Abstract
Nonalcoholic fatty liver disease (NAFLD) is a fast growing, chronic liver disease affecting ∼25% of the global population. Nonalcoholic fatty liver disease severity ranges from the less severe simple hepatic steatosis to the more advanced nonalcoholic steatohepatitis (NASH). The presence of NASH predisposes individuals to liver fibrosis, which can further progress to cirrhosis and hepatocellular carcinoma. This makes hepatic fibrosis an important indicator of clinical outcomes in patients with NASH. Hepatic stellate cell activation dictates fibrosis development during NASH. Here, we discuss recent advances in the analysis of the profibrogenic pathways and mediators of hepatic stellate cell activation and inactivation, which ultimately determine the course of disease in nonalcoholic fatty liver disease/NASH.
Keywords: Hepatic Stellate Cell, NAFLD, Fibrosis, NASH
Summary.
Nonalcoholic fatty liver disease (NAFLD) frequently progresses to liver fibrosis, an important indicator of clinical outcomes. As hepatic stellate cell activation dictates fibrosis development during NAFLD, pathways that mediate hepatic stellate cell activation and inactivation ultimately determine course of disease in NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease worldwide with global prevalence rates of around 25% to nearly 30% in the adult population, which continue to increase.1,2 Because targeted therapies are still lacking, NAFLD represents a high burden for those affected and for health care systems around the world.3,4 NAFLD is considered the hepatic manifestation of the metabolic syndrome and is closely associated with diabetes mellitus, obesity, and dyslipidemia.5 NAFLD encompasses several liver pathologies ranging from simple hepatic lipid accumulation (hepatic steatosis) to the more severe nonalcoholic steatohepatitis (NASH).6 NASH is characterized by cellular stress, hepatocyte death, and inflammation, and may further progress to fibrosis, cirrhosis, and hepatocellular carcinoma.7 The transition to fibrosis and cirrhosis is crucial because fibrosis is the key determinant of adverse outcomes and mortality and rates of liver-related complications increase with higher fibrosis stages.3,8 The impact of NASH as a health burden is demonstrated by the fact that NASH is the fastest growing cause of hepatocellular carcinoma in patients listed for liver transplantation in the United States.9 A key mechanism driving fibrosis development during NASH is the activation of hepatic stellate cells; once activated they differentiate into highly proliferative, extracellular matrix–producing myofibroblasts.10 Therefore, understanding the underlying mechanisms and pathways of hepatic stellate cell activation is fundamental for developing effective therapies for NAFLD/NASH. This review provides an overview of the pathologic functions of hepatic stellate cells in the context of NAFLD and discusses the mechanisms involved in their activation and deactivation, and therapeutic approaches targeting these mechanisms.
Role of Hepatic Stellate Cells in Nonalcoholic Fatty Liver Disease
Etiology of Nonalcoholic Fatty Liver Disease
NAFLD is an umbrella term encompassing various liver pathologies including simple hepatic steatosis or nonalcoholic fatty liver and the more advanced NASH.11 The pathogenesis of NASH is multifactorial, resulting from numerous conditions acting in parallel, such as abnormal lipid metabolism, genetic predisposition, lipotoxicity, oxidative stress, gut dysbiosis, endoplasmic reticulum stress, mitochondrial dysfunction, and inflammation.11,12 NASH is associated with obesity and metabolic dysregulation, during which the adipose tissue exhibits low-grade inflammation and secretes adipokines and inflammatory cytokines, such as leptin, tumor necrosis factor (TNF), and interleukin (IL)6.13 Furthermore, hepatic lipid accumulation correlates with insulin resistance, endoplasmic reticulum stress, mitochondrial dysfunction, and reactive oxygen species generation.13,14 Additionally, NASH and NAFLD are associated with microbiota dysbiosis and a dysfunctional gut barrier resulting in an increased accumulation of gut-derived bacterial products, such as lipopolysaccharides and inflammation in the liver.13,15
During NAFLD, various intrahepatic and extrahepatic triggers lead to activation of liver-resident immune cells and to recruitment of additional immune cells of the innate and adaptive immune systems.16,17 In particular, the liver resident macrophages named Kupffer cells play a major role in promoting inflammation during NAFLD.18 The activation of Kupffer cells leads to the secretion of proinflammatory chemokines and cytokines, such as chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL5, TNF-α, IL1β, and IL6, which further aggravates inflammation by recruiting immune cells, such as monocyte-derived macrophages, neutrophils, and lymphocytes.19, 20, 21 Although inflammation is an important mechanism of pathogenesis, patients tend to be mostly asymptomatic with respect to their liver disease at the earlier stages of NAFLD because the incidence for clinical symptoms increases with fibrosis stages.3 This is in line with the findings that histopathologic inflammation alone is not a reliable predictor of NAFLD progression.22 Fibrosis severity is the critical indicator of mortality during NASH, with higher risk of liver-related complications and death in NASH patients with F3 or F4 stage fibrosis.3,23
General Description of Hepatic Stellate Cells
Hepatic stellate cells are a nonparenchymal cell population, which represent the main fibrogenic cell type in the liver and account for approximately 5%–8% of all liver cells in the normal liver.24,25 Hepatic stellate cells are localized in the space of Disse, between the basolateral region of hepatocytes and the antiluminal surface of the sinusoidal endothelial cells.26 In the healthy liver, the resting, quiescent hepatic stellate cells have several cytoplasmic processes that aid in making contact with the surrounding cells, such as endothelial cells and hepatocytes, and assist in the intercellular transport of cytokines and soluble mediators.26 A distinct feature of quiescent hepatic stellate cells is the storage of retinoids (vitamin A and its metabolites) within their cytoplasmic lipid droplets.26,27 Under healthy conditions, approximately 80%–90% of the liver retinoids are stored in the lipid droplets of hepatic stellate cells.26,27
There is extensive crosstalk between hepatic stellate cells and immune cells, such as macrophages.28,29 In vitro experiments using human macrophages and hepatic stellate cells show that soluble molecules derived from activated hepatic stellate cells induce the differentiation of macrophages to a proinflammatory phenotype.28 Furthermore, recent single-cell RNA sequencing data suggest that hepatic stellate cells communicate with their surrounding endothelial cells and immune cells by secreting a variety of soluble factors or “stellakines,” many of which are upregulated during NASH and liver injury. Thus, enhanced secretion of stellakines by hepatic stellate cells may be linked to NASH pathogenesis.29
Activation of Hepatic Stellate Cells
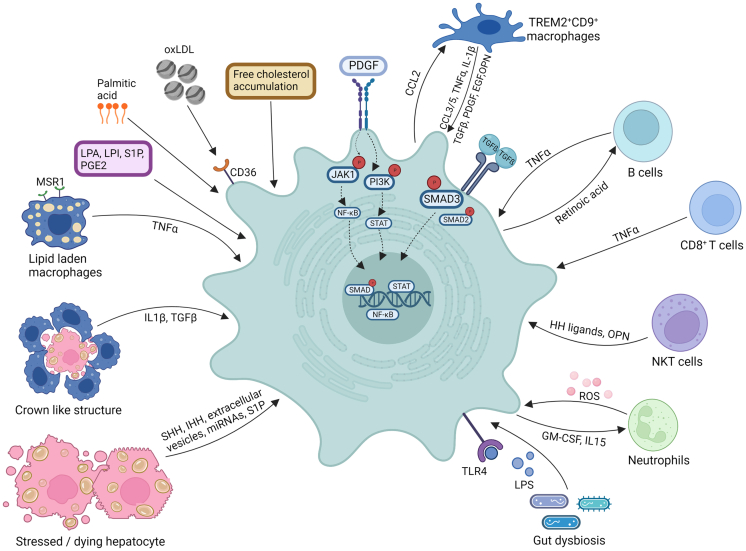
Hepatic stellate cells can be activated by various stimuli during liver injury and NASH (Figure 1).30 Hepatic stellate cells produce most myofibroblasts during liver injury, as shown in rodent models of liver fibrosis and NASH.25,31 The activation of quiescent hepatic stellate cells leads to their transdifferentiation into highly proliferative, extracellular matrix–producing activated hepatic stellate cells or myofibroblasts, with marked changes in their gene expression profile.10,32 These activated hepatic stellate cells exhibit a contractile, proliferative, and fibrogenic phenotype, which can be further distinguished from quiescent hepatic stellate cells by the loss of their retinol-containing lipid droplets.33 Although such activation represents a useful mechanism in acute injury, sustained activation of hepatic stellate cells as seen in NASH results in an excess accumulation of extracellular matrix that ultimately leads to fibrosis.10 Activated hepatic stellate cells secrete a wide range of proinflammatory cytokines, such as CCL2, CCL5, IL8, chemokine (C-X-C motif) ligand-12 (CXCL12), and express adhesion molecules, such as intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 and chemokine receptors (CCR5), resulting in increased recruitment and infiltration of immune cells into the liver.34
Figure 1.
Pathways of hepatic stellate cell activation and survival during NAFLD. Various factors, such as lipid mediators, free cholesterol accumulation, oxLDL, palmitic acid, LPS, and immune cell–derived profibrotic molecules and growth factors, promote hepatic stellate cell activation and survival during NAFLD. OPN, osteopontin; HH ligands, hedgehog ligands; ROS, reactive oxygen species; GM-CSF, granulocyte macrophage colony-stimulating factor; LPS, lipopolysaccharide; TLR4, toll like receptor-4; SHH, sonic hedgehog; IHH, Indian hedgehog; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; S1P, sphingosine-1-phosphate; oxLDL, oxidized low-density lipoprotein; LPA, lysophosphatidic acid; LPI, lysophosphatidylinositol; MSR1, macrophage scavenger receptor 1; miRNA, microRNA. Created with Biorender.com.
Although quiescent hepatic stellate cells are rather homogeneous, activated hepatic stellate cells/myofibroblasts are much more heterogeneous.29,35 Single-cell RNA sequencing studies performed in mouse and human livers have identified distinct subsets of resting and activated hepatic stellate cells/myofibroblasts in fibrotic and cirrhotic livers.35, 36, 37, 38, 39 Functionally, quiescent hepatic stellate cells express high levels of growth factors and can protect hepatocytes from injury, whereas activated hepatic stellate cells mainly produce extracellular matrix proteins, such as different types of collagen I, but also collagen III, VI, and XIV, and cytokines and chemokines, promoting inflammation and fibrogenesis.29,39 Furthermore, activated hepatic stellate cells can also exhibit immunoregulatory effects: hepatic stellate cells from human livers can induce apoptosis of activated T cells via programmed cell death 1 ligand 1; influence B-cell activity via the same mechanism in mice; and exert a positive influence on tolerancing immune, cells such as FoxP3+ regulatory T cells or myeloid-derived suppressor cells in different mouse models.40, 41, 42, 43 Currently, it is not yet clear under which conditions hepatic stellate cells are proinflammatory and under which conditions they are tolerogenic. Several mechanisms trigger the activation of hepatic stellate cells during NAFLD pathogenesis, including metabolic injury with hepatocyte death and inflammation with activation and recruitment of diverse immune cells.33 The various triggers of hepatic stellate cell activation are described in detail in the later sections.
The activation of hepatic stellate cells is mainly mediated by growth factors, including transforming growth factor (TGF)β, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) (Figure 1). TGFβ is the most potent activator of hepatic stellate cells and a key driver of fibrogenesis.10 Of note, TGFβ levels are elevated in the serum of patients with NAFLD.44,45 In the context of NAFLD, TGFβ originates mainly from immune cells, such as macrophages, and from activated hepatic stellate cells in a feed-forward loop.46 During NAFLD, hepatocyte death and the resulting recruitment of macrophages is a critical driver of hepatic stellate cell activation and fibrosis via producing TGFβ.10 When hepatocyte death occurs, recruited macrophages clear the apoptotic cells via the process of efferocytosis, which induces the production of immune regulatory factors, such as TGFβ by macrophages, leading to the activation of hepatic stellate cells.10,47 Accordingly, inhibition of the TGFβ pathway in a mouse model of NAFLD results in reduced activation of hepatic stellate cells and attenuates fibrosis, an effect that was most pronounced when IL13 was inhibited simultaneously.48 Mechanistically, TGFβ-induced hepatic stellate cell activation is mediated by the phosphorylation of SMAD proteins, such as SMAD 3, which ultimately leads to the upregulation of collagen I and III synthesis by complexes of phosphorylated SMAD molecules acting as transcription factors.49, 50, 51 Genes that are upregulated by SMAD also include α-smooth muscle actin (α-SMA) and connective tissue growth factor (CTGF).46 Furthermore, TGFβ can also induce activation of hepatic stellate cells in a SMAD-independent manner via mitogen-activated protein kinase-1, p38, and c-Jun N-terminal kinase mediated mechanisms, among others.52,53 In addition to these direct effects, latent TGFβ can be deposited in the extracellular matrix and become subsequently activated by contraction of hepatic stellate cells through integrin-αV mediated mechanisms.54,55 Concordantly, inhibition of integrin-αV has antifibrotic effects during liver injury.54,55 Furthermore, the epigenetic regulator TET3 is involved in the TGFβ/SMAD2/3 activation pathway, which can be inhibited by microRNA-488-5p in mice with liver fibrosis induced by CCl4 treatment, high-fat diet, or bile duct ligation.56 Moreover, CTGF, also known as CCN2, is 1 of the target genes of TGFβ, which in addition to an increased secretion of extracellular matrix also promotes the proliferation and migration of hepatic stellate cells.57 The expression of CTGF is relatively low in the healthy liver, but markedly increased in biopsies from patients with NAFLD.58 Furthermore, silencing CTGF attenuates fibrosis development in a mouse model of CCl4-induced liver injury.59 In addition to TGFβ-induced upregulation of CTGF, an additional TGFβ-independent upregulation via IL-13 has been described.60,61
PDGF is another profibrogenic growth factor. Under physiological conditions, PDGF is mainly produced by platelets; however, during liver injury PDGF is produced by macrophages, injured endothelial cells, and activated hepatic stellate cells.33,62 During liver injury, there is also an increased expression of PDGF receptor-β (PDGFRβ) in hepatic stellate cells, which can promote increased signaling and activation of hepatic stellate cells.63 Accordingly, in animal models of liver fibrosis, depletion of PDGFRβ leads to a reduction in fibrosis, conversely autoactivation of the receptor increases fibrosis development.64,65 Moreover, PDGFRα is increased in biopsies of patients with severe NAFLD, and levels of circulating PDGFRβ may help predict liver fibrosis.66,67 PDGF signaling is mediated by dimerization and subsequent autophosphorylation of the 2 PDGFRs after binding of a PDGF dimer.68 PDGFRs are receptor tyrosine kinases and on activation trigger subsequent upregulation of mediators, such as phosphoinositide 3-kinase and Janus kinase-1.62 Activation of PDGFR in hepatic stellate cells promotes their proliferation and migration; promotes cell survival; and increases the production of hedgehog pathway ligands, such as sonic hedgehog, which have been proposed to induce hepatic stellate cell activation.69, 70, 71 Moreover, in patients with NASH, hedgehog activity correlates with the inflammation and fibrosis stage, whereas inhibition of hedgehog signaling reduces fibrosis in a mouse model of NASH.72,73 Moreover, vascular endothelial growth factor has been shown to induce hepatic stellate cell activation and proliferation, thereby leading to enhanced fibrosis development.74,75 The EGF receptor also seems to be involved in profibrotic pathways and is more strongly expressed in activated hepatic stellate cells.76 Inhibition of EGF signaling results in decreased activation of hepatic stellate cells.76,77
Triggers of Hepatic Stellate Cell Activation in Nonalcoholic Fatty Liver Disease
Lipotoxicity
Hepatic lipotoxicity, caused by dysregulated lipid metabolism and enhanced influx of free fatty acids from peripheral tissues, is associated with NAFLD severity and progression.78 Several toxic lipids, such as fatty acids, cholesterol, oxidized low-density lipoproteins, and others, promote macrophage activation, upregulate their proinflammatory phenotype, induce hepatic stellate cell activation (either directly or indirectly via increased inflammation, Figure 1), and promote NASH pathogenesis.79, 80, 81, 82 Free cholesterol is a key lipotoxic molecule in the context of NASH.81,83 In animal models of NAFLD, several studies have demonstrated the importance of dietary cholesterol in promoting inflammation, fibrosis, and NASH pathogenesis.83, 84, 85 Interestingly, dietary cholesterol and dietary fat act synergistically to aggravate the metabolic features of NASH in mice and trigger a hepatic pathology resembling that of advanced NASH in humans, which is characterized by increased macrophage recruitment, cholesterol dysregulation, production of oxidized low-density lipoproteins, and significant fibrosis.84,85 Furthermore, free cholesterol is significantly increased in human NASH livers, whereas only a mild nonsignificant increase is seen in NAFLD samples.86,87 Remarkably, cholesterol crystals accumulate in lipid droplets of steatotic hepatocytes, in mouse models of high fat, high cholesterol diet–induced NASH, and in human NASH, an event that is absent in samples with simple steatosis.81,88 Steatotic, dead, or dying hepatocytes containing cholesterol crystals are encircled by Kupffer cells/monocyte-derived macrophages in an attempt to process the lipids, thereby resulting in the formation of activated, lipid-laden foamy macrophages and in the formation of the characteristic “crown like structures.”81,88,89 In this context, the Kupffer cells/monocyte-derived macrophages within the “crown like structures” get activated by cholesterol crystals leading to NLR family pyrin domain containing 3 (NLRP3) inflammasome activation and the production of proinflammatory factors, such as CCL2, IL1β, and TGFβ, which, along with amplifying inflammation, also promote hepatic stellate cell activation.81,88,89 Furthermore, accumulation of free cholesterol in hepatic stellate cells leads to enhanced cell activation because of sensitization of hepatic stellate cells to TGFβ.90 Free cholesterol accumulation in hepatic stellate cells promotes toll-like receptor-4 (TLR4) levels and signal transduction, which results in the suppression of hepatic stellate cell–specific BAMBI (a pseudoreceptor for TGFβ) and consequently TGFβ-induced hepatic stellate cell activation is boosted.90
In addition to directly activating hepatic stellate cells, toxic lipids can also be indirect activators of hepatic stellate cells, for instance via increasing inflammation. Lipid accumulation in macrophages enhances their inflammatory function.80,82,91,92 Specifically, accumulation of lipid droplets in macrophages is important for the production of proinflammatory molecules, such as prostaglandin E2 (PGE2), IL1β, and IL6.91 Fatty acids, such as palmitic acid, can activate the TLR4-MD2 complex in macrophages to generate reactive oxygen species and pro-IL1β.93 Additionally, palmitic acid impairs autophagy in macrophages via activation of hypoxia inducible factor-1α; the decreased autophagy together with upregulation of the hypoxia inducible factor-1α pathway leads to enhanced inflammation through the activation of nuclear factor-κB and increased production of CCL2 and IL1β.94 Uptake of oxidized low-density lipoproteins by macrophages leads to lysosomal accumulation of these lipids and to increased inflammation.82 In addition to macrophages, oxidized low-density lipoproteins also promote the activation of hepatic stellate cells, in cultured rat and human cells.95,96 Oxidized low-density lipoproteins-induced hepatic stellate cell activation is mediated by the scavenger receptor CD36 (Figure 1).96 Furthermore, in mouse macrophages, the scavenger receptor “macrophage scavenger receptor 1” (MSR1) facilitates palmitic acid–induced lipid accumulation and enhances Tnfa and Il6 mRNA expression.92 The macrophage-derived cytokines, such as TNFα and IL1β, in turn promote survival of activated hepatic stellate cells or myofibroblasts, as shown by in vitro and in vivo experiments.97 Moreover, in a mouse model of NAFLD, the PGE2/PGE2 receptor 4 (EP4) axis promotes hepatic stellate cell activation and fibrosis via activating the extracellular signal-regulated kinase pathway and enhancing autophagy in hepatic stellate cells.98
Although hepatocytes can accumulate cholesterol without necessary ballooning, this specific form of hepatocyte cell degeneration is an important histologic hallmark of NASH and is associated with fibrosis development.99 Ballooned hepatocytes are profibrogenic and promote hepatic stellate cell activation possibly by producing sonic hedgehog.100,101 Furthermore, hedgehog signaling has been suggested to promote glycolysis in hepatic stellate cells thereby regulating the myofibroblast phenotype.102 Moreover, a direct link between NASH and hepatic cholesterol levels is well established.81,103 Cholesterol accumulation in hepatocytes leads to hepatic stellate cell activation and NASH by stabilizing the transcriptional regulator TAZ (also known as WWTR1) in hepatocytes, resulting in the secretion of the profibrotic factor Indian hedgehog.104 Sonic hedgehog, Indian hedgehog, and TAZ are upregulated in human livers affected by NASH but not simple steatosis,105,106 suggesting a role of hepatocyte TAZ in promoting the transition from simple steatosis to NASH. Furthermore, fatty acids, such as palmitic acid, promote the release of extracellular vesicles from hepatocytes, which can be taken up by hepatic stellate cells resulting in their activation, both in human and mouse cells.107 Hepatic stellate cells that internalize extracellular vesicles exhibit increased proliferation, chemotaxis, and enhanced expression of profibrotic genes, such as collagen, α-SMA, and tissue inhibitor of metalloproteinases-2.107 Mechanistically, the extracellular vesicles contain microRNAs, such as miR-128-3p, and once transferred to the hepatic stellate cells, these microRNAs inhibit peroxisome proliferator activated receptor (PPAR)γ expression, ultimately leading to a switch from quiescent to activated hepatic stellate cell states.107 Moreover, palmitate treatment of hepatocytes also results in the enhanced accumulation and secretion of the profibrotic lipid mediator, sphingosine-1-phosphate.108 Of note, in addition to acting on hepatocytes, palmitic acid can also directly induce hepatic stellate cell activation via the inflammasome and possibly hedgehog signaling pathways.109 Furthermore, steatotic hepatocytes upregulate Notch signaling, which correlates with NASH severity and results in the production of soluble mediators, such as osteopontin, which in turn activate hepatic stellate cells.110,111 Consistently, inhibition of Notch signaling in hepatocytes reduces liver fibrosis. Additionally, dying hepatocytes also release damage-associated molecular patterns, such as P2Y14 ligands and the alarmin IL33, which activate mouse and human hepatic stellate cells through direct and indirect mechanisms and thereby promote fibrogenesis.112,113
Lipid Mediators
Profibrotic lipid mediators, such as lysophosphatidylinositol and lysophosphatidic acid, have been implicated in hepatic stellate cell activation and fibrogenesis (Figure 1).114,115 Inhibition of the major lysophosphatidic acid generating enzyme autotaxin (ectonucleotide pyrophosphatase/phosphodiesterase family member 2) using a selective inhibitor leads to significant improvement in fibrosis development in mouse models of liver injury and NASH.116 In addition, autotaxin levels are elevated in the serum of patients with NAFLD and patients with liver cirrhosis as compared with healthy individuals and correlate with the stage of fibrosis.117,118 Consistently, in a model of CCl4-induced liver injury in rats, circulating levels of lysophosphatidic acid and autotaxin activity are increased and correlate with the extent of liver fibrosis as well.119 Moreover, circulating levels of lysophosphatidylinositol are increased in patients who have advanced fibrosis as compared with healthy individuals and the lysophosphatidylinositol-G protein-coupled receptor 55 axis has been shown to play a role in hepatic stellate cell activation.115,120 Interestingly, the occurrence of the MBOAT7 rs641738C>T risk variant (which reduces membrane-bound O-acyltransferase domain containing 7 [MBOAT7] mRNA and protein) is associated with increased hepatic lysophosphatidylinositol levels and accordingly with enhanced NASH fibrosis.121,122 Importantly, even in the absence of the MBOAT7 rs641738C>T risk variant, obesity suppresses hepatic MBOAT7 levels.120 Therefore, the increased production and secretion of lysophosphatidylinositol because of reduced MBOAT7 levels may promote hepatic stellate cell activation, although further studies are required to demonstrate the underlying mechanisms.
The sphingosine-1-phosphate axis is also involved in hepatic stellate cell activation and hepatic fibrosis development.108,123, 124, 125 In cultured human hepatic stellate cells, sphingosine-1-phosphate treatment directly induces cell activation, as shown by the upregulation of α-SMA expression.108 Furthermore, sphingosine-1-phosphate treatment also induces the migration of human hepatic myofibroblasts in culture.124 Moreover, in rodent models of bile duct ligation- or CCl4-induced liver injury, treatment with the sphingosine-1-phosphate receptor 1/3 antagonist or with an inhibitor of sphingosine kinase (an enzyme that generates sphingosine-1-phosphate) suppress liver injury and hepatic fibrosis.123 In addition, in a mouse model of diet-induced NASH, treatment with an sphingosine-1-phosphate antagonist abrogates NASH development125 with reduced ballooning, fibrosis, and inflammation following feeding with a diet high in fat, fructose, and cholesterol.125 Moreover, palmitate treatment of hepatocytes results in the increased accumulation of intracellular and extracellular sphingosine-1-phosphate levels.108 Interestingly, conditioned medium derived from palmitate-treated hepatocytes promotes activation of hepatic stellate cells in culture and this activation can be blocked by cotreatment with an sphingosine-1-phosphate receptor 1/3 antagonist; suggesting that palmitate-treated hepatocytes release extracellular sphingosine-1-phosphate that can function in a paracrine manner to activate hepatic stellate cells.108 In addition to promoting hepatic stellate cell activation, sphingosine-1-phosphate can also directly act on immune cells, such as macrophages, to promote NASH pathogenesis.126 In a rodent model of diet-induced NASH, genetic or pharmacologic inhibition of the sphingosine-1-phosphate receptor 4 significantly reduces inflammation and NASH fibrosis via mechanisms involving NLR family pyrin domain containing 3 (NLRP3) inflammasome activation in macrophages.126
Autophagy
Autophagy is a stress response mechanism that involves the degradation of cellular components and organelles through a lysosome-dependent pathway to generate energy and nutrients,30 and plays a critical role in hepatic stellate cell activation and NASH fibrogenesis.30,33,98,127, 128, 129 Autophagy is required to sustain an activated phenotype in hepatic stellate cells.127,128 In culture-induced activation of mouse hepatic stellate cells a significant induction of autophagic flux is seen.128 Moreover, treatment with an autophagy inhibitor reduces proliferation and expression of cellular activation markers, as shown in mouse and human hepatic stellate cells.128 In mice containing autophagy defective hepatic stellate cells, CCl4-induced liver injury results in abrogated extracellular matrix accumulation and fibrosis development.127 Furthermore, the suppressed activation of hepatic stellate cells seen during autophagy deficiency can be partially rescued by the addition of exogenous fatty acids, such as oleic acid, therefore suggesting that the free fatty acids generated during autophagy are required to fuel cellular activation.127 Several profibrotic/proinflammatory molecules, such as TGFβ and lipopolysaccharides, are known to upregulate autophagy in hepatic stellate cells.129,130 TGFβ induced autophagy plays a role in hepatic stellate cell activation, possibly via the c-Jun N-terminal kinase and extracellular signal-regulated kinase signaling pathways.129 Moreover, lipopolysaccharides-induced upregulation of autophagy mediates the suppression of the TGFβ pseudoreceptor BAMBI, thereby sensitizing hepatic stellate cells to TGFβ-induced cell activation.130 Furthermore, macrophage-derived PGE2 can promote hepatic stellate cell activation and fibrosis by inducing autophagy.98 In a mouse model of diet-induced NAFLD, M2-polarized macrophages induce hepatic stellate cell autophagy by producing PGE2, which acts via its receptor EP4 on hepatic stellate cell, consequently enhancing hepatic stellate cell activation, extracellular matrix production, and fibrosis development.98 Blocking the PGE2/EP4 axis using an antagonist inhibits hepatic stellate cell autophagy and improves liver fibrosis.98
Endoplasmic reticulum stress has been shown to be upstream of autophagy in the hepatic stellate cell activation cascade.30,131,132 In cultured hepatic stellate cells, overexpression of X-box binding protein 1, one of the unfolded protein response pathways, results in the upregulation of collagen expression; however, this induction of collagen is inhibited by deletion of an important autophagy mediator, autophagy related 7.131 These data suggest that the X-box binding protein 1-mediated pathway contributes to fibrogenic activation of hepatic stellate cells and is linked to autophagy. Moreover, inositol-requiring enzyme 1, another component of the unfolded protein response pathway, induces hepatic stellate cell activation and autophagy, mediated via the p38 mitogen-activated protein kinase pathway.132 Some of the key features of hepatic stellate cell activation are summarized in Figure 1.
Inflammation
During NAFLD, in addition to the metabolic triggers described previously, inflammation is a major cause of hepatic stellate cell activation.10,33 Macrophages play a key role in the pathogenesis of NAFLD and are 1 of the cell types that interact with hepatic stellate cells.10 Proinflammatory/profibrotic macrophages send strong activation and survival signals to hepatic stellate cells via secreting cytokines, such as TGFβ, PDGF, TNF-α, osteopontin, and IL1β, among others (Figure 1).33,38,133 The chemokines CCL3 and CCL5 are also involved in hepatic stellate cell activation and fibrosis development during liver injury.20,21 In mouse models of liver fibrosis, induced by carbon tetrachloride (CCl4) treatment or by feeding with a methionine and choline-deficient diet, blocking CCL3 or CCL5 results in decreased activation of hepatic stellate cells and reduced fibrosis development.20,21 Furthermore, in mouse and human tissues, single-cell RNA sequencing studies have identified the presence of a subset of macrophages that are TREM2+CD9+ and named as NASH-associated macrophages, lipid-associated macrophages, or scar-associated macrophages.29,38,134 These TREM2+CD9+ scar-associated macrophages are particularly abundant in injured and cirrhotic human livers and secrete high levels of cytokines and growth factors including, EGF and PDGF-BB, osteopontin, and IL1β thereby providing a profibrogenic niche for hepatic stellate cells.38 Furthermore, macrophages promote the survival of myofibroblasts via IL1 and TNF-dependent activation of the nuclear factor kappa-light-chain-enhancer of activated B cells pathway.97 Moreover, the interaction between macrophages and hepatic stellate cells is not 1-sided; hepatic stellate cells also influence the differentiation of macrophages in the liver.135 Activated hepatic stellate cells aggravate inflammation by inducing the proinflammatory polarization of macrophages via the p38 pathway and further promote the recruitment of monocyte-derived macrophages through secretion of CCL2.28,136
In addition to macrophages, other immune cells also provide activation signals to hepatic stellate cells (Figure 1). Immunodeficient animals with severe combined immunodeficiency or B cell deficiency show lower activation of hepatic stellate cells during liver injury. Transfer of CD8+ T cells can reverse this effect.137,138 Furthermore, B cells can promote NAFLD pathogenesis and hepatic stellate cell activation by secreting TNF-α and IL6, as shown in a mouse model of diet-induced NAFLD.139 Of note, hepatic stellate cell-derived retinoic acid has been shown to augment B-cell survival.140 Moreover, CD8+ T cells are increased in different mouse models of NASH and in patients and can also have an activating effect on hepatic stellate cells through the secretion of profibrogenic cytokines, such as TNFα.141,142 However, certain subsets of CD8+ T cells have been shown to have an opposite effect, where CD69+CD103-CD8+ tissue resident memory T cells can induce apoptosis of activated hepatic stellate cells via the FasL-Fas pathway.143 T helper type 17 cells induce hepatic stellate cell activation through the production of IL17 and IL22, which stimulate TGFβ production in the liver and enhance TGFβ signaling and production of extracellular matrix molecules by hepatic stellate cells.144 Natural killer T cell–derived hedgehog ligands and osteopontin can activate hepatic stellate cells.145 Genetic deletion of natural killer T cells leads to a decrease in fibrogenic factors in methionine-choline-deficient diet fed mice.145 Neutrophil granulocytes can also trigger activation of hepatic stellate cells via secretion of their typical granules (eg, via myeloperoxidase).146 With respect to the neutrophil-hepatic stellate cell crosstalk a positive feedback loop has also been described, in which hepatic stellate cells once activated by neutrophil-derived reactive oxygen species in turn improve neutrophil survival by producing granulocyte-macrophage colony-stimulating factor and IL15, thereby amplifying inflammation and fibrosis.147 Additionally, extrahepatic proinflammatory signals can act as triggers for hepatic stellate cell activation, because quiescent hepatic stellate cells express various receptors of the innate immune system including TLR4, which can be activated by lipopolysaccharides.148 This is of particular relevance, because patients with NAFLD often show alterations of the gut-liver-axis with intestinal dysbiosis and increased translocation of intestinal bacteria.149 Furthermore, it has been shown in mice that stimulation of quiescent hepatic stellate cells with lipopolysaccharides induces cell activation resulting in increased chemokine secretion, positively affecting chemotaxis of Kupffer cells/macrophages, and increasing the susceptibility to TGFβ signaling through downregulation of the BMP and activin membrane-bound inhibitor (BAMBI; TGFβ pseudoreceptor).150 Although the most relevant aspect of TGFβ signaling in the context of this review may be activation of hepatic stellate cells and therefore its profibrogenic role as described previously, it is also worth mentioning the interactions TGFβ shows with various immune cells. TGFβ has been described to promote the differentiation of FoxP3+CD4+ regulatory T cells, which was also associated with the progression of hepatocellular carcinoma.151 This immunosuppressive effect of TGFβ seems to be mediated via retinoid metabolism by hepatic stellate cells.152 Because activated hepatic stellate cells lose their lipid droplets this may explain why this mechanism of liver-induced tolerance by TGFβ is not sufficiently protective against inflammation in the presence of continuous stimuli as in NASH. TGFβ can also promote a more anti-inflammatory/immunosuppressive phenotype in macrophages, sometimes called M2 or alternately activated macrophages.153
Inactivation of Hepatic Stellate Cells During Nonalcoholic Fatty Liver Disease
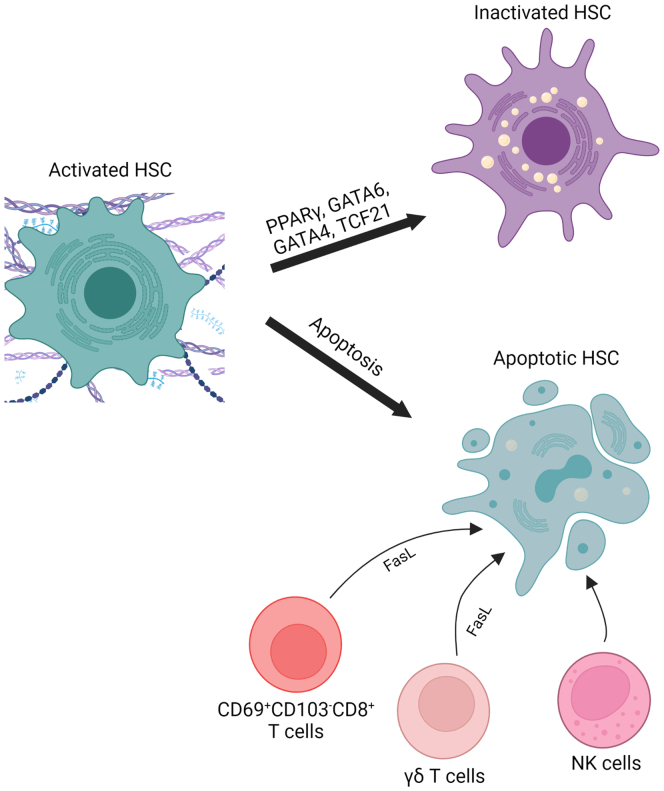
Reversal of hepatic stellate cell activation has been demonstrated in rodent models of fibrosis resolution.154,155 During fibrosis regression activated hepatic stellate cells are removed either via apoptosis or by reverting to a quiescent-like phenotype (Figure 2), where they remain in a primed state and respond more rapidly to new fibrogenic stimuli, thereby contributing to faster fibrosis development on reinjury.154, 155, 156 Several transcription factors, such as PPARγ, GATA-binding factor 6 (GATA6), GATA-binding factor 4 (GATA4), and transcription factor 21 (TCF21), have been implicated in regulating the deactivation of hepatic stellate cells.157, 158, 159 In a model of CCl4-induced liver injury, mice with hepatic stellate cell–specific deletion of GATA6 or PPARγ are more susceptible to fibrosis development.159 Moreover, regression of liver fibrosis is significantly reduced in hepatic stellate cell–specific GATA6-deficient mice and in hepatic stellate cell–specific PPARγ-deficient mice as compared with wild-type mice, therefore suggesting that both GATA6 and PPARγ are critical for maintaining the inactivated phenotype of hepatic stellate cells.159 Furthermore, the expression of the transcription factor TCF21 is diminished in activated hepatic stellate cells of methionine and choline-deficient diet or CCl4-treated fibrotic mouse livers as compared with normal livers.158 This reduced TCF21 expression in hepatic stellate cells is restored during regression of liver fibrosis.158 Overexpression of TCF21 in activated hepatic stellate cells/myofibroblasts downregulates the mRNA expression of profibrotic genes, such as Col1a1 (encoding for collagen, type I, alpha 1), Pdgfrb (encoding for PDGFR-beta), and Acta2 (encoding for α-SMA); in contrast, the expression of hepatic stellate cell quiescence genes, such as Gfap (encoding for glial fibrillary acidic protein) and Ngfr (encoding for nerve growth factor receptor), are upregulated.158 Therefore, TCF21 acts as a deactivation factor in fibrogenic hepatic stellate cells. Furthermore, GATA4 is another transcription factor that is involved in the suppression of hepatic stellate cell activation.157 Accordingly, overexpression of GATA4 in the liver promotes the regression of CCl4-induced liver fibrosis.157
Figure 2.
Inactivation of hepatic stellate cells during NAFLD. Activated hepatic stellate cells that accumulated during NASH fibrosis are removed during fibrosis resolution either by apoptosis or by reverting to an inactive phenotype. Additionally, activated hepatic stellate cells are removed by cell death induced by immune cells such as CD69+CD103-CD8+ T cells, γδ T cells and NK cells. The transcription factors PPARγ, GATA6, GATA4 and TCF21 play an important role in promoting and maintaining the inactivated phenotype of hepatic stellate cells. FasL, Fas ligand; HSC, hepatic stellate cells; NK, natural killer; TCF21, transcription factor 21. Created with Biorender.com.
Immune cells are also involved in suppressing hepatic stellate cell activity by inducing cell death (Figure 2).143,160 Specifically, γδ T cells, certain CD8+ T cells, and natural killer cells can have a proapoptotic effect on hepatic stellate cells and thus an antifibrotic effect.143,160,161 Hepatic γδ T cells induce apoptosis of hepatic stellate cells in a cell-to-cell contact-dependent mechanism and involving Fas-ligand, as shown in mouse models of liver injury.160 Similarly, CD69+CD103-CD8+ tissue resident memory T cells promote apoptosis of activated hepatic stellate cells in Fas-ligand dependent fashion.143 Moreover, natural killer cells also mediate an antifibrotic effect through inducing apoptosis of hepatic stellate cells.162 Activation of natural killer cells in mice with liver fibrosis attenuates fibrosis development by inducing cell death of activated hepatic stellate cells, whereas depletion of natural killer cells reverses this effect, a mechanism mediated by retinoic acid early inducible 1 and TNF.161
Targeting Hepatic Stellate Cells
Despite the increasing prevalence rates and intensive research, there is still no approved NASH-specific therapy available. The agents investigated in advanced clinical trials so far aim at improving metabolic injury (eg, PPAR agonists, farnesoid X receptor agonists, thyreomimetics, glucagon-like peptide agonists and others), reducing hepatocyte injury (eg, ASK-1 inhibitors and caspase inhibitors) and inhibiting immune cell activation and recruitment (eg, CCR2/5 antagonists, PPAR agonists, farnesoid X receptor agonists). As described previously, these factors are important triggers for hepatic stellate cell activation, and pharmacologic manipulation of the upstream mediators or cells may lead to reduced activation of hepatic stellate cells. Specific effects on hepatic stellate cells have also been described for some of these substances; relevant clinical trials are summarized in Table 1. As mentioned, CCL5 is involved in the activation of hepatic stellate cells163 and CCR-5 inhibitors, such as maraviroc, might therefore have antifibrotic effects. In vitro experiments with human hepatic stellate cells showed a reduction of collagen and extracellular matrix and TGFβ as an important mediator of hepatic stellate cell activation.164 However, clinical trials have only been conducted in a small cohort of patients with HIV with NAFLD, because maraviroc is already approved as a combination therapy in HIV treatment. In this study, no reduction in hepatic fat could be detected radiographically, but the applicability to other patient populations is uncertain.165 Although PPAR agonists are thought to act primarily by influencing metabolism in hepatocytes, PPARβ, γ and δ are also expressed in hepatic stellate cells. Their effects in hepatic stellate cells are not yet comprehensively understood, but there is evidence that PPARγ, for example, has inhibitory effects on hepatic stellate cell activation and proliferation and can promote their inactivation.159,166,167 Reduced synthesis of extracellular matrix has also been described for glucagon-like peptide agonists, at least in NASH animal models.168
Table 1.
Clinical Trials With Antifibrotic Agents in Liver Fibrosis
| Compound | Type | Phase | Trial number | Patients | Status | Treatment duration | Main result |
|---|---|---|---|---|---|---|---|
| Maraviroc | CCR-5 inhibitor | IV | NCT03129113 | NAFLD and HIV | Completed | 48 wk | No reduction of hepatic fat by MR-PDFF165 |
| Belapectin | Galectin 3 inhibitor | IIb | NCT02462967 | NASH F4 cirrhosis with portal hypertension | Completed | 52 wk | No reduction of HPVG, no histologic improvement169 |
| Simtuzumab (GS-6624) | Monoclonal antibody against LOXL2 | IIb | NCT01672866 | NASH F3/4 fibrosis | Completed | 96 wk | No histologic improvement of hepatic collagen170 |
| Simtuzumab (GS-6624) | Monoclonal antibody against LOXL2 | IIb | NCT01672879 | NASH F4 cirrhosis | Completed | 96 wk | No reduction of HPVG170 |
| Pirfenidone | Antifibrotic agent | II | NCT02161952. | Chronic hepatitis C | Completed | 24 mo | Significant histologic improvement171 |
| Pirfenidone | Antifibrotic agent | II | NCT04099407 | Advanced fibrosis (mixed etiologies) | Completed | 12 mo | Significant reduction of fibrosis by noninvasive measurement172 |
| BMS-986263 | HSP47 siRNA | II | NCT03420768 | Hepatitis C F3/4 fibrosis | Completed | 12 wk | Association with histologic improvement173 |
| BMS-986263 | HSP47 siRNA | II | NCT04267393 | NASH compensated F4 cirrhosis | Recruiting | ||
| PLN-1474 | Integrin αVβ1 inhibitor | I | not available | NASH F3/4 fibrosis | Recruiting |
CCR-5, C-C chemokine receptor type 5; HPVG, hepatic venous pressure gradient; HSP47, Heat shock protein 47; LOXL2, Lysyl oxidase homolog 2; MR-PDFF, magnetic resonance proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
In addition, substances with a more primary effect on hepatic stellate cells are also investigated. Galectin 3 is a cytosolic protein in cells of the innate immune system, especially in macrophages. Increased expression of galectin 3 causes activation of myofibroblasts and galectin 3 has profibrotic effects in various organs.174 In animal studies, galectin 3 inhibitors have been shown to reduce fibrosis.175 Belapectin is a pharmacologic inhibitor of galectin 3 that has been previously studied in clinical trials in patients with NASH. In a phase IIb study, no histologic improvement in fibrosis was achieved after 1 year.169 However, subgroup analysis showed improvement in hepatic stellate cells in patients without preexisting varices, and further studies are now ongoing for this subgroup. Another antifibrotic agent currently in clinical trials is BMS-986263, a nanoparticle containing HSP47 siRNA. HSP47 for its part is a serine proteinase inhibitor, a protein that specifically binds collagen as a so-called chaperone.176 Thus, by binding of the siRNA, collagen synthesis can be negatively affected. First results of the phase II study in patients with cured hepatitis C but advanced fibrosis (stage 3 or 4) were published in early 2022 and showed histologic improvement of fibrosis.173 Currently, recruitment is underway for NCT04267393, a phase II study to test BMS-986263 in patients with NASH with compensated cirrhosis (F4). After synthesis, collagen is cross-linked by lysyl oxidases, resulting in greater stability. Therefore, another antifibrotic therapeutic approach is the inhibition of these enzymes. Simtuzumab is a monoclonal antibody against Lysyl Oxidase Like 2 (LOXL2), which prevents LOXL2-mediated stabilization of the extracellular matrix and was able to accelerate fibrosis resolution in mouse models.177 However, in 2 phase IIb studies in patients with advanced NASH fibrosis and NASH cirrhosis, respectively, no significant effect was observed after 96 weeks of treatment.170 It is possible that other lysyl oxidases may play a more relevant role.
Other fibrosis-specific therapeutic approaches are based on influencing TGFβ. This can be activated in its latent form in an integrin-mediated manner, as already mentioned. Inhibition of integrin-αV by a pan αV inhibitor causes attenuation of fibrosis in various organ models including liver.178,179 Other integrin antibodies are already approved for different diseases (eg, vedolizumab for inflammatory bowel disease) and clinical trials are ongoing for idiopathic pulmonary fibrosis, but clinical trials in liver fibrosis are still lacking. Currently, only a phase I trial of the selective αVβ1 inhibitor PLN-1474 in patients with NASH has been announced.180 Further interference with TGFβ may be feasible with pirfenidone, an oral antifibrotic agent already approved for the treatment of idiopathic pulmonary fibrosis. The exact mechanism of action is not yet clear, but preclinical studies show reduced collagen synthesis and lower TGFβ levels.181,182 A small clinical trial in patients with chronic hepatitis C showed histologic improvement in fibrosis and also reduced TGFβ levels after 2 years of treatment.171 In the more recent PROMETEO study in patients with advanced fibrosis, of which the largest group was NASH-related, a reduction in fibrosis was also seen, but noninvasive measurement was used.172 Other growth mediators may also be modulated. Angiotensin receptor antagonists are an established therapy option for cardiovascular disease and there is some evidence from rat models that these agents attenuate activation of hepatic stellate cells.183,184 Angiotensin II has also been shown to cause upregulation of CTGF and downstream activation of SMAD2/3,185 but human studies on the effect of these agents in NAFLD are lacking. An ameliorating effect on the development of NAFLD has also been described for EGF receptor. In NAFLD mouse models, continuous administration of canertinib, an EGF receptor inhibitor, prevented the development of steatosis. Additionally, in animals with preexisting NASH, this therapy significantly reduced fibrosis.186 Other studies show a reduced number of activated hepatic stellate cells after administration of an EGF receptor inhibitor in animal models of progressive cirrhosis.76 However, human studies on these compounds are also lacking to date.
Recently, obeticholic acid has been resubmitted to the Food and Drug Administration for approval as the first specific drug to treat NASH fibrosis.187 However, specific antifibrotic therapies are still lacking. It remains to be seen how the new insights into hepatic stellate cells from single cell analyses described previously can be translated into therapeutic concepts.
Conclusions and Future Perspectives
The prevalence of NAFLD is growing rapidly, with fibrosis severity as the critical determinant of disease progression and mortality. Hepatic stellate cells are the precursors for most profibrogenic, extracellular matrix producing myofibroblasts during NAFLD. Various factors, such as inflammation, lipotoxicity, lipid mediators, and growth factors, can promote hepatic stellate cell activation during NAFLD (Figure 1). Additionally, the recruitment and activation of profibrogenic macrophages in response to hepatocyte death is crucial for triggering hepatic stellate cell activation and survival as outlined in the accompanying review by Vonderlin and colleagues. In summary, recent studies have expanded the knowledge on the pathways and mechanisms of hepatic stellate cell activation and NASH fibrosis. However, further studies are required to establish the best intervention to inhibit and reverse the fibrogenic process.
Acknowledgments
Leke Wiering is a participant in the BIH Charité Junior Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH).
CRediT Authorship Contributions
Leke Wiering, MD (Conceptualization: Equal; Data curation: Equal; Visualization: Lead; Writing – original draft: Equal; Writing – review & editing: Equal)
Pallavi Subramanian, PhD (Conceptualization: Equal; Visualization: Supporting; Writing – original draft: Equal; Writing – review & editing: Supporting)
Linda Hammerich, PhD (Conceptualization: Equal; Visualization: Supporting; Writing – original draft: Equal; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by the German Research Foundation (CRC1382, SPP2306).
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., et al. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Le M.H., Yeo Y.H., Li X., et al. Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;2022(20):2809–2817. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal A.J., Van Natta M.L., Clark J., et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Blissett D., Blissett R., et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 5.Diehl A.M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 6.Musso G., Cassader M., Gambino R. Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov. 2016;15:249–274. doi: 10.1038/nrd.2015.3. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Baker R.D., Bhatia T., et al. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol Life Sci. 2016;73:1969–1987. doi: 10.1007/s00018-016-2161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angulo P., Kleiner D.E., Dam-Larsen S., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi Z., Stepanova M., Ong J.P., et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 10.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caligiuri A., Gentilini A., Marra F. Molecular pathogenesis of NASH. Int J Mol Sci. 2016;17:1575. doi: 10.3390/ijms17091575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nati M., Chung K.J., Chavakis T. The role of innate immune cells in nonalcoholic fatty liver disease. J Innate Immun. 2022;14:31–41. doi: 10.1159/000518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarthy M.V., Neuschwander-Tetri B.A. The metabolic basis of nonalcoholic steatohepatitis. Endocrinol Diabetes Metab. 2020;3 doi: 10.1002/edm2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aron-Wisnewsky J., Vigliotti C., Witjes J., et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 16.Cai J., Zhang X.J., Li H. The role of innate immune cells in nonalcoholic steatohepatitis. Hepatology. 2019;70:1026–1037. doi: 10.1002/hep.30506. [DOI] [PubMed] [Google Scholar]
- 17.Sutti S., Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81–92. doi: 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazankov K., Jorgensen S.M.D., Thomsen K.L., et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 19.Krenkel O., Tacke F. Macrophages in nonalcoholic fatty liver disease: a role model of pathogenic immunometabolism. Semin Liver Dis. 2017;37:189–197. doi: 10.1055/s-0037-1604480. [DOI] [PubMed] [Google Scholar]
- 20.Heinrichs D., Berres M.L., Nellen A., et al. The chemokine CCL3 promotes experimental liver fibrosis in mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berres M.L., Koenen R.R., Rueland A., et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–4140. doi: 10.1172/JCI41732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagstrom H., Nasr P., Ekstedt M., et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Vilar-Gomez E., Calzadilla-Bertot L., Wai-Sun Wong V., et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–457. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 24.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 25.Mederacke I., Hsu C.C., Troeger J.S., et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sufletel R.T., Melincovici C.S., Gheban B.A., et al. Hepatic stellate cells - from past till present: morphology, human markers, human cell lines, behavior in normal and liver pathology. Rom J Morphol Embryol. 2020;61:615–642. doi: 10.47162/RJME.61.3.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang J., Hisamatsu T., Shimamura K., et al. Activated hepatic stellate cells mediate the differentiation of macrophages. Hepatol Res. 2013;43:658–669. doi: 10.1111/j.1872-034X.2012.01111.x. [DOI] [PubMed] [Google Scholar]
- 29.Xiong X., Kuang H., Ansari S., et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell. 2019;75:644–660. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trivedi P., Wang S., Friedman S.L. The power of plasticity-metabolic regulation of hepatic stellate cells. Cell Metab. 2021;33:242–257. doi: 10.1016/j.cmet.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman S.L., Roll F.J., Boyles J., et al. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z.Y., Keogh A., Waldt A., et al. Single-cell and bulk transcriptomics of the liver reveals potential targets of NASH with fibrosis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-98806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 34.Carter J.K., Friedman S.L. Hepatic stellate cell-immune interactions in NASH. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.867940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krenkel O., Hundertmark J., Ritz T.P., et al. Single cell RNA sequencing identifies subsets of hepatic stellate cells and myofibroblasts in liver fibrosis. Cells. 2019;8:503. doi: 10.3390/cells8050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobie R., Wilson-Kanamori J.R., Henderson B.E.P., et al. Single-cell transcriptomics uncovers zonation of function in the mesenchyme during liver fibrosis. Cell Rep. 2019;29:1832–1847. doi: 10.1016/j.celrep.2019.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenthal S.B., Liu X., Ganguly S., et al. Heterogeneity of HSCs in a mouse model of NASH. Hepatology. 2021;74:667–685. doi: 10.1002/hep.31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandran P., Dobie R., Wilson-Kanamori J.R., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filliol A., Saito Y., Nair A., et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature. 2022;610:356–3565. doi: 10.1038/s41586-022-05289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles R., Chou H.S., Wang L., et al. Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway. Transplantation. 2013;96:17–24. doi: 10.1097/TP.0b013e318294caae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Lu L., Qian S., et al. Hepatic stellate cells directly inhibit b cells via programmed death-ligand 1. J Immunol. 2016;196:1617–1625. doi: 10.4049/jimmunol.1501737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang G., Yang H.R., Wang L., et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou H.S., Hsieh C.C., Yang H.R., et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sepulveda-Flores R.N., Vera-Cabrera L., Flores-Gutierrez J.P., et al. Obesity-related non-alcoholic steatohepatitis and TGF-beta1 serum levels in relation to morbid obesity. Ann Hepatol. 2002;1:36–39. [PubMed] [Google Scholar]
- 45.Tarantino G., Conca P., Riccio A., et al. Enhanced serum concentrations of transforming growth factor-beta1 in simple fatty liver: is it really benign? J Transl Med. 2008;6:72. doi: 10.1186/1479-5876-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewidar B., Meyer C., Dooley S., et al. TGF-beta in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells. 2019;8:1419. doi: 10.3390/cells8111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kourtzelis I., Hajishengallis G., Chavakis T. Phagocytosis of apoptotic cells in resolution of inflammation. Front Immunol. 2020;11:553. doi: 10.3389/fimmu.2020.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart K.M., Fabre T., Sciurba J.C., et al. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-beta. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3694. [DOI] [PubMed] [Google Scholar]
- 49.Furukawa F., Matsuzaki K., Mori S., et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K., Matsuzaki K., Mori S., T, et al. Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am J Pathol. 2005;166:1029–1039. doi: 10.1016/s0002-9440(10)62324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breitkopf K., Godoy P., Ciuclan L., et al. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44:57–66. doi: 10.1055/s-2005-858989. [DOI] [PubMed] [Google Scholar]
- 52.Engel M.E., McDonnell M.A., Law B.K., et al. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 53.Hanafusa H., Ninomiya-Tsuji J., Masuyama N., et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 54.Wipff P.J., Rifkin D.B., Meister J.J., et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Annes J.P., Chen Y., Munger J.S., et al. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu J., Wu S., Wang P., et al. miR-488-5p mitigates hepatic stellate cell activation and hepatic fibrosis via suppressing TET3 expression. Hepatol Int. 2022 doi: 10.1007/s12072-022-10404-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang G., Brigstock D.R. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci (Landmark Ed) 2012;17:2495–2507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- 58.Paradis V., Perlemuter G., Bonvoust F., et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34(4 Pt 1):738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 59.Hao C., Xie Y., Peng M., et al. Inhibition of connective tissue growth factor suppresses hepatic stellate cell activation in vitro and prevents liver fibrosis in vivo. Clin Exp Med. 2014;14:141–150. doi: 10.1007/s10238-013-0229-6. [DOI] [PubMed] [Google Scholar]
- 60.Sakai K., Jawaid S., Sasaki T., et al. Transforming growth factor-beta-independent role of connective tissue growth factor in the development of liver fibrosis. Am J Pathol. 2014;184:2611–2617. doi: 10.1016/j.ajpath.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Meyer C., Muller A., et al. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-beta-independent Smad signaling. J Immunol. 2011;187:2814–2823. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- 62.Ying H.Z., Chen Q., Zhang W.Y., et al. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review) Mol Med Rep. 2017;16:7879–7889. doi: 10.3892/mmr.2017.7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong L., Yamasaki G., Johnson R.J., et al. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. The J Clin Invest. 1994;94:1563–1569. doi: 10.1172/JCI117497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Czochra P., Klopcic B., Meyer E., et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–428. doi: 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 65.Kocabayoglu P., Lade A., Lee Y.A., et al. beta-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63:141–147. doi: 10.1016/j.jhep.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moylan C.A., Pang H., Dellinger A., et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology. 2014;59:471–482. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambrecht J., Verhulst S., Mannaerts I., et al. A PDGFRbeta-based score predicts significant liver fibrosis in patients with chronic alcohol abuse, NAFLD and viral liver disease. EBioMedicine. 2019;43:501–512. doi: 10.1016/j.ebiom.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heldin C.H., Ernlund A., Rorsman C., et al. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989;264:8905–8912. [PubMed] [Google Scholar]
- 69.Yang L., Wang Y., Mao H., et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sicklick J.K., Li Y.X., Choi S.S., et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368–1380. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- 71.Kikuchi A., Pradhan-Sundd T., Singh S., et al. Platelet-derived growth factor receptor alpha contributes to human hepatic stellate cell proliferation and migration. Am J Pathol. 2017;187:2273–2287. doi: 10.1016/j.ajpath.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guy C.D., Suzuki A., Zdanowicz M., et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirsova P., Ibrahim S.H., Bronk S.F., et al. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Y., Feng H., Kan T., et al. Bevacizumab attenuates hepatic fibrosis in rats by inhibiting activation of hepatic stellate cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang L., Kwon J., Popov Y., et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339–1350. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuchs B.C., Hoshida Y., Fujii T., et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qian Y., Han J., Zhou L., et al. Inhibition of epidermal growth factor receptor (EGFR) reduces lipopolysaccharide (LPS)-induced activation and inflammatory cytokines in hepatic stellate cells in vitro. Med Sci Monit. 2018;24:5533–5541. doi: 10.12659/MSM.909901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rada P., Gonzalez-Rodriguez A., Garcia-Monzon C., et al. Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis. 2020;11:802. doi: 10.1038/s41419-020-03003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanian P., Hampe J., Tacke F., et al. Fibrogenic pathways in metabolic dysfunction associated fatty liver disease (MAFLD) Int J Mol Sci. 2022;23:6996. doi: 10.3390/ijms23136996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leroux A., Ferrere G., Godie V., et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57:141–149. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 81.Ioannou G.N. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab. 2016;27:84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 82.Bieghs V., Walenbergh S.M., Hendrikx T., et al. Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver Int. 2013;33:1056–1061. doi: 10.1111/liv.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Rooyen D.M., Larter C.Z., Haigh W.G., et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393–1403. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savard C., Tartaglione E.V., Kuver R., et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGettigan B., McMahan R., Orlicky D., et al. Dietary lipids differentially shape nonalcoholic steatohepatitis progression and the transcriptome of Kupffer cells and infiltrating macrophages. Hepatology. 2019;70:67–83. doi: 10.1002/hep.30401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puri P., Baillie R.A., Wiest M.M., et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 87.Caballero F., Fernandez A., De Lacy A.M., et al. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol. 2009;50:789–796. doi: 10.1016/j.jhep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 88.Ioannou G.N., Haigh W.G., Thorning D., et al. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J Lipid Res. 2013;54:1326–1334. doi: 10.1194/jlr.M034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ioannou G.N., Subramanian S., Chait A., et al. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J Lipid Res. 2017;58:1067–1079. doi: 10.1194/jlr.M072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teratani T., Tomita K., Suzuki T., et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142:152–164. doi: 10.1053/j.gastro.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 91.Castoldi A., Monteiro L.B., van Teijlingen Bakker N., et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nature Commun. 2020;11:4107. doi: 10.1038/s41467-020-17881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Govaere O., Petersen S.K., Martinez-Lopez N., et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J Hepatol. 2022;76:1001–1012. doi: 10.1016/j.jhep.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 93.Kim S.Y., Jeong J.M., Kim S.J., et al. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nature Commun. 2017;8:2247. doi: 10.1038/s41467-017-02325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X., de Carvalho Ribeiro M., Iracheta-Vellve A., et al. Macrophage-specific hypoxia-inducible factor-1alpha contributes to impaired autophagic flux in nonalcoholic steatohepatitis. Hepatology. 2019;69:545–563. doi: 10.1002/hep.30215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang Q., Chen A. Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1. Lab Invest. 2009;89:1275–1290. doi: 10.1038/labinvest.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schneiderhan W., Schmid-Kotsas A., Zhao J., et al. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology. 2001;34(4 Pt 1):729–737. doi: 10.1053/jhep.2001.27828. [DOI] [PubMed] [Google Scholar]
- 97.Pradere J.P., Kluwe J., De Minicis S., et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao Y., Mai W., Li R., et al. Macrophages evoke autophagy of hepatic stellate cells to promote liver fibrosis in NAFLD mice via the PGE2/EP4 pathway. Cell Mol Life Sci. 2022;79:303. doi: 10.1007/s00018-022-04319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kakisaka K., Suzuki Y., Fujiwara Y., et al. Evaluation of ballooned hepatocytes as a risk factor for future progression of fibrosis in patients with non-alcoholic fatty liver disease. J Gastroenterol. 2018;53:1285–1291. doi: 10.1007/s00535-018-1468-9. [DOI] [PubMed] [Google Scholar]
- 100.Rangwala F., Guy C.D., Lu J., et al. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–410. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chung S.I., Moon H., Ju H.L., et al. Hepatic expression of Sonic Hedgehog induces liver fibrosis and promotes hepatocarcinogenesis in a transgenic mouse model. J Hepatol. 2016;64:618–627. doi: 10.1016/j.jhep.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Chen Y., Choi S.S., Michelotti G.A., et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Min H.K., Kapoor A., Fuchs M., et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X., Cai B., Yang X., et al. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 2020;31:969–986. doi: 10.1016/j.cmet.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Zheng Z., Caviglia J.M., et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khajehahmadi Z., Mohagheghi S., Nikeghbalian S., et al. Downregulation of hedgehog ligands in human simple steatosis may protect against nonalcoholic steatohepatitis: is TAZ a crucial regulator? IUBMB Life. 2019;71:1382–1390. doi: 10.1002/iub.2068. [DOI] [PubMed] [Google Scholar]
- 107.Povero D., Panera N., Eguchi A., et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol. 2015;1:646–663. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al Fadel F., Fayyaz S., Japtok L., et al. Involvement of sphingosine 1-phosphate in palmitate-induced non-alcoholic fatty liver disease. Cell Physiol Biochem. 2016;40:1637–1645. doi: 10.1159/000453213. [DOI] [PubMed] [Google Scholar]
- 109.Duan N.N., Liu X.J., Wu J. Palmitic acid elicits hepatic stellate cell activation through inflammasomes and hedgehog signaling. Life Sci. 2017;176:42–53. doi: 10.1016/j.lfs.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 110.Zhu C., Kim K., Wang X., et al. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aat0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu J., Zhu C., Wang X., et al. Hepatocyte TLR4 triggers inter-hepatocyte Jagged1/Notch signaling to determine NASH-induced fibrosis. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abe1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mederacke I., Filliol A., Affo S., et al. The purinergic P2Y14 receptor links hepatocyte death to hepatic stellate cell activation and fibrogenesis in the liver. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abe5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McHedlidze T., Waldner M., Zopf S., et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaffe E., Katsifa A., Xylourgidis N., et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology. 2017;65:1369–1383. doi: 10.1002/hep.28973. [DOI] [PubMed] [Google Scholar]
- 115.Fondevila M.F., Fernandez U., Gonzalez-Rellan M.J., et al. The L-alpha-Lysophosphatidylinositol/G protein-coupled receptor 55 system induces the development of nonalcoholic steatosis and steatohepatitis. Hepatology. 2021;73:606–624. doi: 10.1002/hep.31290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Booijink R., Salgado-Polo F., Jamieson C., et al. A type IV Autotaxin inhibitor ameliorates acute liver injury and nonalcoholic steatohepatitis. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202216333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujimori N., Umemura T., Kimura T., et al. Serum autotaxin levels are correlated with hepatic fibrosis and ballooning in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2018;24:1239–1249. doi: 10.3748/wjg.v24.i11.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pleli T., Martin D., Kronenberger B., et al. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis: a prospective cohort study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watanabe N., Ikeda H., Nakamura K., et al. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 2007;81:1009–1015. doi: 10.1016/j.lfs.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 120.Helsley R.N., Venkateshwari V., Brown A.L., et al. Obesity-linked suppression of membrane-bound O-Acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. eLife. 2019;8 doi: 10.7554/eLife.49882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mancina R.M., Dongiovanni P., Petta S., et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150:1219–1230. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thangapandi V.R., Knittelfelder O., Brosch M., et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut. 2021;70:940–950. doi: 10.1136/gutjnl-2020-320853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang L., Yue S., Liu X., et al. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol. 2013;59:114–123. doi: 10.1016/j.jhep.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 124.Li C., Zheng S., You H., et al. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J Hepatol. 2011;54:1205–1213. doi: 10.1016/j.jhep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 125.Mauer A.S., Hirsova P., Maiers J.L., et al. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2017;312:G300–G313. doi: 10.1152/ajpgi.00222.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hong C.H., Ko M.S., Kim J.H., et al. Sphingosine 1-phosphate receptor 4 promotes nonalcoholic steatohepatitis by activating NLRP3 inflammasome. Cell Mol Gastroenterol Hepatol. 2022;13:925–947. doi: 10.1016/j.jcmgh.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hernandez-Gea V., Ghiassi-Nejad Z., Rozenfeld R., et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thoen L.F., Guimaraes E.L., Dolle L., et al. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55:1353–1360. doi: 10.1016/j.jhep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J., Jiang N., Ping J., et al. TGFbeta1induced autophagy activates hepatic stellate cells via the ERK and JNK signaling pathways. Int J Mol Med. 2021;47:256–266. doi: 10.3892/ijmm.2020.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen M., Liu J., Yang W., et al. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy. 2017;13:1813–1827. doi: 10.1080/15548627.2017.1356550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim R.S., Hasegawa D., Goossens N., et al. The XBP1 arm of the unfolded protein response induces fibrogenic activity in hepatic stellate cells through autophagy. Sci Rep. 2016;6 doi: 10.1038/srep39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hernandez-Gea V., Hilscher M., Rozenfeld R., et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol. 2013;59:98–104. doi: 10.1016/j.jhep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pastore M., Caligiuri A., Raggi C., et al. Macrophage MerTK promotes profibrogenic cross-talk with hepatic stellate cells via soluble mediators. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Daemen S., Gainullina A., Kalugotla G., et al. Dynamic shifts in the composition of resident and recruited macrophages influence tissue remodeling in NASH. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bonnardel J., T'Jonck W., Gaublomme D., et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51:638–654. doi: 10.1016/j.immuni.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xi S., Zheng X., Li X., et al. Activated hepatic stellate cells induce infiltration and formation of CD163(+) macrophages via CCL2/CCR2 pathway. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.627927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Novobrantseva T.I., Majeau G.R., Amatucci A., et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Safadi R., Ohta M., Alvarez C.E., et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 139.Zhang F., Jiang W.W., Li X., et al. Role of intrahepatic B cells in non-alcoholic fatty liver disease by secreting pro-inflammatory cytokines and regulating intrahepatic T cells. J Dig Dis. 2016;17:464–474. doi: 10.1111/1751-2980.12362. [DOI] [PubMed] [Google Scholar]
- 140.Thapa M., Chinnadurai R., Velazquez V.M., et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology. 2015;61:2067–2079. doi: 10.1002/hep.27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolf M.J., Adili A., Piotrowitz K., et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 142.Bhattacharjee J., Kirby M., Softic S., et al. Hepatic natural killer T-cell and CD8+ T-cell signatures in mice with nonalcoholic steatohepatitis. Hepatol Commun. 2017;1:299–310. doi: 10.1002/hep4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koda Y., Teratani T., Chu P.S., et al. CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat Commun. 2021;12:4474. doi: 10.1038/s41467-021-24734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fabre T., Molina M.F., Soucy G., et al. Type 3 cytokines IL-17A and IL-22 drive TGF-beta-dependent liver fibrosis. Sci Immunol. 2018;3:eaar7754. doi: 10.1126/sciimmunol.aar7754. [DOI] [PubMed] [Google Scholar]
- 145.Syn W.K., Agboola K.M., Swiderska M., et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pulli B., Ali M., Iwamoto Y., et al. Myeloperoxidase-hepatocyte-stellate cell cross talk promotes hepatocyte injury and fibrosis in experimental nonalcoholic steatohepatitis. Antioxid Redox Signal. 2015;23:1255–1269. doi: 10.1089/ars.2014.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou Z., Xu M.J., Cai Y., et al. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018;5:399–413. doi: 10.1016/j.jcmgh.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bigorgne A.E., John B., Ebrahimkhani M.R., et al. TLR4-dependent secretion by hepatic stellate cells of the neutrophil-chemoattractant CXCL1 mediates liver response to gut microbiota. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brandl K., Schnabl B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:128–133. doi: 10.1097/MOG.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Seki E., De Minicis S., Osterreicher C.H., et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 151.Shen Y., Wei Y., Wang Z., et al. TGF-beta regulates hepatocellular carcinoma progression by inducing Treg cell polarization. Cell Physiol Biochem. 2015;35:1623–1632. doi: 10.1159/000373976. [DOI] [PubMed] [Google Scholar]
- 152.Dunham R.M., Thapa M., Velazquez V.M., et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol. 2013;190:2009–2016. doi: 10.4049/jimmunol.1201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen B., Mu C., Zhang Z., et al. The love-hate relationship between TGF-beta signaling and the immune system during development and tumorigenesis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.891268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Troeger J.S., Mederacke I., Gwak G.Y., et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kisseleva T., Cong M., Paik Y., et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zisser A., Ipsen D.H., Tveden-Nyborg P. Hepatic stellate cell activation and inactivation in NASH-fibrosis-roles as putative treatment targets? Biomedicines. 2021;9:365. doi: 10.3390/biomedicines9040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arroyo N., Villamayor L., Diaz I., et al. GATA4 induces liver fibrosis regression by deactivating hepatic stellate cells. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakano Y., Kamiya A., Sumiyoshi H., et al. A deactivation factor of fibrogenic hepatic stellate cells induces regression of liver fibrosis in mice. Hepatology. 2020;71:1437–1452. doi: 10.1002/hep.30965. [DOI] [PubMed] [Google Scholar]
- 159.Liu X., Xu J., Rosenthal S., et al. Identification of lineage-specific transcription factors that prevent activation of hepatic stellate cells and promote fibrosis resolution. Gastroenterology. 2020;158:1728–1744. doi: 10.1053/j.gastro.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hammerich L., Bangen J.M., Govaere O., et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. 2014;59:630–642. doi: 10.1002/hep.26697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Radaeva S., Sun R., Jaruga B., et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 162.Melhem A., Muhanna N., Bishara A., et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 163.Seki E., De Minicis S., Gwak G.Y., et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coppola N., Perna A., Lucariello A., et al. Effects of treatment with Maraviroc a CCR5 inhibitor on a human hepatic stellate cell line. J Cell Physiol. 2018;233:6224–6231. doi: 10.1002/jcp.26485. [DOI] [PubMed] [Google Scholar]
- 165.Mascolini M. 18th European AIDS Conference; London, United Kingdom: 2021. Maraviroc or metformin does not lower liver fat in people with HIV and NAFLD. [Google Scholar]
- 166.Boyer-Diaz Z., Aristu-Zabalza P., Andres-Rozas M., et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2021;74:1188–1199. doi: 10.1016/j.jhep.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 167.Staels B., Rubenstrunk A., Noel B., et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 168.Kim E.R., Park J.S., Kim J.H., et al. A GLP-1/GLP-2 receptor dual agonist to treat NASH: targeting the gut-liver axis and microbiome. Hepatology. 2022;75:1523–1538. doi: 10.1002/hep.32235. [DOI] [PubMed] [Google Scholar]
- 169.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158:1334–1345. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 170.Harrison S.A., Abdelmalek M.F., Caldwell S., et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 171.Flores-Contreras L., Sandoval-Rodriguez A.S., Mena-Enriquez M.G., et al. Treatment with pirfenidone for two years decreases fibrosis, cytokine levels and enhances CB2 gene expression in patients with chronic hepatitis C. BMC Gastroenterol. 2014;14:131. doi: 10.1186/1471-230X-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Poo J.L., Torre A., Aguilar-Ramirez J.R., et al. Benefits of prolonged-release pirfenidone plus standard of care treatment in patients with advanced liver fibrosis: PROMETEO study. Hepatol Int. 2020;14:817–827. doi: 10.1007/s12072-020-10069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lawitz E.J., Shevell D.E., Tirucherai G.S., et al. BMS-986263 in patients with advanced hepatic fibrosis: 36-week results from a randomized, placebo-controlled phase 2 trial. Hepatology. 2022;75:912–923. doi: 10.1002/hep.32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Slack R.J., Mills R., Mackinnon A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int J Biochem Cell Biol. 2021;130 doi: 10.1016/j.biocel.2020.105881. [DOI] [PubMed] [Google Scholar]
- 175.Traber P.G., Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ito S., Nagata K. Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J Biol Chem. 2019;294:2133–2141. doi: 10.1074/jbc.TM118.002812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ikenaga N., Peng Z.W., Vaid K.A., et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66:1697–1708. doi: 10.1136/gutjnl-2016-312473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bouvet M., Claude O., Roux M., et al. Anti-integrin alpha(v) therapy improves cardiac fibrosis after myocardial infarction by blunting cardiac PW1(+) stromal cells. Sci Rep. 2020;10 doi: 10.1038/s41598-020-68223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Henderson N.C., Arnold T.D., Katamura Y., et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Slack R.J., Macdonald S.J.F., Roper J.A., et al. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov. 2022;21:60–78. doi: 10.1038/s41573-021-00284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Escutia-Gutierrez R., Rodriguez-Sanabria J.S., Monraz-Mendez C.A., et al. Pirfenidone modifies hepatic miRNAs expression in a model of MAFLD/NASH. Sci Rep. 2021;11 doi: 10.1038/s41598-021-91187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salah M.M., Ashour A.A., Abdelghany T.M., et al. Pirfenidone alleviates concanavalin A-induced liver fibrosis in mice. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.116982. [DOI] [PubMed] [Google Scholar]
- 183.Liu J., Gong H., Zhang Z.T., et al. Effect of angiotensin II and angiotensin II type 1 receptor antagonist on the proliferation, contraction and collagen synthesis in rat hepatic stellate cells. Chin Med J (Engl) 2008;121:161–165. [PubMed] [Google Scholar]
- 184.Hirose A., Ono M., Saibara T., et al. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375–1381. doi: 10.1002/hep.21638. [DOI] [PubMed] [Google Scholar]
- 185.Li A., Zhang J., Zhang X., et al. Angiotensin II induces connective tissue growth factor expression in human hepatic stellate cells by a transforming growth factor beta-independent mechanism. Sci Rep. 2017;7:7841. doi: 10.1038/s41598-017-08334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bhushan B., Banerjee S., Paranjpe S., et al. Pharmacologic inhibition of epidermal growth factor receptor suppresses nonalcoholic fatty liver disease in a murine fast-food diet model. Hepatology. 2019;70:1546–1563. doi: 10.1002/hep.30696. [DOI] [PubMed] [Google Scholar]
- 187.Intercept Pharmaceuticals I. Morristown; New Jersey, U.S.: 2022. Intercept Resubmits New Drug Application to U.S. FDA for Obeticholic Acid in Patients with Liver Fibrosis due to NASH. [Google Scholar]