Abstract
Arbitrarily primed (AP)-PCR can be used to generate characteristic DNA fingerprint patterns. However, small changes in reaction conditions can cause band irreproducibility. In this study, a single methodology encompassing triplicate reactions, which were intentionally exposed to three different annealing temperatures, enabled bands that were reproducibly generated to be recognized. A single triplicate AP-PCR (TAP-PCR) procedure, using an 18-mer primer, was developed and used to fingerprint representative isolates from the major genera of lactic acid bacteria and Bifidobacterium to the strain level.
Lactic acid bacteria (LAB) are a large group of phylogenetically related, lactic-acid-producing bacteria used primarily in the production of fermented foodstuffs. They encompass many genera, including Lactococcus, Lactobacillus, Streptococcus, Leuconostoc, Enterococcus, Oenococcus, and Carnobacterium (14). In its broadest sense, LAB may also encompass other phylogenetically unrelated, lactic-acid-producing bacteria also used in foods, such as Bifidobacterium. While the primary role of LAB in fermented foods is to produce lactic acid, many are now often added to foods as probiotics for their potential health benefits to the gastrointestinal tract (reviewed in references 11 and 15). The ever increasing use of specialized strains of LAB requires careful attention to strain identification. While classical morphological and biochemical identification methods will always have an important role in culture houses, they are labor intensive and not very definitive. Consequently, more definitive and less time-consuming identification approaches would enable culture houses to verify the identity of each organism on a more routine basis. There are many recently developed approaches for the molecular fingerprinting of bacteria, including the use of specific molecular probes, monitoring plasmid profiles, and pulsed-field gel electrophoresis (reviewed in reference 2). These approaches offer extra flexibility and increase confidence in strain identification programs, but they are not suitable for routine use. Another alternative genomic-based identification methodology involves the use of PCR. The use of PCR for fingerprinting purposes has been refined by using a single arbitrarily chosen primer at low-stringency annealing conditions, which permit it to bind to genomic DNA at places to which it has full or partial homology, resulting in PCR product formation if two sites are within a few thousand base pairs of one another and are on opposite DNA strands (3, 17, 18). This arbitrarily primed PCR (AP-PCR) method is also referred to as random amplified polymorphic DNA PCR or DNA-amplified fingerprinting (DAF) PCR. Unfortunately, reproducibility can be a problem for fingerprints generated by a single primer, because small changes in annealing conditions can affect banding pattern production. Factors that can influence annealing conditions have been well documented and include thermocycler program and model (7, 10, 12, 16) as well as components of the reaction mixture: DNA concentration (3, 9) and purity (8); thermostable polymerase type, source, and concentration (6, 10, 16); and other buffer conditions (1, 5). While many of these factors can be controlled for through the use of a detailed methodology, minute changes in reaction mixture components and slight variations in thermocycler temperature profiles between trials cannot. In this study, we present a detailed methodology that uses three different annealing temperatures simultaneously in a triplicate reaction, allowing the identification of bands that are sensitive to small changes in temperature. This fingerprinting methodology, which we have designated triplicate AP-PCR (TAP-PCR), uses a primer specific to a conserved region within the 16S rRNA gene and was evaluated for the molecular fingerprinting of a wide range of genera of LAB.
Bacterial strains and growth conditions.
All bacterial strains used in this study were obtained from commercial culture collections as indicated or from the collection of L. L. McKay at University of Minnesota. For fingerprinting studies, bacteria were grown to stationary phase (typically overnight) in the following conditions and media. Bifidobacteria were grown in reinforced clostridial medium at 37°C under anaerobic conditions, lactococci and streptococci were grown in M17 medium supplemented with 0.5% glucose at 30°C under microaerophilic conditions, and all other bacteria were grown with MRS medium at 30°C under microaerophilic conditions. All media were purchased from Difco Laboratories (Detroit, Mich.). Bacteria for fingerprinting were then aliquoted to fill 1.5 ml-tubes and then were pelleted, decanted, and frozen (−70°C) until needed.
Evaluation of a possible candidate primer for fingerprinting diverse organisms.
An 18-mer primer was designed that had homology to a universally conserved region of the 16S rRNA gene (5′-CAGCAGCCGCGGTAATWC-3′) and was designated P32. This primer contained a degenerate base, a W (A/T) at the seventeenth nucleotide. Since at least one, and sometimes many, specific binding sites are already present in an organism for this primer, it is possible that it may have an increased likelihood of generating polymorphisms from organisms of diverse genetic backgrounds. Other studies have substantiated this hypothesis by using primers homologous to the eukaryotic 18S rRNA gene (7) and to sequences encoding hydroxyproline-rich glycoproteins (3). To evaluate if this primer possessed this capacity, it was tested with the gram-positive bacterium Lactococcus lactis and the gram-negative bacterium Escherichia coli. Purified genomic DNA was isolated from each organism and used as the template for primer P32 in a PCR mixture (1 ng of genomic DNA in a total reaction volume of 20 μl, using a final magnesium concentration of 1.5 mM and a single annealing temperature of 45°C). This PCR generated five bands of sizes 700 to >3,000 bp for L. lactis cells and 11 bands from 350 to 1,100 bp for E. coli cells (data not shown). These data suggested that P32 may be a useful primer for generating discriminatory polymorphisms from different genera of bacteria.
Refining conditions for rapidity and reproducibility.
To reduce setup time and avoid excess damage to the DNA during extraction and purification, we attempted to use a crude bacterial lysate produced by breaking cells apart by controlled agitation. After removing pelleted cells from the freezer, 200 μl of water was quickly added, followed by a 0.5 total volume of acid-washed glass beads (diameter of bead, ≤106 microns; Sigma, St. Louis, Mo.). Cells were lysed by using a mini-beadbeater-8 (Biospec, Bartlesville, Okla.) on maximum setting for 10 s. Longer agitation periods were not found to release more DNA from a Lactobacillus strain tested (data not shown). Dilutions of lysates were tested by PCR for band reproducibility, as changes in template concentration as little as twofold have been found to affect certain bands (4), while 100-fold changes have been found not to affect other bands (13). Some bands generated in this study using primer P32 were stable over a 10−3 template dilution, while others were disrupted by only a 10-fold change in template concentration (data not shown). A template dilution of 10−2 in sterile water was selected as optimum for fingerprinting LAB.
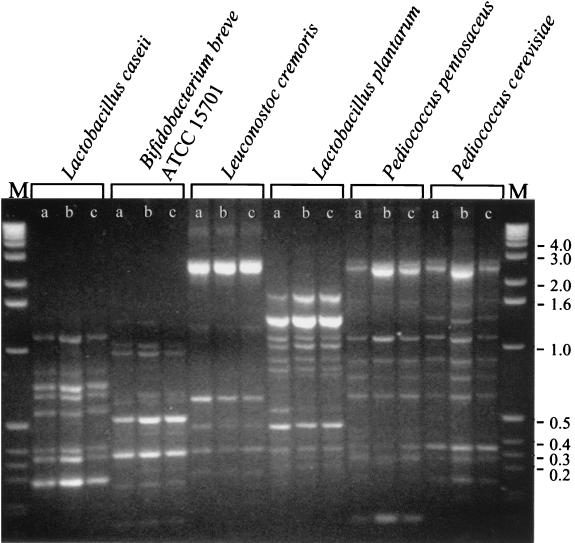
Bands that are sensitive to small changes in annealing conditions are typically less reproducible. Since small changes in the annealing temperature during PCR can greatly affect banding pattern production (4), bands that remain constant at three slightly different annealing temperatures should be more reproducible. This hypothesis was tested by setting up three 10-μl reaction mixtures in 0.5-ml thin-walled PCR tubes (Fisher Scientific, Pittsburgh, Pa.), from a single 30-μl master reaction mix, which contained the following: 200 μM concentrations of each deoxynucleoside triphosphate (Applied Biosystems, Foster City, Calif.), 1.8 U of AmpliTaq (Applied Biosystems), 14 μM P32 primer, 1.5 μl of diluted DNA lysate in reaction buffer (Applied Biosystems), adjusted to 5 mM MgCl2. A 10-μl reaction mixture containing all of the above except DNA was also set up. All reaction mixtures were overlaid with 20 μl of mineral oil. Amplification was carried out by using a Hybaid “omnigene” thermal cycler (containing three separate heating blocks) as follows: one cycle of 92°C for 2 min, followed by 40 cycles of 92°C for 30 s and 38, 40, or 42°C for 1 min, followed by 68°C for 1 min 30 s, followed by a final 10-min incubation at 68°C. Figure 1 shows a representative set of fingerprints of six bacteria representing four genera which were generated by TAP-PCR. Approximately 8 to 11 bands were observed, ranging in size from 150 bp to 2.5 kb. Stable products were assumed to be those found in at least two of the three temperatures. Using this criterion, 51 stable bands and no unstable bands were identified (Table 1).
FIG. 1.
TAP-PCR-generated fingerprints of various LAB by using primer P32 and 1.5 mM MgCl2. Lanes a, b, and c, annealing temperatures of 38, 40, and 42°C, respectively; lane M, 1-kb ladder (BRL). Molecular sizes (right) are in kilobases.
TABLE 1.
Analysis of bands generated for each bacterium shown in Fig. 1
| Bacterium | No. of bands formed at:
|
No. of bands that were:
|
|||
|---|---|---|---|---|---|
| 38°C | 40°C | 42°C | Kept | Eliminateda | |
| Lactobacillus caseii | 8 | 8 | 8 | 8 | 0 |
| Bifidobacterium breve ATCC 15701 | 8 | 8 | 8 | 8 | 0 |
| Leuconostoc cremoris | 6 | 6 | 6 | 6 | 0 |
| Lactobacillus plantarum | 8 | 8 | 8 | 8 | 0 |
| Pediococcus pentosaceus | 10 | 10 | 10 | 10 | 0 |
| Pediococcus cerevisiae | 11 | 11 | 11 | 11 | 0 |
Bands are excluded if present in only one of the three lanes.
Susceptibility of the degenerate primer to batch variation.
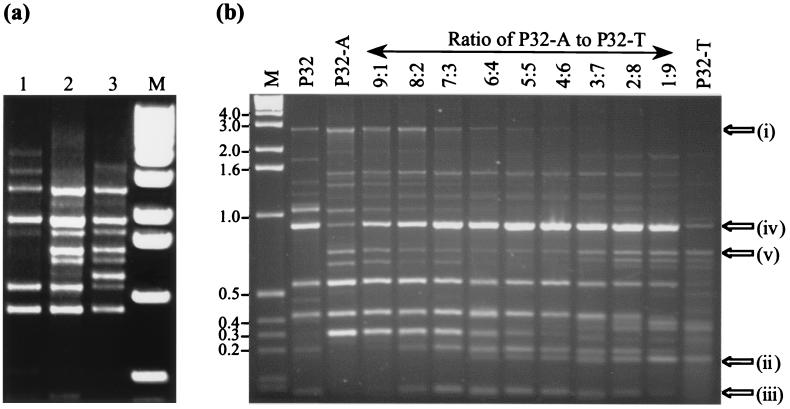
Degenerate sites in a primer are created during primer production by the addition of equal ratios (theoretically) of the nucleosides of interest to the nascent oligonucleotide. The nucleosides are assumed to compete effectively with one another during synthesis, ideally resulting in equal ratios of the two in the finished oligonucleotide. In this study, three different batches of primer P32 were ordered and tested for fingerprinting with identical replicates of L. lactis JS102 lysate by using conditions described above (except 1.5 mM MgCl2 and a single annealing temperature of 45°C). Batches 2 and 3 generated similar fingerprints, except for the generation of an extra band from batch 3 (Fig. 2a). Batch 1 had four bands in common with batches 2 and 3, but differed significantly with the rest of the profile. These data suggested that the degenerate W residue at position 17 of the P32 primer may not represent equal ratios of A and T in each of the batches, as substitution of nucleotides close to the 3′ end of a primer causes more of a change in banding pattern compared with those created by modifying the 5′ end of the primer (13). To ascertain if changing the ratio of nucleotides at a site in the primer close to the 3′ end would have a noticeable effect on the banding pattern, two primers identical to P32 except for containing one of the two degenerate bases (A or T) at position 17 were synthesized. These primers (P32-A and P32-T) were combined in different ratios and used to fingerprint L. lactis JS102, which we compared with a fingerprint generated by using primer P32. Conditions were the same, but the MgCl2 concentration was increased to 5 mM in an attempt to increase the number of products generated. Approximately 11 polymorphisms were amplified in each reaction mixture, and the A-to-T ratio at position 17 was clearly critical for band reproducibility (Fig. 2b). Bands affected by changes in the ratio can be divided into five classes: (i) those present at high ratios of P32-A, (ii) those present at high ratios of P32-T, (iii) those present only at near-equal ratios of P32-A and P32-T, (iv) those that were most intense when P32-A and P32-T were combined in any ratio but less intense when used individually, and (v) those that decreased in intensity at near-equal ratios, but were intense at either extreme. It was noted that none of the fingerprints generated by using any of these ratios were identical to that produced with P32. The results depicted here show that the intensity of a product does not necessarily correlate with sensitivity to changes in primer ratio and therefore suggests that differences in intensities between bands are not solely the result of differences in primer binding site homology.
FIG. 2.
(a) Fingerprints generated from three different batches (1, 2, and 3) of degenerate primer P32 by using L. lactis JS102 DNA. The arrow shows a major product difference between batches 2 and 3. Lane M, 1-kb ladder (BRL). (b) Fingerprints generated with P32, P32-A, P32-T, and various ratios of P32-A and P32-T by using L. lactis JS102. Arrows indicate different classes of bands (see text). Lane M, 1-kb ladder (BRL).
Final fingerprinting methodology.
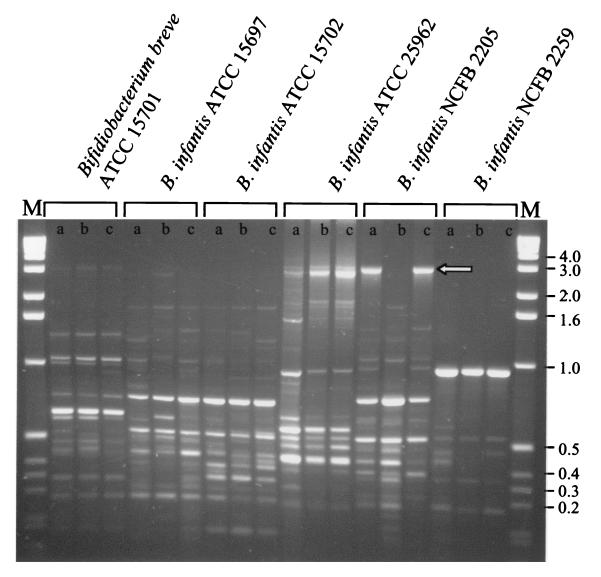
Based on the above studies, a 1-to-1 ratio of P32-A and P32-T was arbitrarily chosen for TAP-PCR, as was a magnesium concentration of 5 mM. It was also determined that the purification of primers by isolation from a polyacrylamide gel was necessary for the reproducibility of fingerprints between batches of primers (data not shown). Using this optimized TAP-PCR protocol, we fingerprinted a variety of LAB encompassing Lactococcus, Lactobacillus, Streptococcus, Pediococcus, Leuconostoc, and Enterococcus and LAB-associated bacteria Bifidobacterium and Propionibacterium. All showed discernible polymorphisms using this single methodology and conditions. To evaluate the discriminatory power of this TAP-PCR methodology, fingerprints were generated from five different strains of Bifidobacterium infantis. All patterns generated were unique and were capable of discriminating all strains tested (Fig. 3). Strain differentiation was also confirmed for L. lactis (data not shown).
FIG. 3.
TAP-PCR-generated fingerprints of various Bifidobacterium breve and B. infantis strains using a 1-to-1 ratio of P32-A to P32-T. Lanes a, b, and c, annealing temperatures of 38, 40, and 42°C, respectively; lane M, 1-kb ladder (BRL). Molecular sizes (left) are in kilobases. The arrow points to a band which is discussed in the text.
An interesting observation from Fig. 3 was that changes in annealing temperature did not affect all bands in a predictable fashion. One band (see arrow) was produced at the lowest and highest annealing temperatures, but not at the intermediate temperature. The underlying reasons for this phenomenon are presently unknown. Changes in annealing temperatures as small as 1°C (12) and changes in the ramp time between temperature changes in the PCR (16) have been found to affect polymorphism production. The data in this study showed that some bands were unaffected by annealing temperature changes of up to 6°C while other bands ceased to be amplified after changes of only 2°C, thus highlighting the value of the TAP-PCR procedure.
TAP-PCR is a fast, technically simple and inexpensive technology that was used to differentiate even closely related strains of many different genera of LAB by using a single protocol. By incorporating triplicate reactions at three different annealing temperatures, bands that are temperature sensitive can be eliminated. This technique holds promise as a tool to quickly and routinely confirm the identities of bacteria used in the food fermentation industry.
Acknowledgments
This study was funded in part by the Biological Processing Technology Institute (BPTI) at University of Minnesota and the Minnesota Agricultural Experimental Station.
Footnotes
Published as paper number 001180009 of the Scientific Journal Series of the Minnesota Agricultural Experiment Station based on research conducted under project 18-055.
REFERENCES
- 1.Bassam B J, Caetano-Anolles G, Gresshoff P M. DNA amplification fingerprinting of bacteria. Appl Microbiol Biotechnol. 1992;38:70–76. doi: 10.1007/BF00169422. [DOI] [PubMed] [Google Scholar]
- 2.Busse H J, Denner E B M, Lubitz W. Classification and identification of bacteria—current approaches to an old problem—overview of methods used in bacterial systematics. J Biotechnol. 1996;47:3–38. doi: 10.1016/0168-1656(96)01379-x. [DOI] [PubMed] [Google Scholar]
- 3.Caetano-Anolles G, Bassam B J, Gresshoff P M. DNA amplification fingerprinting using very short arbitrary primers. Bio/Technology. 1991;9:553–557. doi: 10.1038/nbt0691-553. [DOI] [PubMed] [Google Scholar]
- 4.Ellsworth D L, Rittenhouse K D, Honeycutt R L. Artifactual variation in randomly amplified polymorphic DNA banding patterns. BioTechniques. 1993;14:214–217. [PubMed] [Google Scholar]
- 5.Liu J, Berry R E. Determination of PCR conditions for RAPD analysis in enomopathogenic nematodes (Rhabditida: Heterorhabditidae and Steinernematidae) J Invertebr Pathol. 1995;65:79–81. doi: 10.1006/jipa.1995.1013. [DOI] [PubMed] [Google Scholar]
- 6.Loudon K W, Coke A P, Burnie J P. “Pseudoclusters” and typing by random amplification of polymorphic DNA in Aspergillis fumigatus. J Clin Pathol. 1995;48:183–184. doi: 10.1136/jcp.48.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacPherson J M, Eckstein P E, Scoles G J, Gajadhar A A. Variability of the random amplified polymorphic DNA assay among thermal cyclers, and effect of primer and DNA concentration. Mol Cell Probes. 1993;7:293–299. doi: 10.1006/mcpr.1993.1043. [DOI] [PubMed] [Google Scholar]
- 8.Marshall D G, Chua A, Keeling P W N, Sullivan D J, Coleman D C, Smyth C J. Molecular analysis of Helicobacter pylori populations in antral biopsies from individual patients using randomly amplified polymorphic DNA (RAPD) fingerprinting. FEMS Immunol Med Microbiol. 1995;10:317–324. doi: 10.1111/j.1574-695X.1995.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 9.McClelland M, Welsh J. DNA fingerprinting by arbitrarily primed PCR. PCR Methods Appl. 1994;4:S59–S65. doi: 10.1101/gr.4.1.s59. [DOI] [PubMed] [Google Scholar]
- 10.Meunier J R, Grimont P A D. Factors affecting reproducibility of random amplified polymorphic DNA fingerprinting. Res Microbiol. 1993;144:373–379. doi: 10.1016/0923-2508(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 11.O'Sullivan D J, Kullen M J. Tracking of probiotic bifidobacteria in the intestine. Int Dairy J. 1998;8:513–525. [Google Scholar]
- 12.Penner G A, Bush A, Wise R, Kim W, Domier L, Kasha K, Laroche A, Scoles G, Molnar S J, Fedak G. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. PCR Methods Appl. 1993;2:341–345. doi: 10.1101/gr.2.4.341. [DOI] [PubMed] [Google Scholar]
- 13.Sakallah S A, Lanning R W, Cooper D L. DNA fingerprinting of crude bacterial lysates using degenerate RAPD primers. PCR Methods Appl. 1995;4:265–268. doi: 10.1101/gr.4.5.265. [DOI] [PubMed] [Google Scholar]
- 14.Stiles M E, Holzapfel W H. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 1997;36:1–29. doi: 10.1016/s0168-1605(96)01233-0. [DOI] [PubMed] [Google Scholar]
- 15.Tannock G W. Probiotic properties of lactic-acid bacteria: plenty of scope for fundamental R & D. Trends Biotechnol. 1997;15:270–274. doi: 10.1016/s0167-7799(97)01056-1. [DOI] [PubMed] [Google Scholar]
- 16.Tommerup I C, Barton J E, O'Brien P A. Reliability of RAPD fingerprinting of three basidiomycete fungi, Laccari, Hydnangium and Rhizoctinia. Mycol Res. 1995;99:179–186. [Google Scholar]
- 17.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams J G K, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]