second, compared with 17% in the Euro-Endo registry [14, 29, 30]. Thus, it seems to continue with a trend towards a lower mortality, but these data should be confirmed at 6-month of follow-up. Unfortunately, as with the ICE cohort, the majority of hospitals reporting to the Euro-Endo registry are tertiary reference centers, and consequently may not accurately reflect the overall epidemiology of IE in Europe (including smaller hospitals from smaller cities, with no cardiac surgery).
Our study has several strengths. First, the large number of episodes allows a reliable analysis and provides adequate statistical power. Moreover, no previous large studies of this type have been performed in Europe since the Euro-Endo registry cannot compare two periods of time. To avoid the bias of different prognosis being related to a center’s experience, we have performed a subanalysis with centers participating in both periods, and the main results did not change.
Our article also has several limitations. Firstly, the UN geoscheme for Europe is a statistical and not a meaningful healthcare classification. Most of the countries included in our study belong to the World Health Organization (WHO) regions with low or extremely low childhood mortality, and thus, no comparison was possible between countries with high and low sanitary standards. Moreover, the classification of Southern versus Northern–Central is arbitrary. As a multicenter study, the use of health administrative data would have eliminated the bias due to the selective reporting of cases from reference centers, although it would likely have yielded less granular data. Moreover, there are large differences in practices even within the same country; for instance, a center from Marseille could have been considered as part of SE if only a geographical classification was applied. Conversely, a center from Milan could have been considered as part of the NCE region [14]. Furthermore, some countries are largely over-represented (such as France, Spain, or Italy), and the situation in other countries of the same region may be different. Unfortunately, Eastern Europe was excluded due to lack of data, which might have impacted on the epidemiology and outcomes of IE, as previously discussed. In addition, the retrospective nature of the study and the missing data existing for some variables may affect results of the analyses, particularly the subanalysis with the centers reporting cases in both periods due to the attrition in the number of cases. However, this is a problem observed with all retrospective cohorts. There is a bias of IE selection cases, since mostly large university tertiary centers provided data to ICE. The microbiology, predisposing conditions, and outcome of IE in smaller centers in the same countries or regions could differ considerably. Last but not least, data collection in the ICE cohort finished in 2012, more recent data was not available.
Conclusion
The complexity of IE cases increased in Europe between 2000 and 2012, accompanied by an increase in the proportion of patients undergoing surgical treatment. Survival improved in the latest period, particularly in SE countries. Although the percentage decrease of in-hospital and 6-month mortality is modest, considering the increased age and case complexity of patients with IE, it may represent a significant improvement in the overall treatment, prognosis, and potential public health implications for the management IE in Europe.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We want to express our appreciation to all Cardiovascular Infectious Diseases and infective endocarditis team members for their contribution to this work and their precious task and effort in clinical care and daily practice with all our patients.
Funding
No funding was received for the study, rapid service fee or the support from the Cardiovascular Infectious Diseases and infective endocarditis team members.
Author Contributions
Juan Ambrosioni, Marta Hernández-Meneses, Emanuele Durante-Mangoni, Pierre Tattevin, Lars Olaison, Tomas Freiberger, John Hurley, Margaret M. Hannan, Vivian Chu, Bruno Hoen, Asunción Moreno, Guillermo Cuervo, Jaume Llopis and José M. Miró contributed to the design and development of the study and to the acquisition, analysis, and interpretation of the results. All authors contributed to producing the article, by drafting the work or revising it critically. All authors have had access to the final version and have approved it to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were properly investigated and resolved.
Prior Presentation
This work was presented, in part, at the 14th International Symposium on Modern Concepts in Endocarditis and Cardiovascular Infections, Dublin, Ireland, 22-24 June 2017; abstract 51.
Disclosures
José M. Miró received a personal 80:20 research grant from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–23. Margaret M. Hannan held a Rio Hortega Research Grant (CM17/00062) from the “Instituto de Salud Carlos III” and the “Ministerio de Economia y Competitividad”, Madrid (Spain) in 2018-20. Juan Ambrosioni, Marta Hernández-Meneses, Emanuele Durante-Mangoni, Pierre Tattevin, Lars Olaison, Tomas Freiberger, John Hurley, Vivian Chu, Bruno Hoen, Asunción Moreno, Guillermo Cuervo and Jaume Llopis have nothing to declare.
Compliance with Ethics Guidelines
The Institutional Review Board (IRB) of the Hospital Clinic of Barcelona approved the implementation of this study (ERB number HCB/2004/4629). The study’s retrospective nature waived the requirement for informed written consent. Patient identification was encoded, complying with the needs of the Organic Law on Data Protection 15/1999.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
The ICE investigators are listed in the “Acknowledgements” section.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juan Ambrosioni and Marta Hernández-Meneses equally contributed as first authors.
Jaume Llopis and José M. Miró equally contributed as senior authors.
Change history
12/15/2023
A Correction to this paper has been published: 10.1007/s40121-023-00898-8
Contributor Information
Juan Ambrosioni, Email: jambrosioni@intramed.net.
José M. Miró, Email: jmmiro@ub.edu
International Collaboration for Endocarditis (ICE) Investigators:
Liliana Clara, Marisa Sanchez, José Casabé, Claudia Cortes, Francisco Nacinovich, Pablo Fernandez Oses, Ricardo Ronderos, Adriana Sucari, Jorge Thierer, Javier Altclas, Silvia Kogan, Denis Spelman, Eugene Athan, Owen Harris, Karina Kennedy, Ren Tan, David Gordon, Lito Papanicolas, Tony Korman, Despina Kotsanas, Robyn Dever, Phillip Jones, Pam Konecny, Richard Lawrence, David Rees, Suzanne Ryan, Michael P. Feneley, John Harkness, Phillip Jones, Suzanne Ryan, Phillip Jones, Suzanne Ryan, Phillip Jones, Jeffrey Post, Porl Reinbott, Suzanne Ryan, Rainer Gattringer, Franz Wiesbauer, Adriana Ribas Andrade, Ana Cláudia Passos de Brito, Armenio Costa Guimarães, Max Grinberg, Alfredo José Mansur, Rinaldo Focaccia Siciliano, Tania Mara Varejao Strabelli, Marcelo Luiz Campos Vieira, Regina Aparecida de Medeiros Tranchesi, Marcelo Goulart Paiva, Claudio Querido Fortes, Auristela de Oliveira Ramos, Clara Weksler, Giovanna Ferraiuoli, Wilma Golebiovski, Cristiane Lamas, James A. Karlowsky, Yoav Keynan, Andrew M. Morris, Ethan Rubinstein, Sandra Braun Jones, Patricia Garcia, M. Cereceda, Alberto Fica, Rodrigo Montagna Mella, Ricardo Fernandez, Liliana Franco, Javier Gonzalez, Astrid Natalia Jaramillo, Bruno Barsic, Suzana Bukovski, Vladimir Krajinovic, Ana Pangercic, Igor Rudez, Josip Vincelj, Tomas Freiberger, Jiri Pol, Barbora Zaloudikova, Zainab Ashour, Amani El Kholy, Marwa Mishaal, Dina Osama, Hussien Rizk, Neijla Aissa, Corentine Alauzet, Francois Alla, Catherine Campagnac, Thanh Doco-Lecompte, Christine Selton-Suty, Jean-Paul Casalta, Pierre-Edouard Fournier, Gilbert Habib, Didier Raoult, Franck Thuny, Francois Delahaye, Armelle Delahaye, Francois Vandenesch, Erwan Donal, Pierre Yves Donnio, Erwan Flecher, Christian Michelet, Matthieu Revest, Pierre Tattevin, Florent Chevalier, Antoine Jeu, Jean Paul Rémadi, Dan Rusinaru, Christophe Tribouilloy, Yvette Bernard, Catherine Chirouze, Bruno Hoen, Joel Leroy, Patrick Plesiat, Christoph Naber, Carl Neuerburg, Bahram Mazaheri, Christoph Naber, Carl Neuerburg, Sophia Athanasia, Ioannis Deliolanis, Helen Giamarellou, Tsaganos Thomas, Efthymia Giannitsioti, Elena Mylona, Olga Paniara, Konstantinos Papanicolaou, John Pyros, Athanasios Skoutelis, Elena Mylona, Olga Paniara, Konstantinos Papanikolaou, John Pyros, Athanasios Skoutelis, Gautam Sharma, Johnson Francis, Lathi Nair, Vinod Thomas, Krishnan Venugopal, Margaret M. Hannan, John P. Hurley, Maor Wanounou, Dan Gilon, Sarah Israel, Maya Korem, Jacob Strahilevitz, Ethan Rubinstein, Jacob Strahilevitz, Emanuele Durante-Mangoni, Domenico Iossa, Serena Orlando, Maria Paola Ursi, Pia Clara Pafundi, Fabiana D’Amico, Mariano Bernardo, Susanna Cuccurullo, Giovanni Dialetto, Franco Enrico Covino, Sabrina Manduca, Alessandro Della Corte, Marisa De Feo, Marie Françoise Tripodi, Enrico Cecchi, Francesco De Rosa, Davide Forno, Massimo Imazio, Rita Trinchero, Paolo Grossi, Mariangela Lattanzio, Antonio Toniolo, Antonio Goglio, Annibale Raglio, Veronica Ravasio, Marco Rizzi, Fredy Suter, Giampiero Carosi, Silvia Magri, Liana Signorini, Zeina Kanafani, Souha S. Kanj, Ahmad Sharif-Yakan, Imran Abidin, Syahidah Syed Tamin, Eduardo Rivera Martínez, Gabriel Israel Soto Nieto, Jan T. M. van der Meer, Stephen Chambers, David Holland, Arthur Morris, Nigel Raymond, Kerry Read, David R. Murdoch, Stefan Dragulescu, Adina Ionac, Cristian Mornos, O. M. Butkevich, Natalia Chipigina, Ozerecky Kirill, Kulichenko Vadim, Tatiana Vinogradova, Jameela Edathodu, Magid Halim, Yee-Yun Liew, Ru-San Tan, Tatjana Lejko-Zupanc, Mateja Logar, Manica Mueller-Premru, Patrick Commerford, Anita Commerford, Eduan Deetlefs, Cass Hansa, Mpiko Ntsekhe, Manel Almela, Juan Ambrosioni, Manuel Azqueta, Merce Brunet, Pedro Castro, Elisa De Lazzari, Carlos Falces, David Fuster, Guillermina Fita, Cristina Garcia-de-la-Maria, Javier Garcia-Gonzalez, Jose M. Gatell, Jaume Llopis, Francesc Marco, José M. Miró, Asuncion Moreno, José Ortiz, Salvador Ninot, J. Carlos Paré, Juan M. Pericas, Eduard Quintana, Jose Ramirez, Irene Rovira, Elena Sandoval, Marta Sitges, Adrian Tellez, José M. Tolosana, Barbara Vidal, Jordi Vila, Ignasi Anguera, Bernat Font, Joan Raimon Guma, Javier Bermejo, Emilio Bouza, Miguel Angel Garcia Fernández, Victor Gonzalez-Ramallo Mercedes Marín, Patricia Muñoz, Miguel Pedromingo, Jorge Roda, Marta Rodríguez-Créixems, Jorge Solis, Benito Almirante, Nuria Fernandez-Hidalgo, Pilar Tornos, Arístides de Alarcón, Ricardo Parra, Eric Alestig, Magnus Johansson, Lars Olaison, Ulrika Snygg-Martin, Orathai Pachirat, Pimchitra Pachirat, Burabha Pussadhamma, Vichai Senthong, Anna Casey, Tom Elliott, Peter Lambert, Richard Watkin, Christina Eyton, John L. Klein, Suzanne Bradley, Carol Kauffman, Roger Bedimo, Vivian H. Chu, G. Ralph Corey, Anna Lisa Crowley, Pamela Douglas, Laura Drew, Vance G. Fowler, Thomas Holland, Tahaniyat Lalani, Daniel Mudrick, Zaniab Samad, Daniel Sexton, Martin Stryjewski, Andrew Wang, Christopher W. Woods, Stamatios Lerakis, Robert Cantey, Lisa Steed, Dannah Wray, Stuart A. Dickerman, Hector Bonilla, Joseph DiPersio, Sara-Jane Salstrom, John Baddley, Mukesh Patel, Gail Peterson, Amy Stancoven, Donald Levine, Jonathan Riddle, Michael Rybak, Christopher H. Cabell, Khaula Baloch, Vivian H. Chu, G. Ralph Corey, Christy C. Dixon, Vance G. Fowler, Tina Harding, Marian Jones-Richmond, Lawrence P. Park, Bob Sanderford, Judy Stafford, Kevin Anstrom, Eugene Athan, Arnold S. Bayer, Christopher H. Cabell, Vivian H. Chu, G. Ralph Corey, Vance G. Fowler, Bruno Hoen, A. W. Karchmer, José M. Miró, David R. Murdoch, Daniel J. Sexton, Andrew Wang, Arnold S. Bayer, Christopher H. Cabell, Vivian Chu, G. Ralph Corey, David T. Durack, Susannah Eykyn, Vance G. Fowler, Bruno Hoen, José M. Miró, Phillipe Moreillon, Lars Olaison, Didier Raoult, and Daniel J. Sexton
References
- 1.Ambrosioni J, Hernandez-Meneses M, Téllez A, et al. The Changing Epidemiology of Infective Endocarditis in the Twenty-First Century. Curr Infect Dis Rep. 2017;19:21. doi: 10.1007/s11908-017-0574-9. [DOI] [PubMed] [Google Scholar]
- 2.Olmos C, Vilacosta I, Fernández-Pérez C, et al. The evolving nature of infective endocarditis in Spain. J Am Coll Cardiol. 2017;70:2795–2804. doi: 10.1016/j.jacc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA. 2018;320:72–83. doi: 10.1001/jama.2018.7596. [DOI] [PubMed] [Google Scholar]
- 4.Benito N, Miró JM, de Lazzari E, et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med. 2009;150:586–594. doi: 10.7326/0003-4819-150-9-200905050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noubiap JJ, Nkeck JR, Kwondom BS, et al. Epidemiology of infective endocarditis in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2022;10(1):e77–e86. doi: 10.1016/S2214-109X(21)00400-9. [DOI] [PubMed] [Google Scholar]
- 6.Asgeirsson H, Thalme A, Weiland O. Low mortality but increasing incidence of Staphylococcus aureus endocarditis in people who inject drugs. Medicine (Baltimore) 2016;95:e5617. doi: 10.1097/MD.0000000000005617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54:1230–1239. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 8.Giannitsioti E, Skiadas I, Antoniadou A, et al. Nosocomial vs. community-acquired infective endocarditis in Greece: changing epidemiological profile and mortality risk. Clin Microbiol Infect. 2007;13:763–769. doi: 10.1111/j.1469-0691.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 9.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation. 2010;121:1005–1013. doi: 10.1161/CIRCULATIONAHA.109.864488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang D-H, Kim Y-J, Kim S-H, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366:2466–2473. doi: 10.1056/NEJMoa1112843. [DOI] [PubMed] [Google Scholar]
- 12.Mestres CA, Paré JC, Miró JM, Working Group on Infective Endocarditis of the Hospital Clínic de Barcelona Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985–2014) Rev Esp Cardiol (Engl Ed) 2015;68:363–368. doi: 10.1016/j.recesp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Habib G, Lancellotti P, Erba P-A, et al. The ESC-EORP EURO-ENDO (European Infective Endocarditis) registry. Eur Hear J Qual Care Clin Outcomes. 2019;5:202–207. doi: 10.1093/ehjqcco/qcz018. [DOI] [PubMed] [Google Scholar]
- 14.United Nations Geoscheme. 2023. https://unstats.un.org/unsd/methodology/m49/.
- 15.Habib G, Lancellotti P, Antunes MJ, et al. ESC Guidelines for the management of infective endocarditis. Eur Heart J. 2015;2015:36. [Google Scholar]
- 16.Pericàs JM, Llopis J, Jiménez-Exposito MJ, et al. Infective endocarditis in patients on chronic hemodialysis. J Am Coll Cardiol. 2021;77:1629–1640. doi: 10.1016/j.jacc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Wilson W, Taubert KA, Gewitz M, Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 18.Habib G, Hoen B, Tornos P, et al. ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30(19):2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery DC, Peck EA, Vining GG, editors. Introduction to linear regression analysis. 6. Hoboken: John Wiley & Sons Inc; 2021. [Google Scholar]
- 20.Kong WKF, Salsano A, Giacobbe DR, et al. Outcomes of culture-negative vs culture- positive infective endocarditis: the ESC-EORP EURO-ENDO registry. Eur Heart J. 2022;43(29):2770–2780. doi: 10.1093/eurheartj/ehac307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tissot-Dupont H, Casalta JP, Gouriet F, et al. International experts’ practice in the antibiotic therapy of infective endocarditis is not following the guidelines. Clin Microbiol Infect. 2017;23:736–739. doi: 10.1016/j.cmi.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112:1171–1176. doi: 10.1016/j.amjcard.2013.05.060. [DOI] [PubMed] [Google Scholar]
- 23.Eurosurveillance Editorial Team The European Monitoring Centre for Drugs and Drug Addiction publishes the European Drug Report 2013: trends and developments. Euro Surveill. 2013;18:20491. [PubMed] [Google Scholar]
- 24.Sousa C, Botelho C, Rodrigues D, et al. Infective endocarditis in intravenous drug abusers: an update. Eur J Clin Microbiol Infect Dis. 2012;31:2905–2910. doi: 10.1007/s10096-012-1675-x. [DOI] [PubMed] [Google Scholar]
- 25.Forestier E, Fraisse T, Roubaud-Baudron C, et al. Managing infective endocarditis in the elderly: new issues for an old disease. Clin Interv Aging. 2016;11:1199–1206. doi: 10.2147/CIA.S101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pericàs JM, Corredoira J, Moreno A, et al. Relationship between Enterococcus faecalis infective endocarditis and colorectal neoplasm: preliminary results from a Cohort of 154 patients. Rev Española Cardiol (Engl Ed) 2017;70:451–458. doi: 10.1016/j.recesp.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 27.Escolà-Vergé L, Peghin M, Givone F, et al. Prevalence of colorectal disease in Enterococcus faecalis infective endocarditis: results of an observational multicenter study. Rev Esp Cardiol (Engl Ed) 2020;73(9):711–717. doi: 10.1016/j.recesp.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Pericàs JM, Ambrosioni J, Muñoz P, GAMES Investigators et al. Prevalence of colorectal neoplasms among patients with Enterococcus faecalis endocarditis in the GAMES Cohort (2008–2017) Mayo Clin Proc. 2021;96(1):132–146. doi: 10.1016/j.mayocp.2020.06.056. [DOI] [PubMed] [Google Scholar]
- 29.Habib G, Erba PA, Iung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40:3222–3232. doi: 10.1093/eurheartj/ehz620. [DOI] [PubMed] [Google Scholar]
- 30.Miro JM, Ambrosioni J. Infective endocarditis: an ongoing global challenge. Eur Heart J. 2019;40(39):3233–3236. doi: 10.1093/eurheartj/ehz694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.