Abstract
The development of wearable non-invasive glucose sensors provides a convenient technical means to monitor the glucose concentration of diabetes patients without discomfortability and risk of infection. Apart from enzymes as typical catalytic materials, the active catalytic materials of the glucose sensor are mainly composed of polymers, metals, alloys, metal compounds, and various metals that can undergo catalytic oxidation with glucose. Among them, metallic nanomaterials are the optimal materials applied in the field of wearable non-invasive glucose sensing due to good biocompatibility, large specific surface area, high catalytic activity, and strong adsorption capacity. This review summarizes the metallic nanomaterials used in wearable non-invasive glucose sensors including zero-dimensional (0D), one-dimensional (1D), and two-dimensional (2D) monometallic nanomaterials, bimetallic nanomaterials, metal oxide nanomaterials, etc. Besides, the applications of wearable non-invasive biosensors based on these metallic nanomaterials towards glucose detection are summarized in detail and the development trend of the wearable non-invasive glucose sensors based on metallic nanomaterials is also outlook.
Keywords: Wearable sensor, Non-invasive, Glucose sensor, Metallic nanomaterials, Electrochemical biosensor
Graphical abstract
1. Introduction
In recent years, wearable biosensors have attracted great attention from researchers. Wearable devices can collect physiological parameters worn on the human body while analyzing and storing the collected data through intelligent transmission and information processing systems ([[1], [2], [3]]). Furthermore, biosensors gradually achieve data interaction and cloud interaction, which promotes the provision of health monitoring and safety protection services for patients and people in special industries ([[4], [5], [6]]). Among them, wearable non-invasive glucose sensors are one of the most concerned fields because of their ability to continuously monitor glucose levels for diabetes management (see Table 1).
Table 1.
List of abbreviations.
| Abbreviation | Definition | Abbreviation | Definition |
|---|---|---|---|
| POCT | Point-of-Care Testing | ZIF-67 | Zeolitic imidazolate framework |
| GOx | Glucose oxidase | IDME | Interdigitated microelectrode |
| GDH | Glucose dehydrogenase | Pt-PLA | Pt-poly (l-lactic acid) |
| 3-APBA | 3-aminophenylboronic acid | DPV | Differential pulse voltammetry |
| AuNPs | Au nanoparticles | LSG | Laser-scribed graphene |
| AAO | Aluminum oxide | rGO | reduced graphene oxide |
| SF | Silk fibroin | WSNFs | Wrinkled and stretchable nanohybrid fiber |
| LOD | Limit of detection | AgNW | Silver nanowire |
| PET | Polyethylene terephthalate | PDMS | Polydimethylsiloxane |
| MPA | Micropillar array | MNDP | Micro-nano dualporous |
| PB | Prussian blue | LIG | Laser-induced graphene |
| Ch-AuNP | Chitosan-AuNPs composite | PP/LIG | LIG based on poly (3,4-ethylenedioxythiophene)-poly (styrene sulfonate) modification |
| MEM | Microelectromechanical | MOF | Metal−organic framework |
| SWNT | Single-walled carbon nanotubes | PU | Polyurethane |
| CNTs | Carbon nanotubes | NCGP | Ni–Co MOF/Ag/rGO/PU |
| LED | Light-emitting diode | FET | Field-effect transistor |
| PtNPs | Platinum nanoparticles | CF@NiCoO2 | Carbon fiber-based NiCoO2 |
| PdNPs | Pd nanoparticles | AuNCs | Au nanocluster |
According to the data released by some relevant research, diabetes has become one of the most common chronic diseases caused by modern life style ([7,8]). Especially in the context of the global catastrophe, relevant studies have shown that because diabetes patients are under an abnormal metabolic state for a long time, which may result in abnormalities in lung physiology and micrangium and aggravation of inflammatory storm, there is an increased risk of COVID-19 poor prognosis for diabetes patients ([9,10]). Additionally, chronic exposure to diabetes will lead to a variety of complication diseases including diabetic foot, diabetes retinopathy, and diabetes nephropathy, etc. ([[11], [12], [13]]). Therefore, more attention should be paid to the management of the diabetes population by monitoring the glucose concentration of diabetes patients in real-time. However, the commercial way of human blood glucose detection is still through the needle point method to test the collected blood. Such a method can bring physical pain to patients and increase the risk of infection, which also increases the psychological burden ([14,15]). Consequently, wearable non-invasive glucose sensors are in urgent demand for providing convenient, safe, and painless Point-of-Care Testing (POCT) for continuous monitoring of glucose levels in the human biofluid including sweat, tear, saliva, urine, and interstitial fluid ([16,17]).
In wearable non-invasive glucose sensors, the electrode material is the key factor that affects the sensing performances. The typical catalyst materials applied in the wearable non-invasive glucose sensors are mainly enzyme-based materials like glucose oxidase (GOx) and glucose dehydrogenase (GDH) ([[18], [19], [20]]), polymer-based materials like 3-aminophenylboronic acid (3-APBA) ([21]), metal-based materials including Cu2O, NiCeOx, ZnO@Ni (OH)2, etc. ([[22], [23], [24], [25]]). The enzyme-based and polymer-based materials have not only poor conductivity but also relatively low sensitivity. Consequently, metallic materials are an optimal substitution as conductive materials in wearable non-invasive glucose sensors because of their high conductivity. Besides, metallic materials can be applied as catalytic materials with outstanding sensing performance ([[26], [27], [28]]). Furthermore, metallic materials can be modified into nanoscale to remarkably increase the specific area to further improve the catalytic and conductivity properties ([29,30]). Metallic nanomaterials can be divided into zero-dimensional (e.g., nanoparticles), one-dimensional (e.g., nanowires, nanotubes, nanorods, etc.), and two-dimensional (e.g., films, nanosheets) according to their dimensions ([31]). Different nanomaterials with distinct dimensions exhibit specific sensing properties and thus are widely applied in wearable non-invasive glucose sensors. Moreover, there are two categories of metallic nanomaterials including noble metallic nanomaterials and non-noble metallic nanomaterials ([32]). Compared with non-noble metallic nanomaterials, noble metallic nanomaterials possess more active sites and thus higher conductivity and catalytic ability. However, the source of noble metallic nanomaterials is limited and with a high price ([33]). Therefore, both noble metallic nanomaterials and non-noble metallic nanomaterials with satisfactory sensing performance play a significant role in wearable non-invasive glucose sensors.
In this manuscript, the most recent development of wearable non-invasive glucose sensors based on metallic nanomaterials is summarized. The contents are divided into four classifications according to the applied sensing nanomaterials: mono-metal, bimetallic, metallic oxidation, and others (e.g., trimetal, metal compound oxidation, etc.). In addition, the future perspectives of wearable non-invasive glucose sensors based on metallic nanomaterials are discussed and outlooked.
2. Application of metallic nanomaterials in wearable non-invasive glucose sensors
2.1. Wearable non-invasive glucose sensors based on monometallic nanomaterials
2.1.1. Wearable non-invasive glucose sensors based on 0D noble monometallic nanomaterials
Monometallic nanomaterials have the advantages of low resistance, excellent catalytic performance, and high detection sensitivity ([[36], [35], [34]]). The excellent biocompatibility and strong electrical conductivity lead to outstanding electrocatalytic ability, which is a prominent property of the basic materials for wearable non-invasive glucose sensors. Among them, noble metallic nanoparticles exhibit a high specific surface area and activity, which became the most common nanomaterials applied for wearable non-invasive glucose sensors ([[37], [38], [39]]).
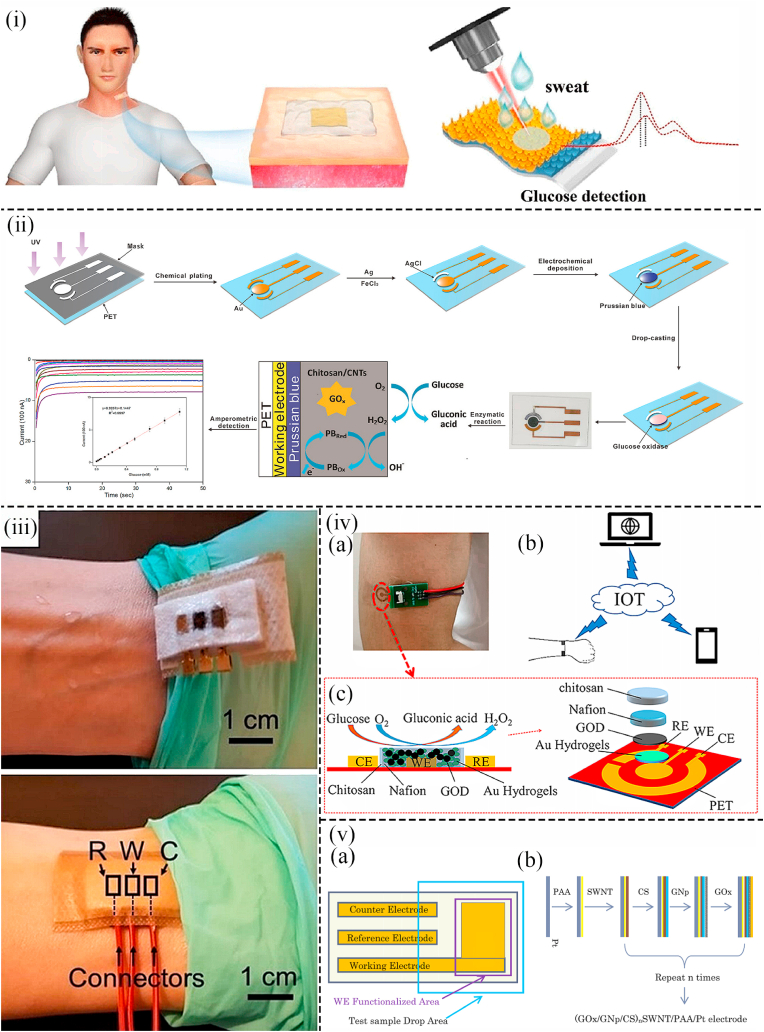
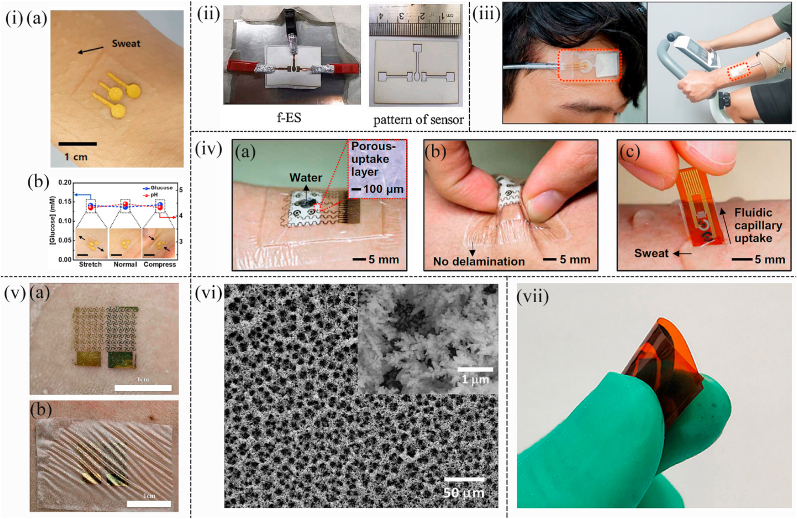
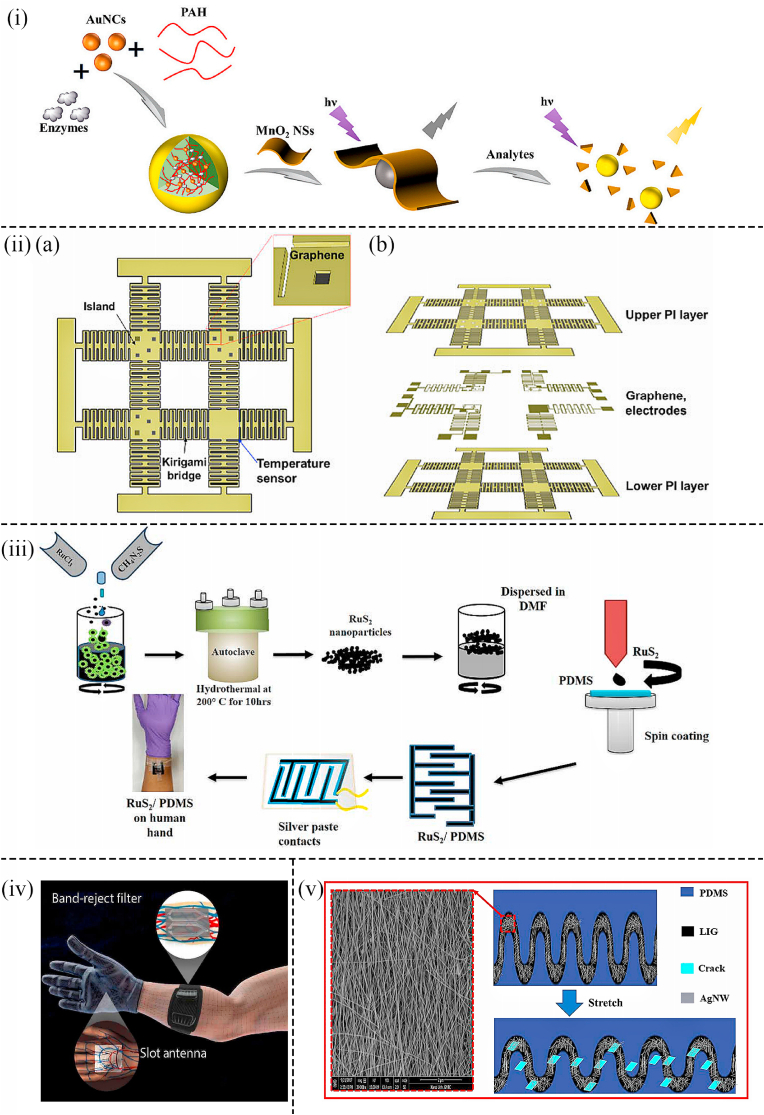
Especially for Au nanoparticles, for instance, Wang et al. proposed an epidermal (skin surface) sensor based on Au nanoparticles (AuNPs) to detect glucose levels in sweat. The ultrathin flexible Anodic Aluminum Oxide (AAO) template was combined with the Silk Fibroin (SF) film at room temperature. AuNPs were deposited on the film to form the silk fibroin-anodic aluminum oxide-Au nanoparticles (SF-AAO-AU) 3D nanostructure (Fig. 1(i)). By attaching the glucose sensor onto the neck to collect sweat, it was found that the limit of detection (LOD) was very low (1.68 × 10−7 M) and the detection range was wide (10−7-10−3 M) (Fig. 1(i)). In addition, due to the 3D periodic porous particle-in-cavity structure and specific materials applied in the sensor, such sensor possessed good stability, sensitivity, and excellent mechanical properties ([40]). Similarly, AuNPs were absorbed into the PET (polyethylene terephthalate) substrate to fabricate a wearable non-invasive patch in the work of Wang et al. The AuNPs-PET working electrode was constructed by the technique of ultraviolet mediated chemical plating (Fig. 1(ii)). As a result, the glucose sensor based on this specific electrode possessed ultralow LOD (2.7 μM), a wide sensing range (0.02–1.11 mM), and prominent sensitivity (22.05 μA mM−1 cm−2). Additionally, the wearable non-invasive sensor was tested on human sweat and commercial drinks, showing high accuracy ([41]). Moreover, in the work of Dervisevic et al. a wearable non-invasive glucose-sensing patch utilizing a high-density silicon micropillar array (MPA) was constructed to detect glucose levels in sweat (Fig. 1(iii) (a)). The glucose-sensing patch had a three-electrode configuration in which the working electrode was modified by a Prussian Blue (PB) layer and a Chitosan-AuNPs composite (Ch-AuNP) (Fig. 1(iii) (b)). The three electrodes were wrapped in wicking cotton, which was moist enough to connect them when the body sweats (Fig. 1(iii)). This sensor can prevent the loss of enzymes and settle the problem of damage to the sensor microenvironment after being worn on the body ([42]). In addition, a wearable, non-invasive, and biocompatible glucose sensor based on Au hydrogels to monitor the glucose concentration in sweat was developed in the study of Li et al. (Fig. 1(iv) (a)). Au hydrogels were fabricated by AuNPs as carriers for enzymes and sensing materials (Fig. 1(iv) (c)). Taking glucose oxidase as the model enzyme, the glucose sensor exhibited high sensitivity, long lifespan, and good selectivity. With the support of a wireless and Bluetooth module, this wearable sensing platform performed real-time, non-invasive glucose monitoring on the human skin (Fig. 1(iv) (b)). Besides, the high reproducibility and low cost of Au sensing electrodes were ensured through the synthesis of soft microelectromechanical (MEM) technology. Therefore, this work is expected to provide a general, high-performance wearable biosensing platform for various biomarkers in sweat and provide reliable diagnostic information for health management ([43]).
Fig. 1.
(i) Schematic diagram showing the flexible glucose SERS sensors based on SF-AAO-Au substrate ([40]). (ii) Schematic illustration of the construction process of the gold electrode glucose sensor as well as the mechanism of amperometric detection towards glucose ([41]). (iii) Optical images illustrating the glucose detection device with reference (R), and counter (C), working (W) electrodes amounted on the arm ([42]). (iv) (a) Photographs of the wearable glucose sensor attached to the skin. (b) Schematic diagram illustrating the working principle of the Internet of Thing. (c) Schematic image showing the wearable glucose sensor with the working electrodes based on Au Hydrogel/GOx/Nafifion/Chitosan ([43]). (v) Schematic illustration showing the (a) wearable glucose sensor; (b) modification process of the electrode in the sensor ([48]).
Further, AuNPs can be combined with graphene quantum dots ([44]) and carbon nanotubes ([45]) to form working electrodes with higher sensing performance than AuNPs themselves, which ascribe to the advantages of strong structural tunability, high conductivity, and large comparative area of carbon nanotubes, both of which provide novel and promising materials for the next-generation wearable glucose sensors ([46,47]).
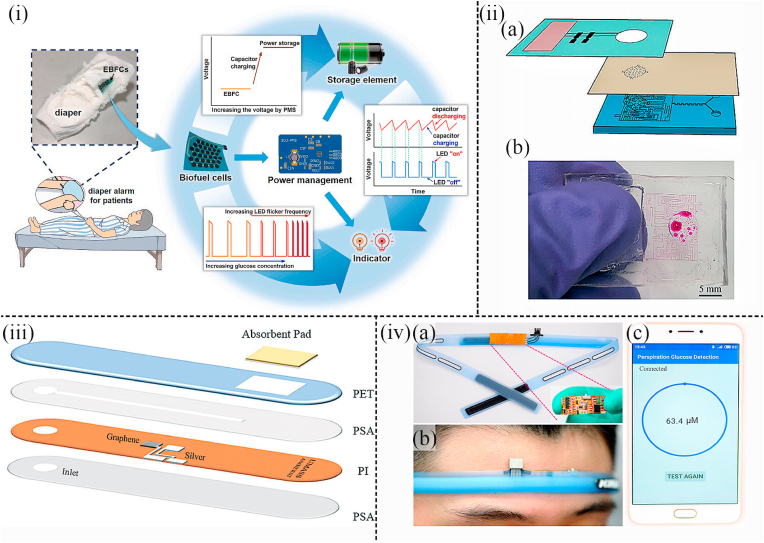
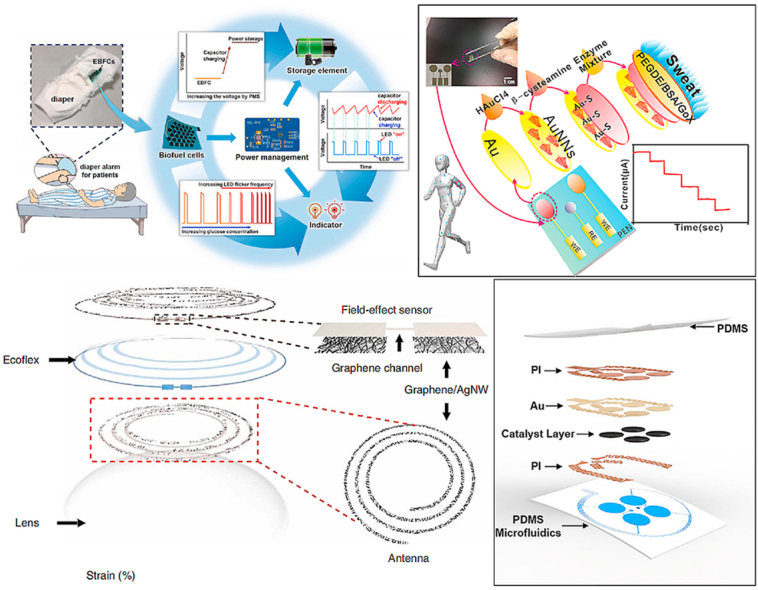
In the study of Zhang et al. a non-invasive disposable saliva nano-biosensor in which the working electrode was functionalized by using single-walled carbon nanotubes (SWNT), AuNPs, and GOx was developed (Fig. 1(v)). The biosensor that displayed low LOD and provided a non-invasive, convenient, rapid, and continuous way to detect salivary glucose levels offered fast and reliable results for clinical decision-making and the prediction of the treatment outcome ([48]). Although the detection of glucose levels in urine can not be continuous, compared with tears and sweat, the detection results towards urine glucose are precise as the volume of urine is much larger. Thus, the detection results are more accurate and the detection devices are more suitable for the disabled and babies. Consequently, A wearable non-invasive self-powered diaper based on carbon nanotubes (CNTs) and AuNPs was fabricated in the work of Zhang et al. for glucose detection in urine. By adding CNTs/AuNPs hybrid on the anode, the electron transfer between the active site of the enzyme and the electrode surface was increased. The electrode array of a flexible battery was buried in a diaper, and when urine was produced, the glucose in the urine was used as fuel to drive a light-emitting diode (LED) to blink (Fig. 2(i)). In the glucose concentration range of 1–5 mM, the blinking frequency of the LED was proportional to the glucose concentration (r = 0.994). At the same time, the sensor exhibited prominent anti-interference ability and fast response time ([49]). As a two-dimensional sheet, graphene has excellent mechanical, optical, and electrical properties, including high carrier mobility, optical transparency, flexibility, and conductivity. As a result, graphene has attracted researchers' attention and is used in glucose sensing in combination with single nanometal materials ([[53], [52], [51], [50]]). In sensing detection, more and more researchers combine graphene and noble metallic nanoparticles to form combined nanocomposite with better electrical analysis performance ([[54], [55], [56]]). In the study of Naik et al. a wearable non-invasive microfluidic platform (smart bandage) based on AuNPs to monitor glucose levels in sweat was developed (Fig. 2(iv)). This platform consisted of inkjet-printed electrodes and silver/graphene electrodes and was printed on polymer substrates. Combined with continuous glucose detection, AuNPs were deposited on the surface of graphene electrodes as the immobilization sites of glucose antibodies for glucose detection. This flexible printed smart bandage microfluidic sensor provided a platform for low-cost biomarker measurements like glucose and cortisol in sweat and showed the potential to be extended to other analytes and electrochemical sensing mechanisms (Fig. 2(iii)) ([57]).
Fig. 2.
(i) Schematic representing the structure and mechanism of the wearable diaper glucose sensors as well as the alarm component. ([49]). (ii) (a) Schematic diagram illustrating the wearable glucose sensor assembled on the synthetic skin. (b) Photograph of the microfluid for the collection of sweat amounted on the synthetic skin ([57]). (iii) Schematic representing the multi-layer structure of the biosensor ([57]). (iv) (a) Optical image representing the sweatband integrated with the nonenzymatic wearable glucose device based on Pd@ZIF-67. (b) Photo showing the sweatband with the wearable glucose sensor amounted on the head. (c) Optical image showing the app on a smartphone that was connected to the wearable glucose sensor by Bluetooth to obtain the glucose concentration in sweat ([58]).
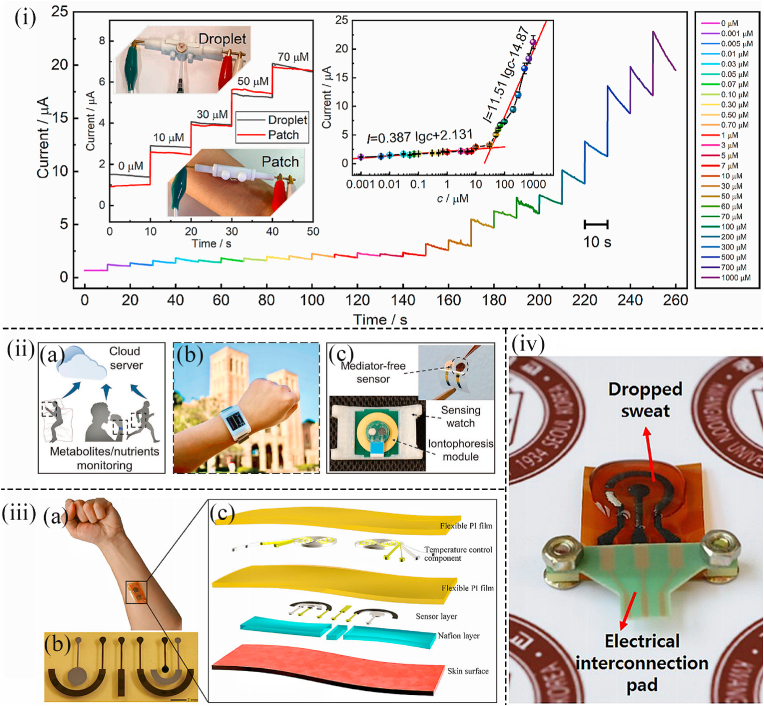
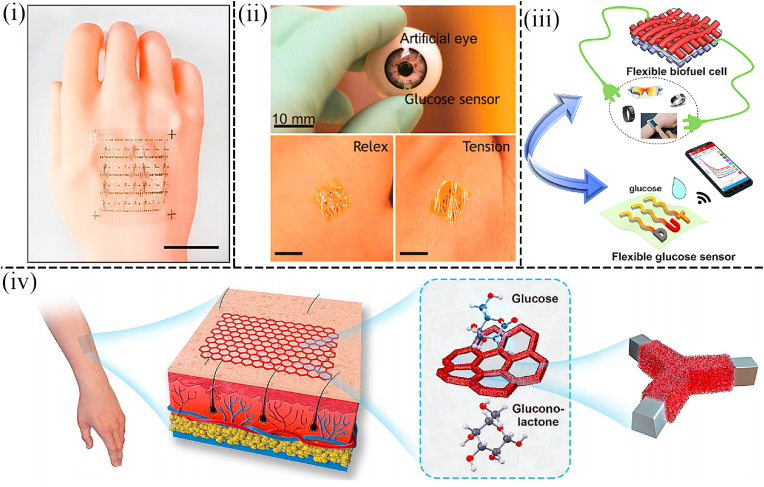
Apart from using AuNPs, platinum nanoparticles (PtNPs) and Pd nanoparticles (PdNPs) are also superior nanomaterials in non-invasive wearable sensors. For instance, a wearable non-invasive and nonenzymatic wearable sweatband based on PdNPs was fabricated by Zhu et al. Glucose levels can be detected in situ on the forehead by attaching a flexible circuit board with electrodes onto a sweatband on which a sweat collection slot existed on the top of the sweatband (Fig. 2(iv)). Furthermore, A co-based Zeolitic Imidazolate Framework (ZIF-67) was decorated by PdNPs to work as the electrocatalyst, which avoided the formation of hydrogen bubbles to ensure the precision of the test. Carbon paste was deposited on the Pd nanoparticle-encapsulated Co-based Zeolitic Imidazolate Framework. The framework was printed on a flexible PET substrate as the working electrode by the screen-printed method. The sensor displayed low LOD (2.0 μM) and stable sensitivity under ambient conditions ([58]). Additionally, Han et al. photoetched the Aluminum interdigitated microelectrode (IDME) on a quartz substrate and developed a miniaturized wearable non-invasive biosensor that incorporated a pair of interdigital Pt-poly (l-lactic acid) (Pt-PLA) IDME microelectrode arrays as working and auxiliary arrays to detect glucose in sweat. The microelectrode packed with Pt-PLA nanoparticles showed great electrochemical performance in the analysis using the DPV (differential pulse voltammetry) and amperometric response. Two linear relationships fitted between the logarithmic glucose concentration and the peak current (0.01–0.105 μM; 0.105–100 μM) indicated the electrode had a high electrocatalytic response to the glucose (Fig. 3(i)) ([59]). Similarly, Cheng et al. designed a medium-free sensing interface including coupled platinum nanoparticle/multi-walled carbon nanotube layers and a permselective membrane, which was suitable for developing a wearable sensor to detect glucose in sweat. Excellent selectivity, high sensitivity, stability, and reliability of sensing operation of this sensor in sweat samples were demonstrated. And integrated into a custom-developed smartwatch, a wireless sensing system for human sweat biomarker data collection and transmission to a cloud server was realized. This work provided a direction for a novel type of sensor for personal health monitoring applications (Fig. 3(ii)) ([60]). The concentration of glucose in the interstitial fluid is highly correlated with that in the blood, but because the interstitial fluid is under the skin, it is difficult to extract the interstitial fluid in a non-invasive manner. However, an epidermal bio-microfluidics technique that improved the efficiency of transdermal interstitial fluid was developed by Pu et al. to detect glucose concentration in interstitial fluid. A 3D nanostructured working electrode decorated with graphene and PtNPs on the surface was used in this wearable non-invasive sensor to eliminate the effect of passive perspiration (Fig. 4(i)). Interstitial fluid extraction electrodes and glucose detector electrodes were integrated into a PI film. When the PI film was attached onto the skin, a weak electric current was added to extract the interstitial fluid which can be collected by a biomicrofluidic device (Fig. 3(iii)). Because of the advantages of metallic nanomaterials and the excellent structure of wearable devices, this sensor showed a desirable linear range of glucose (0–400 mg/dl) and extremely low LOD (0.52 mg/dl) ([61]).
Fig. 3.
(i) Amperometric responses from 20 to 30 s, gotten by applying Pt-PLA microelectrodes in artificial perspiration with distinct glucose levels from 0.001 to 1000 μM (59). (ii) (a) Schematic diagram showing the various applications of the wearable biosensor. (b,c) Smartwatch with complete wearable biosensors in it ([60]). (iii) (a) Photographic image indicating the wearable glucose sensing system worn on the skin. (b) Photograph illustrating the electrode structure in the wearable sensing patch. (c) Schematic illustration showing the integrated sensing patch with glucose sensors and microfluid system ([61]). (iv) Photograph showing the practical application of the proposed wearable glucose sensor for the detection of glucose levels in sweat ([63]).
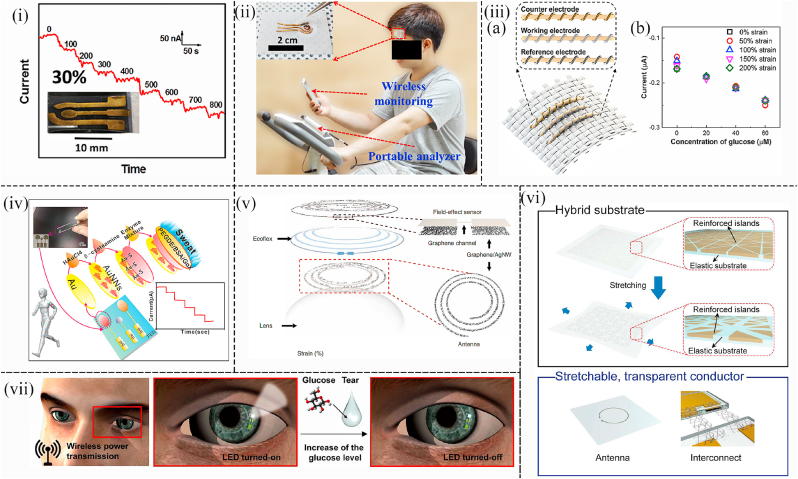
Fig. 4.
(i) Amperometric detection of the constructed biosensor with high stretchability when successive adding glucose at the voltage of −0.1 V under 30% strain. Inset is the optical image showing the structure of the electrodes ([71]). (ii) Photos showing the glucose detection system laminated on the forehead with the WSNF glucose sensor and portable analyzer. Inset is the enlarged photo showing the glucose sensor attached to a fabric with high stretchability ([72]). (iii) (a) Schematic representation showing the working electrode, the counter electrode, and reference electrode sewn on a fabric substrate; (b) Chronoamperometric results of the wearable non-invasive glucose sensor under the distinct strain of between 0 and 200% ([73]). (iv) Schematic illustration showing the glucose sensor with high flexibility and wearability. Photographic images indicate that the sensor amounted on PEN can be bent easily while keeping the integrity of its structure ([74]). (v) Schematic illustration showing the structures of wearable contact lens sensor including the contact lens, antenna, Ecoflex, materials, and filed-effect sensor ([77]). (vi) Schematic illustration of the hybrid substrate with reinforced islands before and after the stretching and the structure of the stretchable and transparent antenna ([78]). (vii) Schematic of the working mechanism of the smart contact lens with the ability to monitor the glucose concentration in tears. Electric power is wirelessly transmitted to the lens through an antenna is applied to wirelessly transmit the electric power to the contact lens with the glucose sensor and LED pixel. The LED pixel will turn off when the glucose concentration in the tear exceeds the threshold ([78]).
Laser-scribed graphene (LSG) is graphene patterned by laser with rich edge plane sites and a large specific surface area, which is the optimal catalyst material by combing with monometal nanomaterials ([62]).
For example, Yoon et al. applied acetic acid treatment to modify the surface of the laser-induced graphene (LIG) electrode to develop a wearable non-invasive sensor in sweat. PtNPs can uniformly disperse on the surface of LIG electrode to decrease the concentration of the electric field on the PtNPs. The glucose sensor based on this specific treatment and PtNPs/LIG nanostructures displayed a large linear sensing range of more than 2.1 mM, low LOD of less than 300 nM, and excellent sensitivity of 4.622 μA/mM. When multiple samples of human sweat were tested, the results were highly consistent with glucose levels in the blood (Fig. 3(iv)). Consequently, those superior sensing performances granted the wearable non-invasive glucose sensor with promising future perspectives ([63]).
2.1.2. Wearable non-invasive glucose sensors based on 1D noble monometallic nanomaterials
In addition to nanoparticles, one-dimensional noble metallic nanomaterials like nanotubes, nanowires, nanostrips, nanoneedles, etc. also play an important role in wearable non-invasive glucose sensors. Those nanomaterials are characterized by anisotropy in morphology, leading to the physical properties of the load and their self-assembly behavior ([[67], [66], [65], [64]]). Thus, 1D metallic materials are not only served as connecting wires of nanodevices but also applied as catalysts ([[68], [69], [70]]).
Among 1D metallic nanomaterials, 1D Au and Ag are the most desirable nanomaterials as their catalytic and conductive abilities are excellent. For example, a wearable flexible biosensor based on the Au nanowire was fabricated in the study of Zhai et al. The stretchable electrodes were constructed with Au nanowires decorated with mushroom-like AuNPs. Then, the Au nanowires grew directly on elastomeric substrates. Finally, the electrodes were modified with glucose oxidase and Prussian blue nanoparticles to sense the glucose levels. The unique high stretchable structure and outstanding materials endowed the biosensor with low LOD (10 μM), and high sensitivity (23.72 μA mM−1 cm−2). Meanwhile, even under 30% strain, a sensitivity of 4.55 μA mM−1 cm−2 toward glucose detection in the artificial sweat was possible (Fig. 4(i)) ([71]). Moreover, Toi et al. partially covered the reduced graphene oxide (rGO) on Au nanowrinkles to form a wrinkled and stretchable nanohybrid fiber (WSNFs). The synergistic effect between the Au nanowrinkles and the oxygen-containing functional groups on the reduced rGO support matrix are able to promote dehydrogenation decomposition in glucose oxidation. Consequently, WSNFs exhibited high electrocatalytic activity, stretchability, splendid sensitivity, low LOD, high selectivity for interferences, and high environmental stability. The wearable non-invasive sensor patch can be sewn onto a stretchable fabric and attached to the human body to continuously measure glucose levels in sweat (Fig. 4(ii)) ([72]). Furthermore, in the work of Zhao et al. a wearable non-invasive glucose sensing platform based on Au nanofibers was fabricated. Glucose oxidase and Prussian blue were applied to functionalize the Au nanofiber as the working electrode (Fig. 5(iii)), and Ag/AgCl was served as the reference electrode. Three electrodes were sewn into the fabric garment to monitor glucose in the sweat (Fig. 4(iii) (a)). This wearable non-invasive glucose sensor showed great sensing performances including high sensitivity of 11.7 μA mM−1 cm−2 and a broad sensing range of 0–500 μM. Meanwhile, this wearable non-invasive glucose fabric sensor had stable output even under 200% deformation (Fig. 4(iii) (b)). As a result, all of those remarkable performances indicated the great potential for this sensor to be applied in the real-world diabetes monitoring system ([73]). Au nanoneedles are also optimal 1D nanomaterials to fabricate glucose sensors besides Au nanowires, Au nanowrinkles, and Au nanofiber. Yu et al. reported a flexible glucose sensing chip based on Au nanoneedles. The wearable non-invasive glucose patch sensor, which was attached to the skin, detected glucose levels continuously in sweat. The working electrode was decorated on Au nanoneedles by the technology of the electrochemical deposition method (Fig. 4(iv)). The Au nanoneedles applied in this work greatly improved the signal of the glucose levels. As a result, this wearable device had the ability to sense glucose levels in sweat with high stability, selectivity, and low LOD of 7 nM. At the same time, this wearable device also can sense lactate. This work provided a novel way for the next-generation of multifunctional wearable sensors towards monitoring human health ([74]).
Fig. 5.
(i) (a) Photo showing the glucose sensor worn on the skin with sweat. (b) Performance of the proposed glucose sensor amounted on the arm under distinct mechanical deformation. Inset is the corresponding photographs under distinct mechanical deformation with the scale bar of 1 cm (82). (ii) Optical images showing the structure of the f-ES glucose sensor and the pattern of the sensor with a ruler to show its scale (83). (iii) Photographs indicating the stretchable wearable non-invasive glucose sensing patch integrated with microfluidic which are worn on the forehead (left) and arm (right) (86). (iv) (a) Photograph illustrating the flexible glucose detection patch with a waterproof band and a sweat-uptake layer. (Inset: Enlarged image of the porous sweat-uptake layer. (b) Photograph showing the flexible glucose detection sensor under deformation with no delamination. (c) Photograph indicating the disposable perspiration detection strip attached to the arm with sweat (87). (v) (a) Optical image showing wearable non-invasive glucose sensing patch amounted on human skin; (b) Optical image of the glucose sensing patch under deformation (88). (vi) SEM images of the working electrode decorated with MNDP gold by electrodeposition technique. The inset is a magnified view of the MNDP gold (89). (vii) Optical image indicating the wearable non-invasive glucose sensor under folding (91).
Apart from sweat, tears are also an easily collected biofluid. Therefore, smart contact lenses with the ability to collect tears and monitor glucose have become a popular wearable POCT glucose detection device in recent years (75). However, previous contact lens electronics used opaque and fragile components to operate, which obstructed the user's vision and may cause damage to the eyes ([75,76]).
Therefore, a wearable smart contact lens was fabricated by Kim et al. for wireless monitoring of glucose and blood pressure. Compared with previous contact lens sensors made of traditional opaque materials, this sensor was fabricated based on a graphene-silver nanowire (AgNW) hybrid structure in a non-invasive manner (Fig. 4(v)). The application of this unique structure enhanced electrical and mechanical properties at the expense of transparency. Furthermore, the transconductivity of this hybrid structure was negligible, which made it useful as an electrode to build passive electronic components. The sensor can monitor disease-related biomarkers and assess ocular and overall health. Consequently, this multiplexed contact lens held great promise for the next generation of ocular diagnostic devices ([77]). Additionally, Park et al. fabricated a wearable non-invasive transparent contact lens by direct electrospinning of 1D ultralong Ag nanofibers, which can promote the reduction of sheet resistance by minimizing the number of junctions between metallic fibers (Fig. 4(vi)). When worn on the eyes, the glucose concentration exceeding the set threshold will cause the resistance of the LED and glucose sensor to decrease, thus causing the LED light to go out (Fig. 4(vii)). After testing it in tears, the smart contact lens based on the Ag nanofibers exhibited excellent transparency and excellent stretchability, which can delay irritation of the eyes and lids due to the sensation of foreign objects (78).
2.1.3. Wearable non-invasive glucose sensors based on 2D noble monometallic nanomaterials
Two-dimensional nanomaterials possess a large specific surface area and are often used as carriers to support metallic nanoparticles ([[79], [80], [81]]). For instance, a skin-attachable and flexible non-invasive glucose sensor based on Au nanosheets was fabricated in the work of Oh et al. to detect glucose in perspiration. First, stretchable Au electrodes were fabricated on Polydimethylsiloxane (PDMS) substrates. A layer-by-layer method was applied to deposit carbon nanotubes onto patterned Au nanosheets prepared by filtration on the top of a stretchable substrate. After the CoWO4/CNT and polyaniline/CNT nanocomposites were coated onto the carbon nanotubes/Au nanosheet electrodes to increase the sensing performance. Meanwhile, a reference electrode was prepared by the chlorination of AgNWs. Encapsulation of the stretchable sensor with sticky silicone led to a skin-attachable sweat sensor (Fig. 5(i)). Therefore, the sensor exhibited high performance with sensitivities of 10.89 μA mM−1 cm−2, outstanding mechanical stability, and air stability ([82]). In addition, Khoshroo et al. fabricated a wearable and flexible biosensor based on silver nanosheets for the detection of glucose (Fig. 5(ii)). By the galvanic replacement technology, the Cu tape was coated on the nano-structural silver to prepare the working electrode, Ag/AgCl reference electrode, and silver-counter electrode. Because of the large electroactive surface area provided by Ag nanosheets, this sensor showed outstanding electro-catalytic performance for glucose determination with a wide linear range of 3 μM–3.3 mM, high sensitivity of 4610 μA mM−1 cm−2, and low LOD of 1.1 μM (S/N = 3). The galvanic replacement reaction which was a cheap, instrument-free, and reproducible technique was applied to provide the nano-structural silver coating on Cu tape for the preparation of the working electrode, silver-counter electrode, and Ag/AgCl reference electrode ([83]).
2.1.4. Wearable non-invasive glucose sensors based on noble monometallic nanostructures
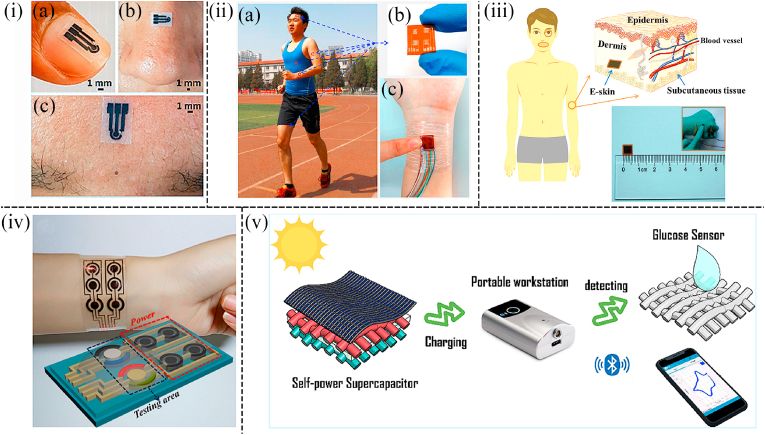
Some monometallic materials are fabricated into nanoporous structures, which are also the key materials for some biosensor applications ([84,85]). For instance, Bae et al. reported a wearable non-invasive glucose sensor patch consisting of an omnidirectional stretchable nanoporous Au electrochemical biosensor and a stretchable passive microfluidic device. The development of wearable continuous monitoring biosensor systems was extremely challenging due to the difficulty of imparting both high stretchability and high performance to materials and components. By embedding stretchable cotton fabrics as capillaries into thin polyurethane nanofiber-reinforced PDMS channels, a thin, stretchable, and durable microfluidic device was fabricated. This sensor was capable of collecting and accurately transporting sweat from the skin to the electrode surface with excellent replacement capability (Fig. 5(iii)). The nanoporous Au glucose sensor showed great sensitivity, great selectivity to interfering biomolecules, satisfactory mechanical durability, and excellent stretchability. The integrated glucose sensor patch exhibited an exceptional ability to continuously and accurately monitor sweat glucose levels ([86]). Similarly, to address the difficulty of sweat collection and changes in glucose oxidase activity due to ambient temperature, Lee et al. proposed a novel closed-loop solution for sweat-based non-invasive diabetes management. A porous Au nanostructure with a high specific surface area was applied as the basic material, so that the sensor can detect small amounts of glucose in sweat with high sensitivity (Fig. 5(iv)). Sweat collection and multiple glucose sensing devices can be efficiently collected through miniaturized sensor design, multiple sweat control, and absorption layers. Multimodal glucose sensors are able to maximize the accuracy of sensing through real-time correction based on pH, temperature, and humidity measurements. Hyaluronic acid hydrogel microneedles can be embedded with two different temperature-sensitive phase transition nanoparticles to load drugs for feedback transdermal therapy, which enabled multistage, spatially patterned, and precisely controlled drug release in response to the glucose level of patients ([87]). Besides, Chen et al. presented an e-skin-like nanostructured biosensor system. The microelectromechanical systems fabrication process was applied to fabricate the sensor on the silicon wafer, and finally, the liquid capillary was transferred off the wafer. The Au electrode was nano-crystallized with 100 nm-size nanostructures to achieve nano-PB deposition, which endowed nano-scaled PB with good performance and robustness. This nanostructure conformed to the skin morphology and facilitated high electrochemical deposition of the mechanically robust nano-transducer layer. This wearable non-invasive ultra-thin electronic tattoo was attached directly to the skin to monitor glucose levels in sweat (Fig. 5(v)) ([88]). Moreover, Xuan et al. developed a low-cost and wearable non-invasive biosensor based on micro-nano dualporous (MNDP) Au to detect glucose levels. MNDP Au was directly synthesized by the electrochemical deposition method on a carbon surface using a hydrogen bubble as the dynamic template, which had a highly porous structure and mechanical stablility (Fig. 5(vi)). This wearable non-invasive sensor showed a wide linear range with the logarithm of glucose concentration between 1.5 and 16 mM, high sensitivity of 48.4 μA·mM-1·cm−2, and LOD of 25 μM. The performance of the sensor was further tested with human serum and satisfactory results was obtained ([89]).
2.1.5. Wearable non-invasive glucose sensors based on non-noble monometallic nanomaterials
Non-noble monometallic nanomaterials are cheaper and possess satisfactory catalytic properties compared with noble monometallic nanomaterials, which are also welcoming sensing materials applied in wearable non-invasive glucose sensors. Li et al. fabricated a transparent, flexible, attachable, and breathable nonenzymatic biosensor based on Cu nanoparticles to detect glucose in sweat. First, a free-standing transparent nickel mesh electrode was fabricated by laser direct writing techniques. The Cu nanoparticles were electrochemically deposited on the free-standing nickel mesh electrode as a working electrode (Fig. 2(iii)). Owing to the unique network structure and the superior properties of Cu nanoparticles, this sensor showed ultra-high sensitivity, an ultra-low detection limit, and excellent stability when attaching tightly to the surface of the skin ([90]). Additionally, LSG was decorated with Ni nanoparticles to be applied as the working electrode by electrochemical deposition technology in the work of Chen et al. Such the glucose device based on the specific materials exhibited outstanding sensing performance towards glucose detection including ultra-low LOD with 0.29 μM, prominent sensitivity of 2040 μA mM−1 cm−2, and a broad sensing range of from 0.50 to 1666 μM. Moreover, this glucose biosensor exhibited great flexibility and deformability, which shows great potential in the application of wearable non-invasive glucose detection devices (Fig. 5(vii)) ([91]).
2.2. Wearable non-invasive glucose sensor based on bimetallic nanomaterials
Bimetallic nanoparticles have the advantages of easy adjustment of structure and better sensing performance compared with monometallic nanoparticles. At the nanoscale, it is of great significance to tune the synthesis of bimetallic catalytic materials. By changing the size, composition, and microstructure of bimetallic materials, the properties of bimetallic catalytic materials with these different parameters are explored ([[94], [93], [92]]).
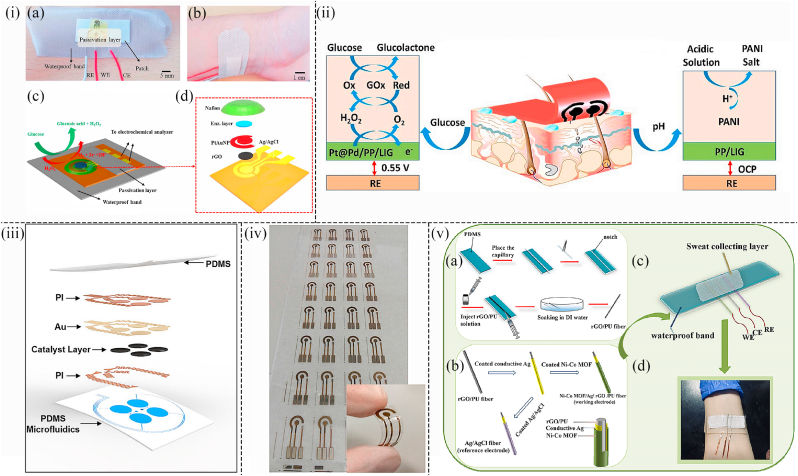
For example, Xuan et al. developed a wearable non-invasive patch-type biosensor based on reduced rGO-based nanostructure to detect the glucose concentration in sweat. By using electrochemical deposition, the surface of microfabricated rGO was decorated with Au and platinum alloy nanoparticles. Next, the chitosan-glucose oxidase composite was integrated into the modified surface of the working electrode. Because of the high quality of the hybrid working electrode (Au/rGO/AuPtNP/GOx/Nafion), this sensor displayed good stability, a fast response time (12s), and high sensitivity. Besides, the three electrodes were bonded to a waterproof band, which was attached to the wrist to monitor the glucose concentration in sweat in real-time (Fig. 6(i)) ([95]). Fabrication of highly conductive biosensing platforms based on LIG remains a challenge as the patterned LIG flakes are usually not interconnected, which results in low uniform conductivity. Zahed et al. reported flexible electrochemistry of 3D stabilized porous LIG based on poly (3,4-ethylenedioxythiophene)-poly (styrene sulfonate) modification (PP/LIG) preparation of wearable biosensors for glucose and pH detection. PtNPs and PdNPs were successfully electrodeposited on PP/LIG (Fig. 8(ii)), markedly enhancing the electrocatalytic activity for glucose detection. Furthermore, the proposed wearable non-invasive biosensor can be integrated with a flexible printed circuit board, which was attached directly to the skin to develop a small sweat monitoring system (Fig. 6(ii)) ([96]).
Fig. 6.
(i) Physical image of (a) the structure of the wearable glucose sensing device and (b) the wearable sensor amounted on the wrist. (c) Schematic diagram showing the structure and sensing mechanism of the wearable glucose sensor. (d) Explored image of the electrodes in the wearable glucose sensor (95). (ii) Conceptual images showing the wearable non-invasive glucose sensing patch laminated on human skin and the reaction mechanism when detecting glucose and pH (96). (iii) Schematic diagram representing the structure of the wearable glucose sensing system with PMDS microfluidics (100). (iv) Photographic image illustrating a series of wearable glucose sensing patches deposited on the substrate of PET (inset is the enlarged photo representing a single flexible glucose sensing patch under deformation (101). (v) (a) Schematic illustration showing the manufacturing procedure of rGO/PU fiber via the technique of improved wet spinning. (b) Schematic representation indicating the fabrication procedure of the reference electrode (Ag/AgCl fiber) and working electrode (NCGP). (c) Schematic image representing the structure of non-invasive sweat glucose sensor sewn onto the substrate of elastic fabric, and (d) the photograph of the wearable non-invasive sweat glucose sensing patch laminated on the arm of the subject ([102]).
Fig. 8.
(i) Optical image showing the miniaturized wearable glucose sensing patch based on CuO/LSG worn on (a) nail, (b) nose, and (c) forehead (109). (ii) (a) The wearable non-invasive glucose sensing patch laminated on the skin of a runner at distinct parts of the body to detect the components of sweat and monitor the physical state of users. Digital photograph showing (b) structure of wearable sensing patch and a human wrist with the sensor amounted on it (110). (iii) Schematic illustrating the miniaturized wearable glucose sensing patch amounted on human skin (111). (iv) Optical image and schematic diagram representing the structure of wearable versatile sensing patch which are attached on the wrist (112). (v) Conceptual image showing the working principle of a self-powered wearable glucose sensing system including a supercapacitor, portable working station, glucose sensor, and a smartphone to receive the glucose concentration data ([113]).
Moreover, in the preparation of bimetallic, noble metallic nanoparticles are replaced by low-valent metallic nanoparticles, which conserve noble metals resources and reduce costs while ensuring high catalytic activity ([[97], [98], [99]]).
Huang et al. reported a newly developed stretchable self-powered epidermal biosensor based on Au and Cr nanoparticles, which are capable of detecting the lactate and glucose concentrations in sweat. This self-powered sensor was associated with lactate and glucose enzymatic biofuel cells and a thin soft polydimethylsiloxane-based microfluidic system (Fig. 6(iii)). Besides, the device was composed of stretchable electronics that can efficiently collect sweat and also provide excellent mechanical properties and stable performance output under 30% stretch. Such a glucose sensor can be integrated into almost any position of the body with a high level of performance. This work offered a promising approach for the development of next-generation sweat sensors for real-time and in-situ sweat analysis ([100]). By using inkjet printing technology, Romeo et al. developed a low-cost and flexible enzyme-free glucose sensor based on Cu nanowires and AuNPs. The working electrode and counter electrode was printed on a PET flexible substrate with Au nanoparticle-based ink. After the wiring and spacer were printed with silver ink. Finally, CuO microparticles synthesized by Cu nanowires were used to decorate the electrodes. Because the performance of the Cu oxide-microparticles-based sensor closely matched the detection of glucose concentration in tears, this device was particularly suitable for detecting glucose concentration in tears. At the same time, the sensor also had the characteristics of all-electrode integration, reusability, and flexibility (Fig. 6(iv)) ([101]). Additionally, a wearable non-invasive sensor based on highly stretchable Ni–Co metal−organic framework/Ag/reduced rGO/polyurethane (Ni–Co MOF/Ag/rGO/PU) fiber was developed by Shu et al. to monitor the glucose concentration in sweat continuously. The rGO/PU fiber was simply produced by an improved wet spinning technology, and the Ni–Co MOF nanosheet was coated on the fiber surface to prepare the Ni–Co MOF/Ag/rGO/PU (NCGP) fiber electrode. The conductive Ag glue and as-synthesized Ni–Co MOF nanosheets were coated on the rGO/PU fiber, respectively, to obtain a stretchable fiber working electrode (Fig. 6(v) (a,b)). Three electrodes were attached to a waterproof band, covered with a sweat-absorbing cloth, and attached to the wrist to measure glucose levels continuously in sweat (Fig. 6(v) (c,d)). Owing to the high electrocatalytic activity of Ni–Co MOF nanosheets for glucose oxidation, the fiber sensor exhibited excellent electrochemical performance for glucose detection with a low LOD of 3.28 μM and a wide linear range of 10 μM−0.66 mM. Meanwhile, the NCGP fiber electrode maintained outstanding mechanical flexibility and stable electrochemical performance under the stretching and bending states ([102]).
2.3. Wearable non-invasive glucose sensor based on metal oxide nanomaterials
In addition to monometals and bimetals, transition metals represented by some metallic oxides are also widely used to construct non-enzymatic glucose sensors, which are inexpensive compared to noble metals ([103,104]).
For instance, Liu et al. demonstrated a highly sensitive and conformal In2O3 nanoribbon FET biosensor with a fully integrated on-chip Au side gate. The sensor used inkjet-printed electrodes modified with glucose oxidase, biocompatible polymer chitosan, and single-walled carbon nanotubes. Consequently, this sensor displayed a broad detection range spanning at least 5 orders of magnitude and a limit of detection down to 10 nM. This sensor was capable of detecting glucose in various body fluids, such as sweat and saliva, and had been laminated to various surfaces like artificial arms and watches (Fig. 7(i)). Besides, it was demonstrated that this sensor can perform well when being amounted to artificial skin and eye replicas. Therefore, this type of device was a highly sensitive platform for glucose detection and many other types of sensing applications ([105]). Furthermore, Rim et al. developed ultrathin (3.5 nm), high-density, and uniform films with large areas by the one-step layering of aqueous In2O3 and specific chemical treatments. Enzyme immobilization on In2O3-based FET biosensors facilitated real-time detection of glucose. This ultrathin biosensor platform had advantages as a conformal sensor through layering and possessed excellent contact capabilities on rough artificial skin surfaces and artificial eye surfaces (Fig. 7(ii)). Therefore, the device opened new opportunities for future wearable human technology ([106]).
Fig. 7.
(i) Physical image of an artificial human hand with In2O3 FET foil amounted on it (105). (ii) Schematic illustration showing integrated contact lens attached to an artificial eye to detect glucose concentration in tears. Wearable glucose sensing patch remained in contact with the skin under relaxation and tension (106). (iii) Conceptual image showing the application of self-powered wearable non-invasive glucose sensing system (107). (iv) Schematic diagram showing the wearable glucose sensor based on Cu2O@Ni micromesh film is attached to the skin for glucose detection in real-time (108).
Cu and its compounds generally show a wide linear range for glucose detection and thus are promising materials to fabricate glucose sensors. Jiang et al. systematically fabricated several facet-controlled cuprous oxide nanostructures to study the facet-dependent electrocatalysis mechanism. After comparing cuboctahedral Cu2O with a hollow structure exhibited the best sensing performance for glucose detection. In addition, a facet-controlled electrochemical sensing mechanism for Cu2O was also demonstrated. It is suggested that facets led to enhanced catalytic activity by getting higher interaction with glucose and accelerating electron transfer, respectively. Moreover, it was also verified that the wearable enzyme-free biofuel cell system can be combined with a portable wireless device and sense glucose through opening circuit potential and power output signals (Fig. 7(iii)). Therefore, this wearable enzyme-free smart sensing concept will facilitate the targeted establishment of biomarker electrocatalysts. As a result, the sensing device provided a promising prospect for the development of biofuel cells in the field of wearable medical monitoring ([107]). Besides, Li et al. reported a conformable, transparent, and gas-permeable skin-like Cu2O@Ni micromesh structural patch for detecting glucose in sweat. A dense and crack-free Cu2O nanofilm composed of 100–300 nm of Cu2O nanoparticles was wrapped around the Ni mesh. This processing method can form a core-shell structure with the Ni mesh as the conductive “core” and the uniformly deposited Cu2O layer as the active “shell”. This wearable non-invasive sensor was small and lightweight, which was attached directly to the finger to monitor glucose levels in sweat (Fig. 7(iv)). Furthermore, because of the synergistic effects of the self-supporting micromesh configuration, the high conductivity of the metallic mesh, and the high reactivity of Cu2O towards glucose, this biosensor exhibited excellent performance including high sensitivity (15420 μA cm−2 mM−1), the fast response time (<2 s), and low LOD (50 nM), while also showed highly shape conformability (thickness ≈11 μm), highly optical transparent (≈82%), and excellent gas permeability (>2500 mm s−1 at 10 Pa) ([108]). Apart from Cu2O, in the work of Prabhakaran et al. CuO nanoparticles were conformally anchored on the surface of LSG to fabricate a wearable non-invasive biosensor. Such sensors exhibited splendid selectivity, a large sensing range (1 μM–5 mM), low LOD (0.1 μM), and fast response time (<0.2s) (Fig. 1(iii)). Meanwhile, this wearable non-invasive biosensor is extremely small and can attach to a nail, nose, and forehead to measure glucose concentration in situ (Fig. 8(i)) ([109]).
Moreover, ZnO is one of the most widely used metallic oxides in non-enzymatic glucose sensors. Han et al. developed a self-power flexible noninvasive electronic skin based on a piezo-biosensing unit matrix of enzyme/ZnO nanoarrays to analyze components in sweat including glucose, uric acid, lactate, and urea. The electronic skin can continuously monitor the physiological state in real-time of a runner by analyzing the perspiration on the human skin. This sensor applied the piezoelectric impulse of the piezo-biosensing units as the power supply and the data biosensor. The enzymes were modified onto the surface of ZnO nanowires (Fig. 1(iv)). Because of the piezoelectric-enzymatic-reaction coupling effect of enzyme/ZnO nanowires, this sensor can actively output the piezoelectric signal (driven by body movement), which was dependent on the analyte concentration in the perspiration. The wearable device possessed low LOD (0.02 mM), small resolution (0.02 ± 0.005 mM), and excellent selectivity. And when the wearable non-invasive sensor was attached to the forehead, the wearable devices can continuously monitor the physiological state during running in real-time (Fig. 8(ii)) ([110]). In addition, Xue et al. constructed a self-powered electronic skin based on the coupled piezoelectric-enzymatic reaction process of GOx@ZnO nanowire arrays to detect glucose levels in body fluids (Fig. 8(iii)). In this work, the e-skin did not need an external power supply and can work for a long time by collecting the mechanical energy of human movement. In the past, field-effect transistor (FET) biosensors were always applied by using bulky Ag/AgCl electrodes and metallic wire grids, which prevented the former FET biosensors from being truly wearable. The unique materials applied in this work enabled the biosensor with excellent wearability ([111]).
When a kind of metal is composited with other metals or nanomaterials, complex oxide nanomaterials exhibit more superior properties including small grain size, uniform distribution of active sites, large specific surface, good stability, etc. Therefore, complex oxide nanomaterials are welcome materials applied in wearable glucose sensors.
Lu et al. fabricated a wearable self-powered-like monitoring system based on NiCo2O4 nanowires to detect glucose concentration in sweat. A hydrothermal method was applied to synthesize urchin-like NiCo2O4 samples using nanowires. After the chitosan solution was ultrasonically combined with the solution containing NiCo2O4 to form a mixed solution. The mixed solution was dropped onto the Au electrode to form the working electrode (Fig. 8(iv)). Because of the advantages of NiCo2O4 nanowires, this biosensor showed high sensitivity (0.5 μA/μM) and low LOD (10 μM). Meanwhile, micro-supercapacitors were integrated into this sensor, which had high energy density (0.64 μW/cm2) and power density (0.09 mW/cm2) ([112]).
A wearable self-powered smart sensor system based on NiCoO2 nanosheets was reported in the work of Sun et al. for the monitoring of glucose levels. Through a simple electrochemical deposition method, carbon fiber-based NiCoO2 (CF@NiCoO2) nanosheets were synthesized. Then the CF@NiCoO2 nanosheets were coated with nitrogen-doped carbon to form CF@NiCoO2@N–C nanocomposites which exhibited excellent behaviors as a supercapacitor and prominent electrocatalytic properties to apply for enzyme-free biosensor. Meanwhile, integrated with Bluetooth, this smart sensor system can be remotely controlled using a smartphone (Fig. 8(v)). Because of the significant electrochemical properties of CF@NiCoO2@N–C, this biosensor exhibited high sensitivity of 0.62 mA/mM, an excellent linear relationship between current density and glucose concentration, and low LOD (34.8 μM). Moreover, the flexible asymmetric supercapacitor maintained 95% capacitance even after 8000 cycles and 10,000 bending cycles ([113]).
2.4. Others
In addition, there are other combinations of metallic catalysts to form metal-based nanocomposite nanomaterials with better sensing performance. A Luminescent Wearable Sweat Tape based on responsive luminophores enzyme-loaded Au nanocluster (AuNCs) nano-networks was fabricated by Zhou et al. to detect glucose, uric acid, and alcohol levels in sweat. The nanoprobes were synthesized with the responsive luminophores and the enzyme-loaded AuNCs nanonetwork. Next, the processed nanoprobes were wrapped with manganese dioxide nanosheets. Finally, the multi-component nanoprobes were embedded on the microwell-patterned paper substrate of hollowed-out double-sided tapes (Fig. 9(i)). After using double-sided tape to attach electrodes to the vest and measure glucose in sweat, the wearable non-invasive sensor had a wide detection range (0–1 mM), and users can also easily understand the glucose concentration with the support of a smartphone ([114]).
Fig. 9.
(i) Scheme illustrating the construction of enzyme/AuNCs@PAH@MnO2 nanosheets as well as the sensing principle of nanoprobes as analytes (114). (ii) (a) Schematic images showing the wearable glucose sensor developed in a structure of kirigami-patterned mesh without strain-sensitive characteristics. (b) Metal electrodes and sensing channels based on graphene are embedded in polyimide (118). (iii) Schematic illustration showing the fabrication process of RuS2 nanoparticles by a one-step hydrothermal method and deposition of RuS2 NPs onto PDMS substrate applying the technique of spin coating (119). (iv) The antenna slots and the filter are inspired by the anatomy of the veins and arteries of the hand and the arm, respectively (120). (v) Top view of AgNWs on the film of LIG after stretching. The inset is the SEM image of AgNWs (121).
By geometrically modifying the rigid conductive components themselves to improve the stretchability and flexibility of rigid electronic components, these strategies fail to achieve fully strain-insensitive electrical performance under biaxial stretching, twisting, and mixed strain states ([[117], [116], [115]]). To address this problem, Lee et al. developed a new platform for temperature and glucose concentration detection based on graphene-based multiaxial stretchable kirigami-patterned grid structures (Fig. 9(ii)). When graphene and metallic electrodes were embedded in the structure, their resistance changed by less than 0.5% under mechanical deformation, e.g., 180° twisting and 100% biaxial stretching due to the specific kirigami structures. The kirigami devices can be integrated with wearable healthcare system electronics in the future, enabling simultaneous monitoring of multiple biological signals, data processing, and data transmission ([118]).
Veeralingam et al. first reported a wearable non-invasive multifunctional sensor platform enabled by artificial intelligence/machine learning. A facile hydrothermal method was applied to synthesize RuS2 nanoparticles, and the RuS2 nanoparticles were deposited on the PDMS film substrates by layer-by-layer spin coating technology (Fig. 9(iii)). The application of K-nearest neighbors which was based on artificial intelligence in the open-source microcontroller board (Quiescence) greatly ensured the precision and fast data acquisition of glucose detection. This wearable sensor was attached to the skin and continuously monitor pH and glucose levels in sweat and the hydration level of the skin with high speed and accuracy ([119]).
Hanna et al. designed two prototypes of a flexible antenna and a band-stop filter on the flexible and rigid substrate as the main sensing elements of a wearable non-invasive glucose monitoring system. As a result, the wearable device can continuously detect glucose concentration with high sensitivity (Fig. 9(iv)). The sensor system with the flexible antennas was fabricated by an inkjet printer with PET and silver nano inks. The biosensor showed a high correlation between the physical parameters of the system and blood glucose levels in the report of non-invasive measurement results in human experiments without any time lag. Besides, the biosensor can target multiple body parts simultaneously, which opened the door for the development of a closed-loop artificial pancreas ([120]).
To solve the problems like the degradation of electrical conductivity caused by tensile deformation, Xuan et al. used 3D porous LIG-silver nanocomposites to prepare highly stretchable and conductive electrodes and added platinum nanoparticles electrodes on the 3D porous LIG (Fig. 9(v)). The unique composite materials applied in this work greatly improved the electrochemical performance of wearable glucose sensor applications. It was experimentally known that the fabricated electrodes exhibited good electrical conductivity even under large mechanical deformations. The glucose sensor had a low detection limit and a wide detection range, which exhibited a good linear response in the pH 4–7 range ([121]).
3. Conclusion and perspective
In this manuscript, the state-of-art works about wearable metallic nanomaterial-based non-invasive glucose sensors are summarized. The comparison among the application of the works is tabulated in Table 2. The glucose in the human body can be detected non-invasively through various body fluids e.g., sweat, urine, saliva, tears, interstitial fluid, etc. and thus the wearable non-invasive glucose sensors can be fabricated into distinct necessaries. Specifically, for the detection of glucose in sweat, the glucose sensor can be constructed into a patch type with high flexibility and also can be made into a watch that is convenient and easy to carry. At the same time, some sensors based on fabric material are applied as substrates so that the sensors can be directly sewn on clothes. As for the monitoring of glucose in tears, sensors can be built into contact lenses with sufficient clarity. Through electrospinning technology, extremely fine and ultra-long nanowires are used as wires to realize wireless transmission. Besides, combining the sensor with the diaper can also easily and quickly detect the glucose level of the human body through urine.
Table 2.
Comparison among the wearable sensors based on different Nano metal-based materials towards glucose monitoring.
| Material | Modification/Functionalization/Fabrication | LOD (μM) | Linear range (μM) | Sensitivity (μA·μM−1·cm−2) | Ref. | |
|---|---|---|---|---|---|---|
| Monomental nanomaterials | Au nanoparticles | Deposited on the SF film | 0.168 | 0.1–1000 | – | (40) |
| The technique of ultraviolet mediated chemical plating | 2.7 | 20–1110 | 22.05 | (41) | ||
| Decoration on the working electrode | 26 ± 5 | 50–1400 | 4.7 ± 0.8 | (42) | ||
| Carrier for enzyme and sensing materials | 17.84 | 0–5000 | 10.51 | (43) | ||
| Decorated with CNTs onto the anode | – | 1000–5000 | – | (49) | ||
| Functionalized working electrodes with single-walled carbon nanotubes | – | 17–1110 | 26.6 | (48) | ||
| Deposited on the surface of graphene electrodes | 10 | 200–10000 | 18 | (57) | ||
| Pd nanoparticles | Decorated on Co-based zeolitic imidazolate framework | 2 | 10–1000 | – | (58) | |
| Pt nanoparticles | Multi-potential step deposition process | 1.9 × 10−4 | 0.01–0.105 0.105–100 |
0.387 11.51 |
(59) | |
| Electrodeposited onto a multiwall carbon nanotube | 0.1 | 1–5000 | – | (60) | ||
| Inkjet printing technology | 28.9 | 0–22222 | – | (61) | ||
| Disperse on the surface of LIG electrode | 0.3 | 0.3–2100 | 4.622 | (63) | ||
| Au nanowires | Growing directly on elastomeric substrates | 10 | 0–1400 | 23.72 | (71) |
| Monomental nanomaterials | Au nanowrinkles | Covered rGO to form a wrinkled | 0.5 | 5 × 10−4-10000 | 140 | (72) |
| Au nanofibers | Functionalized by glucose oxidase and Prussian blue | 6 | 0–500 | 11.7 | (73) | |
| Au nanoneedles | Electrochemical deposition method | 7 | 25–250 | – | (74) | |
| Ag nanowire | Decorated with graphene to form AgNW |
0.4 | 100–600 | – | (77) | |
| Ag nanofibers | Electrospinning | 12.57 | 100–900 | – | (78) | |
| Au nanosheets | Layer-by-layer method | 1.3 | 0–300 | 10.89 | (82) | |
| Ag nanosheets | Coated with Cu tape | 1.1 | 3–3300 | 4610 | (83) | |
| Nanoporous Au | Formed through the dealloying of vacuum-deposited Au |
– | 10–1000 | 253.4 | (86) | |
| Electrodeposition | – | 10–1000 | – | (87) | ||
| Micro-Nano Dualporous Au | Electrochemical deposition method | 25 | 1500–16000 | 48.4 | (89) | |
| Au nanostructures | Electrochemical deposition method | 5 | 5–40 | 130.4 | (88) | |
| Cu nanoparticles | Electrochemically deposited on the working electrode | 2 | 2–600 | 8510 | (90) | |
| Ni nanoparticles | – | 0.29 | 0.5–1666 | 2040 | (91) | |
| bimetallic nanomaterials | Au and Pt alloy nanoparticles | Electrochemical deposition | – | 100–2300 | 82 | (95) |
| Pt and Pd nanoparticles | Electrodeposited on PP/LIG | 3 | 10–9200 | 247.3 | (96) | |
| Cr and Au nanofilm | Deposited on the PI film | – | 0–150 | – | (100) | |
| Cu nanowires and Au nanoparticles | Inkjet printing technology | 2.99 | 3–700 | 850 | (101) | |
| Ni–Co MOF Nanosheet | Coated on rGO/PU fiber surface | 3.28 | 10–660 | 425.9 | (102) | |
| Metal oxide nanomaterials | In2O3 nanoribbon | Functionalized FET | 0.01 | 0.01–1000 | – | (105) |
| In2O3 nanofilm | Decorated with enzyme | – | 100–600 | – | (106) | |
| Cu2O nanoparticles | The hydroxylamine hydrochloride reduction method | 3.3 | 0–6000 | 1.81 | (107) | |
| Cu2O nanofilm | Wrapped around the Ni mesh | 0.05 | 0.05–118.25 118.25–1070 |
15420 5850 |
(108) | |
| CuO nanoparticles | Sunlight aided technique | 0.1 | 1–5000 | – | (109) | |
| ZnO nanowires | Decorated on enzymes | 20 | – | – | (110) |
| Metal oxide nanomaterials | ZnO nanowire arrays |
Wet-chemical method |
0.33 |
– |
– |
(111) |
|---|---|---|---|---|---|---|
| NiCo2O4 nanowires |
Hydrothermal method |
10 |
10–200 |
0.5 |
(112) |
|
| NiCoO2 nanosheets | Electrochemical deposition method | 34.8 | 0–7000 | 592 | (113) | |
| Others | Au nanocluster and MnO2 nanosheets | AuNCs wrapped by MnO2 nanosheets | – | 0–1000 | – | (114) |
| Cr/Au/Ti nanofilm | Deposition to graphene channels | 1 × 10−6 | 1 × 10−6-1000 | – | (118) | |
| RuS2 nanoparticles | Facile hydrothermal method | 4.87 × 10−3 | 0.01–100 | 87.9 ± 0.6 | (119) | |
| Silver nano-ink | Inkjet printing technology | – | – | – | (120) | |
| AgNW and PtAuNP | Electrodeposition | 5 | 0–1100 | 6.4 | (121) |
Metallic nanomaterials are optimal basic materials in wearable non-invasive glucose sensors due to their long-term stability and prominent sensing performance. Among them, 0D, 1D, and 2D noble metallic nanomaterials are always applied to improve the conductivity of electrode materials, while non-noble metallic nanomaterials are used as catalytic materials in wearable non-invasive glucose sensors. Meanwhile, these metallic nanomaterials can be combined together to develop bimetallic nanomaterials with enhanced sensing performance. Furthermore, metallic oxide nanomaterials exhibit higher catalytic properties than metallic non-oxide nanomaterials, which are also promising choices as the basic materials in wearable non-invasive glucose sensors.
To be specific, the application of monometallic nanomaterials in wearable non-invasive glucose sensors mainly focuses on noble metallic elements including Au, Pt, and Ag. Au nanomaterials are applied the most in wearable non-invasive glucose sensors including Au nanoparticles, Au nanowires, Au nanowrinkles, Au nanofibers, Au nanoneedles, and Au nanosheets due to their intrinsic remarkable sensing properties. To further improve the conductivity and catalytic ability of Au, nanoporous Au and some Au nanostructures with the enhanced specific surface area were also developed. In addition, there are also other noble metal nanomaterials, e.g., zero-dimensional Pt nanoparticles, Pd nanoparticles, one-dimensional Ag nanowires, Ag nanofibers, and two-dimensional Ag nanosheets applied in wearable non-invasive glucose sensors. These noble metallic materials have excellent catalytic activity, however, there are also various problems of easy poisoning, poor selectivity, and high price, and thus their large-scale applications are greatly limited. Therefore, several non-noble metallic materials are modified into nanoparticles like Cu and Ni nanoparticles to obtain satisfactory sensing performance as substitution materials in wearable non-invasive glucose sensors.
Apart from monometallic materials, bimetallic materials are also popular materials in glucose sensor electrodes. At present, common bimetallic materials include combinations of different kinds of noble metals, combinations of different kinds of transition metals, and combinations of noble metals and transition metals, e.g., Au and Pt alloy nanoparticles, Pt and Pd nanoparticles, Cr and Au nanofilm, Cu nanowires and Au nanoparticles, Ni–Co MOF nanosheet, etc. When another metallic element is incorporated into the bulk metallic material, the synergistic effect of the two metals enables bimetallic materials with better properties compared to monometallic materials. Doping metallic atoms can change the electronic properties, chemical bond characteristics, atomic distribution, morphology, and structure of bulk metals, all of which grant the reactions occurring on the catalyst surface with novel and specific characteristics. Moreover, the combination of metallic materials allows a more flexible design of the structure, e.g., more precise control of the atomic doping ratio and the distribution of two metallic atoms in the structure, which greatly increases the controllability of catalyst synthesis. Therefore, bimetallic materials are of great value in the synthesis of catalysts.
When it comes to metallic oxide nanomaterials, they have the advantages of strong adsorption capacity, low cost, stable properties, and comparative sensitivity with noble metallic nanomaterials and have received extensive attention in the field of electrocatalysis. Nonetheless, after oxidation, the conductivity of metallic nanomaterials decreases remarkably. Consequently, researchers are trying to develop some strategies including developing nanoarray and growing on the substrate with a large specific surface area like Ni foam to greatly enhance the sensing performance of wearable non-invasive glucose sensors.
In the future, it is believed that non-invasive glucose testing is developing into the mainstream as compared with invasive glucose testing, which will bring less discomfort and risk of being infected. Among the biofluid in which glucose concentration is detected by wearable non-invasive glucose sensors, sweat holds the most promising future. This because the skin is the largest organism of humans and thus sweat can be most easily to be collected and a variety of wearable devices for sweat collection can be conveniently worn on the body. However, most of the diabetes patients are elders with low metabolic rates and low sweat secretion volume. Therefore, wearable non-invasive glucose sensors based on metallic nanomaterials for the continuous monitoring of the glucose level in saliva are also attractive research fields. Furthermore, more sensitive and efficient electrode materials will be developed and applied in wearable non-invasive glucose sensors. Distinct metallic nanomaterials with their specific characteristics will be combined together to form bimetallic or multi-metallic nanomaterials with specific nanostructures which exhibit lower cost and higher glucose sensing performance. Additionally, substrate materials with high flexibility, deformability, and conductivity can be developed to carry the metallic nanomaterials and form specific nanostructures to remarkably improve the sensing performance of metallic nanomaterials. Moreover, the glucose sensor can be developed towards miniaturization and combined with cloud-based technology to transmit glucose information to clinical institutions in real-time. Consequently, wearable non-invasive glucose sensors based on metallic nanomaterials can get in time treatment advice and painlessly help diabetic patients for diabetes management. By combining with microfluid technologies, the glucose levels of diabetes patients can be continuously and real-time monitored by wearable non-invasive glucose sensors based on metallic nanomaterials. In addition to monitoring blood glucose levels, other vital signs can be detected by using other electrode active materials. It is believed that in the near future, scientific researchers will gradually enrich the types of non-invasive and wearable personalized sensors for monitoring more biomarkers and serve medical personnel and scientific researchers in the treatment and research of diseases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded By Key Scientific And Technological Program Of Ningbo City (No. 2021Z108) and Yongjiang Talent Introduction Programme (No. 2021A-154-G).
Contributor Information
Chen Liu, Email: qqwanggood@zju.edu.cn.
Qianqian Wang, Email: liuchen@nit.zju.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Munje R.D., Muthukumar S., Prasad S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sensor. Actuator. B Chem. 2017;238:482–490. [Google Scholar]
- 2.Wang H., et al. 3D-Printed flexible tactile sensor mimicking the texture and sensitivity of human skin. Adv. Mater. Technol. 2019;4 [Google Scholar]
- 3.Kim J., Campbell A.S., de Avila B.E., Wang J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo S., et al. Development of a cloud-based epidermal MoSe2 device for hazardous gas sensing. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 5.Cheng Y., et al. A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring. Biosens. Bioelectron. 2022;203 doi: 10.1016/j.bios.2022.114026. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y., Bariya M., Javey A. Wearable biosensors for body computing. Adv. Funct. Mater. 2020;31 [Google Scholar]
- 7.Xu L., Li Y., Dai Y., Peng J. Natural products for the treatment of type 2 diabetes mellitus: pharmacology and mechanisms. Pharmacol. Res. 2018;130:451–465. doi: 10.1016/j.phrs.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Forouhi N.G., Wareham N.J. Epidemiology of diabetes. Medicine. 2019;47:22–27. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., Chi J., Lv W., Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19) Diabetes Metab. Res. Rev. 2021;37:e3377. doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pranata R., Henrina J., Raffaello W.M., Lawrensia S., Huang I. Diabetes and COVID-19: the past, the present, and the future. Metabolism. 2021;121 doi: 10.1016/j.metabol.2021.154814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin L., Bowling F.L., Armstrong D.G., Boulton A.J.M. Saving the diabetic foot during the COVID-19 pandemic: a tale of two cities. Diabetes Care. 2020;43:1704–1709. doi: 10.2337/dc20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagoo M.K., Gnudi L. Diabetic nephropathy: an overview. Diabetic Nephropathy: Methods and Protocols. 2020;2067:3–7. doi: 10.1007/978-1-4939-9841-8_1. [DOI] [PubMed] [Google Scholar]
- 13.Antonetti D.A., Silva P.S., Stitt A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021;17:195–206. doi: 10.1038/s41574-020-00451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y., et al. Integration of interstitial fluid extraction and glucose detection in one device for wearable non-invasive blood glucose sensors. Biosens. Bioelectron. 2021;179 doi: 10.1016/j.bios.2021.113078. [DOI] [PubMed] [Google Scholar]
- 15.Beck R.W., Bergenstal R.M., Laffel L.M., Pickup J.C. Advances in technology for management of type 1 diabetes. Lancet. 2019;394:1265–1273. doi: 10.1016/S0140-6736(19)31142-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., Liu C., Sun X., Huang W. Current development of materials science and engineering towards epidermal sensors. Prog. Mater. Sci. 2022;128 [Google Scholar]
- 17.Wei M., et al. Electrochemical non-enzymatic glucose sensors: recent progress and perspectives. Chem Commun (Camb) 2020;56:14553–14569. doi: 10.1039/d0cc05650b. [DOI] [PubMed] [Google Scholar]
- 18.Liu J., Agarwal M., Varahramyan K. Glucose sensor based on organic thin film transistor using glucose oxidase and conducting polymer. Sensor. Actuator. B Chem. 2008;135:195–199. [Google Scholar]
- 19.Okuda-Shimazaki J., Yoshida H., Sode K. FAD dependent glucose dehydrogenases - discovery and engineering of representative glucose sensing enzymes. Bioelectrochemistry. 2020;132 doi: 10.1016/j.bioelechem.2019.107414. [DOI] [PubMed] [Google Scholar]
- 20.Li Z.-X., et al. A novel enzyme-responded controlled release electrochemical biosensor for hyaluronidase activity detection. Journal of Analysis and Testing. 2021;5:69–75. [Google Scholar]
- 21.Dorledo de Faria R.A., Iden H., Heneine L.G.D., Matencio T., Messaddeq Y. Non-enzymatic impedimetric sensor based on 3-aminophenylboronic acid functionalized screen-printed carbon electrode for highly sensitive glucose detection. Sensors. 2019;19:1686. doi: 10.3390/s19071686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waqas M., et al. Zn2+ induced self-assembled fabrication of marigold-like ZnO microflower@Ni(OH)2 three-dimensional nanosheets for nonenzymatic glucose sensing. Electrochim. Acta. 2022;410 [Google Scholar]
- 23.Waqas M., et al. Controlled fabrication of nickel and cerium mixed nano-oxides supported on carbon nanotubes for glucose monitoring. Electrochim. Acta. 2023;440 [Google Scholar]
- 24.Wu L.-N., et al. Controllable synthesis of six corner star-like Cu2O/PEDOT-MWCNT composites and their performance toward electrochemical glucose sensing. Electrochim. Acta. 2019;318:837–846. [Google Scholar]
- 25.Waqas M., et al. Cu2O microspheres supported on sulfur-doped carbon nanotubes for glucose sensing. ACS Appl. Nano Mater. 2020;3:4788–4798. [Google Scholar]
- 26.Zhang X., et al. A yolk–albumen–shell structure of mixed Ni–Co oxide with an ultrathin carbon shell for high-sensitivity glucose sensors. Mater. Adv. 2020;1:908–917. [Google Scholar]
- 27.Liu X., et al. A two-dimensional G-CoP/N,P-co-doped carbon nanowire electrode for the simultaneous determination of hydroquinone and catechol in domestic wastewater. Anal. Chim. Acta. 2022;1210 doi: 10.1016/j.aca.2022.339871. [DOI] [PubMed] [Google Scholar]
- 28.Bisht A., Mishra A., Bisht H., Tripathi R.M. Nanomaterial based biosensors for detection of viruses including SARS-CoV-2: a review. J. Anal. Test. 2021;5:327–340. doi: 10.1007/s41664-021-00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang X.Y., et al. Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors. Nat. Commun. 2013;4:2169. doi: 10.1038/ncomms3169. [DOI] [PubMed] [Google Scholar]
- 30.Qiao Y., et al. High-performance non-enzymatic glucose detection: using a conductive Ni-MOF as an electrocatalyst. J. Mater. Chem. B. 2020;8:5411–5415. doi: 10.1039/d0tb00131g. [DOI] [PubMed] [Google Scholar]
- 31.Tiwari J.N., Tiwari R.N., Kim K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012;57:724–803. [Google Scholar]
- 32.Simsek M., Wongkaew N. Carbon nanomaterial hybrids via laser writing for high-performance non-enzymatic electrochemical sensors: a critical review. Anal. Bioanal. Chem. 2021;413:6079–6099. doi: 10.1007/s00216-021-03382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimcheva N. Nanostructures of noble metals as functional materials in biosensors. Curr. Opin. Electrochem. 2020;19:35–41. [Google Scholar]
- 34.Hojaiji H., et al. An autonomous wearable system for diurnal sweat biomarker data acquisition. Lab Chip. 2020;20:4582–4591. doi: 10.1039/d0lc00820f. [DOI] [PubMed] [Google Scholar]
- 35.Moradi O. A review on nanomaterial-based electrochemical sensors for determination of vanillin in food samples. Food Chem. Toxicol. 2022;168 doi: 10.1016/j.fct.2022.113391. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X., Ju Y., Chen J., Liu D., Liu H. Nonenzymatic wearable sensor for electrochemical analysis of perspiration glucose. ACS Sens. 2018;3:1135–1141. doi: 10.1021/acssensors.8b00168. [DOI] [PubMed] [Google Scholar]
- 37.Adeel M., et al. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112331. [DOI] [PubMed] [Google Scholar]
- 38.Lee H., Hong Y.J., Baik S., Hyeon T., Kim D.H. Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201701150. [DOI] [PubMed] [Google Scholar]
- 39.Bariya M., Nyein H.Y.Y., Javey A. Wearable sweat sensors. Nat. Electron. 2018;1:160–171. [Google Scholar]
- 40.Wang D., et al. Dual-functional ultrathin wearable 3D particle-in-cavity SF-AAO-Au SERS sensors for effective sweat glucose and lab-on-glove pesticide detection. Sensor. Actuator. B Chem. 2022;359 [Google Scholar]
- 41.Wang Y., et al. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta. 2019;198:86–92. doi: 10.1016/j.talanta.2019.01.104. [DOI] [PubMed] [Google Scholar]
- 42.Dervisevic M., et al. Silicon micropillar array-based wearable sweat glucose sensor. ACS Appl. Mater. Interfaces. 2022;14:2401–2410. doi: 10.1021/acsami.1c22383. [DOI] [PubMed] [Google Scholar]
- 43.Li G., et al. Integrating highly porous and flexible Au hydrogels with soft-MEMS technologies for high-performance wearable biosensing. Anal. Chem. 2021;93:14068–14075. doi: 10.1021/acs.analchem.1c01581. [DOI] [PubMed] [Google Scholar]
- 44.de Lima L.F., et al. Enzymeless glucose sensor based on disposable Ecoflex®/graphite thermoplastic composite substrate modified with Au@GQDs. Sens. Actuat. Rep. 2022;4 [Google Scholar]
- 45.Zhang W., Du Y., Wang M.L. On-chip highly sensitive saliva glucose sensing using multilayer films composed of single-walled carbon nanotubes, gold nanoparticles, and glucose oxidase. Sens. Bio-Sens. Res. 2015;4:96–102. [Google Scholar]
- 46.Yang D., Li L., Xiao G., Zhang S. Steering charge kinetics in metal-free g-C3N4/melem hybrid photocatalysts for highly efficient visible-light-driven hydrogen evolution. Appl. Surf. Sci. 2020;510 [Google Scholar]
- 47.Gao S., et al. Electrochemical chloramphenicol sensors-based on trace MoS2 modified carbon nanomaterials: insight into carbon supports. J. Alloys Compd. 2021;872 [Google Scholar]
- 48.Zhang W., Du Y., Wang M.L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Bio-Sens. Res. 2015;4:23–29. [Google Scholar]
- 49.Zhang J., et al. A wearable self-powered biosensor system integrated with diaper for detecting the urine glucose of diabetic patients. Sensor. Actuator. B Chem. 2021;341 [Google Scholar]
- 50.Bonaccorso F., Sun Z., Hasan T., Ferrari A.C. Graphene photonics and optoelectronics. Nat. Photonics. 2010;4:611–622. [Google Scholar]
- 51.Kim K.S., et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457:706–710. doi: 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- 52.Zhang G., et al. Tribological performances of highly dispersed graphene oxide derivatives in vegetable oil. Tribol. Int. 2018;126:39–48. [Google Scholar]
- 53.Lee C., Wei X., Kysar J.W., Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321:385–388. doi: 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- 54.Basiruddin S.K., Swain S.K. Phenylboronic acid functionalized reduced graphene oxide based fluorescence nano sensor for glucose sensing. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;58:103–109. doi: 10.1016/j.msec.2015.07.068. [DOI] [PubMed] [Google Scholar]
- 55.Sedighi A., Montazer M., Mazinani S. Synthesis of wearable and flexible NiP0.1-SnOx/PANI/CuO/cotton towards a non-enzymatic glucose sensor. Biosens. Bioelectron. 2019;135:192–199. doi: 10.1016/j.bios.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 56.He W., et al. Printing graphene-carbon nanotube-ionic liquid gel on graphene paper: towards flexible electrodes with efficient loading of PtAu alloy nanoparticles for electrochemical sensing of blood glucose. Anal. Chim. Acta. 2016;903:61–68. doi: 10.1016/j.aca.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 57.Naik A.R., et al. Printed microfluidic sweat sensing platform for cortisol and glucose detection. Lab Chip. 2021;22:156–169. doi: 10.1039/d1lc00633a. [DOI] [PubMed] [Google Scholar]
- 58.Zhu X., et al. Water splitting-assisted electrocatalytic oxidation of glucose with a metal-organic framework for wearable nonenzymatic perspiration sensing. Anal. Chem. 2019;91:10764–10771. doi: 10.1021/acs.analchem.9b02328. [DOI] [PubMed] [Google Scholar]
- 59.Han J., et al. Pt-poly(L-lactic acid) microelectrode-based microsensor for in situ glucose detection in sweat. Biosens. Bioelectron. 2020;170 doi: 10.1016/j.bios.2020.112675. [DOI] [PubMed] [Google Scholar]
- 60.Cheng X., et al. A mediator-free electroenzymatic sensing methodology to mitigate ionic and electroactive interferents' effects for reliable wearable metabolite and nutrient monitoring. Adv. Funct. Mater. 2019;30 [Google Scholar]
- 61.Pu Z., et al. A thermal activated and differential self-calibrated flexible epidermal biomicrofluidic device for wearable accurate blood glucose monitoring. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Q., et al. 3D printing stretchable core-shell laser scribed graphene conductive network for self-powered wearable devices. Compos. B Eng. 2022;240 [Google Scholar]
- 63.Yoon H., et al. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sensor. Actuator. B Chem. 2020;311 [Google Scholar]
- 64.Yang L., et al. Graphene oxide glue-electrode for fabrication of vertical, elastic, conductive columns. ACS Nano. 2017;11:2944–2951. doi: 10.1021/acsnano.6b08323. [DOI] [PubMed] [Google Scholar]
- 65.Yao H.B., et al. A flexible and highly pressure-sensitive graphene-polyurethane sponge based on fractured microstructure design. Adv. Mater. 2013;25:6692–6698. doi: 10.1002/adma.201303041. [DOI] [PubMed] [Google Scholar]
- 66.Ai Y., et al. All rGO-on-PVDF-nanofibers based self-powered electronic skins. Nano Energy. 2017;35:121–127. [Google Scholar]
- 67.Choi S., Jiang Z. A novel wearable sensor device with conductive fabric and PVDF film for monitoring cardiorespiratory signals. Sensor Actuator Phys. 2006;128:317–326. [Google Scholar]
- 68.Pantelopoulos A., Bourbakis N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Syst. Man Cybernet. Part C (Appl. Rev.) 2010;40:1–12. [Google Scholar]
- 69.Bandodkar A.J., Jeerapan I., Wang J. Wearable chemical sensors: present challenges and future prospects. ACS Sens. 2016;1:464–482. [Google Scholar]
- 70.Ma D., et al. Wearable, antifreezing, and healable epidermal sensor assembled from long-lasting moist conductive nanocomposite organohydrogel. ACS Appl. Mater. Interfaces. 2019;11:41701–41709. doi: 10.1021/acsami.9b15412. [DOI] [PubMed] [Google Scholar]
- 71.Zhai Q., et al. Enokitake mushroom-like standing gold nanowires toward wearable noninvasive bimodal glucose and strain sensing. ACS Appl. Mater. Interfaces. 2019;11:9724–9729. doi: 10.1021/acsami.8b19383. [DOI] [PubMed] [Google Scholar]
- 72.Toi P.T., Trung T.Q., Dang T.M.L., Bae C.W., Lee N.E. Highly electrocatalytic, durable, and stretchable nanohybrid fiber for on-body sweat glucose detection. ACS Appl. Mater. Interfaces. 2019;11:10707–10717. doi: 10.1021/acsami.8b20583. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y., et al. Highly stretchable and strain-insensitive fiber-based wearable electrochemical biosensor to monitor glucose in the sweat. Anal. Chem. 2019;91:6569–6576. doi: 10.1021/acs.analchem.9b00152. [DOI] [PubMed] [Google Scholar]
- 74.Yu M., et al. Gold nanostructure-programmed flexible electrochemical biosensor for detection of glucose and lactate in sweat. J. Electroanal. Chem. 2021;882 [Google Scholar]
- 75.Guo S., et al. Integrated contact lens sensor system based on multifunctional ultrathin MoS2 transistors. Matter. 2021;4:969–985. doi: 10.1016/j.matt.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng B., et al. Evaluation of enzyme-based tear glucose electrochemical sensors over a wide range of blood glucose concentrations. Biosens. Bioelectron. 2013;49:204–209. doi: 10.1016/j.bios.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Kim J., et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017;8 doi: 10.1038/ncomms14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park J., et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aap9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zucca A., et al. Tattoo conductive polymer nanosheets for skin-contact applications. Adv. Healthc. Mater. 2015;4:983–990. doi: 10.1002/adhm.201400761. [DOI] [PubMed] [Google Scholar]
- 80.Zhang S., et al. Full review: the progress and developing trends of nanosheet-based sensing applications. Coord. Chem. Rev. 2021;433 [Google Scholar]
- 81.Zhang S., et al. Current advances and challenges in nanosheet-based wearable power supply devices. iScience. 2021;24 doi: 10.1016/j.isci.2021.103477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh S.Y., et al. Skin-attachable, stretchable electrochemical sweat sensor for glucose and pH detection. ACS Appl. Mater. Interfaces. 2018;10:13729–13740. doi: 10.1021/acsami.8b03342. [DOI] [PubMed] [Google Scholar]
- 83.Khoshroo A., Sadrjavadi K., Taran M., Fattahi A. Electrochemical system designed on a copper tape platform as a nonenzymatic glucose sensor. Sensor. Actuator. B Chem. 2020;325 [Google Scholar]
- 84.Gong S., et al. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014;5:3132. doi: 10.1038/ncomms4132. [DOI] [PubMed] [Google Scholar]
- 85.Liao M., Liao H., Ye J., Wan P., Zhang L. Polyvinyl alcohol-stabilized liquid metal hydrogel for wearable transient epidermal sensors. ACS Appl. Mater. Interfaces. 2019;11:47358–47364. doi: 10.1021/acsami.9b16675. [DOI] [PubMed] [Google Scholar]
- 86.Bae C.W., et al. Fully stretchable capillary microfluidics-integrated nanoporous gold electrochemical sensor for wearable continuous glucose monitoring. ACS Appl. Mater. Interfaces. 2019;11:14567–14575. doi: 10.1021/acsami.9b00848. [DOI] [PubMed] [Google Scholar]
- 87.Lee H., et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y., Lu S., Feng X. IEEE; 2017. IEEE International Electron Devices Meeting (IEDM) pp. 18.12. 11–18.12. 14. 2017. [Google Scholar]
- 89.Xuan Viet N. Enzyme-free glucose sensor based on micro-nano dualporous gold-modified screen-printed carbon electrode. Int. J. Electrochem. Sci. 2018:8633–8644. [Google Scholar]
- 90.Li Y., et al. vol. X. 2020. (Paper Presented at the Advanced Sensor Systems and Applications). [Google Scholar]
- 91.Chen H., Mei Z., Qi K., Wang Y., Chen R. A wearable enzyme-free glucose sensor based on nickel nanoparticles decorated laser-induced graphene. J. Electroanal. Chem. 2022;920 [Google Scholar]
- 92.Bae C.W., Chinnamani M.V., Lee E.H., Lee N.E. Stretchable non-enzymatic fuel cell-based sensor patch integrated with thread-embedded microfluidics for self-powered wearable glucose monitoring. Adv. Mater. Interfac. 2022;9 [Google Scholar]
- 93.He C., et al. Noble metal construction for electrochemical nonenzymatic glucose detection. Adv. Mater. Technol. 2022 [Google Scholar]
- 94.Radhakrishnan S., et al. Recent developments and future perspective on electrochemical glucose sensors based on 2D materials. Biosensors. 2022;12:467. doi: 10.3390/bios12070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xuan X., Yoon H.S., Park J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018;109:75–82. doi: 10.1016/j.bios.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 96.Zahed M.A., et al. Highly flexible and conductive poly (3, 4-ethylene dioxythiophene)-poly (styrene sulfonate) anchored 3-dimensional porous graphene network-based electrochemical biosensor for glucose and pH detection in human perspiration. Biosens. Bioelectron. 2020;160 doi: 10.1016/j.bios.2020.112220. [DOI] [PubMed] [Google Scholar]
- 97.Lisi F., Peterson J.R., Gooding J.J. The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens. Bioelectron. 2020;148 doi: 10.1016/j.bios.2019.111835. [DOI] [PubMed] [Google Scholar]
- 98.O'Malley E.G., et al. A prospective evaluation of point-of-care measurements of maternal glucose for the diagnosis of gestational diabetes mellitus. Clin. Chem. 2020;66:316–323. doi: 10.1093/clinchem/hvz005. [DOI] [PubMed] [Google Scholar]
- 99.Galindo R.J., et al. Comparison of the FreeStyle libre pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43:2730–2735. doi: 10.2337/dc19-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang X., et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manufactur. 2021;5:201–209. [Google Scholar]
- 101.Romeo A., et al. Inkjet printed flexible non-enzymatic glucose sensor for tear fluid analysis. Appl. Mater. Today. 2018;10:133–141. [Google Scholar]
- 102.Shu Y., et al. Highly stretchable wearable electrochemical sensor based on Ni-Co MOF nanosheet-decorated Ag/rGO/PU fiber for continuous sweat glucose detection. Anal. Chem. 2021;93:16222–16230. doi: 10.1021/acs.analchem.1c04106. [DOI] [PubMed] [Google Scholar]
- 103.Kim S.-Y., et al. Alcohol gas sensors capable of wireless detection using In2O3/Pt nanoparticles and Ag nanowires. Sensor. Actuator. B Chem. 2018;259:825–832. [Google Scholar]
- 104.Pu F., et al. High-performance non-enzymatic glucose sensor based on flower-like Cu2O-Cu-Au ternary nanocomposites. Appl. Surf. Sci. 2022;581 [Google Scholar]
- 105.Liu Q., et al. Highly sensitive and wearable In2O3 nanoribbon transistor biosensors with integrated on-chip gate for glucose monitoring in body fluids. ACS Nano. 2018;12:1170–1178. doi: 10.1021/acsnano.7b06823. [DOI] [PubMed] [Google Scholar]
- 106.Rim Y.S., et al. Printable ultrathin metal oxide semiconductor-based conformal biosensors. ACS Nano. 2015;9:12174–12181. doi: 10.1021/acsnano.5b05325. [DOI] [PubMed] [Google Scholar]
- 107.Jiang Y., et al. Facet-dependent Cu2O electrocatalysis for wearable enzyme-free smart sensing. ACS Catal. 2021;11:2949–2955. [Google Scholar]
- 108.Li Y.L., Liu Y.H., Chen L.S., Xu J.L. A conformable, gas-permeable, and transparent skin-like micromesh architecture for glucose monitoring. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202100046. [DOI] [PubMed] [Google Scholar]
- 109.Prabhakaran A., Nayak P. Surface engineering of laser-scribed graphene sensor enables non-enzymatic glucose detection in human body fluids. ACS Appl. Nano Mater. 2019;3:391–398. [Google Scholar]
- 110.Han W., et al. A self-powered wearable noninvasive electronic-skin for perspiration analysis based on piezo-biosensing unit matrix of enzyme/ZnO nanoarrays. ACS Appl. Mater. Interfaces. 2017;9:29526–29537. doi: 10.1021/acsami.7b07990. [DOI] [PubMed] [Google Scholar]
- 111.Xue X., et al. Self-powered electronic-skin for detecting glucose level in body fluid basing on piezo-enzymatic-reaction coupling process. Nano Energy. 2016;26:148–156. [Google Scholar]
- 112.Lu Y., Jiang K., Chen D., Shen G. Wearable sweat monitoring system with integrated micro-supercapacitors. Nano Energy. 2019;58:624–632. [Google Scholar]
- 113.Sun T., et al. Wearable textile supercapacitors for self-powered enzyme-free smartsensors. ACS Appl. Mater. Interfaces. 2020;12:21779–21787. doi: 10.1021/acsami.0c05465. [DOI] [PubMed] [Google Scholar]
- 114.Zhou Z., et al. Luminescent wearable biosensors based on gold nanocluster networks for "turn-on" detection of Uric acid, glucose and alcohol in sweat. Biosens. Bioelectron. 2021;192 doi: 10.1016/j.bios.2021.113530. [DOI] [PubMed] [Google Scholar]
- 115.Sonner Z., Wilder E., Gaillard T., Kasting G., Heikenfeld J. Integrated sudomotor axon reflex sweat stimulation for continuous sweat analyte analysis with individuals at rest. Lab Chip. 2017;17:2550–2560. doi: 10.1039/c7lc00364a. [DOI] [PubMed] [Google Scholar]
- 116.Choi D.H., et al. Sweat test for cystic fibrosis: wearable sweat sensor vs. standard laboratory test. J. Cyst. Fibros. 2018;17:e35–e38. doi: 10.1016/j.jcf.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 117.Zhai Q., et al. Vertically aligned gold nanowires as stretchable and wearable epidermal ion-selective electrode for noninvasive multiplexed sweat analysis. Anal. Chem. 2020;92:4647–4655. doi: 10.1021/acs.analchem.0c00274. [DOI] [PubMed] [Google Scholar]
- 118.Lee H.C., Hsieh E.Y., Yong K., Nam S. Multiaxially-stretchable kirigami-patterned mesh design for graphene sensor devices. Nano Res. 2020;13:1406–1412. [Google Scholar]
- 119.Veeralingam S., Khandelwal S., Badhulika S. AI/ML-Enabled 2-D - RuS2 nanomaterial-based multifunctional, low cost, wearable sensor platform for non-invasive point of care diagnostics. IEEE Sensor. J. 2020;20:8437–8444. [Google Scholar]
- 120.Hanna J., et al. Noninvasive, wearable, and tunable electromagnetic multisensing system for continuous glucose monitoring, mimicking vasculature anatomy. Sci. Adv. 2020;6:eaba5320. doi: 10.1126/sciadv.aba5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xuan X., et al. A highly stretchable and conductive 3D porous graphene metal nanocomposite based electrochemical-physiological hybrid biosensor. Biosens. Bioelectron. 2018;120:160–167. doi: 10.1016/j.bios.2018.07.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.