Abstract
The main etiological agent of periodontitis is the anaerobic bacterium Porphyromonas gingivalis. Virulence of this pathogen is controlled by various mechanisms and executed by major virulence factors including the gingipain proteases, peptidylarginine deiminase (PPAD), and RagB, an outer membrane macromolecular transport component. Although the structures and functions of these proteins are well characterized, little is known about their posttranslational maturation. Here, we determined the phosphoproteome of P. gingivalis in which phosphorylated tyrosine residues constitute over 80% of all phosphoresidues. Multiple phosphotyrosines were found in gingipains, PPAD, and RagB. Although mutation of phosphorylated residues in PPAD and RagB had no effect on secretion or activity, site-directed mutagenesis showed that phosphorylation in hemagglutinin/adhesin domains of RgpA and Kgp, and in the catalytic domain of RgpB, had a strong influence on secretion, processing, and enzymatic activity. Moreover, preventing phosphorylation of one gingipain influenced the others, suggesting multiple phosphorylation-dependent pathways of gingipain maturation in P. gingivalis. Various candidate kinases including Ptk1 BY kinase and ubiquitous bacterial kinase 1 (UbK1) may be involved, but their contribution to gingipain processing and activation remains to be confirmed.
Keywords: gingipains, phosphoproteome, phosphorylation, Porphyromonas gingivalis, virulence factors
1 |. INTRODUCTION
Numerous reports indicate a pivotal role for Porphyromonas gingivalis in the development of periodontal disease (Ximenez-Fyvie et al., 2000). The pathogenetic potential of this anaerobic and asaccharolytic bacterium is a result of the production of numerous virulence factors, including peptidylarginine deiminase (PPAD), the lipoprotein RagB, a component of the nutrient acquisition system, and cysteine proteases named gingipains (Curtis et al., 1999; McGraw et al., 1999; Potempa et al., 2000). Two gingipains (RgpA and RgpB) are arginine-specific proteases and one (Kgp) is a lysine-specific enzyme. They are produced as latent proenzymes composed of a signal peptide, with an N-terminal prodomain (NTD), a catalytic domain, and conserved C-terminal domain (CTD) that targets the progingipain for secretion via the type 9 secretion system (T9SS) (Lasica et al., 2017; Li & Collyer, 2011). In the case of RgpA and Kgp, a large segment between the catalytic domain and the CTD encompasses hemagglutinin/adhesin (HA) domains. Biogenesis of gingipains is a complex process occurring during or just after translocation across the outer membrane via the T9SS (Lasica et al., 2017). It involves degradation of an inhibitory NTD, cleavage of the CTD, and attachment of an acidic lipopolysaccharide for anchorage of mature enzymes onto the bacterial surface. In addition, Kgp and RgpA are proteolytically processed into catalytic and HA domains, and assembled into a multidomain noncovalent complex (Bhogal et al., 1997). Considering the complexity of the process, it is unsurprising that the mechanism of functional maturation of gingipains is only partially understood (Veith et al., 2002). Elucidating this mechanism is especially challenging in the context of posttranslational modifications (PTMs) as it was shown that putative acylation (Mishra et al., 2018) and phosphorylation might have a substantial impact on gingipain secretion and processing (Maeda et al., 2008; Shen et al., 2020).
In bacteria, phosphorylation targets the side chains of tyrosine, threonine, serine, histidine, aspartate, arginine, lysine, and cysteine (Mijakovic et al., 2016). Although phosphorylation of histidine and aspartate is mostly reserved for two-component systems, the best characterized kinases target serine, threonine, and tyrosine residues (Whitmore & Lamont., 2012). Recently, a new class of bacterial kinases belonging to the ubiquitous bacterial kinase (UbK) family has been described (Nguyen et al., 2017; Perpich et al., 2021). UbKs have dual specificity and phosphorylate both Ser/Thr and Tyr residues. The best characterized kinase expressed by P. gingivalis is Ptk1, a BY kinase (bacterial tyrosine kinase) that, together with its cognate phosphatases Ltp1 and Php1, controls different aspects of P. gingivalis virulence (Jung et al., 2019; Maeda et al., 2008; Wright et al., 2014). Phosphorylation of proteins can have a significant impact on capsule biogenesis, but it also plays an important role in activation and maturation of non-capsule-related virulence factors. Autolysin LytA from Streptococcus pneumoniae is phosphorylated on Tyr264 by the CpsD BY kinase, which enhances the affinity for choline and elevates amidase activity (Standish & Morona, 2014). Phosphorylation of RgpA, RgpB, and Kgp gingipains, which most likely occurs in the cytoplasm immediately after translation (Kadowaki et al., 1998), was demonstrated using phosphotyrosine-specific antibodies and verified by in vitro dephosphorylation using the Lpt1 phosphatase. An Lpt1 deletion mutant (Δlpt1) showed decreased Rgp activity in culture supernatants, but not in whole cells. On the other hand, cell-associated Kgp activity was increased in Δlpt1, suggesting that dephosphorylation diminishes the proteolytic activity of Kgp. The reason for this discrepancy is unclear; however, it has been hypothesized that changes in gingipain activity might not be determined directly by phosphorylation status, but rather by an indirect effect mediated through other pathways controlled by Ltp1 (Maeda et al., 2008).
Herein, to cast more light on the role of phosphorylation in the pathophysiology of P. gingivalis and the biogenesis of gingipains, we analyzed the phosphoproteome and located several phosphorylated Tyr residues in multiple proteins, including highly expressed virulence factors (gingipains, PPAD, and RagB). Using site-directed mutagenesis, we show that phosphorylation of specific Tyr residues in gingipains but not PPAD and RagB directly affects the maturation and function of these proteins.
2 |. MATERIALS AND METHODS
2.1 |. Bacterial strains and growth conditions
Bacterial strains used in this study are listed in Table S3. Porphyromonas gingivalis ATCC 33277, W83, and derived mutant strains were grown anaerobically (80% N2, 10% CO2, and 10% H2) in eTSB medium (30 g/L tryptic soy broth, 5 g/L yeast extract). After sterilization, supplements (0.25 μg/L L-cysteine, 5 μg/L hemin, and 1 μg/ml vitamin K) were added. For blood agar plates, 5% defibrinated sheep blood and 1.5% agar were added. Selection of clones was performed using appropriate antibiotics: tetracycline (1 μg/ml), erythromycin (5 μg/ml), or ampicillin (2 μg/ml). Escherichia coli strains were cultured in Luria Bertani (LB) medium with ampicillin (100 μg/ml). Analysis of growth rate was conducted using triplicate cultures in eTSB medium inoculated with overnight cultures starting from an absorbance at 600 nm (OD600) of 0.2. Growth was monitored spectrophotometrically at 600 nm at predetermined time points. Experiments were conducted independently three times in triplicate.
2.2 |. Sample preparation for phosphoproteomic analysis
For phosphoproteomic studies, P. gingivalis strain ATCC 33277 was employed. Bacteria from three independent cultures were grown in eTSB under anaerobic conditions to mid-log phase. Cultures were pelleted by centrifugation and resuspended in 5 ml of 50 mM TRIS lysis buffer (pH 8.0, 0.125% RapiGest, 1% 2 and 3 phosphatase inhibitor cocktails [Sigma], 20 μM bestatin, 3 μM pepstatin A, 3 μM E-64c) and 30 μl of 1 M dithiothreitol was added. The suspension was heated in boiling water for 7 min, and Universal Nuclease (Pierce) was used to digest DNA and RNA for 15 min. After adjusting the concentration, proteins were reduced, alkylated, and digested with trypsin in 50:1 5% acetonitrile (ACN):35 mM NH4HCO3 at 37°C overnight. Samples were acidified and desalted with a Sep-Pak tC18 cartridge (Waters). Peptides were eluted into two fractions: the first was eluted with 0.1% trifluoroacetic acid (TFA) in 30% ACN and the second with 0.1% TFA in 50% ACN. Phosphopeptide enrichment using the TiO2 method developed by Ishihama’s group was performed with some modifications (Sugiyama et al., 2007). Enriched peptides dissolved in 30%–50% ACN were eluted by 1 M NH4OH, concentrated by SpeedVac to 5 μl, and stored at −80°C. Phosphoenrichment was also performed using Fe-magnetic beads (Ficarro et al., 2011).
2.3 |. Two-dimensional liquid chromatography–mass spectrometry analysis
The RP–RP two-dimensional HPLC separation system developed by Marto’s group (Ficarro et al., 2011) was employed with some modifications. An Alcott 718 autosampler (GP Instruments Inc.) was used to inject eluents, and an Advance nano-HPLC (Microm) was used for separation. An LTQ mass spectrometer (Thermo Electron) was used for MS analysis with an in-house micro-ESI interface. Briefly, a POROS 10 R2 column was used for first dimension separation in 5 mM tetrapropylammonium (TPA) in 3% ACN with 6 mM NH4Ac (pH 9.9, solvent C). A 5-μm Magic C18AQ column (Michrom) was used for second dimension separation with 0.5% acetic acid (solvent A) and 0.5% acetic acid in ACN (solvent B).
The enriched samples were reconstituted in 30 μl of 5 mM TPA, 6 mM NH4Ac, 0.5 mM Ethylenediaminetetraacetic acid (EDTA), and 0.5 mM Na2HPO4 in 5%–10% ACN. For samples eluted with ammonium acetate, 0.6 μl of 1 M NH4OH was added to reach pH 9.5–10. Samples were loaded onto the first dimension column, and during loading the trapping column was connected with solvent B to lower the pH to 3.5. For the elution step, eluent was loaded for 15 min, and then gradients for second dimension separation were started. A four-step elution (5%, 15%, 20%, and 50% ACN) was used for first dimension separation.
Xcalibur 1.4 data acquisition software (Thermo) was used with an MS1 scanning rate from 350 to 1600 m/z as default. Each MS1 scan was followed by seven MS2 scans and each MS2 scan was followed by a neutral loss (32.7, 40.0, and 49.0) MS3 scan for the top five fragment ions. The maximum injection time was 300 ms. For dynamic exclusion, repeat count 2, repeat duration 15 s, exclusion duration 60 s, and exclusion mass width low 0.70 m/z and high 1.30 m/z were employed. For MS2 scans, minimum signal threshold 500, isolation width 2.5 m/z, and normalized collision energy 40.0% were used. For MS3 scans, minimum signal threshold 50, isolation width 3.0 m/z, and normalized collision energy 35.0% were selected.
Raw mass spectral data were processed using TransProteomic Pipeline (TPP, version 4.8.0) with the Comet (2016 release) search engine (Eng et al., 2013). The open reading frame (ORF) database with decoy included sequences from P. gingivalis, Fusobacterium nucleatum, Streptococcus gordonii, human, bovine, and yeast. Full tryptic peptide and variable phosphorylation (D, H, S, T, and Y for MS2; S and T-18 Da for MS3) and oxidation of methionine were applied. A peptide was identified when the probability score was 0.8 (false-positive errors 1.9%–2.8%) or higher.
2.4 |. Mutant construction
All P. gingivalis mutants were constructed in the wild-type (WT) W83 strain and its derivatives by homologous recombination (Nguyen et al., 2007) and are listed in Table S3. Modification of gingipains and introduction of point mutations required a complex approach due to the large size of the gene. Due to the location of mutations, it was necessary to modify both N- and C-terminal ends of Kgp and RgpA. Mutations in PPAD and RagB were introduced using previously constructed master plasmids (Madej et al., 2020; Wegner et al., 2010). Details of mutant construction are available in the Supporting Information. Modified plasmids were transformed into selected P. gingivalis strains (Park & McBride, 1993) and plated on eTSB blood agar plates containing appropriate antibiotics. After 10 days, clones were selected, and desired mutations were confirmed by PCR and DNA sequencing. All plasmids and primers used in this study are listed in Tables S4 and S5.
2.5 |. Bacterial fractionation
Porphyromonas gingivalis strains were grown overnight in eTSB broth under anaerobic conditions. The next day, all cultures were adjusted to OD600 = 1.0, and 1 mM tosyl-L-lysyl-chloromethane hydrochloride (TLCK) and 5 mM Aldrithiol (Sigma) were added. After whole culture (WC) fraction collection, cells were centrifuged for 15 min at 6000 × g at 4°C. The supernatant was collected as medium fraction. Pelleted cells were washed twice with phosphate-buffered saline (PBS), resuspended in the initial volume of PBS with added inhibitors, and sonicated. The lysate was collected as whole cell extract and the remainder was centrifuged (150,000 × g for 60 min, 4°C). The resulting supernatant was assigned as the periplasmic/cytoplasmic fraction. Pellets were briefly resuspended in PBS by sonication and referred to as membranes.
2.6 |. Sample preparation and western blotting analysis
Fractionated samples were treated with 4× SDS polyacrylamide gel electrophoresis (SDS-PAGE) Sample Buffer (Bio-Rad) containing 5 mM Aldrithiol and boiled at 95°C for 5 min. Next, β-mercaptoethanol was added to 2.5% concentration, and after re-boiling samples were subjected to SDS-PAGE using 4%–20% Mini-Protean TGX Precast Gels (Bio-Rad) in TRIS/glycine/SDS buffer (Laemmli, 1970). Proteins were transferred onto a nitrocellulose membrane in 25 mM TRIS, 192 mM glycine, and 20% methanol at 100 V for 60 min. The amount of protein was estimated by standard Ponceau S staining. Membranes were blocked with 2% bovine serum albumin (BSA) in TTBS (TBS + 0.1% Tween-20) at room temperature for 1 h and further labeled with specific antibodies, namely, anti-GP1 (anti-Rgp), anti-Kgp, anti-RgpB, anti-PPAD, anti-CTD, and anti-RagB diluted in blocking solution. Signals were detected using Pierce ECL Western Blotting Substrate (Thermo) and visualized on X-Ray film (AGFA).
2.7 |. Proteolytic activity assays
Gingipain activity was measured by the spectrophotometric method with specific chromogenic substrates N-(p-Tosyl)-Gly-Pro-Lys-pNA (Tos-GPK-pNA) and Nα-benzoyl-Arg-pNA (L-BApNA, Sigma). Briefly, P. gingivalis strains were grown overnight under anaerobic conditions. Cultures were adjusted to OD600 = 1.0 and cells were collected by centrifugation (10,000 × g for 15 min). Supernatants served as cell-free culture medium samples. The detailed protocol was described previously (Pomowski et al., 2017). PPAD activity was measured in PBS-washed bacterial cells using a modified Boyde method with N-acetyl-L-arginine substrate (Bereta et al., 2019; Boyde & Rahmatullah, 1980).
2.8 |. Flow cytometry analysis
Wild-type W83 and mutant P. gingivalis strains were grown in eTSB until they reached the late exponential or early stationary growth phase (OD600 ~ 1.2–1.5). Bacterial cells were harvested by centrifugation (6000 × g for 15 min), washed twice with PBS, and adjusted to OD600 = 0.2 with buffer (PBS supplemented with 0.2% BSA and protease inhibitors: 1 mM TLCK, 1× Complete EDTA-free). Then, 150 μl of cell suspension was transferred to a 96-well plate and incubated for 30 min with the buffer. Cells were collected by centrifugation (1000 × g, 5 min) and the pellet was resuspended in the previous buffer containing mouse anti-RgpB mAb at concentration of 20 μg/ml, and incubated for 30 min. Thereafter, cells were centrifuged (1000 × g, 5 min) and the newly obtained pellet was resuspended in the previous buffer containing goat anti-mouse antibody conjugated with fluorescein isothiocyanate (Abcam) at 1:150 dilution and incubated for 30 min. Cells were washed twice with PBS after each incubation with antibodies. The whole staining procedure was performed on ice. After staining, one-color flow cytometry analyses were performed using a FACSCalibur apparatus (BD Biosciences) operating with CellQuest software (BD Biosciences). Graphs were prepared using the FLOWJO v.10 program (Ashland, USA).
2.9 |. Quantitative real-time polymerase chain reaction analysis of gingipains gene expression
Expression of gingipain genes was analyzed by quantitative real-time polymerase chain reaction using gene-specific primers. Selected P. gingivalis strains were grown from overnight cultures up to OD600 = 1.0. RNA was isolated from 1 × 109 of bacteria using TRI-reagent (Invitrogen) and digested with DNase I (Ambion). For cDNA synthesis, 400 ng of RNA was used and diluted 50× in nuclease-free water. The reaction was performed for 16S rRNA and gingipain genes using SYBR Green (Sigma). Expression levels were normalized against 16S rRNA by the ΔΔCT method with the reference strain. Primers used for analysis are listed in Table S5.
3 |. RESULTS
3.1 |. Major virulence factors of P. gingivalis are phosphorylated
PTMs of bacterial proteins in response to environmental cues occur in a rapid, efficient, and specific manner. PTMs are intensively investigated because they affect protein folding and activity, and often impact the virulence of pathogenic bacteria and their interactions with hosts (Mijakovic et al., 2016). Little information is available on posttranslational phosphorylation of proteins in P. gingivalis, or how it affects the secretion, maturation, and activity of major virulence factors (gingipains). Therefore, we determined the phosphoproteome of the ATCC 33277 strain (in triplicate). Samples were prepared carefully with addition of phosphatase inhibitors, and phosphorylated peptides were enriched using TiO2 and Fe-magnetic beads and pooled for MS analysis. In total, 88 phosphorylated peptides representing 87 distinct phosphorylation sites were identified (Table S1).
Unlike eukaryotes, in which almost 90% of phosphorylation sites are serine (Sharma et al., 2014), P. gingivalis favors tyrosine, accounting for over 88% of all identified phosphoresidues. Consistent with analysis of other bacterial phosphoproteomes (Pan et al., 2015), phosphopeptides derived from translation machinery proteins, primarily ribosomal proteins, but also elongation factors and a tRNA synthetase, were enriched in the present work (Table S1). Uniquely for P. gingivalis, several identified phosphotyrosine-bearing peptides were derived from highly expressed proteins including gingipains, lipoprotein RagB, and PPAD. Gingipain-derived phosphopeptides and their equivalent amino acid residues from the W83 strain are listed in Table 1. Interestingly, no phosphopeptides originating from components of the T9SS were detected. Of note, all major virulence factors of P. gingivalis including gingipains and PPAD are secreted, processed, and anchored to the bacterial cell surface by the T9SS (Lasica et al., 2017).
TABLE 1.
Phosphorylated amino acid residues in gingipains. The table contains all gingipain-related phosphopeptides identified by phosphoproteomic analysis of the P.gingivalis ATCC 33277 strain. The corresponding amino acid residues present in the W83 strain are listed in brackets. Phosphoresidues are marked in red
| Protein | ORF | Phosphorylated aa in ATCC 33277 strain [corresponding aa in W83 strain] | Phosphorylated peptide | Localization |
|---|---|---|---|---|
| RgpA | PGN_1970 | Tyr320 [Tyr323] Tyr329 [Tyr332] |
K.SDQVY*GQIVGNDHY*NEVFIGR.F K.SDQVYGQIVGNDHY*NEVFIGR.F |
Catalytic domain |
| Tyr830 [Tyr833] | K.EDDY*VFEAGK.K | Hemagglutinin/adhesin domain | ||
| Tyr1542 [Tyr1545] Thr1546 [Thr1549] |
R.Y*DDFT*FEAGK.K R.Y*DDFTFEAGK.K R.Y*DDFTFEAGKK.Y |
Hemagglutinin/adhesin domain | ||
| RgpB | PGN_1466 | Tyr325 [Tyr325] Tyr334 [Tyr334] |
K.SDQVY*GQIVGNDHY*NEVFIGR.F K.SDQVYGQIVGNDHY*NEVFIGR.F |
Catalytic domain |
| Kgp | PGN_1728 | Tyr231 [Tyr231] Thr232 [Thr232] Tyr238 [Tyr238] |
R.DVY*T*DHGDLY*NTPVR.M R.DVY*TDHGDLYNTPVR.M R.DVYT*DHGDLYNTPVR.M |
Catalytic domain |
| Tyr662 [Tyr662] | K.QITENGNY*DVVITR.S | Catalytic domain | ||
| Tyr851 [Tyr851] Thr855 [Thr855] |
R.Y*DDFT*FEAGK.K R.Y*DDFTFEAGK.K R.Y*DDFTFEAGKK.Y |
Hemagglutinin/adhesin domain |
3.2 |. Impact of mutated phosphotyrosines on HA-containing gingipains
Because gingipains are one of the main factors responsible for virulence in P. gingivalis, we investigated the role of phosphorylation of these enzymes on their processing and activity. To this end, we replaced Tyr residues identified as being phosphorylated in gingipains with Phe using site-directed mutagenesis. Due to the high similarity between Tyr and Phe, this modification does not tend to disrupt protein structure and function (Dong et al., 1997). We generated four RgpA mutants (RgpAY323F, RgpAY332F, RgpAY833F, and RgpAY1545F), four Kgp mutants (KgpY231F, KgpY238F, KgpY662F, and KgpY851F), and two RgpB mutants (RgpBY325F and RgpBY334F). Moreover, to eliminate the effects of other Rgp gingipain on the analysis, we additionally prepared the same mutations in the appropriate deletion background (ΔrgpA for RgpB mutants and ΔrgpB for RgpA mutants).
None of the mutations had any effect on the growth rate of the strains (Figure S1). Although Y231F and Y238F substitutions in Kgp had no effect or caused only a slight (~20%) reduction in Kgp activity in WC samples, Y662F and Y851F mutations caused the residual activity to decrease by more than 50% relative to the parental strain. The amount of Kgp activity released into the growth medium by these mutants was reduced almost to the background level (Figure 1a). In the case of the Y662F mutation, reduction of Kgp activity was correlated with the presence of unprocessed, single-chain proKgp (220 kDa) associated with the cell membrane fraction of the mutant strain (Figure 2a). Overall, Rgp activity in Kgp mutants was slightly but statistically significantly increased. Interestingly, however, the activity in medium was twice as high in strains expressing KgpY231F and KgpY238F (Figure 1b).
FIGURE 1.
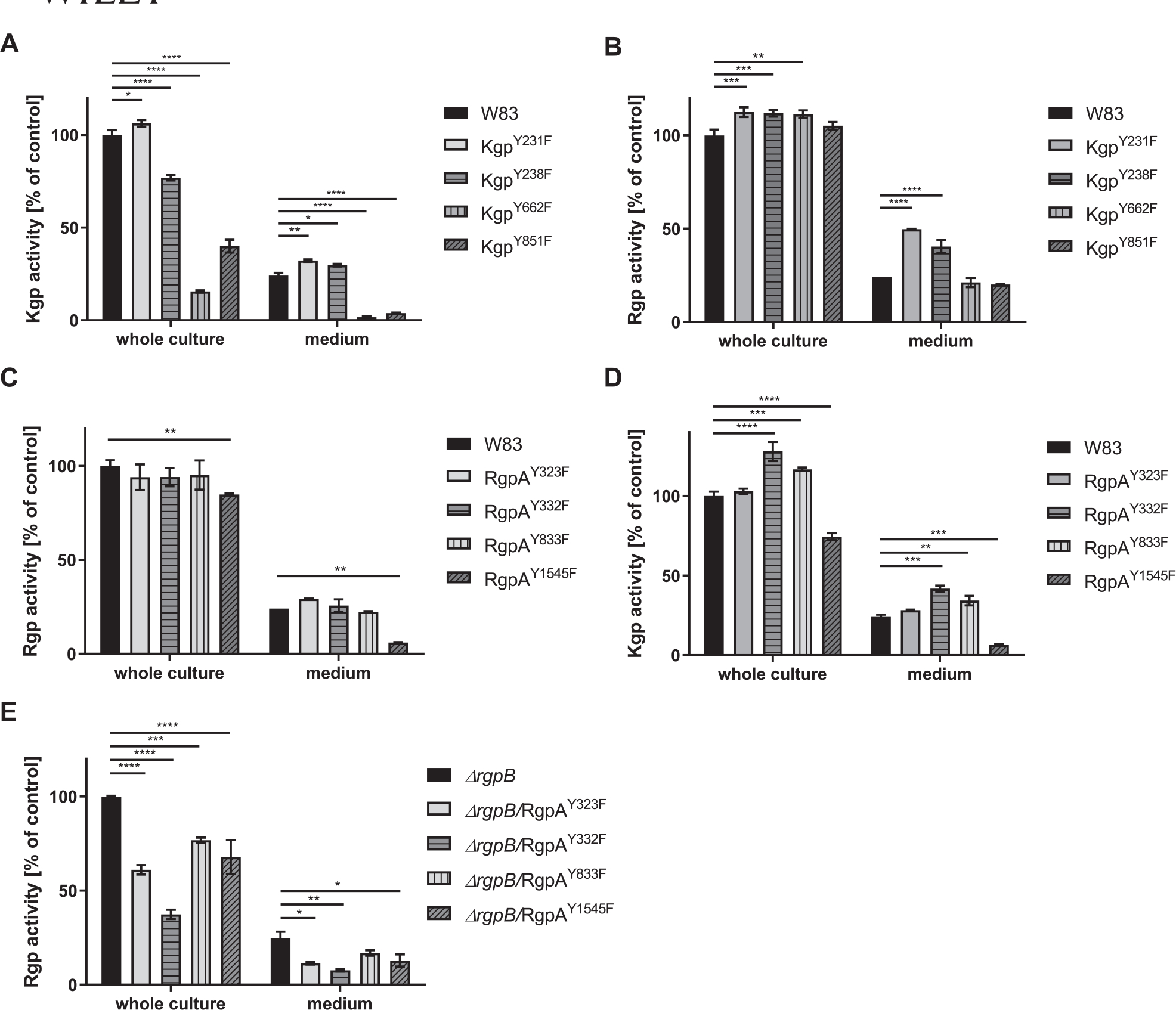
Effect of substitution of phosphorylated Tyr residues with Phe in Kgp (a and b) and RgpA (c–e) on gingipain activity. Rgp and Kgp activity was measured with L-BApNA and Tos-GPK-pNA as the substrate, respectively, in both whole culture and growth medium after bacterial cells were removed by centrifugation. Activity of W83 and ΔrgpB was arbitrarily taken as 100%. ****p < 0.0001; ***p < 0.0005; **p < 0.001; *p < 0.05
FIGURE 2.
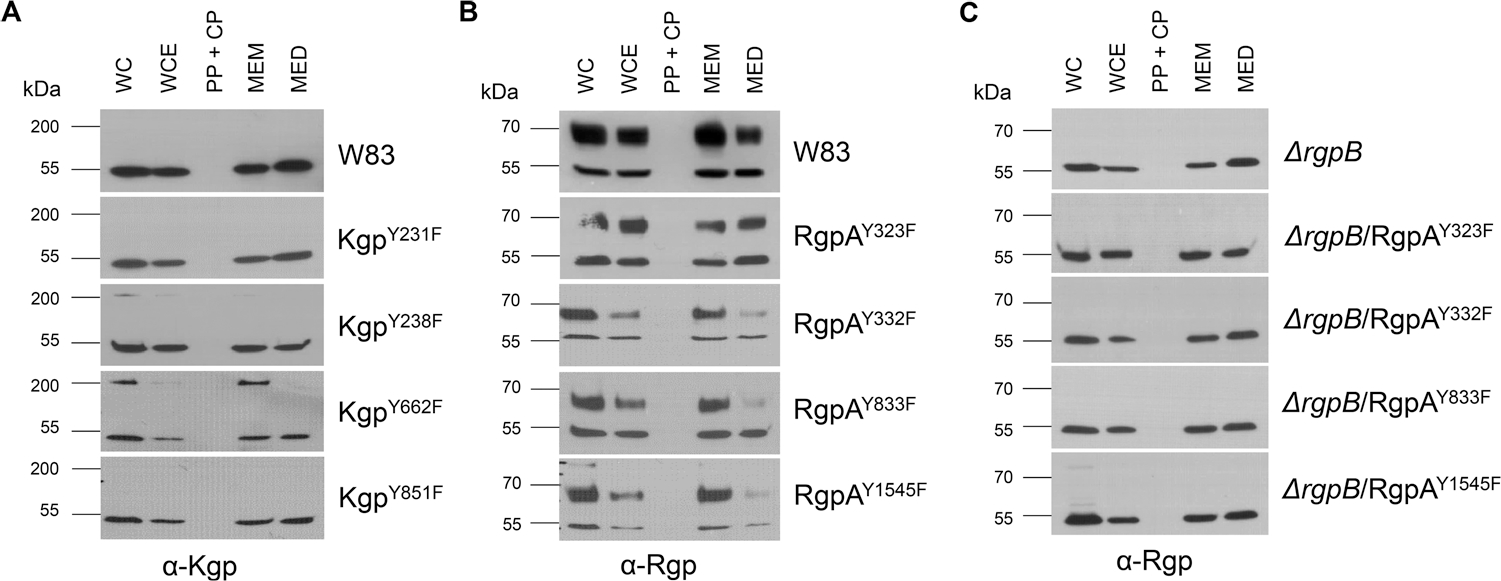
Effect of modification of phosphorylated Tyr residues in Kgp and RgpA on their processing. Processing and secretion were analyzed by western blotting using specific anti-Kgp antibodies for Kgp mutants (a) and anti-Rgp for RgpA mutants (b and c) WC, whole culture; WCE, whole cell extract; PP + CP, periplasm/cytoplasm; MEM, membranes; MED, medium.
The Tyr to Phe substitutions in RgpA had a negligible effect on Arg-specific gingipains activity in WC samples of the mutant strain with the exception of the RgpAY1545F mutant, for which activity was significantly reduced both in whole cell samples and in the medium (Figure 1c). In this mutant, a very subtle band corresponding to unprocessed proRgpA was visible (Figure 2b). Interestingly, the Y332F and Y833F mutations in RgpA increased the level of Kgp activity, whereas the mutant expressing RgpAY1545F showed significantly decreased activity, both in WC and growth medium samples (Figure 1d). The same mutations but in the ΔrgpB background exerted much stronger effects on RgpA activity, which declined by at least 30% (Figure 1e). Nevertheless, there was no accumulation of unprocessed proRgpA in these mutants (Figure 2c).
Collectively, these results indicate that phosphorylation-abrogating mutation of selected Tyr residues decreased gingipain activity of RgpA and Kgp, presumably due to aberrant processing of latent progingipains. In the case of RgpA, the NTD that is an effective inhibitor of mature RgpA likely persisted and was responsible for the reduced activity of the Tyr mutants of this gingipain (Veillard et al., 2013).
3.3 |. Phosphorylation has a strong impact on RgpB activity and processing
In contrast to RgpA and Kgp, which are assembled as noncovalent complexes of catalytic and HA domains (Li & Collyer., 2011), RgpB is a single-chain enzyme composed of catalytic and immunoglobulin-like domains (Zhou et al., 2013). The mature form of RgpB is generated by removal of the CTD by PorU sortase during translocation to the bacterial surface via the T9SS (Gorasia et al., 2015) and degradation of the N-terminal, inhibitory prodomain in a process not yet understood. Two Tyr residues of RgpB in the catalytic domain, both found to be phosphorylated, were replaced with Phe. Regardless of the mutation (RgpBY325F or RgpBY334F), mutated RgpB was present on the bacterial surface of the ΔrgpA strain at the higher level than native RgpB detected in the WT-Pg (Figure 3). Although the overall Rgp activity was increased in the RgpBY325F mutant by 30%, it was decreased by 50% in the RgpBY334F mutant (Figure 4a) without any clear difference in the intensity and distribution of bands immunoreactive with anti-Rgp catalytic domain polyclonal antibodies (Figure 5a). A similar trend was observed with respect to Kgp activity (Figure 4b). The residual Rgp activity in the RgpBY334F mutant was apparently mostly due to RgpA because the same mutation in the ΔrgpA strain reduced the activity to less than 10% of the parental strain (ΔrgpA) (Figure 4c). Consistent with low Rgp activity, western blotting using mAb anti-RgpB revealed a relatively weak, diffused band above 70 kDa apparently representing the mature membrane-type RgpB (Figure 5b). On the other hand, the western blotting analysis using anti-CTD polyclonal antibodies showed that Y334F mutation in the ΔrgpA background rendered proRgpB incompatible for processing by the PorU sortase. Instead, as indicated by the presence of the intact CTD (Figure S2), the membrane-bound proRgpB undergoes truncation at the N-terminus apparently yielding RgpB forms with the partially cleaved NTD, without the NTD and a C-terminal fragment of proRgpB. This resembles processing of proRgpB expressed in yeast (Mikolajczyk et al., 2003). Of note, a side chain of Tyr325 is located on the protein surface of the catalytic domain and does not interact with other residues (Eichinger et al., 1999). On the other hand, the side chain of Tyr334 points toward a protein core, but nevertheless, its replacement by Phe is very unlikely to affect the RgpB structure. Interestingly, activity of Rgps (Figure 4a) and Kgp (Figure 4b) in RgpBY325F and RgpBY334F mutants presented a similar pattern, but the same mutations in the ΔrgpA background totally abrogated Kgp activity (Figure 4d). Moreover, no trace of proKgp or proKgp fragments was detected either in culture/WC lysates or any of the analyzed subcellular fractions (Figure 5c). Importantly, absence of the Kgp protein was not a result of impaired gene expression because rgpB and kgp gene transcripts were present at the same levels in parental and mutant strains (Figure S3). This excludes the possibility that the lack of Y325 and Y334 phosphorylation in RgpB in the ΔrgpA background affected expression of the kgp gene, and implies degradation of the Kgp polypeptide either in the cytoplasm or after nascent proKgp is exported to the periplasm. Thus, it is tempting to speculate that phosphorylation of tyrosines regulates the folding and stability and/or the processing and secretion of RgpB as well as Kgp.
FIGURE 3.
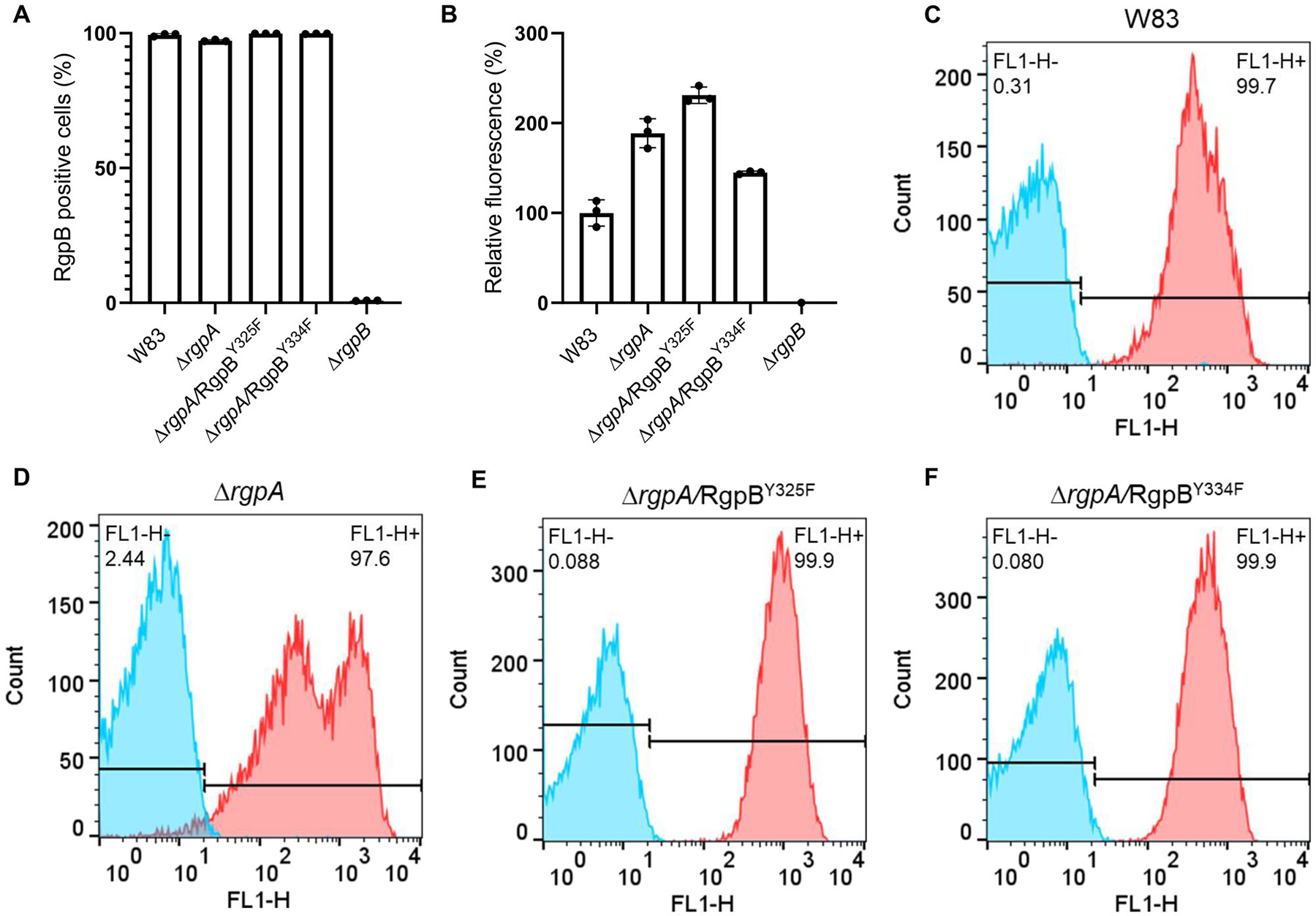
Exposure of RgpB on the P. gingivalis surface. FACS analysis of the presence of RgpB variants on the P. gingivalis surface as the percentage of RgpB-positive bacterial cells (a) and relative fluorescence analysis of the signal (b) with respect to the wild-type parental P. gingivalis W83 strain normally producing RgpB (100%) and the ΔrgpB knockout (0%). Representative histograms (c–f) showing the percentage of RgpB-labeled cells (shown in red) relative to the negative antibody isotype control (shown in blue). Results represent the mean of three independent experiments ± SD
FIGURE 4.

Effect of substitution of phosphorylated Tyr residues with Phe in RgpB in P. gingivalis wild-type W83 (a and b) and ΔrgpA (c and d) strains on gingipain activity. Rgp and Kgp activity was measured with L-BApNA and Tos-GPK-pNA as the substrate, respectively, in both whole culture and growth medium after bacterial cells were removed by centrifugation. Activity of W83 and ΔrgpA was arbitrarily taken as 100%. ****p < 0.0001; ***p < 0.0005; **p < 0.001
FIGURE 5.
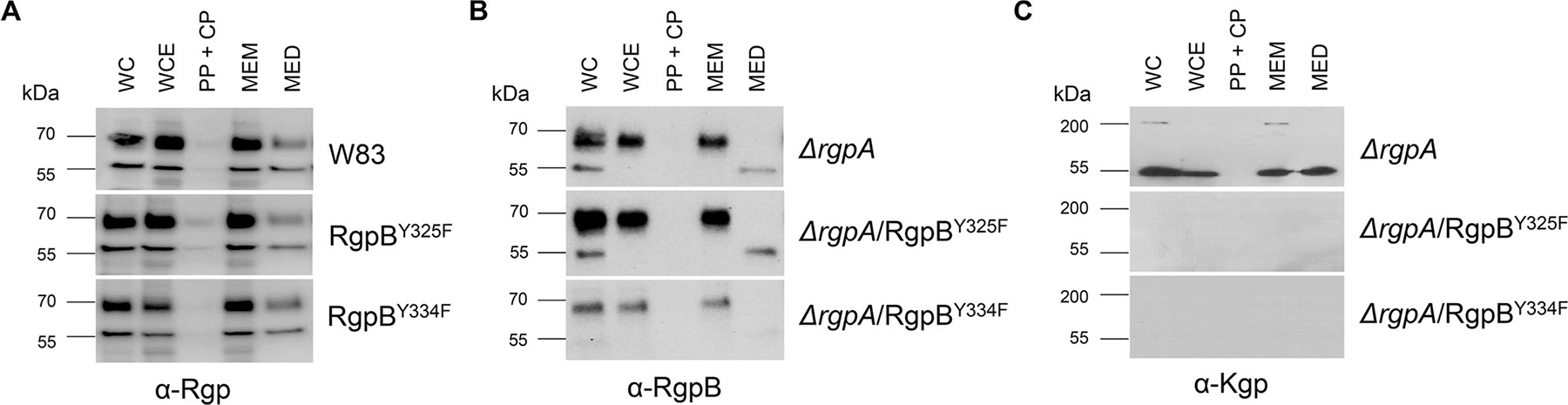
Effect of modification of phosphorylated Tyr residues in RgpB on its processing. Processing and secretion in RgpB mutants were analyzed by western blotting using specific anti-Rgp (a), anti-RgpB (b), and anti-Kgp (c) antibodies WC, whole culture; WCE, whole cell extract; PP + CP, periplasm/cytoplasm; MEM, membranes; MED, medium.
3.4 |. Phosphorylation patterns in P. gingivalis may be strain specific
In addition to gingipains, phosphoproteomic analysis revealed multiple phosphorylated Tyr residues in other major P. gingivalis enzymes and components of the peptide acquisition system (Table S1). The sequence of PPAD is highly conserved among P. gingivalis strains, and Y378 is likely targeted for phosphorylation regardless of the strain (Table S2). At least in the W83 background, PPADY378F modification did not result in any changes in activity, processing, or secretion of proPPAD (Figure S4).
In contrast to PPAD and gingipains, RagB from ATCC 33277 and W83 shares only 49% amino acid sequence identity (Hall et al., 2005), and phosphorylated Y341 in ATCC 33277 does not have an equivalent in the W83 strain (Table S2). In addition, this lipoprotein is secreted independently of T9SS. We obtained several RagB Tyr/Phe mutants (RagBY136F, RagBY191F, RagBY249F, and RagBY278F) and compared secretion of RagB in all strains. None of the mutated strains showed any changes in the level of protein and location in the outer membrane indicating no significant impact of identified phosphotyrosines on RagB posttranslational processing and secretion (Figure S5).
4 |. DISCUSSION
In this study, we attempted to gain insight into the phosphoproteomic status of P. gingivalis and identified specific amino acid residues phosphorylated in major virulence factors (Table S1). The analysis revealed that most of the phosphorylated residues were tyrosines (over 80%). By contrast, phosphoproteomic studies on Mycobacterium bovis bacillus Celmette–Guérin (Zheng et al., 2015), Lactococcus lactis (Soufi et al., 2008), Campylobacter jejuni (Voisin et al., 2007), Clostridium acetobutyicum (Bai & Ji., 2012), and Streptomyces coelicolor (Parker et al., 2010) all reported that tyrosine accounted for ~10% of the identified phosphorylation sites. Due to several factors, including the relative inefficiency of enrichment methods and the often incomplete and highly transient nature of biological phosphorylation processes in general, a large proportion of phosphopeptides was detected by only one spectral count, and threonines were found to be phosphorylated in only a small fraction of peptides.
In the P. gingivalis phosphoproteome, we identified several phosphotyrosine residues in gingipains, and using site-directed mutagenesis we linked phosphorylation to maturation of these essential virulence factors. Tyr to Phe substitutions in gingipains revealed a significant impact of phosphorylation of specific Tyr residues on gingipain activity, secretion, and processing. In the case of Kgp, phosphorylated Tyr (Y662 and Y851) affect the activity of this gingipain and are located within HA domains. The results suggest that lack of phosphorylation hinders the processing of proKgp, the proenzyme associated with the cell envelope, which was most evident for KgpY662F (Figure 2a). Similarly, RgpBY334F was only partially processed, and the latent progingipain was detected in the cell membrane fraction. Most interestingly, the Kgp protein was totally absent in the ΔrgpA mutant expressing RgpY325F or RgpBY334F (Figure 5c), but was normally processed and active in WT P. gingivalis expressing the Y/F RgpB mutants. This result suggests that RgpA stabilizes Kgp, consistent with the finding that these two gingipains form a dimer in the P. gingivalis periplasm (Glew et al., 2014). Furthermore, it is tempting to speculate that nonphosphorylated proRgpB is responsible for proKgp protein degradation. This assumption is supported by the perplexing difference in the latency of the recombinant proRgpB expressed in yeast (Mikolajczyk et al., 2003) and the native protein (Veillard et al., 2019) isolated from P. gingivalis periplasm. Although the former has very low latency and almost instantly undergoes self-processing to the fully active protease, the latter is a very stable zymogen, likely due to phosphorylation. There may also be other factors such as VimA, an acyltransferase, which regulates progingipain biogenesis (Dou et al., 2015). Although progingipains effectively autoprocess themselves, in the absence of the VimA protein, the maturation/activation pathway for proKgp and proRgpB is interdependent. On the other hand, processing of proRgpA is dependent on VimA. Furthermore, activation of gingipains is probably additionally reliant on a response regulator RprY (PGN_1186) that was found to be phosphorylated (Y41) in our phosphoproteomic analysis (Table S1) in accordance with recent findings (Perpich et al., 2021; Shen et al., 2020). Regulation was observed at the mRNA level and included other T9SS cargo proteins such as PPAD (Shen et al., 2020). Together, those results along with the present work clearly suggest the existence of multiple gingipain activation pathways in P. gingivalis that are dependent on PTM.
Among the 57 of the identified phosphorylated proteins of P. gingivalis, we also investigated whether the functions of PPAD and RagB were phosphorylation dependent. Only one phosphotyrosine was found in PPAD, and its substitution did not alter activity or processing of PPAD. Although PPAD is secreted via the T9SS, similarly to gingipains, posttranslational processing is dependent only on PorU sortase activity, and does not appear to be regulated by phosphorylation (Goulas et al., 2015; Konig et al., 2015). By contrast, as many as five phosphotyrosines were found in the lipoprotein RagB, which is abundant on the bacterial surface (Table S2) as a component of the RagAB system essential for peptide acquisition and bacterial growth (Madej et al., 2020). There are four major variants of RagB sharing ~50% sequence identity, and strains expressing them have different requirements for protein to grow in minimal medium, in which proteins are the sole source of peptides (Hall et al., 2005; Nagano et al., 2007). However, our results showed it is unlikely that phosphorylation significantly alters RagB processing and function, which was also confirmed by absence of any phosphorylated amino acids in RagB structure (Goulas et al., 2016). On the other hand, major RagB sequence differences between ATCC 33277 and W83 strains can lead to variations in PTMs and their further impact on peptide acquisition.
The high-resolution atomic structures of RgpB, PPAD, and the catalytic domain of Kgp have been determined. Tyr phosphorylation was not observed (de Diego et al., 2014; Eichinger et al., 1999; Goulas et al., 2015), because phosphorylation occurs in the cytoplasm and is reversible. Dephosphorylation is likely to take place once proteins are exported to the periplasm or after being secreted to the extracellular environment. We hypothesize that gingipain phosphorylation facilitates the protein folding in the periplasm needed for maintenance of the latency and/or intramolecular interactions of progingipains, which are essential for proper maturation. During the folding process, progingipains are reportedly dephosphorylated by a periplasmic alkaline phosphatase (PG_0890), Ltp1 phosphatase, or other enzymes yet to be characterized (Maeda et al., 2008; Yamashita et al., 1990). This novel mechanism controlling gingipain maturation requires more detailed study, because it may also contribute to the formation of the electron-dense layer composed of T9SS cargo proteins on the P. gingivalis surface (Gorasia et al., 2020).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by National Science Centre, Poland grants UMO-2016/23/N/NZ1/01513 for ZN, UMO-2015/19/N/NZ1/00322 for MM, and UMO-2018/31/B/NZ1/03968 for JP and NIH/NIDCR grants DE012505, DE01111, and DE023193 for RJL, DE014372 for MH, and DE026939 and DE028346 for DPM. The funding sources had no input in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/omi.12354.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
All relevant data are in the article and Supporting Information.
REFERENCES
- Bai X, & Ji Z (2012). Phosphoproteomic investigation of a solvent producing bacterium Clostridium acetobutylicum. Applied Microbiology and Biotechnology, 95, 201–211. 10.1007/s00253-012-4156-3 [DOI] [PubMed] [Google Scholar]
- Bereta G, Goulas T, Madej M, Bielecka E, Solà M, Potempa J, & Gomis-Rüth FX (2019). Structure, function, and inhibition of a genomic/clinical variant of Porphyromonas gingivalis peptidylarginine deiminase. Protein Science, 28(3), 478–486. 10.1002/pro.3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal PS, Slakeski N, & Reynolds EC (1997). A cell-associated protein complex of Porphyromonas gingivalis W50 composed of Arg- and Lysspecific cysteine proteinases and adhesins. Microbiology, 143(7), 2485–2495. 10.1099/00221287-143-7-2485 [DOI] [PubMed] [Google Scholar]
- Boyde TR, & Rahmatullah M (1980). Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Analytical Biochemistry, 107(2), 424–431. 10.1016/0003-2697(80)90404-2 [DOI] [PubMed] [Google Scholar]
- Curtis MA, Hanley SA, & Aduse-Opoku J (1999). The rag locus of Porphyromonas gingivalis: A novel pathogenicity island. Journal of Periodontal Research, 34(7), 400–405. 10.1111/j.1600-0765.1999.tb02273.x [DOI] [PubMed] [Google Scholar]
- de Diego I, Veillard F, Sztukowska MN, Guevara T, Potempa B, Pomowski A, Huntington JA, Potempa J, & Gomis-Rüth FX (2014). Structure and mechanism of cysteine peptidase gingipain K (Kgp), a major virulence factor of Porphyromonas gingivalis in periodontitis. Journal of Biological Chemistry, 289, 32291–32302. 10.1074/jbc.M114.602052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LQ, Farris S, Christal J, & Liu F (1997). Site-directed mutagenesis and yeast two-hybrid studies of the insulin and insulin-like growth factor-1 receptors: The Src homology-2 domain-containing protein hGrb10 binds to the autophosphorylated tyrosine residues in the kinase domain of the insulin receptor. Molecular Endocrinology, 11(12), 1757–1765. 10.1210/mend.11.12.0014 [DOI] [PubMed] [Google Scholar]
- Dou Y, Robles A, Roy F, Aruni W, Sandberg L, Nothnagel E.u., & Fletcher HM (2015). The roles of RgpB and Kgp in late onset gingipain activity in the vimA-defective mutant of Porphyromonas gingivalis W83. Molecular Oral Microbiology, 30, 347–360. 10.1111/omi.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger A, Beisel HG, Jacob U, Huber R, Medrano FJ, Banbula A, Potempa J, Travis J, & Bode W (1999). Crystal structure of gingipain R: An Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO Journal, 18, 5453–5462. 10.1093/emboj/18.20.5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JK, Jahan TA, & Hoopmann MR (2013). Comet: An open-source MS/MS sequence database search tool. Proteomics, 13(1), 22–24. 10.1002/pmic.201200439 [DOI] [PubMed] [Google Scholar]
- Ficarro SB, Zhang Y, Carrasco-Alfonso MJ, Garg B, Adelmant G, Webber JT, Luckey CJ, & Marto JA (2011). Online nanoflow multidimensional fractionation for high efficiency phosphopeptide analysis. Molecular and Cellular Proteomics, 10, O111.011064. 10.1074/mcp.O111.011064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew MD, Veith PD, Chen D, Seers CA, Chen YY, & Reynolds EC (2014) Blue native-PAGE analysis of membrane protein complexes in Porphyromonas gingivalis. Journal of Proteomics, 110, 72–92. 10.1016/j.jprot.2014.07.033 [DOI] [PubMed] [Google Scholar]
- Gorasia DG, Veith PD, Chen D, Seers CA, Mitchell HA, Chen YY, Glew MD, Dashper SG, & Reynolds EC (2015). Porphyromonas gingivalis type IX secretion substrates are cleaved and modified by a sortase-like mechanism. PLoS Pathogens, 11(9), e1005152. 10.1371/journal.ppat.1005152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorasia DG, Veith PD, & Reynolds EC (2020). The type IX secretion system: Advances in structure, function and organisation. Microorganisms, 8(8), 1173. 10.3390/microorganisms8081173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas T, Garcia-Ferrer I, Hutcherson JA, Potempa BA, Potempa J, Scott DA, & Xavier Gomis-Rüth F (2016). Structure of RagB, a major immunodominant outer-membrane surface receptor antigen of Porphyromonas gingivalis. Molecular Oral Microbiology, 31(6), 472–485. 10.1111/omi.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas T, Mizgalska D, Garcia-Ferrer I, Kantyka T, Guevara T, Szmigielski B, Sroka A, Millán C, Usón I, Veillard F, Potempa B, Mydel P, Solà M, Potempa J, & Gomis-Rüth FX (2015). Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Scientific Reports, 5, 11969. 10.1038/srep11969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LM, Fawell SC, Shi X, Faray-Kele MC, Aduse-Opoku J, Whiley RA, & Curtis MA (2005). Sequence diversity and antigenic variation at the rag locus of Porphyromonas gingivalis. Infection and Immunity, 73(7), 4253–4262. 10.1128/IAI.73.7.4253-4262.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Miller DP, Perpich JD, Fitzsimonds ZR, Shen D, Ohshima J, & Lamont RJ (2019). Porphyromonas gingivalis tyrosine phosphatase Php1 promotes community development and pathogenicity. mBio, 10(5), e02004–19. 10.1128/mBio.02004-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Nakayama K, Yoshimura F, Okamoto K, Abe N, & Yamamoto K (1998). Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. Journal of Biological Chemistry, 273(44), 29072–29076. 10.1074/jbc.273.44.29072 [DOI] [PubMed] [Google Scholar]
- Konig MF, Paracha AS, Moni M, Bingham CO 3rd, & Andrade F (2015). Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Annals of the Rheumatic Diseases, 74(11), 2054–2061. 10.1136/annrheumdis-2014-205385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Lasica AM, Ksiazek M, Madej M, & Potempa J (2017). The type IX secretion system (T9SS): Highlights and recent insights into its structure and function. Frontiers in Cellular and Infection Microbiology, 7, 215. 10.3389/fcimb.2017.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, & Collyer CA (2011). Gingipains from Porphyromonas gingivalis – Complex domain structures confer diverse functions. European Journal of Microbiology and Immunology, 1, 41–58. 10.1556/EuJMI.1.2011.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madej M, White JBR, Nowakowska Z, Rawson S, Scavenius C, Enghild JJ, Bereta GP, Pothula K, Kleinekathoefer U, Baslé A, Ranson NA, Potempa J, & van den Berg B (2020). Structural and functional insights into oligopeptide acquisition by the RagAB transporter from Porphyromonas gingivalis. Nature Microbiology, 5(8), 1016–1025. 10.1038/s41564-020-0716-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Tribble GD, Tucker CM, Anaya C, Shizukuishi S, Lewis JP, Demuth DR, & Lamont RJ (2008). A Porphyromonas gingivalis tyrosine phosphatase is a multifunctional regulator of virulence attributes. Molecular Microbiology, 69, 1153–1164. 10.1111/j.1365-2958.2008.06338.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw WT, Potempa J, Farley D, & Travis J (1999). Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infection and Immunity, 67(7), 3248–3256. 10.1128/IAI.67.7.3248-3256.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijakovic I, Grangeasse C, & Turgay K (2016). Exploring the diversity of protein modifications: Special bacterial phosphorylation systems. FEMS Microbiology Reviews, 40, 398–417. 10.1093/femsre/fuw003 [DOI] [PubMed] [Google Scholar]
- Mikolajczyk J, Boatright KM, Stennicke HR, Nazif T, Potempa J, Bogyo M, & Salvesen GS (2003). Sequential autolytic processing activates the zymogen of Arg-gingipain. Journal of Biological Chemistry, 278(12), 10458–10464. 10.1074/jbc.M210564200 [DOI] [PubMed] [Google Scholar]
- Mishra A, Roy F, Dou Y, Zhang K, Tang H, & Fletcher HM (2018). Role of acetyltransferase PG1842 in gingipain biogenesis in Porphyromonas gingivalis. Journal of Bacteriology, 200(24), e00385–18. 10.1128/JB.00385-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K, Murakami Y, Nishikawa K, Sakakibara J, Shimozato K, & Yoshimura F (2007). Characterization of RagA and RagB in Porphyromonas gingivalis: Study using gene-deletion mutants. Journal of Medical Microbiology, 56(11), 1536–1548. 10.1099/jmm.0.47289-0 [DOI] [PubMed] [Google Scholar]
- Nguyen HA, El Khoury T, Guiral S, Laaberki MH, Candusso MP, Galisson F, Foucher AE, Kesraoui S, Ballut L, Vallet S, Orelle C, Zucchini L, Martin J, Page A, Attieh J, Aghajari N, Grangeasse C, & Jault JM (2017). Expanding the kinome world: A new protein kinase family widely conserved in bacteria. Journal of Molecular Biology, 429(20), 3056–3074. 10.1016/j.jmb.2017.08.016 [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Travis J, & Potempa J (2007). Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-Negative bacteria? Journal of Bacteriology, 189, 833–843. 10.1128/JB.01530-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Wang B, Zhang Y, Wang Y, Ullah S, Jian R, Liu Z, & Xue Y (2015) dbPSP: A curated database for protein phosphorylation sites in prokaryotes. Database, 2015, bav031. 10.1093/database/bav031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, & McBride BC (1993). Characterization of the tpr gene product and isolation of a specific protease-deficient mutant of Porphyromonas gingivalis W83. Infection and Immunity, 61, 4139–4146. 10.1128/iai.61.10.4139-4146.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JL, Jones AM, Serazetdinova L, Saalbach G, Bibb MJ, & Naldrett MJ (2010). Analysis of the phosphoproteome of the multicellular bacterium Streptomyces coelicolor A3(2) by protein peptide fractionation, phosphopeptide enrichment and high-accuracy mass spectrometry. Proteomics, 10, 2486–2497. 10.1002/pmic.201000090 [DOI] [PubMed] [Google Scholar]
- Perpich JD, Yakoumatos L, Johns P, Stocke KS, Fitzsimonds ZR, Wilkey DW, Merchant ML, Miller DP, & Lamont RJ (2021). Identification and characterization of a UbK family kinase in Porphyromonas gingivalis which phosphorylates the RprY response regulator. Molecular Oral Microbiology, 36(5), 258–266. 10.1111/omi.12347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomowski A, Usón I, Nowakowska Z, Veillard F, Sztukowska MN, Guevara T, Goulas T, Mizgalska D, Nowak M, Potempa B, Huntington JA, Potempa J, & Gomis-Rüth FX (2017). Structural insights unravel the zymogenic mechanism of the virulence factor gingipain K from Porphyromonas gingivalis, a causative agent of gum disease from the human oral microbiome. Journal of Biological Chemistry, 292, 5724–5735. 10.1074/jbc.M117.776724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Banbula A, & Travis J (2000). Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology 2000, 24, 153–192. 10.1034/j.1600-0757.2000.2240108.x [DOI] [PubMed] [Google Scholar]
- Sharma K, D’Souza RCJ, Tyanova S, Schaab C, Wiśniewski J, Cox J, & Mann M (2014). Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Reports, 8, 1583–1594. 10.1016/j.celrep.2014.07.036 [DOI] [PubMed] [Google Scholar]
- Shen D, Perpich JD, Stocke KS, Yakoumatos L, Fitzsimonds ZR, Liu C, Miller DP, & Lamont RJ (2020). Role of the RprY response regulator in P. gingivalis community development and virulence. Molecular Oral Microbiology, 35(6), 231–239. 10.1111/omi.12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi B, Gnad F, Jensen PR, Petranovic D, Mann M, Mijakovic J, & Macek B (2008). The Ser/Thr/Tyr phosphoproteome of Lactococcus lactis IL1–403 reveals multiple phosphorylated proteins. Proteomics, 8, 3486–3493. 10.1002/pmic.200800069 [DOI] [PubMed] [Google Scholar]
- Standish AJ, & Morona R (2014). The role of bacterial protein tyrosine phosphatases in the regulation of the biosynthesis of secreted polysaccharides. Antioxidants & Redox Signaling, 20(14), 2274–2289. 10.1089/ars.2013.5726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Masuda T, Shinoda K, Nakamura A, Tomita M, & Ishihama Y (2007). Phosphopeptide enrichment by aliphatic hydroxyl acid-modified metal oxide chromatography for nano-LC-MS/MS in proteomics applications. Molecular and Cellular Proteomics, 6(6), 1103–1109. 10.1074/mcp.T600060-MCP200 [DOI] [PubMed] [Google Scholar]
- Veillard F, Sztukowska M, Mizgalska D, Ksiazek M, Houston J, Potempa B, Enghild JJ, Thogersen IB, Gomis-Rüth FX, Nguyen KA, & Potempa J (2013). Inhibition of gingipains by their profragments as the mechanism protecting Porphyromonas gingivalis against premature activation of secreted proteases. Biochimica Et Biophysica Acta, 1830(8), 4218–4228. 10.1016/j.bbagen.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillard F, Sztukowska M, Nowakowska Z, Mizgalska D, Thøgersen IB, Enghild JJ, Bogyo M, Potempa B, Nguyen KA, & Potempa J (2019). Proteolytic processing and activation of gingipain zymogens secreted by T9SS of Porphyromonas gingivalis. Biochimie, 166, 161–172. 10.1016/j.biochi.2019.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veith PD, Talbo GH, Slakeski N, Dashper SG, Moore C, Paolini RA,& Reynolds EC (2002). Major outer membrane proteins and proteolytic processing of RgpA and Kgp of Porphyromonas gingivalis W50. Biochemical Journal, 363(1), 105–115. 10.1042/0264-6021:3630105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin S, Watson DC, Tessier L, Ding W, Foote S, Bhatia S, Kelly JF, & Young NM (2007). The cytoplasmic phosphorproteome of the Gram-negative bacterium Camplyobacter jejuni: Evidence for modification by unidentified protein kinases. Proteomics, 7, 4338–4348. 10.1002/pmic.200700483 [DOI] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, Kinloch A, Culshaw S, Potempa J, & Venables PJ (2010). Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis and Rheumatism, 62, 2662–2672. 10.1002/art.27552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore SE, & Lamont RJ (2012). Tyrosine phosphorylation and bacterial virulence. International Journal of Oral Science, 4, 1–6. 10.1038/ijos.2012.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Xue P, Hirano T, Liu C, Whitmore S, Hackett M, & Lamont RJ (2014). Characterization of a bacterial tyrosine kinase in Porphyromonas gingivalis involved in polymicrobial synergy. MicrobiologyOpen, 3, 383–394. 10.1002/mbo3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenez-Fyvie LA, Haffajee AD, & Socransky SS (2000). Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. Journal of Clinical Periodontology, 27, 648–657. 10.1034/j.1600-051x.2000.027009648.x [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Toyoshima K, Yamazaki M, Hanada N, & Takehara T (1990). Purification and characterization of alkaline phosphatase of Bacteroides gingivalis 381. Infection and Immunity, 58(9), 2882–2887. 10.1128/iai.58.9.2882-2887.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Liu L, Liu B, & Jin Q (2015). Phosphoproteomic analysis of bacillus Celmette-Guérin using gel-based and gel-free approaches. Journal of Proteomics, 126, 189–199. 10.1016/j.jprot.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Zhou XY, Gao JL, Hunter N, Potempa J, & Nguyen KA (2013). Sequence-independent processing site of the C-terminal domain (CTD) influences maturation of the RgpB protease from Porphyromonas gingivalis. Molecular Microbiology, 89(5), 903–917. 10.1111/mmi.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are in the article and Supporting Information.


