Abstract
Background
Xihuang pill, a Traditional Chinese Medicine (TCM) prescription and a representative of Huoxuehuayu therapy (one of the TCM therapies to promote blood circulation and remove blood stasis), has been widely used in breast cancer treatments. Although some evidence suggests the efficacy and safety of the Xihuang pill in treating certain cancer, the overall efficacy of the Xihuang pill in other cancer treatment is uncertain.
Objective
This study aimed to summarize the current clinical literature and provided evidence support for addressing the research question of whether the Xihuang pill is safe and effective in the treatment of various cancers as Huoxuehuayu therapy, and possibly identify the clinical dosage range and therapeutic effect of Xihuang pills.
Method
Seven Chinese and English databases such as PubMed, CNKI, and Google Scholar were searched to collect the publications on Xihuang pill and cancer. Then the researchers extracted data from the articles that met the inclusion criteria and used SAS statistical program version 9.4 (by SAS Institute, Cary, North Carolina, USA) for statistical statistics.
Results
Our search identified 78 studies, including 69 RCTs (randomized control trials), 6 NRCCs (non-randomized concurrent control trials), and 3 BAS (before-after study), evaluating 3151 patients in total. The daily doses of Xihuang pills/capsule were between 2 g and 60 g, and duration between 2 weeks and 5 years, mostly used in the middle or late stage of cancer. The therapeutic effect of the Xihuang pill was mainly reflected in improving Complete Response (CR, a term from The Response Evaluation Criteria In Solid Tumors) or Partial Response (PR, a term from The Response Evaluation Criteria In Solid Tumors), reducing adverse reactions, promoting quality of life (QoL), regulating immunity, alleviating pain, prolonging survival, reducing metastasis and recurrence, reducing inflammation, regulating estrogen levels, decreasing hypercoagulative status, and reducing tumor markers.
Conclusion
Xihuang pill representing Huoxuehuayu therapy has a good prospect in the treatment of cancer.
Keywords: Xihuang pill, Safety and effectiveness, Cancer, Huoxuehuayu therapy, Traditional Chinese medicine
1. Introduction
Cancer incidence and mortality are rapidly growing worldwide. In 2020, an estimated 19.3 million new cancer cases had been diagnosed worldwide, with around 10.0 million cancer deaths. In 2040, there will be an estimated 28.4 million cancer cases expected to happen globally [1]. Given its relapsing and fatal features, curing cancer seems to be insurmountable for the patients. Currently, common cancer therapeutics include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. However, treatment with curative intent by these methods is mainly eligible for patients with the localized disease or treatment-sensitive cancers, so their contributions to cancer curability are relatively limited [2]. Therefore, finding more safe and effective methods for cancer treatment has always been one of the focuses of researchers around the world.
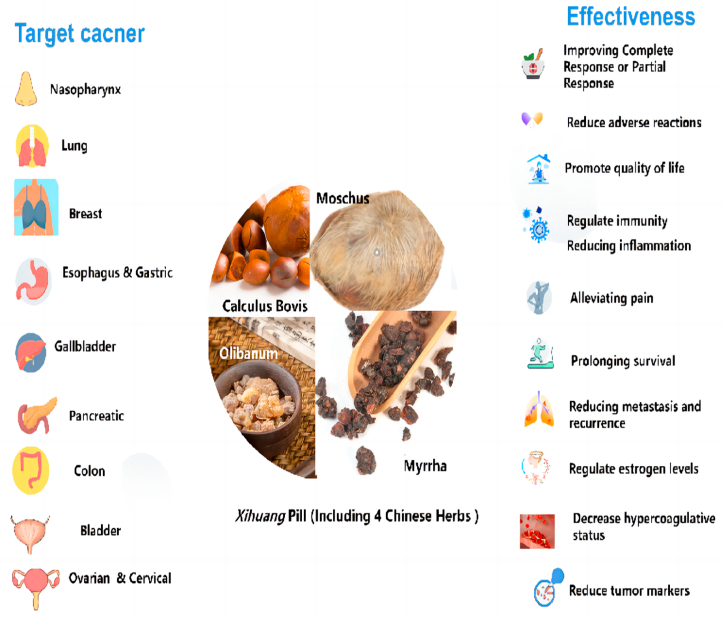
It should be noticed that Traditional Chinese Medicine (TCM) has been taking advantage of treating cancer due to its various biological activities, including fitting well within the whole system and multi-target cancer treatment [3]. The diagnosis and treatment of tumors were discussed in the literature of ancient Chinese medicine, where there is a wealth of content [4]. Among them, the Xihuang pill was first recorded in ‘The Encyclopedia of Surgical Diagnosis and Treatment (外科证治全书)’ by Wang Hongxu in the Qing Dynasty (Fig. 1), which is traditionally used to treat “Ru Yan” (considered as breast cancer nowadays) and to treat breast cancer or other cancers in today [[5], [6], [7], [8], [9]]. It contains four Chinese herbal medicines: Calculus Bovis (from Bos taurus domesticus Gmelin), Moschus (from Moschus berezovskii Flerov), Olibanum (Boswellia carterii Birdw.), and Myrrha (from Commiphora myrrha Engl.) [10], which typically represents Huoxuehuayu therapy, one of the TCM therapies to promote blood circulation and remove blood stasis. In the previous study [11], we demonstrated that Huoxuehuayu therapy showed promise in cancer treatment, improving quality of life, addressing cancer-related symptoms, and reducing toxicity in a secure way. However, it is unsure that the secure dosage of herbs or prescription with Huoxuehuayu functions. Moreover, the Xihuang pill as an example of Huoxuehuayu therapy is lacking sufficient evidence to suggest it could also work for more cancer treatments. In this study, we tried to give a specific example of Huoxuehuayu therapy, the Xihuang pill, to discuss whether it is safe and effective in the treatment of various cancers and to explore the securely clinical dosage and comprehensively therapeutic effect of the Xihuang pill.
Fig. 1.
Xihuang pill recorded in The Encyclopedia of Surgical Diagnosis and Treatment (外科证治全书)*. *The picture derives from Guoyi Diancang Database http://v2.gydc.ac.cn:82/.
2. Materials and methods
2.1. Evidence acquisition
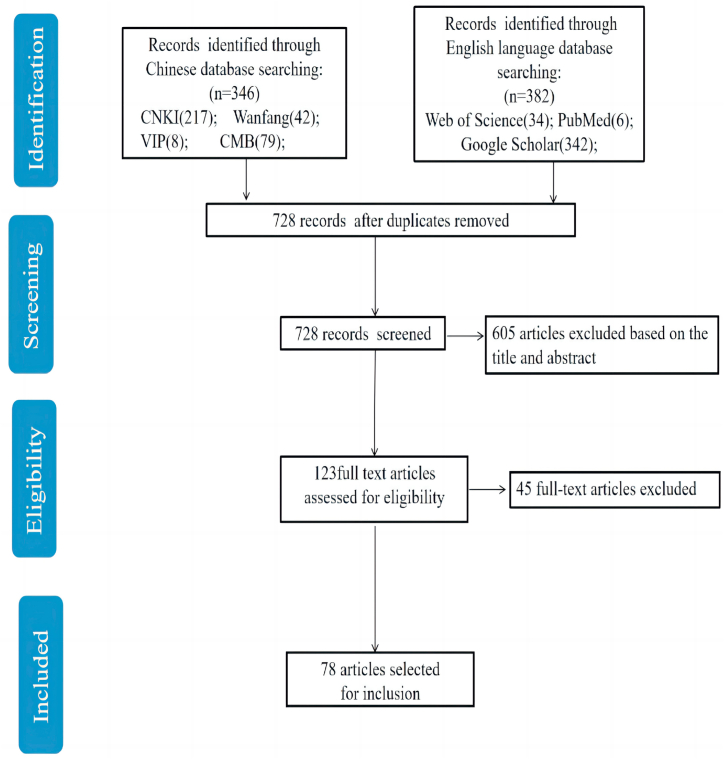
We used Chinese databases, including CNKI Database, Wanfang Database, VIP Database, and China Biology Medicine disc (CMB disc), and English databases, including PubMed, Google Scholar, and Web of Science to perform electronic searches for publications on Xihuang pill and cancer. Our search included Chinese or English language entries from inception until July 2022 and incorporated the following keywords: Xihuang/cancer/tumor in all fields. The search formula of relevant literature was listed in Table 1. Only human studies were eligible for inclusion; case reports, editorials, letters, commentaries, and reviews were excluded. Repeatedly published research, research based on extractive or components of the Xihuang pill, the use of other herbal medicine combination therapy, unclear diagnosis, and treatment period were also excluded. The initial search resulted in 728 publications. After title and abstract screening, 123 studies remained, and 78 were finally selected for the present review after full-text screening (Fig. 2).
Table 1.
Search formula of relevant literature.
| Search mode | Search fomula |
|---|---|
| #1 | cancer |
| #2 | malignant neoplasm |
| #3 | carcinoma |
| #4 | malignant tumor |
| #5 | #1 or #2 or #3 or #4 |
| #6 | Xihuang pill |
| #7 | Xihuang capsule |
| #8 | Xihuang wan |
| #9 | #6 or #7 or #8 |
| #10 | #5 and #9 |
Fig. 2.
Summary of the flow of our literature search.
3. Results
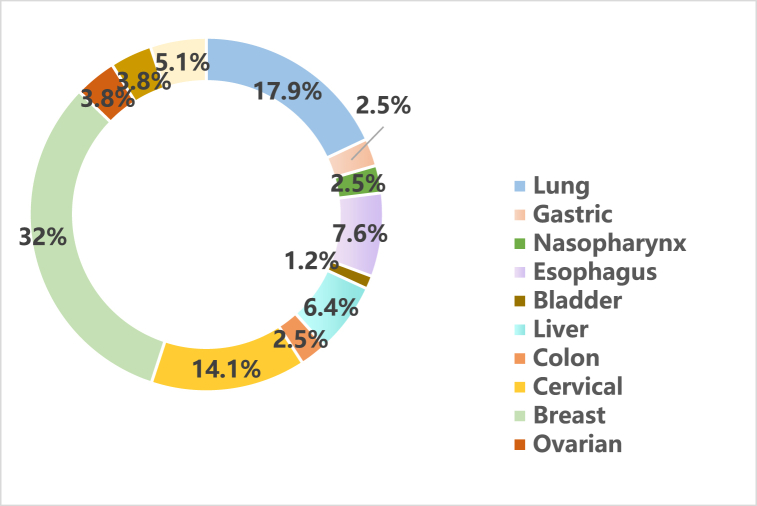
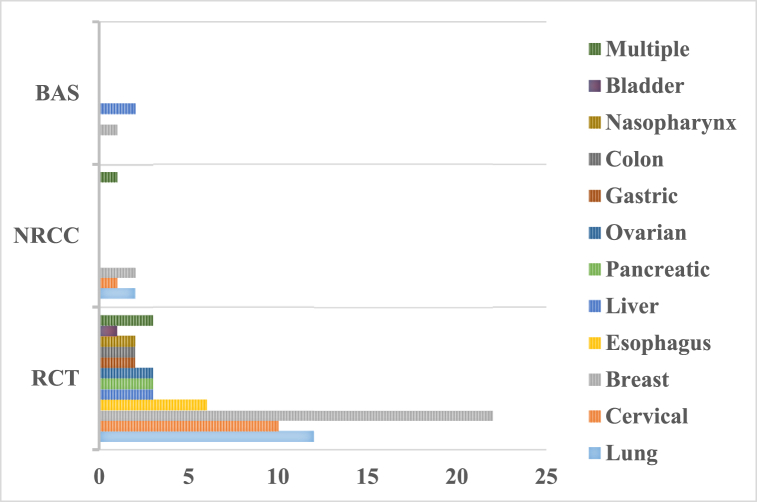
We identified 78 studies on clinical aspects of Xihuang pills/capsules in treating cancer, including 11 specific types of cancer and multiple types of cancer. Twenty-five studies reported in treating breast cancer (32%) [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]] (Table 2), 14 lung cancer (17.9%) [6,[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]] (Table 3), 11 cervical cancer (14.1%) [5,[50], [51], [52], [53], [54], [55], [56], [57], [58], [59]] (Table 4), 6 esophagus cancer (7.6%) [[60], [61], [62], [63], [64], [65]] (Table 5), 5 liver cancer (6.4%) [8,66 = 69], (Table 6), 3 pancreatic cancer (3.8%) [[66], [67], [68]] (Table 7), 3 ovarian cancer (3.8%) [7,69,70] (Table 7), 2 gastric cancer (2.5%) [9,71] (Table 7), 2 colon cancer (2.5%) [72,73] (Table 7), 2 nasopharynx cancer (2.5%) [74,75] (Table 7), 1 bladder cancer (1.2%) [76] (Table 7), and 4 multiples of cancer (5.1%) [[77], [78], [79], [80]] (Table 8). Among 78 studies, 69 types of research were RCTs, 6 NRCCs, and 3 BAS (Fig. 3, Fig. 4).
Table 2.
General study characteristics of included studies for Breast Cancer.
| Ref. | Stage | Research Type | Number of patients | Control group | Trial group | Daily dose | Treatment Period (week) |
Outcome |
|---|---|---|---|---|---|---|---|---|
| Chen [12] | Ⅱ-Ⅳ | RCT | 50 | XH + chemotherapy | chemotherapy | 1 g/bid | 6 | improve CR/PR, reduce adverse reactions, prolong survival |
| Hong et al. [13] | Ⅱ-Ⅳ | RCT | 42 | XH + chemotherapy | chemotherapy | 3 g/bid | 18 | improve CR/PR, QoL, prolong survival |
| Jia et al. [14] | Advanced | RCT | 40 | XH + chemotherapy | chemotherapy | 3 g/bid | 8 | alleviate pain |
| Jin et al. [15] | Ⅰ-Ⅳ | RCT | 30 | XH + chemotherapy | chemotherapy | 3 g/tid | 12 | improve CR/PR, regulate immunity |
| Li et al. [16] | Ⅰ-Ⅲ | RCT | 40 | XH + chemotherapy | chemotherapy | 1 g/bid | 48 | improve QoL, reduce tumor marker, regulate estrogen levels |
| Mao et al. [17] | Ⅱ-Ⅲ | RCT | 34 | XH + chemotherapy, surgery | chemotherapy, surgery | 2 g/bid | 5 years | improve CR/PR, QoL, lessen metastasis and recurrence |
| Peng et al. [18] | Ⅱ-Ⅲ | RCT | 46 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 2 g/bid | 12 | improve CR/PR, regulate immunity, reduce adverse reactions |
| Qi et al. [19] | Ⅲ-Ⅳ | RCT | 48 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 3 g/bid | 12 | improve CR/PR, regulate immunity, prolong survival |
| Qian et al. [20] | Ⅱ-Ⅳ | RCT | 45 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 3 g/bid | 12 | improve CR/PR, QoL, reduce adverse reactions |
| Wang [21] | Ⅲ-Ⅳ | RCT | 64 | XH + chemotherapy | chemotherapy | 3 g/bid | 12 | improve CR/PR, QoL, prolong survival, reduce tumor marker |
| Wang [22] | Ⅲ | RCT | 15 | XH + chemotherapy | chemotherapy | 2 g/bid | 24 | reduce adverse reactions |
| Wang et al. [23] | Ⅳ | RCT | 57 | XH + endocrine therapy | endocrine therapy | 3 g/bid | 12 | improve CR/PR, prolong survival, reduce tumor marker, regulate immunity |
| Wang [24] | Ⅱ-Ⅳ | RCT | 40 | XH + chemotherapy | chemotherapy | 1 g/bid | 6 | improve CR/PR, prolong survival, reduce adverse reactions |
| Wang [25] | Advanced | RCT | 49 | XH + chemotherapy | chemotherapy | 3 g/bid | 8 | improve CR/PR, regulate immunity, reduce tumor marker, improve hypercoagulative status |
| Wu et al. [26] | Ⅲ | RCT | 39 | XH + chemotherapy | chemotherapy | 2 g/bid | 18 | improve CR/PR, regulate immunity, reduce adverse reactions |
| Wu [27] | Ⅱ-Ⅳ | RCT | 45 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | regulate immunity, reduce adverse reactions and inflammation |
| Xu et al. [28] | Ⅲ-Ⅳ | RCT | 55 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | regulate immunity |
| Xu et al. [29] | Ⅲ-Ⅳ | RCT | 55 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | improve QoL, reduce inflammation |
| Xu et al. [30] | Ⅲ-Ⅳ | RCT | 55 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | improve CR/PR, reduce adverse reactions, prolong survival |
| Yue et al. [31] | Ⅲ | RCT | 39 | XH + chemotherapy | chemotherapy | 3 g/bid | 18 | improve CR/PR, regulate estrogen levels |
| Zhang et al. [32] | Ⅰ-Ⅲ | RCT | 45 | XH + chemotherapy | chemotherapy | 2 g/bid | 12 | improve QoL, reduce adverse reactions |
| Zhang et al. [33] | Ⅱ-Ⅳ | BAS | 32 | XH + chemotherapy | chemotherapy | 1.5 g/bid | 8 | improve QoL, reduce adverse reactions, regulate immunity |
| Zhang et al. [34] | Advanced | RCT | 40 | XH + chemotherapy | chemotherapy | 1.5 g/bid | 9 | improve CR/PR, QoL, reduce tumor marker, alleviate pain |
| Zhou et al. [35] | Ⅱ-Ⅳ | RCT | 22 | XH + radiotherapy | radiotherapy | 1 g/bid | 6 | improve QoL, regulate immunity |
| Zhou et al. [36] | Ⅲ | RCT | 44 | XH + chemotherapy | chemotherapy | 1 g/bid | 6 | improve CR/PR, reduce tumor marker, reduce adverse reactions |
Table 3.
General study characteristics of included studies for Lung Cancer.
| Ref. | Stage | Research Type | Number of patients | Control group | Trial group | Daily dose | Treatment Period (week) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Zeng et al. [37] | Advanced | NRCC | 40 | XH + chemotherapy | chemotherapy | 1–2g/bid | 6 | improve CR/PR, reduce adverse reactions |
| He et al. [38] | IV | RCT | 36 | XH + chemotherapy | chemotherapy | 1.5 g/bid | 8 | improve CR/PR, reduce adverse reactions |
| Chen et al. [6] | Advanced | RCT | 29 | XH + chemotherapy | chemotherapy | 1 g/bid | 6 | improve CR/PR, reduce adverse reactions |
| Chi et al. [39] | Medium and Advanced | RCT | 55 | XH + morphine | morphine | 2 g/bid | 2 | alleviate pain |
| Guo et al. [40] | Ⅲ-Ⅳ | RCT | 30 | XH + chemotherapy | chemotherapy | 3 g/bid | 8 | improve CR/PR, QoL, reduce adverse reactions |
| He et al. [41] | Ⅲ-Ⅳ | RCT | 40 | XH + chemotherapy | chemotherapy | 3 g/bid | 3 | improve QoL, reduce tumor marker |
| Liu et al. [42] | Ⅲ-Ⅳ | RCT | 25 | XH + chemotherapy | chemotherapy | 2 g/bid | 16 | improve CR/PR, regulate immunity, reduce adverse reactions |
| Du et al. [43] | Ⅲ-Ⅳ | RCT | 32 | XH + chemotherapy | chemotherapy | 1 g/bid | 16 | alleviate symptoms |
| Wu et al. [44] | Medium and Advanced | RCT | 49 | XH + chemotherapy | chemotherapy | 2 g/bid | 2 | improve QoL, reduce adverse reactions, alleviate pain |
| Xiao et al. [45] | Medium and Advanced | RCT | 30 | XH + radiotherapy, chemotherapy | chemotherapy, radiotherapy | 20 g/tid | 8 | improve CR/PR, QoL, reduce adverse reactions |
| Xu et al. [46] | Medium and Advanced | RCT | 40 | XH + heteropathy | heteropathy | 20 g/tid | 8 | improve PFS, OS, reduce adverse reactions |
| Yan et al. [47] | Ⅲ-Ⅳ | RCT | 50 | XH + oxycodone hydrochloride | oxycodone hydrochloride | 3 g/bid | 2 | improve QoL, alleviate pain, reduce adverse reactions |
| Zhang et al. [48] | Ⅲ-Ⅳ | RCT | 30 | XH + chemotherapy | chemotherapy | 2 g/bid | 6 | improve CR/PR, QoL, reduce adverse reactions |
| Zhang et al. [49] | Ⅰ-Ⅲ | NRCC | 20 | XH + chemotherapy | chemotherapy | 2 g/bid | 24 | improve CR/PR, reduce adverse reactions |
Table 4.
General study characteristics of included studies for Cervical cancer.
| Ref. | Stage | Research Type | Number of patients | Control group | Trial group | Daily dose | Treatment Period (week) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Bao et al. [5] | Ⅱ-Ⅲ | NRCC | 40 | XH + chemotherapy | chemotherapy | 1 g/tid | 8 | improve CR/PR, QoL, reduce adverse reactions |
| Chang [50] | Ⅲ-Ⅳ | RCT | 25 | XH + radiotherapy | radiotherapy | 1 g/bid | 4 | reduce adverse reactions |
| Chen et al. [51] | Ⅱ-Ⅳ | RCT | 77 | XH + chemotherapy+3D-CRT | chemotherapy+3D-CRT | 3 g/bid | 6 | reduce adverse reactions, prolong survival |
| Li et al. [52] | Ⅱ-Ⅲ | RCT | 45 | XH + radiotherapy | radiotherapy | 1 g/bid | 8 | improve CR/PR, reduce adverse reactions |
| Peng et al. [53] | Ⅲ-Ⅳ | RCT | 93 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 1.5 g/bid | 8 | improve CR/PR, reduce adverse reactions, lessen metastasis and recurrence |
| Qiu et al. [54] | Ⅱ-Ⅲ | RCT | 52 | XH + radiotherapy | radiotherapy | 2 g/bid | 3 | improve CR/PR, reduce adverse reactions, prolong survival |
| Sang et al. [55] | Ⅰ-Ⅲ | RCT | 40 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | reduce adverse reactions, regulate immunity |
| Shao [56] | Ⅰ-Ⅲ | RCT | 41 | XH + chemotherapy | chemotherapy | 3 g/tid | 12 | improve CR/PR, reduce adverse reactions, tumor marker |
| Shi [57] | Ⅲ-Ⅳ | RCT | 48 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | improve CR/PR, regulate immunity |
| Wang et al. [58] | Ⅲ-Ⅳ | RCT | 50 | XH + chemotherapy | chemotherapy | 3 g/tid | 12 | improve CR/PR, QoL, regulate immunity, reduce tumor marker |
| Zhang [59] | Ⅲ-Ⅳ | RCT | 39 | XH + chemotherapy | chemotherapy | 3 g/bid | 12 | improve QoL, reduce tumor marker, prolong survival |
Table 5.
General study characteristics of included studies for Esophagus cancer.
| Ref. | Stage | Research Type | Number of patients | Control group | Trial group | Daily dose | Treatment Period (week) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Cheng et al. [60] | Advanced | RCT | 18 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | improve CR/PR, reduce adverse reactions |
| Ma et al. [61] | Ⅱ-Ⅳ | RCT | 40 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 2 g/bid | 24 | improve CR/PR, reduce adverse reactions |
| Shao et al. [62] | Medium and Advanced | RCT | 30 | XH + chemotherapy | chemotherapy | 1.5 g/tid | 3 | improve CR/PR and QoL |
| Xu et al. [63] | Ⅰ-Ⅲ | RCT | 30 | XH + radiotherapy | radiotherapy | 2 g/bid | 4 | improve CR/PR and QoL |
| Xue [64] | Ⅰ-Ⅲ | RCT | 34 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 1.5 g/bid | 6 | improve CR/PR and QoL, reduce adverse reactions |
| Zhang et al. [65] | Medium and Advanced | RCT | 106 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 2 g/tid | 12 | improve QoL, reduce adverse reactions |
Table 6.
General study characteristics of included studies for Liver.
| Ref. | Stage | Research Type | Number of patients | Control group | Trial group | Daily dose | Treatment Period (week) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Cheng [8] | Advanced | BAS | 23 | XH + heteropathy + acesodyne | 3 g/bid | 18 | improve QoL, alleviate pain, reduce adverse reactions | |
| Feng et al. [81] | Ⅱ-Ⅲ | RCT | 49 | XH + chemotherapy | chemotherapy | 1–2g/bid | 8 | improve CR/PR, reduce adverse reactions, alleviate renal or hepatic impairment |
| Liu et al. [82] | Ⅱ-Ⅲ | RCT | 40 | XH + chemotherapy | chemotherapy | 3 g/bid | 2 | improve CR/PR and QoL, prolong survival |
| Wu et al. [83] | Advanced | RCT | 70 | XH + TACE | TACE | 3 g/bid | 2 | improve CR/PR, prolong survival |
| Zhang [84] | Ⅲ | BAS | 28 | XH + heteropathy | 3 g/bid | 6 | reduce adverse reactions, alleviate hepatic impairment | |
Table 7.
General study characteristics of included studies for Pancreatic cancer, Ovarian cancer, Gastric cancer, Colon cancer, Nasopharynx Cancer, Bladder Cancer.
| Ref. | Stage | Research Type | Number of patients | Control group | Trial group | Daily dose | Treatment Period (week) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Pancreatic cancer | ||||||||
| Yang et al. [66] | Medium and Advanced | RCT | 20 | XH + morphine | morphine | 2 g/bid | 4 | improve QoL, alleviate pain, |
| Zhang et al. [67] | Ⅱ-Ⅳ | RCT | 16 | XH + chemotherapy | chemotherapy | 6 g/qd | 6 | improve CR/PR and QoL, reduce tumor marker and adverse reactions |
| Zhu et al. [68] | Advanced | RCT | 17 | XH + chemotherapy | chemotherapy | 3 g/bid | 8–24 | improve QoL, reduce adverse reactions |
| Ovarian cancer | ||||||||
| Cheng et al. [7] | Advanced | RCT | 35 | XH + chemotherapy | chemotherapy | 3 g/bid | 12 | improve CR/PR, reduce adverse reactions |
| Guan et al. [69] | Ⅲ-Ⅳ | RCT | 35 | XH + chemotherapy | chemotherapy | 3 g/tid | 12 | improve CR/PR and QoL, regulate immunity, reduce adverse reactions |
| Zhang [70] | Ⅲ-Ⅳ | RCT | 45 | XH + chemotherapy | chemotherapy | 3 g/tid | 12 | improve CR/PR and QoL, regulate immunity, reduce adverse reactions |
| Gastric cancer | ||||||||
| Jiang [9] | Ⅳ | RCT | 20 | XH + chemotherapy | chemotherapy | 3 g/bid | 6 | improve CR/PR and QoL |
| Zhu et al. [71] | Advanced | RCT | 40 | XH + chemotherapy | chemotherapy | 2 g/bid | 12 | improve CR/PR, regulate immunity, prolong survival |
| Colon cancer | ||||||||
| Li et al. [72] | Advanced | RCT | 35 | XH + chemotherapy | chemotherapy | 1 g/bid | 6 | improve CR/PR and QoL, reduce adverse reactions |
| Zhang et al. [73] | Ⅳ | RCT | 60 | XH + chemotherapy | chemotherapy | 2 g/tid | 6–12 | reduce tumor marker and adverse reactions |
| Nasopharynx Cancer | ||||||||
| Ren et al. [74] | Advanced | RCT | 30 | XH + chemotherapy, radiotherapy | chemotherapy, radiotherapy | 2 g/bid | 12 | improve CR/PR and QoL, regulate immunity |
| Zhang et al. [75] | Ⅲ-Ⅳ | RCT | 40 | XH + radiotherapy | radiotherapy | 1–2g/bid | 2 | improve QoL, regulate immunity, reduce adverse reactions |
| Bladder Cancer | ||||||||
| Wang et al. [76] | 0-Ⅰ | RCT | 28 | XH + chemotherapy | chemotherapy | 3 g/bid | 12 | lessen recurrence, reduce adverse reactions |
Table 8.
General study characteristics of included studies for Multiple Cancer.
| Ref. | Stage | Research Type | Number of patients | Control group | Trial group | Daily dose | Treatment Period (week) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [77] | Advanced | RCT | 24 | XH + radiotherapy | radiotherapy | 3 g/tid | 3 | alleviate pain |
| Huang et al. [78] | Advanced | NRCC | 47 | XH + heteropathy | heteropathy | 3 g/bid | 3 | improve QoL |
| Liu et al. [79] | Medium and Advanced | RCT | 40 | XH + acesodyne | acesodyne | 2 g/bid | 2 | alleviate pain, reduce adverse reactions |
| Yang [80] | Ⅳ | RCT | 24 | XH + morphine | morphine | 2 g/bid | 2 | improve QoL, alleviate pain |
Fig. 3.
The proportion of different cancer types in the included studies.
Fig. 4.
Different categories of studies in treating cancer by Xihuang pills/capsules.
3.1. The stage of cancer treated by Xihuang pills/capsule
Among 78 studies, 11 types of cancer were mentioned to be treated by Xihuang pills/capsules in various stages. Stage Ⅲ (57 studies) and Ⅳ (50 studies) were the major target periods for the application of Xihuang pills/capsules, in contrast to Stage 0 (1 study) and I (9 studies). At the initial stage, bladder cancer was reported to be treated with Xihuang pills (Fig. 5).
Fig. 5.
The staging of different cancer treated by Xihuang pills/capsules.
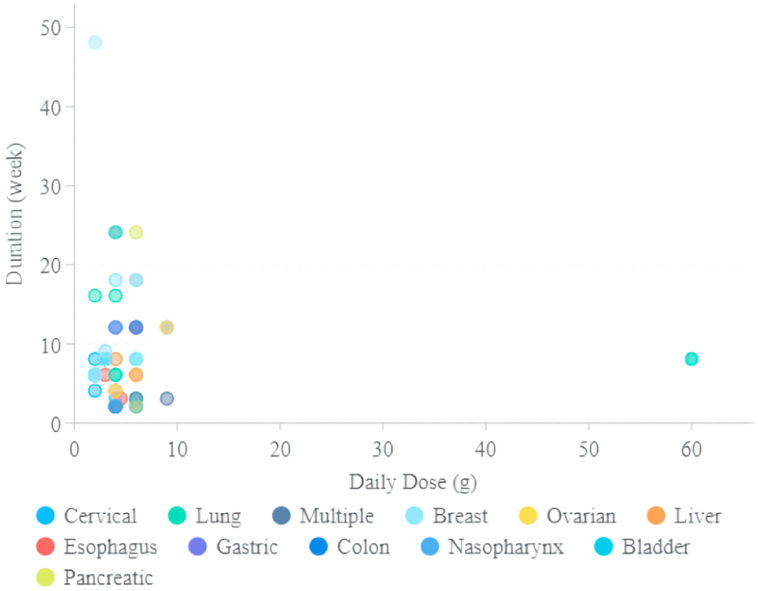
3.2. The daily doses and duration of Xihuang pills/capsule for cancer treatment
In terms of the daily dose, 32 studies indicated the daily dose of Xihuang pills/capsules was 6 g, including 2 g/tid, 3 g/bid, and 6 g/qd. Twenty-one studies showed the usage of Xihuang pills/capsule was 4 g/day and 2 g/day for 13 studies. The maxim daily doses of Xihuang pills/capsule reached 60 g, which was recorded in 2 studies, treating lung cancer. Regarding the treatment period, most studies revealed the duration was ≤12 weeks (6 weeks for 20 studies, 8 weeks for 11 studies, 12 weeks for 18 studies), while 12 studies demonstrated the treatment period was≥16 weeks (16 weeks for 2 studies, 18 weeks for 4 studies, 24 weeks for 4 studies, 48 weeks for 1 study), and one treatment of breast cancer was maintained for five years (Fig. 6).
Fig. 6.
The daily doses and duration of Xihuang pills/capsule for cancer treatment.
3.3. The therapeutic effect of Xihuang pills/capsules
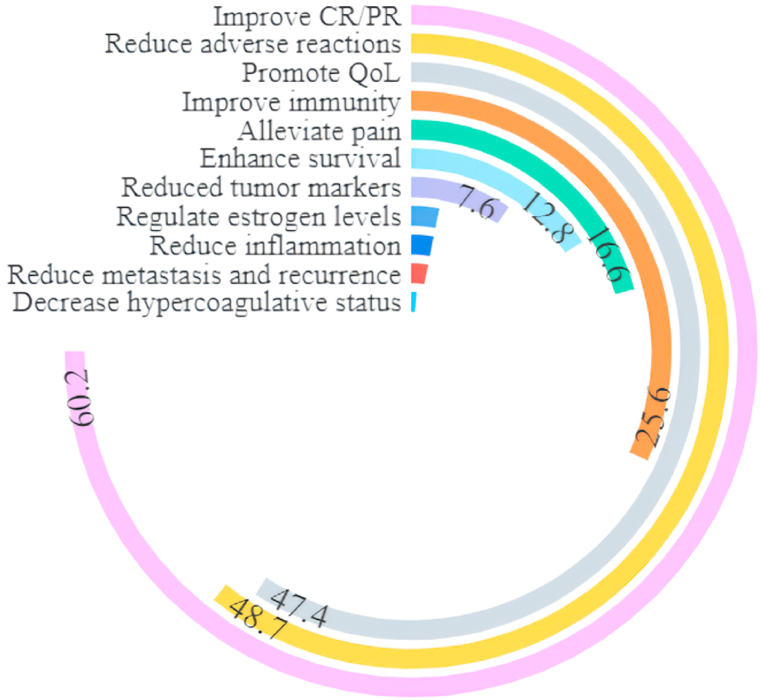
We summarized the outcome of RCTs studies and non-RCTs, comparing the control group and trial group or before and after treatment. Radiotherapy, chemotherapy, heteropathy, analgetic, and endocrine therapy were used in the control group, and Xihuang pills/capsules combined with which were applied in the trial group. According to the outcomes of included studies, improving CR or PR was demonstrated in 47 studies, and Xihuang pills/capsule show promise in reducing adverse reactions (38 studies) and promoting QoL (37 studies). In addition, improving immunity (20 studies) and alleviating pain (13 studies) were also reported as the effectiveness of Xihuang pills/capsules. Typically, it was conducive to applying Xihuang pills/capsules to prolong survival (10 studies) and reduce metastasis and recurrence (2 studies) (Fig. 7).
Fig. 7.
The proportion of literature related to the therapeutic effect of Xihuang pills/capsule.
4. Discussion
Xihaung pill is composed of Bovis Calculus Sativus, Moschus, Boswellia carterii Birdw. (Frankincense), and Commiphora myrrha (Nees) Engl. (Myrrha), with ratio 1:1:37:37 [10]. Involving three kinds of blood-stasis removing herbs (Huoxuehuayu function) and one kind of heat-clearing and detoxicate herb, the Xihaung pill has many beneficial effects such as heat-clearance and detoxification, activating blood circulation to dissipate blood stasis, and disintegrating scleroma, which was recorded to treat furunculosis, scrofula, and neoplasms in ancient China [85], and is still used today, especially for the treatment of Ruyan (Traditional Chinese medical diagnosis of breast cancer). Although breast cancer is the most common targeting cancer for the Xihuang pill, we witnessed Xihuang pill can be widely used to treat 11 types of cancer including lung, liver, ovarian, colon, nasopharynx, etc. We believed the Xihuang pill has a curative effect on different kinds of cancer, which means that it has good popularization in tumor treatment.
Compared with the initial stage of cancer, advanced cancer meets the dilemma of limited therapies, the constitution of patients, and the cachexia caused by invasion cancer. According to our results, the Xihuang pill became a promising option for the treatment of middle and advanced cancers and was used more often. This finding has not been focused on before because most studies are inclined to discuss the therapeutic effect of the Xihuang pill but not its targeting stage or dosage of it. The main therapeutic approach for advanced cancer is combined surgery with neoadjuvant chemotherapy, targeted therapy, or immunotherapy [86], which also brings many side effects such as multi-drug resistance, fatigue, weakened immune system, and gastrointestinal side effect [87]. Xihuang pill is used frequently in the middle and late stage with the above-mentioned therapy for cancer treatment, which may play the role of enhancing effect and reducing toxicity.
In addition, we paid special attention to the dosage of Xihuang pills because the safe dosage of drugs with HuoxueHuayu function is still unclear. Based on the Chinese Pharmacopoeia edition 2020 [88], the regular dose of the Xihuang pill is 3 g at a time, twice a day. Only 29 (37.1%) studies showed that the dosage of Xihuang pills was following the pharmacopoeia instructions, most of the studies were based on self-prescribed doses and did not exceed the standard dose, which means the clinical use of the Xihuang pill is relatively cautious. We should emphasize that the maximum dose of the Xihuang pill was 60 g/day, treating stage III lung cancer in 2 studies without records of adverse reactions. However, more evidence is needed to determine whether this dose is optimal, safe, and generally applicable to other cancer treatments.
Apart from the daily dose of Xihuang pills, the treatment period of Xihuang pills was also summarized to assess the medication safety. Due to the categories of studies (RCTs), the duration was limited to about 20 weeks in most cases. Even at the maximum dose (60 g/day), it did not exceed 8 weeks. Notably, two studies revealed that the Xihuang pill could be used as a supplement for 1 year or more, and the outcome of 5 consecutive years of use was tracked. The optimal duration of immunotherapy and chemotherapy treatment for cancer patients is unknown [89,90], while the Xihuang pill is usually combined with such therapies, which is difficult to evaluate the ideal treatment period. Meanwhile, without regular therapies, long-time intake of the Xihuang pill as a complementary therapy is acceptable for patients with cancer in China. Although there is rarely compelling evidence to demonstrate the beneficial effects of Xihuang pills on cancer patients when taken for a long time, according to the results of the current studies, Xihuang pills seem harmless to cancer patients when taken in small doses for a long time.
Xihuang pill combined with chemotherapy in comparison with chemotherapy alone could significantly enhance the tumor response, improve QoL, alleviate toxicity induced by chemotherapy [91], decrease hypercoagulability [92], regulate immunity, reduce tumor markers [10], and relieve the side effects of modern therapies [93], which is convinced by previous studies. We tried to summarize the specific effects of Xihuang pills more comprehensively than just those mentioned above. In terms of side effects, Xihuang pills enable to alleviate myelosuppression (leukopenia, anemia, thrombocytopenia), gastrointestinal reactions (nausea, vomiting, diarrhea, constipation, anorexia), renal or hepatic impairment, skin lesions (hand-foot syndrome, pruritus, alopecia), neurological reactions (dizziness, somnolence, insomnia, blurry vision), and has an analgesic effect. The treatment for cancer-related pain remains largely unchanged, so most patients with cancer-related pain are managed using opioids [94]. While in patients responding to strong opioids, long-term use of opioids will lead to many undesired side effects such as constipation, tolerance, and addiction [95]. We found that Xihuang pills could achieve the same or better pain relief as opioids and avoid the possibility of addiction, which provides a viable replacement for opioids. However, Xihuang pills should be used with caution in pregnant women because excessive Huoxuehuayu therapy may lead to abortion [96]. For non-pregnant women with cancer, 2 studies referred that Xihuang pills can not only improve the symptoms of hectic fever and night sweat but also regulate estrogen equalization. Besides that, Xihuang pills also have a positive effect on the prognosis of tumor treatment, reflected in extending survival by 1–2 years (10 studies) and reducing the 5-year recurrence and metastasis rate (2 studies), which was not mentioned in the previous review. Despite the relative lack of evidence, We believe that the Xihuang pill, the representative medicine for Huoxuehuayu therapy, tends to inhibit tumor metastasis and recurrence to prolong the lifetime in the treatment of tumors, which is relatively safe under the condition of rational use (Fig. 8).
Fig. 8.
Target cancer and effectiveness of the Xihuang Pill.
We tried to answer the question of whether the effect of the Xihuang pill on cancer treatment varies according to the type of cancer through relative literature, even though breast cancer is the most frequently treated cancer. Network pharmacology-based identification predicted that the potential therapeutic targets of Xihuang pills might be VEGFA, EGFR [97], IL-17, toll-like receptor, and tumor necrosis factor signaling pathways [98] (Akt/mTOR pathway [99], MEKK1/SEK1/JNK1/AP-1 pathway [100]), which can be found in most cancer. Hence, it is difficult to assess which cancer Xihuang pill is better for treatment. We surmised that the therapeutic effect of the Xihuang pill on different cancers may be similar due to its multi-target properties.
5. Suggestions for future research
We confronted some limitations based on published literature so that our conclusion might be conserved, and further research is deeply needed. First, the dosage forms of Xihuang pills included in the literature were divided into capsules and pills. Even though the ingredients and compatibility ratios are the same among the two types, in the theory of TCM formulations, different dosage forms will lead to certain differences in drug effects, reflected in the duration and the speed of effectiveness. Therefore, it will be beneficial to learn potential differences in dosage forms for the treatment of cancer patients. Second, we attempted to explore the possible differences in the targeting effect of the Xihuang pill on different tumors, such as improving CP or PR of breast cancer or prolonging the survival time of lung cancer. But unfortunately, due to the inclusion of a few references, Xihuang pills treat more types of cancer, and the experimental results do not have a certain tendency. Similarly, the included literature suggests that the Xihuang pill is mainly used for the treatment of stage III-IV cancer. We are not sure whether publication bias leads to rare literature in the initial stage of treatment of the Xihuang pill, and therefore the difference in treatment effect of different stages cannot be obtained. Besides, we found that there was a great span in the dosage and time of Xihuang pill application, and there was no significant difference in the therapeutic effect, so the optimal dosage and treatment time of the Xihuang pill could not be determined. In future studies, we need more evidence on the efficacy of the Xihuang pill in treating different tumor stages (especially the initial stage) and different tumor types (especially tumors other than breast cancer). Third, treatment based on syndrome differentiation is one of the distinctive features of TCM. Xihuang pills are used to treat patients with the syndrome of blood stasis and heat toxicity. In this study, almost all literature did not indicate that patients with cancer undergo TCM syndrome differentiation, as long as they met the cancer diagnosis. Based on the treatment effect, we speculated that cancer patients had the syndrome of blood stasis and heat toxicity, especially in patients with advanced cancer, which needs more compelling evidence in the future.
6. Conclusion
In conclusion, the highlight of this study is to provide relatively comprehensive insight into the application of Xihuang pills in cancer treatment. Xihuang pill has a therapeutic effect on various kinds of cancer, and the therapeutic effect may not be different in terms of tumor stage and types, which is reflected in enhancing the tumor response, reducing adverse reactions/toxicity induced by modern therapies, promoting QoL, alleviating pain, prolonging survival, lessening metastasis and recurrence, reducing inflammation, regulating estrogen levels, improving hypercoagulative status, and decreasing tumor markers. The optimal dose and duration of Xihuang pills have not yet been determined. Given the present study, the Xihuang pill is generally applicable and safe in the treatment of middle and advanced cancer, demonstrating the safety and effectiveness of HuoxueHuayu therapy in the treatment of tumors.
Author contribution statement
Zehui Chen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shuo Yang: Conceived and designed the experiments.
Jing An: Analyzed and interpreted the data.
Yue Wei: Analyzed and interpreted the data.
Zhiming Li: Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
Consent for publication
The authors all agreed for publication of this paper.
Funding
This work was supported by Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences, No: CI2021A05309.
Declaration of interest's statement
The authors declare no competing interests.
Contributor Information
Zehui Chen, Email: chenzh@mail.cintcm.ac.cn.
Shuo Yang, Email: y1y6y3@139.com.
Abbreviations
- CR
Complete Response
- BAS
before-after study
- NRCC
non-randomized concurrent control trial
- OS
Overall Survival
- PFS
progression-free survival
- PR
Partial Response
- QoL
Quality of life
- RCT
randomized control trial
- TACE
Transhepatic Arterial Chemotherapy And Embolization
- TCM
Traditional Chinese Medicine
- XH
Xihuang pill or capsule
- 3D-CRT
3 dimensional conformal radiation therapy
References
- 1.Ali A., Manzoor M.F., Ahmad N., et al. The burden of cancer, government strategic policies, and challenges in Pakistan: a comprehensive review. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.940514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S.C., Liu Y.W., Feng Y.B., et al. A review on curability of cancers: more efforts for novel therapeutic options are needed. Cancers. 2019;11(11) doi: 10.3390/cancers11111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H., Fang J.S., Fan X.D., et al. Advances in molecular mechanisms for traditional Chinese medicine actions in regulating tumor immune responses. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Wang S., Zhang Y., Fan H.T., Lin H.S. Traditional Chinese medicine and cancer: history, present situation, and development. THORACIC CANCER. 2015;6(5):561–569. doi: 10.1111/1759-7714.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao Y., Jiang X. Clinical summary of Xihuang pill combined with chemotherapy in the treatment of advanced cervical cancer. J. Aerospace Medicine. 2017;28(8):995–997. [Google Scholar]
- 6.Chen Y. Clinical efficacy and safety of Xihuang capsule combined with chemotherapy in the treatment of advanced lung adenocarcinoma. Medical Diet and Health. 2020;18(11):12–13. [Google Scholar]
- 7.Cheng X., Yi Y., Shen C., Yu Y., Liao Z., Wang Q. Effect of Xihuang pill combined with TP chemotherapy regimen on tumor markers and Th1/Th2 cytokines in patients with advanced ovarian cancer. Medical Innovation of China. 2021;18(36):76–80. [Google Scholar]
- 8.Cheng Z. Clinical observation on Xihuang Pill in treating 23 cases of advanced primary hepatic cancer. China J. Traditional Chinese Medicine. 2010;25(1):52–54. [Google Scholar]
- 9.Jiang J. Clinical analysis of Xihuang pill combined with Teggio regimen in the treatment of advanced gastric cancer. Contemporary Medicine. 2021;27(27):115–116. [Google Scholar]
- 10.Ge A., Yang K., Deng X., Zhao D., Ge J., Liu L. The efficacy and safety of Xihuang Pill/capsule in adjuvant treatment of breast cancer: a systematic review and meta-analysis of 26 randomized controlled trials. J. Ethnopharmacol. 2022;295 doi: 10.1016/j.jep.2022.115357. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z., Wang A., Wei Y., Zhu Y., An J., Li Z. Effectiveness and safety of the traditional Chinese medicine treatment (HuoxueHuayu therapy) for malignant tumors: a systematic review and meta-analysis. Evid. base Compl. Alternative Med. 2022;2022 doi: 10.1155/2022/7944063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L. Clinical observation of Xihuang capsule combined with chemotherapy in the treatment of advanced breast cancer. China Health Standard Management. 2016;7(13) [Google Scholar]
- 13.Hong R., Wu Y., Wu Y. Clinical observation of Xihuang pill in adjuvant treatment of advanced breast cancer. China J. Chin. Mater. Med. 2014;39(6) [Google Scholar]
- 14.Jia W., Tian F., Xing X., Zhang L. Clinical study of Xihuang pill combined with Zoleic acid injection in the treatment of bone metastasis of breast cancer. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2009;(3) [Google Scholar]
- 15.Jin J., Li Z. Xihuang pill combined with chemotherapy in the treatment of 30 cases of breast cancer. China Journal of Traditional Chinese Medicine and Pharmacy. 2010;(5) [Google Scholar]
- 16.Li J., Han B. Effect of Xihuang capsule adjuvant therapy on estrogen receptor, tumor markers and estrogen in breast cancer patients. Clinical Journal of Traditional Chinese Medicine. 2020;32(7) [Google Scholar]
- 17.Mao D., Huang L., Zhou C., et al. Clinical observation of Xihuang capsule in the treatment of 34 cases of triple negative breast cancer after postoperative chemotherapy. New Chinese Medicine. 2014;46(4):155–157. [Google Scholar]
- 18.Peng G., Tang Y., Li B. Clinical efficacy and safety of conventional chemotherapy combined with Xihuang capsule in the treatment of advanced breast cancer. Chinese Journal of Clinical Rational Drug Use. 2021;14(21) [Google Scholar]
- 19.Qi A., Yan F., Lin L. Efficacy and safety of Xihuang pill combined with paclitaxel and cisplatin in the treatment of breast cancer. Modern Practical Medicine. 2020;32(10) [Google Scholar]
- 20.Qian W., Li Z., Li R., et al. Effect analysis of Xihuang pill combined with TP regimen in the treatment of advanced breast cancer. Liaoning J. Traditional Chinese Medicine. 2020;47(6):115–117. [Google Scholar]
- 21.Wang B. Clinical study of Xihuang pill combined with TP regimen in the treatment of advanced breast cancer. Drugs & Clinic. 2018;33(7) [Google Scholar]
- 22.Wang H. Clinical efficacy and safety of Xihuang capsule in the treatment of advanced breast cancer. Healthy women. 2021;(40):13. [Google Scholar]
- 23.Wang J., Yang H. Drugs & Clinic; 2019. Clinical Study of Xihuang Pill Combined with Letrozole in the Treatment of Advanced Breast Cancer. [Google Scholar]
- 24.Wang L. Clinical observation of Xihuang capsule combined with chemotherapy in the treatment of advanced breast cancer. Acta Chinese Medicine and Pharmacology. 2015;43(6) [Google Scholar]
- 25.Wang P., Liu C., Zhu H., Wu Y. Effect of Xihuang pill combined with CAF regimen in the treatment of patients with advanced breast cancer. Pharmacology and Clinics of Chinese Materia Medica. 2017;33(5) [Google Scholar]
- 26.Wu J., Wang D. Clinical study of Xihuang capsule combined with TEC chemotherapy regimen in the treatment of stage ⅲ breast cancer and its effect on immune function. New Chinese Medicine. 2020;52(12) [Google Scholar]
- 27.Wu G. Efficacy of Xihuang pill in adjuvant treatment of breast cancer and its influence on immune function of patients. Shanxi Medical Journal. 2016;45(21) [Google Scholar]
- 28.Xu W., Ding S., Shi L. Effect of Xihuang pill combined with GP regimen on cellular immune function in patients with advanced breast cancer. Hebei Medical Journal. 2021;43(15) [Google Scholar]
- 29.Xu W., Ding S., Shi L. Effect of Xihuang pill combined with GP regimen on inflammatory factors, oxidative stress and expression of proliferation and invasion genes in patients with advanced breast cancer. Hebei Medical Journal. 2021;43(18) [Google Scholar]
- 30.Xu W., Ding S., Xu N., Shi L., Qi B. Clinical observation of Xihuang pill combined with GP regimen in the treatment of advanced breast cancer. Hebei Medical Journal. 2020;42(9) [Google Scholar]
- 31.Yue Y., Zeng Y., Zheng H., He J., Sun Z. Effect of TAC regimen combined with Xihuang pill on P53,HER-2 and TOP ⅱ levels in patients with stage ⅲ breast cancer. Prog. Mod. Biomed. 2017;17(8):1505–1508. [Google Scholar]
- 32.Zhang J., Zhang Y., Meng H., et al. Clinical study of Xihuang capsule adjuvant chemotherapy in breast cancer patients. Global Traditional Chinese Medicine. 2015;8(1) [Google Scholar]
- 33.Zhang X., Huo D., Ma Y., Hu L., Chen S., Li H. Effect of Xihuang capsule on immune function and quality of life in patients with triple negative breast cancer. Chinese and Foreign Medical Research. 2019;17(5) [Google Scholar]
- 34.Zhang X., Li Z., Bai J., et al. Clinical study of Xihuang capsule combined with albumin paclitaxel in the treatment of advanced triple negative breast cancer. Chin. Tradit. Herb. Drugs. 2020;51(24) [Google Scholar]
- 35.Zhou H., Chen X., Wang Z., Yu Z. Effect of Xihuang capsule on immune function and quality of life in postoperative radiotherapy patients with breast cancer. China Practical Medicine. 2014;(20) [Google Scholar]
- 36.Zhou X., Liao P., Wu W., Jiang H., Jiang H. Effect of Xihuang capsule combined with GP regimen on serum levels of TNF-α,VEGF,MMP-2 and MMP-9 in patients with advanced breast cancer. Prog. Mod. Biomed. 2017;17(23) [Google Scholar]
- 37.Zeng Y. Efficacy of Xihuang capsule combined with DP regimen in the treatment of advanced non-small cell lung cancer. Chinese Journal of Clinical Rational Drug Use. 2020;13(31):93–94. [Google Scholar]
- 38.He F., Yi H., Zhou F., Jian X., Chen S. To observe the clinical efficacy and side effects of Xihuang capsule combined with gefitinib in the treatment of advanced lung adenocarcinoma. The Journal of Medical Theory and Practice. 2018;31(16):2384–2385. [Google Scholar]
- 39.Chi F. Clinical effect of Xihuang capsule combined with morphine hydrochloride sustained-release tablets in the treatment of advanced lung cancer pain. Health Protection and Promotion. 2017;(16):141. [Google Scholar]
- 40.Guo H., Tian F., Jia W., Xing X. Clinical observation of Xihuang pill combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Journal of Emergency in Traditional Chinese. 2008;17(1) [Google Scholar]
- 41.He S., Gao D., Jin X., Wang Y., Zhang F., Liu B. Efficacy of vinorelbine + cisplatin chemotherapy combined with Xihuang pill in the treatment of lung squamous cell carcinoma. Clin. J. Med. Off. 2020;48(3):305–306. [Google Scholar]
- 42.Liu T., Chen X., Qin X. Effects of Xihuang capsule combined with chemotherapy on adverse reactions and immune function in patients with non-small cell lung cancer. Systems Medicine. 2018;3(1):1–3+6. [Google Scholar]
- 43.Du F., Min X. Clinincal effect of chemotherapy alone or in combination with Xihuang capsule in treatment of advanced lung adenocarcinoma. Journal of Anhui University of Chinese Medicine. 2018;37(2):34–36. [Google Scholar]
- 44.Wu S., Wu B. Clinical observation of Xihuang capsule combined with morphine hydrochloride sustained-release tablets in the treatment of pain in middle and advanced lung cancer. China Medicine and Pharmacy. 2012;2(3) [Google Scholar]
- 45.Xiao L., Li Q., Yan P. Observation on the clinical efficacy of Xihuang capsule in enhancing chemoradiotherapy of lung cancer. Tianjin Pharmacy. 2007;19(6) [Google Scholar]
- 46.Xu H., Wei D. Clinical efficacy of Xihuang capsule combined with chemoradiotherapy in the treatment of advanced lung cancer. China Health Care & Nutrition. 2016;26(27):249. [Google Scholar]
- 47.Yan Q., Yang W., Xiong W. Efficacy evaluation and mechanism analysis of Xihuang pill combined with oxycodone sustained-release tablets in the treatment of lung cancer pain. The Journal of Medical Theory and Practice. 2021;34(22) [Google Scholar]
- 48.Zhang J., Niu Y. Efficacy analysis of Xihuang capsule combined with DP regimen chemotherapy in the treatment of advanced non-small cell lung cancer. Chinese Medicine Modern Distance Education of China. 2019;17(3) [Google Scholar]
- 49.Zhang C., Yan L., Li H. Clinical effect of Xihuang capsule on reducing toxicities and side effects of chemotherapy in lung cancer. Guangxi Medical Journal. 2016;38(5) [Google Scholar]
- 50.Chang X. Effect of pelvic radiotherapy combined with Xihuang capsule on alleviating myelosuppression of cervical cancer. Practical Journal of Medicine & Pharmacy. 2017;34(11):980–981. [Google Scholar]
- 51.Chen J., Xu Y., Wang J., Fan H., Jiang C., Wang Z. Effect of Xihuang pill on the short-term efficacy of three-dimensional conformal radiotherapy combined with intracavitary afterloading radiotherapy in the treatment of advanced cervical cancer. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2016;25(26):2916–2918. [Google Scholar]
- 52.Li L., Ke X. Clinical observation of 45 cases of advanced cervical cancer treated by Xihuang capsule combined with radiotherapy. Acta Chinese Medicine and Pharmacology. 2009;37(4):78–79. [Google Scholar]
- 53.Peng M., Ma L., Wang Y., Zhang J., Wang X. Clinical observation of Xihuang capsule combined with gemcitabine in the treatment of advanced cervical cancer. New Chinese Medicine. 2018;50(11):169–171. [Google Scholar]
- 54.Qiu G., Liu M., Fang B., Zhang H. Clinical observation of Xihuang capsule combined with image-guided radiotherapy in the treatment of advanced cervical cancer. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2017;26(27):3007–3009. [Google Scholar]
- 55.Sang X., Liu G. Effect of Xihuang pill combined with paclitaxel and platinum neoadjuvant chemotherapy on postoperative efficacy in patients with advanced cervical cancer. Drug Evaluation Research. 2017;40(8):1112–1116. [Google Scholar]
- 56.Shao X. Effect of Xihuang pill combined with neoadjuvant chemotherapy on prognosis and Th1/Th2 cytokines in patients with advanced cervical cancer. Chinese Journal of Rational Drug Use. 2020;17(6):92–96. [Google Scholar]
- 57.Shi Z. Effect of Xihuang pill combined with chemotherapy on the immune function of patients with advanced cervical cancer undergoing surgery. Strait Pharmaceutical Journal. 2018;30(9):150–152. [Google Scholar]
- 58.Wang X., Shen H., Xu D., Ye L., Cheng S. Effect of Xihuang pill combined with TP neoadjuvant chemotherapy on chemotherapy efficacy, immune function and tumor markers in patients with advanced cervical cancer. Hebei Journal of Traditional Chinese Medicine. 2018;40(6):827–831+836. [Google Scholar]
- 59.Zhang G. Effect analysis of Xihuang pill combined with TP regimen chemotherapy on advanced cervical cancer. China & Foreign Medical Treatment. 2020;39(19):11–14. [Google Scholar]
- 60.Cheng Z., Zhu W. Clinical observation of Xihuang pill combined with chemotherapy in the treatment of 18 cases of advanced esophageal carcinoma. China Journal of Traditional Chinese Medicine and Pharmacy. 2010;25(8):1302–1304. [Google Scholar]
- 61.Ma H., Luo Q., Zhang X. Improving effect of Xihuang capsule on postoperative side effects of radiotherapy and chemotherapy in patients with esophageal squamous cell carcinoma. Guangxi Medical Journal. 2019;41(9):1116–1118. [Google Scholar]
- 62.Shao Y., Sun H. Clinical observation of Xihuang capsule combined with chemotherapy in the treatment of 30 cases of advanced esophageal cancer. China Modern Medicine. 2009;16(17):61–62. [Google Scholar]
- 63.Xu H., Zhang L. Short-term efficacy of Xihuang capsule assisted intensity-modulated radiotherapy for esophageal cancer reradiotherapy. J. Pract. Oncol. 2018;33(1):61–65. [Google Scholar]
- 64.Xue X. Application value of Xihuang capsule in neoadjuvant chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Henan Medical Research. 2019;28(1):130–132. [Google Scholar]
- 65.Zhang N., Wang J., Cheng G., He M. Efficacy evaluation of Xihuang capsule adjuvant chemoradiotherapy in patients with advanced esophageal cancer. J. Jilin Univ. (Sci. Ed.) 2017;43(4):812–817. [Google Scholar]
- 66.Yang J., Cui L. Analgesic effect of Xihuang capsule combined with morphine sulfate sustained-release tablets on patients with advanced pancreatic cancer. Practical Journal of Medicine & Pharmacy. 2014;31(6):486–487. [Google Scholar]
- 67.Zhang Y., Jia Y., Sun Y., Chen J. Clinical benefit analysis of Xihuang pill combined with gemcitabine for advanced pancreatic cancer. Chin. Tradit. Pat. Med. 2010;32(1):13–15. [Google Scholar]
- 68.Zhu S., Zhu W., Cheng Z. 2009. Clinical Observation of Xihuang Pill Combined with Chemotherapy in the Treatment of 17 Cases of Advanced Pancreatic Cancer. Paper presented at: Xihuang pill clinical application expanded research academic activities 20092009; Beijing China. [Google Scholar]
- 69.Guan Y., Liu J., Wu Q. Effect of Xihuang pill combined with TP regimen on postoperative efficacy and immune function of advanced ovarian cancer. Hebei Medical Journal. 2015;37(22):3429–3431. [Google Scholar]
- 70.Zhang C. Postoperative effect of Xihuang pill combined with TP regimen in the treatment of advanced ovarian cancer and its influence on immune function. Journal of China Prescription Drug. 2018;16(3):76–77. [Google Scholar]
- 71.Zhu X., Tang Z., Hu P., Huang G. Clinical observation of Xihuang capsule assisted with oxaliplatin and tegano chemotherapy in the treatment of advanced gastric cancer. Shaanxi J. Tradit. Chin. Med. 2016;37(3):321–323. [Google Scholar]
- 72.Li R., Hu J. Clinical study of Xihuang capsule combined with pemetrexed in the treatment of advanced colon cancer. Drugs & Clinic. 2019;34(9):2790–2793. [Google Scholar]
- 73.Zhang X., Xing R., Miao Z., Guo Z., Han G. Effect of Xihuang capsule adjuvant chemotherapy on postoperative liver metastasis of colon cancer and its influence on peripheral blood IL-17 and IL-6. Chin. Tradit. Herb. Drugs. 2015;46(6):871–874. [Google Scholar]
- 74.Ren Q., Xiong R., He Y., Zhuge J., Lu D., Jiang X. Clinical study of Xihuang capsule combined with CIK cells in the treatment of advanced nasopharyngeal carcinoma. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2021;30(6):584–587+592. [Google Scholar]
- 75.Zhang S., Wang Y., Yang Z., Yu J., Liu H. Clinical efficacy of Xihuang capsule combined with radiotherapy in the treatment of nasopharyngeal carcinoma. Chin. J. Exp. Tradit. Med. Formulae. 2015;21(23):193–196. [Google Scholar]
- 76.Wang Y., Jiang W. Effect of Xihuang pill combined with intravesical chemotherapy on postoperative superficial bladder cancer. China Practical Medicine. 2016;11(31):131–132. [Google Scholar]
- 77.Chen G., Peng B., Zhu J., et al. Clinical observation of Xihuang pill combined with three dimensional conformal radiotherapy in the treatment of bone metastatic cancer pain. Clinical Journal of Chinese Medicine. 2017;9(32):120–121. [Google Scholar]
- 78.Huang S., Zhu Y. Clinical observation of Xihuang pill in the treatment of patients with advanced hepatic, bile duct and pancreatic cancer. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 1995;(2):6–7. [Google Scholar]
- 79.Liu H., Zhou Y., Zeng S., Xiong S. Clinical observation of Xihuang capsule combined with oxycodone hydrochloride sustained release tablets in the treatment of gynecological malignant tumor cancer pain. Jiangxi Medical Journal. 2016;51(7):669–671. [Google Scholar]
- 80.Yang J. Clinical study of Xihuang capsule combined with western medicine in the treatment of advanced cancer pain. Asia-Pacific Traditional Medicine. 2015;11(4):113–115. [Google Scholar]
- 81.Xue F., Yi L., Chen T. Effects of Xihuang capsule combined with sorafenib toluenesulfonic acid tablets on efficacy, adverse reactions and liver function in patients with liver cancer treated with chemotherapy. Chinese Archives of Traditional Chinese. 2020;38(12):221–224. [Google Scholar]
- 82.Liu B., Yu s, Xing L., Zhao X., Lv Y., Gao Q. Analysis of therapeutical effects of Xihuang Pills with intraarterial intervention chemotherapy on 80 cases of advanced primary hepatic carcinoma. China Journal of Traditional Chinese Medicine. 2010;25(6):947–948. [Google Scholar]
- 83.Wu X., Zhu L. Effect of Xihuang pill combined with transcatheter arterial chemoembolization on the survival of patients with advanced liver cancer. Jiangxi Medical Journal. 2016;51(8):774–775. [Google Scholar]
- 84.Zhang Z. Clinical observation of Xihuang pill in treating 28 cases of advanced primary liver cancer. Hebei Journal of Traditional Chinese Medicine. 2012;34(4):581–582. [Google Scholar]
- 85.Wu R., Chen J., Li S., et al. Research progress on antitumor mechanism of Xihuang pill. Hunan Journal of Traditional Chinese Medicine. 2021;37(12):190–192. [Google Scholar]
- 86.Chen Z-d, Zhang P.-F., Xi H.-Q., Wei B., Chen L., Tang Y. Recent advances in the diagnosis, staging, treatment, and prognosis of advanced gastric cancer: a literature review. Front. Med. 2021;8 doi: 10.3389/fmed.2021.744839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang K.-L., Yu Y.-C., Chen H.-Y., et al. Recent advances in Glycyrrhiza glabra (Licorice)-Containing herbs alleviating radiotherapy- and chemotherapy-induced adverse reactions in cancer treatment. Metabolites. 2022;12(6) doi: 10.3390/metabo12060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Commission C.P. Chinese Pharmacopoeia. China Medical Science and Technology Press; Beijing: 2020. Chinese pharmacopoeia; p. 876. [Google Scholar]
- 89.Smith A., Shaghayegh G., Menzies A.M., Roberts-Thomson R. Duration of immunotherapy - should we continue ad infinitum? Intern. Med. J. 2020;50(7):865–868. doi: 10.1111/imj.14919. [DOI] [PubMed] [Google Scholar]
- 90.Bisogno G., Hawkins D.S. An unresolved issue in rhabdomyosarcoma treatment: the duration of chemotherapy. Pediatr. Blood Cancer. 2020;67(5) doi: 10.1002/pbc.28174. [DOI] [PubMed] [Google Scholar]
- 91.Mao D., Feng L., Huang S., Zhang S., Peng W., Zhang S. Meta-analysis of Xihuang pill efficacy when combined with chemotherapy for treatment of breast cancer. Evid. base Compl. Alternative Med. 2019;2019 doi: 10.1155/2019/3502460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu D., An G.Y. Clinical effects of Xihuang pill combined with chemotherapy in patients with advanced colorectal cancer. Evid. base Compl. Alternative Med. 2017;2017 doi: 10.1155/2017/5936086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo Q., Lin J., Liu R., et al. Review on the applications and molecular mechanisms of Xihuang pill in tumor treatment. Evid. base Compl. Alternative Med. 2015 doi: 10.1155/2015/854307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Irwin M., Petersen K.S., Smith M.A. Rapid buprenorphine induction for cancer pain in pregnancy. J. Palliat. Med. 2021;24(8):1257–1262. doi: 10.1089/jpm.2020.0524. [DOI] [PubMed] [Google Scholar]
- 95.Zhang H. Cancer pain management-new therapies. Curr. Oncol. Rep. 2022;24(2):223–226. doi: 10.1007/s11912-021-01166-z. [DOI] [PubMed] [Google Scholar]
- 96.Li Y. Nanjing University of Chinese Medicine, Nanjing University of Chinese Medicine; Nanjing: 2017. Literature Research of Pregnant Forbidden Chinese Medicine. [Google Scholar]
- 97.Zhao X., Hao J., Chen S. Network pharmacology-based strategy for predicting therapy targets of traditional Chinese medicine Xihuang pill on liver cancer. Evid. base Compl. Alternative Med. 2020:2020. doi: 10.1155/2020/6076572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu J., Luo D., Li S. Network pharmacology-oriented identification of key proteins and signaling pathways targeted by Xihuang pill in the treatment of breast cancer. Breast Cancer. 2020;12:267–277. doi: 10.2147/BCTT.S284076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fu J., Zhu S.-H., Xu H.-B., et al. Xihuang pill potentiates the anti-tumor effects of temozolomide in glioblastoma xenografts through the Akt/mTOR-dependent pathway. J. Ethnopharmacol. 2020:261. doi: 10.1016/j.jep.2020.113071. [DOI] [PubMed] [Google Scholar]
- 100.Su L., Jiang Y., Xu Y., et al. Xihuang pill promotes apoptosis of Treg cells in the tumor microenvironment in 4T1 mouse breast cancer by upregulating MEKK1/SEK1/JNK1/AP-1 pathway. Biomed. Pharmacother. 2018;102:1111–1119. doi: 10.1016/j.biopha.2018.03.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.