Abstract
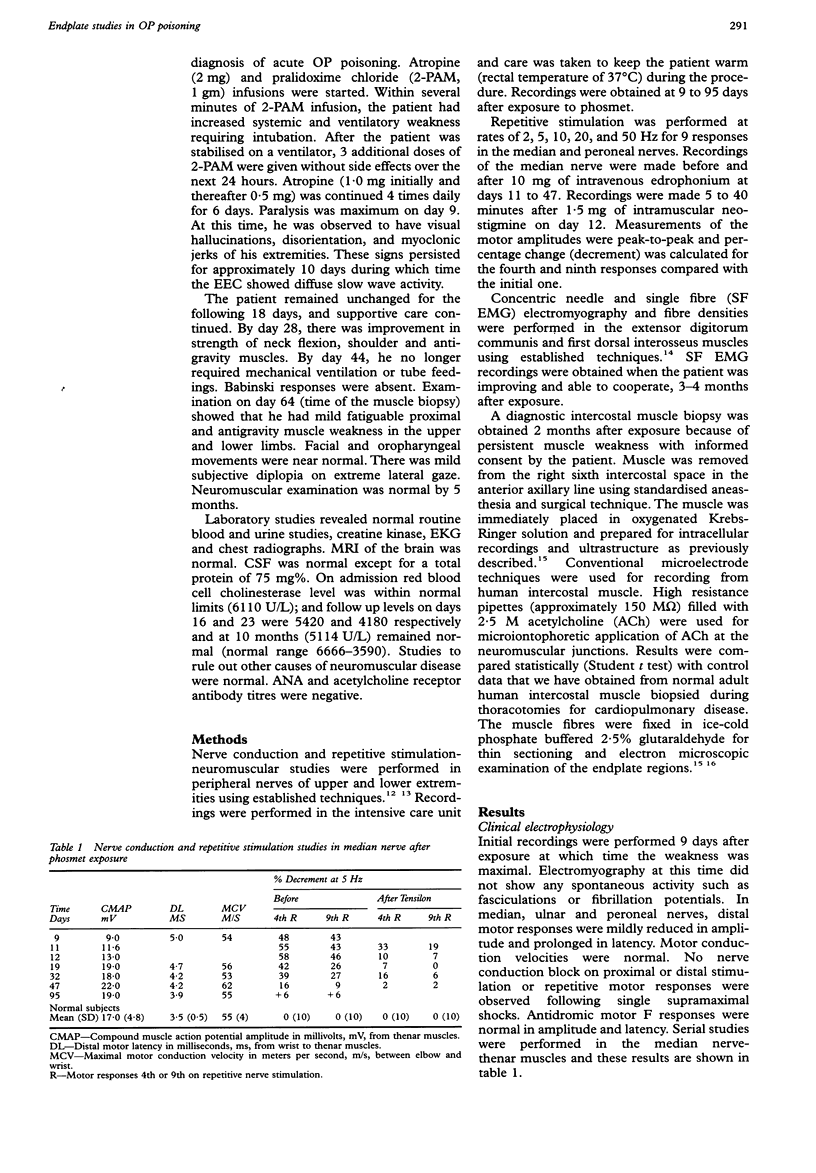
A 51 year old man developed progressive cranial and proximal muscle weakness, hyperreflexia and mental change. The disorder progressed over 9 days following the fifth weekly spraying with the organophosphate (OP) insecticide, phosmet, with limited symptoms of acute toxicity. Marked decremental responses of 50-80% on slow and fast rates of stimulation were improved to 15% by edrophonium or neostigmine. Intracellular recordings at the endplate region of intercostal muscle revealed small miniature endplate potentials (mepps), reduced mean acetylcholine sensitivity and normal membrane potentials. Electronmicroscopy revealed degeneration and regeneration of the endplates. This study demonstrates that OP poisoning due to phosmet can produce a subacute postsynaptic neuromuscular syndrome without marked symptoms of acute toxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Donia M. B., Lapadula D. M. Mechanisms of organophosphorus ester-induced delayed neurotoxicity: type I and type II. Annu Rev Pharmacol Toxicol. 1990;30:405–440. doi: 10.1146/annurev.pa.30.040190.002201. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Aracava Y., Cintra W. M., Brossi A., Schönenberger B., Deshpande S. S. Structure-activity relationship of reversible cholinesterase inhibitors: activation, channel blockade and stereospecificity of the nicotinic acetylcholine receptor-ion channel complex. Braz J Med Biol Res. 1988;21(6):1173–1196. [PubMed] [Google Scholar]
- Albuquerque E. X., Rash J. E., Mayer R. F., Satterfield J. R. An electrophysiological and morphological study of the neuromuscular junction in patients with myasthenia gravis. Exp Neurol. 1976 Jun;51(3):536–563. doi: 10.1016/0014-4886(76)90179-5. [DOI] [PubMed] [Google Scholar]
- Alkondon M., Albuquerque E. X. The nonoxime bispyridinium compound SAD-128 alters the kinetic properties of the nicotinic acetylcholine receptor ion channel: a possible mechanism for antidotal effects. J Pharmacol Exp Ther. 1989 Sep;250(3):842–852. [PubMed] [Google Scholar]
- Besser R., Gutmann L., Dillmann U., Weilemann L. S., Hopf H. C. End-plate dysfunction in acute organophosphate intoxication. Neurology. 1989 Apr;39(4):561–567. doi: 10.1212/wnl.39.4.561. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. End-plate currents and acetylcholine noise at normal and myasthenic human end-plates. J Physiol. 1979 Feb;287:247–265. doi: 10.1113/jphysiol.1979.sp012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek W., Grahl R., Schmidt R. A comparative study of guanine N7-alkylation in mice in vivo by the organophosphorus insecticides trichlorphon, dimethoate, phosmet and bromophos. Acta Pharmacol Toxicol (Copenh) 1984 Aug;55(2):104–109. doi: 10.1111/j.1600-0773.1984.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Hudson C. S., Rash J. E., Tiedt T. N., Albuquerque E. X. Neostigmine-induced alterations at the mammalian neuromuscular junction. II. Ultrastructure. J Pharmacol Exp Ther. 1978 May;205(2):340–356. [PubMed] [Google Scholar]
- Leonard J. P., Salpeter M. M. Agonist-induced myopathy at the neuromuscular junction is mediated by calcium. J Cell Biol. 1979 Sep;82(3):811–819. doi: 10.1083/jcb.82.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER R. F. NERVE CONDUCTION STUDIES IN MAN. Neurology. 1963 Dec;13:1021–1030. doi: 10.1212/wnl.13.12.1021. [DOI] [PubMed] [Google Scholar]
- MacNeil J. D., Hikichi M. Phosmet residues in an orchard and adjacent recreational area. J Environ Sci Health B. 1986 Oct;21(5):375–385. doi: 10.1080/03601238609372531. [DOI] [PubMed] [Google Scholar]
- Maselli R., Jacobsen J. H., Spire J. P. Edrophonium: an aid in the diagnosis of acute organophosphate poisoning. Ann Neurol. 1986 May;19(5):508–510. doi: 10.1002/ana.410190517. [DOI] [PubMed] [Google Scholar]
- Namba T., Nolte C. T., Jackrel J., Grob D. Poisoning due to organophosphate insecticides. Acute and chronic manifestations. Am J Med. 1971 Apr;50(4):475–492. doi: 10.1016/0002-9343(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Pascuzzo G. J., Akaike A., Maleque M. A., Shaw K. P., Aronstam R. S., Rickett D. L., Albuquerque E. X. The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. I. Agonist, desensitizing, and binding properties. Mol Pharmacol. 1984 Jan;25(1):92–101. [PubMed] [Google Scholar]
- Rash J. E., Albuquerque E. X., Hudson C. S., Mayer R. F., Satterfield J. R. Studies of human myasthenia gravis: electrophysiological and ultrastructural evidence compatible with antibody attachment to acetylcholine receptor complex. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4584–4588. doi: 10.1073/pnas.73.12.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. W., Overstreet D. H. Mechanisms underlying sensitivity to organophosphorus anticholinesterase compounds. Prog Neurobiol. 1987;28(2):97–129. doi: 10.1016/0301-0082(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Sawada Y., Nishide K., Sadamitsu D., Yoshioka T., Sugimoto T., Nishii S., Kishi H. Delayed neurotoxicity produced by an organophosphorus compound (Sumithion). A case report. Arch Toxicol. 1984 Dec;56(2):136–138. doi: 10.1007/BF00349087. [DOI] [PubMed] [Google Scholar]
- Senanayake N., Karalliedde L. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med. 1987 Mar 26;316(13):761–763. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- Tiedt T. N., Albuquerque E. X., Hudson C. S., Rash J. E. Neostigmine-induced alterations at the mammalian neuromuscular junction. I. Muscle contraction and electrophysiology. J Pharmacol Exp Ther. 1978 May;205(2):326–339. [PubMed] [Google Scholar]
- Wadia R. S., Chitra S., Amin R. B., Kiwalkar R. S., Sardesai H. V. Electrophysiological studies in acute organophosphate poisoning. J Neurol Neurosurg Psychiatry. 1987 Nov;50(11):1442–1448. doi: 10.1136/jnnp.50.11.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia R. S., Sadagopan C., Amin R. B., Sardesai H. V. Neurological manifestations of organophosphorous insecticide poisoning. J Neurol Neurosurg Psychiatry. 1974 Jul;37(7):841–847. doi: 10.1136/jnnp.37.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]



