Abstract
Lactococcus lactis subsp. lactis strains show glutamate decarboxylase activity, whereas L. lactis subsp. cremoris strains do not. The gadB gene encoding glutamate decarboxylase was detected in the L. lactis subsp. cremoris genome but was poorly expressed. Sequence analysis showed that the gene is inactivated by the frameshift mutation and encoded in a nonfunctional protein.
Lactococcal strains are essential to cheese manufacture and in the early stages of ripening. The species Lactococcus lactis is subdivided into L. lactis subsp. lactis and L. lactis subsp. cremoris on the basis of physiological properties. L. lactis strains can be classed as two phylogenetic groups by Southern hybridization (2) and by DNA sequence analyses (8, 11). It has been proposed that the subspecies diagnoses be redefined to reflect these natural relationships (2). However, the classification of subspecies based on phenotypes is of primary importance in the dairy industry. Phenotypes to distinguish two subspecies of L. lactis have been reported (1, 5), and classification is based on the following criteria: growth in 4% NaCl, pH 9.2, at 40°C; the ability to hydrolyze arginine; and sensitivity to lithium chloride. These criteria, however, have not yet been elucidated at the molecular level. Recently, a novel criterion for distinguishing L. lactis subsp. lactis from L. lactis subsp. cremoris was reported; glutamate decarboxylase (GAD; EC 4.1.1.15) activity was observed in L. lactis subsp. lactis and not in L. lactis subsp. cremoris (6).
GAD catalyzes the irreversible decarboxylation of glutamate to γ-aminobutyric acid (GABA). GAD constitutes a glutamate-dependent acid resistance mechanism with a glutamate-GABA antiporter (10). The introduction of a defect in the gadC and gadB genes, which encode the glutamate-GABA antiporter and GAD, has been shown to reduce acid resistance in L. lactis (9). Based on the sequence analysis, it has been suggested that L. lactis gadCB forms an operon present in one copy in the chromosome (7, 9). Here we studied the sequence of gadB genes in L. lactis subsp. lactis and in L. lactis subsp. cremoris.
L. lactis subsp. lactis biovar diacetylactis ATCC 13675, L. lactis subsp. lactis ATCC 19435, and L. lactis subsp. cremoris ATCC 19257 were obtained from the American Type Culture Collection (Manassas, Va.). Other strains tested were from laboratory collections and had been isolated from dairy products or cheese starters (6). The bacteria were maintained in sterile litmus milk and subcultured once a week. TYG medium consisted of 0.5% tryptone (Difco), 0.5% yeast extract (Difco), and 1.0% glucose, the pH of which was adjusted to 7.0 with 1 M NaOH. Actively growing cultures were obtained by transferring 1% inoculum to TYG or modified M17 (9) and incubating at 30°C for 16 h.
Genomic DNA from L. lactis was isolated by the method described previously (7). Total RNA was isolated as follows: actively growing culture (0.5 ml) was transferred to 50 ml of TYG medium or M17 supplemented with 50 mM l-glutamate and 0.3 M NaCl. Cells were harvested at late log phase by centrifugation at 1,800 × g for 20 min and resuspended with 100 μl of 50 mM Tris-Cl (pH 7.4) containing 25% (wt/vol) sucrose, 3 mM MgCl2, and 0.1 mg of lysozyme per ml (12). The suspension was incubated at 4°C for 10 min. Total RNA was extracted from the suspension with 1.5 ml of ISOGEN (Nippongene, Toyama, Japan) according to the manufacturer's instructions. The RNA fractions were treated with DNase I (3.5 U) in 50 μl of 100 mM sodium acetate buffer (pH 5.0) containing 5 mM MnCl2 at 37°C for 10 min to remove any contaminating DNA. RNA was extracted with phenol-chloroform, precipitated with 2-propanol, and stored at −80°C until use.
Total DNAs from L. lactis were digested, separated on 1.0% agarose, and transferred to Hybond-N (Amersham). The Southern blots were hybridized with the 2.4-kb XbaI-EcoRI fragment of L. lactis 01-7 gadCB (7). Hybridization was performed at 68°C. The filter was washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1% sodium dodecyl sulfate at room temperature and then twice in 0.1× SSC–0.1% sodium dodecyl sulfate at 68°C.
The gadCB fragments were amplified by PCR, using the total L. lactis DNA or the first-strand cDNA as the template. The PCR primers were designed from the published sequence of L. lactis gadCB (7). The sense primer was 5′-GTTTTGTTGTGACTGCTATCTTGCCA-3′ (nucleotides 704 to 729), and the antisense primer was 5′-TTTTTGGGAAGTGGATAAGCAGGCA-3′ (nucleotides 2136 to 2112). The length of the expected amplified fragment was 1,433 bp. Fifty microliters of each PCR mixture contained 200 ng of DNA, 20 pmol of each of the primers, reagent mix, and AmpliTaq Gold DNA polymerase (Perkin-Elmer, Foster City, Calif.). PCR amplification was conducted with a GeneAmp PCR System 2400 (Perkin-Elmer). The following 35 or 45 cycles of amplification were performed: denaturation for 30 s at 94°C, annealing for 30 s at 55°C, and extension for 90 s at 72°C. Amplified double-stranded DNA was purified by electrophoresis on a 1.5% agarose gel for direct sequencing. Both strands of purified DNA were sequenced with a Taq Dye Terminator cycle sequencing kit and a model 373A DNA sequencer (both from Applied Biosystems, Foster City, Calif.). From the nucleotide sequence, the amino acid sequence was deduced. The amplification of ldh was carried out as described previously (3).
Total RNA (200 ng) was added to RTG reverse transcription (RT)-PCR reagent with the d(N)6 random hexamer (Amersham Pharmacia Biotech, Piscataway, N.J.). The first-strand synthesis reaction was carried out at 42°C for 30 min. The mixture was heated at 95°C for 5 min, and then the gadB-specific primer was added. The gadB amplification was performed as described above.
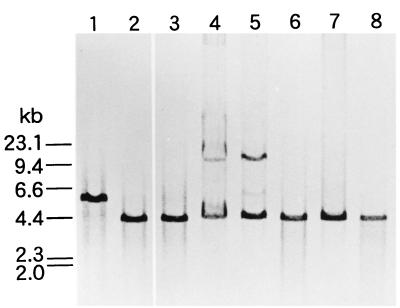
In order to study the genetic basis of non-GABA productivity, three L. lactis subsp. cremoris strains were compared with six L. lactis subsp. lactis strains by Southern hybridization. A fragment of L. lactis 01-7 gadCB was used as a probe. Positive hybridizing bands were observed with all strains (Fig. 1). Restriction fragment length polymorphism was observed among the strains examined. PCR amplification of six L. lactis subsp. lactis and three L. lactis subsp. cremoris strains was performed. The 1.4-kb fragments were amplified from each of the samples (data not shown). The sizes of the amplified products corresponded to the sizes predicted from the published gadCB sequence (7), and no nonspecific amplification was observed. We believe that the pair of primers designed was useful for the amplification of L. lactis gadB. The results indicated that the gadCB genes are also present in L. lactis subsp. cremoris and that they are not grossly rearranged by insertions or deletions of large fragments.
FIG. 1.
Southern blot analysis of L. lactis gadB. The DNA was digested with EcoRI. The blot was probed with a digoxigenin-labeled 2.4-kb XbaI-EcoRI fragment (7). Lanes: 1, L. lactis subsp. lactis biovar diacetylactis 01-7; 2, L. lactis subsp. lactis biovar diacetylactis ATCC 13675; 3, L. lactis subsp. lactis ATCC 19435; 4, L. lactis subsp. lactis 712; 5, L. lactis subsp. lactis SlN; 6, L. lactis subsp. cremoris ATCC 19257; 7, L. lactis subsp. cremoris H-61; 8, L. lactis subsp. cremoris HP.
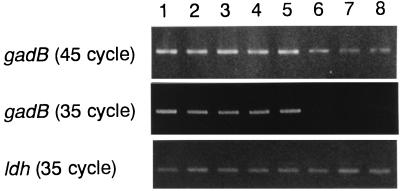
To detect a gadB transcription, RT-PCR analysis was performed with the successful primer pair by using the first-strand cDNA as a template. The 1.4-kb positive signals were observed with both L. lactis subsp. lactis and L. lactis subsp. cremoris when amplification was performed with 45 cycles (Fig. 2). The signals of L. lactis subsp. cremoris could not be detected with 35 cycles of amplification, while that of L. lactis subsp. lactis was clearly observed. The amplifications of ldh were observed at equivalent intensities, indicating that the RNAs from L. lactis subsp. cremoris were not degraded. L. lactis gadB was transcribed to mRNA not only in L. lactis subsp. lactis but also in L. lactis subsp. cremoris, although the amount of the latter was slight. When the cells were cultured in the medium without glutamate and NaCl, no amplification was observed with any of the strains investigated (data not shown).
FIG. 2.
RT-PCR analysis for gadB mRNA. Lanes: 1, L. lactis subsp. lactis biovar diacetylactis 01-7; 2, L. lactis subsp. lactis biovar diacetylactis ATCC 13675; 3, L. lactis subsp. lactis ATCC 19435; 4, L. lactis subsp. lactis 712; 5, L. lactis subsp. lactis SlN; 6, L. lactis subsp. cremoris ATCC 19257; 7, L. lactis subsp. cremoris H-61; 8, L. lactis subsp. cremoris HP.
The gadB gene of L. lactis subsp. cremoris ATCC 19257 was sequenced and compared with that of L. lactis subsp. lactis (7). The DNA sequence from ATCC 19257 revealed close homology to L. lactis subsp. lactis 01-7 (95.4%). However, a one-base deletion of adenine and a one-base insertion of thymine were detected within the coding region, resulting in frameshift mutations. The regions around these two mutations were subsequently sequenced in other L. lactis subsp. cremoris strains to confirm that the mutations are common. The adenine deletion was conserved in six of seven strains (the exception was H-61), and the thymine insertion was detected in all seven strains (Fig. 3). The resulting frameshift from these mutations created stop codons and was thought to affect GadB of L. lactis subsp. cremoris. Among the adenine-deleted strains and in strain H-61, the GadB proteins were truncated to 102 and 405 amino acids, respectively, while the counterpart of L. lactis subsp. lactis typically contains 466 amino acids. Given that the coding region of strain H-61 gadB was not sequenced completely, the product might be further truncated by additional mutations within the unsequenced region.
FIG. 3.
Comparison of the nucleotide sequences of gadB from L. lactis subsp. lactis and L. lactis subsp. cremoris. The first line is the sequence from L. lactis subsp. lactis strain 01-7 (7). The second and the following lines are from L. lactis subsp. cremoris. The insertion and the deletion sites are indicated with arrows. The stop codons created by the frameshift are boxed. Numbers are counted from the translational start point of GadB of strain 01-7.
The GAD gene has been observed to exist in the L. lactis subsp. cremoris genome and to transcribe to mRNA. The transcription was not induced by acid and chloride stress, which do induce the expression of gadCB in L. lactis (9). The transcription was therefore insufficient. Because of the frameshift resulting from a one-base insertion or deletion within the coding region, the translated protein was not functional. Given that a 55-residue segment around the active-center lysine of GAD is highly conserved (4), the segment is considered important to the expression of enzymatic activity. An adenine deletion, which creates a stop codon, was observed in six of seven GAD-negative strains. Protein synthesis was terminated by the stop codon, and the truncated protein was considered to show no activity because the active-center lysine was not synthesized. Although the remaining strain, H-61, was also GAD negative (6), the adenine deletion was not detected in it. In H-61 GadB, the active-center lysine could be translated but the C-terminal region was not synthesized as a result of the thymine insertion. As a result of the deformation, GadB in strain H-61 could not be folded exactly and thus could not fulfill its function. For expression of GAD activity, it is necessary that the conserved segment exist not only around the active center but also in the sequence of the C-terminal region. Given that the thymine insertion was observed in all L. lactis subsp. cremoris strains, such a mutation would be more remarkable than the adenine deletion.
This study indicated that gadB of L. lactis subsp. cremoris was present but was poorly expressed and encoded in a nonfunctional protein. To our knowledge, this is the first study to establish the nature of lesions affecting the criteria that distinguish L. lactis subsp. lactis and L. lactis subsp. cremoris at the molecular level.
Nucleotide sequence accession numbers.
The DDBJ accession numbers for the sequences reported in this paper are AB033218 to AB033230.
REFERENCES
- 1.Al-Zoreky N, Sandine W E. Lactococcus genus: a selective and differential agar medium. J Food Sci. 1991;56:1729–1734. [Google Scholar]
- 2.Godon J J, Delorme C, Ehrlich S D, Renault P. Divergence of genomic sequences between Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1992;58:4045–4047. doi: 10.1128/aem.58.12.4045-4047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin H G, I'Anson K J, Gasson M J. Rapid isolation of genes from bacterial lambda libraries by direct polymerase chain reaction screening. FEMS Microbiol Lett. 1993;112:49–54. doi: 10.1111/j.1574-6968.1993.tb06422.x. [DOI] [PubMed] [Google Scholar]
- 4.Maras B, Sweeney G, Barra D, Bossa F, John R A. The amino acid sequence of glutamate decarboxylase from Escherichia coli. Eur J Biochem. 1992;204:93–98. doi: 10.1111/j.1432-1033.1992.tb16609.x. [DOI] [PubMed] [Google Scholar]
- 5.Mundt J O. Lactic acid streptococci. In: Sneath P H A, et al., editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1065–1066. [Google Scholar]
- 6.Nomura M, Kimoto H, Someya Y, Suzuki I. Novel characteristic for distinguishing Lactococcus lactis subsp. lactis from subsp. cremoris. Int J Syst Bacteriol. 1999;49:163–166. doi: 10.1099/00207713-49-1-163. [DOI] [PubMed] [Google Scholar]
- 7.Nomura M, Nakajima I, Fujita Y, Kobayashi M, Kimoto H, Suzuki I, Aso H. Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology. 1999;145:1375–1380. doi: 10.1099/13500872-145-6-1375. [DOI] [PubMed] [Google Scholar]
- 8.Salama M, Sandine W, Giovannoni S. Development and application of oligonucleotide probes for identification of Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1991;57:1313–1318. doi: 10.1128/aem.57.5.1313-1318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders J W, Leenhouts K, Burghoorn J, Brands J R, Venema G, Kok J. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol. 1998;27:299–310. doi: 10.1046/j.1365-2958.1998.00676.x. [DOI] [PubMed] [Google Scholar]
- 10.Small P L C, Waterman S R. Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol. 1998;6:214–216. doi: 10.1016/s0966-842x(98)01285-2. [DOI] [PubMed] [Google Scholar]
- 11.Swindell S R, Griffin H G, Gasson M J. Cloning, sequencing and comparison of three lactococcal l-lactate dehydrogenase genes. Microbiology. 1994;140:1301–1305. doi: 10.1099/00221287-140-6-1301. [DOI] [PubMed] [Google Scholar]
- 12.van Rooijen R J, de Vos W M. Molecular cloning, transcriptional analysis, and nucleotide sequence of lacR, a gene encoding the repressor of the lactose phosphotransferase system of Lactococcus lactis. J Biol Chem. 1990;265:18499–18503. [PubMed] [Google Scholar]