Key Points
Question
What is the association of prediagnostic cigarette smoking and alcohol consumption with all-cause and breast cancer–specific mortality among Black or African American breast cancer survivors?
Findings
In this population-based cohort study of 1926 Black breast cancer survivors, current smoking at the time of breast cancer diagnosis was associated with a 52% increased risk for all-cause mortality compared with never smoking. This association was most pronounced for women with greater pack-years of smoking and who regularly consumed alcohol.
Meaning
This study suggests that smoking at the time of diagnosis was associated with a higher risk of mortality among Black breast cancer survivors.
Abstract
Importance
There are limited data about how lifestyle factors are associated with breast cancer prognosis among Black or African American women because most of the evidence is based on studies of White breast cancer survivors.
Objective
To examine the association of prediagnostic cigarette smoking and alcohol consumption with all-cause mortality and breast cancer–specific mortality in a cohort of Black breast cancer survivors.
Design, Setting, and Participants
This population-based cohort study included 1926 Black or African American breast cancer survivors who received a diagnosis from June 6, 2005, to May 21, 2019, identified in 10 counties in New Jersey through rapid case ascertainment by the New Jersey State Cancer Registry. Statistical analysis was conducted from January 1, 2021, to August 1, 2022.
Exposures
Information on prediagnostic cigarette smoking, alcohol consumption, and additional covariates was collected during in-person interviews. The covariates examined included smoking status at the time of breast cancer diagnosis (currently smoking at the time of breast cancer diagnosis, formerly smoking, or never smoking), smoking duration (number of years smoking), smoking intensity (cigarettes smoked per day), number of pack-years of smoking, and regular alcohol consumption the year before diagnosis (categorized as nondrinkers, ≤3 drinks per week, or >3 drinks per week).
Main Outcomes and Measures
Primary outcomes included breast cancer–specific mortality and all-cause mortality.
Results
Among the 1926 women in the study, the mean (SD) age at breast cancer diagnosis was 54.4 (10.8) years. During 13 464 person-years of follow-up (median follow-up, 6.7 years [range, 0.5-16.0 years]), there were 337 deaths, of which 187 (55.5%) were breast cancer related. Compared with never smokers, current smokers at the time of breast cancer diagnosis had a 52% increased risk for all-cause mortality (hazard ratio [HR], 1.52; 95% CI, 1.15-2.02), which was most pronounced for those with 10 or more pack-years of smoking (HR, 1.84; 95% CI, 1.34-2.53). Similar findings were observed for breast cancer–specific mortality (current smokers vs never smokers: HR, 1.27; 95% CI, 0.87-1.85), although they were not statistically significant. There was no statistically significant association between alcohol consumption and all-cause mortality (>3 drinks per week vs nondrinkers: HR, 1.05; 95% CI, 0.73-1.51) or breast cancer–specific mortality (>3 drinks per week vs nondrinkers: HR, 1.06; 95% CI, 0.67-1.67).
Conclusions and Relevance
This population-based cohort study of Black breast cancer survivors suggests that current smoking at the time of diagnosis was associated with an increased risk of all-cause mortality, particularly among women with greater pack-years of smoking.
This cohort study uses data from the Women’s Circle of Health Follow-up Study to examine the association of prediagnostic cigarette smoking and alcohol consumption with all-cause mortality and breast cancer–specific mortality in a cohort of Black breast cancer survivors.
Introduction
Despite advances in treatment and decreasing cancer mortality rates, survival disparities for breast cancer persist.1,2,3 Having a healthy lifestyle that includes not smoking and limited alcohol consumption has been associated with improved overall survival for women with breast cancer.4 Although smoking at the time of a breast cancer diagnosis is consistently associated with increased risk of breast cancer–specific and all-cause mortality,5,6,7 it is unclear whether associations vary by race and ethnicity. Only 3 studies have examined this association for Black or African American breast cancer survivors.8,9,10 The Carolina Breast Cancer Study9 and a pooled study of over 10 000 breast cancer survivors in California10 reported an elevated risk of breast cancer–specific mortality for African American women who were current or ever smokers (vs never smokers), but not for non–African American women.10 Conversely, another study reported smoking to be associated with worse survival among White breast cancer survivors, while Black women had similar survival regardless of smoking status.8
In contrast to smoking, less is known about how alcohol use is associated with breast cancer prognosis,11 despite alcohol use being an established risk factor for breast cancer incidence.11,12,13,14 The evidence linking the association between alcohol intake and breast cancer prognosis has been inconclusive, likely owing to different prognostic outcomes evaluated, variation in drinking patterns, differences in exposure assessment, and failure to adjust for important confounders.15,16,17,18 Moreover, most studies have been conducted with White women, so there is limited information on how drinking may be associated with breast cancer prognosis for Black women. Only 1 study presented stratified results by race and ethnicity, and it was limited to models examining lifetime wine consumption and breast cancer–specific mortality, for which no evidence of increased risk was observed.19
Furthermore, while smoking prevalence has decreased among cancer survivors,20 there has been a persistent trend of increased alcohol consumption among cancer survivors over the past decade.20,21 In addition, there are known differences across racial and ethnic groups in patterns of behaviors and adherence to healthy lifestyle recommendations.22 Understanding how these modifiable lifestyle factors are associated with breast cancer prognosis for Black or African American women (referred to hereafter as Black women) is important for clinical recommendations and management after a breast cancer diagnosis, especially given the 41% higher mortality rate for Black women compared with their White counterparts after a breast cancer diagnosis.1 To this end, we examined the association of cigarette smoking and alcohol consumption with all-cause and breast cancer–specific mortality among participants in the Women’s Circle of Health Follow-up Study (WCHFS), an ongoing population-based cohort study of Black breast cancer survivors in New Jersey.
Methods
Study Population
The WCHFS23 includes 1928 Black breast cancer survivors who received a diagnosis from June 6, 2005, to May 21, 2019, identified in 10 counties in New Jersey through rapid case ascertainment by the New Jersey State Cancer Registry (NJSCR), as previously described.23,24 In brief, eligibility criteria include women who self-identify as Black or African American; have incident, histologically confirmed ductal carcinoma in situ or invasive breast cancer; are aged 20 to 75 years at breast cancer diagnosis; are English speaking; and have no prior history of cancer except nonmelanoma skin cancer. As previously described, distributions of tumor stage and grade were similar for participants and all eligible breast cancer cases identified by the NJSCR, suggesting that the tumor characteristics of WCHFS participants are representative of Black breast cancer survivors in New Jersey.23 All participants provided written informed consent. The study protocol was approved by the institutional review boards at Rutgers University and Roswell Park Comprehensive Cancer Center. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
At the initial home visit, approximately 10 months after breast cancer diagnosis, participants completed an interviewer-administered questionnaire that collected information on sociodemographic factors and breast cancer risk factors, including family history of breast cancer, reproductive factors, hormone use, medical history, medication use, and dietary and lifestyle factors (alcohol intake, smoking history, and physical activity). Anthropometric measurements (weight, height, and body composition), blood pressure measurements, and biospecimens were also collected during the home visits at the time of interview. Information on clinical and tumor characteristics, such as stage and hormone receptor status (eg, estrogen receptor [ER]), were abstracted from pathology reports and NJSCR files. In the present analyses, we excluded 2 women who had missing information on alcohol consumption or smoking status, leaving 1926 women to be included in this study.
Exposure Assessment
For cigarette smoking, we defined smokers as those who reported smoking at least 1 cigarette per day for 1 year and current smokers as women who reported smoking at the time of their breast cancer diagnosis (hereafter referred to as current smokers). Smoking duration was calculated from the age the participants started smoking to the age the participant quit smoking, accounting for periods of time that the women did not smoke. Smoking intensity was caclulated as the mean number of cigarettes smoked per day, during the period when women smoked. We estimated pack-years as the product of smoking intensity (cigarette packs smoked per day) by smoking duration (years smoked). For former and current smokers, we categorized pack-years (<10 or ≥10 pack-years of smoking), smoking duration (<25 or ≥25 years), and smoking intensity (<8 or ≥8 cigarettes per day). For former smokers, we also examined the recency of smoking cessation (≤10 or >10 years).
We asked Black women about their regular consumption of alcoholic beverages during the year before diagnosis. For drinkers, we collected additional information about the amount, type (beer, wine, or liquor), and frequency of alcohol consumed. We defined nondrinkers as women who did not report drinking or drank zero alcoholic beverages per week. For regular drinkers, we estimated the number of alcoholic drinks consumed per week as 3 or less or more than 3 drinks per week, where 1 serving was defined as 355 mL (12 fl oz) of beer, 177 mL (6 fl oz) of wine (1 medium glass), or 45 mL (1.5 fl oz) of liquor (1 shot). We estimated alcoholic drinks per week as the sum of the intake of each of the 3 different types of alcoholic beverages consumed. Because the overall drinking level was low in our cohort, we could not examine drinking levels above the current recommendations of 1 drink per day for women.25
Outcome Ascertainment
Primary outcomes of interest included breast cancer–specific and all-cause mortality. Outcomes were ascertained through linkage with the NJSCR, which collected information on vital status, date of death, and cause of death. The NJSCR is updated using multiple sources, including the National Death Index, hospital discharge files, Medicare and Medicaid files, and Social Security administrative data, allowing for virtually complete ascertainment of mortality.
Statistical Analysis
Statistical analysis was conducted from January 1, 2021, to August 1, 2022. We used Cox proportional hazards regression models to estimate hazard ratios (HRs) and corresponding 95% CIs for the association of each smoking and alcohol variable with all-cause mortality. We estimated the number of person-years from date of diagnosis to death or the end of follow-up (September 3, 2021). When examining breast cancer–specific mortality, we used the Fine-Gray subdistribution hazard model26 to account for competing risks, and death from other causes was considered the competing event. We assessed the proportional hazards assumption through Schoenfeld residuals. We selected covariates for the final model based on directed acyclic graphs and the prior literature. These covariates included age at diagnosis, tumor stage, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), educational level, inflation-adjusted household income, marital status, menopausal status, physical activity, alcohol consumption (for smoking variables), and smoking status (for alcohol consumption). For models examining recency of smoking cessation, the covariates included age at diagnosis, tumor stage, waist-to-hip ratio, history of diabetes, history of hypertension, educational level, inflation-adjusted household income, and physical activity. Treatment variables (type of surgery, chemotherapy, radiotherapy, and hormone therapy) were considered in the directed acyclic graphs; they were not included in the final models because they were not determined to be confounders. As a sensitivity analysis, we adjusted for treatment variables that were associated with our exposures (surgery, chemotherapy, and radiotherapy); this adjustment did not substantially change our results. We also examined alcohol and smoking combined by examining the statistical interaction of the 2 variables on the multiplicative and additive scale. We assessed a multiplicative interaction by including a cross-product term in the model and assessing the corresponding β coefficient using the Wald test, and we assesed an additive interaction by estimating the relative excess risk due to the interaction.27 For all variables, we examined a multiplicative interaction by age group (<40, 40 to ≤60, and >60 years), menopausal status (premenopausal or postmenopausal), ER status, BMI (<30 and ≥30), waist-to-hip ratio (≤0.85 and >0.85), and family history of breast cancer (yes or no), as well as stratified models. We performed sensitivity analyses by excluding ductal carcinoma in situ cases and excluding women with a diagnosis of stage IV tumors. We also performed sensitivity analyses estimating follow-up time starting from date of interview (instead of date of diagnosis) to account for potential immortal time bias; this did not change our estimates. All statistical tests were 2-sided, and P < .05 was considered statistically significant. All statistical analyses were performed in SAS, version 9.4 (SAS Institute Inc).
Results
Among the 1926 women in the study, the mean (SD) age at breast cancer diagnosis was 54.4 (10.8) years (Table 1). During 13 464 person-years of follow-up (median follow-up, 6.7 years [range, 0.5-16.0 years]), we observed 337 deaths, of which 187 (55.5%) were breast cancer specific. Other causes of death included other cancers, not including breast cancer (39 of 337 [11.6%]); cardiovascular, vascular, or atherosclerotic disease (32 of 337 [9.5%]); infections including COVID-19 (12 of 337 [3.6%]); respiratory conditions (10 of 337 [3.0%]); kidney failure (5 of 337 [1.5%]); metabolic disorder, diabetes, or obesity-related death (5 of 337 [1.5%]); pregnancy-related death (5 of 337 [1.5%]); and other causes (42 of 337 [12.5%]). Descriptive characteristics of the cohort overall and by smoking status are shown in Table 1. Of the 1926 women in this study, 16.5% (n = 317) reported smoking at the time of their breast cancer diagnosis, 24.3% (n = 468) reported former smoking, and more than half reported never smoking (59.2% [n = 1141]). Less than half of the women (41.5% [n = 799]) reported regularly consuming alcohol in the year before their breast cancer diagnosis. Of regular drinkers, 70.6% (n = 564) reported consuming 3 or fewer alcoholic drinks per week, and less than one-third of regular drinkers drank more than 3 drinks per week (28.3% [n = 226]). Current smokers consumed more alcoholic beverages per week (19.9% [63 of 317] consuming >3 drinks per week) than did former smokers (14.3% [67 of 468] consuming >3 drinks per week) or never smokers (8.4% [96 of 1141] consuming >3 drinks per week). Estrogen receptor status was similar across levels of smoking status, with 70.3% of the overall cohort (n = 1354) having an ER-positive tumor.
Table 1. Descriptive Characteristics of Participants in the Women’s Circle of Health Follow-up Study, by Cigarette Smoking.
| Characteristic | Participants, No. (%) | P valuea | |||
|---|---|---|---|---|---|
| Never smokers (n = 1141) | Former smokers (n = 468) | Current smokers (n = 317) | Total (N = 1926) | ||
| Age at breast cancer diagnosis, mean (SD), y | 53.0 (11.2) | 58.3 (9.4) | 53.9 (9.6) | 54.4 (10.8) | .001 |
| Age at breast cancer diagnosis, y | |||||
| <40 | 149 (13.1) | 18 (3.8) | 26 (8.2) | 193 (10.0) | .001 |
| 40 to ≤60 | 669 (58.6) | 243 (51.9) | 214 (67.5) | 1126 (58.5) | |
| >60 | 323 (28.3) | 207 (44.2) | 77 (24.3) | 607 (31.5) | |
| Menopausal status | |||||
| Premenopausal | 526 (46.1) | 112 (23.9) | 109 (34.4) | 747 (38.8) | .001 |
| Postmenopausal | 615 (53.9) | 356 (76.1) | 208 (65.6) | 1179 (61.2) | |
| Smoking | |||||
| Never smokers | 1141 (100) | NA | NA | 1141 (59.2) | NA |
| Former smokers, pack-years | |||||
| <10 | NA | 284 (60.7) | NA | 284 (14.7) | |
| ≥10 | NA | 184 (39.3) | NA | 184 (9.6) | |
| Current smokers, pack-years | |||||
| <10 | NA | NA | 128 (40.4) | 128 (6.6) | |
| ≥10 | NA | NA | 189 (59.6) | 189 (9.8) | |
| Smoking intensity | |||||
| Never smokers | 1141 (100) | NA | NA | 1141 (59.2) | NA |
| Former smokers, cigarettes/d | |||||
| <8 | NA | 232 (49.6) | NA | 232 (12.0) | |
| ≥8 | NA | 236 (50.4) | NA | 236 (12.3) | |
| Current smokers, cigarettes/d | |||||
| <8 | NA | NA | 144 (45.4) | 144 (7.5) | |
| ≥8 | NA | NA | 173 (54.6) | 173 (9.0) | |
| Smoking duration | NA | ||||
| Never smokers | 1141 (100) | NA | NA | 1141 (59.2) | |
| Former smokers, y | |||||
| <25 | NA | 316 (67.5) | NA | 316 (16.4) | |
| ≥25 | NA | 152 (32.5) | NA | 152 (7.9) | |
| Current smokers, y | |||||
| <25 | NA | NA | 69 (21.8) | 69 (3.6) | |
| ≥25 | NA | NA | 248 (78.2) | 248 (12.9) | |
| Recency of cessation for former smokers, y | |||||
| ≤10 | NA | 152 (32.5) | NA | 152 (7.9) | NA |
| >10 | NA | 316 (67.5) | NA | 316 (16.4) | |
| Age at smoking initiation, mean (SD), y | NA | 19.5 (5.7) | 19.2 (5.8) | 19.4 (5.8) | .47 |
| Smoking duration, mean (SD), y | NA | 19.5 (12.0) | 33.3 (11.2) | 25.1 (13.5) | <.001 |
| Smoking intensity, mean (SD), cigarettes/d | NA | 10.3 (8.5) | 9.4 (6.1) | 10.0 (7.7) | .09 |
| Alcohol consumption | |||||
| Nondrinkerb | 716 (62.8) | 263 (56.2) | 148 (46.7) | 1127 (58.5) | .001 |
| ≤3 drinks/wk | 324 (28.4) | 137 (29.3) | 103 (32.5) | 564 (29.3) | |
| >3 drinks/wk | 96 (8.4) | 67 (14.3) | 63 (19.9) | 226 (11.7) | |
| Missing | 5 (0.4) | 1 (0.2) | 3 (0.9) | 9 (0.5) | |
| BMI | |||||
| <30 | 511 (44.8) | 173 (37.0) | 160 (50.5) | 844 (43.8) | .001 |
| ≥30 | 628 (55.0) | 294 (62.8) | 156 (49.2) | 1078 (56.0) | |
| Missing | 2 (0.2) | 1 (0.2) | 1 (0.3) | 4 (0.2) | |
| BMI, mean (SD) | 31.7 (6.9) | 33.1 (7.0) | 30.9 (7.3) | 31.9 (7.0) | .001 |
| Waist-to-hip ratio | |||||
| ≤0.85 | 383 (33.6) | 110 (23.5) | 83 (26.2) | 576 (29.9) | .001 |
| >0.85 | 729 (63.9) | 348 (74.4) | 224 (70.7) | 1301 (67.5) | |
| Missing | 29 (2.5) | 10 (2.1) | 10 (3.2) | 49 (2.5) | |
| Physical activity, MET h/wk | |||||
| <11.7 | 350 (30.7) | 154 (32.9) | 131 (41.3) | 635 (33.0) | .007 |
| 11.8-37.9 | 402 (35.2) | 148 (31.6) | 91 (28.7) | 641 (33.3) | |
| ≥38 | 389 (34.1) | 163 (34.8) | 94 (29.7) | 646 (33.5) | |
| Missing | 0 | 3 (0.6) | 1 (0.3) | 4 (0.2) | |
| Physical activity, mean (SD), MET h/wk | 38.5 (49.7) | 39.1 (46.9) | 34.2 (48.2) | 37.9 (48.8) | .32 |
| Educational level | |||||
| High school graduate or less | 391 (34.3) | 181 (38.7) | 164 (51.7) | 736 (38.2) | .001 |
| Some college or vocational school | 346 (30.3) | 155 (33.1) | 104 (32.8) | 605 (31.4) | |
| Bachelor’s or graduate degree | 403 (35.3) | 132 (28.2) | 48 (15.1) | 583 (30.3) | |
| Missing | 1 (0.1) | 0 | 1 (0.3) | 2 (0.1) | |
| Household income per capita, adjusted for inflation, $ | |||||
| <15 000 | 349 (30.6) | 137 (29.3) | 144 (45.4) | 630 (32.7) | .001 |
| 15 000-29 999 | 376 (33.0) | 132 (28.2) | 97 (30.6) | 605 (31.4) | |
| ≥30 000 | 331 (29.0) | 162 (34.6) | 56 (17.7) | 549 (28.5) | |
| Unknown | 85 (7.4) | 37 (7.9) | 20 (6.3) | 142 (7.4) | |
| Insurance | |||||
| Private | 691 (60.6) | 255 (54.5) | 130 (41.0) | 1076 (55.9) | .001 |
| Medicare or Medicaid | 254 (22.3) | 153 (32.7) | 134 (42.3) | 541 (28.1) | |
| Uninsured | 115 (10.1) | 39 (8.3) | 43 (13.6) | 197 (10.2) | |
| Other or missing | 81 (7.1) | 21 (4.5) | 10 (3.2) | 112 (5.8) | |
| Marital status | |||||
| Married or living as married | 461 (40.4) | 169 (36.1) | 79 (24.9) | 709 (36.8) | .001 |
| Widow | 101 (8.9) | 69 (14.7) | 19 (6.0) | 189 (9.8) | |
| Divorced or separated | 263 (23.0) | 121 (25.9) | 84 (26.5) | 468 (24.3) | |
| Single or never married | 314 (27.5) | 107 (22.9) | 135 (42.6) | 556 (28.9) | |
| Missing | 2 (0.2) | 2 (0.4) | 0 | 4 (0.2) | |
| Estrogen receptor status | |||||
| Positive | 806 (70.6) | 324 (69.2) | 224 (70.7) | 1354 (70.3) | .63 |
| Negative | 294 (25.8) | 132 (28.2) | 89 (28.1) | 515 (26.7) | |
| Missing | 41 (3.6) | 12 (2.6) | 4 (1.3) | 57 (3.0) | |
| Tumor stage | |||||
| 0 | 195 (17.1) | 107 (22.9) | 42 (13.2) | 344 (17.9) | .001 |
| I | 408 (35.8) | 183 (39.1) | 104 (32.8) | 695 (36.1) | |
| II | 368 (32.3) | 124 (26.5) | 115 (36.3) | 607 (31.5) | |
| III and IV | 147 (12.9) | 46 (9.8) | 51 (16.1) | 244 (12.7) | |
| Missing | 23 (2.0) | 8 (1.7) | 5 (1.6) | 36 (1.9) | |
| Breast cancer subtype | |||||
| Luminal A | 538 (47.2) | 220 (47.0) | 166 (52.4) | 924 (48.0) | .38 |
| ERBB2 (formerly HER2) positive | 208 (18.2) | 65 (13.9) | 54 (17.0) | 327 (17.0) | |
| Triple negative | 191 (16.7) | 85 (18.2) | 57 (18.0) | 333 (17.3) | |
| Unknown or missing | 204 (17.9) | 98 (20.9) | 40 (12.6) | 342 (17.8) | |
| Surgery | |||||
| No surgery | 30 (2.6) | 15 (3.2) | 21 (6.6) | 66 (3.4) | .001 |
| Lumpectomy | 576 (50.5) | 267 (57.1) | 136 (42.9) | 979 (50.8) | |
| Mastectomy | 535 (46.9) | 185 (39.5) | 160 (50.5) | 880 (45.7) | |
| Unknown or missing | 0 | 1 (0.2) | 0 | 1 (0.1) | |
| Chemotherapy | |||||
| No | 495 (43.4) | 247 (52.8) | 132 (41.6) | 874 (45.4) | .001 |
| Yes | 644 (56.4) | 219 (46.8) | 184 (58.0) | 1047 (54.4) | |
| Unknown or missing | 2 (0.2) | 2 (0.4) | 1 (0.3) | 5 (0.3) | |
| Radiotherapy | |||||
| No | 376 (33.0) | 134 (28.6) | 117 (36.9) | 627 (32.6) | .04 |
| Yes | 765 (67.0) | 333 (71.2) | 196 (61.8) | 1294 (67.2) | |
| Unknown or missing | 0 | 1 (0.2) | 4 (1.3) | 5 (0.3) | |
| Hormone therapy | |||||
| No | 399 (35.0) | 169 (36.1) | 121 (38.2) | 689 (35.8) | .48 |
| Yes | 741 (64.9) | 296 (63.2) | 192 (60.6) | 1229 (63.8) | |
| Unknown or missing | 1 (0.09) | 3 (0.6) | 4 (1.3) | 8 (0.4) | |
| History of diabetes | |||||
| No | 805 (70.6) | 277 (59.2) | 209 (65.9) | 1291 (67.0) | .001 |
| Yes | 222 (19.5) | 136 (29.1) | 72 (22.7) | 430 (22.3) | |
| Unknown or missing | 114 (10.0) | 55 (11.8) | 36 (11.4) | 205 (10.6) | |
| History of hypertension | |||||
| No | 370 (32.4) | 107 (22.9) | 75 (23.7) | 552 (28.7) | .001 |
| Yes | 657 (57.6) | 306 (65.4) | 206 (65.0) | 1169 (60.7) | |
| Unknown or missing | 114 (10.0) | 55 (11.8) | 36 (11.4) | 205 (10.6) | |
| First-degree family history of breast cancer | |||||
| No | 916 (80.3) | 374 (79.9) | 255 (80.4) | 1545 (80.2) | .85 |
| Yes | 198 (17.4) | 87 (18.6) | 54 (17.0) | 339 (17.6) | |
| Missing | 27 (2.4) | 7 (1.5) | 8 (2.5) | 42 (2.2) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MET, metabolic equivalent task; NA, not applicable.
From the χ2 test, t test, or analysis of variance when appropriate.
Nondrinkers defined as women who did not report drinking or drank zero alcoholic beverages per week.
Smoking at the time of diagnosis was associated with an increased risk of all-cause mortality; compared with never smokers, current smokers had a 52% increased risk for all-cause mortality (HR, 1.52; 95% CI, 1.15-2.02) after adjusting for covariates (Table 2). When we examined this association within levels of pack-years, we found an 84% increased risk of all-cause mortality for current smokers who smoked 10 or more cumulative pack-years (HR, 1.84; 95% CI, 1.34-2.53) and no association for current smokers who smoked fewer than 10 pack-years (HR, 1.06; 95% CI, 0.67-1.66). We also observed an increased risk of breast cancer–specific mortality for current smokers, although less substantial than all-cause mortality and with wider 95% CIs, likely due to limited power. Compared with never smokers, current smokers had a 27% increased risk for breast cancer–specific mortality (HR, 1.27; 95% CI, 0.87-1.85), which was greater for women who were heavier smokers, at 41% (≥10 pack-years: HR, 1.41; 95% CI, 0.88-2.26). In addition, recency of smoking cessation was not associated with either all-cause or breast cancer–specific mortality. The overall findings for smoking variables were similar in sensitivity analyses of models excluding stage IV tumors (eTable 1 in Supplement 1), models limited to only invasive breast cancers (eTable 2 in Supplement 1), and models adjusted for treatment factors including chemotherapy, surgery, and radiotherapy (current smokers [vs never smokers]: HR, 1.45; 95% CI, 1.09-1.93).
Table 2. Associations of Cigarette Smoking With Mortality After First Breast Cancer in the Women’s Circle of Health Follow-up Study.
| Characteristic | All-cause mortality | Breast cancer–specific mortality | ||||
|---|---|---|---|---|---|---|
| Person-years | Total deaths | HR (95% CI) | Person-years | Breast cancer deaths | HR (95% CI) | |
| Smoking statusa | ||||||
| Never smokers | 7897.2 | 168 | 1 [Reference] | 7897.2 | 102 | 1 [Reference] |
| Former smokers | 3261.5 | 82 | 1.25 (0.95-1.65) | 3261.5 | 41 | 1.22 (0.82-1.81) |
| Current smokers | 2094.2 | 80 | 1.52 (1.15-2.02) | 2094.2 | 42 | 1.27 (0.87-1.85) |
| No. of pack-years of smokinga | ||||||
| Never smokers | 7897.2 | 168 | 1 [Reference] | 7897.2 | 102 | 1 [Reference] |
| Former smokers | ||||||
| <10 | 1951.2 | 49 | 1.19 (0.86-1.65) | 1951.2 | 27 | 1.21 (0.77-1.89) |
| ≥10 | 1310.3 | 33 | 1.39 (0.94-2.05) | 1310.3 | 14 | 1.24 (0.67-2.31) |
| Current smokers | ||||||
| <10 | 886.4 | 23 | 1.06 (0.67-1.66) | 886.4 | 15 | 1.06 (0.62-1.81) |
| ≥10 | 1207.8 | 57 | 1.84 (1.34-2.53) | 1207.8 | 27 | 1.41 (0.88-2.26) |
| Duration of smoking, ya | ||||||
| Never smokers | 7897.2 | 168 | 1 [Reference] | 7897.2 | 102 | 1 [Reference] |
| Former smokers | ||||||
| <25 | 2244.4 | 48 | 1.08 (0.78-1.50) | 2244.4 | 24 | 1.01 (0.63-1.64) |
| ≥25 | 1017.1 | 34 | 1.67 (1.13-2.47) | 1017.1 | 17 | 1.87 (1.06-3.29) |
| Current smokers | ||||||
| <25 | 448.2 | 17 | 1.43 (0.85-2.42) | 448.2 | 8 | 0.80 (0.39-1.65) |
| ≥25 | 1645.9 | 63 | 1.56 (1.15-2.11) | 1645.9 | 34 | 1.48 (0.97-2.25) |
| Intensity of smokinga | ||||||
| Never smokers | 7897.2 | 168 | 1 [Reference] | 7897.2 | 102 | 1 [Reference] |
| Former smokers, cigarettes/d | ||||||
| <8 | 1583.4 | 41 | 1.22 (0.86-1.74) | 1583.4 | 22 | 1.26 (0.78-2.03) |
| ≥8 | 1678.1 | 41 | 1.28 (0.90-1.82) | 1678.1 | 19 | 1.17 (0.68-2.03) |
| Current smokers, cigarettes/d | ||||||
| <8 | 982.1 | 31 | 1.35 (0.91-2.02) | 982.1 | 19 | 1.34 (0.82-2.21) |
| ≥8 | 1112.1 | 49 | 1.65 (1.18-2.30) | 1112.1 | 23 | 1.21 (0.74-2.00) |
| Recency of cessationb | ||||||
| Never smokers | 6889.7 | 136 | 1 [Reference] | 6889.7 | 81 | 1 [Reference] |
| Current smokers | 1830.3 | 65 | 1.55 (1.15-2.10) | 1830.3 | 32 | 1.21 (0.79-1.84) |
| Former smokers, y | ||||||
| ≤10 | 856.3 | 23 | 1.40 (0.89-2.20) | 856.3 | 11 | 1.26 (0.63-2.51) |
| >10 | 1878.6 | 44 | 1.19 (0.84-1.70) | 1878.6 | 23 | 1.22 (0.74-2.00) |
Abbreviation: HR, hazard ratio.
Cox proportional hazards regression models adjusted for age at diagnosis, tumor stage, body mass index, alcohol consumption, educational level, household income, marital status, menopausal status, and physical activity. Competing risk models (Fine-Gray subdistribution hazard model) were used for breast cancer–specific mortality.
Cox proportional hazards regression models adjusted for age at diagnosis, tumor stage, waist-to-hip ratio, history of diabetes, history of hypertension, educational level, household income, and physical activity. Competing risk models (Fine-Gray subdistribution hazard model) were used for breast cancer–specific mortality.
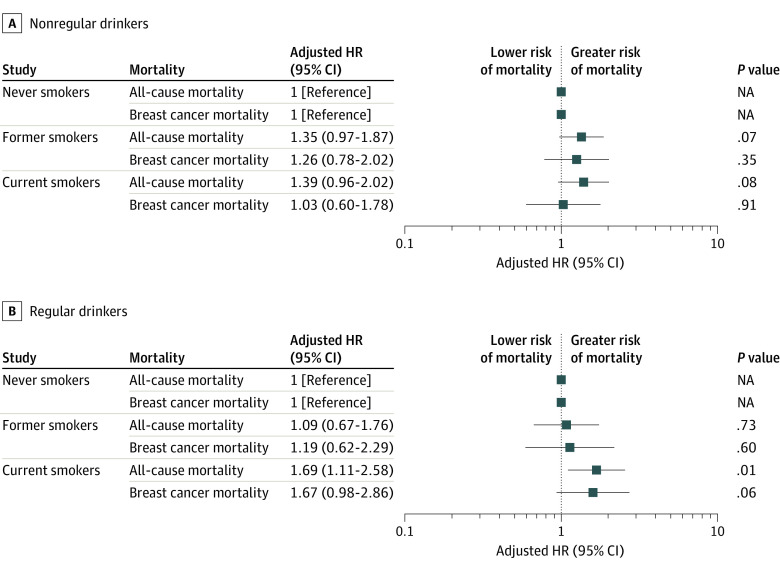
As shown in Table 3, regular consumption of alcohol the year before diagnosis was not associated with all-cause mortality (>3 drinks per week vs nondrinkers: HR, 1.05; 95% CI, 0.73-1.51) or breast cancer–specific mortality mortality (>3 drinks per week vs nondrinkers: HR, 1.06; 95% CI, 0.67-1.67). To further assess the combination of smoking and drinking combined, we examined the interaction of the 2 exposures on a multiplicative and additive scale. Although the multiplicative and additive interaction of smoking and alcohol were not statistically significant, compared with never smokers who regularly drank alcohol, current smokers who regularly drank alcohol had a 69% increased risk of all-cause mortality (HR, 1.69; 95% CI, 1.11-2.58), whereas the association for current smokers who did not regularly drink alcohol (compared with never smokers who did not regularly drink alcohol) was less substantial (HR, 1.39; 95% CI, 0.96-2.02) (Figure 1). We observed similar associations for breast cancer–specific mortality, with a 67% increased risk for current smokers who regularly drank alcohol compared with never smokers who regularly drank alcohol (HR, 1.67; 95% CI, 0.98-2.86), but no association for those who were not regular drinkers (Figure 1).
Table 3. Associations of Prediagnostic Alcohol Consumption With Mortality After First Breast Cancer in the Women's Circle of Health Follow-up Study.
| Total alcoholic drinks per week | All-cause mortality | Breast cancer–specific mortality | ||||
|---|---|---|---|---|---|---|
| Person-years | Total deaths | HR (95% CI) | Person-years | Breast cancer deaths | HR (95% CI)a | |
| Nondrinkerb | 7928.6 | 211 | 1 [Reference] | 7928.6 | 114 | 1 [Reference] |
| ≤3 | 3870.7 | 81 | 0.83 (0.64-1.09) | 3870.7 | 47 | 0.79 (0.55-1.12) |
| >3 | 1453.6 | 38 | 1.05 (0.73-1.51) | 1453.6 | 24 | 1.06 (0.67-1.67) |
Abbreviation: HR, hazard ratio.
Cox proportional hazards regression models adjusted for age at diagnosis, tumor stage, body mass index, cigarette smoking, educational level, household income, marital status, menopausal status, and physical activity. Competing risk models (Fine-Gray subdistribution hazard model) were used for breast cancer–specific mortality.
Defined as women who did not report drinking or drank zero alcoholic beverages per week.
Figure 1. Association of Smoking Status With All-Cause Mortality and Breast Cancer–Specific Mortality, by Drinking Status.
Hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards regression models adjusted for age at diagnosis, tumor stage, body mass index, educational level, household income, marital status, menopausal status, and physical activity. NA indicates not applicable.
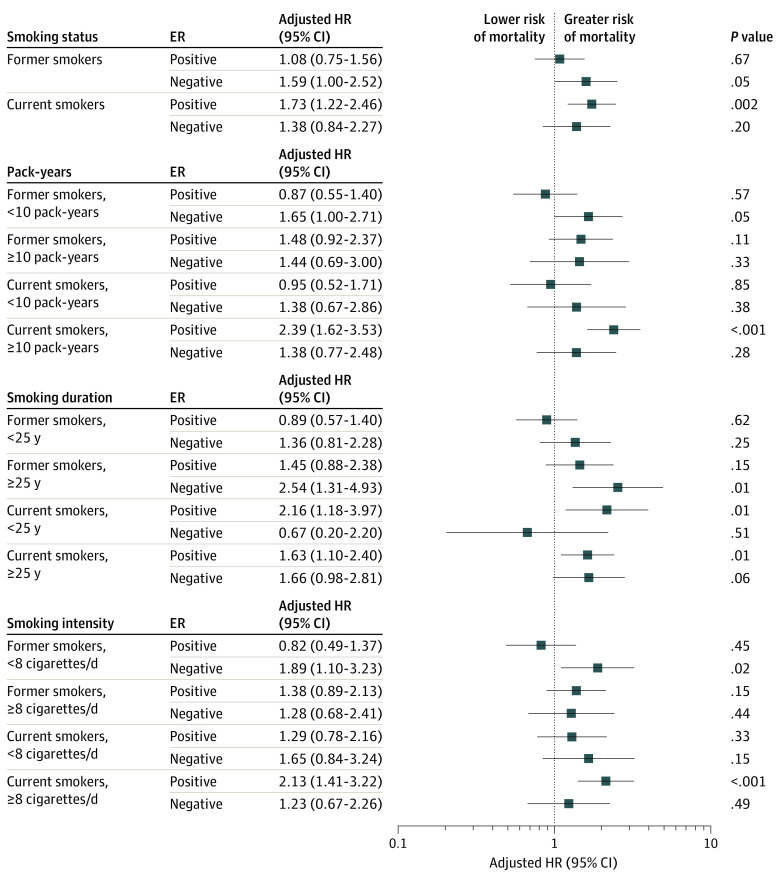
We did not find evidence of a multiplicative interaction with any of the smoking or alcohol variables and ER status, age group, menopausal status, BMI, waist-to-hip ratio, or family history of breast cancer. In stratified analyses by ER status, we observed an elevated risk of all-cause mortality for both ER-positive and ER-negative tumors for current smokers compared with never smokers (ER positive: HR, 1.73; 95% CI, 1.22-2.46; ER negative: HR, 1.38; 95% CI, 0.84-2.27), although this finding was not statistically significant for ER-negative tumors (Figure 2).
Figure 2. Association of Smoking Variables With All-Cause Mortality, Stratified by Estrogen Receptor (ER) Status.
The reference category is never smokers. Hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards regression models adjusted for age at diagnosis, tumor stage, body mass index, alcohol consumption, educational level, household income, marital status, menopausal status, and physical activity.
Discussion
In this large population-based cohort study of Black breast cancer survivors, smoking at the time of diagnosis was associated with an increased risk of all-cause mortality. Our findings of elevated risk of all-cause mortality for current smokers are consistent with prior findings from studies primarily comprising White women.6,28 Three prior studies examined these associations for Black women8,9,10 but had limited information on detailed smoking behaviors and did not examine smoking and alcohol consumption combined. John et al10 reported a 47% increased risk of all-cause mortality for African American women with ER-positive or progesterone receptor–positive tumors who ever smoked (compared with never smokers) but no increased risk for non-Hispanic White women or African American ever smokers with ER-negative tumors. On the other hand, 2 studies reported an association of elevated risk of all-cause mortality among current smokers, but only for non–African American women.8,9
Parada et al9 reported an association of current smoking and all-cause mortality for current smokers, but only for women who survived at least 5 years after diagnosis; however, when stratified by race, this association was not observed among African American women. This study reported a 69% increased risk of breast cancer–specific mortality for African American women who survived at least 5 years after diagnosis, but not for non–African American women. This finding contrasts with our findings of an elevated risk of all-cause mortality but not breast cancer–specific mortality for current smokers vs never smokers. The study by Parada et al9 had a longer follow-up time than our study (median follow-up, 13.6 vs 6.7 years), so our findings for breast cancer–specific mortality may be limited by the fewer number of deaths and shorter follow-up.
In our study, we found a 41% increased risk for breast cancer–specific mortality for current smokers with 10 or more pack-years (vs never smokers), but the 95% CI included the null value. In a study using large administrative databases in Florida, current smoking was associated with worse survival for White and Hispanic women but not for Black women8; however, that study had information only on smoking status and intensity, had a short mean follow-up of 4.9 years, and did not directly ask women about their smoking history. Although an overall association was not observed between smoking and breast cancer–specific mortality, possibly due to insufficient follow-up time, the association between smoking and poorer prognosis for breast cancer survivors is biologically plausible, and several mechanisms have been proposed. Smoking can enhance the metastatic ability of breast cancer cells and induce tumor growth,29,30 is associated with other comorbidities that affect survival, and may increase resistance to cancer therapies.31
Our finding of no association between alcohol consumption and all-cause mortality or breast cancer–specific mortality is consistent with prior literature.18,19,32,33 However, our cohort had low levels of alcohol consumption. A meta-analysis reported an association between 20 g per day of alcohol consumption, but not lower levels of consumption, and increased breast cancer mortality.15 In addition, there are known differences in alcohol consumption patterns between racial and ethnic subgroups,34,35 with lower levels of drinking reported for Black women compared with White women.
Even though we did not observe an association with drinking and all-cause mortality or breast cancer–specific mortality, we did find a stronger association with mortality for current smokers who regularly drank alcohol. In stratified analyses, we observed a 69% increased risk for all-cause mortality and a 67% increased risk for breast cancer–specific mortality for women who both regularly drank and were current smokers compared with never smokers who regularly drank, but we observed no association for current smokers who were nonregular drinkers. To our knowledge, no other study has examined the association of mortality with smoking and drinking combined among Black women. Consistent with our findings, Din et al36 reported a 92% increased risk of breast cancer–specific mortality among current smokers who consumed alcohol, but the study did not report stratified models. In addition, similar to the differences in alcohol patterns by race and ethnicity, a recent study using National Health and Nutrition Examination Survey data for female cancer survivors reported that Black women were less likely than non-Hispanic White women to report smoking.22 However, Black cancer survivors with at least 1 comorbidity were more likely to smoke within their lifetime compared with non-Hispanic White survivors. Considering the high prevalence of comorbidities among Black breast cancer survivors, drinking history should be captured along with smoking history during clinical management, and there may be a benefit associated with targeted prevention efforts for women who both smoke and regularly consume alcohol. Moreover, recent alcohol consumption was found to be associated with continued smoking after a cancer diagnosis among African American cancer survivors,37 underscoring the importance of examining these behaviors together.
Limitations and Strengths
Our study has some limitations. We could not assess the association of smoking or alcohol consumption after diagnosis because we had limited power given that only women recruited after 2014 (when additional funding was obtained) were invited to the follow-up study.23 However, of the subset of women with smoking and drinking information at follow-up, 86% of those who reported smoking since their breast cancer diagnosis were also current smokers at breast cancer diagnosis, suggesting that prediagnosis and postdiagnosis smoking are associated. We observed similar findings for alcohol consumption. We also did not have information on other types of smoking, including passive smoking, e-cigarette use, and hookah smoking. In addition, both alcohol and smoking exposures were based on self-report and may be underreported. Finally, we had limited power to examine interactions, especially for breast cancer–specific mortality or associations by breast cancer subtype.
Our study also has some strengths. A major strength is the cohort because the WCHFS is one of the few population-based epidemiologic studies available of Black breast cancer survivors that has extensive epidemiologic data, with data on outcomes after breast cancer diagnosis. This allowed us to examine the association of several smoking exposures and of alcohol and smoking combined, as well as adequately control for important confounders.
Conclusions
Our cohort study suggests that smoking at the time of a breast cancer diagnosis is associated with an increased risk of all-cause mortality, particularly for women who also drank and had 10 or more pack-years of smoking. Although we observed a similar elevated risk associated with smoking for breast cancer–specific mortality, risk estimates were not statistically significant, likely due to limited power. Future studies among Black breast cancer survivors are warranted to understand the role of smoking and alcohol in prognosis, including its role in recurrence and mortality due to breast cancer and by tumor subtype, as well as factors associated with continued smoking. Our findings add to the evidence of the detrimental health effects of smoking and underscore the need of tailored and targeted survivorship care for breast cancer survivors, particularly women with heavier levels of smoking.
eTable 1. Hazard Ratios and 95% Confidence Intervals for Associations of Cigarette Smoking and Mortality After First Breast Cancer in the Women’s Circle of Health Follow-up Study (WCHFS), Excluding Stage IV Tumors
eTable 2. Hazard Ratios and 95% Confidence Intervals for Associations of Cigarette Smoking and Mortality After First Breast Cancer in the Women’s Circle Of Health Follow-up Study (WCHFS), Excluding Ductal Carcinoma In-Situ
Data Sharing Statement
References
- 1.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi: 10.3322/caac.21555 [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363-385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Lesseur C, Neugut AI, et al. The associations of healthy lifestyle index with breast cancer incidence and mortality in a population-based study. Breast Cancer. 2022;29(6):957-966. doi: 10.1007/s12282-022-01374-w [DOI] [PubMed] [Google Scholar]
- 5.Bérubé S, Lemieux J, Moore L, Maunsell E, Brisson J. Smoking at time of diagnosis and breast cancer–specific survival: new findings and systematic review with meta-analysis. Breast Cancer Res. 2014;16(2):R42. doi: 10.1186/bcr3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan W, Li S, Meng X, Sun Y, Jia C. Smoking and survival of breast cancer patients: a meta-analysis of cohort studies. Breast. 2017;33:117-124. doi: 10.1016/j.breast.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 7.Passarelli MN, Newcomb PA, Hampton JM, et al. Cigarette smoking before and after breast cancer diagnosis: mortality from breast cancer and smoking-related diseases. J Clin Oncol. 2016;34(12):1315-1322. doi: 10.1200/jco.2015.63.9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padron-Monedero A, Tannenbaum SL, Koru-Sengul T, et al. Smoking and survival in female breast cancer patients. Breast Cancer Res Treat. 2015;150(2):395-403. doi: 10.1007/s10549-015-3317-3 [DOI] [PubMed] [Google Scholar]
- 9.Parada H Jr, Sun X, Tse CK, Olshan AF, Troester MA, Conway K. Active smoking and survival following breast cancer among African American and non–African American women in the Carolina Breast Cancer Study. Cancer Causes Control. 2017;28(9):929-938. doi: 10.1007/s10552-017-0923-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.John EM, McGuire V, Kurian AW, et al. Racial/ethnic disparities in survival after breast cancer diagnosis by estrogen and progesterone receptor status: a pooled analysis. Cancer Epidemiol Biomarkers Prev. 2021;30(2):351-363. doi: 10.1158/1055-9965.EPI-20-1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36(1):83-93. doi: 10.1200/jco.2017.76.1155 [DOI] [PubMed] [Google Scholar]
- 12.Gapstur SM, Bandera EV, Jernigan DH, et al. Alcohol and cancer: existing knowledge and evidence gaps across the cancer continuum. Cancer Epidemiol Biomarkers Prev. 2022;31(1):5-10. doi: 10.1158/1055-9965.Epi-21-0934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Cancer Research Fund; American Institute for Cancer Research . Alcoholic drinks and the risk of cancer. 2018. Accessed July 1, 2022. https://www.wcrf.org/wp-content/uploads/2021/02/Alcoholic-Drinks.pdf
- 14.Zeinomar N, Knight JA, Genkinger JM, et al. ; kConFab Investigators . Alcohol consumption, cigarette smoking, and familial breast cancer risk: findings from the Prospective Family Study Cohort (ProF-SC). Breast Cancer Res. 2019;21(1):128. doi: 10.1186/s13058-019-1213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gou YJ, Xie DX, Yang KH, et al. Alcohol consumption and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14(8):4785-4790. doi: 10.7314/apjcp.2013.14.8.4785 [DOI] [PubMed] [Google Scholar]
- 16.Weaver AM, McCann SE, Nie J, et al. Alcohol intake over the life course and breast cancer survival in Western New York exposures and breast cancer (WEB) study: quantity and intensity of intake. Breast Cancer Res Treat. 2013;139(1):245-253. doi: 10.1007/s10549-013-2533-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali AMG, Schmidt MK, Bolla MK, et al. Alcohol consumption and survival after a breast cancer diagnosis: a literature-based meta-analysis and collaborative analysis of data for 29,239 cases. Cancer Epidemiol Biomarkers Prev. 2014;23(6):934-945. doi: 10.1158/1055-9965.Epi-13-0901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeinomar N, Thai A, Cloud AJ, McDonald JA, Liao Y, Terry MB. Alcohol consumption and breast cancer–specific and all-cause mortality in women diagnosed with breast cancer at the New York site of the Breast Cancer Family Registry. PLoS One. 2017;12(12):e0189118. doi: 10.1371/journal.pone.0189118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H, Malone KE, McDonald JA, et al. Pre-diagnosis alcohol consumption and mortality risk among Black women and White women with invasive breast cancer. BMC Cancer. 2019;19(1):800. doi: 10.1186/s12885-019-5991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arem H, Mama SK, Duan X, Rowland JH, Bellizzi KM, Ehlers DK. Prevalence of healthy behaviors among cancer survivors in the United States: how far have we come? Cancer Epidemiol Biomarkers Prev. 2020;29(6):1179-1187. doi: 10.1158/1055-9965.Epi-19-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanford NN, Sher DJ, Xu X, et al. Alcohol use among patients with cancer and survivors in the United States, 2000-2017. J Natl Compr Canc Netw. 2020;18(1):69-79. doi: 10.6004/jnccn.2019.7341 [DOI] [PubMed] [Google Scholar]
- 22.Dibble KE, Connor AE. Evaluation of disparities in maintaining healthy lifestyle behaviors among female cancer survivors by race/ethnicity and US nativity. Cancer Epidemiol. 2022;80:102235. doi: 10.1016/j.canep.2022.102235 [DOI] [PubMed] [Google Scholar]
- 23.Bandera EV, Demissie K, Qin B, et al. The Women’s Circle of Health Follow-Up Study: a population-based longitudinal study of Black breast cancer survivors in New Jersey. J Cancer Surviv. 2020;14(3):331-346. doi: 10.1007/s11764-019-00849-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandera EV, Qin B, Lin Y, et al. Association of body mass index, central obesity, and body composition with mortality among Black breast cancer survivors. JAMA Oncol. 2021;7(8):1-10. doi: 10.1001/jamaoncol.2021.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245-271. doi: 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 27.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3:33-72. doi: 10.1515/em-2013-0005 [DOI] [Google Scholar]
- 28.Izano M, Satariano WA, Hiatt RA, Braithwaite D. Smoking and mortality after breast cancer diagnosis: the Health and Functioning in Women study. Cancer Med. 2015;4(2):315-324. doi: 10.1002/cam4.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Cello F, Flowers VL, Li H, et al. Cigarette smoke induces epithelial to mesenchymal transition and increases the metastatic ability of breast cancer cells. Mol Cancer. 2013;12:90. doi: 10.1186/1476-4598-12-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallicchio L, Flaws JA, Sexton M, Ioffe OB. Cigarette smoking and metallothionein expression in invasive breast carcinomas. Toxicol Lett. 2004;152(3):245-253. doi: 10.1016/j.toxlet.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 32.Minami Y, Kanemura S, Kawai M, et al. Alcohol consumption and survival after breast cancer diagnosis in Japanese women: a prospective patient cohort study. PLoS One. 2019;14(11):e0224797. doi: 10.1371/journal.pone.0224797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowry SJ, Kapphahn K, Chlebowski R, Li CI. Alcohol use and breast cancer survival among participants in the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev. 2016;25(8):1268-1273. doi: 10.1158/1055-9965.Epi-16-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delker E, Brown Q, Hasin DS. Alcohol consumption in demographic subpopulations: an epidemiologic overview. Alcohol Res. 2016;38(1):7-15. [PMC free article] [PubMed] [Google Scholar]
- 35.Wu AH, Gomez SL, Vigen C, et al. The California Breast Cancer Survivorship Consortium (CBCSC): prognostic factors associated with racial/ethnic differences in breast cancer survival. Cancer Causes Control. 2013;24(10):1821-1836. doi: 10.1007/s10552-013-0260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Din N, Allen IE, Satariano WA, Demb J, Braithwaite D. Alcohol consumption and mortality after breast cancer diagnosis: the Health And Functioning in Women Study. Breast Dis. 2016;36(2-3):77-89. doi: 10.3233/bd-150202 [DOI] [PubMed] [Google Scholar]
- 37.Malburg CM, Fucinari J, Ruterbusch JJ, et al. Continued smoking in African American cancer survivors: the Detroit Research on Cancer Survivors Cohort. Cancer Med. 2020;9(20):7763-7771. doi: 10.1002/cam4.3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Hazard Ratios and 95% Confidence Intervals for Associations of Cigarette Smoking and Mortality After First Breast Cancer in the Women’s Circle of Health Follow-up Study (WCHFS), Excluding Stage IV Tumors
eTable 2. Hazard Ratios and 95% Confidence Intervals for Associations of Cigarette Smoking and Mortality After First Breast Cancer in the Women’s Circle Of Health Follow-up Study (WCHFS), Excluding Ductal Carcinoma In-Situ
Data Sharing Statement