Summary
Alzheimer’s disease (AD) is characterized by neurodegeneration, memory loss, and social withdrawal. Brain inflammation has emerged as a key pathogenic mechanism in AD. We hypothesized that oxytocin, a pro-social hypothalamic neuropeptide with anti-inflammatory properties, could have therapeutic actions in AD. Here, we investigated oxytocin expression in experimental models of AD, and evaluated the therapeutic potential of treatment with oxytocin. Amyloid-β peptide oligomers (AβOs) reduced oxytocin expression in vitro and in vivo, and treatment with oxytocin prevented microglial activation induced by AβOs in purified microglial cultures. Treatment of aged APP/PS1 mice, a mouse model of AD, with intranasal oxytocin attenuated microglial activation and favored deposition of Aβ in dense core plaques, a potentially neuroprotective mechanism. Remarkably, treatment with oxytocin alleviated social and non-social memory impairments in aged APP/PS1 mice. Our findings point to oxytocin as a potential therapeutic target to reduce brain inflammation and correct memory deficits in AD.
Subject areas: Molecular physiology, Neuroscience, Immunology
Graphical abstract
Highlights
-
•
Oxytocin expression is downregulated in Alzheimer’s disease models
-
•
Intranasal oxytocin increases hippocampal oxytocin immunoreactivity
-
•
Oxytocin attenuates Alzheimer’s disease-related microglial activation
-
•
Intranasal oxytocin reverses social and non-social memory deficits in APP/PS1 mice
Molecular physiology; Neuroscience; Immunology
Introduction
Dementia affects approximately 50 million people worldwide with nearly 10 million new cases per year, and AD is responsible for 60–70% of those cases.1 Among other features, AD is characterized by brain accumulation of the Aβ peptide and by prominent brain inflammation, comprising aberrant production and release of pro-inflammatory cytokines, and activation of astrocytes and microglia to toxic phenotypes.2,3 The inflammatory hypothesis for AD4 has stimulated a large number of recent studies investigating the role of brain inflammation in AD pathogenesis and cognitive dysfunction.5,6 In particular, studies have aimed to attenuate microglial activation in an attempt to arrest or delay the development of the disease.5,7,8 However, despite encouraging results in preclinical studies,2 none of those potential treatments has reached or been successful in clinical trials to date.9 Thus, it is critical to identify therapeutic agents that can regulate microglial activation and inflammation, to prevent or reduce disease progression in AD patients.
A promising anti-inflammatory therapeutic molecule is oxytocin, a nonapeptide hormone produced in the supraoptic nucleus and paraventricular nucleus (PVN) of the hypothalamus.10 In the periphery, oxytocin is well-known for its role in lactation and parturition.10 In the brain, oxytocin plays important roles in social behavior, including bonding and reproduction,11 and recent studies have described its neuroprotective and anti-inflammatory actions.12,13 Oxytocin has been found to prevent microglial activation induced by lipopolysaccharide (LPS) in vitro and in vivo,12 and recent work suggests that oxytocin could be protective in early stages of AD.14,15 Although there is evidence of the anti-inflammatory actions of oxytocin in the brain, its possible role and therapeutic potential in AD, notably at later stages of the disease, remains to be established. Here, we first investigated changes in oxytocin expression in AD models. Next, we developed a chronic intranasal treatment protocol to increase oxytocin levels in the brain, and finally tested oxytocin as a potential approach to attenuate microglial activation and reverse memory deficits in the APP/PS1 mouse model.
Results
Hypothalamic expression of oxytocin is reduced in AD models
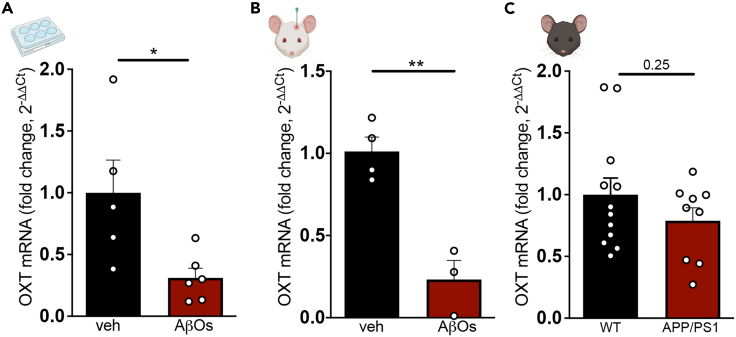
We first examined whether hypothalamic expression of oxytocin was affected in AD models. Oxytocin expression was reduced in mouse hypothalamic slices exposed to amyloid-β oligomers (AβOs) (Figure 1A), toxins that accumulate in AD brains and are proximally implicated in pathogenesis.16,17 Reduced hypothalamic expression of oxytocin was further verified in vivo in 3-month-old wild-type mice that received intracerebroventricular infusions of AβOs for 5 consecutive days (Figure 1B). Aged APP/PS1 mice (a genetic mouse model of AD) presented a non-statistically significant trend of reduction of OXT mRNA compared to WT littermates (Figure 1C), in agreement with a report of reduced oxytocin mRNA in young (4-month-old) APP/PS1 mice.18 These observations indicate that oxytocin expression is defective in AD models.
Figure 1.
Hypothalamic expression of oxytocin is reduced in AD models
Oxytocin expression was determined by qPCR.
(A) Acute hypothalamic slices (from 3-month-old wild-type male mice) exposed to AβOs (0.5 μM) or vehicle for 3 h; p = 0.0238, N = 5–6 mice per group, unpaired two-tailed Student’s ttest.
(B) 3-month-old wild-type male mice received intracerebroventricular infusions of AβOs (10 pmol) or vehicle for 5 consecutive days, and oxytocin expression was assessed in the hypothalamus; p = 0.0027, N = 3–4 mice per group, unpaired two-tailed Student’s ttest.
(C) Hypothalamic OXT mRNA levels in 12–13.5-month-old male APP/PS1 mice or wild-type littermates; p = 0.2546, unpaired two-tailed Student’s ttest. Bars represent means ± SEM. Symbols represent data from individual mice.
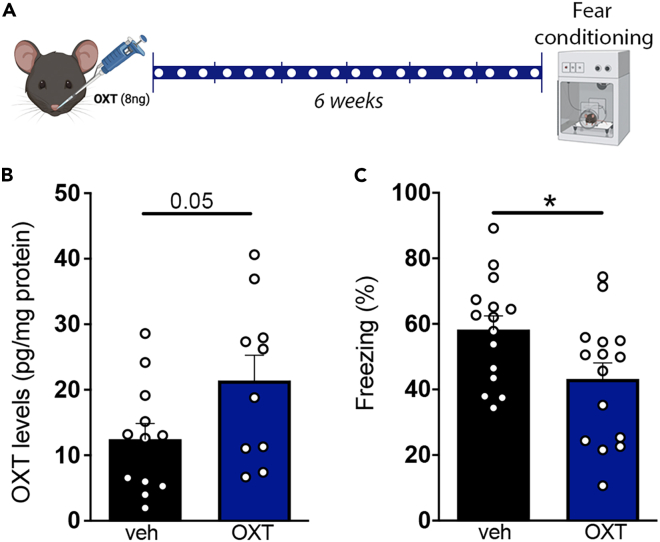
Chronic intranasal treatment with oxytocin increases hippocampal oxytocin levels
To test the hypothesis that reduced brain oxytocin could be a contributing factor in the pathogenesis of AD, we developed a protocol to treat mice with intranasal oxytocin aiming to increase oxytocin levels in the brain. We first treated 9–10-month-old C57Bl/6 wild-type mice intranasally with 8 ng oxytocin three times a week for 6 weeks (Figure 2A). This led to ∼70% increase in hippocampal oxytocin peptide levels in treated mice (Figure 2B). In addition, and consistent with the known decrease in fear response induced by oxytocin,19 mice treated intranasally with oxytocin showed decreased freezing behavior in contextual fear conditioning paradigm (Figure 2C). These results demonstrate that intranasal administration of oxytocin effectively increases oxytocin levels in the mouse hippocampus.
Figure 2.
Chronic intranasal administration of oxytocin increases hippocampal oxytocin and attenuates fear response in mice
(A) Experimental design used for intranasal treatment with oxytocin. White dots indicate intranasal administration of vehicle (veh) or oxytocin (OXT).
(B) Hippocampal oxytocin peptide levels (OXT) in 9–10-month-old wild-type male mice treated intranasally with veh or OXT; p = 0.0546, N =10–12 mice per group, unpaired two-tailed Student’s ttest.
(C) Effect of intranasal treatment with veh or OXT on freezing behavior in the contextual fear conditioning paradigm; p = 0.0256, N = 15 mice per group, unpaired two-tailed Student’s ttest. Bars represent means ± SEM. Symbols represent individual mice.
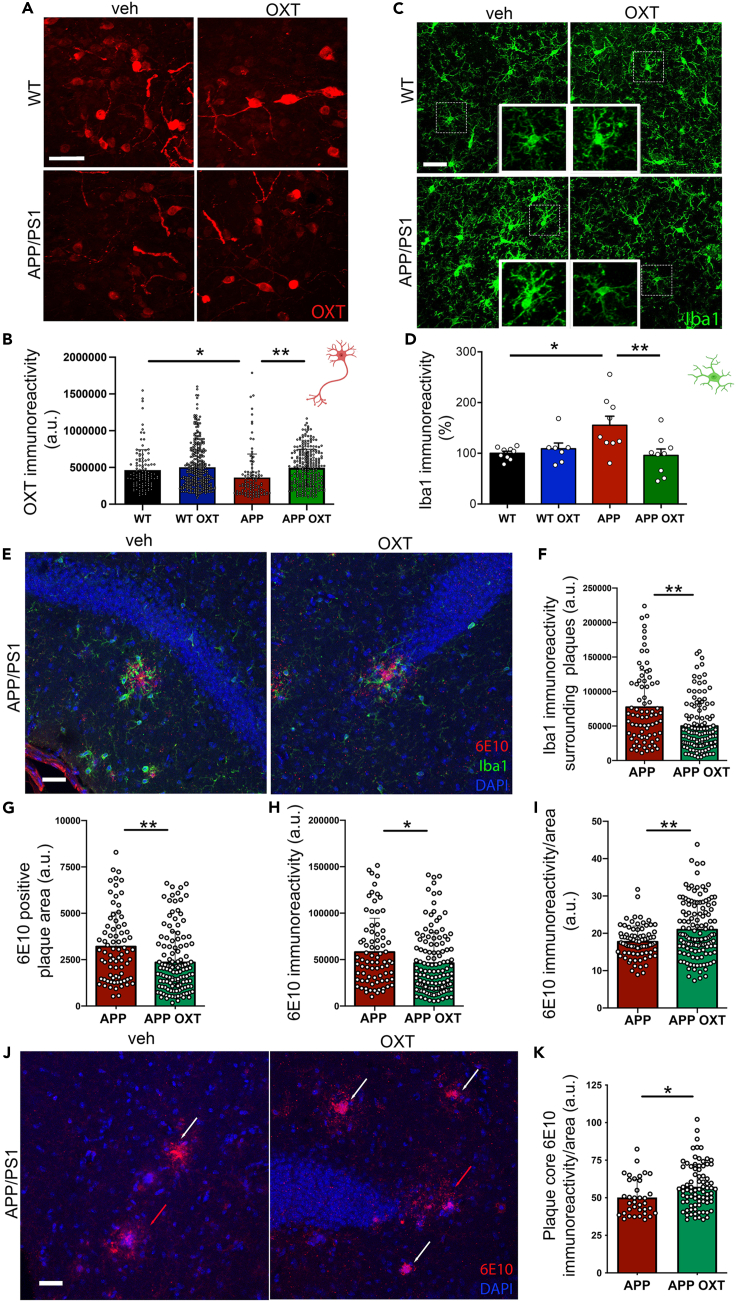
Intranasal oxytocin normalizes reduced hypothalamic oxytocin peptide levels in APP/PS1 mice
We next determined oxytocin peptide levels within hypothalamic PVN oxytocinergic neurons in wild-type or APP/PS1 mice treated intranasally with oxytocin. We found decreased hypothalamic oxytocin immunoreactivity in APP/PS1 mice compared to wild-type littermates (Figures 3A and 3B). Of interest, oxytocinergic cells from APP/PS1 mice treated intranasally with oxytocin exhibited higher oxytocin immunoreactivity, similar to WT mice (Figure 3B). Although oxytocin has been shown to induce endogenous oxytocin release,20 to our knowledge this is the first piece of evidence indicating that treatment with exogenous oxytocin could induce endogenous hypothalamic production of oxytocin in APP/PS1 mice.
Figure 3.
Cellular and molecular impact of intranasal oxytocin in APP/PS1 mouse brains
(A) Representative images for oxytocin (OXT) immunoreactivity in the PVN of wild-type (WT) or APP/PS1 (APP) mice treated with intranasal OXT or vehicle.
(B) Quantification of OXT immunoreactivities. Symbols represent individual cells. N = 2–3 mice per group; WT versus APP, p < 0.0001; APP versus APP OXT, p < 0.0001. Bars represent means ± SD.
(C) Representative images for Iba1 immunohistochemistry in the CA1 region of the hippocampus of wild-type or APP/PS1 mice treated with intranasal OXT or vehicle.
(D) Quantification of Iba1 immunoreactivity. Symbols represent mean values from individual mice; WT versus APP, p = 0.0100; APP versus APP OXT, p = 0.0054. Bars represent means ± SEM.
(E) Representative double immunofluorescence image showing amyloid plaques (6E10 immunoreactivity, red) and Iba1 immunoreactivity (green) in the hippocampus of an APP/PS1 mouse.
(F) Iba1 immunoreactivity surrounding plaques, p = 0.0001. Bars represent means ± SD.
(G) 6E10-positive area of individual plaques, p = 0.0004. Bars represent means ± SD.
(H) 6E10 immunofluorescence intensity per plaque, p = 0.0124. Bars represent means ± SD.
(I) 6E10 immunofluorescence intensity normalized per plaque area, p = 0.0030. Bars represent means ± SD.
(J) Immunofluorescence (6E10, red) showing diffuse plaques (6E10 immunofluorescence/area ratio <35 a.u., red arrows) and dense core plaques (6E10 immunofluorescence/area ratio >35 a.u., white arrows) in the hippocampi of APP/PS1 mice that received intranasal treatment with OXT or vehicle.
(K) Plaque core density (6E10 immunofluorescence intensity normalized per plaque core area) in dense core plaques, p = 0.0107. Symbols represent individual plaques. N = 3–5 mice per group. Bars represent means ± SD. (B) Kruskal-Wallis test, (D) One-way ANOVA, (F-K) Mann-Whitney test, Scale bars: 50 μm.
Intranasal oxytocin attenuates microglial activation in the hippocampus of APP/PS1 mice
Although previous studies have investigated the regulation of neuronal function by oxytocin signaling,11,20,21 much less is known on the effects of oxytocin on other cell types of the central nervous system, including microglia. Yuan et al. (2016) showed that oxytocin prevents LPS-mediated microglial activation in vitro and in vivo.112 That study was followed by description of the anti-inflammatory actions of oxytocin in neurological conditions, including post-traumatic stress disorder and early AD.14,22,23 Because microglial activation is thought to play a major role in AD pathogenesis, we tested whether intranasal treatment with oxytocin could attenuate microglial responses in aged APP/PS1 mice.
In line with a recent report,24 we first confirmed that microglia exhibited increased Iba1 immunoreactivity (indicative of activation) in the hippocampi of aged APP/PS1 mice (Figures 3C and 3D). Treatment with intranasal oxytocin reduced hippocampal Iba-1 immunoreactivity in APP/PS1 mice. Of note, Iba1 immunoreactivity surrounding hippocampal amyloid plaques was decreased in APP/PS1 mice treated with oxytocin compared to control APP/PS1 mice treated with saline (Figures 3E and 3F).
Intranasal oxytocin alters amyloid plaque morphology in the hippocampus of APP/PS1 mice
To assess possible consequences of oxytocin treatment on brain amyloid load, we quantified amyloid plaque area and immunoreactivity using the Aβ-specific 6E10 antibody in the hippocampi of APP/PS1 mice. We observed the presence of smaller amyloid plaques and reduced total Aβ immunoreactivity in the hippocampi of APP/PS1 mice treated with oxytocin (Figures 3G and 3H). Of interest, plaques in oxytocin-treated mice had higher densities of Aβ immunoreactivity per unit area than plaques in control APP/PS1 mice (Figure 3I). Huang and collaborators (2021) recently reported results suggesting that microglia act as “trash compactors” and promote dense core plaque formation,25 a mechanism that could decrease soluble Aβ levels and be neuroprotective in AD.26,27 This prompted us to evaluate Aβ immunoreactivities within plaque cores. For this analysis, we calculated core density as the ratio of 6E10 immunoreactivity per unit area in plaques presenting a dense core (defined as core 6E10 immunoreactivity/area ratio greater than 35 arbitrary units). We found that APP/PS1 mice treated with oxytocin had higher density plaque cores than vehicle-treated mice (Figures 3J and 3K). These results suggest that oxytocin treatment favors Aβ deposition in the form of dense core plaques.
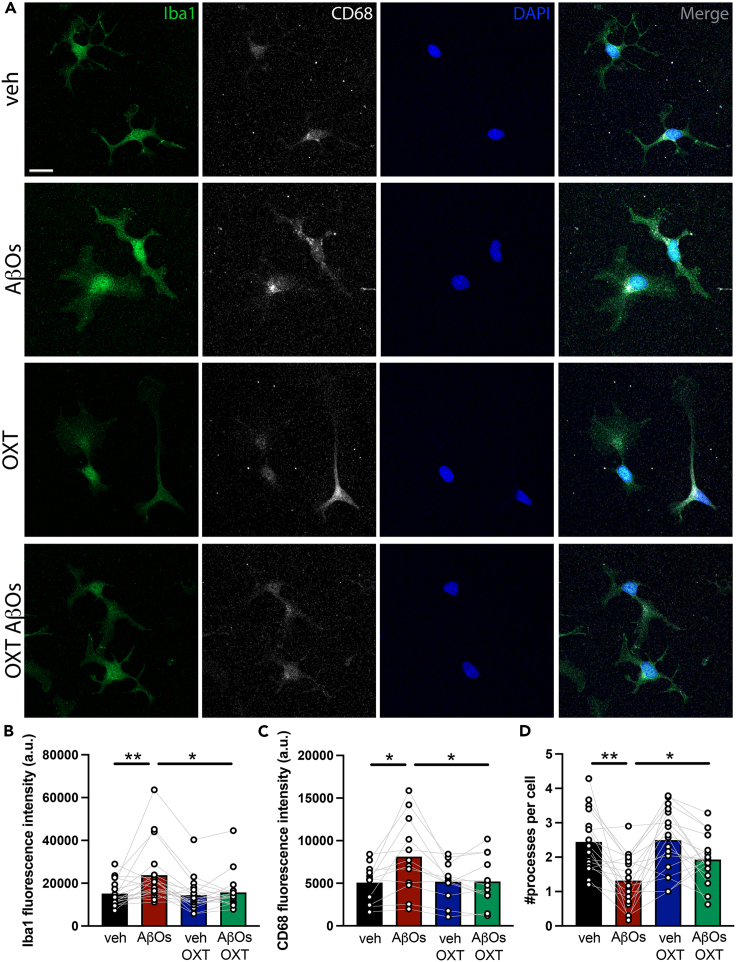
Oxytocin reduces AβO-induced microglial activation in vitro
To determine whether the attenuation of microglial activation by oxytocin observed in vivo reflected a direct regulatory action of oxytocin, we used purified microglial cultures.28 Exposure of cultures to AβOs (500 nM) induced increases in Iba1 (Figures 4A and 4B) and CD68 (Figures 4A and 4C) immunoreactivities, and a decrease in the number of microglial processes (Figures 4A and 4D), consistent with induction of an activated phagocytic microglial phenotype. Treatment with oxytocin (10 nM) blocked the increases in Iba1 and CD68 immunoreactivities, and attenuated the reduction in microglial ramification (Figure 4). These results indicate that oxytocin directly attenuates microglial activation induced by AβOs.
Figure 4.
Oxytocin attenuates AβO-induced microglial activation in vitro
Microglia were immunopanned from P18 rat pups, kept in culture for 5 DIV and treated with oxytocin (OXT, 10 nM) and/or AβOs (500 nM) for 3h.
(A) Representative Iba1 immunocytochemistry images.
(B), Iba1 immunoreactivity; Veh versus AβOs, p = 0.0077; AβOs versus OXT AβOs, p = 0.0171.
(C) CD68 immunoreactivity; Veh versus AβOs, p = 0.0323; AβOs versus OXT AβOs, p = 0.0167.
(D) Number of primary microglial processes per cell; Veh versus AβOs, p = 0.0009; AβOs versus OXT AβOs, p = 0.0190. N = 12–19 independent cultures. (B) Friedman test and (C and D) repeated measures ANOVA followed by Dunnett’s post-hoc test. Bars represent means ± SEM. Symbols represent mean values from independent microglial cultures. Scale bar: 50 μm.
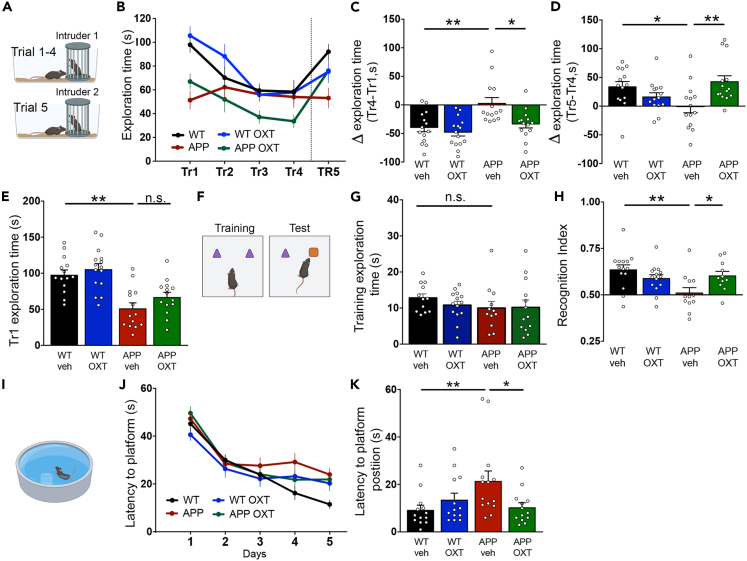
Intranasal treatment with oxytocin reverses social, object recognition and spatial memory deficits in aged APP/PS1 mice
Finally, we tested whether oxytocin could comprise a therapeutic strategy for AD by assessing the effects of intranasal oxytocin on memory in APP/PS1 mice. We first studied the effect of intranasal treatment with oxytocin on social memory in APP/PS1 mice. For this, we used a 5-trial social memory test, which evaluates habituation to an intruder mouse during the first 4 trials, and novelty response to a novel intruder on the fifth trial29 (Figure 5A). Although wild-type animals showed the expected decrease in exploration times across trials 1–4, APP/PS1 mice did not (Figures 5B and 5C). In addition, APP/PS1 mice failed to exhibit increased exploration in trial 5, when a novel mouse was presented to the test mouse (Figures 5B and 5D). These observations show that APP/PS1 mice present impairments in social behaviors that are corrected by treatment with oxytocin, suggesting that oxytocin rescues social memory deficits in APP/PS1 mice (Figures 5B–5D).
Figure 5.
Intranasal oxytocin reverses social and non-social memory deficits in aged APP/PS1 mice
(A) Wild-type (WT) or APP/PS1 (APP) male mice (10-month-old) treated with intranasal oxytocin (OXT) or vehicle (veh) were tested in the 5-trial social test. The same stranger mouse was used in Trials 1 to 4. A novel stranger mouse was introduced in Trial 5. Time exploring the stranger mouse was quantified at 5-min intervals along each trial.
(B) Total time spent exploring the stranger mouse in each of the 5 trials.
(C) Difference in exploration time between trials 4 and 1; WT versus APP, p = 0. 0052; APP versus APP OXT, p = 0.0224.
(D) Difference in exploration time between trials 5 and 4; WT versus APP, p = 0.0341; APP versus APP OXT, p = 0.0058.
(E) Total exploration time in the first trial; WT versus APP, p < 0.0001; APP versus APP OXT, p = 0.2959.
(F) Mice were tested in the Novel Object Recognition task.
(G) Total exploration time of the two objects in the 5 min training phase of the Novel Object Recognition task; WT versus APP, p = 0.3525; APP versus APP OXT, p>0.9999.
(H) Short-term memory evaluated using the Novel Object Recognition task. Recognition index calculated as time exploring the new object/total exploration time. Values higher than 0.5 indicate novelty preference; WT versus APP, p = 0.0025; APP versus APP OXT, p = 0.0444.
(I) Mice were tested in the Morris water maze.
(J) Latencies to reach the hidden platform in the training sessions.
(K) Probe trial latencies to reach the area where the platform used to be; WT versus APP, p = 0.0095; APP versus APP OXT, p =0.0483. (C, G, K) Kruskal-Wallis test, (D, E, H) One-way ANOVA followed by Dunnett’s post-hoc test. Symbols represent individual mice; N = 11–15 mice per group. Bars represent means ± SEM.
Because oxytocin treatment had no effect on the decreased social exploration time exhibited by APP/PS1 mice during the first trial in the 5-trial test (Figures 5B and 5E), we asked whether this could reflect a general reduction in exploratory behavior in APP/PS1 mice. To investigate this possibility, we quantified the total amount of time mice spent exploring the two objects presented in the 5-min training phase of a novel object recognition task (Figure 5F). There were no differences in total exploration times between APP/PS1 and wild-type mice (Figure 5G), suggesting that the reduced exploration time exhibited by APP/PS1 mice in the 5-trial social task is not caused by a generalized reduction in exploratory activity but rather reflects decreased sociability. Collectively, results from the 5-trial social recognition test indicated that oxytocin treatment corrected social memory deficits but did not rescue sociability in APP/PS1 mice, suggesting that oxytocin could be protective against social memory loss in AD.
Oxytocin has been shown to regulate hippocampal memory consolidation30 and to prevent Aβ-induced impairment in synaptic long-term potentiation.31 We thus hypothesized that oxytocin treatment might rescue non-social memory impairments in APP/PS1 mice. Remarkably, intranasal oxytocin treatment restored memory of aged APP/PS1 mice in both the novel object recognition test (Figure 5H) and in the Morris water maze (MWM) spatial memory test (Figures 5I and 5K). Control measurements showed that oxytocin had no effect on the MWM learning curve (Figure 5J). Together, these results show that oxytocin not only rescues social memory deficits in the APP/PS1 AD mouse model, but it also reverses deficits in other types of non-social memory, including object recognition and spatial memory.
Discussion
Despite significant efforts in preclinical research and clinical trials, to date no drugs have demonstrated clear clinical benefits to AD patients. Repurposing currently approved drugs may comprise a faster, safer and cost-effective pathway to the development of novel treatments for AD. Moreover, although the majority of therapeutic approaches tested in AD models use spatial and recognition memory tasks as readouts of efficacy,32 it is important to note that AD severely impacts social memory.33 Potential therapeutic approaches for social memory deficits have been much less investigated despite the fact that the inability of AD patients to recognize family members and friends is one of the heaviest emotional burdens for relatives. Here, we evaluated the therapeutic potential of the pro-social, anti-inflammatory hormone, oxytocin, using social and non-social memory tasks in APP/PS1 mice.
Based on our initial finding of reduced hypothalamic expression of oxytocin in in vitro and in vivo models of AD, we developed a protocol for chronic intranasal treatment with oxytocin with the aim to increase oxytocin peptide levels in the brain. Intranasal administration of oxytocin is a translational, non-invasive route to deliver oxytocin to the brain; however, results of this approach in the literature are quite variable. This could be due, among other reasons, to the use of different mouse strains, ages, sex and experimental protocols.34,35,36 We found that our protocol effectively increased oxytocin peptide levels in the hippocampus and decreased fear response, an effect that has been described for central oxytocin19
Treatment with intranasal oxytocin normalized oxytocin immunoreactivity in the hypothalamus, attenuated hippocampal microglial activation, modulated amyloid plaque deposition by favoring formation of dense core plaques, and corrected memory deficits in APP/PS1 male mice. The promotion of dense core plaque formation by oxytocin may, at least in part, underlie its beneficial actions, because sequestration of soluble Aβ species into dense core plaques has been suggested recently as a mechanism by which microglia protect the brain from AD-related degeneration,25,26,27 and we and others have shown that neutralizing soluble Aβ oligomers rescues memory deficits in APP/PS1 mice.37
Current results demonstrate, for the first time, that oxytocin modulates microglial reactivity and rescues social and non-social cognitive impairments in aged AD model mice. These results are in line with recent reports of protective actions of oxytocin in AD mouse and rat models.14,15 Using purified microglial cultures, we further found that oxytocin directly prevents the activation of microglia by AβOs. Microglial cells express oxytocin receptors,12,38 and oxytocin prevents LPS-induced microglial activation in vitro and in vivo in mice.12 These results pave the way for more detailed investigation of the cellular mechanisms and functional consequences of oxytocin signaling in AD.
Finally, we performed behavioral experiments to evaluate the therapeutic potential of intranasal oxytocin. Most preclinical studies in AD focus on spatial memory and other non-social behaviors. Given the impact of social memory loss on AD patients and families, we decided to evaluate both non-social and social memory. Remarkably, we found that treatment with intranasal oxytocin reversed both social and non-social (object recognition and spatial) memory deficits in aged APP/PS1 mice.
Oxytocin is approved by the US Food and Drug Administration for intramuscular and intravenous use (NDA #018248), and intranasal administration of oxytocin is currently in clinical trials as treatment for obesity (NCT03043053), post-traumatic stress disorder (NCT03238924, NCT01466127), age-related inflammation (NCT02069431) and frontotemporal dementia (NCT01386333, NCT01937013, NCT01002300, NCT03260920). However, to date, there are no studies registered in the ClinicalTrials.gov database to evaluate the actions of oxytocin in AD. Considering that oxytocin is a commercially available endogenous hormone, safe and approved for clinical use by the FDA and other regulatory agencies, our findings suggest that repurposing oxytocin could comprise a novel therapeutic strategy for AD.
Limitations of the study
First, this study is focused on the effect of oxytocin in an aged male mouse model of Alzheimer’s disease. The effects and/or mechanisms described here might be different in female mice, and might also be age/disease state dependent. Second, we found that treatment with exogenous (intranasal) oxytocin caused an increase in endogenous oxytocin immunoreactivity in hypothalamic oxytocin-producing cells, suggesting that the effects we observed after delivering exogenous oxytocin could be at least in part indirectly mediated by hypothalamic oxytocin. Last, we found intranasal oxytocin favored Aβ deposition in the form of dense core plaques, which have been proposed to sequester Aβ as a neuroprotective mechanism. However, this is a recent proposal that remains under investigation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-oxytocin (1:500) | Millipore | MAB5296; RRID:AB_2157626 |
| Anti-Iba1 (1:200) | Wako or Abcam | 019-19741; RRID:AB_839504/ab5076; RRID:AB_2224402 |
| 6E10 (1:1000) | Biolegend | 803001; RRID:AB_2564653 |
| Alexa Fluor 647/555/594/488 | Abcam | ab150131; RRID:AB_2732857 /ab150075; RRID:AB_2752244/ab150106; RRID:AB_2857373 /ab150108; RRID:AB_2732073 /ab150129; RRID:AB_2687506 /ab150105; RRID:AB_2732856 /ab150133; RRID:AB_2832252 |
| AntiCd11b (OX-42) | BIO-RAD | MCA275GA; RRID:AB_566455 |
| Anti-CD68 (1:200) | Abcam | ab125212; RRID:AB_10975465 |
| Chemicals, peptides, and recombinant proteins | ||
| Aβ1–42 | California Peptide (Echelon Biosciences) | 641-15 |
| Oxytocin | Sigma | 50-56-6 |
| Critical commercial assays | ||
| Oxytocin ELISA | Enzo | ADI-900-153A-001 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Charles River | NA |
| Swiss wild-type mice | Anilab, Brazil | NA |
| APP/PS1 mice | The Jackson Laboratory | #034832-JAX |
| Sprague Dawley pups | Hiltop | NA |
| Oligonucleotides | ||
| OXT Fw: GAGGAGAACTACCTGCCTTCG | Fisher | NA |
| OXT Rv: TCCCAGAAAGTGGGCTCAG | Fisher | NA |
| Actin Fw: TGTGACGTTGACATCCGTAAA | Fisher | NA |
| Actin Rv: GTACTTGCGCTCAGGAGGAG | Fisher | NA |
| Software and algorithms | ||
| Prism 9 | GraphPad | https://www.GraphPad.com |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Maria Clara Selles (maria.sellesjapas@nyulangone.org).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Animals were housed in groups with free access to food and water, under a 12 h light/dark cycle with controlled room temperature and humidity. All procedures followed the Principles of Laboratory Animal Care from the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the Federal University of Rio deJaneiro (protocol #IBqM 050/20) and the Institutional animal care and use committee (IACUC) for NYU Langone Health (protocol #IA16-01096).
Mice
Three-month-old male C57BL/6 and Swiss wild-type mice were used for hypothalamic slice cultures and cannula implantation, respectively. Male 12-13 month-old APP/PS1 (or wild-type littermates) mice were used to determine oxytocin mRNA levels in the hypothalamus, and 9-10 month-old APP/PS1 (or wild-type littermates) mice were used for intranasal oxytocin treatment.
Rats
P18 male Sprague Dawley rats were used for isolation of microglia cells.
Method details
Preparation and characterization of AβOs
AβOs were prepared from synthetic Aβ1–42 (California Peptide/Echelon Biosciences). Aβ was dissolved in HFIP and aliquoted after 1h incubation at room temperature. HFIP was evaporated by keeping the tubes open in a laminar flow hood overnight. The dried films were resuspended in DMSO when necessary, followed by dilution in PBS and 24h incubation at 4°C. After centrifugation, protein concentration was determined in the supernatant, and characterization of the oligomers was performed by size exclusion HPLC.39,40 Occasionally, characterization also included Western blots using NU4, an oligomer-sensitive monoclonal antibody.41 AβO preparations comprise a mixture of Aβ dimers, trimers, tetramers, and higher molecular weight oligomers.42
Hypothalamic slice cultures
C57Bl/6 mice (3 month-old) were euthanized and their brains were removed. Hypothalami were dissected and sectioned in 400 μM slices using a McIlwain Tissue Chopper. Slices were exposed to vehicle or 500 nM AβOs for 3h after 1h of incubation in aCSF. After treatment, samples were collected for qPCR analysis of oxytocin mRNA levels.
Implantation of intracerebroventricular cannula
This protocol was adapted from Boschi et al. (1981).43 Briefly, 3 month-old Swiss mice were anesthetized with ketamine(100mg/kg)/xylazin(10mg/kg and placed in a stereotaxic frame. The coordinates used to implant a cannula in the lateral ventricle were: -0,6 mm Antero-Posterior; -1,2 mm Medial-Lateral; -2,6 mm Dorsal-Ventral.(Allen Brain Atlas). Acrylic cement and stainless steel screws were used to fix the cannula to the bone. After implantation, mice were allowed to recover for 5 days and then received 5 consecutive daily injections of 10 pmol (in 3 μL) AβOs or vehicle.
RNA extraction and quantitative real-time PCR
RNA was extracted from homogenized samples with the SV total RNA isolation kit (Promega) or RNAeasy Minikit (Qiagen). mRNA purity was determined using the 260/280 nm absorbance ratio and one μg mRNA was used for cDNA synthesis using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) or QuantiTect Rev. Transcription Kit (Qiagen). PCR was performed on an Applied Biosystems 7500 RT–PCR system or StepOnePlus™ Real-Time PCR System using the Power SYBR kit (Applied Biosystems). Fold changes in gene expression (2-ΔΔCt) were calculated using cycle threshold (Ct) values.44 Values were normalized to the mean value of controls. The following primers were used to detect oxytocin mRNA: Forward: GAGGAGAACTACCTGCCTTCG; Reverse: TCCCAGAAAGTGGGCTCAG; actin-β was used as a normalizer, using the following primers: Forward: 5′TGTGACGTTGACATCCGTAAA-3′; Reverse: 5′GTACTTGCGCTCAGGAGGAG-3′.
Intranasal administration of oxytocin
Mice were manually and gently restrained and received 10 μL of a solution containing oxytocin or vehicle in both nostrils. This procedure was repeated 3 times a week for 6 weeks. The optimal dose of oxytocin used was determined in a pilot experiment in which mice received 3 different doses of oxytocin (0.8 μg, 8 ng and 8 pg) 3 times a week for 4 weeks prior to an intracerebroventricular injection of AβOs, followed by evaluation of cognitive impairment using the Novel Object Location test. The dose selected for subsequent studies (8 ng) was found to protect mice from AβO-induced memory loss and did not per se induce any cognitive impairment.
Oxytocin ELISA
Oxytocin was quantified in the hippocampi of animals treated with vehicle or oxytocin using an ELISA kit (Enzo) and following manufacturer instructions. Briefly, hippocampi were dissected from the brains after euthanasia, and were homogenized in PBS containing protease and phosphatase inhibitor cocktail (Thermo Scientific Pierce). After sample centrifugation, supernatants were used for oxytocin determination.
Contextual fear conditioning
In the training phase, mice were presented to the conditioning cage (40 x 25 x 30 cm), which they were allowed to freely explore for 2 minutes followed by application of two sequential foot shocks (0.8 mA) spaced by 30 sec. Mice were kept for another minute in the cage and removed. In the next day, mice were presented to the same cage for 5 minutes, without shocks. Freezing behavior was recorded automatically using the Freezing software (Panlab; Cornella, Spain) and was used as a fear memory index.45
Immunohistochemistry
Brains were fixed by perfusing anesthetized animals with saline, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were removed, cryoprotected using increasing concentrations of sucrose, frozen in dry ice and stored at -80°C. Brains were subsequently sectioned (40 μm) in a cryostat (Leica Microsystems) and stored in PBS. After washing with PBS, coronal sections were blocked for 2 hours with 0.3% Triton X-100 and 5% BSA in PBS at room temperature, and incubated overnight a 4 C with anti- oxytocin (1:500, Millipore), anti-Iba1 (1:200; Wako or Abcam) or 6e10 (1:1,000; Biolegend) primary antibodies diluted in blocking buffer. On the following day, sections were washed and incubated for 2 hat room temperature with Alexa Fluor 647-, 555-, 594- or 488-conjugated secondary antibodies (1:1,000; Abcam) and mounted with Prolong with DAPI (Invitrogen). Images were acquired using a Zeiss Axio Observer Z1 microscope, area and intensity levels were quantified on FIJI.
Microglia isolation and culture
Microglial cells were isolated from P18 rat pups.28 Tissue was dissociated using DNAseI (Worthington) and myelin and debris removal. Microglial cells were isolated by immunopanning with the OX-42 anti-Cd11b antibody (BIO-RAD). Cells were plated in 12-well chamber slides (Ibidi) and cultured during 5 DIV at 37°C and 10% CO2. A 50% media change was performed every 2 days.
Immunocytochemistry
Microglial cultures were treated with AβOs (500nM) and/or oxytocin (10nM) for 3h. Cells were fixed with 4% paraformaldehyde in DPBS (Gibco) for 10 minutes. Cells were permeabilized with Triton X-100 diluted 0.1% in DPBS for 10 minutes and blocked using Normal Donkey Serum (NDS) 10% in DPBS for 1h at room temperature. Iba1 and CD68 primary antibodies (Abcam) were diluted 1:500 and 1:200, respectively, in blocking buffer and incubated at 4°C overnight. Alexa-conjugated secondary anti-goat and anti-rabbit antibodies (Abcam) were prepared 1:1,000 in 1% NDS and incubated for 1h at room temperature. Cells were washed 3 times with DPBS between steps. Cells were mounted in Prolong with DAPI (Invitrogen) and imaged in a Zeiss LSM 800 confocal microscope. Iba1 and CD68 intensity levels were quantified on FIJI and process number were quantified manually.
Five-trial social test
Mice in experimentation were allowed to socially interact with a stranger mouse for 5 minutes during 4 sessions. In the 5th and last session, the stranger mouse was replaced by a new one. The interaction times of the experimental mice with the introduced mouse in trials 1 to 5 were quantified.46
Novel object recognition task
An open field arena measuring 30 × 30 × 45 cm (W x L x H) was used. Before training, each animal was allowed to freely explore the arena for 5 min. During the training phase, the mice were placed in the arena in the presence of two identical objects which they could explore for 5 min. For the test, one of the objects was replaced by a novel one. The experiment was recorded and the recognition index (RI) was calculated as RI = time exploring the novel object/(time exploring the novel object + time exploring the familiar object).42
Morris Water Maze
This task was adapted from Vorhees and Williams (2006).47 Animals were placed in a pool with a hidden platform. During 5 days, mice were trained in 4 trials/day, beginning a different starting points. After each 60 second-long trials or once they reached the platform, the animals were allowed to remain for 40 seconds in the platform. Latencies to find the platform were quantified. On the 6th day, the platform was removed for the probe trial, and the latency to reach the region where the platform used to be was quantified.
Quantification and statistical analysis
Statistical analyses were performed using GraphPad Prism. Normality was tested using the D'Agostino & Pearson test and the Shapiro-Wilk test for low-n datasets. Sample sizes and tests used are detailed in the figure legends.
Acknowledgments
We thank Drs. Eric Klann and Martin Sadowski for donation of mice, and NYU Langone Microscopy Core for experimental and technical support. We thank Dr. Luis Eduardo Santos and Jessica Minder for critical discussions.
Funding: National Council for Scientific and Technological Development CNPq/Brazil; 406436/2016-9 (STF), Fundação deAmparo à Pesquisa do Estado do Rio deJaneiro FAPERJ/Brazil; E-26/200.998/2021 (STF), National Institute of Translational Neuroscience Brazil; 465346/2014-6 (STF), Simons Foundation Explorer grant 555504 (MVC), NIHR01 MH110136-01 (MVC), NINDS R25 NS107178 Training Grant (MVC), U19 NS107616(MVC, RCF), National Council for Scientific and Technological Development CNPq/Brazil pre-doctoral Fellowship (MCS), Fundação deAmparo à Pesquisa do Estado do Rio deJaneiro FAPERJ/Brazil pre-doctoral Fellowship (MCS), International Society for Neurochemistry travel grant (MCS), IUBMB travel grant (MCS) and Pew Charitable Trusts post-doctoral fellowship (MCS).
Author contributions
Conceptualization: M.C.S., R.C.F., M.V.C., and S.T.F. Methodology: M.C.S., J.T.S.F., Y.P.R.F., L.D., R.L.F., and B.M.L. Funding acquisition: R.C.F., M.V.C., and S.T.F. Project administration: M.C.S., R.C.F., M.V.C., and S.T.F. Supervision: R.C.F., M.V.C., and S.T.F. Writing – original draft: M.C.S. Writing – review and editing: R.C.F., M.V.C., and S.T.F.
Declaration of interests
Authors declare that they have no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper received support from a program designed to increase minority representation in their field of research.
Published: March 31, 2023
Contributor Information
Robert C. Froemke, Email: robert.froemke@med.nyu.edu.
Moses V. Chao, Email: moses.chao@nyulangone.org.
Sergio T. Ferreira, Email: ferreira@bioqmed.ufrj.br.
Data and code availability
This study did not generate new code.
References
- 1.WHO WHO facts sheets, dementia. 2019. https://www.who.int/news-room/fact-sheets/detail/dementia
- 2.Spangenberg E.E., Green K.N. Inflammation in Alzheimer's disease: lessons learned from microglia-depletion models. Brain Behav. Immun. 2017;61:1–11. doi: 10.1016/j.bbi.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad M.H., Fatima M., Mondal A.C. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer's disease: rational insights for the therapeutic approaches. J. Clin. Neurosci. 2019;59:6–11. doi: 10.1016/j.jocn.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calsolaro V., Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Nichols M.R., St-Pierre M.K., Wendeln A.C., Makoni N.J., Gouwens L.K., Garrad E.C., Sohrabi M., Neher J.J., Tremblay M.E., Combs C.K. Inflammatory mechanisms in neurodegeneration. J. Neurochem. 2019;149:562–581. doi: 10.1111/jnc.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGeer P.L., Rogers J., McGeer E.G. Inflammation, antiinflammatory agents, and Alzheimer's disease: the last 22 years. J. Alzheimers Dis. 2016;54:853–857. doi: 10.3233/JAD-160488. [DOI] [PubMed] [Google Scholar]
- 8.Hemonnot A.L., Hua J., Ulmann L., Hirbec H. Microglia in Alzheimer disease: well-known targets and new opportunities. Front. Aging Neurosci. 2019;11:233. doi: 10.3389/fnagi.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozben T., Ozben S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer's disease. Clin. Biochem. 2019;72:87–89. doi: 10.1016/j.clinbiochem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Jurek B., Neumann I.D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 2018;98:1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 11.Froemke R.C., Young L.J. Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci. 2021;44:359–381. doi: 10.1146/annurev-neuro-102320-102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan L., Liu S., Bai X., Gao Y., Liu G., Wang X., Liu D., Li T., Hao A., Wang Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflammation. 2016;13:77. doi: 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M., Saito S., Inoue T., Satoh-Asahara N., Ihara M. Potential therapeutic approaches for cerebral amyloid angiopathy and Alzheimer's disease. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21061992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye C., Cheng M., Ma L., Zhang T., Sun Z., Yu C., Wang J., Dou Y. Oxytocin nanogels inhibit innate inflammatory response for early intervention in Alzheimer's disease. ACS Appl. Mater. Interfaces. 2022;14:21822–21835. doi: 10.1021/acsami.2c00007. [DOI] [PubMed] [Google Scholar]
- 15.El-Ganainy S.O., Soliman O.A., Ghazy A.A., Allam M., Elbahnasi A.I., Mansour A.M., Gowayed M.A. Intranasal oxytocin attenuates cognitive impairment, β-amyloid burden and Tau deposition in female rats with Alzheimer's disease: interplay of ERK1/2/GSK3β/caspase-3. Neurochem. Res. 2022;47:2345–2356. doi: 10.1007/s11064-022-03624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira S.T., Lourenco M.V., Oliveira M.M., De Felice F.G. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer's disease. Front. Cell. Neurosci. 2015;9:191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson H.M., Soto I., Graham L.C., Carter G.W., Howell G.R. Clustering of transcriptional profiles identifies changes to insulin signaling as an early event in a mouse model of Alzheimer's disease. BMC Genom. 2013;14:831. doi: 10.1186/1471-2164-14-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knobloch H.S., Charlet A., Hoffmann L.C., Eliava M., Khrulev S., Cetin A.H., Osten P., Schwarz M.K., Seeburg P.H., Stoop R., Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Lopatina O., Inzhutova A., Salmina A.B., Higashida H. The roles of oxytocin and CD38 in social or parental behaviors. Front. Neurosci. 2012;6:182. doi: 10.3389/fnins.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitre M., Minder J., Morina E.X., Chao M.V., Froemke R.C. Oxytocin modulation of neural circuits. Curr. Top. Behav. Neurosci. 2018;35:31–53. doi: 10.1007/7854_2017_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S.C., Lin C.C., Chen C.C., Tzeng N.S., Liu Y.P. Effects of oxytocin on fear memory and neuroinflammation in a rodent model of posttraumatic stress disorder. Int. J. Mol. Sci. 2018;19:3848. doi: 10.3390/ijms19123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue T., Yamakage H., Tanaka M., Kusakabe T., Shimatsu A., Satoh-Asahara N. Oxytocin suppresses inflammatory responses induced by lipopolysaccharide through inhibition of the eIF-2-ATF4 pathway in mouse microglia. Cells. 2019;8:527. doi: 10.3390/cells8060527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Rodriguez A.B., Hennessy E., Murray C.L., Nazmi A., Delaney H.J., Healy D., Fagan S.G., Rooney M., Stewart E., Lewis A., et al. Acute systemic inflammation exacerbates neuroinflammation in Alzheimer's disease: IL-1β drives amplified responses in primed astrocytes and neuronal network dysfunction. Alzheimers Dement. 2021;17:1735–1755. doi: 10.1002/alz.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y., Happonen K.E., Burrola P.G., O'Connor C., Hah N., Huang L., Nimmerjahn A., Lemke G. Microglia use TAM receptors to detect and engulf amyloid β plaques. Nat. Immunol. 2021;22:586–594. doi: 10.1038/s41590-021-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fyfe I. Dense-core plaques could be beneficial in AD. Nat. Rev. Neurol. 2021;17:328. doi: 10.1038/s41582-021-00513-9. [DOI] [PubMed] [Google Scholar]
- 27.Lemke G., Huang Y. The dense-core plaques of Alzheimer's disease are granulomas. J. Exp. Med. 2022;219 doi: 10.1084/jem.20212477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohlen C.J., Bennett F.C., Bennett M.L. Isolation and culture of microglia. Curr. Protoc. Immunol. 2019;125:e70. doi: 10.1002/cpim.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson J.N., Young L.J., Hearn E.F., Matzuk M.M., Insel T.R., Winslow J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y.T., Hsu K.S. Oxytocin receptor signaling in the hippocampus: role in regulating neuronal excitability, network oscillatory activity, synaptic plasticity and social memory. Prog. Neurobiol. 2018;171:1–14. doi: 10.1016/j.pneurobio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi J., Yamada D., Ueta Y., Iwai T., Koga E., Tanabe M., Oka J.I., Saitoh A. Oxytocin reverses Aβ-induced impairment of hippocampal synaptic plasticity in mice. Biochem. Biophys. Res. Commun. 2020;528:174–178. doi: 10.1016/j.bbrc.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 32.Nunez J. Morris water maze experiment. J. Vis. Exp. 2008:897. doi: 10.3791/897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alzheimer’s Association 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Veening J.G., Olivier B. Intranasal administration of oxytocin: behavioral and clinical effects, a review. Neurosci. Biobehav. Rev. 2013;37:1445–1465. doi: 10.1016/j.neubiorev.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintana D.S., Lischke A., Grace S., Scheele D., Ma Y., Becker B. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol. Psychiatry. 2021;26:80–91. doi: 10.1038/s41380-020-00864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quintana D.S., Smerud K.T., Andreassen O.A., Djupesland P.G. Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Ther. Deliv. 2018;9:515–525. doi: 10.4155/tde-2018-0002. [DOI] [PubMed] [Google Scholar]
- 37.Selles M.C., Fortuna J.T.S., Cercato M.C., Santos L.E., Domett L., Bitencourt A.L.B., Carraro M.F., Souza A.S., Janickova H., Azevedo C.V., et al. AAV-mediated neuronal expression of an scFv antibody selective for Aβ oligomers protects synapses and rescues memory in Alzheimer models. Mol. Ther. 2023;31:409–419. doi: 10.1016/j.ymthe.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karelina K., Stuller K.A., Jarrett B., Zhang N., Wells J., Norman G.J., DeVries A.C. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke. 2011;42:3606–3611. doi: 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chromy B.A., Nowak R.J., Lambert M.P., Viola K.L., Chang L., Velasco P.T., Jones B.W., Fernandez S.J., Lacor P.N., Horowitz P., et al. Self-assembly of Abeta(1-42) into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- 41.Lambert M.P., Velasco P.T., Chang L., Viola K.L., Fernandez S., Lacor P.N., Khuon D., Gong Y., Bigio E.H., Shaw P., et al. Monoclonal antibodies that target pathological assemblies of Abeta. J. Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- 42.Figueiredo C.P., Clarke J.R., Ledo J.H., Ribeiro F.C., Costa C.V., Melo H.M., Mota-Sales A.P., Saraiva L.M., Klein W.L., Sebollela A., et al. Memantine rescues transient cognitive impairment caused by high-molecular-weight aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J. Neurosci. 2013;33:9626–9634. doi: 10.1523/JNEUROSCI.0482-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boschi G., Launay N., Rips R. Implantation of an intracerebral cannula in the mouse. J. Pharmacol. Methods. 1981;6:193–198. doi: 10.1016/0160-5402(81)90108-x. [DOI] [PubMed] [Google Scholar]
- 44.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Lourenco M.V., Clarke J.R., Frozza R.L., Bomfim T.R., Forny-Germano L., Batista A.F., Sathler L.B., Brito-Moreira J., Amaral O.B., Silva C.A., et al. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's β-amyloid oligomers in mice and monkeys. Cell Metab. 2013;18:831–843. doi: 10.1016/j.cmet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Choleris E., Gustafsson J.A., Korach K.S., Muglia L.J., Pfaff D.W., Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc. Natl. Acad. Sci. USA. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate new code.