Summary
B(C6F5)3·H2O has been long recognized as a common Brønsted acid. The lack of X-ray crystal structure of B(C6F5)3·H2O with other substrates has greatly limited the development of a new catalytic mode. In this work, a complex of B(C6F5)3·H2O and amide 2-phenyl-3,4-dihydroisoquinolin-1(2H)-one with hydrogen bonds and π-π interactions is characterized by X-ray diffraction. Such noncovalent interactions in solution also exist, as verified by NMR, UV-Vis absorption, and fluorescence emission measurements. Moreover, the mixture of amide 2-phenyl-3,4-dihydroisoquinolin-1(2H)-one and B(C6F5)3·H2O, instead of other tested Brønsted acids, shows a tailing absorption band in the visible light region (400–450 nm). Based on the photoactive properties of the complex, a photoredox catalysis is developed to construct α-aminoamides under mild conditions.
Subject areas: Organic reaction, Physical organic chemistry, Chemical phenomena
Graphical abstract

Highlights
-
•
Aerobic oxidation under visible light conditions
-
•
No need for an external photocatalyst
-
•
Hydrogen bonds and π-π interactions between B(C6F5)3·H2O and amides were identified
Organic reaction; Physical organic chemistry; Chemical phenomena
Introduction
Tris(pentafluorophenyl)borane, B(C6F5)3, as a main group Lewis acid, has been deeply investigated during the last decade.1,2,3,4,5,6,7,8 Besides, with the discovery of crystal structure of B(C6F5)3 with various molecules,9,10,11,12,13,14,15,16,17,18 the catalytic mode of B(C6F5)3 was further revealed.19,20,21,22,23,24,25,26,27,28,29,30,31On the other hand, owing to the moisture sensitivity of B(C6F5)3, it is likely that the Brønsted acid B(C6F5)3·H2O acts as the real catalyst.32,33 Although, there are some reports indicating that this effect can be attenuated in frustrated Lewis pairs (FLP) chemistry,34,35,36,37 the strict anhydrous conditions are still necessary in most B(C6F5)3 catalyzed transformations.
B(C6F5)3·H2O is a strong Brønsted acid38,39,40 and the strength is similar to that of HCl in acetonitrile.38 It has been reported that B(C6F5)3·H2O shows superior activity than other Brønsted acids in some transformations.41,42,43,44,45 However, it is difficult to identify the real catalytic species under moist conditions, because Lewis acid and water-mediated pathways are competitive.34,35,36,37,41,42,43,44,45,46,47 In the water-tolerant FLP-catalyzed hydrogenation, for instance, ethers could competitively replace water to coordinate with B(C6F5)3 (Figure 1A).34,35,36,37 Meanwhile, B(C6F5)3·H2O are mainly considered as a hydrogen bond donor. For instance, in the B(C6F5)3·H2O catalyzed ring opening reactions of epoxides,46,47 hydrogen bond interactions between B(C6F5)3·H2O and epoxides/alcohols are revealed by microkinetic modeling and density functional theory calculations (Figure 1B).44 Even though existing studies could account for the hydrogen bond interactions, other noncovalent interactions are barely considered. Thus, the direct and intuitive evidences such as X-ray single crystal diffraction are highly desirable.
Figure 1.
Competitive boron Lewis acid, water-and alcohol-mediated pathways
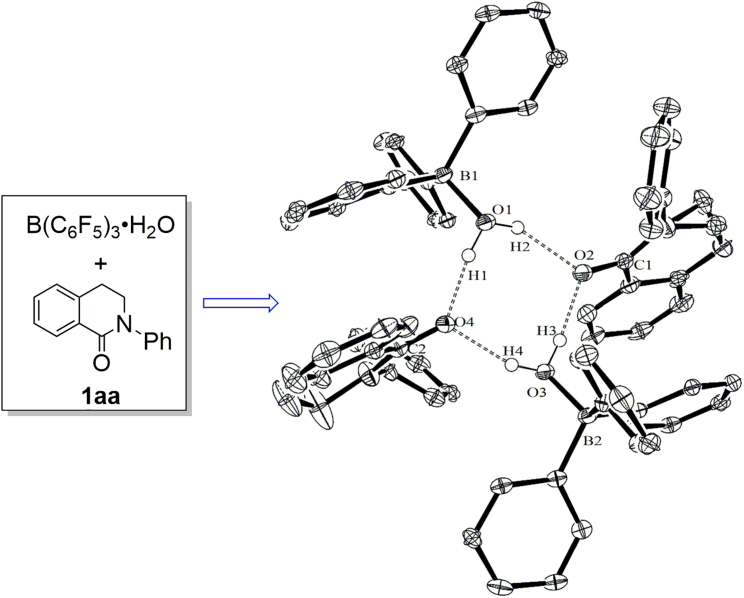
A myriad of X-ray structures of four-coordinated complex via anhydrous B(C6F5)3 were reported.9,10,11,12,13,14,15,16,17,18 In contrast, there have been very limited reports involving X-ray structures of B(C6F5)3·H2O with other molecules,38,39,40 resulting in difficulties understanding the difference between B(C6F5)3·H2O and other Brønsted acids. Herein, we wish to report an interesting X-ray crystal structure (C1) of B(C6F5)3·H2O and 2-phenyl-3,4-dihydroisoquinolin-1(2H)-one (1aa), forming through intermolecular hydrogen bonds and π-π interactions. Meanwhile, the mixture of B(C6F5)3·H2O and 1aa in tetrahydrofuran (THF) solution showed reinforcement both in UV-Vis absorption and fluorescence emission. On the basic of these observations, a photoredox oxidation reaction was developed using the combination of B(C6F5)3·H2O and 1aa as the photocatalyst.
Results and discussion
Identify noncovalent interactions between B(C6F5)3·H2O and amide 1aa
The crystals of C1 suitable for X-ray crystal structure analysis were obtained by slowly solvent evaporation of the solution of 1aa and B(C6F5)3·H2O (1:1) in dichloromethane (Figure 2). The B-O bond lengths in C1 are 1.5692(19) and 1.5557(19) Å, slightly shorter than that in aqua complexes of B(C6F5)3.38,39,40 Correspondingly, the C=O bond lengths are elongated to be 1.2579(19) and 1.2622(19), respectively.48,49
Figure 2.
X-ray crystal structure of C1 (B(C6F5)3·H2O + 1aa)
F atoms and part of the hydrogen atoms are omitted for clarity. Selected bond distances (Å): B1–O1 1.5692(19), B2–O3 1.5557(19), C1–O2 1.2579(19), C2–O4 1.2622(19). CCDC (2207426).
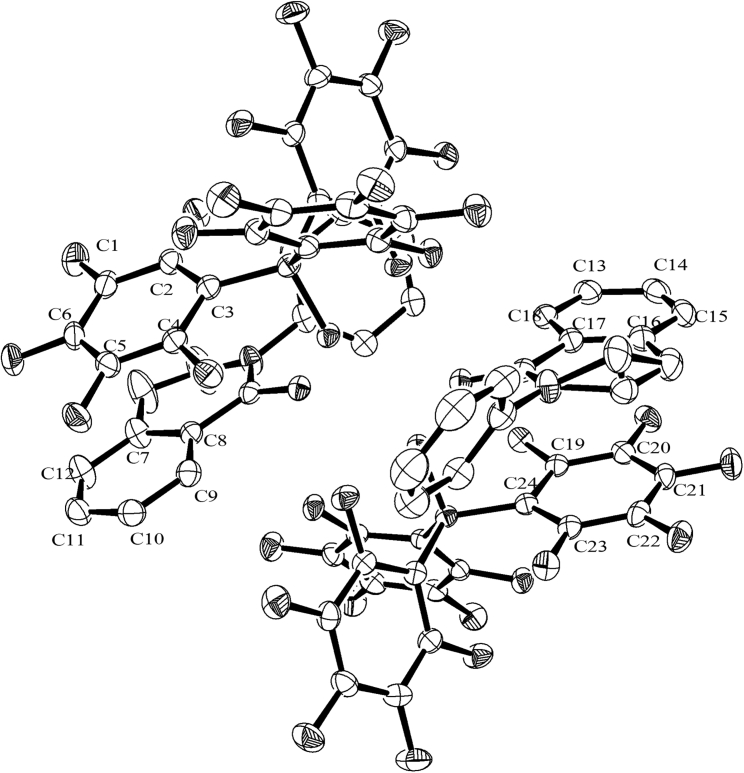
As can be seen from Figure 3, there are two π-π interactions between –C6F5 of B(C6F5)3 and phenyl moiety of 1aa. The twist angles of aromatic rings are 21.21(14) and 1.32(16) degree respectively. Besides, the distances between centroids are in the range of 3.30–3.80 Å.50
Figure 3.
π-π interactions
Hydrogen atoms are omitted for clarity. Selected distances (Å) and angles (deg): plane centroid of C1–C6 to plane centroid of C7–C12 3.8065(10), plane centroid of C1–C6 to plane C7–C12 3.4126(16), plane to plane twist angle 21.21(14); plane centroid of C13–C18 to plane centroid of C19–C24 3.5511(10), plane centroid of C13–C18 to plane C19-C24 3.3748(12), plane to plane twist angle 1.32(16).
There are four oxygen atoms and four hydrogen atoms, forming an eight-membered ring in the conformation of boat-chair via hydrogen bond possessing the lowest transannular strain and energy (Figure 4).51,52 The O … O distances in O–H … O hydrogen bond system range is 2.5627(15)−2.7583(15) Å, relatively shorter than the reported intermolecular O … O distances (2.70–3.00 Å),53,54 suggesting the existence of stronger hydrogen bonds in crystal C1.
Figure 4.
Structure analysis of eight-membered rings of hydrogen bond
Selected bond distances (Å): O1-H1 0.89(3), O1–H2 0.86(3), O3–H3 0.86(3), O3–H4 0.90 (3), O4 … H1 1.69(3), O2 … H2 1.97(3), O2 … H3 1.71(3), O4 … H4 1.83(3), O1 … O2 2.7583(15), O2 … O3 2.5627(15), O3 … O4 2.6578(15), O1 … O4 2.5659(15).
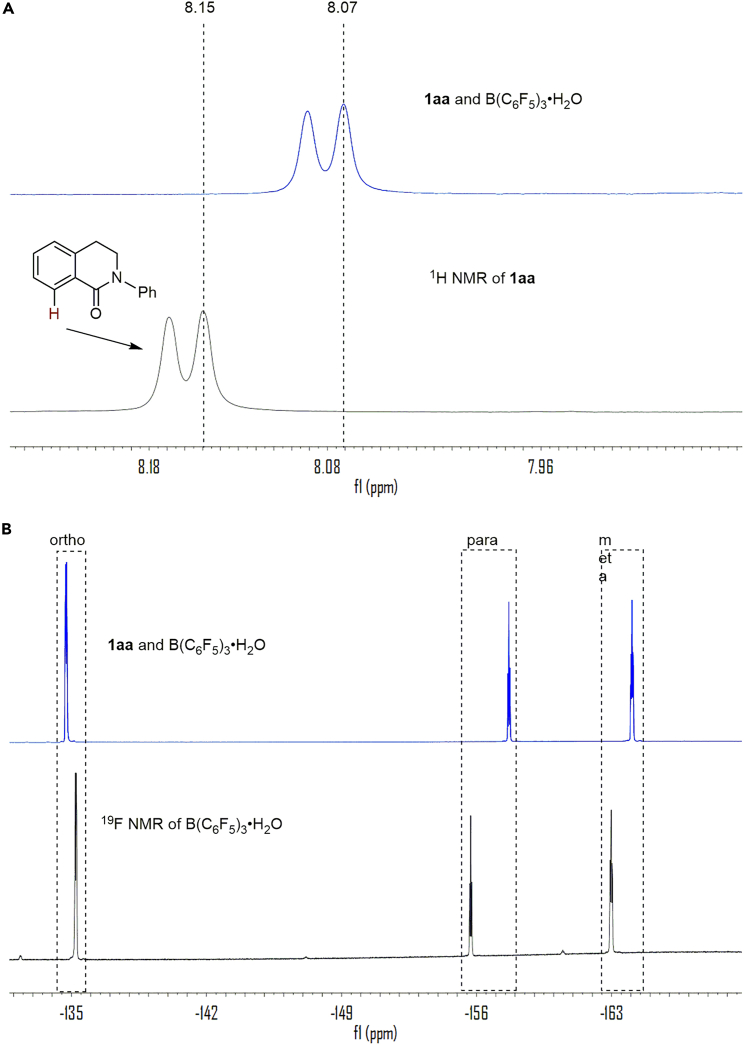
Encouraged by the above discoveries in C1, we considered that similar interactions might exist in solution. Compound 1aa showed a1H NMR resonance at 8.15 ppm in CDCl3 (Figure 5A, black curve). With the addition of B(C6F5)3·H2O (1.0 equiv), the resonance shifted to 8.07 ppm (Figure 5A, blue curve), indicating the formation of π-π interactions in solution.55,56 The 19F NMR of B(C6F5)3·H2O also changed on the addition of 1aa. The original peaks of B(C6F5)3·H2O were observed at −135.2, −155.7 and −163.0 ppm (Figure 5B, black curve).41 Upon addition of 1aa (1.0 equiv), the characteristic peaks changed to −134.7, −157.7 and −164.0 ppm, respectively (Figure 5B, blue curve). According to previous reports,36,37,38,41,42 intermolecular hydrogen bonds should exist between 1aa and B(C6F5)3·H2O in solution.
Figure 5.
NMR experiments
0.05 mmol 1aa was dissolved in 0.6 mL deuterated chloroform (CDCl3).
(A) 1aa (black); 1aa/B(C6F5)3·H2O (1:1) (blue).
(B) B(C6F5)3·H2O (black); 1aa/B(C6F5)3·H2O (1:1) (blue).
In tetrahydrofuran solution, 1aa as well as the mixture of 1aa and p-toluenesulfonic acid (TsOH), show no absorption in visible light region, respectively (Figure 6A, black and blue curves). Similarly, using other Brønsted acids instead of TsOH as hydrogen bond donors, such as phenylboronic acid, cyclohexanecarboxylic acid, and methanesulfonic acid, the corresponding mixtures also show no absorption in the visible light region (see supplmental information for detail, Figure S4). However, the mixture of 1aa and B(C6F5)3·H2O exhibits a tailing band from 400 to 450 nm (Figure 6A, red curve).
Figure 6.
UV-Vis absorption and fluorescence emission measurements
(A) 1aa in THF (10−3 M, black); 1aa and B(C6F5)3·H2O (1:1) in THF (10−3 M, red); 1aa and p-toluenesulfonic acid (TsOH) (1:1) in THF (10−3 M, blue).
(B) Fluorescence emission spectra of C1, excitation wavelength, 330 nm.
(C) Fluorescence emission spectra of 1aa in THF (10−4 M), B(C6F5)3·H2O were added to the solution of 1aa, excitation wavelength, 330 nm.
(D) Emission intensity, 387 nm and 402 nm.
The fluorescence emission measurements of C1 and 1aa/B(C6F5)3·H2O were attempted. The solid fluorescence emission spectrum of C1 showed three peaks at 381 nm, 390 nm, and 401 nm (Figure 6B). Similar emission peaks were observed in solution. There were two fluorescent peaks at 387 nm and 402 nm, which are enhanced in terms of the emission intensity by increasing the B(C6F5)3·H2O fraction (Figure 6C). When the ratio of B(C6F5)3·H2O/1aa is 1.5:1, the emission intensity was comparable at 387 nm and 402 nm. With the addition of B(C6F5)3·H2O, the emission intensity at 402 nm gradually became stronger than the emission intensity at 387 nm, indicating that the rotation of aromatic rings was more restricted because of increased π-π interactions.55,56 In comparison to B(C6F5)3·H2O/1aa (5:1) and 1aa, the fluorescence emission intensities at 387 nm and 402 nm were enhanced by 23% and 38%, respectively (Figure 6D).
To further explore the properties of mixture of B(C6F5)3·H2O and 1aa, electron paramagnetic resonance (EPR) measurements were conducted. As can be seen in Figure 7, directly subjecting the THF solution of 1aa, B(C6F5)3·H2O and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) to EPR, a weak radical signal was detected (Figure 7A). A strong signal appeared when the above solution was irradiated by blue light for 2 min (Figure 7B). As the irradiation time was extended to 5 min, further enhancement was observed (Figure 7C). According to previous reports, DMPO-O2- species is formed (αN = 13.1 G, αH = 9.1 G), indicating that the mixture of B(C6F5)3·H2O and 1aa can activate molecular oxygen probably through energy transfer on visible light irradiation.57,58,59,60
Figure 7.
EPR measurements
0.05 mmol scale, 1aa/DMPO/B(C6F5)3·H2O (1:1:1) in THF (1 mL) under air.
(A) in the dark.
(B) irradiated by blue LEDs for 2 min.
(C) irradiated by blue LEDs for 5 min.
These observations of the complex of B(C6F5)3·H2O and 1aa inspired us to further exploit its catalytic potential in photoredox catalysis. Therefore, the reaction of N-phenyl-tetrahydroisoquinoline (1a) and 4-isocyano-1,1′-biphenyl (2a) was investigated. As expected, the reaction gave corresponding product 3aa in 58% yield in THF using 10 mol % of B(C6F5)3·H2O/1aa as photocatalyst (Figure 8A). Besides, in the absence of isocyanide, 1a could be converted to 1aa in 30% yield catalyzed by B(C6F5)3·H2O, with 61% 1a recovered (Figure 8B). Thus, when 1a and 2a were treated by B(C6F5)3·H2O, the desired product 3aa could still be obtained in 51% yield (Figure 8C). Meanwhile, only trace amount of 3aa was formed in the dark (Figure 8C).
Figure 8.
Catalytic properties of B(C6F5)3·H2O and 1aa
(A) C1 Catalysis Experiment: 1a (0.15 mmol), 2a (0.1 mmol).
(B) generate 1aa via 1a direct oxidation, 1a (0.1 mmol).
(C) 1a was found to generate to 1aa, subsequently combined with B(C6F5)3·H2O to catalyze the reaction. 1a (0.15 mmol), 2a (0.1 mmol).
In addition, the UV-Vis absorption measurements of reaction mixture showed a tailing band from 400 to 450 nm (Figure 9A), similar to the absorption curve of B(C6F5)3·H2O/1aa (Figure 6A). The mixture of 1a and B(C6F5)3·H2O in THF showed no absorption in visible light region (Figure 9B), different from the result that an Electron Donor-Acceptor (EDA) complex was formed between 1a and anhydrous B(C6F5)3.61 EPR measurement of the reaction mixture with DMPO showed similar signals (Figure 9D) as those of the mixture of 1a/DMPO/B(C6F5)3·H2O (Figure 9C), further confirming that the combination of B(C6F5)3·H2O and 1aa (in situ formed from 1a) acts the photocatalyst of the reaction.
Figure 9.
UV-Vis absorption spectra and EPR measurements
(A) 1a, 2a and B(C6F5)3·H2O (1:1:1) in THF (10−3 M) irradiated by blue LEDs for 2 h.
(B) 1a in THF (10−3 M, blue); 1a and B(C6F5)3·H2O (1:1) in THF (10−3 M, red).
(C) 0.1 mmol 1a/DMPO and B(C6F5)3·H2O (0.01 mmol) THF (1 mL) irradiated by blue LEDs for 30 s.
(D) 0.1 mmol 1a/2a/DMPO (1:1:1) and B(C6F5)3·H2O (0.01 mmol) in THF (1 mL), irradiated by blue LEDs for 30 s.
Substrate Scope
Under the optimized reaction conditions (See supplmental information), the generality of the reaction was tested by variation of different tetrahydroisoquinolines (Figure 10). All reactions proceeded smoothly to give corresponding products 3 in moderate to good yields. 3aa was obtained in 62% isolated yield in gram scale. Substrate with a β-naphthyl group was compatible with the reaction conditions, and the desired product 3ba could be obtained in 83% yields. For tetrahydroisoquinoline with a hydrogen bond acceptor CN group, the reaction furnished product 3ca in 57% yield. For substrate bearing a Cl atom on the N-aryl ring, the reaction delivered 3da in 34% yield. Substrates having electron-donating methoxy substituent were also suitable for this transformation, giving 3ea and 3fa in 66% and 37% yields, respectively. For substrates with a para- and meta-Me on the N-aryl ring, corresponding products 3ga and 3ha were isolated in moderate yields. When substrates had two methoxy groups on the benzene ring (R1), the reaction could still perform smoothly to give 3ia in 52% yields. To our delight, bupivacaine analogue 3ja could be obtained in 32% yield from N-Ph piperidine, and the reaction of N-Ph proline also provided corresponding product 3ka in 39% yield.
Figure 10.
Substrate scope of tetrahydroisoquinolines
Reaction condition: 1 (0.15 mmol), 2a (0.1 mmol), B(C6F5)3 (10 mol %), THF: H2O = 10: 1 (1 mL), blue LED, O2 atmosphere at room temperature. a, Gram scale reaction. b, Reaction time: 36 h.
Next, a variety of isocyanides were examined and the results are presented in Figure 11. For isocyanide bearing a methoxy group on the benzene ring, the reaction produced the desired product 3 ab in 86% yield. The reactions of electron-deficient aromatic isocyanides provided the corresponding amides 3ac-3ag in moderate yields. 2-biphenylyl isocyanide led to amide product 3ah in 77% yield. Similarly, for other 2-biarylyl substituted isocyanides, products 3ai-3ak were obtained in moderate to good yields (73%–84%). Aliphatic isocyanides were also suitable for this transformation and gave the desired products 3 aL-3ar in 56–90% yields. Notably, ester tethered isocyanide furnished the target product 3as in 92% yield.
Figure 11.
Substrate scope of Isocyanide
Reaction conditions: 1a (0.1 mmol), 2 (0.15 mmol), B(C6F5)3·H2O (10 mol %). a, Reaction time: 14 h.
Mechanistic studies
Light on/off experiments were conducted to investigate the effect of light. The mixture of 1a, 2b and B(C6F5)3·H2O in THF/H2O was stirred for 2 h, alternating between 10-min periods of blue LED irradiation and 30 min in the dark. It was noted that the reaction proceeded smoothly on light irradiation, but the consumption of the isocyanide 2b immediately stalled without light, indicating that continuous light irradiation is essential for the reaction. (Figure 12).
Figure 12.
Light on/off experiment
1a (0.15 mmol), 2b (0.1 mmol), B(C6F5)3·H2O (0.01 mmol), THF/H2O 10:1, the conversion of 2b was monitored by GC.
The reaction mixture was monitored by 19F NMR after completion of the reaction. As shown in Figure 13, the resonance shifts appeared at −135.7, −160.7 and −165.2 ppm, suggesting that B(C6F5)3·H2O is remained after the reaction.62 Moreover, potassium iodide-starch test showed discoloration, implying that oxidizing substances were formed in the reaction. Subsequently, H2O2 was observed by a reported method using Ti(SO4)2 as the chromogenic agent, the characteristic peak was monitored at 405 nm (see supplmental information for detail).63
Figure 13.
19F NMR measurement after completion of the reaction
Plausible reaction mechanism
Based on the aforementioned results and reported photoredox aerobic oxidations,64,65,66 a plausible mechanism is proposed (Figure 14). Initially, amide is formed via autoxidation of 1.67 Then, the photocatalyst species PC is formed via hydrogen bond and π-π interactions between B(C6F5)3·H2O and amide. In addition, for 1j and 1k, N-phenyl group of the corresponding amides might take part in the π-π interactions. Next, singlet oxygen (1O2) is generated via energy transfer (EnT) by excited PC∗. Subsequent oxidation of compounds 1 by1O2 gives imine cation intermediate, together with the formation of H2O2 as a byproduct. Finally, the multiple component reaction occurs between imine cation, isocyanide and H2O delivers the final products 3.
Figure 14.
Plausible mechanism
Conclusion
In summary, the crystal structure of B(C6F5)3·H2O and amide 1aa was obtained, from which hydrogen bond and π-π interactions are observed. This crystal was composed of six molecules with an eight-membered ring via hydrogen bonds. The boat-chair conformation of the eight-membered ring possesses the lowest energy and the hydrogen bond strength is stronger than normal ones reported in the literature.50,51,52 Moreover, similar noncovalent interactions were also observed in solution as confirmed by NMR measurements, UV-Vis absorption and fluorescence emission spectra. The π-π interactions demonstrate the specific characteristics of B(C6F5)3·H2O among other Brønsted acids. Based on the synergistic effect of hydrogen bonds and π-π interactions in B(C6F5)3·H2O/amides complex, a photoredox catalysis under visible light is developed using the complex as the photocatalyst.
Limitations of the study
The reaction works well with N-aryl tetrahydroisoquinolines; for N-alkyl substituted tetrahydroisoquinolines, very low yields were obtained.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 1,2,3,4-Tetrahydro-2-isoquinoline | Meryer | CAS: 91-21-4 |
| 1,2,3,4-tetrahydro-6,7-dimethoxy-isoquinolin | Meryer | CAS: 1745-07-9 |
| 4-Aminobiphenyl | Meryer | CAS: 92-67-1 |
| 4-Chlorobromobenzene | Energy Chemical | CAS: 106-39-8 |
| 4-Bromobenzonitrile | Energy Chemical | CAS: 623-00-7 |
| 2-Bromoanisole | Energy Chemical | CAS: 578-57-4 |
| 4-Bromoanisole | Adamas | CAS: 104-92-7 |
| 3-Bromoanisole | Adamas | CAS: 2398-37-0 |
| 4-Bromotoluene | Adamas | CAS: 106-38-7 |
| 3-Bromotoluene | Adamas | CAS: 591-17-3 |
| 4-Bromoaniline | Adamas | CAS: 106-40-1 |
| 4-Iodoaniline | Adamas | CAS: 540-37-4 |
| p-Anisidine | Adamas | CAS: 104-94-9 |
| 4-Aminobenzonitrile | Adamas | CAS: 873-74-5 |
| 4-Nitroaniline | Adamas | CAS: 100-01-6 |
| 2-Aminobiphenyl | Adamas | CAS: 90-41-5 |
| 4′-Methyl-Bpphenyl-2-ylamine | Adamas | CAS: 1204-43-9 |
| 4′-Methoxy-Bpphenyl-2-ylamine | Adamas | CAS: 38089-03-1 |
| Deposited data | ||
| Complex of B(C6F5)3.H2O and amide 1aa. | CCDC | CCDC-2207426 |
| Other | ||
| Silica gel (200-300 mesh) | Huanghai | https://www.aladdin-e.com/ |
| AVIII 400 MHz | Bruker | https://www.bruker.com |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Xiang-Ying Tang (xtang@hust.edu.cn).
Materials availability
All materials generated in this study are available within the article and the supplemental information or from the lead contact upon reasonable request.
Method details
General information
The nuclear magnetic resonance spectra were recorded on the Bruker Avance III 400 MHz with tetramethylsilane (TMS) as an internal standard. High resolution mass spectra were recorded using analyses by BrukerDaltonics SolariX 7.0T. Organic solvents used were dried by standard methods when necessary. Commercially obtained reagents were used without further purification. Flash column chromatography was performed using 300-400 mesh silica gel. For thin-layer chromatography (TLC), silica gel plates (Huanghai GF254) were used. EPR (electron paramagnetic resonance) spectra were recorded by Bruker EMXmicro-6/1 instrument. X-Ray diffraction were collected by XtaLAB PRO MM007HF Cu (Rigaku, Japan). UV-vis spectra were obtained on a UV-2600 (Shimadzu). Fluorescence spectroscopic studies were performed with a RF-5301PC (Shimadzu). All heat sources are oil bath. All light sources are 3 W blue LED bands and the wavelength of peak is 427 nm. The distance from the light source to the container is 3-5 cm.
General procedure
Synthesis of substrate isoquinolines
A mixture of Pd2(dba)3 (3 mol%) and ligand (2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl) (8 mol%) were placed into an oven dried reaction tube. Subsequently, the reaction tube was degassed with N2 for three times. Then, dry toluene, bromoarene (1.0 equiv.), 1,2,3,4-tetrahydroisoquinoline (1.2 equiv.) and tBuONa (1.4 equiv.) were sequentially added under nitrogen protection. Then the reaction mixture was heated to 100 °C for 12 h. After completion, the resulting reaction mixture was slowly cooled to room temperature, quenched by brine and extracted with ethylacetate. The organic layer was dried overNa2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel. In addition, 1a-1h are known compound.
Synthesis of isocayanides
A mixture of HCOOH (2.0 equiv) and (CH3CO)2O (2.0 equiv.) were stirred at 55 °C for 2 h. Before aniline (1.0 equiv.) was added the reaction mixture was cooled down at 0 °C, then continued stirring for 2hat room temperature. After consumption of aniline, all the volatiles were removed under reduced pressure and the crude product was directly used in the next step without further purification.
To a stirred solution of crude product in dry THF at 0 °C were added Et3N (5.0 equiv.) and POCl3 (1.2 equiv.) dropwise sequentially. After stirring for 2hat 0 °C, the reaction was quenched with water and extracted with ethylacetate (EA) for three times. The organic layer was washed with brine and dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel to observed pure product 2. In addition, 2a-2s are known compound.
Synthesis of compounds 3
To a stirred solution of 1 (0.15 mmol, 1.5 equiv.) in mixed solvent (1 mL, THF: H2O = 10:1) were added 2 (0.1 mmol, 1.0 equiv.) and boron catalyst (10 mol%). The resulting solution was stirred in the presence of O2 under blue light irradiation. The reaction was monitored by TLC, the crude products were purified by flash column chromatography to provide a series of amide compounds 3. In addition, 3aa was known compound.
Gram scale reaction
To a stirred solution of 2a (6 mmol, 1.074 g) in mixed solvent (50 mL, THF: H2O = 10:1) were added to 1a (7 mmol, 1.463 g) and boron catalyst (10 mol%, 0.307 g). The resulting solution was stirred at blue LED and O2 for 36 h. The crude product was purified by flash column chromatography to provide a series of amide compound 3aa (1.502 g, 62%).
Oxidation reaction of 1a
To a stirred solution of 1a (0.1 mmol, 20.9 mg, 1.0 equiv.) in mixed solvent (1 mL, THF: H2O = 10:1) was added to boron catalyst (10 mol%). The resulting solution was stirred at blue LEDs and 1 atm O2 for 12 h. The crude product was purified by flash column chromatography to provide 1aa (6.6 mg, 30%) and 1a (12.7 mg, 61%).
X-ray crystal structure analysis for 1aa
X-ray crystal structure analysis of C1. A clear colorless plate. Formula C33H15BF15NO2, M = 753.27, 0.25∗0.2∗0.05 mm, a = 12.0787(1), b = 11.9169(1), c = 41.6237(3) Å, β = 97.510(1)°, V = 5939.95(8) Å3, μ= 1.518 mm-1, 0.651 ≤ T ≤ 1.000, Theta(max) = 74.296°, R = 0.0381, wR2 = 0.1036, temperature = 293 K, hydrogen atoms calculated and refined as riding atoms.
Light on/off experiments
To a stirred solution of the 1-isocyano-4-methoxybenzene (2b, 0.1 mmol, 1.0 equiv.) and boron catalyst (10 mol%) in mixed solvent (1mL, THF: H2O = 10:1) was added the N-phenyl-1,2,3,4-tetrahydroisoquinoline (1a, 0.15 mmol, 1.5 equiv.). Besides, 0.1 mmol dodecane was added as internal standard. The light was kept on for ten minutes then kept off for thirty minutes. The reaction mixture 0.05 mL was taken and diluted with 1,2-dichloroethane (DCE) to 2 mL for gas chromatography (GC) monitoring.
Spectroscopic data
N-([1,1'-biphenyl]-4-yl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3aa)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (33.7 mg, 84% yield). m.p.: 192−194 °C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.88 (s, 1H), 7.65 (d, J = 6.8 Hz, 1H), 7.57−7.49 (m, 6H), 7.36−7.27 (m, 4H), 7.26−7.23 (m, 1H), 7.18 (d, J = 7.2 Hz, 1H), 7.02 (d, J = 8.0 Hz, 2H), 6.95 (dd, J1 = J2 = 7.6 Hz, 1H), 5.09 (s, 1H), 3.94 (dt, J = 9.6 Hz, J = 4.4 Hz, 1H), 3.40 (td, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.16-3.00 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.6, 149.4, 140.5, 137.3, 136.7, 134.5, 132.1, 129.6, 129.1, 127.8, 127.7, 127.5, 127.1, 126.9, 120.4, 120.1, 115.3, 66.5, 45.4, 28.8. IR ν 3298, 3034, 2251, 2228, 1662, 1287, 858, 690 cm-1. HRMS (ESI) m/z: calcd for C28H25N2O [M+H]+ 405.1961; found 405.1960.
N-([1,1'-biphenyl]-4-yl)-2-(naphthalen-2-yl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ba)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (37.6 mg, 83% yield). m.p.: 141−143 °C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.89 (s, 1H), 7.79 (d, J = 8.8 Hz, 1H), 7.75−7.67 (m, 3H), 7.54−7.45 (m, 6H), 7.44−7.35 (m, 3H), 7.33−7.24 (m, 6H), 7.17 (d, J = 7.2 Hz, 1H), 5.24 (s, 1H), 4.03 (dt, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.50 (td, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.18−3.01 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 147.0, 140.5, 137.4, 136.7, 134.5, 134.4, 132.0, 129.5, 129.2, 128.8, 128.6, 127.9, 127.8, 127.6, 127.5, 127.1, 126.94, 126.9, 126.8, 126.7, 123.9, 120.1, 117.5, 66.2, 45.9, 28.8. IR ν 3303, 3052, 2924, 1667, 1596, 1386, 1112, 834 cm-1. HRMS (ESI) m/z: calcd for C32H27N2O [M+H]+ 455.2118; found 455.2117.
N-([1,1'-biphenyl]-4-yl)-2-(4-cyanophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ca)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (24.5 mg, 57% yield). m.p.: 137−139 °C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.37 (s, 1H), 7.61−7.58 (m, 1H), 7.55−7.48 (m, 8H), 7.40 (dd, J1 = J2 = 8.0 Hz, 2H), 7.33−7.28 (m, 3H), 7.23−7.20 (m, 1H), 6.95 (d, J = 8.8 Hz, 2H), 5.18 (s, 1H), 4.06−4.00 (m, 1H), 3.43 (td, J = 11.2 Hz, J = 3.6 Hz, 1H), 3.25−3.16 (m, 1H), 3.07 (dt, J = 15.6 Hz, J = 3.6 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 169.4, 152.0, 140.3, 137.8, 136.3, 134.4, 133.8, 131.7, 128.8, 128.4, 127.8, 127.6, 127.3, 127.27, 126.9, 120.4, 119.8, 113.7, 101.5, 65.6, 44.5, 28.7. IR ν 3319, 3026, 2831, 2246, 1662, 1318, 904, 722 cm-1. HRMS (ESI) m/z: calcd for C29H24N3O [M+H]+ 430.1914; found 430.1907.
N-([1,1'-biphenyl]-4-yl)-2-(4-chlorophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3da)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (14.9 mg, 34% yield). m.p.: 188−190 °C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.72 (s, 1H), 7.63 (d, J = 6.8 Hz, 1H), 7.55−7.48 (m, 6H), 7.42−7.37 (m, 2H), 7.33−7.24 (m, 5H), 7.17 (d, J = 6.8 Hz, 1H), 6.92 (d, J = 9.2 Hz, 2H), 5.03 (s, 1H), 3.94−3.88 (m, 1H), 3.35 (td, J = 10.8 Hz, J = 3.6 Hz, 1H), 3.16−3.08 (m, 1H), 3.01 (dt, J = 12.0 Hz, J = 3.2 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.2, 148.0, 140.4, 137.5, 136.6, 134.3, 131.8, 129.4, 129.0, 128.8, 127.9, 127.8, 127.6, 127.2, 127.0, 126.9, 125.5, 120.1, 116.4, 66.4, 45.6, 28.8. IR ν 3255, 3061, 2920, 2851, 1596, 1331, 1052, 840 cm-1. HRMS (ESI) m/z: calcd for C28H24N2OCl [M+H]+ 439.1572; found 439.1571.
N-([1,1'-biphenyl]-4-yl)-2-(2-methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ea)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (28.6 mg, 66% yield). m.p.: 66-68°C. 1H NMR (400 MHz, CDCl3, TMS) δ 9.98 (s, 1H), 7.72 (d, J =7.2 Hz, 1H), 7.60-7.50 (m, 6H), 7.40 (dd, J1 = J2 = 7.6 Hz, 2H), 7.32-7.22 (m, 3H), 7.17-7.10 (m, 3H), 6.97-6.87 (m, 2H), 5.08 (s, 1H), 4.00 (s, 3H), 3.48-3.31 (m, 2H), 3.03-2.84 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.5, 153.8, 140.6, 139.0, 137.6, 136.6, 134.6, 132.1, 129.0, 128.7, 128.6, 127.5, 127.2, 127.0, 126.8, 126.2, 125.6, 123.3, 121.3, 119.5, 111.5, 65.2, 55.6, 47.7, 28.0. IR ν 3298, 3026, 2849, 2228, 1490, 1035, 904, 720 cm-1. HRMS (ESI) m/z: calcd for C29H27N2O2[M+H]+ 435.2067; found 435.2066.
N-([1,1′-biphenyl]-4-yl)-2-(3-methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3fa)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (16.1 mg, 37% yield). m.p.: 64-66°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.81 (s, 1H), 7.63 (d, J = 7.2 Hz, 1H), 7.57-7.48 (m, 6H), 7.42-7.38 (m, 2H), 7.33-7.21 (m, 4H), 7.17 (d, J = 6.8 Hz, 1H), 6.62-6.56 (m, 2H), 5.10 (s, 1H), 3.95-3.89 (m, 1H), 3.80 (s, 3H), 3.39 (td, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.15-2.99 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.4, 160.8, 150.8, 140.5, 137.3, 136.7, 134.5, 132.1, 130.3, 129.1, 128.8, 127.7, 127.5, 127.1, 126.8, 120.1, 108.0, 105.0, 101.9, 66.4, 55.3, 45.3, 28.8. IR ν 3319, 3025, 2920, 2847, 1662, 1445, 1166, 757 cm-1. HRMS (ESI) m/z: calcd for C29H27N2O2[M+H]+ 435.2067; found 435.2066.
N-([1,1'-biphenyl]-4-yl)-2-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ga)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (22.9 mg, 55% yield). m.p.: 160-162°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.94 (s, 1H), 7.65 (d, J = 7.2 Hz, 1H), 7.56-7.47 (m, 6H), 7.41-7.37 (m, 2H), 7.32-7.22 (m, 3H), 7.17-7.11 (m, 3H), 6.93 (d, J = 8.8 Hz, 2H), 5.04 (s, 1H), 3.88 (dt, J = 10.4 Hz, J = 4.4 Hz, 1H), 3.35 (td, J = 11.2 Hz, J = 4.0 Hz, 1H), 3.13-2.96 (m, 2H), 2.28 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.7, 147.3, 140.5, 137.2, 136.8, 134.5, 132.1, 130.1, 129.0, 128.8, 127.8, 127.6, 127.5, 127.1, 126.8, 126.77, 120.1, 115.8, 66.5, 45.9, 28.8, 20.4. IR ν 3303, 3022, 2920, 2850, 1913, 1671, 1499, 1149 cm-1. HRMS (ESI) m/z: calcd for C29H27N2O [M+H]+ 419.2118; found 419.2119.
N-([1,1'-biphenyl]-4-yl)-2-(m-tolyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ha)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (19.3 mg, 46% yield). m.p.: 137-139°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.88 (s, 1H), 7.66-7.63 (m, 1H), 7.58-7.49 (m, 6H), 7.42-7.37 (m, 2H), 7.33-7.15 (m, 5H), 6.84-6.76 (m, 3H), 5.09 (s, 1H), 3.92 (dt, J = 11.2 Hz, J = 4.4 Hz, 1H), 3.40 (td, J = 10.4 Hz, J = 4.0 Hz, 1H), 3.14-2.99 (m, 2H), 2.35 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.6, 149.5, 140.5, 139.4, 137.3, 136.8, 134.6, 132.2, 129.4, 129.2, 128.8, 127.7, 127.66, 127.5, 127.1, 126.85, 126.8, 121.3, 120.1, 116.0, 112.4, 66.4, 45.4, 28.8, 21.9. IR ν 3326, 3031, 2917, 2827, 1671, 1487, 1113, 835 cm-1. HRMS (ESI) m/z: calcd for C29H26N2ONa [M+Na]+ 441.1937; found 441.1935.
N-([1,1'-biphenyl]-4-yl)-6,7-dimethoxy-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ia)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 2:1 as the eluent). Yellow solid (24.1 mg, 52% yield). m.p.: 197-198°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.85 (s, 1H), 7.56-7.48 (m, 6H), 7.42-7.37 (m, 2H), 7.35-7.28 (m, 3H), 7.18 (s, 1H), 7.03 (d, J = 8.0 Hz, 1H), 6.95 (dd, J1 = J2 = 7.2 Hz, 1H), 6.65 (s, 1H), 5.01 (s, 1H), 3.93-3.86 (m, 7H), 3.44-3.37 (m, 1H), 3.07-2.90 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.8, 149.5, 148.5, 147.8, 140.5, 137.3, 136.8, 129.6, 128.8, 127.6, 127.1, 126.8, 126.6, 123.8, 120.5, 120.2, 115.6, 111.8, 110.6, 65.9, 56.1, 56.0, 45.6, 28.2. IR ν 3263, 3021, 2911, 2828, 1657, 1217, 1114, 992 cm-1. HRMS (ESI) m/z: calcd for C30H29N2O3[M+H]+ 465.2173; found 465.2172.
N-([1,1'-biphenyl]-4-yl)-1-phenylpiperidine-2-carboxamide (3ja)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (11.4 mg 32% yield). m.p.: 139-141°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.45 (s, 1H), 7.55-7.48 (m, 6H), 7.41 (dd, J1 = J2 = 7.6 Hz, 2H), 7.33-7.29 (m, 3H), 7.07 (d, J =8.0 Hz, 2H), 6.94 (dd, J1 = J2 = 7.2 Hz, 1H), 4.19 (t, J = 5.2 Hz, 1H), 3.36-3.34 (m, 2H), 2.21-2.17 (m, 1H), 2.01-1.94 (m, 1H), 1.80-1.61 (m, 5H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 171.3, 150.7, 140.6, 137.1, 137.0, 129.6, 128.8, 127.6, 127.1, 126.8, 121.2, 120.0, 117.8, 62.8, 49.5, 26.4, 24.0, 21.7. IR ν 3323, 3025, 1671, 1484, 1241, 830, 759, 695. HRMS (ESI) m/z: calcd for C24H25N2O [M+H]+ 357.1961; found 357.1958.
([1,1'-biphenyl]-4-yl)-1-phenylpyrrolidine-2-carboxamide (3ka)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (13.3 mg, 39% yield). m.p.: 207-208°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.44 (s, 1H), 7.59-7.51 (m, 6H), 7.41 (dd, J1 = J2 = 7.6 Hz, 2H), 7.33-7.27 (m, 3H), 6.89-6.84 (m, 1H), 6.73 (d, J = 8.0 Hz, 2H), 4.12-4.08 (m, 1H), 3.79-3.75 (m, 1H), 3.31-3.24 (m, 1H), 2.42-2.28 (m, 2H), 2.10-1.99 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.3, 147.6, 140.5, 137.4, 136.6, 129.5, 128.8, 127.6, 127.2, 126.9, 120.3, 118.9, 113.5, 65.4, 50.1, 31.6, 24.4. IR ν 3326, 2967, 2818, 1663, 1497, 1005, 755, 713. HRMS (ESI) m/z: calcd for C23H23N2O [M+H]+ 343.1875; found 343.1803.
N-(4-methoxyphenyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ab)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (30.7 mg, 86% yield). m.p.: 132-134°C . 1H NMR (400 MHz, CDCl3, TMS) δ 8.70 (s, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.38-7.29 (m, 4H), 7.28-7.21 (m, 2H), 7.16 (d, J = 6.8 Hz, 1H), 6.99 (d, J = 8.0 Hz, 2H), 6.93 (dd, J1 = J2 = 6.8 Hz, 1H), 6.81-6.76 (m, 2H), 5.06 (s, 1H), 3.94-3.89 (m, 1H), 3.74 (s, 3H), 3.37 (td, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.14-2.97 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.3, 156.5, 149.5, 134.6, 132.2, 130.6, 129.5, 129.1, 127.7, 127.6, 126.8, 121.6, 120.2, 115.1, 114.0, 66.3, 55.5, 45.3, 28.8. IR ν 3298, 3029, 2900, 2850, 1498, 888, 814 cm-1. HRMS (ESI) m/z: calcd for C23H23N2O2[M+H]+ 359.1754; found 359.1753.
N-(4-bromophenyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ac)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (22.7 mg, 56% yield). m.p.: 154-155°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.83 (s, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.36-7.22 (m, 8H), 7.17 (d, J = 6.8 Hz, 1H), 7.00-6.92 (m, 3H), 5.06 (s, 1H), 3.91 (dt, J =11.2 Hz, J = 4.4 Hz, 1H), 3.38 (td, J =10.8 Hz, J = 4.0 Hz, 1H), 3.12-2.97 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.7, 149.3, 136.5, 134.5, 131.9, 131.8, 129.6, 129.1, 127.81, 127.8, 126.9, 121.4, 120.5, 117.0, 115.3, 66.3, 45.4, 28.7. IR ν 3298, 3028, 2915, 2228, 1597, 1320, 992, 690 cm-1. HRMS (ESI) m/z: calcd for C22H20N2OBr [M+H]+ 407.0754; found 407.0752.
N-(4-iodophenyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ad)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (19.0 mg, 42% yield). m.p.: 122-124°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.80 (s, 1H), 7.63-7.59 (m, 1H), 7.57-7.53 (m, 2H), 7.35-7.22 (m, 6H), 7.18-7.15 (m, 1H), 7.00-6.92 (m, 3H), 5.06 (s, 1H), 3.90 (dt, J = 11.2 Hz, J = 4.8 Hz, 1H), 3.42-3.35 (m, 1H), 3.11-2.97 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ. 170.6, 149.3, 137.8, 137.2, 134.5, 131.8, 129.6, 129.1, 127.8, 127.77, 126.9, 121.7, 120.6, 115.4, 87.6, 66.4, 45.4, 28.7. IR ν 3323, 3027, 2919, 2242, 2242, 1597, 1178, 818 cm-1. HRMS (ESI) m/z: calcd for C22H20N2OI [M+H]+ 455.0615; found 455.0614.
N-(4-cyanophenyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ae)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (18.1 mg, 51% yield). m.p.: 74-76°C. 1H NMR (400 MHz, CDCl3, TMS) δ 9.05 (s, 1H), 7.62-7.58 (m, 3H), 7.55-7.51 (m, 2H), 7.36-7.30 (m, 2H), 7.28-7.24 (m, 2H), 7.19-7.16 (m, 1H), 7.02-6.94 (m, 3H), 5.09 (s, 1H), 3.91 (dt, J = 11.2 Hz, J = 4.8 Hz, 1H), 3.43-3.36 (m, 1H), 3.11-2.98 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 171.2, 149.2, 141.4, 134.5, 133.2, 131.4, 129.7, 129.1, 129.0, 128.0, 127.9, 126.9, 120.9, 119.7, 118.8, 115.6, 107.3, 66.4, 45.6, 28.7. IR ν 3323, 3030, 2915, 2224, 1921, 1672, 1402, 933 cm-1. HRMS (ESI) m/z: calcd for C23H20N3O [M+H]+ 354.1601; found 354.1602.
N-(3-cyanophenyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3af)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (15.5 mg, 43% yield). m.p.: 138-140°C. 1H NMR (400 MHz, CDCl3, TMS) δ 8.97 (s, 1H), 7.88-7.87 (m, 1H), 7.67-7.60 (m, 2H), 7.37-7.31 (m, 4H), 7.30-7.23 (m, 2H), 7.19-7.16 (m, 1H), 7.02-6.94 (m, 3H), 5.09 (s, 1H), 3.92 (dt, J = 11.2 Hz, J = 4.4 Hz, 1H), 3.42-3.35 (m, 1H),3.13-2.99 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 171.2, 149.3, 138.3, 134.5, 131.5, 129.8, 129.7, 129.0, 127.94, 127.9, 127.8, 126.9, 123.9, 123.0, 120.8, 118.3, 115.5, 113.0, 66.3, 45.6, 28.7. IR ν 3298, 3043, 2899, 2251, 2227, 1499, 1036, 888 cm-1. HRMS (ESI) m/z: calcd for C23H20N3O [M+H]+ 354.1601; found 354.1599.
N-(4-nitrophenyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ag)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 5:1 as the eluent). Yellow oil (20.8 mg, 56% yield). 1H NMR (400 MHz, CDCl3, TMS) δ 9.19 (s, 1H), 8.13 (d, J = 9.2 Hz, 2H), 7.67-7.60 (m, 3H), 7.34-7.25 (m, 5H), 7.18 (d, J = 6.8 Hz, 1H), 7.02-6.95 (m, 3H), 5.11 (s, 1H), 3.96-3.89 (m, 1H), 3.43-3.38 (m, 1H), 3.11-3.01 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 171.3, 149.2, 143.7, 143.2, 134.5, 131.3, 129.7, 129.0, 128.04, 128.0, 127.0, 121.0, 119.3, 115.7, 66.4, 45.7, 28.6.
N-([1,1'-biphenyl]-2-yl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ah)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (31.1 mg, 77% yield). m.p.: 53-54°C. 1H NMR (400 MHz, CDCl3, TMS) δ 9.18 (s, 1H), 8.50 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 7.2 Hz, 1H), 7.34-7.24 (m, 4H), 7.23-7.13 (m, 5H), 7.09 (d, J = 7.2 Hz, 1H), 7.05-7.02 (m, 3H), 6.91 (dd, J1 = J2 = 7.2 Hz, 1H), 6.77 (d, J = 8.0 Hz, 2H), 4.91 (s, 1H), 3.28 (dt, J = 10.8 Hz, J = 3.6 Hz, 1H), 2.98 (td, J = 10.8 Hz, J = 3.2 Hz, 1H), 2.67 (dt, J = 10.2 Hz, J = 3.2 Hz, 1H), 2.46-2.38 (m, 1H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.5, 148.9, 137.8, 135.0, 134.4, 132.1, 131.7, 129.6, 129.3, 129.2, 129.1, 128.9, 128.8, 128.5, 127.7, 127.6, 127.5, 126.9, 123.8, 119.7, 119.3, 114.4, 66.6, 44.3, 28.6. IR ν 3323, 3028, 2916, 2242, 1672, 1489, 1295, 1037 cm-1. HRMS (ESI) m/z: calcd for C28H25N2O [M+H]+ 405.1961; found 405.1960.
N-(4'-methoxy-[1,1'-biphenyl]-2-yl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ai)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (36.4 mg, 84% yield). m.p.: 128-130°C. 1H NMR (400 MHz, CDCl3, TMS) δ 9.17 (s, 1H), 8.46 (dd, J1 = 8.0 Hz, J2 = 0.8 Hz, 1H), 7.58 (d, J = 7.2 Hz, 1H), 7.32-7.26 (m, 3H), 7.25-7.17 (m, 2H), 7.15-7.12 (m, 1H), 7.10-7.05 (m, 2H), 6.98-6.89 (m, 3H), 6.77 (d, J = 8.0 Hz, 2H), 6.71-6.67 (m, 2H), 3.82 (s, 3H), 3.34 (dt, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.03 (td, J = 11.2 Hz, J = 3.6 Hz, 1H), 2.73 (dt, J = 16.0 Hz, J = 3.2 Hz, 1H), 2.58-2.50 (m, 1H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.4, 159.0, 148.9, 135.1, 134.5, 132.2, 131.4, 130.3, 129.8, 129.7, 129.3, 128.8, 128.2, 127.6, 127.4, 126.9, 123.7, 119.6, 119.3, 114.3, 114.1, 66.6, 55.3, 44.3, 28.6. IR ν 3316, 3061, 2914, 2846, 2247, 1598, 1035, 834 cm-1. HRMS (ESI) m/z: calcd for C29H27N2O2[M+H]+ 435.2067; found 435.2068.
N-(4'-methyl-[1,1'-biphenyl]-2-yl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3aj)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (30.5 mg, 73% yield). m.p.: 136-138°C. 1H NMR (400 MHz, CDCl3, TMS) δ 9.17 (s, 1H), 8.46 (d, J = 9.6 Hz, 1H), 7.56 (d, J = 7.2 Hz, 1H), 7.32-7.12 (m, 6H), 7.10 -7.04 (m, 2H), 6.98 (d, J = 8.0 Hz, 2H), 6.94-6.88 (m, 3H), 6.73 (d, J = 8.0 Hz, 2H), 4.90 (s, 1H), 3.29 (dt, J = 10.4 Hz, J = 4.4 Hz, 1H), 3.02 (td, J = 11.2 Hz, J = 3.2 Hz, 1H), 2.70 (dt, J = 15.6 Hz, J = 3.6 Hz, 1H), 2.54-2.45 (m, 1H), 2.37 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.3, 148.9, 137.4, 135.1, 134.7, 134.5, 132.2, 131.8, 129.6, 129.5, 129.2, 129.0, 128.9, 128.3, 127.6, 127.5, 126.9, 123.7, 119.7, 119.3, 114.3, 66.5, 44.3, 28.5, 21.3. IR ν 3327, 3057, 2950, 2917, 1683, 1493, 1271, 930 cm-1. HRMS (ESI) m/z: calcd for C29H27N2O [M+H]+ 419.2118; found 419.2116.
N-([1,1':4',1''-terphenyl]-2-yl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ak)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (38.4 mg, 80% yield). m.p.: 119-121°C. 1H NMR (400 MHz, CDCl3, TMS) δ 9.23 (s, 1H), 8.50 (dd, J1 = 8.8 Hz, J2 = 1.2 Hz, 1H), 7.65-7.61 (m, 2H), 7.58 (d, J = 7.2 Hz, 1H), 7.54-7.49 (m, 2H), 7.44-7.39 (m, 3H), 7.36-7.31 (m, 1H), 7.28-7.16 (m, 5H), 7.13-7.08 (m, 3H), 7.04 (d, J = 7.2 Hz, 1H), 6.90 (dd, J1 = J2 = 7.2 Hz, 1H), 6.75 (d, J = 8.0 Hz, 2H), 4.92 (s, 1H), 3.28 (dt, J = 10.4 Hz, J = 4.4 Hz, 1H), 2.98 (td, J = 11.2 Hz, J = 3.2 Hz, 1H), 2.68 (dt, J = 15.6 Hz, J = 3.6 Hz, 1H), 2.55-2.46 (m, 1H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 170.4, 148.9, 140.4, 140.3, 136.7, 135.0, 134.4, 132.2, 131.4, 129.6, 129.54, 129.5, 129.3, 129.0, 128.9, 128.6, 127.7, 127.6, 127.53, 127.5, 127.4, 127.1, 127.0, 126.9, 123.8, 119.8, 119.4, 114.4, 66.6, 44.4, 28.5. IR ν 3300, 3025, 2849, 2247, 1685, 1111, 906, 841 cm-1. HRMS (ESI) m/z: calcd for C34H29N2O [M+H]+ 481.2274; found 481.2273.
N-benzyl-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3al)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 10:1 as the eluent). White solid (29.3 mg, 86% yield). m.p.: 154-156°C. 1H NMR (400 MHz, CDCl3, TMS) δ 7.64-7.61 (m, 1H), 7.32-7.14 (m, 9H), 7.04-7.00 (m, 2H), 6.93-6.88 (m, 3H), 5.06 (s, 1H), 4.43-4.32 (m, 2H), 3.82 (dt, J = 10.8 Hz, J = 4.4 Hz, 1H), 3.28 (td, J = 10.4 Hz, J = 3.6 Hz, 1H), 3.07-2.91 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.4, 149.4, 138.1, 134.5, 132.6, 129.4, 128.9, 128.6, 127.7, 127.5, 127.4, 127.3, 126.8, 119.7, 114.9, 65.6, 45.2, 43.4, 29.0. IR ν 3319, 3025, 2831, 2247, 1489, 1055, 903, 756 cm-1. HRMS (ESI) m/z: calcd for C23H23N2O [M+H]+ 343.1805; found 343.1803.
N-phenethyl-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3am)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 5:1 as the eluent). White solid (19.9 mg, 56% yield). m.p.: 123-125°C. 1H NMR (400 MHz, CDCl3, TMS) δ 7.60-7.57 (m, 1H), 7.32-7.23 (m, 4H), 7.19-7.11 (m, 4H), 6.93-6.84 (m, 6H), 4.94 (s, 1H), 3.71 (dt, J = 11.2 Hz, J = 4.0 Hz, 1H), 3.52-3.36 (m, 2H), 3.19 (td, J = 11.2 Hz, J = 3.6 Hz, 1H), 2.89-2.60 (m, 4H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.3, 149.2, 138.7, 134.6, 132.6, 129.4, 128.8, 128.7, 128.5, 127.5, 127.46, 126.8, 126.4, 119.4, 114.3, 65.6, 44.6, 40.6, 35.4, 28.8. IR ν 3298, 3028, 2899, 2228, 1663, 1516, 943, 791 cm-1. HRMS (ESI) m/z: calcd for C24H25N2O [M+H]+ 357.1961; found 357.1960.
N-(4-methylphenethyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3an)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 5:1 as the eluent). White solid (28.5 mg, 77% yield). m.p.: 97-99°C. 1H NMR (400 MHz, CDCl3, TMS) δ 7.59-7.56 (m, 1H), 7.31-7.21 (m, 4H), 7.14-7.11 (m, 1H), 6.97 (d, J = 7.6 Hz, 2H), 6.90-6.79 (m, 6H), 4.93 (s, 1H), 3.71 (dt, J = 11.2 Hz, J = 4.8 Hz, 1H), 3.49-3.34 (m, 2H), 3.20 (td, J = 10.4 Hz, J = 4.4 Hz, 1H), 2.90-2.75 (m, 2H), 2.71-2.55 (m, 2H), 2.29 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.3, 149.3, 135.8, 135.6, 134.6, 132.7, 129.4, 129.2, 128.9, 128.7, 128.6, 127.5, 127.4, 126.7, 119.4, 114.3, 65.6, 44.7, 40.7, 35.0, 28.8, 21.0. IR ν 3262, 3063, 2831, 2241, 1598, 1442, 1426, 987 cm-1. HRMS (ESI) m/z: calcd for C25H27N2O [M+H]+ 371.2118; found 371.2120.
N-(3,4-dimethoxyphenethyl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ao)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 2:1 as the eluent). White solid (23.3 mg, 56% yield). m.p.: 105-106°C. 1H NMR (400 MHz, CDCl3, TMS) δ 7.58-7.55 (m, 1H), 7.31-7.20 (m, 4H), 7.13-7.10 (m, 1H), 6.93-6.82 (m, 4H), 6.66 (d, J = 8.4 Hz, 1H), 6.54 (d, J = 2.0 Hz, 1H), 6.46 (dd, J = 8.0 Hz, J = 1.6 Hz, 1H), 4.93 (s, 1H), 3.84 (s, 3H), 3.72-3.65 (m, 4H), 3.48-3.41 (m, 2H), 3.20 (td, J = 11.2 Hz, J = 3.6 Hz, 1H), 2.89-2.57 (m, 4H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.3, 149.3, 148.9, 147.5, 134.5, 132.7, 131.1, 129.4, 128.9, 127.5, 127.4, 126.7, 120.6, 119.4, 114.2, 111.6, 111.2, 65.7, 55.9, 55.7, 44.6, 40.5, 35.0, 28.8. IR ν 3028, 2915, 2850, 2251, 1662, 1061, 889, 752 cm-1. HRMS (ESI) m/z: calcd for C26H29N2O3[M+H]+ 417.2173; found 417.2171.
2-phenyl-N-(4-phenylbutyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ap)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 5:1 as the eluent). White solid (23.8 mg, 62% yield). m.p.: 146-148°C. 1H NMR (400 MHz, CDCl3, TMS) δ 7.60-7.57 (m, 1H), 7.31-7.19 (m, 6H), 7.17-7.12 (m, 2H), 7.05-7.02 (m, 2H), 6.91-6.84 (m, 4H), 4.96 (s, 1H), 3.83 (dt, J = 11.2 Hz, J = 4.4 Hz, 1H), 3.31-3.11 (m, 3H), 3.06-2.91 (m, 2H), 2.50 (t, J = 6.8 Hz, 2H), 1.51-1.36 (m, 4H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.2, 149.4, 142.1, 134.4, 132.8, 129.4, 128.9, 128.3, 127.5, 127.4, 126.8, 125.7, 119.6, 114.5, 65.6, 45.0, 39.2, 35.4, 29.1, 29.0, 28.5. IR ν 3254, 3061, 2941, 2828, 1676, 1559, 1217, 827 cm-1. HRMS (ESI) m/z: calcd for C26H29N2O [M+H]+ 385.2274; found 385.2272.
N-octyl-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3aq)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 5:1 as the eluent). White solid (32.8 mg, 90% yield). m.p.: 129-130°C. 1H NMR (400 MHz, CDCl3, TMS) δ 7.60-7.57 (m, 1H), 7.32-7.27 (m, 2H), 7.25-7.19 (m, 2H), 7.15-7.12 (m, 1H), 6.92-6.87 (m, 4H), 4.97 (s, 1H), 3.85 (dt, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.28 (td, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.23-3.11 (m, 2H), 3.10-2.94 (m, 2H), 1.41-1.32 (m, 2H), 1.26-1.10 (m, 11H), 0.86 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.1, 149.4, 134.4, 132.8, 129.4, 128.9, 127.5, 127.4, 126.7, 119.5, 114.5, 65.6, 45.0, 39.5, 31.7, 29.5, 29.2, 29.1, 29.0, 26.8, 22.6, 14.1. IR ν 3264, 3060, 2950, 2847, 1673, 1425, 1220, 1053 cm-1. HRMS (ESI) m/z: calcd for C24H33N2O [M+H]+ 365.2587; found 365.2585.
N-dodecyl-2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carboxamide (3ar)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 5:1 as the eluent). White solid (32.3 mg, 77% yield). m.p.: 101-103°C. 1H NMR (400 MHz, CDCl3, TMS) δ 7.61-7.57 (m, 1H), 7.33-7.20 (m, 4H), 7.16-7.13 (m, 1H), 6.92-6.87 (m, 4H), 4.97 (s, 1H), 3.86 (dt, J = 10.8 Hz, J = 4.4 Hz, 1H), 3.30 (td, J = 10.8 Hz, J = 4.0 Hz, 1H), 3.25-3.11 (m, 2H), 3.10-2.94 (m, 2H), 1.43-1.12 (m, 20H), 0.88 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.1, 149.4, 134.4, 132.8, 129.4, 128.9, 127.5, 127.4, 126.7, 119.5, 114.5, 65.6, 44.9, 39.5, 31.9, 29.65, 29.64, 29.54, 29.51, 29.49, 29.4, 29.2, 29.0, 26.8, 22.7, 14.2. IR ν 3317, 3027, 2918, 2246, 1663, 1488, 904, 723 cm-1. HRMS (ESI) m/z: calcd for C28H41N2O [M+H]+ 421.3213; found 421.3212.
Ethyl (2-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carbonyl)glycinate (3as)
Purified by silica gel chromatography (petroleum ether/ethyl acetate 5:1 as the eluent). Colorless oil (31.1 mg, 92% yield). 1H NMR (400 MHz, CDCl3, TMS) δ 7.55-7.52 (m, 1H), 7.45-7.42 (m, 1H), 7.33-7.22 (m, 4H), 7.17-7.13 (m, 1H), 6.96-6.87 (m, 3H), 5.03 (s, 1H), 4.16-4.06 (m, 3H), 3.91-3.83 (m, 2H), 3.37-3.30 (m, 1H), 3.20-3.11 (m, 1H), 3.02-2.95 (m, 1H), 1.20 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3, TMS) δ 172.6, 169.6, 149.3, 135.0, 132.5, 129.4, 129.1, 127.6, 127.56, 126.7, 119.5, 114.4, 65.4, 61.5, 44.8, 41.3, 28.6, 14.1. IR ν 3298, 2918, 2250, 1598, 1318, 1036, 791, 692 cm-1. HRMS (ESI) m/z: calcd for C20H23N2O3[M+H]+ 339.1703; found 339.1701.
Acknowledgments
We are grateful for the financial support provided by the National Natural Science Foundation of China (22271108, 21871100, 21901079), the Fundamental Research Funds for the Central Universities (2017KFYXJJ166, 2019kfyRCPY096), Huazhong University of Science and Technology (HUST), Hubei Technological Innovation Project (2019ACA125), and the Opening Fund of Hubei Key Laboratory of Bioinorganic Chemistry and Materia Medica (No. BCMM201805). We also thank the Analytical and Testing Center of HUST, Analytical and Testing Center of the School of Chemistry and Chemical Engineering (HUST) for access to their facilities.
Author contributions
X-Y.T. conceived and supervised the study. S-J.W. performed the syntheses, the spectroscopic characterizations and the X-ray crystal diffraction analysis. S-J.W., L.W., and X-Y.T. analyzed the data and wrote the manuscript.
Declaration of interests
The authors declare no competing interest.
Published: March 30, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106528.
Supplemental information
Data and code availability
-
•
The original crystal structure of B(C6F5)3.H2O with 1aa has been deposited at CCDC and is publicly available as of the date of publication. CCDC number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper can be obtained from the lead contact upon request.
References
- 1.Stephan D.W., Erker G. Frustrated Lewis pairs: metal-free hydrogen activation and more. Angew. Chem. Int. Ed. 2010;49:46–76. doi: 10.1002/anie.200903708. [DOI] [PubMed] [Google Scholar]
- 2.Stephan D.W. Frustrated Lewis pairs: from concept to catalysis. Acc. Chem. Res. 2015;48:306–316. doi: 10.1021/ar500375j. [DOI] [PubMed] [Google Scholar]
- 3.Stephan D.W. Frustrated Lewis pairs. J. Am. Chem. Soc. 2015;137:10018–10032. doi: 10.1021/jacs.5b06794. [DOI] [PubMed] [Google Scholar]
- 4.Oestreich M., Hermeke J., Mohr J. A unified survey of Si-H and H-H bond activation catalysed by electron-deficient boranes. Chem. Soc. Rev. 2015;44:2202–2220. doi: 10.1039/C4CS00451E. [DOI] [PubMed] [Google Scholar]
- 5.Meng W., Feng X., Du H. Frustrated Lewis pairs catalyzed asymmetric metal-free hydrogenations and hydrosilylations. Acc. Chem. Res. 2018;51:191–201. doi: 10.1021/acs.accounts.7b00530. [DOI] [PubMed] [Google Scholar]
- 6.Basak S., Winfrey L., Kustiana B.A., Melen R.L., Morrill L.C., Pulis A.P. Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes. Chem. Soc. Rev. 2021;50:3720–3737. doi: 10.1039/D0CS00531B. [DOI] [PubMed] [Google Scholar]
- 7.Kumar G., Roy S., Chatterjee I. Tris(pentafluorophenyl)borane catalyzed C–C and C–heteroatom bond formation. Org. Biomol. Chem. 2021;19:1230–1267. doi: 10.1039/d0ob02478c. [DOI] [PubMed] [Google Scholar]
- 8.Welch G.C., San Juan R.R., Masuda J.D., Stephan D.W. Reversible, metal-free hydrogen activation. Science. 2006;314:1124–1126. doi: 10.1126/science.1134230. [DOI] [PubMed] [Google Scholar]
- 9.Parks D.J., Piers W.E., Parvez M., Atencio R., Zaworotko M.J. Synthesis and solution and solid-state structures of tris(pentafluorophenyl)borane adducts of PhC(O)X (X = H, Me, OEt, NPri2) Organometallics. 1998;17:1369–1377. doi: 10.1021/om9710327. [DOI] [Google Scholar]
- 10.Mömming C.M., Otten E., Kehr G., Fröhlich R., Grimme S., Stephan D.W., Erker G. Reversible metal-free carbon dioxide binding by frustrated Lewis pairs. Angew. Chem. Int. Ed. 2009;48:6643–6646. doi: 10.1002/anie.200901636. [DOI] [PubMed] [Google Scholar]
- 11.Otten E., Neu R.C., Stephan D.W. Complexation of nitrous oxide by frustrated Lewis pairs. J. Am. Chem. Soc. 2009;131:9918–9919. doi: 10.1021/ja904377v. [DOI] [PubMed] [Google Scholar]
- 12.Welch G.C., Coffin R., Peet J., Bazan G.C. Band gap control in conjugated oligomers via Lewis acids. J. Am. Chem. Soc. 2009;131:10802–10803. doi: 10.1021/ja902789w. [DOI] [PubMed] [Google Scholar]
- 13.Sajid M., Klose A., Birkmann B., Liang L., Schirmer B., Wiegand T., Eckert H., Lough A.J., Fröhlich R., Daniliuc C.G., et al. Reactions of phosphorus/boron frustrated Lewis pairs with SO2. Chem. Sci. 2013;4:213–219. doi: 10.1039/C2SC21161K. [DOI] [Google Scholar]
- 14.Henthorn J.T., Agapie T. Dioxygen reactivity with a Ferrocene–Lewis acid pairing: reduction to a boron peroxide in the presence of tris(pentafluorophenyl)borane. Angew. Chem. Int. Ed. 2014;53:12893–12896. doi: 10.1002/anie.201408462. [DOI] [PubMed] [Google Scholar]
- 15.Hansmann M.M., López-Andarias A., Rettenmeier E., Egler-Lucas C., Rominger F., Hashmi A.S.K., Romero-Nieto C. B(C6F5)3: a Lewis acid that brings the light to the solid state. Angew. Chem. Int. Ed. 2016;55:1196–1199. doi: 10.1002/anie.201508461. [DOI] [PubMed] [Google Scholar]
- 16.Ye K.-Y., Kehr G., Daniliuc C.G., Liu L., Grimme S., Erker G. Coupling of carbon monoxide with nitrogen monoxide at a frustrated Lewis pair template. Angew. Chem. Int. Ed. 2016;55:9216–9219. doi: 10.1002/anie.201603760. [DOI] [PubMed] [Google Scholar]
- 17.Tao X., Daniliuc C.G., Janka O., Pöttgen R., Knitsch R., Hansen M.R., Eckert H., Lübbesmeyer M., Studer A., Kehr G., Erker G. Reduction of dioxygen by radical/B(p-C6F4X)3 pairs to give isolable bis(borane)superoxide compounds. Angew. Chem. Int. Ed. 2017;56:16641–16644. doi: 10.1002/anie.201709309. [DOI] [PubMed] [Google Scholar]
- 18.Antoni P.W., Golz C., Holstein J.J., Pantazis D.A., Hansmann M.M. Isolation and reactivity of an elusive diazoalkane. Nat. Chem. 2021;13:587–593. doi: 10.1038/s41557-021-00675-5. [DOI] [PubMed] [Google Scholar]
- 19.Tamke S., Qu Z.-W., Sitte N.A., Flörke U., Grimme S., Paradies J. Frustrated Lewis pair-catalyzed cycloisomerization of 1,5-enynes via a 5-endo-dig cyclization/protodeborylation sequence. Angew. Chem. Int. Ed. 2016;55:4336–4339. doi: 10.1002/anie.201511921. [DOI] [PubMed] [Google Scholar]
- 20.Shang M., Cao M., Wang Q., Wasa M. Enantioselective direct mannich-type reactions catalyzed by frustrated Lewis acid/Brønsted base complexes. Angew. Chem. Int. Ed. 2017;56:13338–13341. doi: 10.1002/anie.201708103. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z.Y., Liu Z.Y., Guo R.T., Zhao Y.Q., Li X., Wang X.C. B(C6F5)3-Catalyzed ring opening and isomerization of unactivated cyclopropanes. Angew. Chem. Int. Ed. 2017;56:4028–4032. doi: 10.1002/anie.201700864. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y., Zhang L., Luo Y., Nishiura M., Hou Z. B(C6F5)3–Catalyzed C–Si/Si–H cross-metathesis of hydrosilanes. J. Am. Chem. Soc. 2017;139:12434–12437. doi: 10.1021/jacs.7b08053. [DOI] [PubMed] [Google Scholar]
- 23.Shang M., Chan J.Z., Cao M., Chang Y., Wang Q., Cook B., Torker S., Wasa M. C–H functionalization of amines via alkene-derived nucleophiles through cooperative action of chiral and achiral Lewis acid catalysts: applications in enantioselective synthesis. J. Am. Chem. Soc. 2018;140:10593–10601. doi: 10.1021/jacs.8b06699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Park S., Chang S. Catalytic access to bridged sila-N-heterocycles from piperidines via cascade sp3 and sp2 C−Si bond formation. J. Am. Chem. Soc. 2018;140:13209–13213. doi: 10.1021/jacs.8b08733. [DOI] [PubMed] [Google Scholar]
- 25.Han Y., Zhang S., He J., Zhang Y. B(C6F5)3-Catalyzed (convergent) disproportionation reaction of indoles. J. Am. Chem. Soc. 2017;139:7399–7407. doi: 10.1021/jacs.7b03534. [DOI] [PubMed] [Google Scholar]
- 26.Cabré A., Rafael S., Sciortino G., Ujaque G., Verdaguer X., Lledós A., Riera A. Catalytic regioselective isomerization of 2,2-disubstituted oxetanes to homoallylic alcohols. Angew. Chem. Int. Ed. 2020;59:7521–7527. doi: 10.1002/anie.201915772. [DOI] [PubMed] [Google Scholar]
- 27.Arslan M., Kiskan B., Yagci Y. Ring-opening polymerization of 1,3-benzoxazines via borane catalyst. Polymers. 2018;10:239. doi: 10.3390/polym10030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatano M., Sakamoto T., Mochizuki T., Ishihara K. Tris(pentafluorophenyl)borane-Assisted chiral phosphoric acid catalysts for Enantioselective Inverse-electron-demand hetero-diels-alder reaction of α,β-substituted acroleins. Asian J. Org. Chem. 2019;8:1061–1066. doi: 10.1002/ajoc.201900104. [DOI] [Google Scholar]
- 29.Retini M., Bartolucci S., Bartoccini F., Mari M., Piersanti G. Concise and convergent enantioselective total syntheses of (+)- and (-)-Fumimycin. J. Org. Chem. 2019;84:12221–12227. doi: 10.1021/acs.joc.9b02020. [DOI] [PubMed] [Google Scholar]
- 30.Cai L., Liu X., Wang J., Chen L., Li X., Cheng J.-P. Enantioselective and regioselective aza-Friedel-Crafts reaction of electron-rich phenols with isatinderived ketimines. Chem. Commun. 2020;56:10361–10364. doi: 10.1039/D0CC04966B. [DOI] [PubMed] [Google Scholar]
- 31.Ishihara H., Huang J., Mochizuki T., Hatano M., Ishihara K. Enantio- and diastereoselective carbonyl-Ene cyclization–acetalization tandem reaction catalyzed by tris(pentafluorophenyl)borane-assisted chiral phosphoric acids. ACS Catal. 2021;11:6121–6127. doi: 10.1021/acscatal.1c01242. [DOI] [Google Scholar]
- 32.Meng S.-S., Tang X., Luo X., Wu R., Zhao J.-L., Chan A.S.C. Borane-catalyzed chemoselectivity-controllable N-alkylation and ortho C-alkylation of unprotected arylamines using benzylic alcohols. ACS Catal. 2019;9:8397–8403. doi: 10.1021/acscatal.9b03038. [DOI] [Google Scholar]
- 33.Chen X., Patel K., Marek I. Stereoselective construction of tertiary homoallyl alcohols and ethers by nucleophilic substitution at quaternary carbon stereocenter. Angew. Chem. Int. Ed. 2023;62:e202212425. doi: 10.1002/anie.202212425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahdi T., Stephan D.W. Enabling catalytic ketone hydrogenation by frustrated Lewis pairs. J. Am. Chem. Soc. 2014;136:15809–15812. doi: 10.1021/ja508829x. [DOI] [PubMed] [Google Scholar]
- 35.Gyömöre Á., Bakos M., Földes T., Pápai I., Domján A., Soós T. Moisture-tolerant frustrated Lewis pair catalyst for hydrogenation of aldehydes and ketones. ACS Catal. 2015;5:5366–5372. doi: 10.1021/acscatal.5b01299. [DOI] [Google Scholar]
- 36.Scott D.J., Simmons T.R., Lawrence E.J., Wildgoose G.G., Fuchter M.J., Ashley A.E. Facile protocol for water-tolerant “frustrated Lewis pair”-catalyzed hydrogenation. ACS Catal. 2015;5:5540–5544. doi: 10.1021/acscatal.5b01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fasano V., Radcliffe J.E., Ingleson M.J. B(C6F5)3-Catalyzed reductive amination using hydrosilanes. ACS Catal. 2016;6:1793–1798. doi: 10.1021/acscatal.5b02896. [DOI] [Google Scholar]
- 38.Bergquist C., Bridgewater B.M., Harlan C.J., Norton J.R., Friesner R.A., Parkin G. Aqua, alcohol, and acetonitrile adducts of tris(perfluorophenyl)borane: evaluation of Brønsted acidity and ligand lability with experimental and computational methods. J. Am. Chem. Soc. 2000;122:10581–10590. doi: 10.1021/ja001915g. [DOI] [Google Scholar]
- 39.Danopoulos A.A., Galsworthy J.R., Green M.L.H., Doerrer L.H., Cafferkey S., Hursthouse M.B. Equilibria in the B(C6F5)3–H2O system: synthesis and crystal structures of H2O·B(C6F5)3 and the anions [HOB(C6F5)3]– and [(F5C6)3B(μ-OH)B(C6F5)3]–. Chem. Commun. 1998:2529–2560. doi: 10.1039/A804918A. [DOI] [Google Scholar]
- 40.Focante F., Camurati I., Resconi L., Guidotti S., Beringhelli T., D’Alfonso G., Donghi D., Maggioni D., Mercandelli P., Sironi A. Synthesis and reactivity of N-heterocycle-B(C6F5)3 complexes. 4. Competition between pyridine- and pyrrole-type substrates toward B(C6F5)3: structure and dynamics of 7-B(C6F5)3-7-azaindole and [7-Azaindolium]+[HOB(C6F5)3]- Inorg. Chem. 2006;45:1683–1692. doi: 10.1021/ic051285p. [DOI] [PubMed] [Google Scholar]
- 41.Dryzhakov M., Hellal M., Wolf E., Falk F.C., Moran J. Nitro-assisted brønsted acid catalysis: application to a challenging catalytic azidation. J. Am. Chem. Soc. 2015;137:9555–9558. doi: 10.1021/jacs.5b06055. [DOI] [PubMed] [Google Scholar]
- 42.Dryzhakov M., Moran J. Autocatalytic friedel–crafts reactions of tertiary aliphatic fluorides initiated by B(C6F5)3·H2O. ACS Catal. 2016;6:3670–3673. doi: 10.1021/acscatal.6b00866. [DOI] [Google Scholar]
- 43.Dryzhakov M., Richmond E., Li G., Moran J. Catalytic B(C6F5)3·H2O-promoted defluorinative functionalization of tertiary aliphatic fluorides. J. Fluor. Chem. 2017;193:45–51. doi: 10.1016/j.jfluchem.2016.11.005. [DOI] [Google Scholar]
- 44.Shibuya M., Okamoto M., Fujita S., Abe M., Yamamoto Y. Boron-catalyzed double hydrofunctionalization reactions of unactivated alkynes. ACS Catal. 2018;8:4189–4193. doi: 10.1021/acscatal.8b00955. [DOI] [Google Scholar]
- 45.San H.H., Wang S.-J., Jiang M., Tang X.-Y. Boron-catalyzed O–H bond insertion of α-aryl α-diazoesters in water. Org. Lett. 2018;20:4672–4676. doi: 10.1021/acs.orglett.8b01988. [DOI] [PubMed] [Google Scholar]
- 46.Bennett C.K., Bhagat M.N., Zhu Y., Yu Y., Raghuraman A., Belowich M.E., Nguyen S.T., Notestein J.M., Broadbelt L.J. Strong influence of the nucleophile on the rate and selectivity of 1,2-epoxyoctane ring opening catalyzed by tris(pentafluorophenyl)borane, B(C6F5)3. ACS Catal. 2019;9:11589–11602. doi: 10.1021/acscatal.9b02607. [DOI] [Google Scholar]
- 47.Bhagat M.N., Chang G.-F., Bennett C.K., Raghuraman A., Belowich M.E., Broadbelt L.J., Nguyen S.T., Notestein J.M. Improving and stabilizing fluorinated aryl borane catalysts for epoxide ring-opening. Applied Catalysis A, General. 2022;636:118601. doi: 10.1016/j.apcata.2022.118601. [DOI] [Google Scholar]
- 48.Bock C.W., Trachtman M., George P. An ab initio study of the influence of substituents and intramolecular hydrogen bonding on the carbonyl bond length and stretching force constant. I. Monosubstituted carbonyl compounds. J. Comput. Chem. 1981;2:30–37. doi: 10.1002/jcc.540020107. [DOI] [Google Scholar]
- 49.Lynch D.E., Reeves C.R. Statistical analysis of the effect of a single OH hydrogen-bonding interaction on carbonyl bond lengths. J. Mol. Struct. 2019;1180:158–162. doi: 10.1016/j.molstruc.2018.11.100. [DOI] [Google Scholar]
- 50.Janiak C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc., Dalton Trans. 2000:3885–3896. doi: 10.1039/B003010O. [DOI] [Google Scholar]
- 51.Hendrickson J.B. Molecular geometry. V. Evaluation of functions and conformations of medium rings. J. Am. Chem. Soc. 1967;89:7036–7043. doi: 10.1021/ja01002a036. [DOI] [Google Scholar]
- 52.Hu Y.-J., Li L.-X., Han J.-C., Min L., Li C.-C. Recent advances in the total synthesis of natural products containing eight-membered carbocycles (2009–2019) Chem. Rev. 2020;120:5910–5953. doi: 10.1021/acs.chemrev.0c00045. [DOI] [PubMed] [Google Scholar]
- 53.Gilli P., Pretto L., Bertolasi V., Gilli G. Predicting hydrogen-bond strengths from Acid−Base molecular properties. The pKaslide rule: toward the solution of a long-lasting problem. Acc. Chem. Res. 2009;42:33–44. doi: 10.1021/ar800001k. [DOI] [PubMed] [Google Scholar]
- 54.Buldashov I.A., Medvedev A.G., Mikhaylov A.A., Churakov A.V., Lev O., Prikhodchenko P.V. Non-covalent interactions of the hydroperoxo group in crystalline adducts of organic hydroperoxides and their potassium salts. CrystEngComm. 2022;24:6101–6108. doi: 10.1039/D2CE01017H. [DOI] [Google Scholar]
- 55.Shetty A.S., Zhang J., Moore J.S. Aromatic π-stacking in solution as revealed through the aggregation of Phenylacetylene macrocycles. J. Am. Chem. Soc. 1996;118:1019–1027. doi: 10.1021/ja9528893. [DOI] [Google Scholar]
- 56.Viglianti L., Leung N.L.C., Xie N., Gu X., Sung H.H.Y., Miao Q., Williams I.D., Licandro E., Tang B.Z. Aggregation-induced emission: mechanistic study of the clusteroluminescence of tetrathienylethene. Chem. Sci. 2017;8:2629–2639. doi: 10.1039/C6SC05192H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harbour J.R., Hair M.L. Detection of superoxide ions in nonaqueous media. Generation by photolysis of pigment dispersions. J. Phys. Chem. 1978;82:1397–1399. doi: 10.1021/j100501a015. [DOI] [Google Scholar]
- 58.Buettner G.R. Spin Trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987;3:259–303. doi: 10.1016/S0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 59.Gray B., Carmichael A.J. Kinetics of superoxide scavenging by dismutase enzymes and manganese mimics determined by electron spin resonance. Biochem. J. 1992;281:795–802. doi: 10.1042/bj2810795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weng M., Zhang M., Shen T. EPR studies of the photodynamic action of mercapto-substituted hypocrellin B derivatives: formation of semiquinone radical anion and activated oxygen on illumination with visible light. J. Photochem. Photobiol., A: Chem. 1997;108:159–167. doi: 10.1016/S1010-6030(97)00072-5. [DOI] [Google Scholar]
- 61.Aramaki Y., Imaizumi N., Hotta M., Kumagai J., Ooi T. Exploiting single-electron transfer in Lewis pairs for catalytic bond-forming reactions. Chem. Sci. 2020;11:4305–4311. doi: 10.1039/D0SC01159B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beringhelli T., Maggioni D., D’Alfonso G. 1H and 19F NMR investigation of the reaction of B(C6F5)3 with water in toluene solution. Organometallics. 2001;20:4927–4938. doi: 10.1021/om010610n. [DOI] [Google Scholar]
- 63.Xu M., Bunes B.R., Zang L. Paper-based vapor detection of hydrogen peroxide: colorimetric sensing with tunable interface. ACS Appl. Mater. Interfaces. 2011;3:642–647. doi: 10.1021/am1012535. [DOI] [PubMed] [Google Scholar]
- 64.Ugi I. Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem. 2001;73:187–191. doi: 10.1351/pac200173010187. [DOI] [Google Scholar]
- 65.Gligorovski S., Strekowski R., Barbati S., Vione D. Environmental implications of hydroxyl radicals (·OH) Chem. Rev. 2015;115:13051–13092. doi: 10.1021/cr500310b. [DOI] [PubMed] [Google Scholar]
- 66.Wang D., Weinstein A.B., White P.B., Stahl S.S. Ligand-Promoted palladium-catalyzed aerobic oxidation reactions. Chem. Rev. 2018;118:2636–2679. doi: 10.1021/acs.chemrev.7b00334. [DOI] [PubMed] [Google Scholar]
- 67.Thapa P., Corral E., Sardar S., Pierce B.S., Foss F.W. Isoindolinone synthesis: selective dioxane-mediated aerobic oxidation of isoindolines. J. Org. Chem. 2019;84:1025–1034. doi: 10.1021/acs.joc.8b01920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The original crystal structure of B(C6F5)3.H2O with 1aa has been deposited at CCDC and is publicly available as of the date of publication. CCDC number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper can be obtained from the lead contact upon request.