Abstract
The pgmG gene of Sphingomonas paucimobilis ATCC 31461, the industrial gellan gum-producing strain, was cloned and sequenced. It encodes a 50,059-Da polypeptide that has phosphoglucomutase (PGM) and phosphomannomutase (PMM) activities and is 37 to 59% identical to other bifunctional proteins with PGM and PMM activities from gram-negative species, including Pseudomonas aeruginosa AlgC. Purified PgmG protein showed a marked preference for glucose-1-phosphate (G1P); the catalytic efficiency was about 50-fold higher for G1P than it was for mannose-1-phosphate (M1P). The estimated apparent Km values for G1P and M1P were high, 0.33 and 1.27 mM, respectively. The pgmG gene allowed the recovery of alginate biosynthetic ability in a P. aeruginosa mutant with a defective algC gene. This result indicates that PgmG protein can convert mannose-6-phosphate into M1P in the initial steps of alginate biosynthesis and, together with other results, suggests that PgmG may convert glucose-6-phosphate into G1P in the gellan pathway.
Bacterial strains of the new genus Sphingomonas (47) are relatively ubiquitous in soil, water, and sediments, have broad catabolic capabilities (12, 17, 33, 35), and produce at least eight extracellular acid heteropolysaccharides that have similar but not identical structures (9, 31). These polysaccharides, the sphingans (after the genus), exhibit properties which make them candidates for food and industrial applications, such as thermoreversible gel formation and solution viscosity (9, 34). The industrial strain Sphingomonas paucimobilis ATCC 31461 (formerly Pseudomonas elodea) synthesizes in high yields from different carbon sources and from cheese whey a new gelling agent, gellan gum (15, 23, 36). The commercial utility of gellan (9, 34) has been a stimulus for the study of its biosynthesis.
The cloning and functional analysis of genes essential for gellan synthesis are indispensable in attempting the genetic and environmental manipulation of its biosynthetic pathway in order to develop new polysaccharides with distinct structural and physical properties. Among the gellan biosynthetic enzymes (29), phosphoglucomutase (PGM; EC 5.4.2.2) plays a pivotal role, being an ideal target for metabolic engineering. Indeed, PGM catalyzes the interconversion of d-glucose-6-phosphate (G6P) and d-glucose-1-phosphate (G1P), representing a branch point in carbohydrate metabolism. G6P enters catabolic processes to yield energy and reducing power, whereas G1P is the precursor of sugar nucleotides that are used by the cells in the synthesis of various polysaccharides. In gellan gum biosynthesis, G1P is required for the synthesis of three sugar nucleotides, UDP-d-glucose, dTDP-l-rhamnose, and UDP-d-glucuronic acid, that are activated precursors for the synthesis of the repeating tetrasaccharide unit in gellan gum (29).
The objective of the present work was to identify the PGM gene from S. paucimobilis ATCC 31461 (pgmG gene) to be used as a target in the metabolic engineering of the gellan pathway.
DNA manipulations.
Plasmid DNA was purified from Escherichia coli cultures by the alkaline lysis procedure (4) or with a QIAprep spin plasmid kit (Qiagen, Santa Clarita, Calif.). DNA restriction, agarose gel electrophoresis, and cloning procedures were carried out by established protocols (43). Nonradioactive probes were used in Southern and colony hybridizations. The Gene Images system (Amersham Pharmacia Biotech, Carnaxide, Portugal) was used for labeling and detecting nucleic acids with fluorescein as the nonradioactive label. Plasmid DNA was introduced into E. coli strains either by a standard transformation procedure or by electrotransformation (43).
Cloning and sequence analysis of the S. paucimobilis pgmG gene.
The pgmG gene was cloned based on the PCR amplification of a DNA fragment of 670 bp from S. paucimobilis ATCC 31461 genomic DNA using degenerate primers from conserved regions of the phosphohexosemutase protein sequences in databases: the synthetic oligonucleotides PGM1 (sense) (5′-ACCGSCAGCCABAAYCCG-3′) and PGM2 (antisense) (5′-BSCGCTCATYTCGCC-3′), purchased from Pharmacia (Uppsala, Sweden) (Table 1). Each PCR mixture contained, in a final volume of 50 μl, 200 ng of S. paucimobilis ATCC 31461 DNA, 10 mM each deoxynucleoside triphosphate (Promega Corporation, Madison, Wis.), 300 pmol of each primer, 2.5 U of Taq DNA polymerase (Boehringer GmbH, Mannheim, Germany), 5 μl of 10× Taq DNA polymerase buffer, and 2.5 mM MgCl2. PCR amplification was performed using a PTC-100 thermocycler (MJResearch Inc., Watertown, Mass.) under the following conditions: 30 cycles consisting of 60 s at 95°C, 30 s at 55.5°C, and 60 s at 72°C. The PCR products yielded were analyzed on a 1% (wt/vol) low-melting-temperature agarose gel (FMC Bioproducts, Rockland, Maine). The amplification product was recovered from the gel, purified by using a WizardPCR Preps DNA purification kit (Promega), and finally cloned into vector plasmid pCR2.1 (Invitrogen, San Diego, Calif.), yielding plasmid pPV1. The PCR product was sequenced, and analysis of the deduced amino acid sequence confirmed that it contained an incomplete open reading frame and that the deduced amino acid sequence was homologous to PGM and phosphomannomutase (PMM) protein sequences in data banks.
TABLE 1.
Degenerate oligonucleotide primers used in this work to amplify a specific pgm probe from S. paucimobilis ATCC 31461 genomic DNA
| Protein and degenerate primera | Amino acid and nucleotide sequences
|
Degeneracy (fold) | Database accession no. | |
|---|---|---|---|---|
| Amino acid | DNA (5′→3′) | |||
| E. coli PGM | T A S H N P | ACC GCC AGC CAT AAT CCG | M77127 | |
| P. aeruginosa AlgC | T G S H N P | ACC GGC AGC CAG AAT CCG | M60873 | |
| X. campestris XanA | T A S H N P | ACC GCC AGC CAC AAC CCG | M83231 | |
| N. gonorrhoeae PGM | T G S H N P | ACC GGC AGC CAC AAT CCG | U02489 | |
| Primer PGM1 (sense) | ACC GSC AGC CAB AAY CCGb | 12 | ||
| E. coli PGM | G E M S A | GGC GAA ATG AGC GCC | M77127 | |
| P. aeruginosa AlgC | G E M S G | GGC GAG ATG AGC GGC | M60873 | |
| X. campestris XanA | G E M S A | GGC GAA ATG AGC GCG | M83231 | |
| N. gonorrhoeae PGM | G E M S G | GGC GAA ATG AGC GGA | U02489 | |
| Primer PGM2 (antisense) | BSC GCT CAT YTC GCCb | 12 | ||
Degenerate primers PGM1 and PGM2 were designed according to homologous nucleotide areas corresponding to, respectively, the active site and the sugar-binding site in the pgm and pgm and pmm genes of the gram-negative bacteria indicated in the table.
The International Union of Pure and Applied Chemistry symbols used to denote multiple nucleotides are as follows: S, G and C; Y, C and T; and B, G, T, and C.
The PGM1 and PGM2 primer sites are located in two conserved regions in the PGM or the PGM and PMM proteins of the gram-negative species listed in Table 1, corresponding to the active-site region and the substrate specificity region, respectively (10, 40). The 670-bp labeled pgmG probe was used to screen, by colony hybridization, the 1,200 clones of the genomic library of S. paucimobilis ATCC 31461 constructed to clone the gellan gum gene cluster(s). To prepare this gene bank, high-molecular-weight DNA was extracted from S. paucimobilis ATCC 31461 by the method of Goldberg and Ohman (19), partially digested with Sau3AI, and size fractionated by continuous sucrose gradient (10 to 40% [wt/vol]) ultracentrifugation (43). Fractions containing DNA fragments of approximately 30 to 40 kb were selected and ligated to the BamHI-digested and dephosphorylated Hypercos.1 vector plasmid (Stratagene, La Jolla, Calif.). The ligation product was packaged in vitro into a lambda phage particle packing kit (Boehringer) and used to infect E. coli NM554 (38). Eight clones of the gene bank thus obtained hybridized with the probe mentioned above; restriction analysis of the inserts and cross-hybridization analysis revealed the presence of overlapping insertions. One of the clones (pF6), with an ≈35-kb insert, was retained for further characterization.
Subcloning of pF6 into pKK233-3 (8) led to the identification of pPV233, a subclone with a 2,900-bp PstI fragment insert. Southern hybridization of S. paucimobilis genomic DNA digested with PstI revealed that the above-mentioned pgmG internal region hybridized under high-stringency conditions with a single 2,900-bp genomic fragment encompassing the complete pgmG gene sequence (data not shown).
Nucleotide sequence accession number.
A nucleotide sequence of 1,563 bp within the pPV233 insert was determined on both strands at the DNA Core Facility, University of Missouri, Columbia. This sequence has been deposited in the GenBank database under accession no. AF167367.
Sequence data were analyzed with DNASIS 3.0 software (Hitachi Software Engineering, Hitachi America, Ltd., Brisbane, Calif.), revealing the presence of a unique open reading frame, designated pgmG and 1,388 nucleotides long, starting at a putative ATG start codon. The average G+C content of pgmG (66.8%) is characteristic of Sphingomonas genes, and a high frequency of G or C in the third codon was also observed (48). Preceding the start codon (6 nucleotides upstream), a putative ribosome-binding site (5′-GGGAGG-3′) was found; possible promoter sequences, TATGCTG for the −35 region and TATTAA for the −10 region, were observed in the 5′-flanking region. The protein deduced from the pgmG sequence is composed of 462 residues, with a calculated molecular mass of 50,059 Da and a predicted pI of 4.85. Analysis of the amino acid composition revealed that there are 67% nonpolar residues and 33% polar residues; among the charged residues, 42% are basic.
The deduced amino acid sequence encoded by pgmG was compared with data in the GenBank database using the BLAST network service (2) at the National Center for Biotechnology Information, Bethesda, Md. Alignments to determine protein similarities and for construction of a phylogenetic tree were performed with the CLUSTAL method (20) (Genetics Computer Group, University of Wisconsin) through a computer link to the European Molecular Biology Network (EMBnet) [Portuguese node] at the Gulbenkian Institute of Science, Oeiras, Portugal. The following high levels of identity and similarity with other PGM and/or PMM proteins from a variety of organisms were found: 59% identity and 71% similarity with PmmA of Prochlorothrix hollandica (13), 53% identity and 63% similarity with ExoC of Azospirillum brasilense (EMBL accession no. 695163), 38% identity and 52% similarity with AlgC of Pseudomonas aeruginosa (51), 38% identity and 53% similarity with the PGM of Neisseria gonorrhoeae (50), and 37% identity and 52% similarity with ExoC of Rickettsia prowazekii (3). Lower levels of homology were noted with phosphoglucosamine mutases, including Staphylococcus aureus GlmM (25% identity and 39% similarity) (21), E. coli GlmM (25% identity and 39% similarity) (11), and Pseudomonas syringae GlmM (23% identity and 39% similarity) (41).
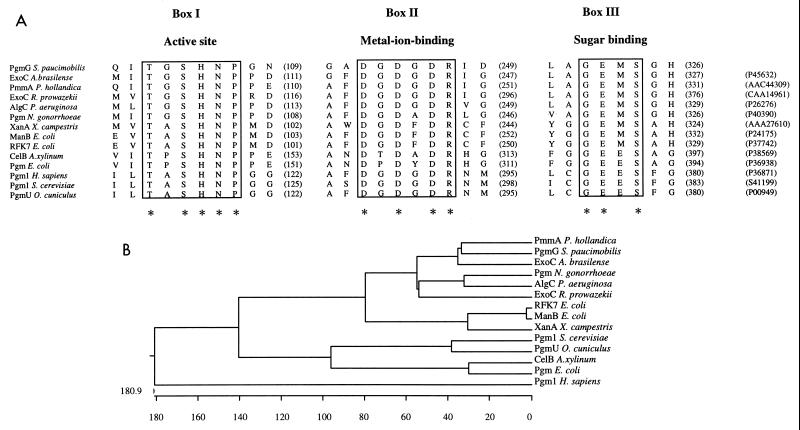
With the CLUSTAL V alignment program (20), the predicted amino acid sequence of S. paucimobilis PgmG was compared with the primary structures of PGM and/or PMM enzymes from prokaryotic and eukaryotic organisms (Fig. 1A). The primary structures of all the proteins compared display a high degree of similarity in length, and the three highly conserved domains were confirmed (box I, box II, and box III) (Fig. 1A). According to the observations first described for rabbit muscle PGM (10, 40), box I (T-X-S-H-N-P; Fig. 1A) was assumed to correspond to the active-site region, in which the serine residue (S 104 for S. paucimobilis PgmG) is phosphorylated during the catalytic action of hexosephosphate mutases (10, 22, 32, 39); box II (D-X-D-X-D-R; Fig. 1A) contains a metal-ion-binding loop; and box III (G-E-X-S; Fig. 1A) may be responsible for interaction with the substrate (10, 39).
FIG. 1.
(A) Multiple amino acid alignment of the three characteristic consensus sequences present in 14 proteins with PGM and/or PMM activities from different organisms. Boxes I to III indicate the functional domains exhibiting highest similarity among the polypeptides. The amino acid sequences in boxes I, II, and III are known to be critical for PGM activity, based on the study of rabbit muscle PGM (10, 40). The box I motif contains the catalytic site of the enzyme; the box II motif contains a metal-ion-binding loop; and the box III motif interacts with the substrate. Asterisks below the sequences indicate residues conserved in all the enzymes. Numbers of intervening amino acids and accession numbers are given in parentheses. (B) Phylogenetic tree based on the multiple-sequence analysis of the fully sequenced PGM and PMM proteins. The CLUSTAL program (20) was used for the sequence alignment and the phylogenetic tree construction.
A phylogenetic tree for the PGM and/or PMM family of 14 proteins was constructed, including S. paucimobilis PgmG described here for the first time (Fig. 1B). S. paucimobilis PgmG was included in the subclass of phosphohexomutases with other bifunctional PMM and/or PGM proteins, such as AlgC from P. aeruginosa (51), XanA from Xanthomonas campestris (24), the N. gonorrhoeae PGM (44, 50), PmmA from P. hollandica (13), the RFK7 and ManB enzymes from E. coli (showing PMM activity) (28, 45), or the putative PMMs from A. brasilense (ExoC) (EMBL accession no. 695163) and R. prowazekii (ExoC) (3). The PGM enzymes of E. coli and Acetobacter xylinum (CelB) (7, 25, 27) were very similar to the PGM enzymes of Saccharomyces cerevisiae (Pgm1) (5) and rabbit muscle (PgmU) (39) and are considered to be highly specific for phosphoglucose. Although these two subclasses share active-site and metal-ion-binding domains (box I and II) in box III [GE(M or E)S] that may control substrate specificity (10), the subclass of specific PGMs exhibits the E residue, while in the subclass of bifunctional enzymes, including PgmG, this residue is substituted by M (Fig. 1A).
Overexpression of pgmG in E. coli and purification of the PgmG protein.
To overexpress the S. paucimobilis pgmG gene in E. coli, plasmid pLC1 was constructed by inserting this gene behind the bacteriophage T7 RNA polymerase promoter in the pET29a translation vector (Novagen Inc., Madison, Wis.) after specific amplification by PCR. Synthetic oligonucleotide primers used for amplification were PGM3 (sense) (5′-GGGGATTCATGACGCACCGTTTCGAT-3′) and PGM4 (antisense) (5′-GGAAGCTTTCAATGCGCCGCCTGCTC-3′), designed also to introduce BamHI and HindIII sites (underlined) at the 5′ ends of the PGM3 and PGM4 primers, respectively. Pwo DNA polymerase (Boehringer) was used for amplification of the pgmG gene. PgmG was overproduced as an N-terminal fusion with the S-tag epitope of the S-protein portion of ribonuclease A in E. coli BL21(DE3) transformed with pLC1. E. coli strain BL21(DE3) carries the gene encoding T7 RNA polymerase under the control of the inducible lacUV5 promoter (46). E. coli transformants were cultivated in 25 ml of Lennox broth containing 0.5% (wt/vol) glucose and 150 mg of ampicillin per liter and grown at 37°C until the culture optical density at 600 nm reached 0.6. pgmG transcription was then induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) (0.1 mM), followed by an additional period of 3 h of growth. Cells were recovered by centrifugation, resuspended in 2.5 ml of cold 20 mM Tris-HCl (pH 7.5) with 0.15 M NaCl and 0.1% (wt/vol) Triton X-100 (Sigma Chemical Co., St. Louis, Mo.), disrupted by sonication (VibraCell; Sonics Material Inc., Danbury, Conn.) (29), and centrifuged at 18,000 × g for 45 min at 4°C. Upon induction with IPTG, PGM and PMM activities in E. coli crude extracts were significantly increased due to recombinant PgmG (Table 2).
TABLE 2.
PGM and PMM activities in crude extracts from cells of E. coli BL21(DE3) harboring the cloning vector pET29a or the recombinant plasmid pLC1, with the S. paucimobilis pgmG gene, induced (3 h) or not induced with IPTG
| Strain | IPTG (mM) | Mean ± SD sp act (U/g of protein) of:
|
|
|---|---|---|---|
| PGM | PMM | ||
| E. coli BL21(DE3)/pET29a | 0.0 | 428.5 ± 18.5 | 52.7 ± 1.8 |
| E. coli BL21(DE3)/pLC1 | 0.0 | 606.0 ± 14.0 | 68.3 ± 3.1 |
| E. coli BL21(DE3)/pLC1 | 0.1 | 1,181 ± 18.5 | 123.0 ± 4.5 |
The specific activities of PGM and PMM were assayed with cell crude extracts and with the sample resulting from PgmG purification under the conditions described by Martins and Sá-Correia (29) and Sá-Correia et al. (42), as modified by Leitão et al. (26). The increase in the optical density at 340 nm due to the reduction of NADP at 30°C in coupled reaction systems was recorded by using a double-beam spectrophotometer (model U-2000; Hitachi Ltd., Tokyo, Japan). Enzyme activities were calculated from the initial linear rates of cofactor reduction after subtraction of endogenous activity (measured in enzyme assays lacking the substrate). Control assays lacking only the extracts were also carried out. All enzymes used in coupled reactions, phosphorylated sugars, sugar nucleotides, NADP, dithiothreitol (DTT), phenylmethylsulfonyl fluoride (PMSF), and bovine serum albumin were from Sigma. Under the assay conditions used, one unit of enzyme activity was defined as the amount of enzyme needed to reduce 1 μmol of NADP per min. Protein concentration was determined by the method described by Bradford (6) with bovine serum albumin fraction V as the standard. PGM and PMM specific activities are the means of the values of at least three enzyme assays and three protein determinations in samples resulting from a growth and purification experiment representative of the two complete independent experiments carried out. Under standard conditions, the reaction mixture for the PGM assay contained 15 mM Tris-Cl buffer (pH 7.6), 10 mM MgCl2, 5 mM DTT, 0.1 mM glucose 1,6-diphosphate, 1 mM G1P, 1 U of G6P dehydrogenase (zwf), and 1 mM NADP. The reaction was started by the addition of 100 μl of the enzyme solution to a final volume of 1 ml. PMM activity was assayed using an identical reaction mixture supplemented with 1 U each of phosphoglucose isomerase and phosphomannose isomerase and with 1 mM mannose-1-phosphate (M1P) instead of G1P. The reaction was started by the addition of 100 μl of the enzyme solution to a final volume of 1 ml.
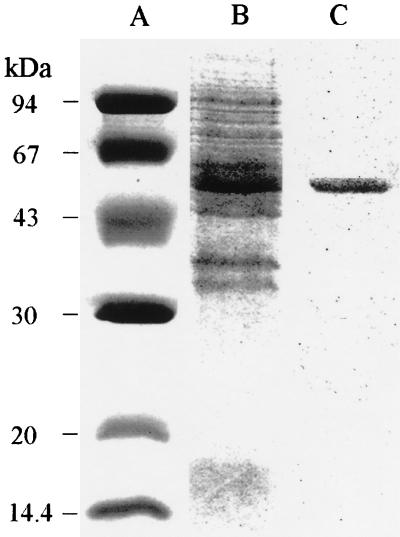
Heterologous PgmG was purified to homogeneity (Table 3) from cell crude extracts prepared from IPTG-induced cells by using the S-tag–thrombin purification system (Novagen). The purified enzyme fraction was eluted with a solution containing 20 mM Tris-HCl (pH 8.4) with 0.15 M NaCl and 2.5 mM CaCl2, dialyzed overnight at 4°C against 300 ml of 100 mM Tris-HCl (pH 7.6) with 2 mM DTT and 0.5 mM PMSF, and immediately used for enzyme assays. The proteins in cell crude extracts obtained by sonication or in the sample obtained after purification were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 12.5% (wt/vol) polyacrylamide gels and samples loaded at a concentration of 30 or 2 μg, respectively (Fig. 2). The S. paucimobilis enzyme, capable of using both G1P and M1P, was purified over 12-fold with a 31% yield (Table 3). Under denaturing conditions, purified PgmG migrated as a single polypeptide with the expected molecular mass of 50 kDa (Fig. 2).
TABLE 3.
Purification of S. paucimobilis PgmGa
| Purification step | Protein (g) | Total activity (U) with:
|
Sp act (U/g) with:
|
Yield (%) | Purification (fold) | ||
|---|---|---|---|---|---|---|---|
| G1P | M1P | G1P | M1P | ||||
| PgmG in crude extractb | 3.6 | 2,709 | 253.1 | 752.5 | 70.3 | 100.0 | |
| S-tag purification | 0.092 | 851.0 | 35.0 | 9,250 | 380.0 | 31.4 | 12.3 |
Yield and fold purification calculations were based on the PGM (G1P) activity of PgmG.
The specific activities of PGM (G1P) and PMM (M1P) of S. paucimobilis PgmG overproduced in crude extracts from cells of E. coli BL21(DE3) harboring pLC1 after 3 h of IPTG (0.1 mM) induction (Table 2) were considered to be the total activities in these extracts subtracted from the background activities in the E. coli host cells harboring the cloning vector (values in Table 2).
FIG. 2.
Coomassie blue-stained proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis during the purification of S. paucimobilis PgmG overproduced in cells of E. coli BL21(DE3) harboring plasmid pLC1 and induced with IPTG. Lanes: A, molecular markers in kilodaltons; B, crude extracts from cells of E. coli BL21(DE3) harboring plasmid pLC1 and harvested after 3 h of IPTG (0.1 mM) induction; C, fraction resulting from S-tag purification of PgmG.
Heterologous complementations.
The functionality of the S. paucimobilis pgmG gene-encoded protein as PGM and PMM enzymes was confirmed by heterologous complementation of both an E. coli mutant defective in the pgm gene (1; kindly provided by the E. coli Genetic Stock Center [CGSC], Yale University, New Haven, Conn.) and a P. aeruginosa mutant defective in alginate synthesis due to an algC gene mutation (51; kindly provided by A. M. Chakrabarty, University of Illinois, Chicago). The recombinant plasmid pPV233 slightly complemented the E. coli PGM1 defect (CGSC 5527) (1). This finding was judged by the ability of PGM1 transformed with pPV233 to grow more efficiently on MacConkey agar base plates (Difco Laboratories) supplemented with 0.5% (wt/vol) galactose, yielding darker purple-red colonies than E. coli PGM1 transformed with the cloning vector pKK233-3 after incubation at 37°C for 2 days (results not shown). The slight change in size and color of the colonies demonstrated the recovery of the ability of the pgm-complemented mutant to produce acid from growth in galactose.
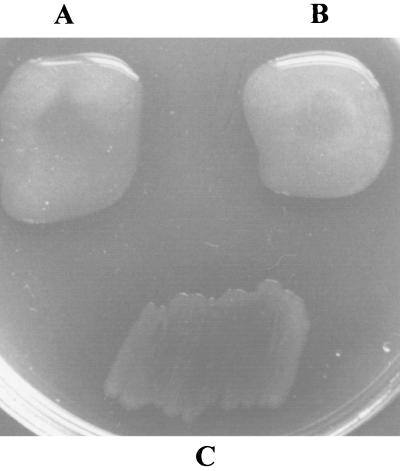
For the complementation experiments with the nonmucoid P. aeruginosa 8858 mutant with a defect in the algC gene (53), the recombinant plasmid pLC100 was constructed by subcloning PCR-amplified pgmG with Pwo DNA polymerase, using primers PGM5 (sense) (5′-GGGAATTCCACGTAACATTTTGCCGG-3′) and PGM4 (antisense) (indicated above), designed also to introduce an EcoRI site (underlined), into the broad-host-range vector pMMB66(EH) (18). This recombinant plasmid was mobilized into P. aeruginosa 8858 by triparental filter mating using E. coli HB101/pRK2013 as the helper strain (14, 16, 43). Individual transconjugants, isolated by spreading the mating mixture onto Pseudomonas isolation agar supplemented with carbenicillin, were examined visually for alginate production on this same medium after 2 days of incubation at 30°C. The introduction of plasmid pLC100 led to the recovery of alginate biosynthetic ability, as did the introduction of plasmid pNZ49 with the P. aeruginosa algC gene into the same cloning vector (51); the nonmucoid phenotype of the host algC mutant was maintained after the introduction of the cloning vector (Fig. 3).
FIG. 3.
(A) Complementation of the nonmucoid algC mutant strain P. aeruginosa 8858 by plasmid pLC100, containing the S. paucimobilis pgmG gene in the cloning vector pMMB66(EH). (B and C) Positive (B) and negative (C) controls were also prepared by the mobilization of pNZ49 or the cloning vector pMMB66(EH) into P. aeruginosa 8858.
Kinetic properties of PgmG.
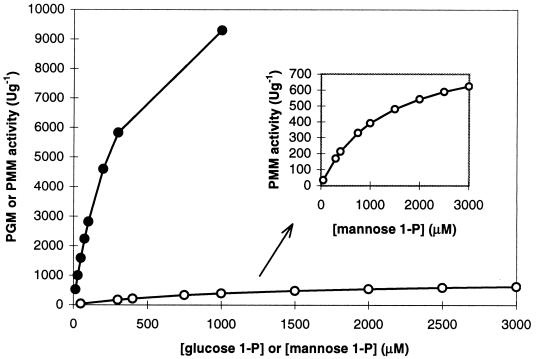
The kinetic constants Km and Vmax, corresponding to the PGM or PMM activities of purified PgmG for G1P (range, 15 to 1,000 μM) or M1P (range, 50 to 3,000 μM), respectively, were calculated based directly on the Michaelis-Menten equation; the rectangular hyperbolic function was solved using iterative procedures (computer program: Solver from Microsoft Excel). kcat values (minute−1) were calculated on the basis of a molecular mass of 50 kDa for PgmG. Thus, kcat values were obtained by dividing Vmax (micromoles minute−1 milligram−1) by 0.02 to convert protein concentration to molarity. The purified PgmG enzyme converted G1P into G6P and M1P into M6P, as indicated by the results shown in Tables 3 and 4 and in Fig. 4. Although S. paucimobilis PgmG is bifunctional, under the assay conditions used here the enzyme exhibits a marked preference for G1P (Fig. 4 and Table 4). Indeed, the catalytic efficiency, based on the kcat/Km ratio, was about 50-fold higher for G1P than it was for M1P (Table 4). The estimated apparent Km values for G1P and M1P were high, 330 and 1,270 μM, respectively.
TABLE 4.
Kinetic parameters of PGM and PMM activities of PgmG
| Sugar | Km (mM) | kcat (min−1)a | kcat/Km (min−1 mM−1) |
|---|---|---|---|
| G1P | 0.33 | 625.0 | 1,871 |
| M1P | 1.27 | 44.5 | 35.0 |
kcat values were calculated on the basis of PgmG as a 50-kDa monomer, being the Vmax values for G1P and M1P, 12.5 and 0.9 μmol min−1 mg−1, respectively.
FIG. 4.
Saturation curves of purified PgmG with the indicated concentrations of G1P or M1P, conforming to Michaelis-Menten kinetics. The apparent Michaelis constant (Km) and Vmax (Table 4) were calculated based directly on the Michaelis-Menten equation.
pgmG gene and the gellan pathway.
The S. paucimobilis pgmG gene identified in the present work is presumably involved in the formation of G1P, required for the synthesis of the three sugar precursors for the synthesis of the repeating tetrasaccharide unit in gellan. However, this gene is not located in the cluster of genes involved in gellan synthesis, recently identified in our laboratory by PCR amplification (P. A. Videira, A. M. Fialho, and I. Sá-Correia, unpublished data), based on the gene cluster involved in the synthesis of the exopolysaccharide (EPS) sphingan S-88 by Sphingomonas S-88 (48). The organization of the gellan gene cluster was found to be essentially identical to that reported for the S-88 gene cluster and includes genes essential for the assembly and secretion of EPS and a four-gene operon needed for the synthesis of dTDP-l-rhamnose. Interestingly, the algC gene of P. aeruginosa, encoding the bifunctional PGM and PMM protein of the alginate biosynthetic pathway, also does not map in the alginate cluster, where algA and algD, the other genes involved in GDP-mannuronic acid formation, are located (30). However, in X. campestris, the gene xanA, encoding the bifunctional PGM and PMM protein of the xanthan gum biosynthetic pathway, is located in the xanthan cluster of biosynthetic genes (24).
The bifunctional protein PgmG exhibits apparent Km values for both substrates (Table 4) higher than those reported for the majority of other PGM or PGM and PMM enzymes characterized before. These values are 8 μM (G1P) for PgmU of rabbit muscle (39), 17 μM (M1P) and 22 μM (G1P) for AlgC of P. aeruginosa (49), and 20 μM (G1P) for the PGM of maize leaves (37). However, these Km values are below the value calculated for the specific PGM of A. xylinum (2,600 μM) [G1P] (25). Interestingly, this protein was included in the subclass of enzymes specific for G1P that is distinct from the subclass of phosphohexosemutases formed by S. paucimobilis PgmG and other bifunctional enzymes, such as AlgC from P. aeruginosa (51), XanA from X. campestris (24), N. gonorrhoeae PGM (44, 50), and PmmA from P. hollandica (13) (Fig. 1B). Although the back reaction has not been studied for A. xylinum PGM, the high Km (G1P) value calculated was considered consistent with the involvement of this enzyme in the production of extracellular cellulose, since a high Km (G1P) favors metabolic flux toward polymer synthesis rather than catabolic pathways (25).
Although the catalytic efficiency (kcat/Km) of S. paucimobilis PgmG was about 50-fold higher for G1P than it was for M1P, the pgmG gene cloned into pMMB66(EH) led to the recovery of alginate biosynthetic ability when introduced into a P. aeruginosa mutant with a defective algC gene. This observation clearly indicates that PgmG can indeed efficiently convert M6P into M1P in the initial steps of alginate biosynthesis and, together with the other results reported, suggests that PgmG may convert G6P into G1P in the gellan pathway. The next step in this work will be the characterization of gellan gum biosynthesis after pgmG disruption in S. paucimobilis ATCC 31461, whether the gene is nonessential and the respective deletion mutant can be obtained, or in recombinant strains in which the expression of this gene is increased.
Acknowledgments
This work was supported by JNICT/FCT, FEDER, and the PRAXIS XXI Program (grant PRAXIS/2/2.1/BIO/1125/95 and Ph.D. and M.Sc. scholarships to P.A.V. and L.L.C., respectively).
The first two authors contributed equally to this work.
REFERENCES
- 1.Adhya S, Schwartz M. Phosphoglucomutase mutants of Escherichia coli K-12. J Bacteriol. 1971;108:621–626. doi: 10.1128/jb.108.2.621-626.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Ponten T, Alsmark U C, Podowski R M, Naslund A K, Eriksson A S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boles E, Liebetran W, Hofman M, Zimmermann F K. A family of hexosephosphate mutases in Saccharomyces cerevisiae. Eur J Biochem. 1994;220:83–96. doi: 10.1111/j.1432-1033.1994.tb18601.x. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brautaset T, Standal R, Fjaervik E, Valla S. Nucleotide sequence and expression analysis of the Acetobacter xylinum phosphoglucomutase gene. Microbiology. 1994;140:1183–1188. doi: 10.1099/13500872-140-5-1183. [DOI] [PubMed] [Google Scholar]
- 8.Brosius J, Holly A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci USA. 1984;81:6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandrasekaran R, Radha A. Molecular architectures and functional properties of gellan gum and related polysaccharides. Trends Food Sci Technol. 1995;6:143–148. [Google Scholar]
- 10.Dai J-B, Liu Y, Ray W J, Konno M. The crystal structure of muscle phosphoglucomutase refined at 2.7 angstrom resolution. J Biol Chem. 1992;267:6322–6337. [PubMed] [Google Scholar]
- 11.Dallas W S, Dev I K, Ray P H. The dihydropteroate synthase gene, folP, is near the leucine tRNA gene, leuU, on the Escherichia coli chromosome. J Bacteriol. 1993;175:7743–7744. doi: 10.1128/jb.175.23.7743-7744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta T K, Selifonov S A, Gunsalus I C. Oxidation of methyl-substituted naphthalenes: pathways in a versatile Sphingomonas paucimobilis strain. Appl Environ Microbiol. 1998;64:1884–1889. doi: 10.1128/aem.64.5.1884-1889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwivedi K, Post A F, Bullerjahn S. Cloning and functional analysis of the pmmA gene encoding phosphomannomutase from the photosynthetic prokaryote Prochlorothrix hollandica. Biochim Biophys Acta. 1996;1290:210–214. doi: 10.1016/0304-4165(96)00061-x. [DOI] [PubMed] [Google Scholar]
- 14.Fialho A M, Monteiro G A, Correia I S. Conjugal transfer of recombinant plasmids into gellan gum producing and non-producing variants of Pseudomonas elodea ATCC 31461. Lett Appl Microbiol. 1991;12:85–87. doi: 10.1111/j.1472-765x.1991.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 15.Fialho A M, Martins L O, Donval M-L, Leitão J H, Ridout M J, Jay A J, Morris V J, Correia I S. Structures and properties of gellan polymers produced by Sphingomonas paucimobilis ATCC 31461 from lactose compared with those produced from glucose and from cheese whey. Appl Environ Microbiol. 1999;65:2485–2491. doi: 10.1128/aem.65.6.2485-2491.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figurski D, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederickson J K, Balkwill D L, Drake G R, Romine M F, Ringelberg D B, White D C. Aromatic-degrading Sphingomonas isolates from the deep subsurface. Appl Environ Microbiol. 1995;61:1917–1922. doi: 10.1128/aem.61.5.1917-1922.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fürste J P, Pansegran W, Frank R, Blöcker H, Sholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg J B, Ohman D E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984;158:1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 21.Jolly L, Wu S, vanHeijenoort J, Lencastre H, Mengin-Lecreulx D, Tomasz A. The femR315 gene from Staphylococcus aureus, the interruption of which results in reduced methicillin resistance, encodes a phosphoglucosamine mutase. J Bacteriol. 1997;179:5321–5325. doi: 10.1128/jb.179.17.5321-5325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi J G, Handler P. Phosphoglucomutase. I. Purification and properties of phosphoglucomutase from Escherichia coli. J Biol Chem. 1964;239:2741–2751. [PubMed] [Google Scholar]
- 23.Kang, K. S., and G. T. Veeder. October 1981. U.S. patent 4,377,636.
- 24.Köplin R, Arnold W, Hötte B, Simon R, Wang G, Pühler A. Genetics of xanthan production in Xanthomonas campestris: the xanA and xanB genes are involved in UDP-glucose and GDP-mannose biosynthesis. J Bacteriol. 1992;174:191–199. doi: 10.1128/jb.174.1.191-199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvam C, Olsvik E S, McKinley-McKee J, Saether O. Studies on recombinant Acetobacter xylinum α-phosphoglucomutase. Biochem J. 1997;326:197–203. doi: 10.1042/bj3260197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitão J H, Fialho A M, Correia I S. Effects of growth temperature on alginate synthesis and enzymes in Pseudomonas aeruginosa variants. J Gen Microbiol. 1992;138:605–610. doi: 10.1099/00221287-138-3-605. [DOI] [PubMed] [Google Scholar]
- 27.Lu M, Kleckner N. Molecular cloning and characterization of the pgm gene encoding phosphoglucomutase of Escherichia coli. J Bacteriol. 1994;176:5847–5851. doi: 10.1128/jb.176.18.5847-5851.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marolda C L, Valvano M A. Identification, expression, and DNA sequence of the GDP-mannose biosynthesis genes encoded by the O7 rfb gene cluster of strain VW187. J Bacteriol. 1993;175:148–158. doi: 10.1128/jb.175.1.148-158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins L O, Correia I S. Gellan gum biosynthetic enzymes in producing and nonproducing variants of Pseudomonas elodea. Biotechnol Appl Biochem. 1991;14:357–364. [PubMed] [Google Scholar]
- 30.May T B, Chakrabarty A M. Pseudomonas aeruginosa: genes and enzymes of alginate biosynthesis. Trends Microbiol. 1994;2:151–157. doi: 10.1016/0966-842x(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 31.Mikolajczak M J, Thorne L, Pollock T J, Armentrout R W. Sphinganase, a new endoglycanase that cleaves specific members of the gellan family of polysaccharides. Appl Environ Microbiol. 1994;60:402–407. doi: 10.1128/aem.60.2.402-407.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milstein C P, Clarke J B, Britton H G. The reactive serine residue in phosphoglucomutase of Micrococcus lysodeikticus. Biochem J. 1973;135:551–553. doi: 10.1042/bj1350551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyauchi K, Suh S-K, Nagata Y, Takagi M. Cloning and sequencing of a 2,5-dichlorohydroquinone reductive dehalogenase gene whose product is involved in degradation of γ-hexachlorocyclohexane by Sphingomonas paucimobilis. J Bacteriol. 1998;180:1354–1359. doi: 10.1128/jb.180.6.1354-1359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moorhouse R. Structure/property relationships of a family of microbial polysaccharides. In: Yalpani M, editor. Industrial polysaccharides: genetic engineering, structure, structure/property relations and applications. Amsterdam, The Netherlands: Elsevier; 1987. pp. 187–206. [Google Scholar]
- 35.Nishikawa S, Sonoki T, Kasahara T, Obi T, Kubota S, Kawai S, Morohoshi N, Katayama Y. Cloning and sequencing of the Sphingomonas (Pseudomonas) paucimobilis gene essential for the O demthylation of vanillate and syringate. Appl Environ Microbiol. 1998;64:836–842. doi: 10.1128/aem.64.3.836-842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock T J. Gellan-related polysaccharides and the genus Sphingomonas. J Gen Microbiol. 1993;139:1939–1945. [Google Scholar]
- 37.Popova T N, Matasova L V, Lapot'ko A A. Purification, separation and characterization of phosphoglucomutase and phosphomannomutase from maize leaves. Biochem Mol Biol Int. 1998;46:461–470. doi: 10.1080/15216549800203982. [DOI] [PubMed] [Google Scholar]
- 38.Raleigh E A, Murray N E, Revel H, Blumenthal R M, Westaway D, Reith A D, Rigby P W J, Ehai J, Hanahan D. McrA and Mcrb restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988;16:1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray W J, Jr, Hermodson M A, Puvathingal J M, Mahoney W C. The complete amino acid sequence of rabbit muscle phosphoglucomutase. J Biol Chem. 1983;258:9166–9174. [PubMed] [Google Scholar]
- 40.Rhyu G I, Ray W J, Jr, Markley J L. Active-site serine phosphate and histidine residues of phosphoglucomutase: pH titration studies monitored by 1H and 31P NMR spectroscopy. Biochemistry. 1985;24:4746–4753. doi: 10.1021/bi00339a006. [DOI] [PubMed] [Google Scholar]
- 41.Rich J J, Willis D K. Multiple loci of Pseudomonas syringae pv. syringae are involved in pathogenicity on bean: restoration of one lesion-deficient mutant requires two tRNA genes. J Bacteriol. 1997;179:2247–2258. doi: 10.1128/jb.179.7.2247-2258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sá-Correia I, Darzins A, Wang S-K, Berry A, Chakrabarty A M. Alginate biosynthetic enzymes in mucoid and nonmucoid Pseudomonas aeruginosa: overproduction of phosphomannose isomerase, phosphomannomutase, and GDP-mannose pyrophosphorylase by overexpression of the phosphomannose isomerase (pmi) gene. J Bacteriol. 1987;169:3224–3231. doi: 10.1128/jb.169.7.3224-3231.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 44.Sandlin R C, Stein D C. Role of phosphoglucomutase in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. J Bacteriol. 1994;176:2930–2937. doi: 10.1128/jb.176.10.2930-2937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevenson G, Andrianopoulos K, Hobbs M, Reeves P R. Organization of the Escherichia coli K-12 gene cluster responsible for the production of the extracellular polysaccharide colanic acid. J Bacteriol. 1996;178:4885–4893. doi: 10.1128/jb.178.16.4885-4893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 47.Yabuuchi E, Yano I, Oyaizu H, Hashimoto Y, Ezaki T, Yamamoto H. Proposals of Sphingomonas gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov. Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus. Microbiol Immunol. 1990;34:99–119. doi: 10.1111/j.1348-0421.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 48.Yamazaki M, Thorne L, Mikolajczak M, Armentrout R W, Pollock T J. Linkage of genes essential for synthesis of a polysaccharide capsule in Sphingomonas strain S88. J Bacteriol. 1996;178:2676–2687. doi: 10.1128/jb.178.9.2676-2687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye R W, Zielinski N A, Chakrabarty A M. Purification and characterization of phosphomannomutase/phosphoglucomutase from Pseudomonas aeruginosa involved in biosynthesis of both alginate and lipopolysaccharide. J Bacteriol. 1994;176:4851–4857. doi: 10.1128/jb.176.16.4851-4857.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou D, Stephens D S, Gibson B W, Engstrom J J, McAllister C F, Lee F K, Apicella M A. Lipooligosaccharide biosynthesis in pathogenic Neisseria. Cloning, identification, and characterization of the phosphoglucomutase gene. J Biol Chem. 1994;269:11162–11169. [PubMed] [Google Scholar]
- 51.Zielinski N A, Chakrabarty A M, Berry A. Characterization and regulation of the Pseudomonas aeruginosa algC gene encoding phosphomannomutase. J Biol Chem. 1991;266:9754–9763. [PubMed] [Google Scholar]