Abstract
Four commercially available fortified sera were compared to fetal bovine serum (FBS) with regard to their ability to maintain or increase the sensitivity of the Buffalo green monkey (BGM) kidney cell line to viral infection. Nine virus strains and five wastewater samples were used. Fortified sera were comparable to FBS for the enumeration of some viruses by the plaque method and for the detection of virus in wastewater by the most-probable-number assay.
Cell culture is currently the standard method for isolating viruses in contaminated water, and the Buffalo green monkey (BGM) kidney cell line is suggested for monitoring the presence of viruses in water by this method (1, 6). A drawback of using cell culture for routine monitoring is the high cost incurred with the use of standard tissue culture practices. The major contributor to the expense of tissue culture is the use of fetal bovine serum (FBS) as an essential additive to culture media. FBS is the standard for cultivating BGM cells, and it has been demonstrated that this cell line is optimized for viral infection when FBS is used in the culture medium (3). Alternatives that could replace FBS in BGM tissue culture medium might greatly reduce the cost of monitoring viruses in the environment.
Commercially available substitutes for FBS called fortified sera contain low amounts of FBS that have been supplemented with growth factors and nutrients known to be essential for cell growth. Fortified serum has been used to cultivate BGM cells for the titration of viruses by the plaque-forming method, and results comparable to those obtained with FBS have been reported (2). Currently, the most-probable-number (MPN) method is the standard for evaluating potable water for viral contamination; fortified sera have not been evaluated by this method (1). In addition, many new alternatives on the market have not been tested.
In this study, four commercially available fortified sera were compared to FBS with regard to their ability to support BGM cell growth. The enumeration of laboratory stock viruses and environmental isolates was done by using BGM cells cultivated with each serum tested. Both the plaque-forming and MPN methods were used for virus titration. The growth rate of BGM cells with each serum was also measured.
BGM cells were cultivated in a 50/50 mixture of Dulbecco modified Eagle medium and L-15 medium supplemented with 100 mM l-glutamine (3). Cells were grown in culture for 3 months using 10% concentrations of each of the following sera: FBS (Gemini Bioproducts, Calabasas, Calif.), CPSR-1 (Sigma Chemical Co., St. Louis, Mo.), Nuserum IV (Becton Dickinson Labware, Bedford, Mass.), Serum Plus (JRH Biosciences, Inc., Lenexa, Kans.) and FetalClone III (HyClone, Logan, Utah). For growth curve and plaque assay analyses, cells were passaged between 96 and 150 times. MPN analysis was done with cells passaged between 169 and 174 times.
Poliovirus (LSC-1), poliovirus type 2, poliovirus type 3 (Sabin), coxsackievirus A7 (Russian), coxsackievirus B3 (Nancy), coxsackievirus B4 (Benschoten), coxsackievirus B5 (Faulkner), echovirus type 7 (Wallace) and echovirus type 11 (Gregory) were propagated using BGM cells grown in basal medium containing 10% FBS. Viruses were harvested after a 2- to 3-day incubation or until complete cytopathogenic effects were observed.
Samples of raw sewage and mixtures of primary and secondary sludge were collected from the Durham, N.H., wastewater treatment plant. Additional sludge samples were obtained from an East Coast wastewater treatment plant. Virus concentration was done on the day of collection when possible by organic flocculation and centrifugation (1, 6).
The growth rate of BGM cells supplemented with each serum type was measured. Twenty Nunclon culture tubes (Becton Dickinson Labware) were seeded with 100,000 cells each for each serum test group in 2 ml of growth medium. Two tubes from each group were selected every 24 h for a period of 10 days. Monolayers were dispersed, and cell concentrations were determined using a hemocytometer (Fisher Scientific, Pittsburgh, Pa.).
The effects of serum type on BGM cell sensitivity to virus infection were determined by titration of laboratory virus strains using the serial-dilution plaque method (3). Agar overlays were prepared using 1% flake agar (Gibco), a 2% concentration of the serum being tested, medium 199 (Sigma Chemical Co.), and neutral red viability stain. Plaques were identified and counted 3 to 4 days after infection. Plaque assay data were compared by a Kruskall-Wallis analysis of variance with ranking data. Sera were ranked by a method previously described (3).
A MPN assay was done to determine serum effects on BGM cell susceptibility to infection by wild-type viruses. Wastewater and sludge grab samples were used as the sources of these viruses. A 400-μl sample of concentrated eluate was inoculated onto BGM monolayers from each serum test group. Cells were maintained in basal media containing a 2% concentration of FBS or fortified serum and were observed for cytopathogenic effects over a 14-day period. Positive flasks were passaged a second or third time for confirmation. Positive samples were each assayed in 18 T-25cm2 tissue culture flasks (Corning) for MPN determination. MPNs were calculated using the Environmental Protection Agency's (EPA's) Most Probable Number Calculator, version 1.0 (http://www.epa.gov/nerlcwww/other.htm). (EPA's Most Probable Number Calculator version 1.00) (EPA, Cincinnati, Ohio.
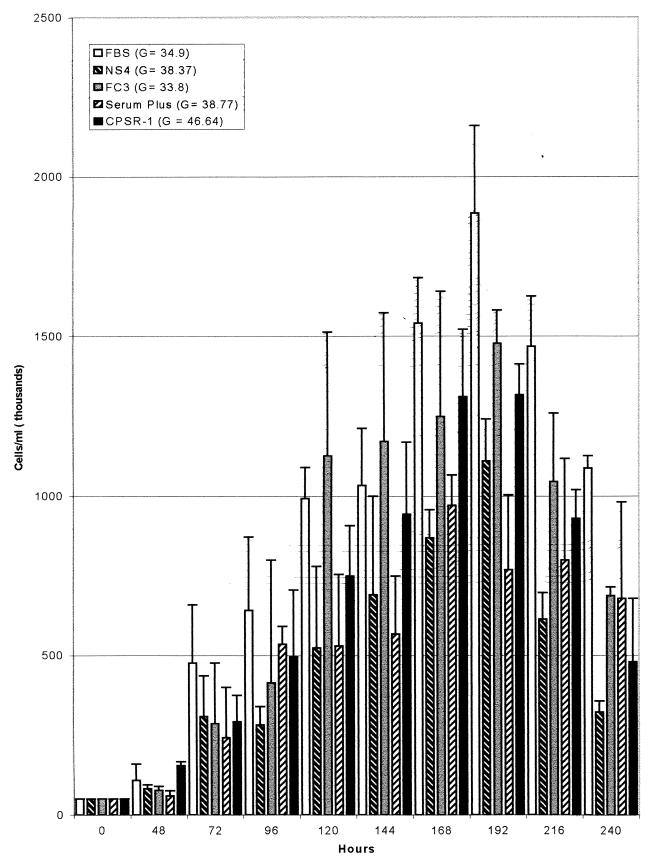
Figure 1 shows the growth rates of BGM cells cultivated with each serum. For each serum type tested, the highest cell density was achieved 192 h after seeding and the cell density declined thereafter. The greatest density of cells was observed with FBS as a medium supplement. The rates at which cell populations doubled are shown in Fig. 1.
FIG. 1.
Growth comparison of BGM kidney cells over a 10-day period. Cells were grown in basal medium supplemented with 10% FBS, Nuserum IV(Becton Dickinson Labware), FetalClone III (HyClone), Serum Plus (Serum +; JRH Biosciences, Inc.) or CPSR-1 (Sigma Chemical Co.). Error bars indicate standard deviations. G, rate (in hours) at which cell population doubled.
A comparison of sera based on plaque assay data collected from nine different viruses is displayed in Table 1. With the exception of Serum Plus, the sera tested supported long-term growth of BGM cells. Generally, none of the cultures grown with the alternative sera were found to be more sensitive to the viruses than cells cultivated with FBS. However, FetalClone III, CPSR-I, and Nuserum IV were found to be applicable for the enumeration of certain viruses by the plaque-forming method. Plaques were not visible when Serum Plus was used as a growth supplement, and therefore the serum was excluded from this study.
TABLE 1.
Average plaque counts
| Serum typea | Avg plaque count (107 PFU/ml) forb:
|
Rank | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PV1 | PV2 | PV3 | CA7 | CB3 | CB4 | CB5 | E7 | E11 | ||
| FBS | 53.8 (16.1) | 2.81 (1.0) | 18.8 (7.46) | 0.296 (0.609) | 3.38 (0.79) | 4.5 (0.94) | 19.6 (6.65) | 21 (9.49) | 14.5 (3.53) | 1.222 |
| CPSR-1 | 50.2 (15.1) | 2.96 (1.39) | 13.7 (5.03) | 0.32 (0.763) | 3.69 (0.99) | 3.49 (1.06) | 10.6 (3.0) | 4.41 (2.33) | 9.48 (2.04) | 1.666 |
| NS4 | 28.6 (5.86) | 1.19 (0.81) | 12.8 (5.29) | 0.136 (0.448) | 1.09 (0.37) | 0.52 (0.34) | 4.40 (2.1) | 18.8 (6.2) | 9.98 (2.69) | 2.333 |
| FC3 | 30.3 (9.18) | 1.54 (0.6) | 16.8 (8.39) | 0.25 (0.371) | 4.76 (1.29) | 3.28 (1.05) | 17.2 (4.17) | 9.61 (2.57) | 8.57 (3.55) | 1.777 |
| Serum + | NAc | NA | NA | NA | NA | NA | NA | NA | NA | NA |
NS4, Nuserum IV; FC3, FetalClone III; Serum +, Serum Plus.
Values in parentheses are standard deviations. PV1, poliovirus type 1; PV2, poliovirus type 2; PV3, poliovirus type 3; CA7, coxsackievirus A7 (Russian); CB3, coxsackievirus B3 (Nancy); CB4, coxsackievirus B4 (Benschoten); CB5, cocksackievirus B5 (Faulkner); E7, echovirus type 7 (Wallace); E11, echovirus type 11 (Gregory).
NA, not available (Serum Plus was not tested in this assay).
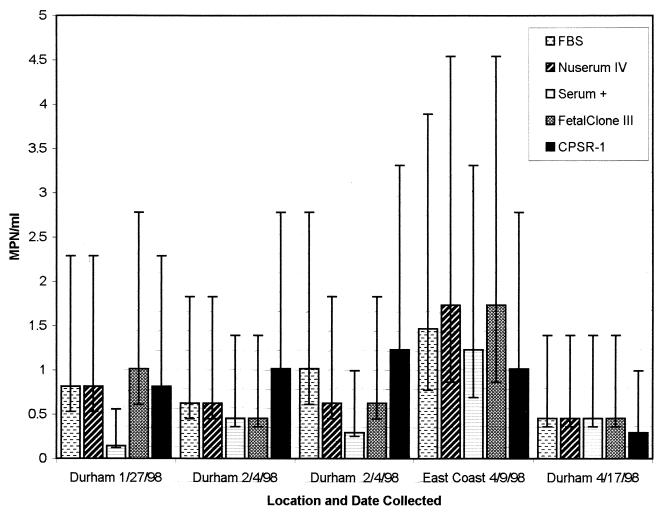
Figure 2 contains MPN values from five wastewater and sludge samples that were evaluated using BGM cells grown with each serum type. BGM cells cultivated with FBS, Nuserum IV, or FetalClone III had on average the highest MPN values for the samples tested. BGM cells grown with CPSR-1 and Serum Plus had lower average MPN values than those grown with the other sera.
FIG. 2.
A comparison of sera based on MPN values of environmental samples. MPN values are based on 18 replicate tests. The 95% confidence limits are represented by error bars. Serum +, Serum Plus.
In this study it was determined that the four fortified sera tested could support the growth of BGM cells. FetalClone III, Nuserum IV, and CPSR-I sustained the growth of BGM cells and were easily adapted to the assays described herein without modification to existing protocols. Additionally, these sera might be sufficient substitutes for FBS for growing BGM cells to enumerate certain viruses by the plaque-forming method. Serum Plus supported the growth of BGM cells only with frequent medium changes, and it was incompatible with the plaque assay. MPN analysis of environmental samples demonstrated that BGM cultures grown with FBS, Nuserum IV, FetalClone III, or CPSR-1 were equally sensitive to infection by wild-type viruses in the samples tested. An average savings of 38% was realized when serum substitutes were used in place of FBS.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 18th ed. Washington, D.C.: American Public Health Association; 1992. [Google Scholar]
- 2.Dahling D R, Wright B A. Comparison of fortified calf serum, serum substitutes and fetal bovine serum with or without extenders for propagation of cell cultures for virus plaque assays. J Virol Methods. 1990;27:287–294. doi: 10.1016/0166-0934(90)90097-y. [DOI] [PubMed] [Google Scholar]
- 3.Dahling D R, Wright B A. Optimization of the BGM cell line culture and viral assay procedures for monitoring viruses in the environment. Appl Environ Microbiol. 1986;51:790–812. doi: 10.1128/aem.51.4.790-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilgen M, Wegmuller B, Burkhalter P, Buhler H P, Muller U, Luthy J, Candrian U. Reverse transcription PCR to detect enteroviruses in surface water. Appl Environ Microbiol. 1995;61:1226–1231. doi: 10.1128/aem.61.4.1226-1231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleaves C A, Rice D H, Meyers J D. Use of serum substitutes for the growth of four cell lines commonly used for virus isolation. J Virol Methods. 1990;28:171–178. doi: 10.1016/0166-0934(90)90032-b. [DOI] [PubMed] [Google Scholar]
- 6.Messer J W, Fout G S, Schaefer III F W, Dahling D R, Stetler R E. ICR microbial laboratory manual. U.S. Cincinnati, Ohio: Environmental Protection Agency; 1995. [Google Scholar]