Abstract
Nonalcoholic fatty liver disease (NAFLD) is the liver manifestation of the metabolic syndrome. NAFLD constitutes a spectrum of pathologies ranging from simple hepatic steatosis (nonalcoholic fatty liver) to the more progressive form of steatohepatitis and fibrosis, which can culminate in liver cirrhosis and hepatocellular carcinoma. Macrophages play multiple roles in the context of NAFLD pathogenesis by regulating inflammatory responses and metabolic homeostasis in the liver and thereby may represent an attractive therapeutic target. Advances in high-resolution methods have highlighted the extraordinary heterogeneity and plasticity of hepatic macrophage populations and activation states thereof. Harmful/disease-promoting as well as beneficial/restorative macrophage phenotypes co-exist and are dynamically regulated, thus this complexity must be taken into consideration in strategies concerning therapeutic targeting. Macrophage heterogeneity in NAFLD includes their distinct ontogeny (embryonic Kupffer cells vs bone marrow–/monocyte-derived macrophages) as well as their functional phenotype, for example, inflammatory phagocytes, lipid- and scar-associated macrophages, or restorative macrophages. Here, we discuss the multifaceted role of macrophages in the pathogenesis of NAFLD in steatosis, steatohepatitis, and transition to fibrosis and hepatocellular carcinoma, focusing on both their beneficial and maladaptive functions at different disease stages. We also highlight the systemic aspect of metabolic dysregulation and illustrate the contribution of macrophages in the reciprocal crosstalk between organs and compartments (eg, the gut–liver axis, adipose tissue, and cardiohepatic metabolic interactions). Furthermore, we discuss the current state of development of pharmacologic treatment options targeting macrophage biology.
Keywords: NASH, macrophage, liver fibrosis, HCC, type 2 diabetes, obesity
Summary.
Distinct macrophage populations are key drivers in promoting but also in attenuating disease progression during all states of nonalcoholic fatty liver disease pathogenesis, making them an attractive therapeutic target. This review provides an overview of the broad spectrum of functionally diverse macrophage phenotypes in nonalcoholic fatty liver disease and related systemic metabolic diseases. The focus was placed on pathogenic relationships and mechanistic interactions of different macrophage populations, as well as on possible therapeutic approaches targeting their (mal)function.
Nonalcoholic fatty liver disease (NAFLD) is the most frequent chronic liver disease in the world, with a prevalence of 25% to 30% in Western societies.1 NAFLD can progress to diseases associated with poor outcome, such as liver cirrhosis and hepatocellular carcinoma, placing an increasing burden on health care systems.1 NAFLD is considered the hepatic manifestation of the metabolic syndrome because it is strongly associated with obesity, type 2 diabetes, dyslipidemia, and cardiovascular disease.2 The collective term NAFLD encompasses various phenotypes of metabolic liver disease, ranging from simple fat accumulation termed hepatic steatosis or nonalcoholic fatty liver, to nonalcoholic steatohepatitis (NASH), displaying additional lobular inflammation and hepatocellular damage. In NASH, a proinflammatory hepatic microenvironment stimulates fibrogenic processes leading to liver fibrosis and, eventually, cirrhosis.3 The only curative therapy for end-stage liver disease and the early stages of hepatocellular carcinoma is liver transplantation. Because of the increasing prevalence and lack of diagnostic and therapeutic strategies, NAFLD is likely to become the leading indication for liver transplantation in the near future.4
The pathogenic processes of NAFLD are incompletely understood. Within the liver, the disruption of the finely regulated interplay between hepatocytes, hepatic stellate cells, endothelial cells, and various immune cell subtypes, driven by secreted cytokines and mediators, can result in a proinflammatory, profibrotic, and protumorigenic hepatic microenvironment.5 In addition, extrahepatic factors such as nutritional intake, gut dysbiosis, and systemic inflammation, as well as genetic disposition influence the disease processes in the liver.5
Macrophages are innate immune cells that are abundant as resident cells in almost all organs of the body.6 They exert key functions in tissue development and homeostasis but also may contribute to pathogenesis of various diseases. Over the past decade, high-resolution methods, such as single-cell RNA sequencing (scRNA-seq), spatial proteogenomics, fate-mapping experiments, and intravital microscopy substantially have increased our knowledge of macrophage origin, activation, and function.7 However, these major advances also have raised many new questions as the impressive complexity of macrophage phenotypes, plasticity, and functional regulation in different organ-specific microenvironments becomes increasingly apparent (Figure 1).
Figure 1.
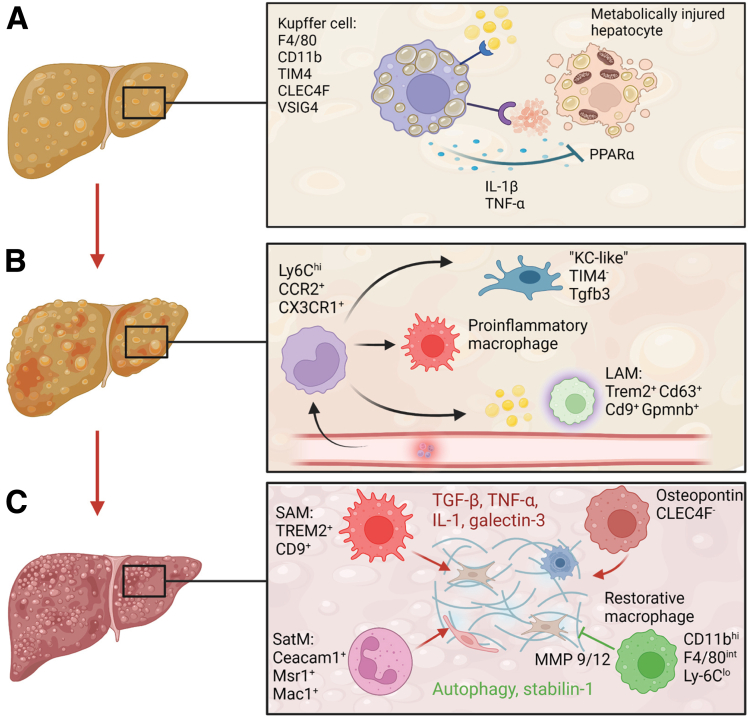
Macrophages foster progression from nonalcoholic fatty liver to NASH and fibrosis. (A) Kupffer cells are activated upon ingestion of apoptotic fat-laden hepatocytes and free cholesterol, triggering proinflammatory activation. (B) The inflammatory microenvironment recruits monocytes that differentiate in heterogeneous monocyte-derived macrophage populations (Kupffer cell-like, inflammatory macrophages, and lipid-associated macrophages [LAMs]) based on signals from the environment. (C) Macrophage populations shape both profibrotic (red) and antifibrotic (green) processes within the fibrotic niche. Important phenotypic markers of the macrophage populations identified in mouse models are shown in the figure. CEACAM1, carcinoembryonic antigen-related cell adhesion molecule 1; CLEC4F, C-Type Lectin Domain Family 4 Member F; Mac1, macrophage-1 antigen; MMP, matrix metalloproteinase; Msr1, macrophage scavenger receptor 1; SAM, scar-associated macrophages; SatM, segregated-nucleus-containing atypical monocytes; Tgfb3, transforming growth factor β; VSIG4, V-set and immunoglobulin domain containing 4. (Figure was created with BioRender).
Sophisticated fate-mapping experiments have highlighted the existence of embryonically derived, long-living, and self-sustaining resident macrophage populations in almost all organs, referred to as Kupffer cells in the liver. In addition to these resident phagocytes, the liver also contains infiltrated bone marrow–derived monocytes and macrophages (termed monocyte-derived macrophages).8, 9, 10 Under homeostatic conditions, resident macrophages exert highly specialized housekeeping functions regulated by the niche-specific microenvironment in which they reside.11,12 Moreover, in the context of disease, the classic dichotomous concept of a proinflammatory M1 and a restorative M2 polarization state has been replaced by a more dynamic view that takes into account the polyfunctional nature and exceptional plasticity of macrophages.13 scRNA-seq of whole human livers has partially elucidated the complexity of co-existing proinflammatory and restorative macrophage phenotypes in the homeostatic steady state.14
Given their complex regulation in homeostasis, it is not surprising that macrophages play a diverse role in various diseases,6,9 including the development of NAFLD, progression to NASH, fibrosis, and end-stage liver disease, as well as in the evolution of associated complications such as hepatocellular carcinoma. In fact, the increase in inflammatory macrophage populations and their spatially distinct accumulation in portal fields and close to (proliferating) bile ducts are a key characteristic of progressive chronic liver diseases, including NAFLD/NASH, but also cholangiopathies or alcohol-associated hepatitis. The exceptional function of macrophage populations in both driving and terminating inflammation, as well as their involvement in local and systemic metabolic regulation, makes them an attractive but challenging therapeutic target. In this review, we summarize the contribution of macrophages to different stages of metabolic liver disease and provide an outlook on open questions and potential therapeutic targets.
Macrophages Regulate Lipid Metabolism in Steatosis and Play a Role in the Initiation of Steatohepatitis
Hepatic steatosis is defined histologically by an accumulation of fat, mainly triglycerides, in more than 5% of hepatocytes.15 Interestingly, there seems to be a nonlinear relationship between simple steatosis and lipotoxicity, the main trigger for the transition to the inflammatory phenotype of steatohepatitis. It has not been determined conclusively whether patients with severe steatosis develop full-blown steatohepatitis more rapidly than subjects with a lesser steatosis burden.15 Lipotoxicity occurs when the liver is stressed by an excessive accumulation of fatty acids in hepatocytes, by which the capacity to use, store, and export them as triglycerides is exceeded.16 This leads to endoplasmic reticulum and oxidative stress, hepatocellular senescence, and lipoapoptosis.3,16 Steatotic hepatocytes secrete cytokines and chemokines, such as C-C motif chemokine ligand (CCL) 2 or C-X-C motif chemokine ligand (CXCL) 10, and extracellular vesicles, which in turn activate nonparenchymal cell types such as hepatic stellate cells, liver sinusoidal endothelial cells, and liver macrophages.16 Another pathway to Kupffer cell activation in NAFLD is mediated by the potent proinflammatory effect of free cholesterol.17 Macrophages are equipped with pattern recognition receptors, promoting proinflammatory signaling, and scavenger receptors, involved in lipid uptake and phagocytosis. In the context of NAFLD, much attention has been focused on the scavenger receptor A and the fatty acid transporter CD36, which mediate the uptake of modified low-density lipoprotein.18,19 Activated, enlarged, fat-laden Kupffer cells show impaired lipid metabolism and recruit other leukocytes owing to their inflammatory phenotype.20 Interestingly, this pathogenic process involves similar receptors and displays resemblance to foam cell-driven atherosclerotic plaque formation,21 underscoring the systemic nature of metabolic diseases.
During homeostasis, bona fide Kupffer cells, defined as F4/80hiCD11bint cells, express T-cell immunoglobulin mucin (TIM) 4, C-Type Lectin Domain Family 4 Member F (CLEC4F), and V-set and immunoglobulin domain containing 4 (VSIG4) in mice,8,9 whereas monocyte-derived macrophages tend to have a CD11bhiF4/80int phenotype and express CX3C motif chemokine receptor (CX3CR) 1 and C-C chemokine receptor type 2 (CCR2).9 Depletion of Kupffer cells in mice fed a high-fat diet reduced hepatic steatosis and inflammation.22 Activated Kupffer cells secrete proinflammatory cytokines such as interleukin (IL)1β and tumor necrosis factor (TNF), which inhibit genes involved in hepatocyte lipid metabolism via the peroxisome proliferator–activated receptor (PPAR)-α pathway, thereby promoting hepatocyte steatosis.23,24 Interestingly, the onset of inflammation may occur at early stages of the disease. Liver samples of obese patients already displayed a gene expression pattern of low-grade inflammation before showing histologic abnormalities.25 Conversely, fat-laden macrophages also can adopt an anti-inflammatory phenotype after the uptake of apoptotic steatotic hepatocytes.26 Moreover, using single-cell transcriptome analyses and fate-mapping approaches, Blériot et al27 recently showed that in addition to the major CD206loESAM- Kupffer cell population, a secondary CD206hiESAM+ subpopulation also is present in healthy and obese mice and is involved in the regulation of lipid metabolism, particularly fatty acid metabolism, via its expression of CD36. Hence, the involvement of Kupffer cells/macrophages in the regulation of metabolic processes and modulation of lobular inflammation is complex.
Macrophages in the Transition to Steatohepatitis
Once the proinflammatory milieu is established, a dramatic shift in macrophage composition occurs. Although the number of embryonically derived TIM4+ mature Kupffer cells decreases as a result of apoptosis and impaired self-renewal, macrophages derived from lymphocyte antigen 6 (Ly6) ChiCCR2-expressing monocytes accumulate in the liver. Specifically, recruited monocytes undergo distinct differentiation programs depending on the signals they receive from the environment and primarily develop into TIM4- macrophages.28, 29, 30 Monocyte-derived macrophages show predominantly immature proinflammatory phenotypes that exacerbate liver injury and drive disease progression.31 Accordingly, Ccr2-/- mice, which lack monocyte recruited macrophages, challenged in dietary NASH models showed less steatosis, inflammatory cell infiltration, and fibrosis.29
An elegant study deciphered the replenishment of Kupffer cells during NASH. Using a Ccr2-/--based chimeric mouse model, Tran et al30 showed that monocyte-derived macrophages also can adopt a Kupffer cell–like phenotype to repopulate the accessible niche in NASH. Interestingly, these monocyte-derived Kupffer cells did not promote hepatic triglyceride storage as efficiently as their embryonic-derived counterparts. However, after disease regression they were able to develop a fully mature Kupffer cell phenotype. These results are important because they strongly suggest a functional difference between Kupffer cells of different origins, at least in the context of NAFLD. Transcriptome and epigenome analyses in human and mouse NASH livers showed that Kupffer cells lose their epigenetic identity by altering their transcriptional signature and up-regulating a NASH-specific enhancer profile with activation of the AFT3, CD9, Arhgap22, and Tgfb3 loci.32
Lipid-Associated Macrophages
The NASH microenvironment is a highly interconnected network of autocrine and paracrine intrahepatic crosstalk between different cell types.33 This microenvironment and its specific microanatomic niches shape the chromatin landscape of myeloid cells, associated with reduced Kupffer cell survival and an increase of monocyte-recruited macrophages, and emergence of a scar-associated macrophage phenotype.32 A subpopulation of TIM4- monocyte-derived macrophages gives rise to lipid-associated macrophages; this designation arises from a characteristic lipid metabolism–related genetic signature of these macrophages and from their localization in the vicinity of sites of steatosis in crown like structures.28 Lipid-associated macrophages are heterogeneous and can be divided into a transitional CX3CR1+CCR2+ lipid-associated macrophage subset, and Trem2+ Cd63+ Cd9+ Gpnmb+ classic lipid-associated macrophages.28
Classic lipid-associated macrophages are not specific to the liver but are an integral part of adipose tissue and can be found in various organs and conditions.34 Indeed, liver macrophages during obesity,34 aortic macrophages in atherosclerosis,35 and even disease-associated microglia in the brain of mice and human beings with neurodegenerative diseases36 display a conserved lipid-associated, macrophage-related gene expression signature. Their function is linked strongly with the expression of triggering receptor expressed on myeloid cells 2 (TREM2), which mediates lipid uptake and metabolism; lipid-associated macrophages in fat thereby prevent adipocyte hypertrophy, adipose tissue dysfunction, and inflammation, as well as systemic metabolic dysregulation.34 Consistently, TREM2 deficiency promotes fat accumulation, dyslipidemia, and glucose intolerance in diet-induced obesity.34 Constitutive TREM2 deficiency accelerates the progression of NASH in a high-fat diet mouse model. The secretion of exosomes by Trem2-/- macrophages has been suggested to disrupt hepatocyte mitochondrial function, although how exosomes specifically target hepatocytes has not been resolved.37 In contrast, the deletion of TREM2 in hematopoietic cells, which also leads to the deletion of TREM2 in monocyte-derived macrophages, protects from steatohepatitis and liver fibrosis in mice.38
Recently, Guilliams et al12 created a liver cell atlas by combining proteogenomic and spatial information to characterize parenchymal und immune cells in healthy and diseased mouse and human livers.39 Interestingly, the investigators found that lipid-associated macrophages present even in healthy livers, where they reside in close proximity to bile ducts. In liver samples with steatosis in greater than 10% of hepatocytes, lipid-associated macrophages not only increased in number but also relocated to the pericentral steatotic areas.12 Comparison of the transcriptional signatures of lipid-associated macrophages between healthy and obese mice showed down-regulation of IL1β, TNF, and IL10 upon high-fat diet feeding.12 This suggests that lipid-associated macrophages may switch toward an anti-inflammatory phenotype as an adaptation to protect against NAFLD progression.38
Presence of Hepatic Crown-Like Structures Indicates Progression of Disease
Phagocytosis of apoptotic steatotic hepatocytes and uptake of free cholesterol is a key characteristic of macrophages in NAFLD.17 However, lipid-laden hepatocytes can swell considerably in size, which makes classic phagocytosis impossible.40 To circumvent this, lipid-associated macrophages may encapsulate dying enlarged hepatocytes by forming multinucleated aggregates through cell membrane fusion and secrete lysosomal contents into these hydrolytic synapses.40,41 These aggregates also occur in visceral adipose tissue and atheromatous plaques.42 In metabolic liver disease, these macrophage aggregates surrounding dying hepatocytes are referred to as hepatic crown-like structures. In fat, crown-like structure formation may prevent adipocyte hypertrophy, massive adipocyte cell death, and preserve systemic lipid homeostasis and tissue integrity.34 TREM2 signaling also appears to play a critical role in crown like structure formation because deletion of Trem2 abolished macrophage recruitment to enlarged adipocytes and the emergence of crown-like structures.34 In the liver, it was proposed that hepatic crown-like structure–forming monocyte-derived macrophages have a unique transitional CX3CR1+CCR2+ lipid-associated macrophage phenotype term that is distinct from classic Trem2+ Cd63+ Cd9+ Gpmnb+ lipid-associated macrophages.28 Importantly, it also has been claimed that hepatic crown-like structures are a significant source of inflammation.43 A specific population of CD11c+ macrophages involved in the formation of hepatic crown-like structures is thought to drive pathogenesis and fibrosis.44 In any case, the presence of hepatic crown-like structures indicates an advanced stage of disease because their number correlates positively with the extent of fibrosis and is associated with transition from simple steatosis to steatohepatitis.42,43 However, further studies are needed to elucidate the heterogeneity of macrophages with respect to their protective and progressive effects on hepatic crown-like structure formation and liver disease.
The Fibrotic Niche Harbors Heterogeneously Regulated Macrophages
Liver fibrosis is a key feature in advanced stages of NASH and is associated closely with its prognosis.45 Approximately 30% of NASH patients are at risk for progressive fibrosis, which can lead to cirrhosis and portal hypertension, impaired organ function, and hepatocellular carcinoma.46 The severity of fibrosis is the most important predictor of liver-related morbidity and mortality.46,47 The fibrotic niche defines the complex interplay between nonparenchymal immune cells, endothelial cells, and mesenchymal cells, including hepatic stellate cells and myofibroblasts.48 Macrophages have been implicated as regulators of liver fibrosis.49 Their plasticity and heterogeneous involvement in profibrotic and healing processes becomes evident in macrophage-depletion models. Depletion of macrophages in early stages of fibrosis results in reduced scar formation and fewer myofibroblasts, whereas macrophage depletion during recovery causes failure of matrix degradation in the carbon tetrachloride mouse fibrosis model.50 In a genome-wide association study examining the effects of genetic variations on liver fibrosis progression, the strongest association was found with a single-nucleotide polymorphism in the MERTK gene, which is part of the core macrophage signature and is involved in phagocytic clearance of apoptotic cells.51,52
Even though a profibrogenic subpopulation of Kupffer cell–derived CD11c+ macrophages has been described,44 RNA sequencing analysis showed that monocyte-derived macrophages rather than Kupffer cells up-regulate growth factors and cytokines associated with fibrosis progression,53 suggesting a predominant role of monocyte-derived macrophages in hepatic scar formation. In line with this, Ccr2-/- mice show lower numbers of monocyte-derived macrophages and less fibrosis.54
However, monocyte-derived macrophages of the fibrotic niche are phenotypically and functionally heterogeneous. scRNA-seq showed a scar-associated profibrogenic TREM2+CD9+ macrophage population.48 The transcriptomic signature of scar-associated macrophages included several genes regulating scar-producing myofibroblasts, such as IL1β, SPP1, LGALS3, CCR2, and TNFSF12.48 Interestingly, they share substantial similarities with lipid-associated macrophages found in healthy and steatotic livers.12,28 Another study identified a Ceacam1+Msr1+Ly6C−F4/80−Mac1+ monocyte population that was termed segregated-nucleus-containing atypical monocytes, based on its segmented nuclear shape and cytoplasmic granules. This population is regulated by CCAAT/enhancer binding protein β and is profibrogenic.55 In addition, a profibrogenic population of osteopontin-expressing CLEC4F- monocyte–derived macrophages was described.56 Although it is not clear how these different macrophage subpopulations relate to each other exactly, the relevance of functionally and phenotypically heterogeneous macrophage populations is supported by clinical studies showing increased levels of osteopontin, monocyte chemoattractant protein (CCL2), and IL8 in serum as well as transcriptional up-regulation of these genes in liver samples of patients with NASH fibrosis.57
Macrophages also are important regulators of fibrosis regression and restoration of tissue integrity because they degrade the extracellular matrix and have immune-regulatory functions. Depletion of CX3CR1+CCR2+ lipid-associated macrophages resulted in reduced hepatic crown-like structure formation but increased liver fibrosis.28 Furthermore, a CD11bhi F4/80intLy-6Clo monocyte-derived macrophage subset expressing high levels of matrix metalloproteinase has been described in liver tissue with pronounced fibrosis. This population arises from a phenotype switch of inflammatory recruited monocytes upon engulfment of cellular debris.58
The fibrotic niche can be understood only by considering the interactions between macrophages, endothelial cells, and collagen-producing mesenchymal cells. Macrophage-derived transforming growth factor β, TNF, IL1β, and galectin-3 are important profibrotic signals that activate the nuclear factor-кB pathway in hepatic stellate cells, inducing collagen production and promoting their survival.49,59 Yes-associated protein signaling in macrophages mediates the crosstalk to a fibrogenic vascular endothelial cell subset.60 Moreover, autophagy61 and the scavenger receptor stabilin-162 have been identified as crucial pathways in restorative macrophages (Figure 1). Together, the molecular mechanisms by which macrophages regulate fibrotic processes represent an active field of research.
Tumor-Associated Macrophages Emerge in the Transition From NASH to Hepatocellular Carcinoma
In addition to liver cirrhosis, the development of hepatocellular carcinoma represents a critical complication of NAFLD/NASH with poor prognosis. Hepatocellular carcinoma is the fourth leading cause of cancer death worldwide,63 and NAFLD is the fastest-growing cause of hepatocellular carcinoma.64 NAFLD patients may develop hepatocellular carcinoma even without existing cirrhosis.65 Potentially curative treatment options include liver transplantation at early stages, surgical resection, and loco-ablative therapies (eg, transarterial chemoembolization or radiofrequency ablation).66 The advent of immunotherapy not only highlights the critical role of the immune system in hepatocarcinogenesis, but also greatly has expanded the available treatment options.66 Nevertheless, treatment success is limited in inflammation-driven tumors such as hepatocellular carcinoma owing to high resistance and disease recurrence, illustrating the need to develop additional therapeutic approaches and biomarkers for early detection and therapy monitoring.67
Hepatocellular carcinoma is a cancer driven by inflammation and fibrogenesis. Patients with hepatocellular carcinoma have an increase in proinflammatory cytokines, such as IL8, IL13, CCL3, CCL4, and CCL5, as well as an increased number of activated blood monocytes compared with patients with NASH.68 The increase of inflammatory mediators in the blood is associated with tumor recurrence after surgery.68,69 CCL2 is highly expressed in liver samples from patients with hepatocellular carcinoma and predicts poor outcome. Furthermore, treatment with a CCR2 antagonist inhibits tumor growth and metastasis and improves survival in a hepatocellular carcinoma mouse model.70 The proinflammatory and profibrotic hepatic microenvironment featuring activated macrophages promotes malignant degeneration of hepatocytes and fosters the development and progression of hepatocellular carcinoma.71 Macrophages are involved in the clearance of premalignant senescent hepatocytes at early stages of hepatocellular carcinoma,72 but progression is characterized by the breakdown of this immune surveillance.64
Monocyte-derived macrophages accumulating at the hepatocellular carcinoma tissue develop into tumor-associated macrophages and adopt a heterogenous phenotype.71 Their transcriptional signature is characterized by high expression of TREM2, GPNMB, SLC40A1, APOE, C1QA, and C1QB, and, interestingly, resembles not only the lipid-associated macrophage signature,34 but also the tumor-associated macrophage signature in lung cancer.73,74 Importantly, lipid-associated macrophages have been implicated in different cancers as immune-suppressive, tumor-promoting macrophages.75,76
The frequency of hepatocellular carcinoma infiltrating tumor-associated macrophages is an independent predictor of poor overall and disease-free survival.77 The mechanisms by which the tumor reprograms macrophages for its own benefit are poorly understood. In any case, proinflammatory phenotypes that drive hepatocellular carcinoma progression and immunosuppressive phenotypes that promote tumor evasion of immune surveillance appear to co-exist. This is reflected in the co-expression of the full spectrum of M1- and M2-related genes within the tumor-associated macrophage population.73 In hepatocellular carcinoma, Gpnmb-/- macrophages produced lower levels of TNF upon Toll-like–receptor stimulation, suggesting a proinflammatory role of transmembrane glycoprotein NMB (GPNMB).73 Simultaneously, tumor-associated macrophages suppress the anti–tumor immune response of cytotoxic T cells and natural killer cells and lead to immunosuppression via activation of regulatory T cells.78 Although GPNMB expression seems to be associated with a proinflammatory phenotype in hepatocellular carcinoma, GPNMB+ TREM2+ lipid-associated macrophages appear to play a rather protective role in steatohepatitis. It is not clear to what extent these populations are related and reflect extensive macrophage plasticity. Thus, further characterization in this context is necessary to understand the full picture of macrophage contributions to hepatocellular carcinoma progression.
One signaling pathway that has attracted much attention is the immunosuppressive programmed cell death protein (ligand) 1 PD-1–PD-L1 interaction.79 After activation by tumor cells, infiltrating monocytes express high levels of PD-L1, and this expression is maintained by autocrine secretion of TNF and IL10.80 PD-L1 expression in monocytes increases with disease progression and is associated with a high mortality rate in patients with hepatocellular carcinoma.80 Mechanistically, PD-L1 signaling promotes suppression of CD8+ cytotoxic immunity against tumor cells.81 Two antibodies directed against PD-1, nivolumab and pembrolizumab, have been widely used for immunotherapy of hepatocellular carcinoma,82,83 although the primary end point, prolongation of survival, was not met in phase III clinical trials.84,85 The anti–PD-L1 antibody atezolizumab in combination with bevacizumab (anti–vascular endothelial growth factor) resulted in improved overall and progression-free survival in patients with advanced hepatocellular carcinoma, making it the current first-line systemic therapy.86 Despite impressive progress in this field, response rates of these so-called immune checkpoint inhibitors in inflammation-driven tumors, including hepatocellular carcinoma, remain rather low.87 In fact, some clinical data indicate that patients with NASH-driven hepatocellular carcinoma may respond less well to immunotherapies compared with other etiologies.88 The mechanisms underlying tumor cell resistance to immunotherapies are poorly understood, but hold great potential for improving treatment efficacy in the future. Even though treatment resistance to immune checkpoint inhibitors has been attributed mainly to T-cell anergy,89 there also is evidence that different macrophage populations are involved.90
Peripheral Macrophages Impact Liver Disease
Because NAFLD represents the hepatic manifestation of the metabolic syndrome, other diseases such as obesity, type 2 diabetes, and cardiovascular diseases are linked closely with this condition. To develop appropriate prevention strategies and treatment options, it is necessary to understand how different functional compartments cooperate across organs (Figure 2). Advances in high-resolution analysis techniques have made this possible. For example, whole-transcriptomic analysis has shown that lipid-associated macrophages found in NAFLD livers are related closely to those in adipose tissue and also to foam cell-forming macrophages in atherosclerosis.34,35 In addition, the systemic nature of metabolic diseases is illustrated by extensive cytokine-mediated crosstalk between organs.
Figure 2.
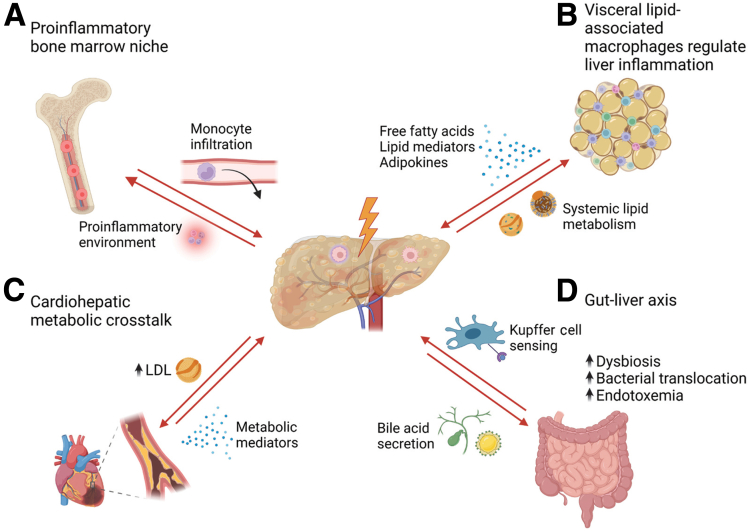
Hepatic inflammation and organ crosstalk. Stress-induced signals from the liver shape metabolic processes and immune cell phenotypes in remote organs and vice versa. (A) NAFLD is associated with a proinflammatory signature of progenitor cells in the bone marrow. (B) Lipid-associated macrophages in visceral adipose tissue that promote liver inflammation. (C) Cardiovascular disease is related to alteration of hepatic lipid metabolism. (D) Intestinal bacterial translocation and endotoxemia promote liver inflammation in NAFLD. LDL, low-density lipoprotein. (Figure was created with BioRender)
In this context, several lines of evidence indicate bidirectional communication between disturbed organs and the bone marrow niche.91, 92, 93, 94 The bone marrow microenvironment senses stress-related and inflammatory signals deriving from the rest of the body, and promotes expansion and differentiation of hematopoietic stem and progenitor cells, thereby enhancing myelopoiesis and mobilization of immune cells into the bloodstream.91,93,94 This phenomenon also has been described in a mouse model of steatohepatitis, in which scRNA-seq showed that the myeloid compartment in the liver and myeloid progenitor cells in the bone marrow adopt a common unique inflammatory phenotype.92
Another important crosstalk exists between visceral adipose tissue and dysfunctional organs. Transplantation of visceral adipose tissue from obese mice to lean low-density lipoprotein-receptor–deficient mice resulted in increased hepatic neutrophil and macrophage accumulation that exacerbated steatohepatitis upon feeding a high-fat diet compared with transplantation of lean adipose tissue.95 Interestingly, this phenotype was partially abrogated by depletion of lipid-associated macrophages in adipose tissue before transplantation. This suggests an important role of lipid-associated macrophages in the visceral adipose tissue in regulating systemic metabolism.
NAFLD patients are at high risk of developing cardiovascular disease.96 Of note, cardiovascular disease is the leading cause of death in patients with NAFLD, even ahead of liver-related complications.97 The liver–heart axis implies the recognition of metabolites as signaling effectors between the heart and liver.98 In a mouse model of hypertrophic cardiomyopathy, alterations in lipid metabolism also have been described in the liver.99 Alterations in hepatic lipid metabolism contribute to dyslipidemia and an increase in low-density lipoprotein, which subsequently promotes foam cell formation and atherogenesis. Whether and how liver macrophages also contribute to the cardiohepatic crosstalk is not fully understood.
Regarding organ interactions in liver disease, much attention has been focused on the gut–liver axis, although many studies are correlational.100 Epidemiologic data on human microbiome profiles suggest a contribution of dysbiosis and impaired intestinal barrier function to the progression of NAFLD and other metabolic diseases.101, 102, 103 Susceptibility to gut leakiness could be the reason for increased endotoxemia owing to translocation of bacterial components and microbially derived metabolites.104 Recently, increased levels of microbially produced ethanol in the portal venous circulation have been associated with progression of NAFLD.105 This pathophysiological relationship, in addition to altered lipid metabolism, is reflected in the multiple hit hypothesis.106 The lamina propria of the intestine harbors large numbers of CX3CR1+ macrophages. These are involved in maintaining a tolerogenic microenvironment to commensal bacteria and regulate intestinal barrier function by stimulating the proliferation of epithelial progenitor cells.107 High-resolution intravital microscopy has highlighted their perivascular location, where they form tight junctions and provide an excellent second barrier underneath the epithelium.108 Lamina propria macrophages scavenged fluorescently labeled bacteria administered into the intestinal lumen. Any disruption of this macrophage barrier, including in a dysbiosis mouse model, resulted in increased translocation of bacteria.108 As a consequence, macrophages in the liver become exposed to bacteria, endotoxin, and bacterial metabolites, which contribute to an inflammatory macrophage phenotype.109 In the intestine of patients with liver cirrhosis, the presence of activated CD14+ Trem1+ inducible nitric oxide synthase+ macrophages releasing IL6 and nitric oxide has been described, which is associated with intestinal barrier dysfunction.110 Moreover, the phagocytic capacity of hepatic macrophages is impaired in patients with cirrhosis, contributing to the clinical observation of an increased susceptibility to infections.111 Taken together, these findings suggest an important interplay between intestinal macrophages, microbiota, and gut epithelial integrity with pathogenic mechanisms related to disease progression in the liver.
Opportunity and Hurdles in Targeting Macrophages for Therapy
In consideration of the increasing burden of the metabolic epidemic on health care systems, new therapeutic approaches urgently are needed. Current pharmacologic treatment for patients with NASH and fibrosis, who are at high risk for disease progression,112,113 is limited because no drugs have been approved by the US Food and Drug Administration or the European Medicines Agency. The crucial involvement of macrophages in all stages of disease, as well as their role in inflammation and metabolic regulation, makes them an attractive and amenable therapeutic target. In the past decade, several promising approaches have found their way into preclinical and clinical evaluation.114,115 Although some clinical trials still are ongoing, some late-stage clinical trials, unfortunately, have failed (Table 1).
Table 1.
Current Status of Drug Development Affecting or Targeting Macrophages in Nonalcoholic Steatohepatitis (Selected Compounds and Trials)
| Drug | Mechanism | Clinical evaluation |
|---|---|---|
| Cenicriviroc | CCR2/CCR5 inhibitor | Failed phase 3 (NCT03028740) |
| Belapectin (GR-MD-02) | Galectin-3 inhibitor | Failed phase 2b (NCT02462967) |
| Selonsertib | ASK1 inhibitor | Failed phase 3 (NCT03053050/NCT03053063) |
| Obeticholic acid | Farnesoid X–receptor agonist | Successful in phase 2 (NCT01265498) Failed phase 3 in NASH cirrhosis (NCT03439254) Phase 3 in NASH fibrosis ongoing (NCT02548351) |
| Liraglutide/semaglutide | GLP-1–receptor agonist | Successful in phase 2 (NCT02970942/NCT01237119) Phase 3 ongoing (NCT04822181/NCT02654665) |
| Pioglitazone | PPAR-γ agonist | Evaluated for patients with concurrent type 2 diabetes |
| Elafibranor | PPAR-α/δ agonist | Failed phase 3 (NCT02704403) |
| Lanifibranor | Pan PPAR-α/γ/δ agonist | Successful in phase 2 (NCT03008070) Phase 3 ongoing (NCT04849728) |
| Resmetirom | THR-β agonist | Successful in phase 2 (NCT02912260) Phase 3 ongoing (NCT03900429) |
ASK1, apoptosis signal-regulating kinase 1; GLP-1, glucagon-like peptide-1; GR-MD-02, belapectin; THR, thyroid hormone receptor.
The most prominent example is probably the dual CCR2/CCR5 antagonist cenicriviroc. Inhibition of monocyte recruitment to the liver showed potent anti-inflammatory and antifibrotic effects in preclinical animal models.53,116 The phase 2b clinical trial involving 289 patients showed no improvement of NASH activity, but a significant improvement in fibrosis after 1 year of therapy.117 However, this antifibrotic efficacy was not sustained over 2 years of treatment with cenicriviroc compared with placebo.118 The subsequent phase 3 clinical study was terminated owing to a lack of efficacy based on a preplanned interim analysis (NCT03028740). Belapectin (GR-MD-02), an inhibitor of galectin-3, also expressed on macrophages, showed no significant effect on fibrosis, NASH activity, or liver-related outcomes in a randomized phase 2b study (NCT02462967).119 Selonsertib, the selective inhibitor of apoptosis signal-regulating kinase 1, which promotes secretion of proinflammatory cytokines in macrophages,120 did not reach the primary end point of fibrosis improvement in the phase III STELLAR trials (NCT03053050 and NCT03053063).121
More encouraging data exist for the farnesoid X–receptor agonist obeticholic acid, which currently is being evaluated in an ongoing phase III trial. This synthetic bile acid analogue significantly regulates hepatic lipid and glucose metabolism. Studies in mice suggest that it furthermore may exert anti-inflammatory effects by inhibiting nuclear factor-κB signaling and promoting phenotype switching to restorative Ly6Clo macrophages.122,123 The phase II clinical trial using obeticholic acid in NASH patients showed convincing improvement in histologic features (NCT01265498).124 Interim results from the ongoing phase III trial showed improvement in histologic fibrosis in 23% of patients treated with 25 mg obeticholic acid daily compared with 12% in the placebo group.125 Final data on corresponding clinical outcomes are eagerly awaited. Of note, the interim analysis of another phase 3 trial evaluating obeticholic acid in patients with NASH-associated liver cirrhosis (NCT03439254) failed to show a benefit of obeticholic acid in this patient population.
Increased attention has been paid to the beneficial effects of glucagon-like peptide-1 (GLP-1)receptor agonists on the progression of NASH.126 These drugs have found widespread use for the treatment of obesity as well as type 2 diabetes owing to their blood glucose–lowering effect by inducing insulin release in pancreatic beta cells and their strong appetite-suppressive effects. The GLP-1/GLP-1–receptor signaling cascade has been reported to foster M2-like polarization of macrophages.127 However, the main actions of GLP-1 or GLP-1/glucose-dependent insulinotropic polypeptide (GIP)–receptor agonists on macrophages are likely to be indirect and rather owing to favoring a metabolically beneficial environment upon weight reduction. Phase II clinical trials also have shown significant improvement in NAFLD resolution for liraglutide and semaglutide (NCT02970942 and NCT01237119).128,129 In particular, liraglutide showed encouraging superiority over placebo. Thirty-nine percent of treated patients met the primary end point of NASH resolution, compared with 9% in the control group.128 Phase III clinical studies currently are underway (NCT04822181 and NCT02654665).
Another antidiabetic drug that has shown a beneficial effect on the resolution of NAFLD is the PPAR-γ–receptor agonist pioglitazone.130,131 PPAR-γ is a transcription factor that activates expression of M2-like anti-inflammatory genes in macrophages and its activation has been shown to restore a high-fat diet–induced imbalance of M1/M2 polarization of Kupffer cells.132,133 The use of pioglitazone in subjects with NAFLD is advocated in the guidelines published by the National Institute for Health and Care Excellence and the American Association for the Study of liver diseases under certain circumstances.112 The European Association for the Study of the Liver guideline is more cautious and recommends that pioglitazone may be considered as a treatment for diabetes with concurrent NAFLD.134 Increasing evidence of a higher risk of bladder cancer in patients treated with pioglitazone also may prompt cautious use.135 As an alternative drug, the PPAR-α/δ agonist elafibranor showed good results in preclinical studies but did not meet the predefined efficacy end point in phase III clinical studies (NCT02704403). In contrast, lanifibranor is a pan-PPAR agonist acting on all 3 PPAR isoforms.136 In preclinical NASH models, lanifibranor potently improved NASH and fibrosis because it acts on hepatocytes (mainly PPAR-α in mice), macrophages (mainly PPAR-δ), and hepatic stellate cells (mainly PPAR-γ in mice) simultaneously.137 A phase 2b clinical trial in patients with NASH and fibrosis showed beneficial treatment effects after only 24 weeks of therapy,138 mandating the further investigation in a large phase III trial.
Encouraging results also have been reported in clinical trials evaluating the thyroid hormone receptor-β–agonist resmetirom in patients with NASH.139 Although its protective effects are mediated primarily through regulation of hepatic lipid metabolism, thyroid receptors also have been reported to promote anti-inflammatory polarization of macrophages.140 Preliminary data from the ongoing phase 3 study suggest a significant reduction of liver steatosis after 52 weeks of treatment. Additional readouts and histologic results from the phase 3 trial are awaited (NCT03900429).
There are a number of molecular targets in macrophages whose manipulation in preclinical studies have suggested pharmacologic benefit, such as macrophage scavenger receptor 1,18 Toll-like receptor 4,141 TREM2,37 and the transcription factor X-box binding protein 1 (XBP1).142 It will be interesting to see which approaches make it into clinical evaluation.
In summary, the development of pharmacologic therapeutic options for NAFLD has seen both encouraging and disappointing results. Of note, translation of animal models to human beings often fails, increasing the need for better models and alternative approaches. From a macrophage perspective, not only identifying important cellular (subsets, organ-specific phagocytes) and molecular (eg, pathways of inflammatory or fibrogenic activation) targets is relevant, but also developing new, safe, and specific drug delivery systems such as hard-shell microbubbles, liposomes, and polymers, in which the phagocytic activity of macrophages could be harnessed for targeted drug administration.114,143 Moreover, macrophage-based adoptive cell transfer therapies, which currently are being evaluated in early clinical phase and tested in different preclinical models, hold great promise.144 A combination of reductionist basic science and translational approaches will be required to elucidate mechanisms behind dysregulated macrophages and identify novel potential therapeutic interventions. Until then, the mainstay of therapy will be lifestyle intervention, including adjusted caloric intake and physical activity.145 In fact, lifestyle modification has been shown not only to reduce intrahepatic lipid content but even to influence monocyte and macrophage activation positively.146,147
Soluble CD163, Soluble TREM2, and Sialic Acid–Binding Immunoglobulin-Like Lectin-7 Are Potential Macrophage-Related Biomarkers
Pharmacologic treatment is desirable in patients with NASH and fibrosis and in patients at high risk for progressive disease.113 However, it is difficult to predict the risk of transition from simple steatosis to more progressive forms including fibrosis and steatohepatitis. Liver biopsy is the gold standard for the diagnosis of present liver fibrosis, but because of its invasiveness is not suitable as a regular follow-up examination. Biomarkers are needed for early detection and disease monitoring in NAFLD. Because of the stage-specific contributions of macrophages to NAFLD progression and their increase during progression, macrophage-related molecules have been suggested as potential circulating biomarkers.115 One potential marker is the hemoglobin–haptoglobin scavenger receptor CD163, which is located on the surface of Kupffer cells and released into blood circulation after activation.148 The soluble form of CD163 has shown promise as a biomarker in various liver diseases, including hepatocellular carcinoma.148 It correlates with the degree of steatosis, but does not predict the severity of fibrosis.149 Although soluble CD163 was not associated with hepatocellular carcinoma size and number, it appears to be useful as a surveillance biomarker because it was associated independently with lower overall survival and cancer progression.148 Another potential biomarker is sialic acid–binding immunoglobulin-like lectin-7, which is expressed by Kupffer cells. Recently, sialic acid–binding immunoglobulin-like lectin-7 was shown to be an independent diagnostic marker with high specificity for advanced fibrosis in patients with NAFLD.150 Based on the recurrent observation that TREM2+ macrophages accumulate in the fibrotic septa of patients with NASH and cirrhosis,12 systemic levels of soluble TREM2 have been suggested as a potential biomarker in NAFLD. Indeed, plasma soluble TREM2 levels mirror NASH severity (no steatosis – steatosis – NASH – fibrosis) in human patients, suggesting that this macrophage-related receptor is a valuable biomarker in NAFLD patients.38 The use of macrophage-derived biomarkers for screening, identification, and disease monitoring in NAFLD needs further (prospective) investigation in broader patient populations.
Future Perspective and Challenges
Different manifestations and numerous parallel pathophysiological metabolic and inflammatory processes make NAFLD an extremely complex clinical condition. The heterogeneously regulated macrophage populations reflect this complexity. Multiple macrophage populations co-exist at different stages in metabolically injured livers, promoting or inhibiting disease progression, and their relationship to each other as well as their crosstalk to further cells in the liver environment still are insufficiently understood. The tremendous advance in biomedical research techniques allows us now to reclassify macrophage subsets based on the transcriptomic and proteomic profile, their spatial organization, their dynamic behavior, as well as based on their functional capacity with respect to disease stage.5 Advances in bioinformatics and data science will allow integration of knowledge on extrahepatic signals (gut, adipose tissue) as well as on patient-specific features (nutrition, genetics, comorbidities), giving rise to the expectation that tailored targeting to modify macrophages as a central immune regulator during NAFLD progression may be a realistic goal.
Acknowledgments
Author contributions
Joscha Vonderlin conceived, designed, and wrote the manuscript; Triantafyllos Chavakis and Michael Sieweke provided intellectual input and edited the manuscript; and Frank Tacke contributed to the conception, provided intellectual input, and edited the manuscript.
Footnotes
Conflicts of interest This author discloses the following: Frank Tacke’s laboratory received research grants from Gilead, Allergan, Bristol-Myers Squibb, and Inventiva. The remaining authors disclose no conflicts.
Funding This work was funded by the German Research Foundation (CRC1382, project ID 403224013, and SFB/TRR 296), and the German Ministry of Education and Research (BMBF DEEP-HCC consortium).
References
- 1.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasper P., Martin A., Lang S., et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921–937. doi: 10.1007/s00392-020-01709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelmann C., Tacke F. The potential role of cellular senescence in non-alcoholic fatty liver disease. Int J Mol Sci. 2022;23:652. doi: 10.3390/ijms23020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pais R., Barritt A.S., Calmus Y., et al. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol. 2016;65:1245–1257. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiseler M., Schwabe R., Hampe J., et al. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–1160. doi: 10.1016/j.jhep.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Krenkel O., Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 7.Wallace S.J., Tacke F., Schwabe R.F., Henderson N.C. Understanding the cellular interactome of non-alcoholic fatty liver disease. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott C.L., Zheng F., de Baetselier P., et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;27 doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillot A., Tacke F. Liver macrophages: old dogmas and new insights. Hepatol Commun. 2019;3:730–743. doi: 10.1002/hep4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nati M., Chung K.-J., Chavakis T. The role of innate immune cells in nonalcoholic fatty liver disease. J Innate Immun. 2022;14:31–41. doi: 10.1159/000518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilliams M., Mildner A., Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49:595–613. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Guilliams M., Bonnardel J., Haest B., et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022;185:379–396.e38. doi: 10.1016/j.cell.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray P.J., Allen J.E., Biswas S.K., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacParland S.A., Liu J.C., Ma X.-Z., et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q., Bengmark S., Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD) Lipids Health Dis. 2010;9:1–9. doi: 10.1186/1476-511X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rada P., González-Rodríguez Á., García-Monzón C., Valverde Á.M. Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis. 2020;11:802. doi: 10.1038/s41419-020-03003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn C.L., Morales A.L., Savard C., et al. Role of cholesterol-associated steatohepatitis in the development of NASH. Hepatol Commun. 2022;6:12–35. doi: 10.1002/hep4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govaere O., Petersen S.K., Martinez-Lopez N., et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J Hepatol. 2022;76:1001–1012. doi: 10.1016/j.jhep.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Bieghs V., Wouters K., van Gorp P.J., et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology. 2010;138:2477–2486. doi: 10.1053/j.gastro.2010.02.051. 2486.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroux A., Ferrere G., Godie V., et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57:141–149. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Kzhyshkowska J., Neyen C., Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Chen L., Ye H., Zhao X., et al. Selective depletion of hepatic Kupffer cells significantly alleviated hepatosteatosis and intrahepatic inflammation induced by high fat diet. Hepatogastroenterology. 2012;59:1208–1212. doi: 10.5754/hge11903. [DOI] [PubMed] [Google Scholar]
- 23.Tosello-Trampont A.-C., Landes S.G., Nguyen V., et al. Kupffer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W., Metlakunta A., Dedousis N., et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertola A., Bonnafous S., Anty R., et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jindal A., Bruzzì S., Sutti S., et al. Fat-laden macrophages modulate lobular inflammation in nonalcoholic steatohepatitis (NASH) Exp Mol Pathol. 2015;99:155–162. doi: 10.1016/j.yexmp.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Blériot C., Barreby E., Dunsmore G., et al. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity. 2021;54:2101–2116.e6. doi: 10.1016/j.immuni.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Daemen S., Gainullina A., Kalugotla G., et al. Dynamic shifts in the composition of resident and recruited macrophages influence tissue remodeling in NASH. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura K., Yang L., van Rooijen N., et al. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran S., Baba I., Poupel L., et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity. 2020;53:627–640.e5. doi: 10.1016/j.immuni.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Morinaga H., Mayoral R., Heinrichsdorff J., et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64:1120–1130. doi: 10.2337/db14-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seidman J.S., Troutman T.D., Sakai M., et al. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity. 2020;52:1057–1074.e7. doi: 10.1016/j.immuni.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong X., Kuang H., Ansari S., et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell. 2019;75:644–660.e5. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaitin D.A., Adlung L., Thaiss C.A., et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178:686–698.e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cochain C., Vafadarnejad E., Arampatzi P., et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 36.Keren-Shaul H., Spinrad A., Weiner A., et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Hou J., Zhang J., Cui P., et al. TREM2 sustains macrophage-hepatocyte metabolic coordination in nonalcoholic fatty liver disease and sepsis. J Clin Invest. 2021;131 doi: 10.1172/JCI135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrikx T., Porsch F., Kiss M.G., et al. Soluble TREM2 levels reflect the recruitment and expansion of TREM2+ macrophages that localize to fibrotic areas and limit NASH. J Hepatol. 2022;77:1373–1385. doi: 10.1016/j.jhep.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Guillot A., Tacke F. Location, location, location - spatial insight into hepatic macrophage populations. Nat Rev Gastroenterol Hepatol. 2022;19:281–282. doi: 10.1038/s41575-022-00600-2. [DOI] [PubMed] [Google Scholar]
- 40.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olona A., Mukhopadhyay S., Hateley C., et al. Adipoclast: a multinucleated fat-eating macrophage. BMC Biol. 2021;19:246. doi: 10.1186/s12915-021-01181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannou G.N., Haigh W.G., Thorning D., Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J Lipid Res. 2013;54:1326–1334. doi: 10.1194/jlr.M034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itoh M., Kato H., Suganami T., et al. Hepatic crown-like structure: a unique histological feature in non-alcoholic steatohepatitis in mice and humans. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh M., Suganami T., Kato H., et al. CD11c+ resident macrophages drive hepatocyte death-triggered liver fibrosis in a murine model of nonalcoholic steatohepatitis. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanyal A.J., van Natta M.L., Clark J., et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekstedt M., Franzén L.E., Mathiesen U.L., et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 47.Angulo P., Kleiner D.E., Dam-Larsen S., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran P., Dobie R., Wilson-Kanamori J.R., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuda M., Seki E. Hepatic stellate cell-macrophage crosstalk in liver fibrosis and carcinogenesis. Semin Liver Dis. 2020;40:307–320. doi: 10.1055/s-0040-1708876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffield J.S., Forbes S.J., Constandinou C.M., et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patin E., Kutalik Z., Guergnon J., et al. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology. 2012;143:1244–1252.e12. doi: 10.1053/j.gastro.2012.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gautier E.L., Shay T., Miller J., et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krenkel O., Puengel T., Govaere O., et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 54.Seki E., de Minicis S., Inokuchi S., et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satoh T., Nakagawa K., Sugihara F., et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 56.Remmerie A., Martens L., Thoné T., et al. Osteopontin expression identifies a subset of recruited macrophages distinct from Kupffer cells in the fatty liver. Immunity. 2020;53:641–657.e14. doi: 10.1016/j.immuni.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glass O., Henao R., Patel K., et al. Serum interleukin-8, osteopontin, and monocyte chemoattractant protein 1 are associated with hepatic fibrosis in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2018;2:1344–1355. doi: 10.1002/hep4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramachandran P., Pellicoro A., Vernon M.A., et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pradere J.-P., Kluwe J., de Minicis S., et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qing J., Ren Y., Zhang Y., et al. Dopamine receptor D2 antagonism normalizes profibrotic macrophage-endothelial crosstalk in non-alcoholic steatohepatitis. J Hepatol. 2022;76:394–406. doi: 10.1016/j.jhep.2021.09.032. [DOI] [PubMed] [Google Scholar]
- 61.Lodder J., Denaës T., Chobert M.-N., et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–1292. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rantakari P., Patten D.A., Valtonen J., et al. Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. Proc Natl Acad Sci U S A. 2016;113:9298–9303. doi: 10.1073/pnas.1604780113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J.D., Hainaut P., Gores G.J., et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang D.Q., El-Serag H.B., Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desai A., Sandhu S., Lai J.-P., Sandhu D.S. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol. 2019;11:1–18. doi: 10.4254/wjh.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou D., Luan J., Huang C., Li J. Tumor-associated macrophages in hepatocellular carcinoma: friend or foe? Gut Liver. 2021;15:500–516. doi: 10.5009/gnl20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gallage S., García-Beccaria M., Szydlowska M., et al. The therapeutic landscape of hepatocellular carcinoma. Med (N Y) 2021;2:505–552. doi: 10.1016/j.medj.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Ponziani F.R., Bhoori S., Castelli C., et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 69.Sasaki A., Iwashita Y., Shibata K., et al. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139:755–764. doi: 10.1016/j.surg.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Li X., Yao W., Yuan Y., et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 71.Tian Z., Hou X., Liu W., et al. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019;9:79. doi: 10.1186/s13578-019-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang T.-W., Yevsa T., Woller N., et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q., He Y., Luo N., et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–845.e20. doi: 10.1016/j.cell.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Lavin Y., Kobayashi S., Leader A., et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–765.e17. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timperi E., Gueguen P., Molgora M., et al. Lipid-associated macrophages are induced by cancer-associated fibroblasts and mediate immune suppression in breast cancer. Cancer Res. 2022;82:3291–3306. doi: 10.1158/0008-5472.CAN-22-1427. [DOI] [PubMed] [Google Scholar]
- 76.Marelli G., Morina N., Portale F., et al. Lipid-loaded macrophages as new therapeutic target in cancer. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding T., Xu J., Wang F., et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40:381–389. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Lindau D., Gielen P., Kroesen M., et al. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lombardi R., Piciotti R., Dongiovanni P., et al. PD-1/PD-L1 immuno-mediated therapy in NAFLD: advantages and obstacles in the treatment of advanced disease. Int J Mol Sci. 2022;23:2707. doi: 10.3390/ijms23052707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuang D.-M., Zhao Q., Peng C., et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yun J., Yu G., Hu P., et al. PD-1 expression is elevated in monocytes from hepatocellular carcinoma patients and contributes to CD8 T cell suppression. Immunol Res. 2020;68:436–444. doi: 10.1007/s12026-020-09155-3. [DOI] [PubMed] [Google Scholar]
- 82.Zhu A.X., Finn R.S., Edeline J., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 83.El-Khoueiry A.B., Sangro B., Yau T., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finn R.S., Ryoo B.-Y., Merle P., et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 85.Yau T., Park J.W., Finn R.S., et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30:v874–v875. [Google Scholar]
- 86.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 87.Roderburg C., Wree A., Demir M., et al. The role of the innate immune system in the development and treatment of hepatocellular carcinoma. Hepat Oncol. 2020;7:HEP17. doi: 10.2217/hep-2019-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfister D., Núñez N.G., Pinyol R., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim T.K., Herbst R.S., Chen L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol. 2018;39:624–631. doi: 10.1016/j.it.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arlauckas S.P., Garris C.S., Kohler R.H., et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rohde D., Vandoorne K., Lee I.-H., et al. Bone marrow endothelial dysfunction promotes myeloid cell expansion in cardiovascular disease. Nat Cardiovasc Res. 2022;1:28–44. doi: 10.1038/s44161-021-00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krenkel O., Hundertmark J., Abdallah A.T., et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut. 2020;69:551–563. doi: 10.1136/gutjnl-2019-318382. [DOI] [PubMed] [Google Scholar]
- 93.Chavakis T., Wielockx B., Hajishengallis G. Inflammatory modulation of hematopoiesis: linking trained immunity and clonal hematopoiesis with chronic disorders. Annu Rev Physiol. 2022;84:183–207. doi: 10.1146/annurev-physiol-052521-013627. [DOI] [PubMed] [Google Scholar]
- 94.Mitroulis I., Hajishengallis G., Chavakis T. Trained immunity and cardiometabolic disease: the role of bone marrow. Arterioscler Thromb Vasc Biol. 2021;41:48–54. doi: 10.1161/ATVBAHA.120.314215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bijnen M., Josefs T., Cuijpers I., et al. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut. 2018;67:1317–1327. doi: 10.1136/gutjnl-2016-313654. [DOI] [PubMed] [Google Scholar]
- 96.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 97.Anstee Q.M., Mantovani A., Tilg H., Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15:425–439. doi: 10.1038/s41575-018-0010-0. [DOI] [PubMed] [Google Scholar]
- 98.Baskin K.K., Bookout A.L., Olson E.N. The heart-liver metabolic axis: defective communication exacerbates disease. EMBO Mol Med. 2014;6:436–438. doi: 10.1002/emmm.201303800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magida J.A., Leinwand L.A. Metabolic crosstalk between the heart and liver impacts familial hypertrophic cardiomyopathy. EMBO Mol Med. 2014;6:482–495. doi: 10.1002/emmm.201302852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Marra F., Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 102.Shen F., Zheng R.-D., Sun X.-Q., et al. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 103.Boursier J., Mueller O., Barret M., et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farhadi A., Gundlapalli S., Shaikh M., et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meijnikman A.S., Davids M., Herrema H., et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med. 2022;28:2100–2106. doi: 10.1038/s41591-022-02016-6. [DOI] [PubMed] [Google Scholar]
- 106.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Bain C.C., Mowat A.M. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Honda M., Surewaard B.G.J., Watanabe M., et al. Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat Commun. 2020;11:1329. doi: 10.1038/s41467-020-15068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bruneau A., Hundertmark J., Guillot A., Tacke F. Molecular and cellular mediators of the gut-liver axis in the progression of liver diseases. Front Med (Lausanne) 2021;28 doi: 10.3389/fmed.2021.725390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Du Plessis J., Vanheel H., Janssen C.E.I., et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125–1132. doi: 10.1016/j.jhep.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 111.Pose E., Coll M., Martínez-Sánchez C., et al. Programmed death ligand 1 is overexpressed in liver macrophages in chronic liver diseases, and its blockade improves the antibacterial activity against infections. Hepatology. 2021;74:296–311. doi: 10.1002/hep.31644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leoni S., Tovoli F., Napoli L., et al. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroenterol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rinella M.E., Tacke F., Sanyal A.J., Anstee Q.M. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol. 2019;71:823–833. doi: 10.1016/j.jhep.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 114.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 115.Kazankov K., Jørgensen S.M.D., Thomsen K.L., et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 116.Lefebvre E., Moyle G., Reshef R., et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Friedman S.L., Ratziu V., Harrison S.A., et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ratziu V., Sanyal A., Harrison S.A., et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 2020;72:892–905. doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 119.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158:1334–1345.e5. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 120.Immanuel C.N., Teng B., Dong B., et al. Apoptosis signal-regulating kinase-1 promotes inflammasome priming in macrophages. Am J Physiol Lung Cell Mol Physiol. 2019;316:L418–L427. doi: 10.1152/ajplung.00199.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harrison S.A., Wong V.W.-S., Okanoue T., et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 122.McMahan R.H., Wang X.X., Cheng L.L., et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem. 2013;288:11761–11770. doi: 10.1074/jbc.M112.446575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yao J., Zhou C.-S., Ma X., et al. FXR agonist GW4064 alleviates endotoxin-induced hepatic inflammation by repressing macrophage activation. World J Gastroenterol. 2014;20:14430–14441. doi: 10.3748/wjg.v20.i39.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Younossi Z.M., Ratziu V., Loomba R., et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 126.Patel Chavez C., Cusi K., Kadiyala S. The emerging role of glucagon-like peptide-1 receptor agonists for the management of NAFLD. J Clin Endocrinol Metab. 2022;107:29–38. doi: 10.1210/clinem/dgab578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang L., Chen L., Li D., et al. Effect of GLP-1/GLP-1R on the polarization of macrophages in the occurrence and development of atherosclerosis. Mediators Inflamm. 2021;2021 doi: 10.1155/2021/5568159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Armstrong M.J., Gaunt P., Aithal G.P., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 129.Newsome P.N., Buchholtz K., Cusi K., et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 130.Belfort R., Harrison S.A., Brown K., et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 131.Sanyal A.J., Chalasani N., Kowdley K.V., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luo W., Xu Q., Wang Q., et al. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;16 doi: 10.1038/srep44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bouhlel M.A., Derudas B., Rigamonti E., et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 134.Sberna A.L., Bouillet B., Rouland A., et al. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice recommendations for the management of non-alcoholic fatty liver disease: evaluation of their application in people with type 2 diabetes. Diabet Med. 2018;35:368–375. doi: 10.1111/dme.13565. [DOI] [PubMed] [Google Scholar]
- 135.He S., Tang Y., Zhao G., et al. Pioglitazone prescription increases risk of bladder cancer in patients with type 2 diabetes: an updated meta-analysis. Tumour Biol. 2014;35:2095–2102. doi: 10.1007/s13277-013-1278-x. [DOI] [PubMed] [Google Scholar]
- 136.Francque S., Szabo G., Abdelmalek M.F., et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021;18:24–39. doi: 10.1038/s41575-020-00366-5. [DOI] [PubMed] [Google Scholar]
- 137.Lefere S., Puengel T., Hundertmark J., et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J Hepatol. 2020;73:757–770. doi: 10.1016/j.jhep.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 138.Francque S.M., Bedossa P., Ratziu V., et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 139.Harrison S.A., Bashir M.R., Guy C.D., et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 140.Furuya F., Ishii T., Tamura S., et al. The ligand-bound thyroid hormone receptor in macrophages ameliorates kidney injury via inhibition of nuclear factor-κB activities. Sci Rep. 2017;7 doi: 10.1038/srep43960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lancaster G.I., Langley K.G., Berglund N.A., et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27:1096–1110.e5. doi: 10.1016/j.cmet.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 142.Wang Q., Zhou H., Bu Q., et al. Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J Hepatol. 2022;77:312–325. doi: 10.1016/j.jhep.2022.02.031. [DOI] [PubMed] [Google Scholar]
- 143.Ergen C., Heymann F., Al Rawashdeh W., et al. Targeting distinct myeloid cell populations in vivo using polymers, liposomes and microbubbles. Biomaterials. 2017;114:106–120. doi: 10.1016/j.biomaterials.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 144.Mass E., Lachmann N. From macrophage biology to macrophage-based cellular immunotherapies. Gene Ther. 2021;28:473–476. doi: 10.1038/s41434-021-00221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hallsworth K., Adams L.A. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 2019;1:468–479. doi: 10.1016/j.jhepr.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vanweert F., Boone S.C., Brouwers B., et al. The effect of physical activity level and exercise training on the association between plasma branched-chain amino acids and intrahepatic lipid content in participants with obesity. Int J Obes (Lond) 2021;45:1510–1520. doi: 10.1038/s41366-021-00815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Blanks A.M., Wagamon T.T., Lafratta L., et al. Impact of physical activity on monocyte subset CCR2 expression and macrophage polarization following moderate intensity exercise. Brain Behav Immun Health. 2020;2 doi: 10.1016/j.bbih.2019.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kazankov K., Rode A., Simonsen K., et al. Macrophage activation marker soluble CD163 may predict disease progression in hepatocellular carcinoma. Scand J Clin Lab Invest. 2016;76:64–73. doi: 10.3109/00365513.2015.1099722. [DOI] [PubMed] [Google Scholar]
- 149.Ragab H.M., El Maksoud N.A., Amin M.A., Elaziz W.A. Performance of serum CD163 as a marker of fibrosis in patients with NAFLD. Diabetes Metab Syndr. 2021;15:87–92. doi: 10.1016/j.dsx.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 150.Yoshio S., Kanto T. Macrophages as a source of fibrosis biomarkers for non-alcoholic fatty liver disease. Immunol Med. 2021;44:175–186. doi: 10.1080/25785826.2020.1868664. [DOI] [PubMed] [Google Scholar]