Abstract
OBJECTIVE
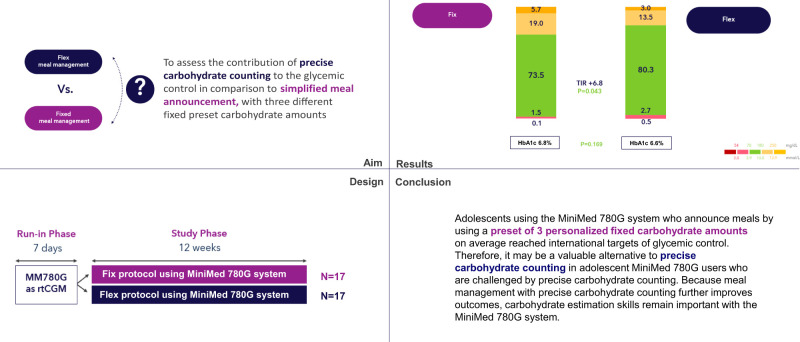
We aimed to compare glucose control in adolescents with type 1 diabetes (T1D) using the MiniMed 780G system who used simplified meal announcement with those who used precise carbohydrate counting.
RESEARCH DESIGN AND METHODS
This randomized controlled trial included 34 participants (age 12–18 years) with T1D who were on multiple daily injections or insulin pump and were scheduled to start using the MiniMed 780G system at Sidra Medicine in Qatar. After a 7-day run-in period, participants were randomly assigned to the fix group (simplified meal announcement by preset of three personalized fixed carbohydrate amounts) or the flex group (precise carbohydrate counting) and followed for 12 weeks. Between-group difference in time in range (TIR) was the primary end point. Secondary end points included HbA1c and other glycometrics.
RESULTS
During the 12-week study phase, TIR was 73.5 ± 6.7% in the fix and 80.3 ± 7.4% in the flex group, with a between-group difference of 6.8% in favor of flex (P = 0.043). Time >250 mg/dL was better in the flex group (P = 0.012), whereas HbA1c (P = 0.168), time below range (P = 0.283), and time between 180 and 250 mg/dL (P = 0.114) did not differ.
CONCLUSIONS
Adolescents using the MiniMed 780G system with a preset of three personalized fixed carbohydrate amounts can reach international targets of glycemic control. Therefore, it may be a valuable alternative to precise carbohydrate counting in users who are challenged by precise carbohydrate counting. Because carbohydrate counting further improves outcomes, these skills remain important for MiniMed 780G users.
Graphical Abstract
Introduction
Carbohydrate counting is an essential component of diabetes management in both adults and children and together with an intensive insulin plan and increased blood glucose monitoring, is associated with lower HbA1c (1,2). Recent meta-analyses concluded that carbohydrate counting is an effective method in reducing HbA1c, recommended carbohydrate counting over alternate advice, and encouraged patients with type 1 diabetes (T1D) to count carbohydrates (3,4). However, carbohydrate counting is perceived as one of the most burdensome tasks in T1D and is frequently done inconsistently and with poor accuracy (5).
Hybrid closed loop (HCL) systems have substantially changed T1D management and have been shown to improve glycemic outcomes across a broad age range of patients, including adolescents (6,7). The MiniMed 780G system is an advanced HCL system, and besides controlling basal insulin, it includes autocorrection boluses. The MiniMed 780G system has been shown to safely achieve internationally recommended glycemic targets in most adolescents and adults with T1D (8,9). The MiniMed 780G system, as are other current HCL systems, is not yet fully automated and still requires patients to enter carbohydrates for meal announcement.
In previous publications from our center (10) and others (11), the use of the MiniMed 780G system with precise carbohydrate counting resulted in improvement of HbA1c and time in range (TIR). However, not all adolescents are able to or willing to perform carbohydrate counting, and data on the effect of simplified meal announcement on glycemic control in users of the MiniMed 780G system are lacking. In this study, we aimed to compare glucose control in adolescents using the MiniMed 780G system with simplified meal announcement with those who used precise carbohydrate counting.
Research Design and MethodS
Study Design and Participants
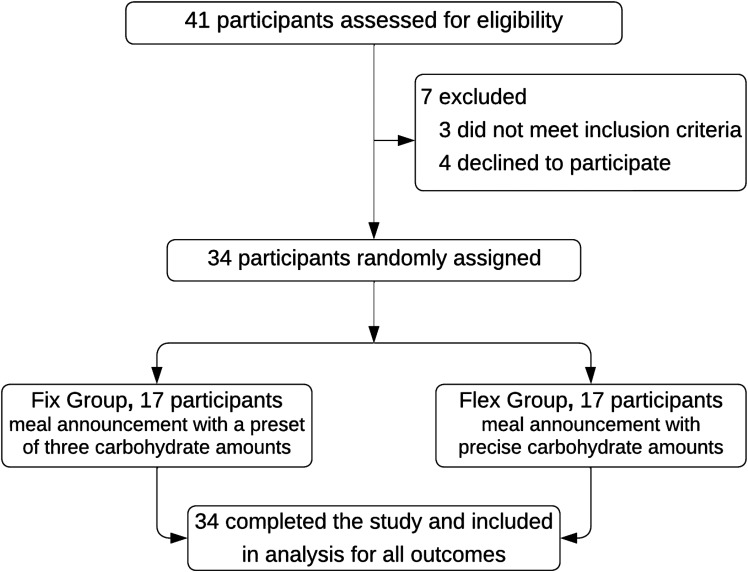
This prospective, open-label, randomized controlled trial was performed at Sidra Medicine in Qatar. Participants were 12–18 years old, had a clinical diagnosis of T1D for at least 12 months, were on multiple daily injection (MDI) or insulin pump therapy, and were scheduled to start using the MiniMed 780G system with Guardian 4 calibration-free sensor. Patients who had significant diabetes complications and/or a severe episode of diabetic ketoacidosis or hypoglycemia in the past 6 months were excluded. Participants were randomly assigned to either using a preset of three personalized fixed carbohydrate amounts (fix group) or precise carbohydrate counting (flex group) and followed for 12 weeks, and glycemic control was compared. Figure 1 depicts a high-level overview of the study design.
Figure 1.
Study design and enrollment scheme.
The study was approved by the institutional review board at Sidra Medicine and the national ethics committee of the Ministry of Public Health in Qatar. All participants and their guardians signed a written informed consent/assent before the start of study-related procedures.
Procedures
Participants were consecutively recruited at regular clinic visits. After obtaining consent/assent, the trial started with a 7-day run-in period to train participants on using the MiniMed 780G system. During this run-in period, participants used the MiniMed 780G system for real-time continuous glucose monitoring (CGM) without any insulin delivery by the MiniMed 780G pump (participants on MDI therapy remained on manual injections, and participants already on insulin pump therapy continued to use their previous model for insulin injections), and these CGM data were used for baseline glycemic metrics. Also, carbohydrate counting skills were assessed during this period by a 7-day food and insulin diary, and participants in whom the announced carbohydrate amount deviated from the actual amount by >20% were excluded from randomization (n = 3).
Participants were randomly assigned to either the fix group or the flex group. A permuted block randomization scheme was used. Block size was fixed at 6. Neither the investigators nor the participants and parents were masked to the treatment allocation.
After randomization, all participants were initiated on the MiniMed 780G system per Sidra Medicine’s validated protocol for sensor-augmented pump (SAP) initiation (12). In brief, participants started using the MiniMed 780G system in open loop for 3 days (SAP period) for the MiniMed 780G algorithm to establish personalized parameters required for system initiation. The MiniMed 780G system was then initiated for all, and the participants used the meal management approach of their allocated group.
Participants in the fix group could choose among a preset of three fixed carbohydrate amounts for each meal announcement. These fixed amounts were personalized based on the 7-day diary; the carbohydrate amounts for a regular meal was set at 40–70 g, for a large meal at 60–90 g, and for a snack at 15–20 g. Fixed amounts for meals were rounded to tens and for snacks, to fives. During individual consultations, a registered dietitian instructed the participants in the fix group to choose the appropriate personalized preset (i.e., the regular meal preset for a regular-sized meal regardless of food type, the large meal preset for eating more than a regular-sized meal, the snack preset for any snack regardless of the amount of carbohydrates). Participants in the flex group were instructed to use precise carbohydrate counting with increments of 1 g. No restriction in dietary intake or daily activities were advised during the study for both groups. Supplementary Table 1 shows the settings at MiniMed 780G system initiation, as well as the methods for calculating the fixed carbohydrate amounts.
Onsite follow-up visits were scheduled 2 and 12 weeks after initiating the MiniMed 780G system, and phone visits were scheduled after weeks 1, 4, and 8. Meal announcement as well as the insulin-to-carbohydrate ratio (ICR) were reassessed after weeks 1 and 2 and adjusted per clinical judgment (if needed) in both groups. Adherence to the correct meal announcement strategy was reviewed (and corrected if needed) at every visit in both groups. Clinical and technical support was available during the study. Standard local hypoglycemia and hyperglycemia treatment guidelines were followed.
Funds to cover devices were secured through medical insurance, self-funding, and donations made by the Qatar Diabetes Association for participants who could not afford the device. There were no rejections because of funding constraints.
Outcomes and Analyses
The primary outcome was the between-group difference in TIR (70–180 mg/dL) in the 12-week study phase. Secondary outcomes were between-group differences in HbA1c, other CGM-derived metrics for glycemic control (including time above range, time below range [TBR], mean sensor glucose, SD, and coefficient of variation [CV]), metrics for meal announcement and insulin use (including number of announced meals, announced grams of carbohydrates, the insulin total daily dose [TDD], and a breakdown of the delivered insulin in autobasal, autocorrection, and manual bolus), metrics for system settings (including ICR, use of optimal settings, and sensor use), and safety. Safety end points included episodes of severe hypoglycemia (defined as a hypoglycemic event that requires medical assistance) and/or diabetic ketoacidosis (defined by a blood glucose >200 mg/dL, venous pH <7.3, or serum bicarbonate <15 mmol/L), and ketonemia (blood β-hydroxybutyrate ≥3 mmol/L or moderate or large ketonuria. An overview of the primary and secondary end points is shown in Tables 2 and 3.
Table 2.
Glycemic control
| Fix group | Flex group | Group difference | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Study | P | Baseline | Study | P | P | |
| HbA1c | |||||||
| % | 8.0 ± 2.1 | 6.8 ± 0.3 | 0.026 | 7.9 ± 1.5 | 6.6 ± 0.5 | 0.001 | 0.168 |
| mmol/mol | 64 ± 26.2 | 51 ± 3.3 | 0.026 | 63 ± 18.6 | 49 ± 5.5 | 0.001 | 0.168 |
| Sensor glucose, mg/dL | 174 ± 26 | 147 ± 23 | 0.002 | 168 ± 29 | 145 ± 18 | 0.005 | 0.804 |
| CV, % | 35.6 ± 8.1 | 34.1 ± 5.0 | 0.520 | 30.1 ± 4.4 | 30.8 ± 4.2 | 0.634 | 0.045 |
| Sensor glucose values in range, % | |||||||
| <54 mg/dL | 0.2 ± 0.4 | 0.1 ± 0.3 | 1.000 | 0.8 ± 1.4 | 0.5 ± 0.3 | 0.275 | 0.167 |
| 54–70 mg/dL | 1.4 ± 0.5 | 1.5 ± 1.5 | 0.605 | 2.0 ± 1.5 | 2.7 ± 1.7 | 0.718 | 0.283 |
| 70–180 mg/dL | 47.5 ± 18.3 | 73.5 ± 6.7 | 0.001 | 49.1 ± 16.8 | 80.3 ± 7.4 | 0.001 | 0.043 |
| 180–250 mg/dL | 22.6 ± 8.1 | 19.0 ± 5.2 | 0.122 | 26.8 ± 8.2 | 13.5 ± 5.9 | 0.001 | 0.114 |
| >250 mg/dL | 28.3 ± 15.9 | 5.7 ± 3.6 | 0.001 | 21.3 ± 11.8 | 3.0 ± 2.4 | 0.001 | 0.012 |
| TDD, units/kg/day | 1.0 ± 0.6 | 1.1 ± 0.4 | 0.517 | 1.0 ± 0.6 | 1.1 ± 0.5 | 0.601 | 0.984 |
| Basal insulin, % of TDD | 43.2 ± 6.7 | 34.2 ± 7.1 | 0.001 | 44.8 ± 5.9 | 32.6 ± 5.2 | 0.001 | 0.459 |
| Weight, kg | 52.2 ± 12.7 | 53.5 ± 11.2 | 0.753 | 51.3 ± 10.7 | 52.4 ± 9.6 | 0.754 | 0.765 |
Data are mean ± SD. Study indicates 12 weeks of MiniMed 780G system use. CGM data at baseline were collected using the Guardian 4 sensor with the MiniMed 780G system for a 1-week period of training (no insulin delivery with pump).
Table 3.
Carbohydrate announcement, insulin, and system settings
| Fix group | Flex group | Group difference | |||||
|---|---|---|---|---|---|---|---|
| Weeks 1 and 2 | Weeks 9–12 | P | Weeks 1 and 2 | Weeks 9–12 | P | P | |
| Meal announcement | |||||||
| Meals per day | 3.9 ± 1.9 | 3.7 ± 0.9 | 0.695 | 5.2 ± 2.7 | 5.1 ± 1.1 | 0.888 | 0.003 |
| Carbohydrates, g/day | 170 ± 84 | 165 ± 66 | 0.873 | 164 ± 82 | 178 ± 65 | 0.585 | 0.566 |
| ICR, g | 7.5 ± 2.2 | 5.8 ± 1.6 | 0.014 | 7.9 ± 2.5 | 6.0 ± 1.3 | 0.009 | 0.619 |
| Insulin delivered, units/day | |||||||
| Manual | 22.6 ± 9.8 | 20.8 ± 9.1 | 0.528 | 23.0 ± 10.4 | 30.8 ± 9.4 | 0.034 | 0.003 |
| Autocorrection | 18.8 ± 14.2 | 17.9 ± 8.6 | 0.824 | 9.3 ± 8.6 | 8.9 ± 3.5 | 0.860 | 0.003 |
| Autobasal | 10.8 ± 10.4 | 22.1 ± 9.3 | 0.792 | 19 ± 7.5 | 20.4 ± 8.1 | 0.604 | 0.573 |
| Total | 52.2 ± 13.4 | 60.8 ± 10.2 | 0.043 | 51.3 ± 9.8 | 60.1 ± 8.8 | 0.009 | 0.813 |
| Insulin delivered, % | |||||||
| Manual | 43 | 45 | 45 | 51 | |||
| Autocorrection | 36 | 29 | 18 | 15 | |||
| Autobasal | 21 | 26 | 37 | 34 | |||
| System use and setting | |||||||
| Sensor wear, % | 93.8 ± 9.6 | 95.8 ± 8.1 | 0.516 | 94.1 ± 10.5 | 94.5 ± 10.9 | 0.913 | 0.695 |
| Advanced HCL usage, % | 89.5 ± 6.9 | 92.1 ± 6.9 | 0.280 | 90.6 ± 8.5 | 93.2 ± 7.8 | 0.359 | 0.666 |
| Advanced HCL exits* | 0.7 ± 0.4 | 0.8 ± 0.5 | 0.524 | 0.7 ± 0.5 | 0.6 ± 0.4 | 0.523 | 0.207 |
| SMBG per day | 0.8 ± 0.6 | 0.4 ± 0.3 | 0.019 | 0.7 ± 0.5 | 0.3 ± 0.5 | 0.026 | 0.338 |
| Set change, days | 2.9 ± 0.7 | 2.8 ± 0.9 | 0.720 | 2.8 ± 1.1 | 2.9 ± 1.4 | 0.813 | 0.805 |
| Reservoir change, days | 2.4 ± 0.4 | 2.3 ± 0.6 | 0.571 | 2.2 ± 0.8 | 2.5 ± 0.9 | 0.312 | 0.451 |
Data are mean ± SD, unless otherwise indicated. SMBG, self-monitoring of blood glucose.
Advanced HCL exits per patient per week.
As a secondary analysis, within-group changes in metrics for glycemic control were evaluated (separately for the fix and the flex group). This analysis was done for the entire 12-week study period and for the different study periods (i.e., run-in; SAP; automated insulin delivery in weeks 1, 2, 3–4, 5–8, and 9–12).
HbA1c was collected by a point-of-care DCA Vantage Analyzer (Siemens Healthineers, Erlangen, Germany). Insulin and CGM data were collected from CareLink therapy management software.
Statistics
The study hypothesis was that the flex group is expected to show better glycemic control compared with the fix group. In addition, we wanted to learn whether the fix group still reaches international targets for glycemic control. On the basis of the hypothesis as well as an α of 0.05, a power of 80%, a TIR in the flex group of 78.9, an SD of 6%, and an effect size of 7% (13), we calculated that 32 participants would be required for this study. To allow for dropouts, we planned to enroll 34.
All analyses were performed for the entire study population. Between-group differences were analyzed using paired Student t test or paired Wilcoxon test (in case of nonnormality). Within-group differences were analyzed using ANOVA. A two-sided α-level of 0.05 was used with no adjustment for multiplicity for secondary outcomes. Statistical analyses were performed using Statistica 12 (StatSoft, Tulsa, OK).
Data and Resource Availability
The data sets generated and/or analyzed during the current study are not publicly available due to institutional policies and regulations but are available from the corresponding author upon reasonable request.
RESULTS
Between November 2021 and February 2022, 34 participants were randomly assigned to either the fix group (n = 17) or the flex group (n = 17). All participants completed the 12-week follow-up and were included in the analyses. The enrollment scheme is shown in Fig. 1, and baseline characteristics are listed in Table 1.
Table 1.
Baseline characteristics
| Fix group | Flex group | |
|---|---|---|
| Participants, n | 17 | 17 |
| Female, n (%) | 9 (53) | 10 (58) |
| Age, years | 14.2 ± 1.5 | 13.9 ± 1.3 |
| BMI, kg/m2 | 20.8 ± 3.2 | 21.4 ± 4.5 |
| Duration of diabetes, years | 5.3 ± 2.4 | 4.3 ± 1.7 |
| HbA1c | ||
| % | 8.0 ± 2.1 | 7.9 ± 1.5 |
| mmol/mol | 64 ± 26.2 | 63 ± 18.6 |
| MDI therapy, n (%) | 12 (71) | 11 (65) |
| Pump therapy, n (%) | 5 (29) | 6 (35) |
| Meals per day | 4.5 ± 1.3 | 4.1 ± 0.9 |
| Carbohydrates, g/day | 165 ± 72 | 169 ± 62 |
Data are mean ± SD, unless otherwise indicated.
Glycemic Control
Table 2 shows the glycemic control during the study. Mean ± SD TIR (70–180 mg/dL) in the fix group increased from 47.5 ± 18.3% at baseline to 73.5 ± 6.7% during the 12-week study phase (P < 0.001, 95% CI 19.1–30.2), and in the flex group, TIR increased from 49.1 ± 16.8% to 80.3 ± 7.4% (P < 0.001, 95% CI 25.2–37.8), with a group difference of 6.8% (P = 0.043, 95% CI 4.1–9.2) during the study phase. HbA1c decreased from 8.0 ± 2.1% (64 ± 26.2 mmol/mol) at baseline to 6.8 ± 0.3% (51 ± 3.3 mmol/mol) at the end of the study in the fix group (P = 0.026, 95% CI −0.6 to −1.6) and from 7.9 ± 1.5% (63 ± 18.6 mmol/mol) to 6.6 ± 0.5% (49 ± 5.5 mmol/mol) in the flex group (P = 0.001, 95% CI −0.7 to −1.9), without a group difference (P = 0.168, 95% CI −0.1 to 0.4). Other CGM-derived metrics did not show a between-group difference except for time >250 mg/dL (P = 0.012, 95% CI −4.6 to −0.4) and CV (P = 0.045, 95% CI −6.5 to −0.1), which were better in the flex group than in the fix group.
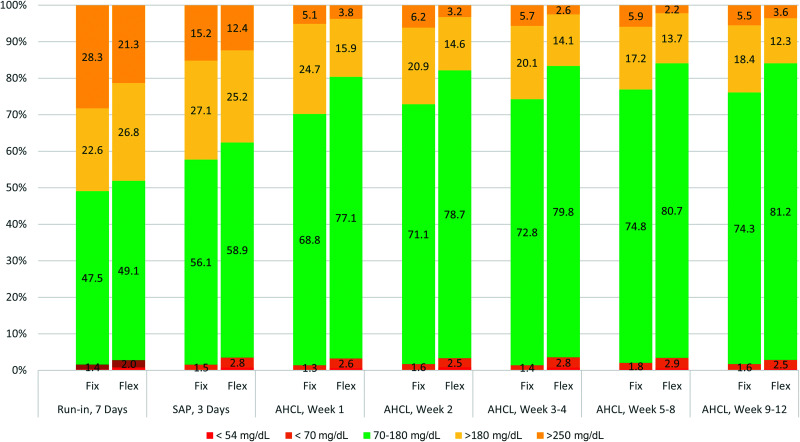
In the fix group, 70% of participants reached an HbA1c of <7% at study end, 67% a TIR of >70%, and 82% a TBR of <4% vs. 75%, 72%, and 86% in the flex group, respectively. Fix group averages over the last 4 weeks were 74.3% for TIR, 6.8% for HbA1c, and 1.7% for TBR (Fig. 2).
Figure 2.
TIR during different study periods. Data are percentage of TIR during the interval. Glucose values <54 mg/dL are not shown on the graph. Baseline data were collected using the Guardian 4 sensor with the MiniMed 780G system for a 1-week period of training. AHCL, advanced hybrid closed loop.
Insulin Delivery and Carbohydrate Announcement
Table 3 shows insulin delivery and carbohydrate announcement during the study. The TDD increased by 0.1 unit/kg in both groups (fix group: 1.0 ± 0.6 to 1.1 ± 0.4 units/kg, P = 0.517, 95% CI −0.6 to 1.3; flex group: 1.0 ± 0.6 to 1.1 ± 0.5 units/kg, P = 0.601, 95% CI −0.7 to 1.4), with no group difference (P = 0.984, 95% CI −0.1 to 0.3) (Table 2). Basal insulin as a percentage of TDD decreased by 9.0% in the fix group (43.2 ± 6.7% to 34.2 ± 7.1%, P = 0.001, 95% CI −6.4 to −9.8) and 12.2% in the flex group (44.8 ± 5.9% to 32.6 ± 5.2%, P = 0.001, 95% CI −8.5 to −13.7), with no group difference (P = 0.459, 95% CI −2.7 to 5.9) (Table 2). At the end of the study, the amount of insulin delivered by autocorrection was almost twice as high in the fix group compared with the flex group (17.9 ± 8.6 vs. 8.9 ± 3.5 units/day, P = 0.003, 95% CI 4.1 to 10.9). The manual bolus amount was lower in the fix group than in the flex group (20.8 ± 9.1 vs. 30.8 ± 9.4 units/day, P = 0.003, 95% CI 3.8 to 12.7).
The total daily announced carbohydrates did not differ between the groups at study end (165 ± 66 vs. 178 ± 65 g in the fix and flex groups, respectively, P = 0.566, 95% CI −11 to 26), though the individual meal carbohydrate quantity distribution did (Supplementary Fig. 1). In addition, participants in the fix group announced fewer meals per day compared with the flex group (3.7 ± 0.9 vs. 5.1 ± 1.1, P = 0.003, 95% CI 0.7–2.1).
Most of the of the participants in the fix group announced snacks by 20 g (94%), regular meal by 60 g (53%), and large meal by 90 g (47%) (Supplementary Table 2). There was no difference in the carbohydrate amounts that individuals announced for snack, regular meal, and large meal at the beginning versus the end of the study.
MiniMed 780G System Use and Settings
Table 3 shows system use at baseline and during the study. More than 90% of both groups wore the sensor and used the MiniMed 780G system during the study, but no group difference was observed (P = 0.695 [95% CI −5.4 to 8.1] and 0.666 [95% CI −6.1 to 4.2] between the fix and flex groups, respectively). The number of exits per patient per week did not differ between the groups and was 0.8 in the fix group and 0.6 in the flex group (P = 0.207, 95% CI −0.1 to 0.5). The number of finger pricks (i.e., self-monitoring of blood glucose) per day significantly decreased from 0.8 ± 0.6 to 0.4 ± 0.3 (P = 0.019, 95% CI −0.3 to −0.5) in the fix group and from 0.7 ± 0.5 to 0.3 ± 0.5 (P = 0.026, 95% CI −0.2 to −0.7) in the flex group, without a between-group difference (P = 0.338, 95% CI −0.1 to 0.3). Infusion sets and reservoirs were changed approximately every 2–3 days.
In terms of optimal settings, 80% of participants initiated the MiniMed 780G system with an active insulin time of 2 h, which increased to 94% of participants at study end. A glucose target of 100 mg/dL was used in 94% of participants at MiniMed 780G system initiation and remained the target during the study.
Safety
No serious adverse events or episodes of severe hypoglycemia or hyperglycemia with ketosis were reported in both groups. Skin irritations related to sensor use occurred in two participants in the fix group and three participants in the flex group. Two participants from the fix group had mild respiratory tract infections. All reported adverse events were resolved without sequelae.
Temporal Trends Analysis
Figure 2 shows the TIR during the different study periods from baseline to using the MiniMed 780G system. TIR increased from baseline to SAP by almost 9% in both groups. In the fix group, TIR reached >70% from the 2nd week and reached a plateau of >74% after ∼5 weeks. In the flex group, TIR also plateaued at ∼5 weeks and reached >80%. TBR <54 mg/dL remained <1% and TBR <70 mg/dL <3% in both groups during the study. Time above range decreased over time.
The ICR in the fix group was adjusted during the study phase from 10.8 g at baseline to 7.5 g at MiniMed 780G initiation (P = 0.001, 95% CI −2.5 to −4.0, adjustment made in 14 participants) with a further decrease to 5.8 g after 2 weeks of MiniMed 780G use (P = 0.014, 95% CI −0.8 to −2.4, 11 participants). In the flex group, adjustments were from 11.2 g at baseline to 7.9 g (P = 0.001, 95% CI −2.3 to −4.2, 12 participants) and then to 6.0 g (P = 0.009, 95% CI −1.1 to −2.5, 10 participants), respectively. All participants used one ICR over a 24-h period, and no further changes were made after the 2nd week of MiniMed 780G usage.
Conclusions
In this study, we investigated glycemic control in adolescents using the MiniMed 780G system with a relatively simple mode of meal announcement, including a preset of three different personalized fixed-carbohydrate amounts (fix group) compared with MiniMed 780G users who performed precise carbohydrate counting (flex group). The key finding of this study is that glycemic control as achieved in the flex group was superior to that achieved in the fix group. This finding is based on a significant 6.8% TIR difference in favor of the flex group during the study phase, as well as a significant −2.7% difference of time >250 mg/dL. The other metrics of glycemic control, such as HbA1c, TBR, and time between 180 and 250 mg/dL, did not show any between-group differences, and there was no between-group difference in safety.
Albeit not as good as the flex group, most participants in the fix group still reached international targets for glycemic control (14). In total, 70% of participants reached an HbA1c of <7% at study end, 67% a TIR of >70%, and 82% a TBR of <4% (vs. 75%, 72%, and 86%, respectively, in the flex group). Also, group averages of HbA1c (6.8%), TIR (74.3%), and TBR (1.6%) were well within targets. These data indicate that meal announcement by using a preset of three personalized fixed-carbohydrate amounts is a valuable alternative to conventional carbohydrate counting.
The MiniMed 780G algorithm with its autocorrection boluses can partly correct for less accurate carbohydrate entries, which is demonstrated by the double amount of insulin delivered by autocorrection bolus in the fix group versus the flex group (17.9% vs. 8.9%, respectively, P = 0.003). The amount delivered autobasally was similar in both groups.
The simplified method for meal announcement as presented in this article may have some room for improvement in terms of both training and defining the personalized presets. First, the fix group announced significantly fewer meals compared with the flex group (3.7 vs. 5.1, respectively), and based on the baseline data, as well as Qatari eating habits, this seems an underrepresentation. Improved and recurrent training might lead to more meal announcements, less need for the MiniMed 780G system to autocorrect, and better glycemic control. Second, although the presets were personalized, the fix method only included three presets (regular meal, large meal, and snack). Further investigation is warranted of whether more than three presets with smaller increments provides better glycemic control without increasing burden. In addition, the high percentage of insulin that was delivered by autocorrection in the fix group may point to an underestimation of announced carbohydrates. Third, carbohydrate consumption may change when young people age, and a timely revision of the personalized presets may be included in the method.
One other meal announcement method has been described for the MiniMed 780G system so far. Unannounced snacks of up to 20 g of carbohydrates can avoid a difference in blood glucose of ≥50 mg/dL in pediatric MiniMed 780G users, and unannounced meals of up to 30 g of carbohydrates are safe (15). However, the MiniMed 780G system is not designed as a full closed loop system; thus, meal announcement is preferred. To our knowledge, other means of carbohydrate counting have not yet been studied in the MiniMed 780G system. Methods such as recommended by American Diabetes Association and International Society for Pediatric and Adolescent Diabetes guidelines, including the use of 10-g portions and 15-g exchanges (16), may also provide safe glycemic control and reduce burden compared with precise carbohydrate counting.
A clear TIR improvement already presented 1 week after MiniMed 780G system initiation, and TIR reached a plateau after ∼5 weeks both in the fix and the flex arms. This rapid and clear increase is in line with a previous study from our center (10) and is partly attributed to the modification of ICR in order to stabilize postmeal glucose levels. The end-of-study TIR of >81% in the flex group was relatively high compared with the MiniMed 780G pivotal study in adolescents (72.7%) (17) and compared with real-world data in users ≤15 years of age (73.9%) (13). However, >90% of participants in our study used the recommended optimal system settings (i.e., a glucose target of 100 mg/dL and active insulin time of 2 h). These settings are known to be predictors for a higher TIR and most probably are attributed to the high TIR (18).
This study has several limitations. First, the follow-up time of 12 weeks is relatively short. Albeit long enough to show changes in TIR and HbA1c, the sustainability of results beyond the duration of this study cannot be confirmed. Second, generalizability was hampered by the fact that patients with limited carbohydrate counting skills were excluded from the study. Third, use of the DCA Vantage Analyzer for the HbA1c analysis is not a laboratory test per se, and some have described the findings from these types of tests as inaccurate (19). On the other hand, the DCA Vantage Analyzer has been frequently used in daily diabetes care and diabetes research and meets NGSP performance criteria (20). Fourth, the study is limited by the window for calculating CGM data. Per Battelino et al. (14), a minimum of 10 days of CGM data is needed to accurately provide CGM metrics, and in our study, this was possible for some but not all studied periods. Finally, we did not adjust for multiplicity on secondary outcomes because of the small sample size.
The main strength of our study is the randomized controlled trial design, which led to two similar groups at baseline. Any difference in glycemic control at study end can therefore mainly be attributed to differences in meal announcement method.
In conclusion, adolescents using the MiniMed 780G system who announce meals through a preset of three personalized fixed-carbohydrate amounts on average reached international targets of glycemic control. Therefore, the preset announcements may be a valuable alternative to precise carbohydrate counting in adolescent MiniMed 780G users who are challenged by precise carbohydrate counting. Because meal management with precise carbohydrate counting further improves outcomes, carbohydrate estimation skills remain important with the MiniMed 780G system.
Article Information
Acknowledgments. The authors thank the participants and their families for their collaboration during the study and diabetes educators Douha AlMajaly, Fareeda Umer, and Manar Hamdan (Sidra Medicine) for contributions to this project. The authors also acknowledge the contributions and efforts of administrative and research staff at Sidra Medicine for data management and analyses.
Funding. This study was funded by Sidra Medicine.
Duality of Interest. G.P. received speakers’ bureau honoraria from Sanofi, Abbott, Novo Nordisk, Medtronic, and Dexcom and travel and accommodation expenses from Dexcom. J.C. received travel and accommodation expenses from Dexcom, Abbott, and Medtronic. T.H. is an employee of Medtronic. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.P. coordinated the study. G.P. and J.C. designed the study and interpreted the results. G.P., J.C., M.P., E.D., and A.K. screened and enrolled participants, arranged informed consent from the participants, and took samples. G.P., J.C., and K.H. contributed to the data and statistical analyses. G.P., J.C., K.H., and T.H. wrote the manuscript. All authors critically reviewed the manuscript. G.P. and J.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Footnotes
Clinical trial reg. no. NCT05069727; clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.21685190.
References
- 1. Mehta SN, Quinn N, Volkening LK, Laffel LM. Impact of carbohydrate counting on glycemic control in children with type 1 diabetes. Diabetes Care 2009;32:1014–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brazeau AS, Mircescu H, Desjardins K, et al. Carbohydrate counting accuracy and blood glucose variability in adults with type 1 diabetes. Diabetes Res Clin Pract 2013;99:19–23 [DOI] [PubMed] [Google Scholar]
- 3. Builes-Montaño CE, Ortiz-Cano NA, Ramirez-Rincón A, Rojas-Henao NA. Efficacy and safety of carbohydrate counting versus other forms of dietary advice in patients with type 1 diabetes mellitus: a systematic review and meta-analysis of randomised clinical trials. J Hum Nutr Diet 2022;35:1030–1042 [DOI] [PubMed] [Google Scholar]
- 4. Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2014;2:133–140 [DOI] [PubMed] [Google Scholar]
- 5. Ruan Y, Thabit H, Leelarathna L, et al.; AP@home Consortium . Variability of insulin requirements over 12 weeks of closed-loop insulin delivery in adults with type 1 diabetes. Diabetes Care 2016;39:830–832 [DOI] [PubMed] [Google Scholar]
- 6. Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 2021;23:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Isganaitis E, Raghinaru D, Ambler-Osborn L, et al.; iDCL Trial Research Group . Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the International Diabetes Closed-Loop Trial. Diabetes Technol Ther 2021;23:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Nimri R, Beck RW, et al.; FLAIR Study Group . A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 2021;397:208–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva JD, Lepore G, Battelino T, et al. Real-world performance of the MiniMed™ 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther 2022;24:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrovski G, Al Khalaf F, Campbell J, et al. Successful transitioning children and adolescents with type 1 diabetes from multiple daily injections to advanced hybrid closed-loop system in 10 days: a prospective intervention study on MiniMed 780G system. Acta Diabetol 2022;59:743–746 [DOI] [PubMed] [Google Scholar]
- 11. Beato-Víbora PI, Gallego-Gamero F, Ambrojo-López A, Gil-Poch E, Martín-Romo I, Arroyo-Díez FJ. Rapid improvement in time in range after the implementation of an advanced hybrid closed-loop system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2021;23:609–615 [DOI] [PubMed] [Google Scholar]
- 12. Petrovski G, Al Khalaf F, Campbell J, Fisher H, Umer F, Hussain K. 10-Day structured initiation protocol from multiple daily injection to hybrid closed-loop system in children and adolescents with type 1 diabetes. Acta Diabetol 2020;57:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arrieta A, Battelino T, Scaramuzza AE, et al. Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: evidence from 12 870 real-world users. Diabetes Obes Metab 2022;24:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tornese G, Carletti C, Giangreco M, Nisticò D, Faleschini E, Barbi E. Carbohydrate tolerance threshold for unannounced snacks in children and adolescents with type 1 diabetes using an advanced hybrid closed-loop system. Diabetes Care 2022;45:1486–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smart CE, Annan F, Higgins LA, Jelleryd E, Lopez M, Acerini CL. ISPAD clinical practice consensus guidelines 2018: nutritional management in children and adolescents with diabetes. Pediatr Diabetes 2018;19(Suppl. 27):136–154 [DOI] [PubMed] [Google Scholar]
- 17. Carlson AL, Sherr JL, Shulman DI, et al. Safety and glycemic outcomes during the MiniMed™ advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24:178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castañeda J, Mathieu C, Aanstoot HJ, et al. Predictors of time in target glucose range in real-world users of the MiniMed 780G system. Diabetes Obes Metab 2022;24:2212–2221 [DOI] [PubMed] [Google Scholar]
- 19. Abildgaard A, Knudsen CS, Bjerg LN, Lund S, Støy J. Lot variation and inter-device differences contribute to poor analytical performance of the DCA Vantage™ HbA1c POCT instrument in a true clinical setting. Clin Chem Lab Med 2021;60:127–134 [DOI] [PubMed] [Google Scholar]
- 20. Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem 2010;56:44–52 [DOI] [PubMed] [Google Scholar]