Abstract
Background:
The disaccharide galactose-α-1,3-galactose (alpha-gal) is expressed in mammals other than humans, apes, and old-world monkeys. In humans, elevated immunoglobulin E (IgE) antibodies specific for alpha-gal can result in allergic hypersensitivity known as alpha-gal syndrome (AGS). Case reports and series suggest that tick bites can induce alpha-gal–specific IgE (sIgE) antibodies.
Objective:
To evaluate tick exposure as a risk factor for AGS and elevated alpha-gal sIgE level.
Methods:
We conducted a case-control study comparing patients with AGS from a North Carolina allergy clinic with controls who were patients at a nearby internal medicine clinic. Cases and controls were administered a questionnaire to obtain information about demographics, home environment, outdoor activities, and recollection of tick bite. Serum samples taken at the time of enrollment were tested for total IgE, alpha-gal sIgE, and antibodies to other tick-borne pathogens.
Results:
The patients with AGS were more likely to recall finding a tick on themselves (odds ratio [OR], 11.20; 95% confidence interval [CI], 4.97–25.15), live near wooded forest (OR, 2.27; 95% CI, 0.92–5.55), and spend 17 or more hours per week outdoors in wooded areas (OR, 5.58; 95% CI, 2.56–12.19). The patients with AGS were also more likely to report 4 or more tick bites (OR, 33.05; 95% CI, 9.92–155.12) and reactions at the site of tick bites (OR, 7.93; 95% CI, 3.74–16.80). Furthermore, elevated alpha-gal sIgE level was observed in 33% of the controls and was associated with tick exposure in the controls (OR, 4.25; 95% CI, 2.21–8.18).
Conclusion:
The results define tick bite as a risk factor for AGS and elevated alpha-gal sIgE level.
Introduction
Alpha-gal syndrome (AGS) is an immunoglobulin E (IgE)-mediated allergy to the disaccharide galactose-α−1,3-galactose (alpha-gal).1 Humans, great apes, and old-world monkeys do not express alpha-gal, but it is found in all other mammals. Humans are, therefore, exposed to alpha-gal when consuming mammalian meat or other products derived from mammals, including pharmaceuticals that contain mammalian components (eg, heparin).2 The patients with AGS typically experience allergic symptoms 2 or more hours after ingestion of products containing alpha-gal, whereas injection of alpha-gal–containing pharmaceuticals results in near immediate hypersensitivity reactions.3
Allergic reactions to alpha-gal are dependent on the presence of alpha-gal–specific IgE (sIgE) antibodies.4 All immunocompetent humans have IgG and IgM antibodies against alpha-gal, which can be elicited by alpha-gal–containing bacteria in the gut microbiota. These antibodies can be beneficial in defense against viruses, bacteria, and some parasites.5 The induction of alpha-gal sIgE is likely a key event in the development of AGS. The patients with AGS have elevated serum levels of alpha-gal sIgE,4 and reactions to alpha-gal include urticaria, pruritus, and anaphylaxis, which are all typical of IgE-mediated type 1 hypersensitivity reactions.4 Furthermore, the patients with AGS often report that they had no reactions to mammalian meat or other products until the sudden onset of AGS symptoms in midlife. 6 This has led to speculation about an environmental trigger for alpha-gal sIgE production in the patients with AGS.
Reactions to alpha-gal in the United States were first noted in 2008 in patients receiving the chemotherapeutic antibody cetuximab.7 This antibody was a chimeric mouse-human IgG1 antibody that contained a glycosylation site on the murine portion that included alpha-gal.8 In 2009, it was first suggested in Australia that meat allergy could be related to tick bites.9 In 2011, it was noted that reactions to cetuximab and meat allergies were occurring in the southeastern United States in areas that overlapped the range of the lone star tick (Amblyomma americanum).10 Since then, accumulated circumstantial evidence has suggested that tick bites lead to AGS development by inducing alpha-gal sIgE.11
Most evidence linking tick bites with AGS has come from case reports and case series in which patients with AGS are asked whether they had experienced a tick bite before AGS onset.9–11 These reports are limited by the lack of control groups, the location of these subjects in areas with high tick activity, and potential recall bias, as patients may be more aware than others of an anticipated link between AGS and tick bites. Other evidence linking tick bites with AGS include demonstration that epitopes reacting with alpha-gal antibodies are found in the saliva of some tick species (A americanum and Ixodes scapularis),12 the finding that A americanum tick salivary gland extract injected into alpha-gal–deficient mice sensitizes mice to alpha-gal and increases alpha-gal sIgE level,13 and correlations between the lone star tick geographic distribution and alpha-gal sIgE-positive testing patterns.14
To better define the relationship between tick bites, the elevation of alpha-gal sIgE level, and development of AGS, we conducted a case-control study and compared tick exposure and environmental risk factors known to be associated with tick-borne disease between patients diagnosed with AGS and controls without AGS. The presence of alpha-gal sIgE in many of the healthy controls also allowed evaluation of tick bite as a risk factor for elevated alpha-gal sIgE level in the absence of AGS.
Methods
Study Design and Subject Enrollment
Case patients were individuals aged above or equal to 18 years presenting at a university-based allergy clinic for diagnosis and treatment of AGS from 2019 to 2020. The patients were approached at the end of a clinic visit for enrollment as a case. Case patients were not required to have confirmatory laboratory evidence of elevated alpha-gal sIgE level at the time of enrollment but were required to report clinical symptoms consistent with the allergy and have blood drawn at the time of visit. Patients aged above or equal to 18 years presenting at a nearby internal medicine clinic from 2019 to 2020 who did not have a history of AGS and who denied symptoms after eating beef, pork, or lamb were eligible for enrollment as a control. Before enrollment, potential case patients and controls were provided a description of the study and asked questions to confirm eligibility using a screening form. Eligible individuals completed an informed consent process (University of North Carolina Institutional Review Board #19–0938). Patients with AGS were approached sequentially by arrival in the allergy clinic regarding possible study participation, whereas controls were recruited as every sixth patient receiving phlebotomy as part of their visit. Case patients and controls were enrolled with a target case:control ratio of 1:2. After informed consent, information on medical, family, dietary, and allergy history, including tick exposure in the year before AGS onset (case patients) or enrollment (controls), were collected by a guided in-person interview, and serum was obtained for serologic testing.
Serology
On the day of enrollment, all participants had venous blood drawn into serum separator tubes. All whole blood samples were separated into serum by centrifugation within 24 hours of collection and stored at 4°C for no more than 24 hours before further processing. Total IgE and alpha-gal sIgE antibodies were measured using commercially available ImmunoCAP assays (Thermo Fisher/Phadia, Portage, Michigan) performed with the ImmunoCAP 250 instrument. The results were expressed as kilounits per liter (kU/L). Sera were assayed for sIgE to alpha-gal (O215) per manufacturer instructions, and the cutoff for a positive test result was the limit of detection, 0.1 kU/L. Serum was tested for antibodies to spotted fever group Rickettsia and Ehrlichia spp. using whole cell Rickettsia rickettsii and Ehrlichia chaffeensis as antigens. Antibodies were detected using the indirect fluorescent antibody (IFA) assay. Whole cell antigens were spotted onto glass slides as described.15 Sera were tested at an initial dilution of 1:32 and then diluted serially to end point. A fluorescein isothiocyanate-labeled goat antihuman conjugate reactive with the heavy (gamma) chains of IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland) was used at 1:150. Titers were expressed as the reciprocal of the last dilution displaying specific fluorescence. Titers above or equal to 64 were considered positive.
Data Collection
Data were abstracted from questionnaire forms and entered into a Research Electronic Data Capture database. Data collected included demographic information, patient medical history (including diagnoses of Lyme disease, Rocky Mountain spotted fever, southern tick-associated rash illness, ehrlichiosis, anaplasmosis, and babesiosis), allergy history before the onset of AGS, and reported history of any tick or chigger bites in the year before symptom onset for case patients and in the year before enrollment for controls. Participants were also asked about environmental factors that could be proxies for tick exposure such as dwelling type, property size, time spent outdoors, the presence of shrubs and brush near the home, the proximity of the home to wooded areas, and frequency of observing deer near the home. Additional data including details regarding onset and severity of AGS reactions, including whether any reaction resulted in an anaphylactic episode and whether emergency care was received, how many reactions were experienced before diagnosis, and the time-of-day reactions typically occurred were collected for the case patients. Data on exposure to specific alpha-gal–containing products and associated self-reported symptoms were also collected for the case patients.
Statistical Analysis
Descriptive statistics were calculated for each variable. Summaries of continuous variables are expressed as medians (first quartile to third quartile [Q1-Q3]) or as geometric means (GMs) and corresponding 95% confidence intervals (CIs), and summaries of categorical variables are expressed as proportions. Differences (95% CIs) were used to compare proportions. Difference in means (95% Student t CI) and medians (95% bootstrap CI) were used to compare continuous variables. The GMs (95% CIs) of alpha-gal sIgE and separately of total IgE were computed and their ratio (95% CIs) was used to compare the case patients with the controls. Comparisons for categorical data between the case patients and the controls were made with the unadjusted odds ratio (OR, 95% CI); in these computations, one-half was added to table cell frequencies when there was a zero cell.16 Receiver operating characteristic (ROC) curves were constructed, for which all alpha-gal sIgE results less than 0.1 kU/L were assigned a value of zero. Data management, analyses, and visualizations were performed using SAS version 9.4 (SAS Institute, Cary, NC, https://www.sas.com) and R version 4.0.3 software (R Foundation, Vienna, Austria, https://r-project.org).
Ethics
This study was reviewed and approved by the University of North Carolina Institutional Review Board (UNC IRB #19–0938).
Results
Patient Demographic Characteristics
In total, 82 case patients and 191 controls were enrolled during 2019 to 2020. The enrolled case patients were more likely to be college or technical school graduates and to have higher income than the controls and were less likely to report Black race or North Carolina residence (Table 1). The median age of the cases and the controls was similar (58.5 vs 54.0 years), and a smaller percentage of the case patients were female (55% vs 62%) (Table 1). All the controls reported having residency in North Carolina, whereas 30% of the case patients were from nearby states (Virginia: 10, Alabama: 4, Tennessee: 3, Maryland: 3, one each from the District of Columbia, Kentucky, New Jersey, Pennsylvania, and Rhode Island). In addition, 2% of the case patients reported Black race compared with 14% of the controls (Table 1).
Table 1.
Number of Case and Control Patients, by Demographic Characteristics
| Characteristic | Case patients, n (%) N = 82 |
Control patients, n (%) N = 191 |
OR | LCL | UCL |
|---|---|---|---|---|---|
|
| |||||
| State of residence | |||||
| North Carolina | 57 (70) | 191 (100) | — | — | — |
| Other state | 25 (30) | 0 (0) | — | — | — |
| Sex | |||||
| Female | 45 (55) | 118 (62) | 0.76 | 0.45 | 1.29 |
| Male | 36 (44) | 72 (38) | Ref | — | — |
| Median age, IQR | 58.5 (45.5–69.8) | 54.0 (43.0–65.5) | |||
| Race | |||||
| American Indian/Alaskan Native | 8 (10) | 6 (3) | 2.80 | 0.97 | 8.04 |
| Asian | 0 (0) | 3 (2) | 0.30 | 0.00 | 2.75 |
| Black | 2 (2) | 26 (14) | 0.20 | 0.04 | 0.63 |
| Native Hawaiian/Pacific Islander | 0 (0) | 2 (1) | 0.42 | 0.00 | 4.12 |
| White | 72 (88) | 151 (79) | Ref | — | — |
| Other | 0 (0) | 1 (1) | — | — | — |
| Unknown | 0 (0) | 1 (1) | — | — | — |
| Ethnicity | |||||
| Hispanic | 1 (1) | 6 (3) | 0.52 | 0.06 | 2.44 |
| Non-Hispanic | 81 (99) | 183 (96) | Ref | — | — |
| Unknown | 0 (0) | 2 (1) | — | — | — |
| Education | |||||
| College or technical school graduate and/or completed graduate school | 57 (70) | 111 (58) | 3.95 | 1.01 | 31.15 |
| HS diploma and/or some college or technical school | 23 (28) | 64 (34) | 2.79 | 0.61 | 25.00 |
| Less than HS diploma | 1 (1) | 11 (6) | Ref | — | — |
| Unknown | 1 (1) | 5 (3) | — | — | — |
| Income | |||||
| Prefer not to say | 16 (20) | 34 (18) | 2.49 | 1.02 | 6.07 |
| >$100,000 | 36 (44) | 47 (25) | 4.06 | 1.83 | 8.97 |
| $50,000–$100,000 | 20 (24) | 57 (30) | 1.86 | 0.81 | 4.28 |
| <$50,000 | 10 (12) | 53 (28) | Ref | — | — |
Abbreviations: HS, high school; IQR, interquartile range; LCL, lower confidence limit; OR, odds ratio; Ref, reference; UCL, upper confidence limit.
Tick Exposure
The case patients and the controls were asked whether they had found a tick on their body in the year before symptom onset (case patients) or past year (controls). The case patients were more likely than the controls to report finding a tick on their body (OR, 11.20; 95% CI, 4.97–25.15) (Table 2). The case patients found ticks more often than the controls and were more likely to have found embedded ticks. Furthermore, 39% of the case patients found ticks 10 or more times compared with 17% of the controls (OR, 4.20; 95% CI, 1.91–9.24) (Table 2), and 48% of the case patients found 4 or more embedded ticks compared with 9% of the controls (OR, 33.05; 95% CI, 9.92–155.12) (Table 2).
Table 2.
Number of Case and Control Patients, by History of Tick Exposure and Antibodies to Rickettsia rickettsii and Ehrlichia chaffeensis
| Characteristic | Case patients, n (%) | Control patients, n (%) | OR | LCL | UCL |
|---|---|---|---|---|---|
|
| |||||
| Tick exposure | |||||
| Found a tick on body in past year (or year before symptom onset) | 72 (88) | 90 (47) | 11.20 | 4.97 | 25.15 |
| Not found a tick on body in past year (or year before symptom onset) | 7 (9) | 98 (51) | Ref | — | — |
| Unsure about finding a tick on body in past year | 3 (4) | 3 (2) | — | — | — |
| Number of times ticks were found in past year | |||||
| 10 or more | 28 (39) | 15(17) | 4.20 | 1.91 | 9.24 |
| 7–9 | 3 (4) | 2 (2) | 3.11 | 0.62 | 18.15 |
| 4–6 | 16 (23) | 16 (18) | 2.25 | 0.97 | 5.21 |
| 1–3 | 24 (34) | 54 (62) | Ref | — | — |
| Number of ticks found biting or stuck on body | |||||
| 4 or more | 34 (48) | 8 (9) | 33.05 | 9.92 | 155.12 |
| 3 | 8 (11) | 6 (7) | 10.65 | 2.63 | 58.14 |
| 2 | 10 (14) | 20 (23) | 4.17 | 1.19 | 17.89 |
| 1 | 16 (23) | 25 (29) | 5.27 | 1.63 | 21.50 |
| 0 | 3 (4) | 28 (32) | Ref | — | — |
| Tick bite reaction | |||||
| Bites from a tick created a mark or took longer than usual to heal | 55 (67) | 20 (11) | 7.93 | 3.74 | 16.80 |
| Bites from a tick did not create a mark or take a long time to heal | 17 (21) | 49 (26) | Ref | — | — |
| Information about tick bite reaction not applicable or missing | 10 (12) | 122 (64) | — | — | — |
| Antibodies reactive to Rickettsia rickettsii and/or Ehrlichia chaffeensis | |||||
| Yes | 14 (17) | 18 (9) | 2.00 | 0.90 | 4.20 |
| No | 68 (83) | 173 (91) | Ref | — | — |
Abbreviations: LCL, lower confidence limit; OR, odds ratio; Ref, reference; UCL, upper confidence limit.
Participants with tick bite history were also asked whether any tick bites had created a mark or a reaction (including rash) or took longer than usual to heal. Case patients who recalled a tick bite were more likely to report a reaction than the controls. Specifically, 76% of the case patients with tick bite reported a reaction vs 29% of the controls (OR, 7.93; 95% CI, 3.74–16.80) (Table 2). As a surrogate measure of tick exposure, the participants were also evaluated to see whether their serum contained antibodies to the tick-borne pathogens R rickettsii and E chaffeensis. There were 17% of the case patients and 9% of the controls who were positive for antibodies (≥64 titer) to one or both pathogens (OR, 2.00; 95% CI, 0.90–4.20) (Table 2), suggesting greater exposure to ticks among the case patients with AGS.
We also inquired about environmental factors that may serve as proxies for tick exposure but be less sensitive to recall bias than the reported tick bite. The case patients were more likely than the controls to report 5 or more acres of land around their primary residence (OR, 2.90; 95% CI, 1.33–6.29), were more likely to have shrubs and brush (OR, 2.32; 95% CI, 1.19–4.48), farmland (OR, 2.05; 95% CI, 1.17–3.60), and woods around their home (OR, 2.27; 95% CI, 0.92–5.55), and to have spent 25 or more hours per week outside (OR, 2.62; 95% CI, 1.16–5.91), and 17 or more hours per week in wooded areas (OR, 5.58; 95% CI, 2.56–12.19) (Table 3). Most case patients reported onset of AGS symptoms from April to September with a peak in June, a period coinciding with the seasonality for ehrlichiosis, a disease transmitted by the lone star tick (Fig 1).17
Table 3.
Number of Case and Control Patients, by Environmental Exposures
| Exposure | Case patients, n (%) | Control patients, n (%) | OR | LCL | UCL |
|---|---|---|---|---|---|
|
| |||||
| Primary residence | |||||
| Apartment | 2 (2) | 11 (6) | 0.48 | 0.10 | 1.68 |
| Duplex | 1 (1) | 2 (1) | 1.33 | 0.14 | 8.70 |
| Townhome | 1 (1) | 4 (2) | 0.74 | 0.08 | 3.81 |
| Single family home | 78 (95) | 174 (91) | Ref | — | — |
| Amount of land around primary residence | |||||
| 5 or more acres | 34 (42) | 43 (23) | 2.90 | 1.33 | 6.29 |
| 2 to <5 acres | 13 (16) | 38 (20) | 1.25 | 0.52 | 3.05 |
| 1 to <2 acres | 13 (16) | 35 (18) | 1.36 | 0.56 | 3.33 |
| 0.5 to <1 acres | 10 (12) | 27 (14) | 1.36 | 0.52 | 3.53 |
| 0 to <0.5 acres | 12 (15) | 44 (23) | Ref | — | — |
| Not sure | 0 (0) | 4 (2) | — | — | — |
| Characteristics of land around primary residence | |||||
| Manicured grass lawn | 77 (94) | 177 (93) | 1.22 | 0.44 | 3.37 |
| No manicured grass lawn | 5 (6) | 14 (7) | Ref | — | — |
| Shrubs and brush | 69 (84) | 133 (70) | 2.32 | 1.19 | 4.48 |
| No shrubs and brush | 13 (16) | 58 (30) | Ref | — | — |
| Farmland | 30 (37) | 42 (22) | 2.05 | 1.17 | 3.60 |
| No farmland | 52 (63) | 149 (78) | Ref | — | — |
| Wooded forest | 76 (93) | 162 (85) | 2.27 | 0.92 | 5.55 |
| No wooded forest | 6 (7) | 29 (15) | Ref | — | — |
| Frequency of seeing deer near residence | |||||
| Monthly or less | 16 (20) | 55 (29) | 0.59 | 0.30 | 1.14 |
| Daily or weekly | 66 (81) | 136 (71) | Ref | — | — |
| Hours outside per week | |||||
| 25 or more | 21 (27) | 38 (20) | 2.62 | 1.16 | 5.91 |
| 19–24 | 12 (15) | 25 (13) | 2.28 | 0.91 | 5.71 |
| 13–18 | 13 (17) | 23 (12) | 2.68 | 1.08 | 6.70 |
| 7–12 | 21 (27) | 48 (25) | 2.08 | 0.93 | 4.62 |
| 0–6 | 12 (15) | 57 (30) | Ref | — | — |
| Hours outside per week in wooded areas | |||||
| 17or more | 21 (26) | 20 (11) | 5.58 | 2.56 | 12.19 |
| 13–16 | 8 (10) | 11 (6) | 3.87 | 1.41 | 10.7 |
| 9–12 | 14 (18) | 21 (11) | 3.54 | 1.55 | 8.13 |
| 5–8 | 18 (23) | 38 (20) | 2.52 | 1.20 | 5.28 |
| 0–4 | 19 (24) | 101 (53) | Ref | — | — |
Abbreviations: LCL, lower confidence limit; OR, odds ratio; Ref, reference; UCL, upper confidence limit.
Figure 1.
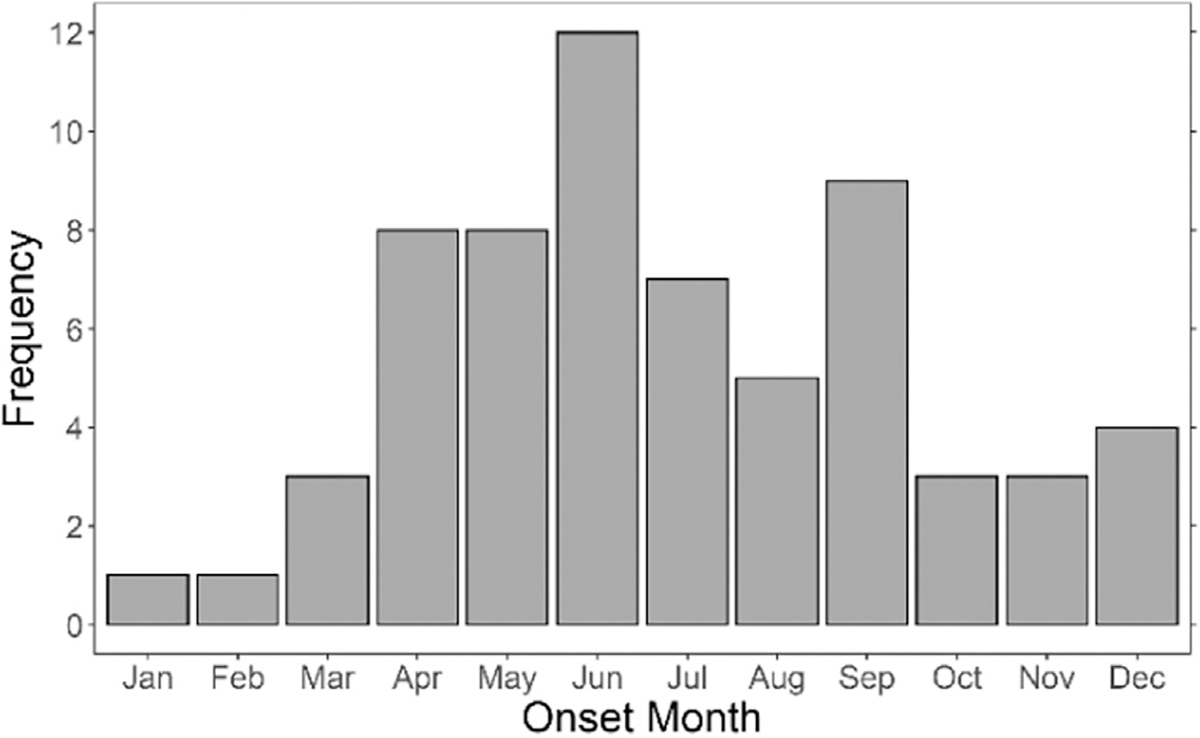
Frequency of AGS cases, by month of onset. The percentage of the case patients with AGS who reported the initial onset of AGS symptoms in each month. AGS, alpha-gal syndrome; Apr, April; Aug, August; Dec, December; Feb, February; Jan, January; Jul, July; Jun, June; Mar, March; Nov, November; Oct, October; Sep, September.
Presence of Immunoglobulin E Antibodies
At the time of enrollment, 98% of the case patients and 33% of the controls were positive for alpha-gal sIgE using a cutoff of 0.1 kU/L, and the GM of the positive sIgE results was higher for the case patients compared with the controls (GM = 4.0 kU/L; 95% CI, 2.7–5.9 vs GM = 0.8 kU/L; 95% CI, 0.6–1.2) (Table 4). However, there was substantial overlap in the sIgE results between the case patients and the controls (Fig 2A). Total IgE level was also higher in case patients with AGS compared with the controls (GM = 145.2 kU/L; 95% CI, 110.4–191.0 vs GM = 44.3 kU/L; 95% CI, 36.2–54.2) (Table 4), and the percentage of total IgE attributable to alpha-gal sIgE was higher in the case patients (6.7%; 95% CI, 4.8–8.7 vs 2.4%; 95% CI, 1.4–3.3) (Table 4). The ROC curve analysis determined that the cutoff value for alpha-gal sIgE that would maximize both sensitivity and specificity is 0.59 kU/L (Fig 2B). This higher cutoff value provides an empirical sensitivity of 83% and a specificity of 81%, with the area under the curve of 0.9. A higher sensitivity of 98% is obtained by using the predetermined cutoff value of 0.1 kU/L, with the specificity dropping to 67%. Many of the patients with AGS had low sIgE titers at the time of enrollment, with 2 cases below 0.1 kU/L. Raising the cutoff to 0.59 kU/L to improve specificity would exclude 17% of the cases.
Table 4.
IgE Antibody Results Among Case and Control Patients
| Antibody Testing Result | Case patients | Control patients | Comparison |
|---|---|---|---|
|
| |||
| Patients with alpha-gal sIgE > 0.1 kU/L | 98% (n = 80) | 33% (n = 63) | 65.16 (21.22, 309.66)a |
| Geo. mean of positive alpha-gal sIgE results (95% CI; range) | 4.0 kU/L (2.7–5.9; 0.11–100) | 0.8 kU/L (0.6–1.2; 0.11–100) | 4.8 (2.8, 8.0)b |
| Geo. mean of total IgE results (95% CI; range) | 145.2 kU/L (110.4–191.0; 4–4404) | 44.3 kU/L (36.2–54.2; 2–2165) | 3.3 (2.3, 4.6)b |
| Alpha-gal sIgE as a percentage of total IgE (95% CI; range) | 6.7% (4.8–8.7; 0.09–42.1) | 2.4% (1.4–3.3; 0.02–16.1) | 4.4 (2.2, 6.6)c |
Abbreviations: CI, confidence interval; Geo., geometric; IgE, immunoglobulin E; sIgE, specific IgE.
Odds ratio with 95% CIs.
Ratio of geometric means with 95% CIs.
Difference on means with 95% CIs.
Figure 2.
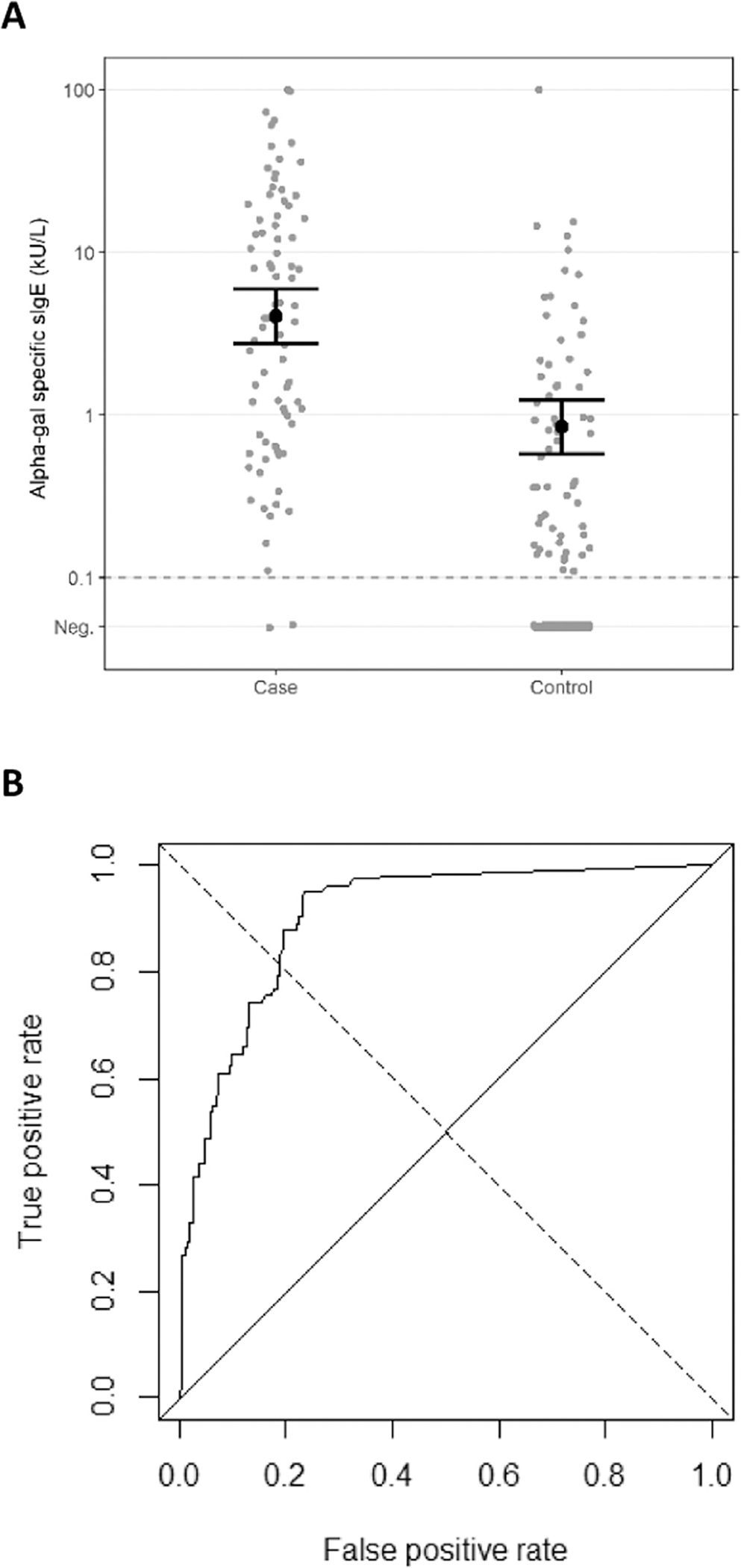
(A) Alpha-gal sIgE among the case patients and the controls. The geometric means and 95% confidence intervals are displayed. (B) ROC curve revealing alpha-gal sIgE by case-control status. The threshold is set to 0.59 kU/L to maximize both sensitivity (83%) and specificity (81%). Neg, negative; ROC, receiver operating characteristic; sIgE, specific immunoglobulin E.
The presence of alpha-gal sIgE in 33% of the controls enabled us to look at risk factors for alpha-gal sIgE in participants who did not have AGS, a group less expected to have recall bias with respect to tick bite. The control participants were presumably not aware that they were alpha-gal sIgE positive. We therefore compared sIgE-positive control participants to sIgE-negative control participants. Finding a tick in the past year was a risk factor for being alpha-gal sIgE positive (OR, 4.25; 95% CI, 2.21–8.18), but the risk did not increase with the number of times ticks were found nor with the numbers of ticks embedded (Table 5). In addition, alpha-gal sIgE-positive controls were not significantly more likely to report a reaction from a tick bite. Control participants who were alpha-gal sIgE positive were more likely to have shrubs/brush (OR, 2.38; 95% CI, 1.16–4.88) and wooded forest (OR, 4.47; 95% CI, 1.56–16.50) near their primary residence. Hours outside also increased the risk of being alpha-gal sIgE positive, with both 25 or more hours outside per week (OR, 2.49; 95% CI, 1.04–5.96) and 17 or more hours in wooded areas (OR, 2.26; 95% CI, 0.87–5.88) associated with the presence of alpha-gal sIgE antibodies.
Table 5.
Number of Control Patients Positive or Negative for Alpha-Gal sIgE Antibodies, by Tick Exposure and Environmental Characteristics
| Characteristic | sIgE+ controls, n (%) | sIgE− controls, n (%) | OR | LCL | UCL |
|---|---|---|---|---|---|
|
| |||||
| Tick exposure | |||||
| Found a tick on body in past year (or year before symptom onset) | 44 (70) | 46 (36) | 4.25 | 2.21 | 8.18 |
| No tick on body in past year (or year before symptom onset) | 18 (29) | 80 (63) | Ref | — | — |
| Unsure about finding a tick on body in past year | 1 (2) | 2 (2) | — | — | — |
| Number of times ticks were found in past year | |||||
| 10 or more | 8 (19) | 7 (16) | 1.54 | 0.50 | 4.25 |
| 7–9 | 2 (5) | 0 (0) | 6.70 | 0.65 | Inf |
| 4–6 | 10 (23) | 6 (14) | 2.25 | 0.73 | 6.90 |
| 1–3 | 23 (54) | 31 (71) | Ref | — | — |
| Number of ticks found biting or stuck on body | |||||
| 4 or more | 4 (6) | 4 (3) | 2.05 | 0.45 | 9.92 |
| 3 | 6 (10) | 0 (0) | 26.68 | 2.77 | Inf |
| 2 | 10 (16) | 10 (8) | 2.21 | 0.65 | 6.85 |
| 1 | 13 (21) | 12 (9) | 2.29 | 0.75 | 6.95 |
| 0 | 9 (14) | 19 (15) | Ref | — | — |
| Tick bite reaction | |||||
| Bites from a tick created a mark or took longer than usual to heal | 12 (19) | 8 (6) | 1.56 | 0.55 | 4.43 |
| Bites from a tick did not create a mark or take a long time to heal | 24 (38) | 25 (20) | Ref | — | — |
| Information about tick bite reaction not applicable or missing | 27 (43) | 95 (74) | — | — | — |
| Primary residence | |||||
| Apartment | 1 (2) | 10 (8) | 0.28 | 0.03 | 1.23 |
| Duplex | 1 (2) | 1 (1) | 1.94 | 0.20 | 19.10 |
| Townhome | 2 (3) | 2 (2) | 1.94 | 0.33 | 11.39 |
| Single family home | 59 (94) | 115 (90) | Ref | — | — |
| Amount of land around primary residence | |||||
| 5 or more acres | 22 (35) | 21 (16) | 6.64 | 2.36 | 18.55 |
| 2 to <5 acres | 16 (25) | 22 (17) | 4.61 | 1.59 | 13.20 |
| 1 to <2 acres | 11 (18) | 24 (19) | 2.90 | 0.97 | 8.66 |
| 0.5 to <1 acres | 6 (10) | 21 (16) | 1.81 | 0.54 | 6.11 |
| 0 to <0.5 acres | 6 (10) | 38 (30) | Ref | — | — |
| Not sure | 2 (3) | 2 (2) | — | — | — |
| Characteristics of land around primary residence | |||||
| Manicured grass lawn | 59 (94) | 118 (92) | 1.17 | 0.39 | 3.94 |
| No manicured grass lawn | 4 (6) | 10 (8) | Ref | — | — |
| Shrubs and brush | 51 (81) | 82 (64) | 2.38 | 1.16 | 4.88 |
| No shrubs and brush | 12 (19) | 46 (36) | Ref | — | — |
| Farmland | 18 (29) | 24 (19) | 1.73 | 0.86 | 3.49 |
| No farmland | 45 (71) | 104 (81) | Ref | — | — |
| Wooded forest | 60 (95) | 102 (80) | 4.47 | 1.56 | 16.50 |
| No wooded forest | 3 (5) | 26 (20) | Ref | — | — |
| Frequency of seeing deer near residence | |||||
| Monthly or less | 13 (21) | 42 (33) | 0.60 | 0.28 | 1.26 |
| Daily or weekly | 50 (79) | 86 (67) | Ref | — | — |
| Hours outside per week | |||||
| 25 or more | 17 (27) | 21 (16) | 2.49 | 1.04 | 5.96 |
| 19–24 | 6 (10) | 19 (15) | 0.97 | 0.33 | 2.86 |
| 13–18 | 10 (16) | 13 (10) | 2.36 | 0.86 | 6.51 |
| 7–12 | 16 (25) | 32 (25) | 1.54 | 0.66 | 3.57 |
| 0–6 | 14 (22) | 43 (34) | Ref | — | — |
| Hours outside per week in wooded areas | |||||
| 17 or more | 10 (16) | 10 (8) | 2.26 | 0.87 | 5.88 |
| 13–16 | 2 (3) | 9 (7) | 0.59 | 0.12 | 2.22 |
| 9–12 | 7 (11) | 14 (11) | 1.13 | 0.42 | 3.02 |
| 5–8 | 13 (21) | 25 (20) | 1.17 | 0.54 | 2.58 |
| 0–4 | 31 (49) | 70 (55) | Ref | — | — |
Abbreviations: Inf, infinity; LCL, lower confidence limit; OR, odds ratio; Ref, reference; sIgE, specific immunoglobulin E; UCL, upper confidence limit.
Discussion
This case-control study evaluated the risk factors for AGS. Neither age nor sex differed substantially between the case patients and the controls, suggesting it is not a significant predictor of AGS. We saw fewer persons of Black race in the case cohort compared with the controls; this may have been influenced by the sites of enrollment or could suggest an underlying host factor contributing to AGS development. Nonetheless, the finding is in stark contrast to other trends in food allergy, where Black race is associated with higher rates of allergy, especially to shellfish, wheat, and nuts.18
Our case-control study also provides substantial epidemiologic evidence implicating tick bite as a risk factor for AGS. The patients with AGS were more likely than the controls to report a tick bite, to have more ticks found on their bodies, and particularly to have found more embedded ticks on their bodies before developing AGS. Furthermore, the patients with AGS reported onset during times of activity of the lone star tick, an aggressive human biting tick in the southeastern United States, which is also a vector of ehrlichiosis and certain spotted fever group Rickettsia species. Deer are a critical host during the lifecycle of the lone star tick, and the presence of deer correlates with tick abundance.19 The patients with AGS were more likely than the controls to have lived in areas with deer, such as larger properties, wooded forest, and properties with shrubs and brush. Furthermore, the patients with AGS reported spending more time outdoors and were more likely to have antibodies reactive to E chaffeensis and R rickettsii, which are both tick-borne pathogens.
The most often suggested mechanism for induction of AGS by tick bite is that ticks induce alpha-gal sIgE. The data from our control group reveal a strong association between tick exposure and alpha-gal sIgE. With 33% of the controls positive for alpha-gal sIgE, we were able to look at tick bite as a risk factor for sIgE in participants who did not have AGS. Recalling a tick bite, living on larger properties, and spending time in the woods were all risk factors for alpha-gal sIgE, suggesting tick bites can cause people to develop alpha-gal antibodies even when they do not result in clinically apparent AGS—a well-recognized state referred to as sensitization. It is unknown why some people develop alpha-gal sIgE but do not present with AGS. Intrinsic factors likely play a role, but we also noted in this study that the association with multiple tick bites was not as strong in the alpha-gal sIgE-positive controls as it was with the patients with AGS. It is possible that a single tick bite may result in sensitization, but that repeated tick bites could be required for some people to develop AGS. A large local reaction at the site of the tick bite could be an initial sign of conversion from benign sensitization to clinical AGS, a point of analysis for future studies.
The high percentage of control patients with alpha-gal sIgE antibodies highlights the difficulty of using IgE antibody results alone for diagnosis. The ROC curve analysis reveals that setting the cutoff for alpha-gal sIgE positive to 0.59 kU/L will maximize the combination of sensitivity (83%) and specificity (81%), but neither is very high at this cutoff. The sensitivity can be increased by lowering the cutoff, but this will result in identifying more patients without AGS as positive. However, keeping the cutoff high at 0.59 kU/L will not only fail to identify 17% of the patients with AGS as alpha-gal sIgE positive but is also complicated by the apparent waning of alpha-gal sIgE over time. For the purpose of diagnosis, where clinical data are available, it is therefore useful to consider sera with sIgE level greater than or equal to 0.1 kU/L as positive, with a compatible clinical picture confirming the diagnosis.
Because AGS cases have been reported most often from the southeastern United States, the lone star tick (A americanum) has been most often implicated as bearing responsibility for AGS. It is the tick most likely to bite humans in the Southeast, and it is expected that most participants in our study were bitten by lone star ticks based on where they live. However, the association of lone star ticks with AGS does not rule out the involvement of other tick species. AGS exists in parts of the world where lone star ticks are not present, so it is likely that other tick species can induce AGS. In vitro studies have also found reaction to alpha-gal antibodies in tick saliva from Ixodes scapularis ticks,12 raising the potential for this species to sensitize people for AGS. Future studies in animal models and better geographic analysis of AGS may shed light on these issues.
Our study has several limitations. Recall bias was possible as patients with AGS may have been aware of the association with tick bite, which may have made them more likely to recall a tick bite. In addition, the cases were asked about tick exposures in the year before illness onset, which may have been several years before the enrollment, whereas the controls were asked about exposures in the year before the enrollment. Other unmeasurable biases were possible because of socioeconomic differences between the case and the control groups and the fact that the patients with AGS were obtained from a referral center and came from a wider geographic area than the controls.
Although tick bites may not be able to induce AGS without contribution from other factors, the present study reveals compelling epidemiologic evidence that tick bites are a critical risk factor for the development of AGS. This highlights the need for tick bite prevention, particularly the use of personal protective measures, which include use of Environmental Protection Agency–registered insect repellents, checking for ticks after spending time outdoors, and prompt removal of ticks if they are found (https://www.cdc.gov/ticks/avoid/on_people.html).20 To avoid allergic reactions, patients with AGS are placed on a diet that excludes mammalian meat and products derived from mammals. There is no clear evidence to say how long patients must maintain an avoidance diet before adding back mammalian products, but many report having to avoid meat for years. Prevention of tick bite is therefore critical to prevent long-term health effects.
Funding:
This work is funded by the US Centers for Disease Control and Prevention.
Footnotes
Disclosures: Dr Commins is on the speaker’s bureau of Genentech and receives royalties from UptoDate. All other authors have no conflicts of interest to report.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nwamara U, Kaplan MC, Mason N, Ingemi AI. A retrospective evaluation of heparin product reactions in patients with alpha-gal allergies. Ticks Tick Borne Dis. 2022;13(1): 101869. [DOI] [PubMed] [Google Scholar]
- 3.Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65(1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platts-Mills TA, Schuyler AJ, Hoyt AE, Commins SP. Delayed anaphylaxis involving IgE to galactose-alpha-1,3-galactose. Curr Allergy Asthma Rep. 2015;15(4):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159(6):1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16(7):667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364(1):8–18. [DOI] [PubMed] [Google Scholar]
- 9.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190(9):510–511. [DOI] [PubMed] [Google Scholar]
- 10.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127 (5):1286–1293.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young I, Prematunge C, Pussegoda K, Corrin T, Waddell L. Tick exposures and alpha-gal syndrome: a systematic review of the evidence. Ticks Tick Borne Dis. 2021;12(3): 101674. [DOI] [PubMed] [Google Scholar]
- 12.Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhary SK, Karim S, Iweala OI, Choudhary S, Crispell G, Sharma SR, et al. Tick salivary gland extract induces alpha-gal syndrome in alpha-gal deficient mice. Immun Inflamm Dis. 2021;9(3):984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder AM, Commins SP, Altrich ML, Wachs T, Biggerstaff BJ, Beard CB, et al. Diagnostic testing for galactose-alpha-1,3-galactose, United States, 2010 to 2018. Ann Allergy Asthma Immunol. 2021;126(4):411–416.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straily A, Stuck S, Singleton J, Brennan S, Marcum S, Condit M, et al. Antibody titers reactive with Rickettsia rickettsii in blood donors and implications for surveillance of spotted fever rickettsiosis in the United States. J Infect Dis. 2020;221(8):1371–1378. [DOI] [PubMed] [Google Scholar]
- 16.Anscombe FJ. On estimating binomial response relations. Biometrika. 1956;43(3–4):461–464. [Google Scholar]
- 17.Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Protudjer JLP, Greenhawt M, Abrams EM. Race and ethnicity and food allergy: remaining challenges. J Allergy Clin Immunol Pract. 2021;9(11):3859–3861. [DOI] [PubMed] [Google Scholar]
- 19.Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr Top Microbiol Immunol. 2007;315:289–324. [DOI] [PubMed] [Google Scholar]
- 20.Available at https://www.cdc.gov/ticks/avoid/on_people.html. Accessed December 12, 2022.


