Abstract
During vascular development, endothelial cells (ECs) undergo arterialization in response to genetic programs and shear stress-triggered mechanotransduction, forming a stable vasculature. Although the Notch receptor is known to sense shear stress and promote EC arterialization, its downstream mechanisms remain unclear. In this study, the Notch downstream miR-342-5p was found to respond to shear stress and promote EC arterialization. Shear stress upregulated miR-342-5p in a Notch-dependent manner in human umbilical vein endothelial cells (HUVECs). miR-342-5p overexpression upregulated the shear stress-associated transcriptomic signature. Moreover, miR-342-5p upregulated arterial markers and promoted EC arterialization in a Matrigel plug assay and retinal angiogenesis model. In contrast, miR-342-5p knockdown downregulated arterial markers, compromised retinal arterialization, and partially abrogated shear stress and Notch activation-induced arterial marker upregulation. Mechanistically, miR-342-5p overexpression suppressed MYC to repress EC proliferation and promote arterialization, achieved by promoting MYC protein degradation by targeting the EYA3. Consistently, EYA3 overexpression rescued miR-342-5p-mediated MYC downregulation and EC arterialization. In vivo, miR-342-5p expression was notably decreased in the ligated artery in a hindlimb ischemia model, and an intramuscular injection of miR-342-5p promoted EC arterialization and improved perfusion. In summary, miR-342-5p, a mechano-miR, mediates the effects of shear stress-activated Notch on EC arterialization and is a potential therapeutic target for ischemic diseases.
Keywords: MT: Non-coding RNAs, endothelial cell, Notch, shear stress, miR-342-5p, arterialization
Graphical abstract

Yan and colleagues report that miR-342-5p mediates the effects of shear stress and Notch activation on promoting EC arterialization by repressing MYC via targeting EYA3, which holds potential in the treatment of ischemic diseases.
Introduction
Angiogenesis, the formation of new blood vessels from existing ones, involves the proliferation, differentiation, and migration of endothelial cells (ECs).1,2,3 Following the fusion of growing angiogenic sprouts, ECs gain an arterial phenotype and undergo further maturation, ultimately forming a stable hierarchical vessel network to perfuse tissues through a process called arterialization.2,4 Failure of arterialization leads to vascular malformation and dysfunction, including hereditary hemorrhagic telangiectasia (HHT) and cerebral cavernous malformations (CCMs).1,4 Therefore, there is a need to clarify the molecular mechanism that underlies and regulates EC arterialization.
Both genetic programs and environmental cues participate in EC arterialization,2 such as shear stress induced by blood flow, vascular endothelial growth factor receptor (VEGFR) signaling, and Notch signaling. Among these factors, the mechanotransduction of shear stress is crucial for arteriovenous specification and is necessary for the maintenance of the arterial identity of ECs.2,5 Indeed, the loss of systemic blood flow in chicks and mice results in defects in vascular remodeling and arteriovenous specification, as shown by decreased arterial gene expression and increased venous gene expression.6,7 Meanwhile, the expression of arterial genes, such as ephrin B2 (EFNB2), connexin 37 (CX37; also known as GJA4), and CX40 (also known as GJA5), is significantly induced in cultured ECs when exposed to physiological laminar shear stress (approximately 15 dyn/cm2).8,9 These findings indicate that EC arterialization is dependent on hemodynamic flow; however, the downstream signaling mechanism underlying shear stress-induced arterialization remains largely unclear.
Previous studies have identified multiple mechanotransduction pathways in ECs. The Notch signaling pathway, which is composed of Notch receptors (Notch1-4), Notch ligands (Delta-like [Dll] 1, 3, 4; Jagged [Jag] 1, 2) on neighboring cells, and the downstream transcription factor recombination signal binding protein Jκ (RBPj) in mammals, plays a central role in regulating arteriovenous specification during embryonic development.10,11 Notch activation cell-autonomously promotes EC arterialization by inducing the expression of arterial genes,12,13 and a lack of Notch signaling leads to the increased expression of venous genes in arterial areas.14 Notably, Notch1 was recently identified as a mechanotransduction sensor of blood flow-derived shear stress in vessel ECs, and shear stress-triggered Notch activation enhances barrier function by upregulating gap junction proteins, such as GJA4, suppressing EC proliferation, and promoting arterial specification.15,16 A recent study demonstrated that Notch activation promotes arterialization by inhibiting MYC-dependent cell cycle progression and metabolism.17 However, the downstream mechanism of the Notch-induced arterialization of ECs is not yet fully understood.
A panel of microRNAs (miRNAs) have been found to mediate the effects of shear stress on regulating EC proliferation, migration, and specification, known as mechano-miRs.18,19 We recently investigated the Notch downstream miRNAs in ECs and found that Notch activation downregulates MYC expression to suppress cell-cycle progression at least partly via miR-218-5p, targeting heterogeneous nuclear ribonucleoprotein1 (hnRNPA1) and eyes absent 3 (EYA3).20 miR-342-5p (denoted miR-342 for convenience) is located in the intron of the Ena-vasodilator-stimulated phosphoprotein-like (EVL) gene and is another Notch downstream miRNA that suppresses EC proliferation and angiogenesis.21,22,23 Hou et al. recently demonstrated that miR-342 can be upregulated by laminar shear stress in EC.24 However, whether the upregulation of miR-342 by shear stress is mediated by Notch signaling, as well as the role of miR-342 in mediating mechanotransduction via Notch signaling to promote EC arterialization, remain unclear.
In the present study, we evaluated the relationship between shear stress, Notch signaling, and miR-342 and their downstream mechanisms in the promotion of EC arterialization. Using cultured ECs under defined flow conditions, postnatal retinal angiogenesis, and a hindlimb ischemia model, miR-342 was identified as a mechano-miR that promotes EC arterialization and improves blood perfusion by attenuating MYC expression by targeting EYA3, a protein phosphatase 2A (PP2A) co-factor that promotes phosphorylation of MYC at S62, thereby stabilizing MYC. The insights provided by these results will aid in efforts to optimize vascular regeneration in ischemic disease.
Results
miR-342 is a mechano-miR and promotes EC response to shear stress
In our previous study, miR-342 was found to act as a downstream effector of Notch, also serving as a mechanotransduction receptor.15,22,25 Thus, we investigated whether miR-342 responds to flow-induced shear stress (FSS) by applying human umbilical vein endothelial cells (HUVECs) with laminar flow that generates 15 dyn/cm2 shear stress for 12 or 24 h using the ibidi system, according to previous studies.15,26,27,28 Compared with HUVECs cultured under static conditions, HUVECs cultured under the FSS condition for 12 h were aligned parallel to the flow direction, accompanied by the upregulation of KLF2 and KLF4, two transcription factors mediating mechanotransduction in ECs (Figures S1A and S1B).29 The results of qRT-PCR showed that miR-342, as well as its host gene EVL, were upregulated significantly under FSS for 12 and 24 h (Figure 1A). Notch activation by Dll4 treatment upregulated miR-342 expression in statically cultured HUVECs, which was blocked by DAPT (Figure S1C). Consistently, FSS activated Notch signaling, as shown by the upregulation of HES1, and upregulated miR-342 and EVL, which could also be abolished by DAPT (Figure 1B). These results indicate that shear stress upregulates miR-342 expression via Notch activation in HUVECs.
Figure 1.
Shear stress upregulates miR-342 via Notch signal
(A) HUVECs were cultured under static or 15 dyne/cm2 FSS for 12 or 24 h. The expression of miR-342 and its host gene EVL was determined using qRT-PCR (n = 3). (B) HUVECs were cultured as in (A) in the presence of DAPT or DMSO for 12 h. The expression of HES1, miR-342, and EVL was determined using qRT-PCR (n = 5). (C) HUVECs were transfected with miR-342 mimic or control for 48 h, and subjected to RNA-seq. GSEA of shear stress responsive transcripts. (D) HUVECs were treated as in (C). The expression level of shear stress related genes was compared using a heatmap. (E) HUVECs were treated as in (C). The expression of KLF4 and KLF2 was determined using qRT-PCR (n = 3). (F) HUVECs were treated as in (C). The expression of KLF4 was determined using western blotting (n = 4). (G) HUVECs were infected with lentivirus expressing miR-342 sponge with GFP or control and exposed to FSS for 12 h. The EC morphology was captured under a fluorescence microscope. The length and width per cell were measured and compared (n represents 16 fields from 3 samples in each group). (H) HUVECs were treated as in (G). The mRNA level of KLF4 and KLF2 was evaluated using qRT-PCR (n = 4). (I) HUVECs were treated as in (G). The protein level of KLF4 was determined using western blotting (n = 4). Bars denote mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. p values were calculated using Student’s t test (A, E, and F) and one-way ANOVA (Tukey post hoc test) (B, G, H, and I).
To evaluate the role of miR-342 in ECs under FSS, HUVECs were transfected with miR-342 mimic or nonsense control for 48 h and subjected to RNA sequencing (RNA-seq) (Figures S1D and S1E). Gene set enrichment analysis (GSEA) and heatmap analyses showed that miR-342 overexpression upregulated a shear stress-related gene signature in statically cultured HUVECs (Figures 1C and 1D). qRT-PCR and western blotting confirmed the upregulation of KLF2 and KLF4 by miR-342 transfection (Figures 1E and 1F). Next, we evaluated whether miR-342 was required for HUVECs to respond to FSS. HUVECs were transduced with lentivirus expressing miR-342 sponge and cultured under FSS for 12 h (Figure S1F). We found that HUVECs with reduced miR-342 expression failed to align in parallel under laminar flow, indicated by differences in the cell-axis ratio (Figure 1G). In addition, miR-342 blockade significantly downregulated KLF4 and KLF2 expression under laminar flow (Figures 1H and 1I). These results suggest that miR-342 mediates the response of ECs to FSS.
miR-342 overexpression upregulates the expression of arterial genes
FSS activates Notch signaling, which upregulates arterial markers, such as GJA4, and promotes arterial specification.15,16 Consistently, the transcriptome of HUVECs overexpressing miR-342 exhibited a markedly increased expression of arterial markers, including EFNB2, GJA4, GJA5, and HEY2, accompanied by a decreased expression of venous markers, including EPHB4, NRP2, and NR2F2 (Figures 2A, 2B, and S2A).8,30 qRT-PCR and western blotting confirmed these findings (Figures 2C and 2D). In addition, we overexpressed miR-342 under FSS. The qRT-PCR results showed that miR-342 overexpression also upregulated the expression of arterial genes and downregulated the expression of venous genes under FSS conditions (Figure S2B). In contrast to the overexpression results, miR-342 blockade downregulated arterial markers and upregulated venous markers at both the mRNA and protein levels in statically cultured HUVECs (Figures 2C and 2D). In vivo, the fluorescence in situ hybridization (FISH) staining of mouse tissues with an miR-342 probe and anti-CD31 showed that the miR-342-positive signal was mostly located in the arteries of the kidney, liver, and lung tissues compared with the capillary vessels (Figure S2C). These results suggest that miR-342 mediates the effects of shear stress on the enhancement of arterial gene expression.
Figure 2.
miR-342 overexpression upregulates arterial gene expression in vitro
(A) HUVECs were transfected with miR-342 mimic or control for 48 h and subjected to RNA-seq 48 h later. GSEA of arterial identity transcripts. (B) Heatmap analysis of the expression level of arterial and venous identity genes between Ctrl and miR-342 overexpression groups. (C and D) HUVECs were transfected with miR-342 mimic or control (Ctrl) or infected with lentivirus expressing miR-342 sponge (anti-342) or nonsense control (NC) for 48 h. The mRNA and protein levels of arterial and venous genes were determined and compared using qRT-PCR (C) (n represents at least 6) and western blotting (D) (n represents at least 5), respectively. Bars denote mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001; n.s, not significant. p values were calculated using one-way ANOVA (Tukey post hoc test) (C and D).
miR-342 promotes EC arterialization in vivo
To further explore the role of miR-342 in regulating EC arterialization in vivo, we implanted Matrigel plugs incorporated with miR-342 agomir or control in mice and examined angiogenesis in Matrigel seven days later. Masson’s staining showed that miR-342 repressed angiogenesis by decreasing the vessel area per field (Figure S3A). Meanwhile, immunostaining showed that miR-342 markedly increased the colocalization of the EC marker CD31 and arterial marker GJA4 or vascular smooth muscle cell (vSMC) marker α-SMA, suggesting increased arterial marker expression and vSMC coverage (Figures 3A and 3B).
Figure 3.
miR-342 enhances EC arterialization in vivo
(A and B) C57BL/6 mice were subcutaneously injected with Matrigel containing miR-342 agomir or control. Seven days later, Matrigel plugs were harvested and sectioned. Sections were immunostained with CD31 plus GJA4 (A) or α-SMA (B). The percentages of GJA4/CD31 and α-SMA/CD31 were quantitatively compared (n = 4). (C) Immunofluorescence staining with IB4 (green) plus α-SMA (red) of P5 retina intravitreally injected with miR-342 agomir or nonsense control. The percentage of α-SMA/IB4 was quantitatively compared (n = 7). (D) Immunofluorescence staining with IB4 (green) plus α-SMA (red) of P5 retina intravitreally injected with miR-342 antagomir or nonsense control (NC). The percentage of α-SMA/IB4 was quantitatively compared (n = 7). Bars denote mean ± SD. ∗p < 0.05, and ∗∗p < 0.01. p values were calculated using Student’s t test (A–D).
We further evaluated miR-342 in regulating arterialization using the retinal angiogenesis model via the intravitreal injection of miR-342 agomir,31 which increased the miR-342 levels significantly compared with the control (Figure S3B). Immunostaining of IB4, together with α-SMA, indicated that, accompanied by reduced vessel density (Figure S3C), miR-342 upregulation markedly increased α-SMA+ vSMCs in the retinal vasculature, suggesting increased vSMC coverage, which is a marker of arterialization (Figure 3C). The expression of arterial markers increased, while the venous markers decreased prominently in miR-342-injected retinas, as determined using qRT-PCR with CD31 as an internal reference (Figure S3D). In contrast, the intravitreal injection of the miR-342 antagomir significantly reduced the α-SMA+ vSMC coverage while slightly increasing the retinal vascular density compared with the control (Figures 3D and S3E). These results suggest that miR-342 promotes arterialization in vivo.
miR-342 mediates the FSS- and Notch-induced upregulation of arterial markers
To evaluate the relationship between miR-342 and FSS in the context of EC arterialization regulation, HUVECs were infected with either the lentivirus expressing miR-342 sponge or the nonsense control and subjected to FSS for 12 h. qRT-PCR showed that miR-342 blockade partially abolished the FSS-induced upregulation of arterial markers (Figure 4A), which was further validated by western blotting (Figure 4B). These results suggest that miR-342 mediates the FSS-induced upregulation of arterial markers in ECs.
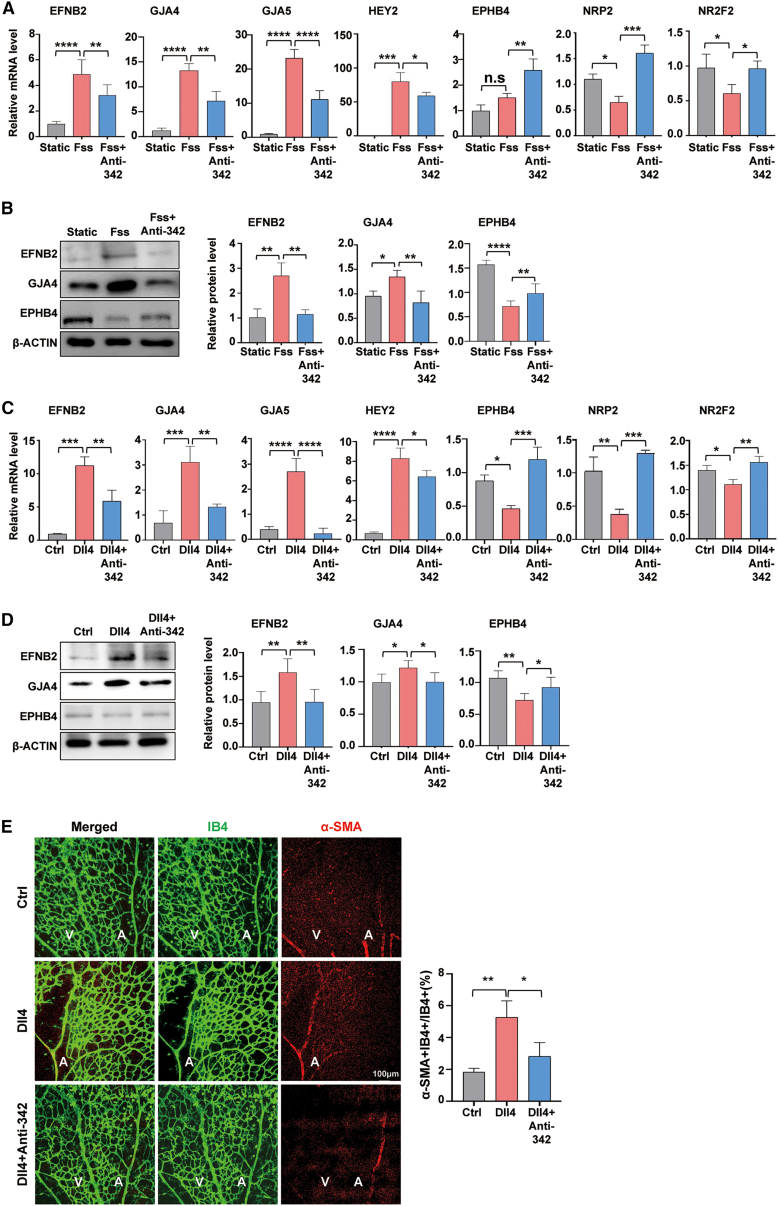
Figure 4.
FSS and Notch activation promote EC arterialization via miR-342
(A and B) HUVECs were infected with lentivirus expressing with miR-342 sponge or control and cultured under static or FSS for 12 h. The expression of arterial and venous genes was determined using qRT-PCR (A) (n = 4) and western blotting (B) (n = 4), respectively. (C and D) HUVECs were infected with lentivirus expressing with miR-342 sponge or control, and then cocultured with Dll4 recombinant protein or PBS for 48 h. The expression of arterial and venous genes was determined using qRT-PCR (C) (n = 6) and western blotting (D) (n = 9). (E) Pups were subcutaneously injected with Dll4 or PBS (Ctrl) from P1 to P3. miR-342 antagomir or control were then intravitreally injected at P3. Retinas were harvested and immunostained with Isolectin B4 plus α-SMA. The ratio of vessels covered with α-SMA positive vSMCs was determined and compared (n = 6). Bars denote mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. p values were calculated using one-way ANOVA (Tukey post hoc test) (A–E).
Notch activation by shear stress promotes the expression of arterial markers and arterialization, and blocking Notch activation abrogates the FSS-induced upregulation of arterial markers (Figure S4A).14,15 To assess the role of miR-342 in mediating Notch activation-induced EC arterialization, we infected HUVECs with lentivirus expressing miR-342 sponge or nonsense control, and cultured cells on Dll4-coated plates to trigger Notch activation. The results showed that while Notch activation upregulated arterial markers and downregulated venous markers at both the mRNA and protein levels, miR-342 knockdown blunted these effects (Figures 4C and 4D). We subcutaneously injected the soluble Dll4 recombination protein in pups from postnatal days 1–3 (P1–P3), and intravitreally injected miR-342 antagomir or control on P3. Retinal whole-mount staining showed that Dll4 injection significantly reduced the vascular density, which was blocked by miR-342 knockdown (Figure S4B). Notably, miR-342 knockdown attenuated arterialization by Dll4-triggered Notch activation, as shown by the reduced α-SMA+ vSMC coverage in the retinal vasculature (Figure 4E). In summary, miR-342 mediates EC arterialization under Notch activation triggered by FSS.
miR-342 suppresses MYC activity and cell cycle progression
Notch activation promotes EC arterialization by suppressing MYC-dependent metabolic and cell cycle activities.17 An analysis of the transcriptome of HUVECs transfected with the miR-342 mimic showed that miR-342 overexpression significantly repressed MYC regulon and cell cycle progression-related genes (Figures 5A and 5B), in addition to attenuated mTORC1 signaling, DNA repair, and glycolysis (Figures S5A–S5C). qRT-PCR and western blotting confirmed that miR-342 overexpression downregulated MYC downstream genes (Figures 5C and 5D). Consistently, cell cycle progression was blunted in HUVECs transfected with miR-342 mimic (Figures S5D and S5E). In addition, transfection with miR-342 sponge rescued the Notch activation-mediated downregulation of MYC downstream genes (Figure S5F). These findings suggest that miR-342 attenuates EC cell cycle progression likely by repressing MYC expression.
Figure 5.
miR-342 suppresses MYC activity and cell cycle progression
(A) GSEAs of MYC activity and cell cycle progression between miR-342 overexpression and control groups. (B) Heatmap shows the MYC and its targets between miR-342 overexpression and control groups. (C) HUVECs were transfected with miR-342 mimic or control for 48 h. The expression level of MYC-dependent cell cycle progression-related genes was determined using qRT-PCR (n = 3). (D) HUVECs were treated as in (C). The protein level of RRM2 and CDK2 was evaluated using western blotting (n = 4). Bars denote mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. p values were calculated using Student’s t test (C and D).
miR-342-induced EC arterialization is abolished by MYC overexpression
To further validate the role of MYC in miR-342-induced EC arterialization, we examined MYC expression in HUVECs overexpressing miR-342. MYC expression was found to decrease under FSS and Notch activation (Figures S6A–S6C).17 Consistently, miR-342 overexpression significantly downregulated the mRNA and protein levels of MYC in HUVECs (Figures 6A and 6B). The intravitreal injection of the miR-342 agomir also downregulated MYC mRNA in the retinas (Figure S6D). In contrast, miR-342 knockdown upregulated the MYC mRNA and protein levels (Figures 6A and 6C). Furthermore, the miR-342 blockade abolished the repression of MYC expression by Notch activation (Figure S6E). These results suggest that miR-342 mediates the inhibition of MYC by Notch activation, which is triggered by FSS.
Figure 6.
MYC overexpression abolishes miR-342-induced EC arterialization
(A) HUVECs were transfected with miR-342 mimic or control or infected with lentivirus expressing miR-342 sponge and control for 48 h. The expression of MYC was determined using qRT-PCR (n = 6). (B) HUVECs were transfected with miR-342 mimic or control for 48 h. The protein level of MYC was evaluated using western blotting (n = 4). (C) HUVECs were infected with lentivirus expressing miR-342 sponge and control for 48 h. The protein level of MYC was measured using western blotting (n = 3). (D and E) HUVECs were treated as in (B) in the presence of adenovirus expressing MYC or control for 48 h and subjected to cell cycle analysis using flow cytometer. The percentage of G0/G1, S, and G2/M phases was determined and compared (n = 3). (F and G) HUVECs were treated as in (D), and the expression level of arterial and venous identity genes was evaluated using qRT-PCR (F) (n represents at least 4) and western blotting (G) (n = 9). Bars denote mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. p values were calculated using Student’s t test (A–C) and one-way ANOVA (Tukey post hoc test) (E–G).
Next, we transfected HUVECs with the miR-342 mimic and simultaneously infected the cells with an adenovirus expressing MYC (Figure S6F). The results showed that MYC overexpression significantly promoted cell cycle progression (Figures 6D and 6E). Moreover, MYC overexpression abrogated miR-342 overexpression-induced EC arterialization, as shown by the repressed mRNA and protein expression of multiple arterial markers, while the expression of venous markers was recovered (Figures 6F and 6G). These results confirm that miR-342 enhances arterialization by inhibiting MYC.
miR-342 attenuates MYC by directly targeting EYA3
MYC expression is regulated at multiple levels.32,33 Bioinformatic analysis has shown that MYC is not a direct target of miR-342. However, the 3′-UTR of EYA3, which increases MYC protein stability by dephosphorylating threonine 58 (Thr58) and phosphorylating serine 62 (Ser62) of MYC via protein phosphatase 2A,34,35 harbors an miR-342 recognition site (Figure S7A). Next, we assessed whether miR-342 influences MYC stability. Indeed, treatment with MG132, a proteasome inhibitor, almost completely rescued the miR-342 overexpression-induced MYC protein downregulation in HUVECs (Figure 7A). Consistently, blocking translation with cycloheximide (CHX) showed that MYC protein decay was accelerated significantly under miR-342 overexpression (Figure 7B).20 Further analysis demonstrated that the half-life of MYC protein and the area under the curve (AUC) were both decreased in the miR-342 overexpression group (Figure S7B). Moreover, miR-342 overexpression markedly increased MYC phosphorylation at Thr58 and decreased MYC phosphorylation at Ser62, accompanied by reduced MYC protein levels in HUVECs (Figure 7C). These results indicate that miR-342 downregulates MYC, at least in part, by promoting MYC degradation via EYA3-mediated MYC phosphorylation.
Figure 7.
miR-342 promotes MYC protein degradation via directly targeting EYA3
(A) HUVEC transfected with miR-342 mimic or control, in the presence of MG132 or DMSO for 48 h. The protein level of MYC was measured using western blotting (n represents at least 4). (B) HUVECs were transfected as in (A) for 48 h. The protein level of MYC was determined using western blotting at different time points after addition of CHX (n represents at least 4). (C) HUVECs were transfected as in (A). The phosphorylation of threonine 58 (pT58) and serine 62 (pS62) sites and MYC protein level were evaluated using western blotting (n = 5). (D and E) HUVECs were transfected with miR-342 mimic or control, or infected with lentivirus expressing miR-342 sponge or control for 48 h. The mRNA and protein levels of EYA3 were determined using qRT-PCR (D) (n = 6) and western blotting (E) (n = 9), respectively. (F) Reporter assay. HEK293T cells were transfected with EYA3-3′UTR-wt or EYA3-3′UTR-mut plus phRL-TK, and co-transfected with miR-342 mimic or control, or infected anti-342 or control lentivirus for 48 h. Luciferase activity in cell lysates was evaluated with a dual-luciferase reporter assay system (n = 12). (G) HUVECs were transfected as in (A) and infected with lentivirus expressing EYA3 or control for 48 h. The phosphorylation of pT58 and pS62 of MYC and total protein level of MYC was measured using western blotting (n = 3). (H) HUVECs were treated as in (G). The protein level of EFNB2, GJA4, and EPHB4 was determined using western blotting (n = 9). Bars denote mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. p values were calculated using Student’s t test (B–E) and one-way ANOVA (Tukey post hoc test) (A and F–H). n.s., not significant.
The transfection of miR-342 mimic downregulated EYA3 expression at both the mRNA and protein levels, evaluated using qRT-PCR and western blotting, while infection with the lentivirus expressing miR-342 sponge showed the opposite effects in HUVECs (Figures 7D and 7E). Consistently, FSS and Notch activation also downregulated EYA3 in HUVECs (Figures S7C–S7E). A reporter assay using the EYA3 3′-UTR showed that miR-342 inhibited reporter gene expression, while the mutation of the putative miR-342-binding site canceled this effect (Figure 7F). These results suggest that EYA3 is directly regulated by miR-342.
To further confirm the role of EYA3 in miR-342-mediated arterialization, we transduced HUVECs overexpressing miR-342 with an adenovirus that overexpressed EYA3 (Figure S7F). Western blotting showed that EYA3 overexpression abrogated miR-342 overexpression-induced Thr58 phosphorylation and Ser62 dephosphorylation of MYC, and increased MYC protein levels in HUVECs (Figure 7G). Furthermore, EYA3 overexpression abolished miR-342-induced EC arterialization, as shown by the downregulation of arterial markers and the upregulation of the venous marker EPHB4 in HUVECs (Figures 7H and S7G). Taken together, these results suggest that miR-342 promotes EC arterialization by directly targeting EYA3 to downregulate MYC.
miR-342 promotes vessel perfusion in the hindlimb ischemia model
EC arterialization is a vital step in vessel perfusion after ischemic injury, a common pathology in many human diseases.4,36 To evaluate whether miR-342 could be used to improve vessel perfusion after tissue ischemia, we established a hindlimb ischemia model by ligating the right femoral artery in mice, with the non-ligated side as a control.37 The operation led to decreased blood flow, as shown with a laser Doppler perfusion imaging system (Figure S8A). The expression of miR-342 and its host gene EVL was measured in the left and right femoral arteries of mice using qRT-PCR. The results showed that the expression of miR-342 and EVL was significantly decreased in the ligated femoral artery (Figure S8B). FISH staining further demonstrated that miR-342 expression in ECs was markedly reduced on the ligated side (Figure 8A). We then intramuscularly injected the miR-342 agomir or control on multiple sides, and measured the blood flow on days 7 and 14 after the operation and injection. The results showed that the miR-342 agomir injection significantly improved vessel perfusion on days 7 and 14 after the insult (Figure 8B). Furthermore, immunostaining of the gastrocnemius and semitendinosus muscles showed that in CD31+ ECs, GJA4 expression and the colocalization of CD31+ ECs and α-SMA+ vSMCs were significantly downregulated upon ligation compared with the non-ligated group, while miR-342 injection notably improved the downregulated arterialization (Figures 8C, 8D, S8C, and S8D). These results suggest that local miR-342 injection may promote vessel perfusion by enhancing EC arterialization in ischemic diseases.
Figure 8.
miR-342 promotes EC arterialization and increases blood perfusion in a hindlimb ischemia model
(A) The right femoral artery of C57BL/6 mice were ligated for 24 h. The femoral arteries were isolated and subjected to FISH staining with miR-342 probes and CD31 antibody. The miR-342 expression level was observed under a fluorescence microscope and compared between the non-ligated and ligated femoral arteries (n = 3). (B) The hindlimb ischemia model was built and the miR-342 agomir or control was intramuscularly injected. The blood flow was detected compared at different time points (n = 5). (C and D) The sections of gastrocnemius (GM) and semitendinousus (SM) muscles were immunostained with the CD31 plus GJA4 or α-SMA. The percentage of GJA4/CD31 (C) or α-SMA/CD31 (D) was determined and compared among the non-ligated, ligated plus Ctrl, and ligated plus miR-342 groups (n = 4). Bars denote mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. p values were calculated using Student’s t test (A and B) and one-way ANOVA (Tukey post hoc test) (C and D).
Discussion
Angiogenesis is the main approach for the remodeling of embryonic vessels and the regeneration of postnatal vessels under both physiological and pathological conditions.1,2 To guarantee the functionality of newly generated vessels, ECs constituting angiogenic sprouts, which are believed to be derived primarily from venous ECs, undergo arterialization to acquire the arterial EC phenotype, which is characterized by higher rates of vSMC recruitment, enhanced barrier function, cell cycle quiescence, and the expression of a panel of arterial markers. Shear stress induced by blood flow is required for arterial differentiation and maintenance.2,5 Although ECs express several types of mechanotransduction receptors, Notch signaling, which is activated by blood flow-derived shear stress, plays a pivotal role in EC arterial differentiation during angiogenesis, as well as embryonic vascularization.15 In this study, the blockade of Notch signaling by DAPT was found to abolish the EC arterialization induced by FSS. However, changes in the arterial and venous genes under FSS or Notch activation by Dll4 treatment did not completely overlap. The reasons for these differences may be two-fold. First, in addition to the Notch receptor, many other membrane mechanosensors can sense FSS and mediate its effects on the EC transcriptome.2 Second, studies that clarify whether FSS activates Notch signaling dependent on Dll4 ligand are currently lacking.38 This suggests that the downstream effects of Notch activation induced by FSS or Dll4 may be different, and more in-depth studies are needed to explore these differences.2
The Notch pathway regulates angiogenesis in almost all steps with different downstream effectors.10 For instance, Notch activation by FSS upregulates GJA4, which increases the expression of p27 to induce cell-cycle arrest and EC arterialization.15 We recently identified a panel of miRNAs that mediate the action of Notch signaling in regulating EC proliferation, migration, and sprouting.19,20,22 In the present study, miR-342 was found to serve as a mechano-miR and mediated Notch activation-induced EC arterialization. In cultured ECs, FSS upregulated miR-342 and its host gene EVL in a Notch-dependent manner. miR-342 overexpression in ECs upregulated FSS-responsive genes and promoted arterialization, whereas miR-342 knockdown attenuated the EC response to FSS and arterialization. Therefore, we concluded that miR-342 functions downstream of Notch signaling in response to FSS and promotes EC arterialization (Figure S9). However, the upregulation of KLF4 by miR-342 seems to be more subtle compared with under the FSS condition, which could be attributed to the multiple downstream miRNAs of FSS and the various targets of a specific miRNA.18 Furthermore, miR-342 regulation by Notch appears to be cell type-dependent, as Notch activation upregulates miR-342 in ECs and macrophages but represses EVL/miR-342 in neural stem cells.22,23 Further studies will be required to elucidate the precise mechanism underlying the Notch-mediated upregulation of miR-342 in ECs.
A recent study attributed Notch activation-induced EC arterialization to attenuated MYC, resulting in metabolic changes and cell-cycle arrest in ECs.17 However, the molecular mechanism underlying Notch activation-mediated MYC downregulation is a topic of controversy. Our results demonstrate that miR-342 mediates the effects of FSS and Notch activation on EC arterialization by attenuating MYC expression (Figure S9).17,39 In terms of the corresponding mechanism, miR-342 promotes MYC protein degradation by targeting EYA3, which serves as a component of PP2A protein phosphatase to dephosphorylate MYC at Thr58 instead of Ser62, thereby enhancing MYC protein stability.34 PP2A, the major serine/threonine phosphatase that dephosphorylates a large array of cell proteins, consists of a catalytic (C) subunit, a structural (A) subunit, and a regulatory/variable B-type subunit. The regulatory subunit is constituted of four subfamilies, and the B56α protein of the B′ subfamily of regulatory subunits directs PP2A to MYC, leading to the dephosphorylation of Ser62 and the proteasome-dependent degradation of MYC. EYA3 counteracts this effect by binding to B55α, leading to the blunted dephosphorylation of MYC at Thr58 and increased MYC stability. miR-342 further downregulates EYA3 by targeting its 3′-UTR, ultimately reducing MYC stability, as shown in the present study. However, the transcription of MYC also decreased significantly under miR-342 overexpression, although the declining level of mRNA was lower than the protein level (26.34% ± 4.317% vs. 37.52% ± 4.012%). Both the literature and our previous studies have shown that miR-342 is a multifunctional miRNA involved in cancer growth, immune responses, and osteoblast development, among others.40,41,42,43 In the cardiovascular system, several reports have shown that miR-342, or that secreted by exosomes, represses angiogenesis and plays a protective role in atherosclerosis.22,24,44 In previous studies, we have shown that endoglin is a direct target of miR-342 in ECs, which is also required for vascular development by regulating flow-induced EC migration and VEGF signaling.45,46 Therefore, it is likely that other mechanisms may also be involved in miR-342-mediated MYC downregulation and EC arterialization, especially the regulation of MYC transcription, which will require further exploration.
We recently reported that miR-218, another Notch downstream miRNA, also targets MYC to repress angiogenesis.20 The miR-218 gene is harbored in intron 14 of the Slit2 gene, and its expression in ECs is upregulated by Notch activation. miR-218 attenuates MYC expression via several parallel mechanisms, including reducing MYC mRNA levels, repressing MYC mRNA translation, and increasing MYC protein degradation.20 Mechanistically, miR-218 can also target EYA3 at its 3′-UTR to compromise the stability of MYC protein via PP2A. Interestingly, our unpublished data indicate that FSS upregulated miR-218. Therefore, it appears that FSS-induced Notch activation can downregulate MYC via both miR-342 and miR-218. This could be of significance in the regulation of EC behaviors by MYC since recent findings have suggested that different levels of MYC determine the output of cellular behavior.17,47 Whether Notch activation stoichiometrically inhibits MYC via these two miRNAs remains to be elucidated.
EC arterialization and the maintenance of arterial characteristics are extremely important for homeostasis, regeneration, and function of blood vessels, which are continuously exposed to various biomechanical stressors.2 In the present study, we evaluated the potential application of miR-342 agomir in treating ischemic diseases using the hindlimb ischemia model in mice. The results are encouraging, as the injection of miR-342 agomir directly into the affected muscles notably promoted EC arterialization and improved blood flow, suggesting that miR-342 could be a potential target for treating ischemia-related diseases.48 However, before translating these findings into clinical applications, the indication, dosage, and possibilities of the vessel-specific delivery and use of lipid nanoparticles (LNPs) as a carrier need to be investigated.49,50 In addition, the maintenance of arterial EC characteristics by laminar shear stress plays a pivotal role in the integrity of large arterial vessels.51 The disruption of EC integrity at the bending and branching sites of large arteries by turbulent shear stress is considered a major factor of atherosclerosis pathology at these sites.5 Given the role of miR-342, and most likely miR-218 as well, in promoting the arterial EC phenotype characterized by more vSMC recruitment, enhanced barrier function, cell cycle quiescence, future studies will need to determine the effect of these miRNAs in the pathogenesis and treatment of atherosclerosis.
Among the limitations of this study, the shear stress level (15 dyn/cm2) loaded artificially in this study has been demonstrated to be high for venous EC and fetal and adult arteries,27,52 and may lead to altered cell viability and an increased expression of inflammatory factors.53,54 Further investigation is needed to evaluate the response of miR-342 under more physiological shear stress levels in vivo. Moreover, the precise expression and function of miR-342 in different EC subtypes, especially arterial and venous ECs in different tissues and organs, requires further validation under different physiological conditions.
Conclusion
Our results reveal a mechanism underlying shear stress- and Notch-mediated endothelial arterialization, namely that miR-342 acts as a mechano-miR that mediates the shear stress-Notch signaling axis to promote endothelial arterialization by downregulating MYC via directly targeting EYA3. Therefore, miR-342 could be a potential target for the treatment of ischemic diseases.
Materials and methods
Animals
For retinal angiogenesis analysis, C57BL/6 pups of P3 were intravitreally injected with synthetic miR-342 agomir or antagomir (0.2 nmol) (RiboBio, Guangzhou, China) in 0.5 μL of RNase-free PBS in one eye and with corresponding nonsense control in the other.20 Retinas were collected on P5, fixed in 4% paraformaldehyde (PFA) for 4 h, and then blocked and permeabilized in PBS containing 1% BSA and 0.5% Triton X-100 at 4°C overnight, followed by immunostaining, as described below. For Notch activation in vivo, recombinant soluble Delta-like 4 protein (1 μg per pup) (R&D, Minneapolis, MN) was subcutaneously (s.c.) injected daily from P1 to P3.
For the Matrigel plug assay, 0.3 mL Matrigel containing 400 ng/mL VEGF, 250 ng/mL basic fibroblast growth factor (bFGF) (Sino Biological, Beijing, China), and miR-342 agomir or nonsense control (8 μg/mL) was implanted subcutaneously in C57BL/6 mice (6–8 weeks old) along the dorsal midline on each side of the body. On day 7, the Matrigel plugs were harvested for subsequent immunostaining.55
A hindlimb ischemia model was established by ligating the right femoral artery of C57BL/6 mice (6–8 weeks old). miR-342 agomir or nonsense control was intramuscularly injected 24 h later at six sites into the ischemic hindlimb distal to the arterial occlusion site.37,39 Three injections were performed in the upper limb in proximity to the semimembranosus muscles, and the remaining three injections were performed in the gastrocnemius and flexor digitorum muscles. Blood flow was evaluated on days 0, 7, and 14 using a laser Doppler perfusion imaging system (RWD, Guangzhou, China). On day 14, the mice were sacrificed and the ischemic muscles were harvested, fixed, and immunostained. All animal experiments were carried out following the guidelines issued by the Animal Experiment Administration Committee of the Fourth Military Medical University (20210182; January 20, 2021).
Cell culture and transfection
Primary HUVECs were isolated and cultured as previously described.22 Briefly, human umbilical cord biopsies obtained from the Department of Gynecology and Obstetrics in Tangdu Hospital were dispersed with 1 mg/mL type I collagenase (Sigma-Aldrich), and HUVECs were isolated and maintained in the EC medium (ECM) (ScienCell, San Diego, CA) containing 5% fetal calf serum (FCS), 1× EC growth supplement (ECGS), 100 U/mL ampicillin, and 100 mg/mL streptomycin. HUVECs were used between passages 3 and 6 for the experiments. The use of human samples was approved by the Ethics Committee of Tangdu Hospital, Fourth Military Medical University (202003-161; March 31, 2020).
To overexpress miR-342, HUVECs were transfected with the miR-342 mimic or nonsense control (50 nM; RiboBio) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. To downregulate miR-342, HUVECs were transfected with the miR-342 inhibitor or nonsense control (100 nM; RiboBio) using Lipofectamine 2000 or infected with a lentivirus expressing miR-342 sponge (anti-342) or control (Ctrl) (HanBio, Shanghai, China) at a multiplicity of infection (MOI) of 10. To overexpress MYC, HUVECs were infected with an adenovirus expressing MYC or control (AdMYC or AdCtrl) (HanBio, Shanghai, China) at an MOI of 100, as previously described.22 To overexpress EYA3, lentivirus expressing EYA3 (NM_001990.3) and control lentivirus were purchased from HanBio and used at an MOI of 10.
To block Notch signaling in vitro, a γ-secretase inhibitor DAPT (Selleck Chemicals, Houston, TX) was used at a concentration of 25 μM, with dimethyl sulfoxide (DMSO) as a control. To activate Notch signaling in vitro, the culture plates were pre-coated with soluble Delta-like 4 proteins (Sino Biological Inc., Beijing, China) at 4°C overnight (0.5 g/well), with PBS as a control. HUVECs (1 × 105/well) were seeded in the culture medium and cultured for 48 h. In some experiments, either DAPT or DMSO was added. For the protein decay assay, HUVECs were treated with 50 mg/mL cycloheximide (Selleck Chemicals), and the cells were harvested at 0, 20, 40, 60, and 80 min. Proteasome inhibitor MG-132 (Selleck Chemicals) was used at a concentration of 50 mM to inhibit protein decay.
Flow-induced shear stress on ECs
HUVECs were plated in a μ-Slide I 0.4 chamber (ibidi, #80176) and unidirectional laminar flow was loaded after 100% confluency with a pumping system (ibidi #10902). The flow device was maintained at 37°C in a humidified atmosphere with 5% CO2, and laminar shear stress was applied at 15 dyn/cm2 for 12 or 24 h, according to previous studies.15,26,27,28 For Notch signaling blockade, DAPT was added to the flow medium at a concentration of 25 μM. For miR-342 knockdown, ECs were infected with a lentivirus expressing a miR-342 sponge for 24 h before plating and flow initiation. The cell-axis ratio was calculated as the length/width (percentage) of each EC.
Immunostaining
For retinal vessel immunostaining, retinas were dissected, fixed in 4% PFA for 4 h at 4°C, blocked, and permeabilized in PBS containing 1% BSA and 0.5% Triton X-100 overnight. Samples were incubated with anti-α-SMA (1:200) (Abcam, Waltham, MA) in PBS containing 1% BSA and 0.5% Triton X-100 overnight at 4°C, followed by incubation with Alexa Fluor 594-conjugated donkey anti-rabbit IgG secondary antibody (1:400) (Invitrogen, Carlsbad, CA) or fluorescein isothiocyanate (FITC)-labeled Isolectin B4 (1:100) (Vector Laboratories, Burlingame, CA). The retinas were flat-mounted under a dissecting microscope (Olympus, Tokyo, Japan) and photographed under a confocal laser scanning microscope (FV1000; Olympus). To quantify vascular density, the equivalent areas of retina with adequate capillary plexus (to avoid vessel-free zone) was selected as a “field” between different samples. The vascular density was calculated as the number of capillary loops, and the arterial area was calculated as the area of vessels covered with α-SMA+ vSMCs.
For the Matrigel plug and hindlimb ischemia assays, mice were anesthetized and cardiac-perfused with PBS at the end of the experiments. Matrigel and muscle tissue samples were harvested, sectioned, and stained with H&E according to the standard procedure. For immunofluorescence, the tissues were fixed in 4% PFA at 4°C for 4 h and then transferred into 30% sucrose in PBS until the tissues sank. Samples were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Inc., Torrance, CA) and sectioned at 8 μm thickness. Sections were air-dried, blocked, permeabilized, and then incubated for 12 h with primary antibodies, including anti-α-SMA (1:200; Abcam), anti-GJA4 (1:100; Abcam), and anti-CD31 (1:200; R&D), followed by incubation with the corresponding secondary antibodies, including Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:400; Invitrogen) and Alexa Fluor 594-conjugated donkey anti-goat IgG secondary antibody (1:400; Invitrogen).
Fluorescence in situ hybridization
The mice were sacrificed and their lungs, livers, and kidneys were dissected, fixed, and embedded in paraffin. Tissue sections were treated with Proteinase K (20 μg/mL) and hybridized with the miR-342 probe at 37°C for 2 h, and then washed in turn with 2× SSC for 10 min at 37°C, 1× SSC twice for 5 min each at 37°C, and 0.5× SSC for 10 min at room temperature. The tissues were then blocked with 5% BSA for 30 min at room temperature and incubated with anti-CD31 antibody (1:200; R&D) at 4°C overnight. After washing, the tissues were incubated with Alexa Fluor 488-conjugated donkey anti-goat IgG for 2 h at room temperature. The sections were observed under a confocal microscope.
Cell cycle analysis
Cell cycle analysis was performed as previously described.20,22 Briefly, HUVECs were trypsinized, washed with PBS, and fixed in 75% ethanol at 4°C for 2 h. The cells were then washed and incubated with PBS containing 0.02% Triton X-100, RNase A (3,000 U/mL), and propidium iodide (50 μg/mL) at 37°C for 30 min. The cells were analyzed by fluorescence-activated cell sorting using a FACSCalibur flow cytometer (BD Immunocytometry Systems), and the number of cells in each phase was calculated using Modfit software.
RNA sequencing
RNA-seq was performed using a commercial service (Gene Denovo Biotechnology Corporation, Guangzhou, China), as previously described.20 Briefly, HUVECs transfected with the miR-342 mimic or control for 48 h were lysed with TRIzol reagent (Invitrogen), and total RNA was extracted and evaluated for quality. The mRNA was then enriched, fragmented, and reverse-transcribed into complementary DNA (cDNA). The cDNA fragments were sequenced using an Illumina NovaSeq 6000. The original RNA-seq data were deposited in the Genome Sequence Archive of the BIG Data Center (Beijing) (accession no. HRA002650). Online OmicShare tools (http://www.omicshare.com/tools) and TBtools were used for subsequent bioinformatics analysis.
qRT-PCR
HUVECs and minced tissues were lysed with TRIzol reagent and total RNA was extracted. cDNA was synthesized using a reverse transcription kit (Takara, Dalian, China). qRT-PCR was performed using a TBGreen kit (Takara) and QuantStudio 5 real-time PCR instrument (Life Technologies, Waltham, MA, USA). The gene expression levels are represented by the relative fold-change using β-actin or CD31 (retinal angiogenesis model) as an internal reference. The miR-342 level was measured using a miRNA qRT-PCR kit (Takara) with U6 as an internal reference. The sequences of the primers used are shown in Table S1.
Western blotting
Total proteins in HUVECs were extracted with radioimmunoprecipitation assay (RIPA) b00uffer (Beyotime, Shanghai, China) containing phenylmethylsulfonyl fluoride (PMSF) (1 mM) (Sigma-Aldrich) and quantified using a bicinchoninic acid (BCA) kit (Beyotime). Protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The resulting membranes were probed with primary antibodies, followed by the corresponding secondary antibodies. The primary antibodies used were anti-EFNB2 (1:1,000) (SAB, Greenbelt, Maryland), anti-GJA4 (1:1,000) (SAB), anti-EPHB4 (1:800) (Cell Signaling, Boston, MA), anti-MYC (1:1,000) (Cell Signaling), anti-EYA3 (1:1,000) (Proteintech, Wuhan, China), anti-CDK2 (1:1,000) (Proteintech), anti-RRM2 (1:1,000) (Proteintech), anti-MYC (pT58) (1:1,000) (SAB), anti-MYC (pS62) (1:1,000) (SAB), and anti-β-ACTIN (1:5,000) (Proteintech). The secondary antibodies included horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG (1:5,000) (Proteintech). The bands were detected using a chemiluminescence system (Tanon, Shanghai, China). Quantification was performed using ImageJ2 (Rawak Software, Stuttgart, Germany).
Reporter assay
The reporter pGL-EYAUTR3 with the wild-type EYA3 3′-UTR (pGL-EYAUTR-wt) was used as previously described,20 and a mutation was introduced at the seed sequence of miR-342 to create pGL-EYAUTR-342mut. HEK293T cells were co-transfected with miR-342 mimic or control, or infected with an miR-342 sponge (anti-342) or control lentivirus for 24 h, together with EYA3-3′-UTR-wt (100 ng) or EYA3-3′-UTR-mut (100 ng) and phRL-TK (5 ng). The cells were lysed 48 h after transfection, and firefly and Renilla luciferase activities were measured and compared using the dual-luciferase reporter assay system (Promega).
Statistical analysis
Image-Pro Plus (version 6.0) and ImageJ2× were used for the image analysis. Statistical analyses were conducted using the GraphPad Prism 8 software. Quantitative data were expressed as the mean ± SD. Continuous variables between the two groups were compared using Student’s t test. One-way ANOVA followed by Tukey’s post hoc test was used to compare the continuous variables of more than two groups for one independent variable. p values <0.05 were considered to indicate statistical significance.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (82003110, 31730041, 81900870, and 32270966) and the Young Talent Fund of the University Association for Science and Technology in Shaanxi, China (20210301 and 20220305).
Author contributions
X.Z., J.S., P.Z., T.W., and R.W. performed the experiments and collected the data. L.L., Z.Y., and J.L. assisted with experiments and data collection. J.Z., B.C., and X.F. performed experiments. X.L., H.H., and X.Y. designed the experiments and supervised the writing of the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2023.03.022.
Contributor Information
Xiaowei Liu, Email: liuxiaow@fmmu.edu.cn.
Hua Han, Email: huahan@fmmu.edu.cn.
Xianchun Yan, Email: yanxianchun@fmmu.edu.cn.
Supplemental information
Data availability
The original RNA-seq data were deposited in the Genome Sequence Archive of the BIG Data Center (Beijing) (accession no. HRA002650). The remaining data and materials are available from the corresponding authors upon request.
References
- 1.Marziano C., Genet G., Hirschi K.K. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021;24:213–236. doi: 10.1007/s10456-021-09785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen D., Schwartz M.A., Simons M. Developmental perspectives on arterial fate specification. Front. Cell Dev. Biol. 2021;9:691335. doi: 10.3389/fcell.2021.691335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Red-Horse K., Siekmann A.F. Veins and arteries build hierarchical branching patterns differently: bottom-up versus top-down. Bioessays. 2019;41:e1800198. doi: 10.1002/bies.201800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang J., Hirschi K. Molecular regulation of arteriovenous endothelial cell specification. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.16701.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roux E., Bougaran P., Dufourcq P., Couffinhal T. Fluid shear stress sensing by the endothelial layer. Front. Physiol. 2020;11:861. doi: 10.3389/fphys.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucitti J.L., Jones E.A.V., Huang C., Chen J., Fraser S.E., Dickinson M.E. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buschmann I., Pries A., Styp-Rekowska B., Hillmeister P., Loufrani L., Henrion D., Shi Y., Duelsner A., Hoefer I., Gatzke N., et al. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137:2187–2196. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- 8.Aranguren X.L., Agirre X., Beerens M., Coppiello G., Uriz M., Vandersmissen I., Benkheil M., Panadero J., Aguado N., Pascual-Montano A., et al. Unraveling a novel transcription factor code determining the human arterial-specific endothelial cell signature. Blood. 2013;122:3982–3992. doi: 10.1182/blood-2013-02-483255. [DOI] [PubMed] [Google Scholar]
- 9.Kalucka J., de Rooij L.P.M.H., Goveia J., Rohlenova K., Dumas S.J., Meta E., Conchinha N.V., Taverna F., Teuwen L.A., Veys K., et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Naiche L.A., Villa S.R., Kitajewski J.K. Endothelial cell fate determination: a top Notch job in vascular decision-making. Cold Spring Harb. Perspect. Med. 2022;12:a041183. doi: 10.1101/cshperspect.a041183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamizu K., Matsunaga T., Uosaki H., Fukushima H., Katayama S., Hiraoka-Kanie M., Mitani K., Yamashita J.K. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J. Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasan S.S., Tsaryk R., Lange M., Wisniewski L., Moore J.C., Lawson N.D., Wojciechowska K., Schnittler H., Siekmann A.F. Endothelial Notch signalling limits angiogenesis via control of artery formation. Nat. Cell Biol. 2017;19:928–940. doi: 10.1038/ncb3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitulescu M.E., Schmidt I., Giaimo B.D., Antoine T., Berkenfeld F., Ferrante F., Park H., Ehling M., Biljes D., Rocha S.F., et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 2017;19:915–927. doi: 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen C.M., Cuervo H., Ding V.W., Kong Y., Huang E.J., Wang R.A. Deletion of Rbpj from postnatal endothelium leads to abnormal arteriovenous shunting in mice. Development. 2014;141:3782–3792. doi: 10.1242/dev.108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang J.S., Coon B.G., Gillis N., Chen Z., Qiu J., Chittenden T.W., Burt J.M., Schwartz M.A., Hirschi K.K. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat. Commun. 2017;8:2149. doi: 10.1038/s41467-017-01742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weijts B., Gutierrez E., Saikin S.K., Ablooglu A.J., Traver D., Groisman A., Tkachenko E. Blood flow-induced Notch activation and endothelial migration enable vascular remodeling in zebrafish embryos. Nat. Commun. 2018;9:5314. doi: 10.1038/s41467-018-07732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo W., Garcia-Gonzalez I., Fernández-Chacón M., Casquero-Garcia V., Sanchez-Muñoz M.S., Mühleder S., Garcia-Ortega L., Andrade J., Potente M., Benedito R. Arterialization requires the timely suppression of cell growth. Nature. 2021;589:437–441. doi: 10.1038/s41586-020-3018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rosa S., Iaconetti C., Eyileten C., Yasuda M., Albanese M., Polimeni A., Sabatino J., Sorrentino S., Postula M., Indolfi C. Flow-responsive noncoding RNAs in the vascular system: basic mechanisms for the clinician. J. Clin. Med. 2022;11:459. doi: 10.3390/jcm11020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R., Yang Z., Liang L., Feng X., Che B., Zhang X., Zheng Q., Yan X., Han H. Notch activation suppresses endothelial cell migration and sprouting via miR-223-3p targeting Fbxw7. In Vitro Cell. Dev. Biol. Anim. 2022;58:124–135. doi: 10.1007/s11626-022-00649-y. [DOI] [PubMed] [Google Scholar]
- 20.Sun J.X., Dou G.R., Yang Z.Y., Liang L., Duan J.L., Ruan B., Li M.H., Chang T.F., Xu X.Y., Chen J.J., et al. Notch activation promotes endothelial quiescence by repressing MYC expression via miR-218. Mol. Ther. Nucleic Acids. 2021;25:554–566. doi: 10.1016/j.omtn.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grady W.M., Parkin R.K., Mitchell P.S., Lee J.H., Kim Y.H., Tsuchiya K.D., Washington M.K., Paraskeva C., Willson J.K.V., Kaz A.M., et al. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene. 2008;27:3880–3888. doi: 10.1038/onc.2008.10. [DOI] [PubMed] [Google Scholar]
- 22.Yan X.C., Cao J., Liang L., Wang L., Gao F., Yang Z.Y., Duan J.L., Chang T.F., Deng S.M., Liu Y., et al. miR-342-5p is a Notch downstream molecule and regulates multiple angiogenic pathways including Notch, vascular endothelial growth factor and transforming growth factor beta signaling. J. Am. Heart Assoc. 2016;5:e003042. doi: 10.1161/JAHA.115.003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao F., Zhang Y.F., Zhang Z.P., Fu L.A., Cao X.L., Zhang Y.Z., Guo C.J., Yan X.C., Yang Q.C., Hu Y.Y., et al. miR-342-5p regulates neural stem cell proliferation and differentiation downstream to Notch signaling in mice. Stem Cell Rep. 2017;8:1032–1045. doi: 10.1016/j.stemcr.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Z., Qin X., Hu Y., Zhang X., Li G., Wu J., Li J., Sha J., Chen J., Xia J., et al. Longterm exercise-derived exosomal miR-342-5p: a novel exerkine for cardioprotection. Circ. Res. 2019;124:1386–1400. doi: 10.1161/CIRCRESAHA.118.314635. [DOI] [PubMed] [Google Scholar]
- 25.Lagendijk A.K., Yap A.S., Hogan B.M. Notching a new pathway in vascular flow sensing. Trends Cell Biol. 2018;28:173–175. doi: 10.1016/j.tcb.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Ruter D.L., Liu Z., Ngo K.M., X S., Marvin A., Buglak D.B., Kidder E.J., Bautch V.L. SMAD6 transduces endothelial cell flow responses required for blood vessel homeostasis. Angiogenesis. 2021;24:387–398. doi: 10.1007/s10456-021-09777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reneman R.S., Hoeks A.P.G. Wall shear stress as measured in vivo: consequences for the design of the arterial system. Med. Biol. Eng. Comput. 2008;46:499–507. doi: 10.1007/s11517-008-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora S., Lam A.J.Y., Cheung C., Yim E.K.F., Toh Y.C. Determination of critical shear stress for maturation of human pluripotent stem cell-derived endothelial cells towards an arterial subtype. Biotechnol. Bioeng. 2019;116:1164–1175. doi: 10.1002/bit.26910. [DOI] [PubMed] [Google Scholar]
- 29.Mehta V., Pang K.L., Rozbesky D., Nather K., Keen A., Lachowski D., Kong Y., Karia D., Ameismeier M., Huang J., et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature. 2020;578:290–295. doi: 10.1038/s41586-020-1979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vega-Tapia F., Peñaloza E., Krause B.J. Specific arterio-venous transcriptomic and ncRNA-RNA interactions in human umbilical endothelial cells: a meta-analysis. iScience. 2021;24:102675. doi: 10.1016/j.isci.2021.102675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 32.Dejure F.R., Eilers M. MYC and tumor metabolism: chicken and egg. EMBO J. 2017;36:3409–3420. doi: 10.15252/embj.201796438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hydbring P., Castell A., Larsson L.G. MYC Modulation around the CDK2/p27/SKP2 Axis. Genes. 2017;8:174. doi: 10.3390/genes8070174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Zhou H., Li X., Vartuli R.L., Rowse M., Xing Y., Rudra P., Ghosh D., Zhao R., Ford H.L. Eya3 partners with PP2A to induce c-Myc stabilization and tumor progression. Nat. Commun. 2018;9:1047. doi: 10.1038/s41467-018-03327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu S., Lian Z., Zhang S., Xu Y., Zhang H. CircGNG4 promotes the progression of prostate cancer by sponging miR-223 to enhance EYA3/c-myc expression. Front. Cell Dev. Biol. 2021;9:684125. doi: 10.3389/fcell.2021.684125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricard N., Zhang J., Zhuang Z.W., Simons M. Isoform-specific roles of ERK1 and ERK2 in arteriogenesis. Cells. 2019;9:38. doi: 10.3390/cells9010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limbourg A., Korff T., Napp L.C., Schaper W., Drexler H., Limbourg F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 38.Polacheck W.J., Kutys M.L., Yang J., Eyckmans J., Wu Y., Vasavada H., Hirschi K.K., Chen C.S. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552:258–262. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhelm K., Happel K., Eelen G., Schoors S., Oellerich M.F., Lim R., Zimmermann B., Aspalter I.M., Franco C.A., Boettger T., et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veys C., Benmoussa A., Contentin R., Duchemin A., Brotin E., Lafont J.E., Saintigny Y., Poulain L., Denoyelle C., Demoor M., et al. Tumor suppressive role of miR-342-5p in human chondrosarcoma cells and 3D organoids. Int. J. Mol. Sci. 2021;22:5590. doi: 10.3390/ijms22115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Li K., Yu G., Yu C., Liu C. miR-342-5p inhibits expression of Bmp7 to regulate proliferation, differentiation and migration of osteoblasts. Mol. Immunol. 2019;114:251–259. doi: 10.1016/j.molimm.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Sun X., Wu Y., Gu M., Zhang Y. miR-342-5p decreases ankyrin G levels in Alzheimer's disease transgenic mouse models. Cell Rep. 2014;6:264–270. doi: 10.1016/j.celrep.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 43.Ahmadi R., Heidarian E., Fadaei R., Moradi N., Malek M., Fallah S. miR-342-5p expression levels in coronary artery disease patients and its association with inflammatory cytokines. Clin. Lab. 2018;64:603–609. doi: 10.7754/Clin.Lab.2017.171208. [DOI] [PubMed] [Google Scholar]
- 44.Xing X., Li Z., Yang X., Li M., Liu C., Pang Y., Zhang L., Li X., Liu G., Xiao Y. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging (Albany NY) 2020;12:3880–3898. doi: 10.18632/aging.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugden W.W., Meissner R., Aegerter-Wilmsen T., Tsaryk R., Leonard E.V., Bussmann J., Hamm M.J., Herzog W., Jin Y., Jakobsson L., et al. Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat. Cell Biol. 2017;19:653–665. doi: 10.1038/ncb3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin Y., Muhl L., Burmakin M., Wang Y., Duchez A.C., Betsholtz C., Arthur H.M., Jakobsson L. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat. Cell Biol. 2017;19:639–652. doi: 10.1038/ncb3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo A., Huang H., Zhu Z., Chen M.J., Shi H., Yuan S., Sharma P., Connelly J.P., Liedmann S., Dhungana Y., et al. cBAF complex components and MYC cooperate early in CD8(+) T cell fate. Nature. 2022;607:135–141. doi: 10.1038/s41586-022-04849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee C.Y., Lin S.J., Wu T.C. miR-548j-5p regulates angiogenesis in peripheral artery disease. Sci. Rep. 2022;12:838. doi: 10.1038/s41598-022-04770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalil I.A., Younis M.A., Kimura S., Harashima H. Lipid nanoparticles for cell-specific in vivo targeted delivery of nucleic acids. Biol. Pharm. Bull. 2020;43:584–595. doi: 10.1248/bpb.b19-00743. [DOI] [PubMed] [Google Scholar]
- 50.Maishi N., Sakurai Y., Hatakeyama H., Umeyama Y., Nakamura T., Endo R., Alam M.T., Li C., Annan D.A.M., Kikuchi H., et al. Novel antiangiogenic therapy targeting biglycan using tumor endothelial cell-specific liposomal siRNA delivery system. Cancer Sci. 2022;113:1855–1867. doi: 10.1111/cas.15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanicek L., Lozano-Vidal N., Bink D.I., Hooglugt A., Yao W., Wittig I., van Rijssel J., van Buul J.D., van Bergen A., Klems A., et al. Long non-coding RNA LASSIE regulates shear stress sensing and endothelial barrier function. Commun. Biol. 2020;3:265. doi: 10.1038/s42003-020-0987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saw S.N., Dawn C., Biswas A., Mattar C.N.Z., Yap C.H. Characterization of the in vivo wall shear stress environment of human fetus umbilical arteries and veins. Biomech. Model. Mechanobiol. 2017;16:197–211. doi: 10.1007/s10237-016-0810-5. [DOI] [PubMed] [Google Scholar]
- 53.Ward A.O., Sala-Newby G.B., Ladak S., Angelini G.D., Caputo M., Suleiman M.S., Evans P.C., George S.J., Zakkar M. Nrf2-Keap-1 imbalance under acute shear stress induces inflammatory response in venous endothelial cells. Perfusion. 2022;37:582–589. doi: 10.1177/02676591211012571. [DOI] [PubMed] [Google Scholar]
- 54.Conway D.E., Williams M.R., Eskin S.G., McIntire L.V. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am. J. Physiol. Heart Circ. 2010;298:H367–H374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi L., Fisslthaler B., Zippel N., Frömel T., Hu J., Elgheznawy A., Heide H., Popp R., Fleming I. MicroRNA-223 antagonizes angiogenesis by targeting beta1 integrin and preventing growth factor signaling in endothelial cells. Circ. Res. 2013;113:1320–1330. doi: 10.1161/CIRCRESAHA.113.301824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original RNA-seq data were deposited in the Genome Sequence Archive of the BIG Data Center (Beijing) (accession no. HRA002650). The remaining data and materials are available from the corresponding authors upon request.