Abstract
In orthopedics and dentistry there is an increasing need for novel biomaterials and clinical strategies to achieve predictable bone regeneration. These novel molecular strategies have the potential to eliminate the limitations of currently available approaches. Specifically, they have the potential to reduce or eliminate the need to harvest autogenous bone, and the overall complexity of the clinical procedures. In this review, emerging tissue engineering strategies that have been, or are currently being, developed based on the current understanding of bone biology, development and wound healing will be discussed. In particular, protein/peptide based approaches, DNA/RNA therapeutics, cell therapy, and the use of exosomes will be briefly covered. The review ends with a summary of the current status of these approaches, their clinical translational potentials and their challenges.
Keywords: tissue engineering, bone regeneration, growth factors, gene therapy, exosomes
1. Introduction
Bone is a sophisticated connective tissue that primarily comprises a mineralized organic matrix with remaining contributions from organic components and water. Bone protects the internal organs from external forces and acts as an effective reservoir of key minerals such as calcium and phosphorus that are involved in a multitude of functions in the human body. In addition, native bone cells secrete hormones such as fibroblast growth factor 23 (FGF23) that regulate phosphorous excretion, also making bone an endocrine organ [1]. Further, bone houses the red bone marrow, where hematopoiesis occurs. In humans, bone develops by one of the following two mechanisms: intramembranous or endochondral ossification. In the intramembranous bone formation, the mesenchymal stem cells (MSCs) consolidate and directly differentiate into osteoblasts which will lay down osteoid (composed primarily of type I collagen and other ground substances), that mineralizes first to woven bone and eventually matures into lamellar bone. Flat bones such as bones of the skull, scapula and clavicle develop by intramembranous bone formation. In contrast, in the endochondral bone formation process, the MSCs differentiate first to chondroblasts which generates a cartilaginous analogue of the future bone, which will then be replaced by bone. Most of the long bones in the human body develop by this latter mechanism [2].
Bone modeling is the process by which the bone gets its form and shape. In this process, bone formation is uncoupled, meaning the bone formation and resorption occur at different sites as independent processes. This occurs primarily in childhood but continues throughout the lifetime of an individual. The net effect of bone modeling is an increase in bone mass and attainment of specific shapes [3]. In contrast, bone remodeling is the process that occurs throughout the life time of an individual and in this case, the resorption and formation are coupled, interconnected and occur at the same site. This process actively remodels 2%–5% of the cortical bone every year [4], [5]. Though bone resorption and formation are balanced initially, with age, the resorption dominates, with a net decrease in bone mass. In conditions such as osteoporosis, the resorption is even more pronounced making the individual prone to fractures. Both in modeling and remodeling, key bone cells such as osteoblasts, osteoclasts and osteocytes all play a significant role, orchestrating the events [3], [6].
In addition to conditions such as osteoporosis where the bone formation–resorption balance is affected, there are a myriad of medical and dental conditions such as trauma, malignancy or periodontal disease, where bone loss is imminent. Loss of bone, depending on the condition, can affect humans in several ways, ranging from increasing the fracture tendency to loss of a tooth or multiple teeth with associated social and economic implications. Though bone has significant regenerative potential, surgical intervention and use of a bone replacement grant is warranted in defects that cannot heal on their own. Throughout the world, a significant number of such procedures are done in medicine and dentistry that involve the use of bone replacement grafts. In fact, next to blood, bone is the second most transplanted tissue in humans and according to one estimate, over 2.3 billion dollars were attributed to bone replacement grafts in the United States in the year 2015 and is expected to grow beyond 3.5 billion dollars by 2024 [7]. In dentistry, with the growing number of dental implants being placed globally, the need to predictably regenerate bone has also increased substantially [8].
The inherent limitations of using autogenous bone (from the same patient) are the need to harvest bone from a second surgical site (and its associated morbidity) and the lack of availability. The alternatives to autogenous grafts include allografts, xenografts and alloplasts and they come with their own set of limitations and barriers, including the lack of inherent osteogenic potential leading to unpredictability in attaining clinical outcomes. In addition, in spite of stringent quality and infection control followed by the tissue banks, a small possibility of infection from the use of allografts cannot be completely ruled out [9].
2. Bone tissue engineering
Tissue engineering is an interdisciplinary field where the principles of engineering and life sciences are applied with the main objective of developing biological substitutes that can restore, maintain, or improve tissue function [10]. In order to engineer a tissue, it is critical to understand the biology of the tissue from two different perspectives—development and wound healing. A thorough understanding of the components and key players in the above-mentioned processes has enabled scientists to develop materials and strategies with the potential to achieve specific objectives. This process of going back to nature and being inspired by biology and bringing the time-tested processes to the lab is termed ‘biomimicry’ and the resulting biomaterials termed ‘biomimetic materials’. As of now, the majority of studies that took the biomimetic approach have utilized the deconstructive approach, in which the individual players in a particular process are first identified, followed by elucidation of the functions of each of these players. The major challenge with this approach that focuses on only one entity at a time is to finally stitch all the gained information from these separate investigations together to come up with an all-encompassing strategy that can enhance the outcome. In the following sections, select tissue engineering strategies are discussed broadly (figure 1), in the context of bone regeneration. The advantages and limitations of each of the approaches described in this review are summarized in table 1.
Figure 1.
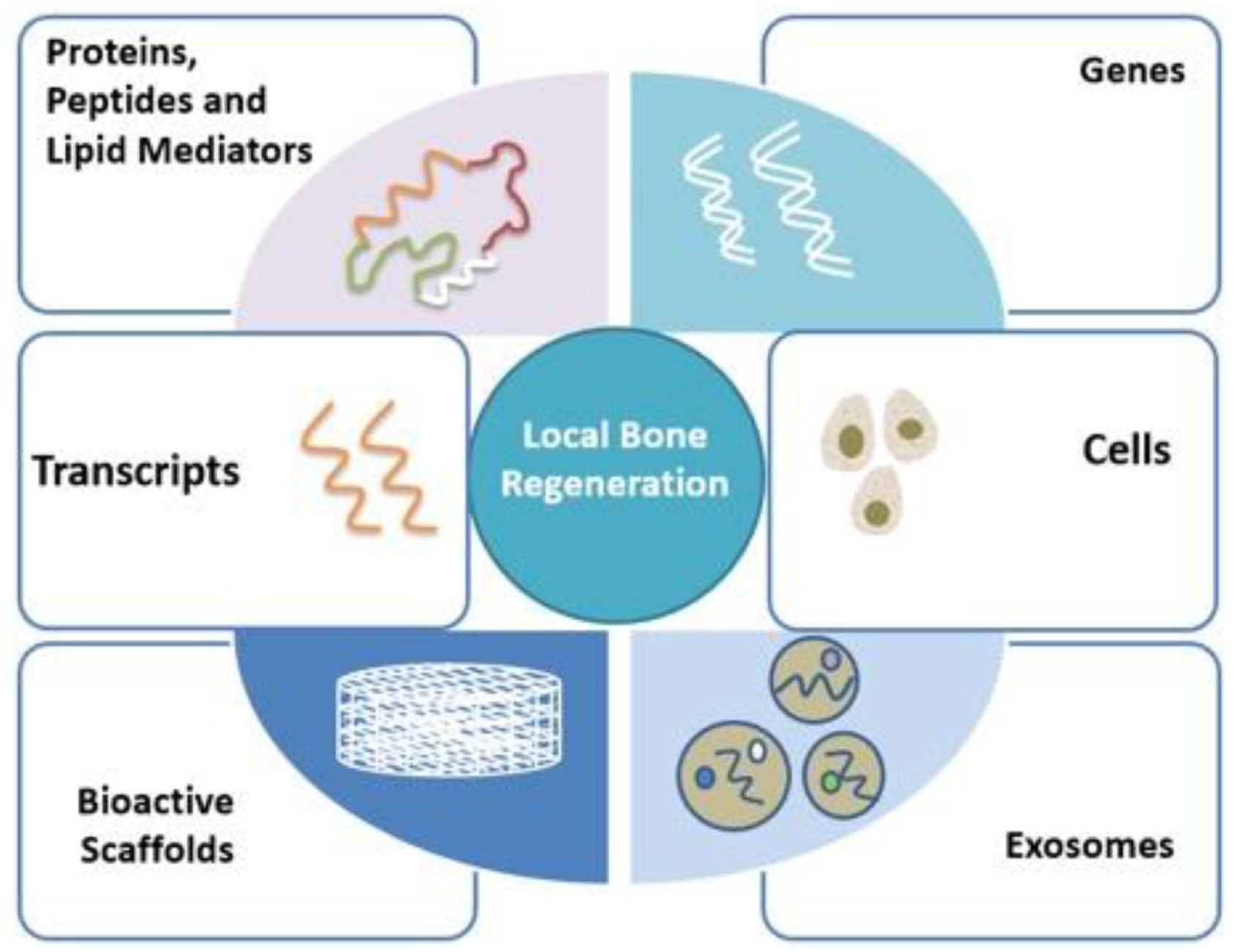
Schematic depicting the locally deliverable tissue engineering approaches for bone regeneration.
Table 1.
Summary of key advantages and limitations of the approaches described in this review.
| Approach | Advantages | Limitations |
|---|---|---|
| Protein-based therapeutics |
|
|
| Gene-based therapeutics viral approaches |
|
|
| Non-viral approaches |
|
|
| Transcript-based approaches |
|
|
| Cell-based approaches |
|
|
| Bioactive scaffolds |
|
|
| Exosomes |
|
|
2.1. Protein- and lipid-based approaches
Bone regeneration is a complex and dynamic process in which several players are involved. Apart from cells and matrix/scaffolds, numerous proteins in the form of multifunctional cytokines and growth factors play their definite roles at specific time points in an organized spatiotemporal fashion. BMPs are one such group of growth factors that belong to the TGF-β superfamily and play an important role in bone formation and bone homeostasis in adults. Though there are approximately 20 different BMPs that have been identified to date, BMP-2 is the most studied and tested for its bone regeneration potential. After the demonstration of bone regenerative capacity of BMP-2 in preclinical studies, several human clinical trials that followed evaluated its clinical efficacy in humans [11], [12]. Currently, recombinant human BMP-2(rhBMP-2) is cleared by the food and drug administration (FDA) of U.S.A for sinus augmentation and ridge preservation indications after its safety and efficacy were demonstrated in clinical trials. The major limitation with the use of rhBMP-2 is the high cost associated with its production. Additionally, in order to compensate for the reduced bioavailability of rhBMP-2 at the target site (due to proteolysis mediated rapid clearance), it is employed in supraphysiological doses, raising safety concerns. Though not reported in dentistry, several adverse effects including death from respiratory difficulty related to postoperative inflammation and edema were reported in the orthopedic field [13], [14]. It is also important to note that in vitro, rhBMP-2 increased the invasiveness and metastatic nature of oral squamous cell carcinoma cell lines [15].
Apart from rhBMP-2, several other proteins and peptides have been explored in preclinical and human clinical research for their role in expediting fracture healing and bone regeneration. Table 2 summarizes some of the explored protein and peptide agents. Another attractive strategy that is currently being explored is antibody mediated osseous regeneration (AMOR). The idea behind this is to immobilize anti-BMP-2 antibodies in the scaffold that could capture endogenous BMP-2 protein molecules at the implant site in vivo and enhance bone regeneration. Few recent reports have shown promising bone regeneration outcomes using this approach in calvarial bone defects in animals [16], [17].
Table 2.
Proteins and peptides explored for their role in bone regeneration.
| Type | Explored proteins and peptides (examples) |
|---|---|
| Growth factors/morphogens | |
| Single factor [16], [17] | Erthropoietin, PDGF-BB, b-FGF, SDF-1, VEGF, wnt-3, β-catenin, BMP-2, and BMP-7. |
| Multiple factors [17] | SDF-1 + BMP-2, BMP-2 + VEGF, and BMP-2 + TGF-β. |
| Mixture of factors [18], [19] | Platelet rich plasma, platelet gels and platelet concentrates. |
| Peptides [16], [20] | PTH-Rp, calcitonin gene-related peptide, osteogenic growth peptide, thrombin peptide 508, PepGen P-15 and RGD containing peptides. |
| Antibodies and inhibitors [17] | Anti-TNF-α antibody, anti-sclerostin antibody, RANKL inhibitor. |
Abbreviations: PDGF-BB: platelet derived growth factor—BB; b-FGF: basic fibroblast growth factor; VEGF: vascular endothelial growth factor; SDF-1: stromal cell derived factor-1; TGF-β: transforming growth factor—beta; PTH-Rp: parathyroid hormone related peptide; RGD: arginine, glycine, and aspartate; TNF-α: tumor necrosis factor—alpha; RANKL: receptor activator of nuclear factor-kappa B ligand.
Apart from proteins and peptides that are currently being studied for their role as active agents of bone regeneration, endogenous lipid mediators that are involved in inflammation resolution are also currently being explored. Lipoxin A4 (LxA4), resolvin E1 (RvE1) and, more recently, benzo-lipoxin A4, a stable analog of lipoxin A, were all shown to be effective in regenerating bone in a preclinical periodontitis model [18–21]. These novel strategies underscore the importance of inflammation resolution in tissue regeneration and will play an important role in bone tissue engineering in the near future.
2.2. Gene (DNA)-based approaches
Gene therapy is the process of introducing exogenous deoxyribonucleic acid (DNA) encoding specific target proteins into cells of various tissues and converting them into protein synthesizing units. Once the DNA is taken up by cells, the DNA has to travel through the cytoplasm and enter into the nucleus, where they go through the process of DNA transcription into RNA, followed by translation of RNA to the protein of interest (in the cytoplasm) [22]. Gene therapy can be accomplished by in vivo or ex vivo approaches (figure 2). In the in vivo approach, the DNA uptake is made to occur inside the body at the target site, while, in the ex vivo approach, the target DNA is introduced first into the target cells in a controlled environment outside the body and then the cells containing the exogenous DNA are introduced into the site of interest, usually along with a scaffold/carrier. The advantage of this latter approach is that the number of cells that take up the delivered DNA molecules can be better controlled and it is targeted to specific cell types. But the downsides of this approach are higher costs and the impracticality of the approach, hampering its clinical translation.
Figure 2.
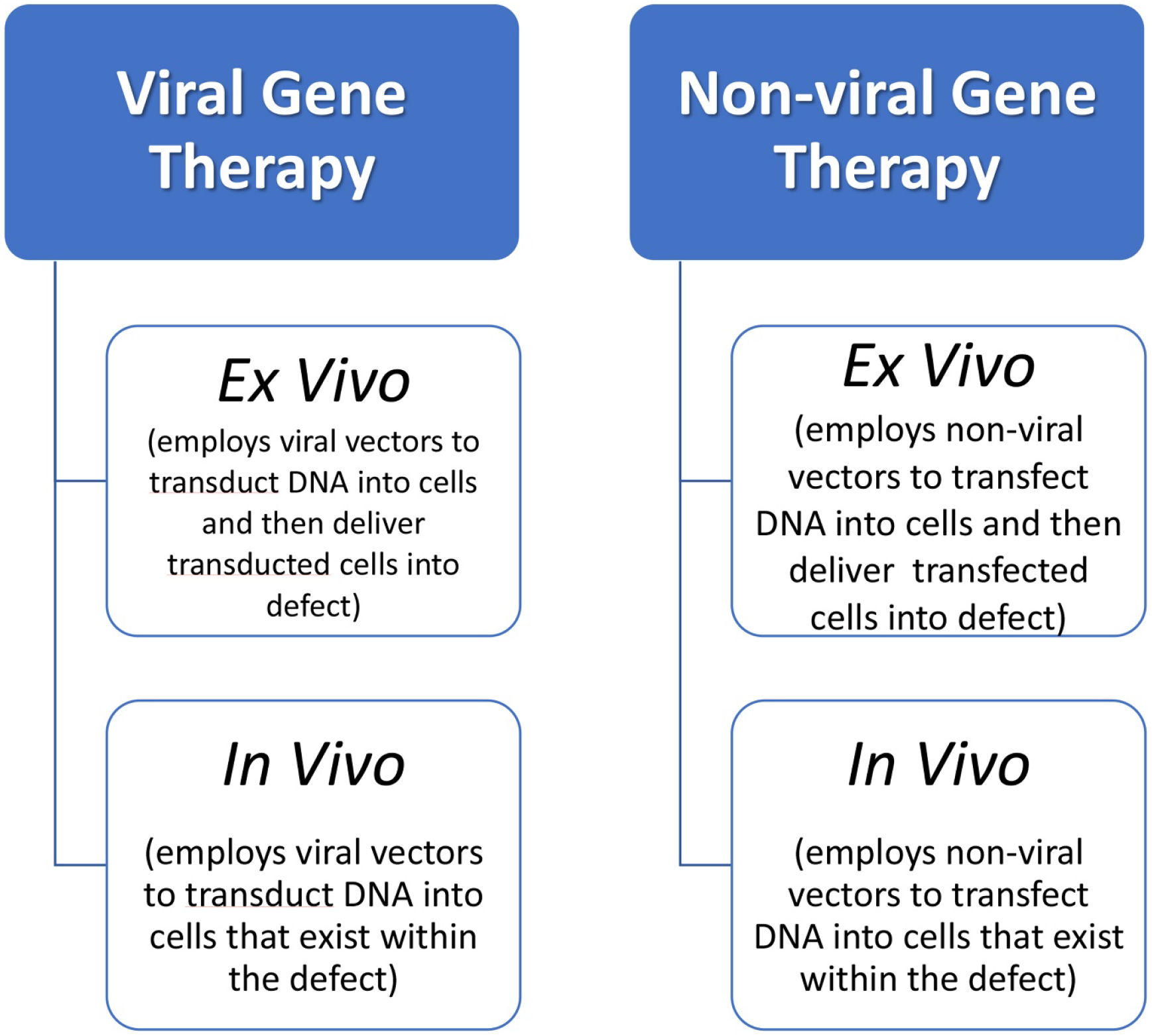
Schematic depicting the types of gene therapy approaches.
The cells can take up the DNA by itself, but to promote its uptake by cells, it is customary to complex DNA with a vehicle or vector that is often cationic to facilitate electrostatic binding with the DNA. Refer to figure 3(a) for an illustration of the underlying mechanism of nonviral gene therapy. The vectors can be of two types: viral and nonviral and, accordingly, the techniques that utilize them are termed viral and nonviral gene therapy, respectively. Viruses have the inherent ability to infect cells and viral gene therapy harnesses this innate efficient capacity of viruses to introduce the target DNA (by a process called ‘transduction’) into cells. The goal in this approach is to eliminate the pathogenicity of the virus but retain its transduction efficacy. To date, in the field of viral gene therapy, different vectors have been explored, including adenovirus, adeno-associated virus, and herpes simplex virus. To achieve bone regeneration, several studies in the past had utilized viral vectors to efficiently deliver DNA encoding growth factors or morphogens such as bone morphogenetic proteins (BMPs) [23], [24], LIM-domain proteins (LMPs) [25], Runx2 [26], and cyclooxygenase-2 [27]. A large majority of these preclinical studies utilized the ex vivo approach of gene delivery and demonstrated a good degree of successful bone regeneration. As mentioned before, though the ex vivo approach is more controlled and targeted, the cost associated with this approach will be much higher, than in vivo approaches. Preclinical studies using viral vectors encoding platelet delivered growth factor (PDGF) and employing an in vivo approach, also demonstrated enhanced bone regeneration in alveolar and peri-implant bony defects [28], [29].For detailed information on viral gene therapy for musculo-skeletal tissue engineering, the reader can refer to detailed reviews on this topic [30], [31].
Figure 3.
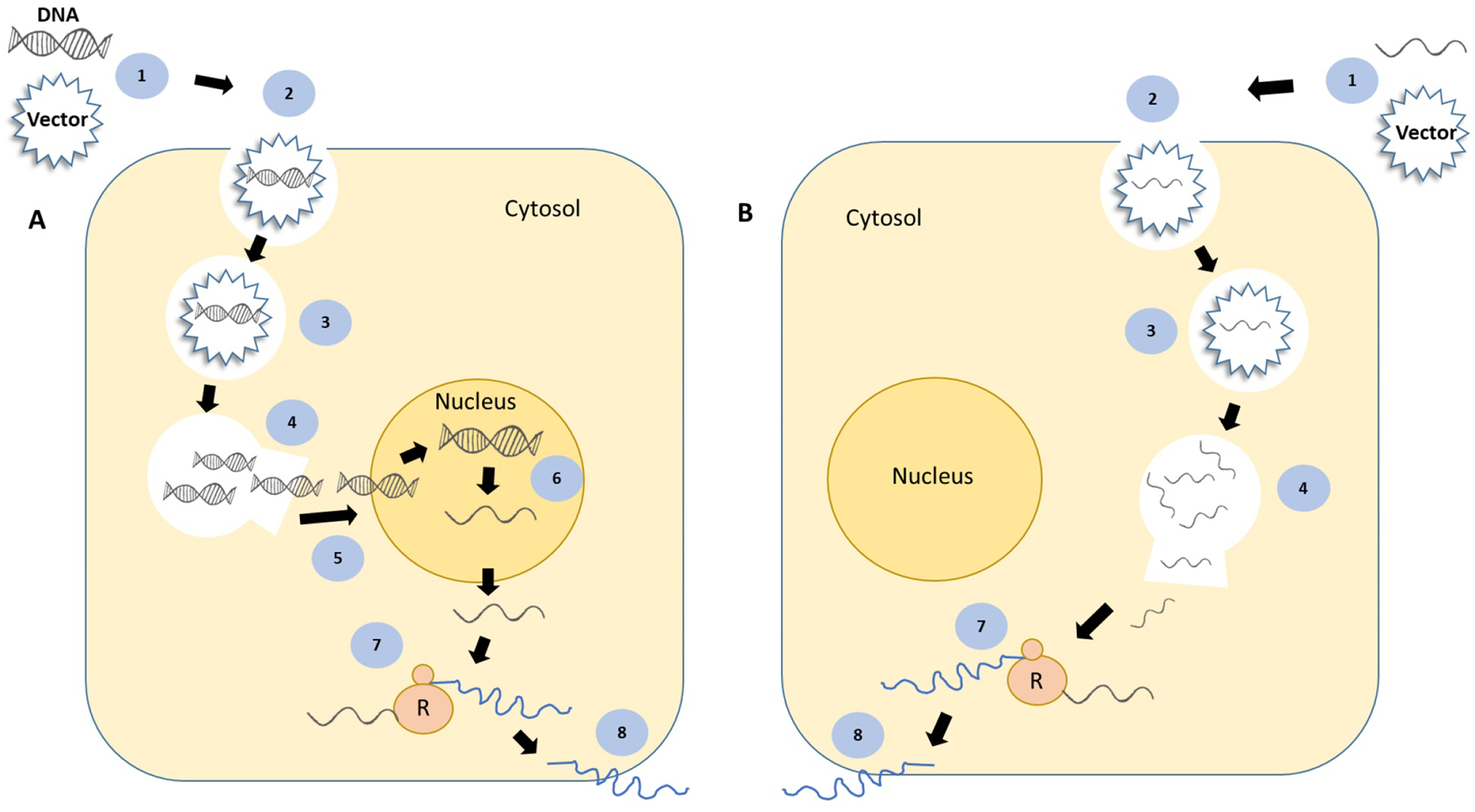
Schematic illustrating the mechanism of action of gene (A) and transcript (B) therapeutics. The steps common to both the strategies are: complexation of DNA or RNA with a vector (1), endocytosis and cellular entry (2), presence in endo-lysosome (3), DNA/RNA escape from endo/lysosome (4), translation of RNA into protein by ribosomes (R) (7) and protein secretion and release (8). The steps unique to gene therapy are nuclear entry of DNA (5) and transcription of DNA into RNA (6).
In nonviral gene therapy, instead of viral particles, several synthetic vectors are being explored, including polymers, liposomes and other poly-cations. The goal here is to select a highly positively charged vector that when allowed to interact with negatively charged DNA, forms stable nano-sized complexes with an overall net positive charge that will enhance its uptake (along with the DNA) by cells (by a process called ‘transfection’), that have negatively charged cell membranes. ‘Transfection’ is a nonviral counterpart of ‘transduction’ and transfection efficiency denotes the efficiency of DNA uptake by cells. An earlier study that explored nonviral gene therapy for bone regeneration utilized plasmid DNAs encoding BMP-4 and/or first 34 amino acids containing peptide of parathyroid hormone and demonstrated successful bone regeneration, when delivered separately and synergistic bone formation, when delivered together [32]. They did not use any vector to deliver the DNA of interest but rather used a collagen scaffold to tether the DNA molecules (‘naked DNA’) that were directly implanted into the bony defects.
Polyethylenimine (PEI), a cationic polymer is one of the most efficient and most commonly employed nonviral gene delivery vectors [33]. A gene activated matrix (GAM) was developed targeting bone regeneration but this time, instead of naked DNA, nano-sized complexes of PEI-DNA was synthesized by allowing the interactions of positively charged PEI molecules with negatively charged DNA molecules. Then, these nano complexes were distributed within a collagen matrix and were thoroughly characterized [34]. When this GAM strategy was applied in vivo in a rat calvarial bone defect model, defects implanted with collagen scaffold containing PEI-DNA (PDGF-B) complexes showed significantly more new bone formation than other control groups tested [35]. Such collagen matrices that carry DNA into the defect are commonly termed gene activated matrices or GAMs. Refer to figure 1 for a mechanistic illustration of GAM. Gene therapy, though promising, has its own challenges. Viral gene therapy, though shown to be safe in the preclinical studies, is plagued by safety concerns, whereas, in nonviral gene therapy, the lower transfection efficiency, compared to its viral counterpart, is the major limiting factor. For detailed information on nonviral gene therapy for periodontal regeneration indication, the reader is referred to a comprehensive review on this topic [36]. In order to enhance transfection efficiency, physical methods such as electroporation [37] and sonoporation [38] are currently being explored in preclinical fracture models with encouraging results, in the context of bone regeneration. Electroporation or sonoporation is known to increase the cellular permeability, which in turn increases the uptake of DNA by cells. Table 3 summarizes some of the preclinical studies that employed viral and nonviral gene therapy for bone regeneration application.
Table 3.
Select preclinical studies that assessed viral and nonviral gene therapy approaches for bone regeneration.
| Viral gene therapy studies | ||||
|---|---|---|---|---|
| Approach (in vivo or ex vivo) | Vector | Animal/model | Gene product | Reference |
| In vivo | Adenovirus | New Zealand white rabbit femoral defect | BMP-2 | [39] |
| Ex vivo (bone marrow cells) | Adenovirus | Rat femoral defect | BMP-2 | [40] |
| Ex vivo (bone marrow stromal cells) | Adenovirus | Rat mandibular defect | BMP-2 | [41] |
| Ex vivo (bone marrow derived MSCs) | Modified adenovirus vector | Rat subcutaneous ectopic bone formation | BMP-2 | [42] |
| In vivo | Adenovirus | Rat femoral defect | VEGF-A | [43] |
| Ex vivo (adipose derived MSCs) | Adenovirus | Rat femoral defect | BMP-2 | [44] |
| Ex vivo (bone marrow derived MSCs) | Adenovirus | Goat tibial defect | BMP-2 | [45] |
| Ex vivo (MSCs) | Adenovirus | Osteoporotic female sheep tibial defect | BMP-2 | [46] |
| In vivo | Adenovirus | Rat femoral defect | BMP-2 | [23] |
| In vivo | Adenovirus | Rat femoral defect | BMP-2 | [47] |
| In vivo | Adenovirus | Rat femoral defect | Runx-2 | [48] |
| Ex vivo (dermal fibroblasts) | Adenovirus | Equine metacarpal/-metatarsal osteotomies | BMP-2 | [49] |
| Ex vivo (gingival fibroblasts) | Adenovirus | Rat cranial defect | BMP-2 | [50] |
| Nonviral gene therapy studies | ||||
| In vivo | Naked plasmids | Rat femoral defect | BMP-4 or PTH (1–34) | [32] |
| In vivo | PEI modified with linoleic acid | Rat subcutaneous implant model | BMP-2 and b-FGF-2 | [51] |
| In vivo | Cationized gelatin microspheres | Rat cranial defect | BMP-2 | [52] |
| In vivo | PEI and nano HA | Rat cranial defect | BMP-2 and VEGF | [53] |
| Ex vivo (bone marrow stromal cells) | (K)16GRGDSPC | Rat segmental femoral defect | TGF- β | [54] |
| In vivo | PEI or FuGENE6 | Rat cranial defect | caALK6 and Runx2 | [55] |
| In vivo | Branched triacrylate/amine polycationic polymer | Rat cranial defect | BMP-2 | [56] |
| In vivo | PEI | Rat cranial defect | PDGF-B | [35] |
| In vivo | PEI | Diabetic rabbit radial defect | FGF-2 and BMP-2 | [57] |
Abbreviations: hydroxyapatite (HA), activin receptor-like kinase 6 (caALK6) and runt-related transcription factor 2 (Runx2).
2.3. Transcript-based approaches
As discussed in section 2.2, in gene therapy, the DNA that enters the cells has to cross the nuclear membrane to reach the nucleus which is the primary site of action (figure 2). Of the different steps involved, the final step of nuclear entry by the DNA molecule is considered to be a rate limiting step in gene therapy, especially in nonviral approaches. The entry occurs effectively during cell division and therefore, in nondividing cells, DNA entry is hindered. To overcome the barriers associated with DNA therapy, several research groups explored the possibility of using RNA encoding the target protein, instead of DNA. As the name suggests, in transcript (RNA) therapy, the site of action is cytoplasm and not the nucleus and therefore nuclear entry is not required. As soon as the RNA enters the cells, they can be directly translated by ribosomes into proteins [58]. Refer to figure 3(b) for a mechanistic illustration of cmRNA therapy.
RNA by itself is unstable, immunogenic and also needs modifications to simulate its post-translational modifications for it to be translated into proteins. Over the last decade, with the help of several investigations, it is clear now that with specific modifications in RNA, we can improve its stability, translation ability and at the same time, reduce its immunogenicity [59]. The resulting RNA is termed by some as chemically modified RNA or simply cmRNA.
It was successfully demonstrated that by implanting collagen scaffolds containing complexes of chemically modified RNA (cmRNA) encoding BMP-2 and PEI (as a vector) in rat calvarial defects, significantly higher new bone formation was observed [60]. In this first-time demonstration of the use of cmRNA for a tissue engineering application, it was shown that the PEI-cmRNA (BMP-2) group outperformed its DNA counterpart [PEI-pDNA (BMP-2)] in several parameters assessed, both in vitro and in vivo. Subsequent publications from different research groups focusing on cmRNA as the regenerative agent further validated this strategy [61–66]. In a follow-up study, we compared cmRNA (BMP-2) with cmRNA (BMP-9) for their effectiveness to regenerate bone. Though cmRNA (BMP-9) demonstrated superior in vitro osteogenic potential, in vivo, the bone volume regenerated was not statistically higher than in the cmRNA (BMP-2) group highlighting that cmRNA delivery is a platform technology that can be used to deliver different growth factors effectively [67]. Apart from cmRNA, RNA interference, a process by which the gene expression is regulated by small RNAs such as small interfering RNA (siRNA) can be successfully harnessed to regenerate bone [68]. The use of siRNA is based on the idea of delivering these small RNA molecules to inhibit the expression of select genes such as noggin to enhance bone regeneration [69]. Additionally, microRNAs, the endogenous small, noncoding RNAs that play a role in gene regulation at the post-transcriptional level can be successfully employed for bone regeneration [70], [71]. Several in vivo studies using a range of bone defect models including calvarial defects and employing a variety of scaffolds to deliver different microRNAs or its antagonists, assessed their efficacy in the context of bone regeneration [72–75].
2.4. Exosomes
Exosomes are extracellular vesicles that are 30–150 nm in size [76]. Their size, nature of formation and composition distinguish these vesicles from other vesicles secreted by the cells. Exosomes are formed in multivesicle compartments by the inward budding of the endosomal membrane and subsequently secreted by fusion of the vesicle to the plasma membrane. Multiple cellular mediators have been shown to trigger this process. Based on their origin and components, several subsets of exosomes could be identified. However, generally exosomes contain RNA, microRNA(miRNA) and DNA, along with plasma membrane and cytosolic proteins [77]. These nano packets of information can be endocytosed by effector cells to trigger a cellular response designated by the parental cell to the effector cell [78]. Although originally believed to be mediators of cellular homeostasis by secreting cellular waste [79], recent studies on exosome function have highlighted their important roles in modulating cellular aspects of immunology, cancer biology and regenerative medicine [79], [80].
Within this exosomal membrane, RNA (both messenger (Mrna) and miRNA), cytosolic proteins as well as trans membrane proteins are present [81]. Amongst the various exosome constituents, miRNA and noncoding RNA have been reported to be in abundance [82]. These miRNA act as regulators of signaling pathways, including those involved in repair and regeneration. Further, the nuclear and cytosolic protein content of the exosomes is selective and representative of the parent cell. Thus, there exists the potential to engineer the parent cell for the release of function specific exosomes, which can in turn be utilized to direct exosome-mediated changes in target cells. Studies have shown the potential of MSC derived exosomes to control various aspects of regeneration including, proliferation, differentiation [83], migration [84], angiogenesis [85] and apoptosis [86].
Depending on the source and target cell type, exosomes are endocytosed by either clathrin or cave-olin mediated endocytosis [87]. This process triggers endocytosis mediated signaling cascades in target cells mediated by the extracellular receptor kinase family (ERK) and mitogen activated protein kinase family (MAPK) [88], [89]. The endocytosis of exosomes also results in the transference of their miRNA and protein cargo intracellularly [78]. After this discovery, there has been an increased focus on their applications in regenerative medicine as inducers of cell proliferation [90], angiogenesis [91], [92] and as immunomodulators for cancer therapy [93–95]. The miRNA and protein composition of the exosome is unique to the parent cell type it is sourced from and can vary in content and activity depending on the state of the source cell [96], [97].
Tissue engineering strategies have aimed at developing novel biomaterials for bone regeneration applications for the past several years. Although many innovative strategies have been developed, none have been able to successfully replace existing bone graft materials. Despite the various drawbacks that bone grafts materials possess including donor and batch dependent variability in consistency [98] that results in unpredictable clinical performance, they continue to remain the primary clinical choice of material for regenerative applications. Emerging evidence indicates that transplanted MSCs act in a paracrine manner [99–101] exerting their influence through extracellular vesicles [94], [102], [103]. Therefore, there exists a possibility for MSCs to serve as a viable alternative to stem cell therapy. As one approach, various cell types, including MSCs can be genetically modified to generate exosomes with function-specific constituents, like specific miRNAs in exosomes.
Such technical innovations differ from conventional exosomal delivery approaches and embrace the innate characteristics of the source exosomes by not considering them as delivery vehicles alone. Recent studies provide positive evidence for the long-term stability of exosomes [95], [104] and their ability to protect against immune rejection and graft versus host disease [94], [105]. Hence, the use of exosomes in medicine can have far-reaching implications not only in the field of regenerative medicine but also in other therapies as well.
2.5. Stem cell-based approaches
Stem cells are defined by their self-renewal and multi-potency (ability to differentiate into specialized cell types) characteristics. Adult stem cells that exist within the bone are what contribute to its inherent regenerative capacity. The idea behind using adult MSCs such as bone marrow derived stromal/stem cells (BMSCs) for bone regeneration is that by increasing the number of these cells (the key players in bone regeneration) at the local target site, we can promote regeneration. Though it sounds simple, there are several challenges that have to be overcome to make this an effective and practical approach. The challenges start with identification of the right kind of cells. It is known that only a small percentage of cells that exist in bone marrow aspirates are true bone progenitor cells and that their number varies significantly [106]. This led to explorations on ways to increase the concentration of progenitor cells that can be used in bone tissue engineering. One approach is to expand the cells ex vivo to increase its numbers and then regraft them into the defect site. Many additional strategies were proposed to enhance the proliferative potential of these cells such as addition of growth factors, extracellular matrix and dynamic culture environment [82], [83], [107], [108]. Over-expressing human telomerase reverse transcriptase gene in adult stem cells was also attempted to eliminate replicative senescence [109]. Ways to increase the differentiation potential of these adult mesenchymal cells and their targeting for bone regeneration are also currently being investigated [108]. Other adult stem cells such as adipose derived stem cells (ADSCs) are also considered good candidate stem cells for bone tissue engineering applications [110]. ADSCs are an attractive alternative to BMSCs as the yield of these cells from the adipose aspirates is higher than BMSCs yield from bone marrow aspirates [111]. The other potential candidate is iPS cells, which are pluripotent and therefore have to be osteo-differentiated before use to avoid tumorigenesis [112], [113]. For more information on stem cells use in tissue engineering, refer to a detailed review on this topic [106].
In dentistry, cell-based therapeutics for bone regeneration is being explored in humans for alveolar bone augmentation/preservation and sinus augmentation indications [114]. In an earlier study, using a combination of PRP, mononuclear cells from bone marrow and bone scaffold, researchers successfully demonstrated the safety and applicability of cell therapy to achieve maxillary bone augmentation in humans, prior to dental implant placement [115]. In a separate first-in-human randomized controlled clinical trial (RCT), implantation of tissue repair cells (mixture of expanded MSCs (CD90 +), monocytes/macrophages (CD14 +) and mononuclear cells from the original bone marrow aspirate) delivered in a gelatin sponge into extraction sockets resulted in significantly more bone regeneration, than the saline soaked sponge (control) group [116]. In a separate human RCT with a one year follow-up, researchers delivered MSCs (CD90 +) with β-tricalcium phosphate (TCP) scaffold, and successfully demonstrated the safety and efficacy of this approach in regenerating new bone in the maxillary sinus lift procedure [117].
2.6. Scaffolds
Scaffolds form an integral component of tissue engineering. They provide the necessary support system and conduit for the cells and the neovasculature to makes its way through, favoring regeneration. Materials to restore bony defects and deformities have evolved over time with first generation materials primarily made out of metals mainly to restore physical functions to a more recent generation of biomaterials that have the capacity to restore both form and function. To be effective, scaffolds for bone tissue engineering should possess certain characteristics and satisfy certain requirements. Being biodegradable and biocompatible are biological requirements of a scaffold, while adequate mechanical properties, space maintenance, pore size and interconnectivity of pores within a scaffold are required physical and structural properties of a scaffold for bone tissue engineering [118].
Apart from the physical characteristics of the scaffold that influence regeneration, it is now clear that the rigidity of the scaffold can directly influence the path of differentiation of MSCs. In a classic study, Engler and coworkers have shown that human MSCs cultured on soft collagen coated gels (similar to brain) committed to a neurogenic lineage, while MSCs cultured on rigid matrices (mimicking bone) committed to an osteogenic lineage [119].
Broadly, scaffolds can be classified based on the source and composition into two kinds: natural and synthetic. Some examples of naturally derived polymers used as scaffolds include collagen, chitosan, silk and alginate. Additionally, a wide range of synthetic materials from polymers such as poly-L-co-D, L-lactide to ceramics such as hydroxyapatite are being explored for their use in bone regeneration. For detailed information on the design characteristics and type of scaffolds, readers are referred to a recent comprehensive review on this topic [120].
Apart from utilizing scaffolds as a passive temporary physical structure, several studies explored the possibility of utilizing them as drug and biomolecule delivery systems, thereby making them biologically active. Three common incorporation strategies include covalent binding, physical entrapment or adsorption and incorporation into micro/nanospheres that are then incorporated into the scaffold [118]. Each of these strategies will result in a specific type of release profile of the active ingredient with the latter approach providing better control over the release rate of the growth factor and also allows for the release of multiple factors, or the sequential release of factors [118]. A study demonstrated enhancement in osteogenesis in the rabbit bone defect model, when they used a hyaluronic hydrogel that physically entrapped growth and differentiation factor-5 (GDF-5) and released the factor in a sustained manner for up to 28 d [121]. In a different study, poly(lactic-co-glycolic acid) (PLGA) nanoparticles and alginate microcapsules delivered from collagen was utilized to deliver BMP-2 and vascular endothelial growth factor (VEGF), that resulted in enhanced bone regeneration in the rat calvarial defect model [122]. The type of delivery system is selected based on the type of factor or biomolecule that is delivered and its biological requirement.
Past studies in this field incorporated the following factors with scaffolds intended for bone regeneration: BMP-2, VEGF, VEGF + FGF-2, VEGF + BMP-2, PDGF, BMP-2+TGFβ, BMP-6+TGFβ3 or BMP-9 [123]. As mentioned in section 2.1, the important issue with proteins such as growth factors is the rapid decline in the therapeutic concentration at the implanted site due to proteolysis and degradation. Scaffolds can be designed to release growth factors gradually rather than releasing them in bulk. This will also reduce the need for higher dosage of growth factors, thereby one can potentially reduce its adverse effects [96], [124]. Several different strategies of incorporating growth factors into scaffolds for bone regeneration have been explored, including bulk incorporation, biomimetic binding, surface adsorption, multilayer coating, nanoparticles, and biomineralization [125].
In addition to GDF, natural and predominantly synthetic scaffolds incorporating several different types of antimicrobials have also been explored both in vitro and in vivo [126]. Some of the antibiotics include gentamycin, tetracycline, vancomycin, and ciprofloxacin. In addition, other pharmacological agents such as dexamethasone, ibuprofen, and bisphosphonates such as alendronate and zolen-dronate were also successfully incorporated into scaffolds [126]. It is also clear from studies that local delivery of systemic bone anabolic medications such as teriparatide and abaloparatide can result in local bone regeneration [127], [128]. A recent study conjugated isoniazid onto synthetic polymer scaffold as a means to achieve bone regeneration and to treat bone tuberculosis [129]. It is therefore clear that there are significant ongoing efforts to make scaffolds bioactive by way of incorporating different biological and pharmaceutical agents . There are also efforts to develop ‘smart biomaterials’, which will respond to external stimuli like light, changes in pH or temperature. The responsiveness of these smart materials can be exploited in vivo to release a specific factor at the appropriate time point [130], but the translatability of these novel drug and biomolecule eluting scaffolds to clinics will depend heavily on their performance in human clinical trials, in the context of their safety and efficacy.
The other exciting and rapidly evolving area in the development of novel scaffolds for bone tissue engineering is 3D bioprinting. It typically involves the following steps [131]: 1. Computer aided design of the construct to be printed for the required dimensions and other specifications (type of cells, type of matrix etc.), 2. Printing the construct. 3. Culturing the construct (if it contains cells) in a bioreactor before implantation. Broadly, there are three ways by which bone bioprinting can be performed [131]. Inkjet printing uses special printer heads to print the scaffold initially as liquid that hardens over time. Bioplotting is similar to inkjet printing but, in this case, a continuous filament of the starting material will be injected out of a syringe [132]. The third utilizes stereo lithography, where the constructs are created one layer at a time and they are made to solidify by curing with an ultra-violet light source. A recent study tested the incorporation of human BMSCs in a 3D bioprinted polysaccharide-based hydrogel and demonstrated that not only did the BMSCs survive but they also successfully differentiated into the osteogenic lineage [133]. Preclinical testing of commercially available 3D printed synthetic bone graft (without cells) and in-house printed synthetic 3D scaffold with bone marrow progenitor cells led to encouraging results [134], [135].
3. Conclusions
Tissue engineering is a rapidly expanding area and products of tissue engineering are making their way into clinical practice after regulatory approvals, including products for bone tissue engineering. One good example is the introduction and current usage of rhBMP-2 for oral and craniomaxillofacial applications. Though the field of bone tissue engineering is growing, several challenges still exist. As mentioned earlier, the deconstructive approach of testing one component at a time has its advantages and limitations. Though such approaches are much more straightforward to evaluate and to get the regulatory clearance, an all-encompassing strategy that includes all the key players such as biomimetic scaffolds, cells and mediators is often required to take the predictability and regenerative potential to the next level [114]. The other challenges with existing strategies are the high manufacturing cost and low efficiency of some of the laboratory techniques such as cell isolation, culture/expansion, seeding, and 3D bioprinting. Therefore, improving the cost-effectiveness of these laboratory techniques is critical for the success of future bone tissue engineering products.
The oral cavity is easily accessible and therefore a good system to test the products of bone tissue engineering but the constant presence of oral microbes during the healing process makes it a challenging model. Therefore, considerations regarding the effect of microbes and their products and the role of inflammation resolution on tissue regeneration should be given, when developing future materials and strategies, especially of dental applications. With regard to novel materials for critical sized defects, striking the right balance between sound mechanical and biological properties is a constant challenge. Though we are successful in regenerating smaller defects, the difficulty is in scaling up the strategy to treat larger critical sized defects with high predictability, in vivo. In addition, when it comes to preclinical testing of novel materials, using larger animal models will enhance the validity and translatability of the findings to human situations and can therefore hasten the rate of clinical translation.
Acknowledgments
The authors (SE and PG) would like to thank the American Academy of Periodontology Foundation for the support provided to pursue a career in academia. This work was supported in part by NIH Grant DE024206 (SE and AS) and DE027404 (PG and SR).
References
- [1].Fukumoto S and Martin TJ 2009. Bone as an endocrine organ Trends Endocrinol. Metab 20 230–6 [DOI] [PubMed] [Google Scholar]
- [2].Maes C and Kronenberg HM 2016. Bone development and remodeling Endocrinology: Adult and Pediatric 7 edn ed Jameson JL et al. (Philadelphia, PA: Saunders; ) 1038–62 [Google Scholar]
- [3].Allen MR and Burr DB 2014. Bone modeling and remodeling Basic and Applied Bone Biology ed Allen MR and Burr DB (New York: Academic; ) 75–90 [Google Scholar]
- [4].Hadjidakis DJ and Androulakis II 2006. Bone remodeling Ann. New York Acad. Sci 1092 385–96 [DOI] [PubMed] [Google Scholar]
- [5].Sims NA and Gooi JH 2008. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption Semin. Cell Dev. Biol 19 444–51 [DOI] [PubMed] [Google Scholar]
- [6].Zaidi M 2007. Skeletal remodeling in health and disease Nat. Med 13 791–801 [DOI] [PubMed] [Google Scholar]
- [7].Grand View Research. Bone Grafts And Substitutes Market Analysis By Material (Natural- Autografts, Allografts; Synthetic- Ceramic, Composite, Polymer, Bone Morphogenetic Proteins (BMP)), By Application (Craniomaxillofacial, Dental, Foot & Ankle, Joint Reconstruction, Long Bone, Spinal Fusion) Forecasts To 2024. 2016.
- [8].Elani HW, Starr JR, Da Silva JD and Gallucci GO 2018. Trends in dental implant use in the U.S., 1999–2016, and projections to 2026 J. Dent Res 97 1424–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Egol KA, Nauth A, Lee M, Pape H-C, Watson JT and Borrelli J Jr 2015. Bone grafting: sourcing, timing, strategies, and alternatives J. Orthop. Trauma 29 S10–S14 [DOI] [PubMed] [Google Scholar]
- [10].Langer R and V J P 1993. Tissue engineering Science 260 920–6 [DOI] [PubMed] [Google Scholar]
- [11].Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, Toljanic J, Jones A and Nevins M 2005. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation J. Periodontology 76 605–13 [DOI] [PubMed] [Google Scholar]
- [12].Kim H, Chung J, Shin S, Shin S, Kye S, Kim N, Kwon T, Paeng J, Kim J and Oh O 2015. Efficacy of rhBMP-2/hydroxyapatite on sinus floor augmentation: a multicenter, randomized controlled clinical trial J. Dent. Res 94 158S–165S [DOI] [PubMed] [Google Scholar]
- [13].Carragee EJ, Hurwitz EL and Weiner BK 2011. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned Spine J. 11 471–91 [DOI] [PubMed] [Google Scholar]
- [14].Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg. Neurol. Int. 2013;4:S343. doi: 10.4103/2152-7806.114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kokorina NA, Zakharkin SO, Krebsbach PH and Nussenbaum B 2011. Treatment effects of rhBMP-2 on invasiveness of oral carcinoma cell lines Laryngoscope 121 1876–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Freire MO, You H-K, Kook J-K, Choi J-H and Zadeh HH 2011. Antibody-mediated osseous regeneration: a novel strategy for bioengineering bone by immobilized anti–bone morphogenetic protein-2 antibodies Tissue Eng. A 17 2911–8 [DOI] [PubMed] [Google Scholar]
- [17].Freire M, Choi J-H, Nguyen A, Chee YD, Kook J-K, You H-K and Zadeh HH 2015. Application of AMOR in craniofacial rabbit bone bioengineering Biomed. Res. Int 2015 628769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN and Van Dyke TE 2007. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo J. Immunol 179 7021–9 [DOI] [PubMed] [Google Scholar]
- [19].Herrera B, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara M, Serhan C, Van Dyke T and Gyurko R 2008. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption Br. J. Pharmacol 155 1214–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Van Dyke TE 2011. Proresolving lipid mediators: potential for prevention and treatment of periodontitis J. Clin. Periodontology 38 119–25 [DOI] [PubMed] [Google Scholar]
- [21].Van Dyke T, Hasturk H, Kantarci A, Freire M, Nguyen D, Dalli J and Serhan C 2015. Proresolving nanomedicines activate bone regeneration in periodontitis J. Dent. Res 94 148–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Somia N and Verma IM 2000. Gene therapy: trials and tribulations Nat. Rev. Genet 1 91–99 [DOI] [PubMed] [Google Scholar]
- [23].Betz OB, Betz VM, Nazarian A, Pilapil CG, Vrahas MS, Bouxsein ML, Gerstenfeld LC, Einhorn TA and Evans CH 2006. Direct percutaneous gene delivery to enhance healing of segmental bone defects J. Bone Joint. Surg 88 355–65 [DOI] [PubMed] [Google Scholar]
- [24].Wright VJ, Peng H, Usas A, Young B, Gearhart B, Cummins J and Huard J 2002. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice Mol. Ther 6 169–78 [DOI] [PubMed] [Google Scholar]
- [25].Lattanzi W, Parrilla C, Fetoni A, Logroscino G, Straface G, Pecorini G, Stigliano E, Tampieri A, Bedini R and Pecci R 2008. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models Gene Ther.15 1330–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Han D and Li J 2013. Repair of bone defect by using vascular bundle implantation combined with Runx II gene-transfected adipose-derived stem cells and a biodegradable matrix Cell Tissue Res. 352 561–71 [DOI] [PubMed] [Google Scholar]
- [27].Rundle CH, Strong DD, Chen ST, Linkhart TA, Sheng MHC, Wergedal JE, Lau KHW and Baylink DJ 2008. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat J. Gene Med 10 229–41 [DOI] [PubMed] [Google Scholar]
- [28].Chang P-C, Seol Y-J, Cirelli JA, Pellegrini G, Jin Q, Franco LM, Goldstein SA, Chandler LA, Sosnowski B and Giannobile WV 2010. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration Gene Ther. 17 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chang P-C, Cirelli JA, Jin Q, Seol Y-J, Sugai JV, D’Silva NJ, Danciu TE, Chandler LA, Sosnowski BA and Giannobile WV 2009. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use Hum. Gene Ther 20 486–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Evans CH and Huard J 2015. Gene therapy approaches to regenerating the musculoskeletal system Nat. Rev. Rheumatol 11 234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Evans CH 2012. Gene delivery to bone Adv. Drug Delivery Rev 64 1331–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fang J, Zhu Y-Y, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, McCauley LK, Davidson BL and Roessler BJ 1996. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes Proc. Natl Acad. Sci. USA 93 5753–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lungwitz U, Breunig M, Blunk T and Göpferich A 2005. Polyethylenimine-based non-viral gene delivery systems Eur. J. Pharm. Biopharm 60 247–66 [DOI] [PubMed] [Google Scholar]
- [34].Tierney EG, Duffy GP, Hibbitts AJ, Cryan S-A and O’Brien FJ 2012. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds J. Control. Release 158 304–11 [DOI] [PubMed] [Google Scholar]
- [35].Elangovan S, D’Mello SR, Hong L, Ross RD, Allamargot C, Dawson DV, Stanford CM, Johnson GK, Sumner DR and Salem AK 2014. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor Biomaterials 35 737–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elangovan S and Karimbux N 2010. DNA delivery strategies to promote periodontal regeneration J. Biomater. Appl 25 3–18 [DOI] [PubMed] [Google Scholar]
- [37].Kimelman-Bleich N, Pelled G, Zilberman Y, Kallai I, Mizrahi O, Tawackoli W, Gazit Z and Gazit D 2011. Targeted gene-and-host progenitor cell therapy for nonunion bone fracture repair Mol. Ther 19 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bez M, Sheyn D, Tawackoli W, Avalos P, Shapiro G, Giaconi JC, Da X, David SB, Gavrity J and Awad HA 2017. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs Sci. Transl. Med 9 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baltzer A, Lattermann C, Whalen J, Wooley P, Weiss K, Grimm M, Ghivizzani S, Robbins PD and Evans CH 2000. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene Gene Ther. 7 734–9 [DOI] [PubMed] [Google Scholar]
- [40].Lieberman JR, Daluiski A, Stevenson S, Jolla L, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA and Berk AJ 1999. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats JBJS 81 905–17 [DOI] [PubMed] [Google Scholar]
- [41].Park J, Ries J, Gelse K, Kloss F, Von Der Mark K, Wiltfang J, Neukam F and Schneider H 2003. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes Gene Ther. 10 1089–98 [DOI] [PubMed] [Google Scholar]
- [42].Tsuda H, Wada T, Ito Y, Uchida H, Dehari H, Nakamura K, Sasaki K, Kobune M, Yamashita T and Hamada H 2003. Efficient BMP2 gene transfer and bone formation of mesenchymal stem cells by a fiber-mutant adenoviral vector Mol. Ther 7 354–65 [DOI] [PubMed] [Google Scholar]
- [43].Tarkka T, Sipola A, Jämsä T, Soini Y, Ylä-Herttuala S, Tuukkanen J and Hautala T 2003. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues J. Gene Med 5 560–6 [DOI] [PubMed] [Google Scholar]
- [44].Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P and Lieberman JR 2005. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue Tissue Eng. 11 120–9 [DOI] [PubMed] [Google Scholar]
- [45].Dai K, Xu X, Tang T, Zhu Z, Yu C, Lou J and Zhang X 2005. Repairing of goat tibial bone defects with BMP-2 gene–modified tissue-engineered bone Calcified Tissue Int. 77 55–61 [DOI] [PubMed] [Google Scholar]
- [46].Egermann M, Baltzer A, Adamaszek S, Evans C, Robbins P, Schneider E and Lill C 2006. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep Hum. Gene Ther 17 507–17 [DOI] [PubMed] [Google Scholar]
- [47].Betz O, Betz V, Nazarian A, Egermann M, Gerstenfeld L, Einhorn T, Vrahas M, Bouxsein M and Evans CH 2007. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects Gene Ther. 14 1039–44 [DOI] [PubMed] [Google Scholar]
- [48].Bhat BM, Robinson JA, Coleburn VE, Zhao W and Kharode Y 2008. Evidence of in vivo osteoinduction in adult rat bone by adeno-Runx2 intra-femoral delivery J. Cell. Biochem 103 1912–24 [DOI] [PubMed] [Google Scholar]
- [49].Ishihara A, Zekas LJ, Litsky AS, Weisbrode SE and Bertone AL 2010. Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model J. Orthop. Res 28 403–11 [DOI] [PubMed] [Google Scholar]
- [50].Shin JH, Kim KH, Kim SH, Koo KT, Kim TI, Seol YJ, Ku Y, Rhyu IC, Chung CP and Lee YM 2010. Ex vivo bone morphogenetic protein-2 gene delivery using gingival fibroblasts promotes bone regeneration in rats J. Clin. Periodontology 37 305–11 [DOI] [PubMed] [Google Scholar]
- [51].Rose LC, Kucharski C and Uludağ H 2012. Protein expression following non-viral delivery of plasmid DNA coding for basic FGF and BMP-2 in a rat ectopic model Biomaterials 33 3363–74 [DOI] [PubMed] [Google Scholar]
- [52].Kasper FK, Young S, Tanahashi K, Barry MA, Tabata Y, Jansen JA and Mikos AG 2006. Evaluation of bone regeneration by DNA release from composites of oligo (poly (ethylene glycol) fumarate) and cationized gelatin microspheres in a critical-sized calvarial defect J. Biomed. Mater. Res. A 78 335–42 [DOI] [PubMed] [Google Scholar]
- [53].Curtin CM, Tierney EG, McSorley K, Cryan SA, Duffy GP and O’Brien FJ 2015. Combinatorial gene therapy accelerates bone regeneration: non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold Adv. Healthcare Mater 4 223–7 [DOI] [PubMed] [Google Scholar]
- [54].Pan H, Zheng Q, Yang S, Guo X, Wu B, Zou Z and Duan Z 2014. A novel peptide-modified and gene-activated biomimetic bone matrix accelerating bone regeneration J. Biomed. Mater. Res. A 102 2864–74 [DOI] [PubMed] [Google Scholar]
- [55].Itaka K, Ohba S, Miyata K, Kawaguchi H, Nakamura K, Takato T, Chung U-I and Kataoka K 2007. Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex nanomicelles Mol. Ther 15 1655–62 [DOI] [PubMed] [Google Scholar]
- [56].Chew SA, Kretlow JD, Spicer PP, Edwards AW, Baggett LS, Tabata Y, Kasper FK and Mikos AG 2011. Delivery of plasmid DNA encoding bone morphogenetic protein-2 with a biodegradable branched polycationic polymer in a critical-size rat cranial defect model Tissue Eng. A 17 751–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Khorsand B, Nicholson N, Do A-V, Femino JE, Martin JA, Petersen E, Guetschow B, Fredericks DC and Salem AK 2017. Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model J. Control. Release 248 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Elangovan S, Kormann MS, Khorsand B and Salem AK 2016. The oral and craniofacial relevance of chemically modified RNA therapeutics Discovery Med. 21 35. [PMC free article] [PubMed] [Google Scholar]
- [59].Sahin U, Karikó K and Türeci Ö 2014. mRNA-based therapeutics—developing a new class of drugs Nat. Rev. Drug Discovery 13 759–80 [DOI] [PubMed] [Google Scholar]
- [60].Elangovan S, Khorsand B, Do A-V, Hong L, Dewerth A, Kormann M, Ross RD, Sumner DR, Allamargot C and Salem AK 2015. Chemically modified RNA activated matrices enhance bone regeneration J. Control. Release 218 22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Balmayor ER, Geiger JP, Aneja MK, Berezhanskyy T, Utzinger M, Mykhaylyk O, Rudolph C and Plank C 2016. Chemically modified RNA induces osteogenesis of stem cells and human tissue explants as well as accelerates bone healing in rats Biomaterials 87 131–46 [DOI] [PubMed] [Google Scholar]
- [62].Badieyan ZS, Berezhanskyy T, Utzinger M, Aneja MK, Emrich D, Erben R, Schüler C, Altpeter P, Ferizi M and Hasenpusch G 2016. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration J. Control. Release 239 137–48 [DOI] [PubMed] [Google Scholar]
- [63].Utzinger M, Jarzebinska A, Haag N, Schweizer M, Winter G, Dohmen C, Rudolph C and Plank C 2017. cmRNA/lipoplex encapsulation in PLGA microspheres enables transfection via calcium phosphate cement (CPC)/PLGA composites J. Control. Release 249 143–9 [DOI] [PubMed] [Google Scholar]
- [64].Balmayor ER, Geiger JP, Koch C, Aneja MK, van Griensven M, Rudolph C and Plank C 2017. Modified mRNA for BMP-2 in combination with biomaterials serves as a transcript-activated matrix for effectively inducing osteogenic pathways in stem cells Stem Cells Dev. 26 25–34 [DOI] [PubMed] [Google Scholar]
- [65].Badieyan ZS, Pasewald T, Mykhaylyk O, Rudolph C and Plank C 2017. Efficient ex vivo delivery of chemically modified messenger RNA using lipofection and magnetofection Biochem. Biophys. Res. Commun 482 796–801 [DOI] [PubMed] [Google Scholar]
- [66].Zhang W, De La Vega RE, Coenen MJ, Müller SA, Peniche Silva CJ, Aneja MK, Plank C, Van Griensven M, Evans CH and Balmayor ER 2019. An improved, chemically modified RNA encoding BMP-2 enhances osteogenesis in vitro and in vivo Tissue Eng. A 25 131–44 [DOI] [PubMed] [Google Scholar]
- [67].Khorsand B, Elangovan S, Hong L, Dewerth A, Kormann MS and Salem AK 2017. A comparative study of the bone regenerative effect of chemically modified RNA encoding BMP-2 or BMP-9 AAPS J. 19 438–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ghadakzadeh S, Mekhail M, Aoude A, Hamdy R and Tabrizian M 2016. Small players ruling the hard game: siRNA in bone regeneration J. Bone Miner. Res 31 475–87 [DOI] [PubMed] [Google Scholar]
- [69].Kowalczewski CJ and Saul JM 2015. Surface-mediated delivery of siRNA from fibrin hydrogels for knockdown of the BMP-2 binding antagonist noggin Acta Biomater.25 109–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hong L, Sharp T, Khorsand B, Fischer C, Eliason S, Salem A, Akkouch A, Brogden K and Amendt BA 2016. MicroRNA-200c represses IL-6, IL-8, and CCL-5 expression and enhances osteogenic differentiation PLoS One 11 e0160915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nakasa T, Yoshizuka M, Andry Usman M, Elbadry Mahmoud E and Ochi M 2015. MicroRNAs and bone regeneration Curr. Genomics 16 441–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Eskildsen T, Taipaleenmäki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S and Kassem M 2011. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo Proc. Natl Acad. Sci. USA 108 6139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu H, Dong Y, Feng X, Li L, Jiao Y, Bai S, Feng Z, Yu H, Li X and Zhao Y 2019. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblastic differentiation of mesenchymal stromal cells in rats Stem Cell Res. Ther 10 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Xue Y, Guo Y, Yu M, Wang M, Ma PX and Lei B 2017. Monodispersed bioactive glass nanoclusters with ultralarge pores and intrinsic exceptionally high miRNA loading for efficiently enhancing bone regeneration Adv. Healthcare Mater 6 1700630. [DOI] [PubMed] [Google Scholar]
- [75].Zhang X, Li Y, Chen YE, Chen J and Ma PX 2016. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects Nat. Commun 7 10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marote A, Teixeira FG, Mendes-Pinheiro B and Salgado AJ 2016. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential Frontiers Pharmacol. 7 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kowal J, Tkach M and Théry C 2014. Biogenesis and secretion of exosomes Curr. Opin. Cell Biol 29 116–25 [DOI] [PubMed] [Google Scholar]
- [78].Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ and Lötvall JO 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells Nat. Cell Biol 9 654–9 [DOI] [PubMed] [Google Scholar]
- [79].Johnstone R, Mathew A, Mason A and Teng K 1991. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins J. Cell. Physiol 147 27–36 [DOI] [PubMed] [Google Scholar]
- [80].Azmi AS, Bao B and Sarkar FH 2013. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review Cancer Metastasis Rev. 32 623–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Théry C, Zitvogel L and Amigorena S 2002. Exosomes: composition, biogenesis and function Nat. Rev. Immunol 2 569–79 [DOI] [PubMed] [Google Scholar]
- [82].Lai RC, Yeo RWY and Lim SK 2015. Mesenchymal stem cell exosomes Semin. Cell Dev. Biol 40 pp 82–8 [DOI] [PubMed] [Google Scholar]
- [83].Wu J-Y, Ji A-L, Wang Z-X, Qiang G-H, Qu Z, Wu J-H and Jiang C-P 2018. Exosome-mimetic nanovesicles from hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo Sci. Rep 8 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W and Qian H 2015. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing Stem Cells 33 2158–68 [DOI] [PubMed] [Google Scholar]
- [85].Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL and Contreras Z 2016. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling Stem Cells 34 601–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lai RC, Yeo RWY, Tan KH and Lim SK 2013. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation Regen. Med 8 197–209 [DOI] [PubMed] [Google Scholar]
- [87].Mulcahy LA, Pink RC and Carter DRF 2014. Routes and mechanisms of extracellular vesicle uptake J. Extracell. Vesicles 3 24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Li C, Liu D-R, Li G-G, Wang H-H, Li X-W, Zhang W, Wu Y-L and Chen L 2015. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway World J. Gastroenterol 21 6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Alcayaga-Miranda F, Varas-Godoy M and Khoury M 2016. Harnessing the angiogenic potential of stem cell-derived exosomes for vascular regeneration Stem Cells Int. 2016 3409169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F and Yan Y 2013. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro Stem Cell Res. Ther 4 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M and Tetta C 2012. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets Cell Transplant. 21 1305–20 [DOI] [PubMed] [Google Scholar]
- [92].Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, Deregibus MC, Tetta C, Segoloni G and Camussi G 2012. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia Int. J. Immunopathol. Pharmacol 25 75–85 [DOI] [PubMed] [Google Scholar]
- [93].Marcus ME and Leonard JN 2013. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver Pharmaceuticals 6 659–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid A-A and Mardani K 2012. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling Immunol. Lett 147 47–54 [DOI] [PubMed] [Google Scholar]
- [95].Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD and Mathivanan S 2013. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma Proteomics 13 3354–64 [DOI] [PubMed] [Google Scholar]
- [96].Narayanan R, Huang C-C and Ravindran S 2016. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells Stem Cells Int. 2016 3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Martins M, Ribeiro D, Martins A, Reis RL and Neves NM 2016. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment Stem Cell Rep. 6 284–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bae H, Zhao L, Zhu D, Kanim LE, Wang JC and Delamarter RB 2010. Variability across ten production lots of a single demineralized bone matrix product J. Bone Joint Surg 92 427–35 [DOI] [PubMed] [Google Scholar]
- [99].Yao Y, Huang J, Geng Y, Qian H, Wang F, Liu X, Shang M, Nie S, Liu N and Du X 2015. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts PloS One 10 e0129164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dai W, Hale SL and Kloner RA 2007. Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats Regen. Med 2 63–8 [DOI] [PubMed] [Google Scholar]
- [101].Gnecchi M, Zhang Z, Ni A and Dzau VJ 2008. Paracrine mechanisms in adult stem cell signaling and therapy Circ. Res 103 1204–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN and El Oakley RM 2010. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury Stem Cell Res 4 214–22 [DOI] [PubMed] [Google Scholar]
- [103].Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R and Schor N 2012. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats PloS One 7 e44092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lee M, Ban -J-J, Im W and Kim M 2016. Influence of storage condition on exosome recovery Biotechnol. Bioprocess Eng 21 299–304 [Google Scholar]
- [105].Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH and Lim SK 2014. Mesenchymal stem cells secrete immunologically active exosomes Stem Cells Dev. 23 1233–44 [DOI] [PubMed] [Google Scholar]
- [106].Bianco P and Robey PG 2001. Stem cells in tissue engineering Nature 414 118–21 [DOI] [PubMed] [Google Scholar]
- [107].Hernigou P, Poignard A, Beaujean F and Rouard H 2005. Percutaneous autologous bone-marrow grafting for nonunions: influence of the number and concentration of progenitor cells J. Bone Joint. Surg 87 1430–7 [DOI] [PubMed] [Google Scholar]
- [108].Dawson JI, Kanczler J, Tare R, Kassem M and Oreffo RO 2014. Concise review: bridging the gap: bone regeneration using skeletal stem cell-based strategies—where are we now? Stem Cells 32 35–44 [DOI] [PubMed] [Google Scholar]
- [109].Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG and Kassem M 2002. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells Nat. Biotechnol 20 592–6 [DOI] [PubMed] [Google Scholar]
- [110].Zhu Y, Liu T, Song K, Fan X, Ma X and Cui Z 2008. Adipose-derived stem cell: a better stem cell than BMSC Cell Biochem. Funct 26 664–75 [DOI] [PubMed] [Google Scholar]
- [111].Liao H-T and Chen C-T 2014. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells World J. Stem Cells 6 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wu Q, Yang B, Hu K, Cao C, Man Y and Wang P 2017. Deriving osteogenic cells from induced pluripotent stem cells for bone tissue engineering Tissue Eng. B 23 1–8 [DOI] [PubMed] [Google Scholar]
- [113].Bastami F, Nazeman P, Moslemi H, Rezai Rad M, Sharifi K and Khojasteh A 2017. Induced pluripotent stem cells as a new getaway for bone tissue engineering: a systematic review Cell Proliferation 50 e12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Pilipchuk SP, Plonka AB, Monje A, Taut AD, Lanis A, Kang B and Giannobile WV 2015. Tissue engineering for bone regeneration and osseointegration in the oral cavity Dent. Mater 31 317–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Cerruti HF, Kerkis I, Kerkis A, Tatsui NH, da Costa Neves A, Bueno DF and Da Silva MCP 2007. Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: clinical case reports Artif. Organs 31 268–73 [DOI] [PubMed] [Google Scholar]
- [116].Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, Tarle SA, Bartel RL and Giannobile WV 2013. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial Cell Transplant. 22 767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, Wang F, Lanis A and Giannobile WV 2015. Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: a randomized clinical trial J. Bone Miner. Res 30 1206–16 [DOI] [PubMed] [Google Scholar]
- [118].De Witte T-M, Fratila-Apachitei LE, Zadpoor AA and Peppas NA 2018. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices Regener. Biomater 5 197–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Engler AJ, Sen S, Sweeney HL and Discher DE 2006. Matrix elasticity directs stem cell lineage specification Cell 126 677–89 [DOI] [PubMed] [Google Scholar]
- [120].Polo-Corrales L, Latorre-Esteves M and Ramirez-Vick JE 2014. Scaffold design for bone regeneration J. Nanosci. Nanotechnol 14 15–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bae MS, Ohe J-Y, Lee JB, Heo DN, Byun W, Bae H, Kwon Y-D and Kwon IK 2014. Photo-cured hyaluronic acid-based hydrogels containing growth and differentiation factor 5 (GDF-5) for bone tissue regeneration Bone 59 189–98 [DOI] [PubMed] [Google Scholar]
- [122].Subbiah R, Hwang MP, Van SY, Do SH, Park H, Lee K, Kim SH, Yun K and Park K 2015. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue Adv. Healthcare Mater 4 1982–92 [DOI] [PubMed] [Google Scholar]
- [123].Blackwood KA, Bock N, Dargaville TR and Woodruff MA 2012. Scaffolds for growth factor delivery as applied to bone tissue engineering Int. J. Polym. Sci 2012 174942 [Google Scholar]
- [124].Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Müller R and Hubbell JA 2003. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices Nat. Biotechnol 21 513–8 [DOI] [PubMed] [Google Scholar]
- [125].Nyberg E, Holmes C, Witham T and Grayson WL 2016. Growth factor-eluting technologies for bone tissue engineering Drug Delivery Transl. Res 6 184–94 [DOI] [PubMed] [Google Scholar]
- [126].Mouriño V and Boccaccini AR 2010. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds J. R. Soc. Interface 7 209–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jacobson JA, Yanoso-Scholl L, Reynolds DG, Dadali T, Bradica G, Bukata S, Puzas EJ, Zuscik MJ, Rosier R and O’Keefe RJ 2011. Teriparatide therapy and beta-tricalcium phosphate enhance scaffold reconstruction of mouse femoral defects Tissue Eng. A 17 389–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ning Z, Tan B, Chen B, Lau DSA, Wong TM, Sun T, Peng S, Li Z and Lu WW 2019. Precisely controlled delivery of abaloparatide through injectable hydrogel to promote bone regeneration Macromol. Biosci 19 1900020. [DOI] [PubMed] [Google Scholar]
- [129].Huang D, Li D, Wang T, Shen H, Zhao P, Liu B, You Y, Ma Y, Yang F and Wu D 2015. Isoniazid conjugated poly (lactide-co-glycolide): long-term controlled drug release and tissue regeneration for bone tuberculosis therapy Biomaterials 52 417–25 [DOI] [PubMed] [Google Scholar]
- [130].Ratner BD and Bryant SJ 2004. Biomaterials: where we have been and where we are going Annu. Rev. Biomed. Eng 6 41–75 [DOI] [PubMed] [Google Scholar]
- [131].Arealis G and Nikolaou VS 2015. Bone printing: new frontiers in the treatment of bone defects Injury 46 S20–S22 [DOI] [PubMed] [Google Scholar]
- [132].Duarte Campos DF, Blaeser A, Buellesbach K, Sen KS, Xun W, Tillmann W and Fischer H 2016. Bioprinting organotypic hydrogels with improved mesenchymal stem cell remodeling and mineralization properties for bone tissue engineering Adv. Healthcare Mater 5 1336–45 [DOI] [PubMed] [Google Scholar]
- [133].Carrel JP, Wiskott A, Scherrer S and Durual S 2016. Large bone vertical augmentation using a three-dimensional printed TCP/HA bone graft: A pilot study in dog mandible Clin. Implant Dent. Relat. Res 18 1183–92 [DOI] [PubMed] [Google Scholar]
- [134].Konopnicki S, Sharaf B, Resnick C, Patenaude A, Pogal-Sussman T, Hwang K-G, Abukawa H and Troulis MJ 2015. Tissue-engineered bone with 3-dimensionally printed β-tricalcium phosphate and polycaprolactone scaffolds and early implantation: an in vivo pilot study in a porcine mandible model J. Oral Maxillofacial Surg 73 1016–e1 [DOI] [PubMed] [Google Scholar]
- [135].Amini AR, Laurencin CT and Nukavarapu SP 2012. Bone tissue engineering: recent advances and challenges Crit. Rev. Biomed. Eng 40 363–408 [DOI] [PMC free article] [PubMed] [Google Scholar]


