Abstract
Introduction
Phytoestrogens found in soy, fruits, peanuts, and other legumes, have been identified as metabolites capable of providing beneficial effects in multiple pathological conditions due to their ability to mimic endogenous estrogen. Interestingly, the health-promoting effects of some phytoestrogens, such as isoflavones, are dependent on the presence of specific gut bacteria. Specifically, gut bacteria can metabolize isoflavones into equol, which has a higher affinity for endogenous estrogen receptors compared to dietary isoflavones. We have previously shown that patients with multiple sclerosis (MS), a neuroinflammatory disease, lack gut bacteria that are able to metabolize phytoestrogen. Further, we have validated the importance of both isoflavones and phytoestrogen-metabolizing gut bacteria in disease protection utilizing an animal model of MS. Specifically, we have shown that an isoflavone-rich diet can protect from neuroinflammatory diseases, and that protection was dependent on the ability of gut bacteria to metabolize isoflavones into equol. Additionally, mice on a diet with isoflavones showed an anti-inflammatory response compared to the mice on a diet lacking isoflavones. However, it is unknown how isoflavones and/or equol mediates their protective effects, especially their effects on host metabolite levels.
Objectives
In this study, we utilized untargeted metabolomics to identify metabolites found in plasma that were modulated by the presence of dietary isoflavones.
Results
We found that the consumption of isoflavones increased anti-inflammatory monounsaturated fatty acids and beneficial polyunsaturated fatty acids while reducing pro-inflammatory glycerophospholipids, sphingolipids, phenylalanine metabolism, and arachidonic acid derivatives.
Conclusion
Isoflavone consumption alters the systemic metabolic landscape through concurrent increases in monounsaturated fatty acids and beneficial polyunsaturated fatty acids plus reduction in pro-inflammatory metabolites and pathways. This highlights a potential mechanism by which an isoflavone diet may modulate immune-mediated disease.
Keywords: phytoestrogen, isoflavone, inflammation, untargeted metabolomics, metabolites, multiple sclerosis
1. Introduction
Recently, diet has emerged as an important regulator of human health. Aside from nutrition, it provides numerous bioactive molecules such as polyphenols, flavonoids, phytoestrogens, carotenoids, and vitamins necessary for maintaining a healthy state (Sun et al., 2019). The trillions of bacteria (microbiota) living in our gut play an important role in mediating the beneficial effects of dietary compounds as they help in metabolizing plant compounds into more potent metabolites (Jensen et al., 2021). Thus, consuming a healthy diet full of vegetables and fruits will enrich the growth of healthy gut microbiota, which in turn will help produce beneficial metabolites necessary for maintaining health. For example, plant-based foods containing phytoestrogen compounds are thought to be beneficial for human health due to their structural similarity to estrogens, which allows them to bind and activate endogenous estrogen receptors (ERs). Phytoestrogen compounds present in plant-based food have a weak affinity for ERs, but numerous bacteria present in the human gut have enzymes that can metabolize these dietary phytoestrogens into secondary metabolites to enhance their ability to bind and activate endogenous ERs (Aboushanab et al., 2021). Certain gut bacteria such as Parabacteroides, Adlercreutzia, Slackia, Lactobacillus, and Bifidobacterium genera can metabolize the isoflavone (phytoestrogen) daidzein into S-equol which has higher activity than daidzein itself (Mayo et al., 2019a). For simplicity, S-equol will be referred to as equol throughout the rest of this work. The loss of these bacteria, and the absence of equol, may create an environment in which non-commensal bacteria dominate and thus predispose the host to disease by inducing the production of pro-inflammatory mediators.
Therefore, it is not surprising that multiple human diseases are linked with the loss of beneficial metabolites and gut microbiota (dysbiosis) which is characterized by an increase in both pro-inflammatory mediators and pathogenic gut bacteria with a concomitant decrease in anti-inflammatory mediators (Mangalam et al., 2021). Interestingly, we have previously shown that people with multiple sclerosis (MS), a chronic inflammatory disease of the central nervous system, lack gut bacteria with the ability to metabolize dietary phytoestrogens (Chen et al., 2016). Besides MS, isoflavones have been associated with protection against other diseases such as cardiovascular disease, osteoporosis, prostate cancer, breast cancer, atherosclerosis, and diabetic conditions (Pabich et al., 2019; Sathyapalan et al., 2018). Isoflavones are also being studied as a treatment strategy for atrophy, menopause, and postmenopausal symptoms (Pabich et al., 2019).
We validated the importance of phytoestrogen-metabolizing gut bacteria in MS utilizing experimental autoimmune encephalomyelitis (EAE), an animal model of MS (Jensen et al., 2021). In this study, we showed that mice on an isoflavone diet developed milder disease compared to those on a phytoestrogen-free (phyto-free) diet. Most importantly, the isoflavone diet-mediated disease protection was dependent on the presence of phytoestrogen metabolizing gut bacteria, specifically those lacking in patients with MS (pwMS). We also observed that compared to mice on a phyto-free diet, mice on an isoflavone diet demonstrated lower inflammatory responses as measured by antigen-specific T cell proliferation and cytokine levels in bone-marrow derived dendritic cells (Ghimire et al., 2022; Jensen et al., 2021). In summary, published studies and our data have established an important role of an isoflavone diet in suppressing inflammatory diseases (Aboushanab et al., 2021; Chen et al., 2016; Jensen et al., 2021; Mayo et al., 2019; Pabich et al., 2019; Sathyapalan et al., 2018).
Due to the structural similarity of equol to endogenous estrogen, it can interact with ERs, which are expressed in most mammalian cells and tissue (Warner et al., 2017). Thus, equol could have systemic effects beyond the gut. Further, recent studies have also highlighted thousands of metabolites present in the human blood which play an important role in maintaining overall health by regulating numerous physiological processes (François et al., 2021; Poisson et al., 2015; Rangel-Huerta et al., 2017; Zhang et al., 2015). Based on these data, we hypothesized that isoflavones and the metabolite equol may mediate their disease-protective effect through modulation of the host metabolome, specifically the balance between pro and anti-inflammatory metabolites.
Therefore, in this study, we investigated whether mice on a diet with isoflavones had a distinct metabolite profile compared to mice on a diet lacking isoflavones (and all other phytoestrogens). We observed significant differences in the metabolome compositions between the two diets. In fact, we observed enrichment of metabolites linked with a healthy state in mice on a diet with isoflavone and a consequent increase in metabolites linked with a disease state in mice on a phyto-free diet. Our study is one of the first to profile the metabolome using untargeted metabolomics in mice on a diet with or without isoflavones. These findings give us an insight into how isoflavone might protect from diseases through the alteration of specific metabolites, which can be harnessed for the development of future therapies for MS as well as other inflammatory diseases.
2. Materials and Methods
2.1. Experimental mice and diet treatment
C57BL/6J male mice (4–6 weeks old) were purchased from the Jackson Laboratories (Bar Harbor, ME) and allowed to adjust to their new environment for 1–2 weeks. Mice (n=10) were then put on a custom diet with the phytoestrogen-free diet, essentially being the purified diet offered by Envigo (Indianapolis, IN) that contains casein as a source of protein, dextrose as a source of sugar, cellulose as a source of fiber, lard as a source of fat, plus additional vitamins and minerals (TD.180434). The only additions to the isoflavone diet were genistein (0.24 g/kg of diet) and daidzein (0.22 g/kg of diet) (TD.180435). Mice were put on their respective diets ad libitum for 6 weeks, as described previously (Jensen et al., 2021).
2.2. Serum sample collection
At the end of the six-week special diet regimen, blood was collected, and the serum was separated by a standard blood clotting method. These serum samples were stored at −80°C and shipped to Metabolon (Morrisville, NC) for metabolite identification.
2.3. Untargeted metabolomics
Metabolic profiling was performed by Metabolon Inc. (Morrisville, NC) using proprietary methods as previously described by our group (Mangalam et al., 2013; Poisson et al., 2015; Zahoor et al., 2022). In brief, Metabolon Inc. precipitates proteins and extracts small molecules from the sample utilizing methanol and intense shaking followed by centrifugation. For metabolite analysis, Metabolon then utilizes reverse-phase ultra-high-performance chromatography-mass spectrometry with negative and positive ion mode electrospray ionization (ESI) and hydrophilic interaction ultra-performance liquid chromatography-mass spectrometry with negative ion mode ESI. Metabolon also ran several types of controls along with our samples to assure quality measurements, including a pooled sample containing a small component of each of our samples, ultra-pure water, a mixture of solvents used in the extraction, and a well-characterized plasma sample maintained by Metabolon Inc. Compounds were then identified based on their retention time/index (RI), mass to charge ratio (m/z), and fragmentation characteristics (MS/MS spectral data) by comparison to a library of compounds created by Metabolon Inc. Their library contains the RI, m/z, and spectral data information for over 3300 commercially available purified standard compounds. The workflow output was a data table containing the abundance of each metabolite present in each sample (Table S1). A total of 876 metabolites were identified. Metabolon Inc. has provided a more detailed explanation of its methods on its website (Metabolon Inc., 2022).
2.4. Statistical analysis of metabolites
Both MetaboAnalyst 5.0 (Pang et al., 2021) and R (R Core Team, 2022) (version 4.1.1) were utilized for statistical analyses of the metabolites observed in the serum samples from both groups. Overall data were first filtered based on missing values. If a given metabolite had missing values for more than 50% of the samples in either group, it was removed. Data were then normalized by sum scaling at the sample level and log (base 10) transforming at the metabolite level. For univariate testing, we utilized a Wilcoxon Rank Sum test, and the p-values were adjusted by utilizing the Benjamini-Hochberg procedure. Results were considered significant if the p-value < 0.05 and the adjusted p-value was < 0.10. Univariate tests were performed and visualized utilizing the R packages: ggplot2 (Wickham, 2016), ggpubr (Kassambara, 2020), tidyverse (Wickham et al., 2019), dplyr (Wickham et al., 2022), and sigminer (Wang et al., 2021). Pathway analysis utilized the Benjamini-Hochberg procedure for p-value adjustment with < 0.10 considered significant.
3. Results
3.1. Isoflavones alter the metabolome
First, we generated a Principal Component Analysis (PCA) plot to determine whether isoflavone affected the overall composition of the mouse plasma metabolome (Fig 1). The mice on a diet with isoflavone clustered separately from mice on a phyto-free diet, suggesting the metabolite composition was distinct between the two diets.
Fig 1.
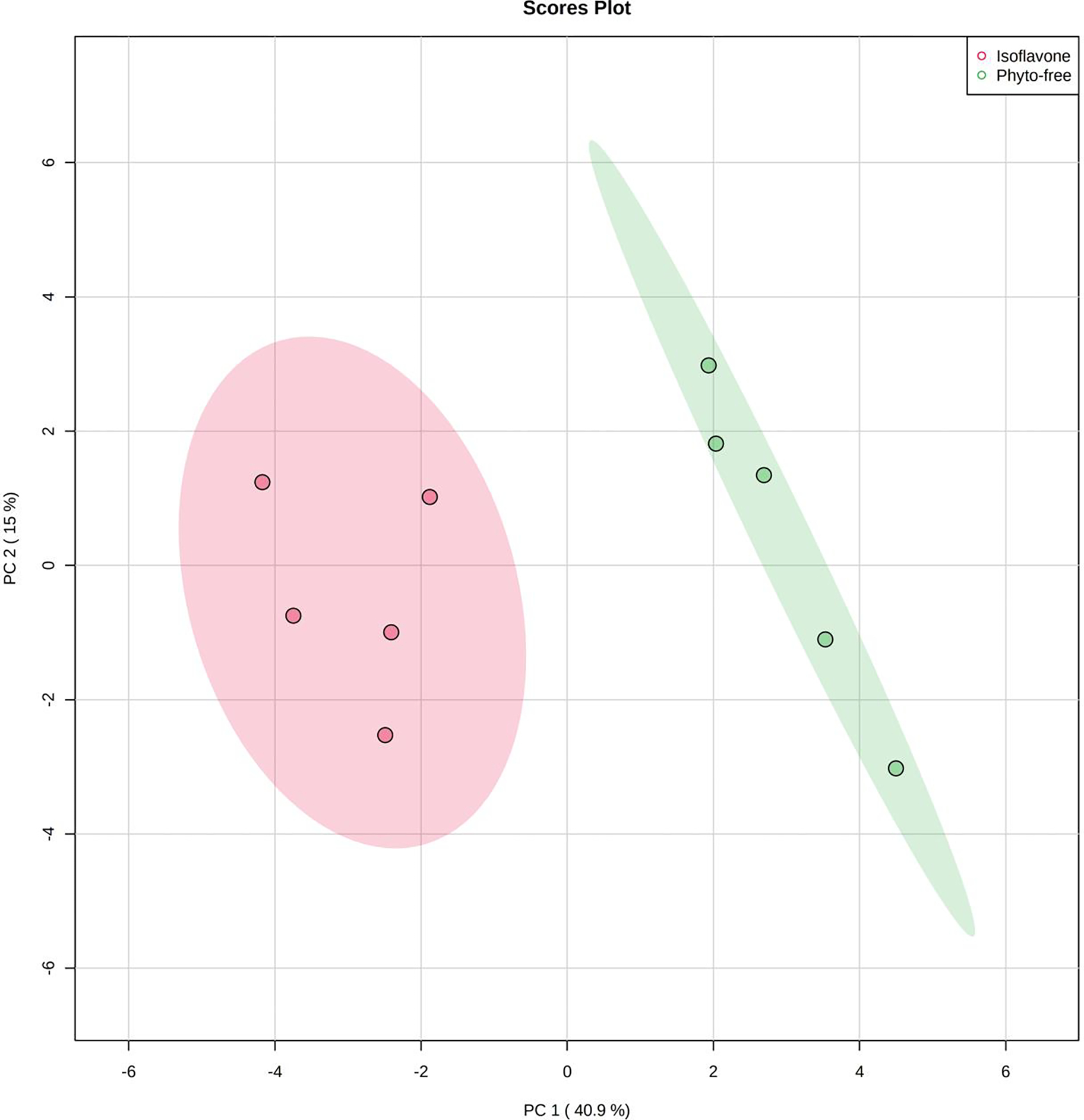
Principal Component Analysis (PCA) of metabolite composition of plasma obtained from mice kept on an isoflavone or phyto-free diet. 876 metabolites were obtained from untargeted metabolomics across all samples.
3.2. Individual metabolites are altered on an isoflavone diet
To identify metabolite-based biomarkers specific to the isoflavone diet, we used the nonparametric Wilcoxon Rank Sum test. While only considering metabolites significant if their p-value was < 0 .05 and their adjusted p-value (FDR) was < 0.10, we found 132 significantly different metabolites, as shown in Fig 2. 109 of these metabolites were significantly increased while 23 were decreased in the isoflavone diet compared to the phyto-free diet. We observed a significant increase in equol sulfate in mice on the isoflavone diet compared to the phyto-free diet (Fig 3A), validating the metabolic changes with respect to change in diet. Two monounsaturated fatty acids, oleate/vaccenate (18:1) and palmitate (16:0), had the largest relative abundances in both groups and were both significantly increased on the isoflavone diet compared to phyto-free (Fig 3B and 3C, respectively). All the significant metabolites with their statistical values are tabulated in Table S2. These results suggest that dietary isoflavones influence the host metabolome by modulating many metabolites.
Fig 2.
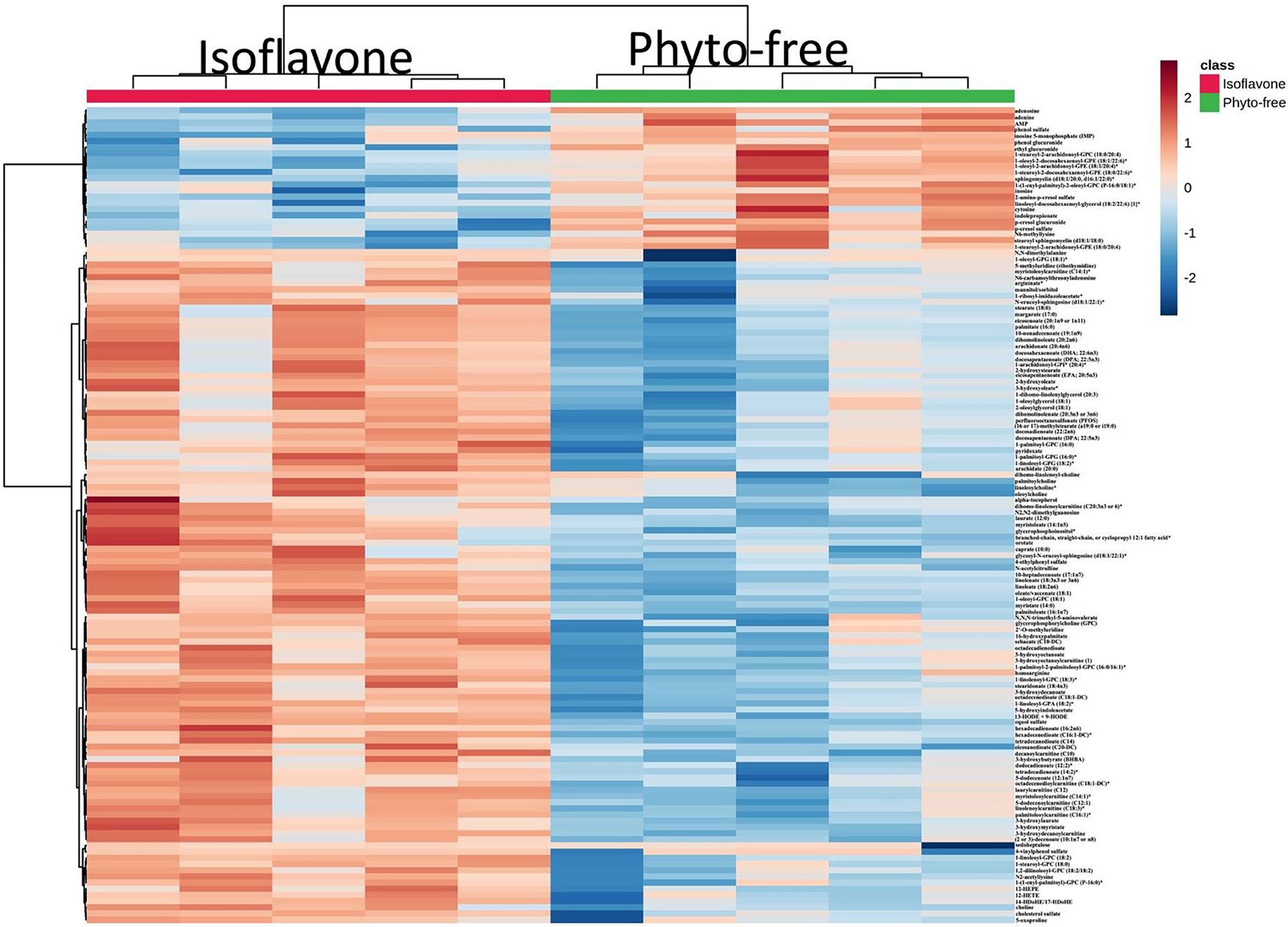
Hierarchical clustering of significantly altered metabolites in the plasma of mice on either isoflavone or phyto-free diet. Significantly altered metabolites were identified using the Wilcoxon test (p < 0.05, q < 0.10). Metabolites were further clustered based on the Euclidean distance and Ward’s linkage clustering algorithm. Each cell color represents the metabolite average intensity on a normalized scale from decreased (blue) to increased (red) abundance in each row.
Fig 3.
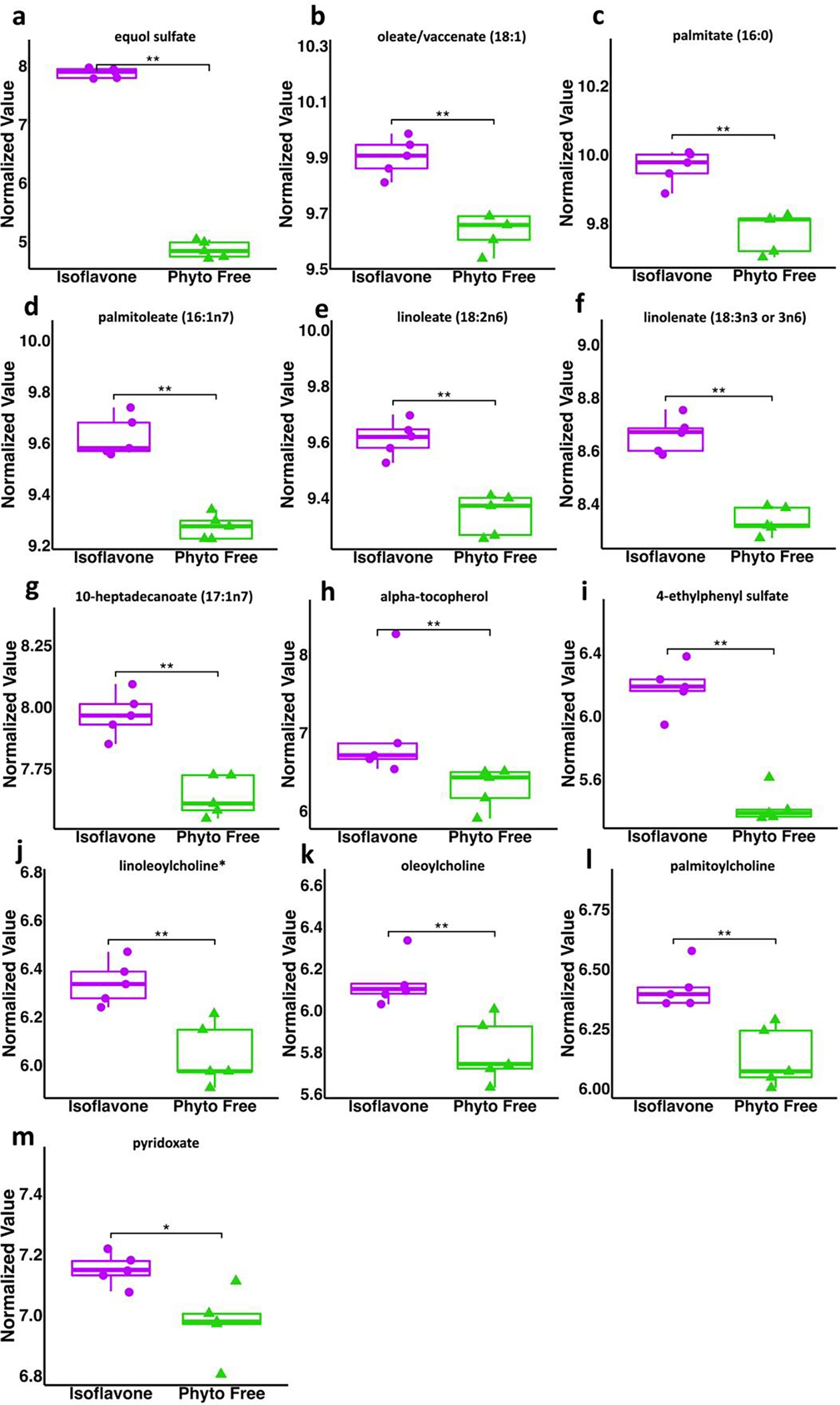
Metabolites increased in mice on an isoflavone diet compared to phyto-free diet. All values are normalized. Wilcoxon Rank Sum test utilized for statistical significance (* p < .05, ** p < .01, *** p < .001).
3.3. Metabolic pathways are altered on an isoflavone diet
As multiple metabolites varied between plasma from mice on a diet with or without isoflavones, we next investigated specific metabolic pathways affected by the diet. This analysis was performed with MetaboAnalyst. All metabolites were input for analysis, and the pathway analysis parameters included the Global Test for the enrichment methods and the Relative-betweenness Centrality for the topology analysis. The KEGG Mus musculus pathway library was chosen for identification. The pathways were considered significant if they had a p-value < 0.05 and an adjusted p-value < 0.10. With these parameters, we found 12 pathways significantly altered: Purine Metabolism, Selenocompound Metabolism, Tryptophan Metabolism, Thiamine Metabolism, Aminoacyl-tRNA Biosynthesis, Galactose Metabolism, Arachidonic Acid Metabolism, Linoleic Acid Metabolism, Alpha-Linolenic Acid Metabolism, Starch and Sucrose Metabolism, Phenylalanine Metabolism, and Vitamin B6 Metabolism. These pathways and their significant values are listed in Table 1. Thus, the pathway analysis showed that dietary isoflavones modulated specific metabolic pathways. The significant metabolites that correspond with the significant pathways are in figures 3 and 4 and are expanded upon in the discussion.
Table 1.
The pathways significantly altered between the mice on an isoflavone diet compared to the mice on a phyto-free diet. The pathway analysis parameters included the Global Test for the enrichment methods and the Relative-betweenness Centrality for the topology analysis.
| Pathway | p-value | FDR |
|---|---|---|
|
| ||
| Purine metabolism | 0.00023888 | 0.012539 |
| Selenocompound metabolism | 0.00041796 | 0.012539 |
| Tryptophan metabolism | 0.0012081 | 0.024162 |
| Thiamine metabolism | 0.0027655 | 0.040851 |
| Aminoacyl-tRNA biosynthesis | 0.0055593 | 0.040851 |
| Galactose metabolism | 0.0065999 | 0.040851 |
| Arachidonic acid metabolism | 0.0067309 | 0.040851 |
| Linoleic acid metabolism | 0.0067309 | 0.040851 |
| alpha-Linolenic acid metabolism | 0.0067309 | 0.040851 |
| Starch and sucrose metabolism | 0.0068085 | 0.040851 |
| Phenylalanine metabolism | 0.0082912 | 0.045225 |
| Vitamin B6 metabolism | 0.012389 | 0.061946 |
Fig 4.
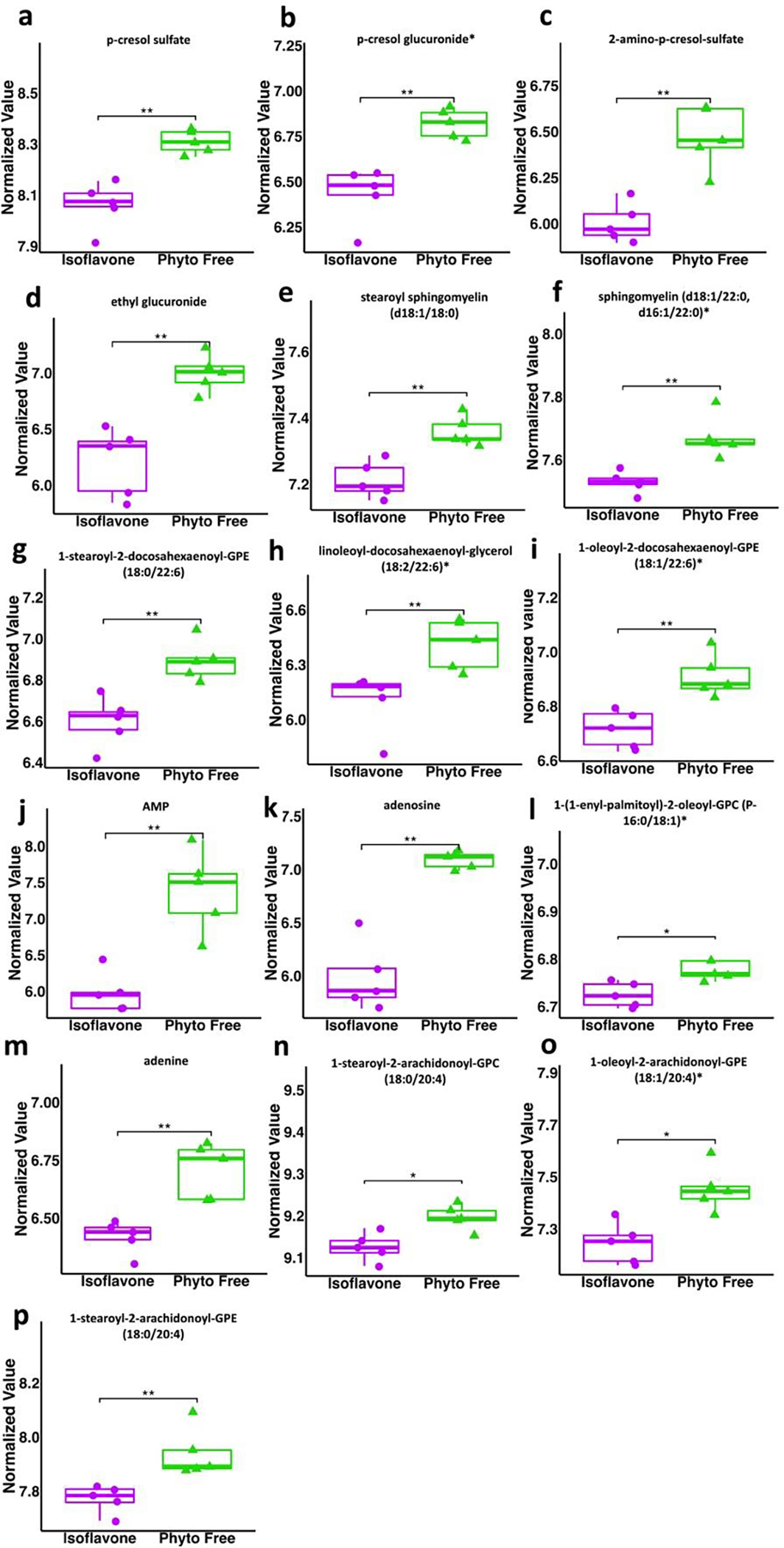
Metabolites decreased in mice on an isoflavone diet compared to phyto-free diet. All values are normalized. Wilcoxon Rank Sum test utilized for statistical significance (* p < .05, ** p < .01, *** p < .001).
4. Discussion
The significance of diet and gut microbiota in the pathobiology of MS is increasingly appreciated, specifically their potential as novel therapeutic agents (Bjørnevik et al., 2017; Yu et al., 2022; Zahoor et al., 2021). We and others have previously shown that MS patients have gut dysbiosis with depletion of beneficial gut bacteria, notably those able to metabolize phytoestrogens (Chen et al., 2016; Storm-Larsen et al., 2019). Further, using the animal model of MS, we have shown that an isoflavone (phytoestrogen) rich diet can protect mice from EAE, but this protection was dependent on the presence of specific gut bacteria and their ability to metabolize dietary phytoestrogens into equol (Jensen et al., 2021). In the present study, we performed untargeted metabolomics in the sera of mice on a diet with or without isoflavones and have shown an increase in anti-inflammatory monounsaturated fatty acids and beneficial polyunsaturated fatty acids while reducing pro-inflammatory glycerophospholipids, sphingolipids, phenylalanine metabolism, and arachidonic acid derivatives.
We observed that an isoflavone diet affected the host metabolism, as highlighted by a distinct metabolite composition in mice on a diet with or without isoflavones. Several studies have shown that the beneficial effects of a diet or a dietary supplement are associated with global change in the host metabolome (Díaz-Rubio et al., 2015; Rangel-Huerta et al., 2017; Torres Santiago et al., 2019). Healthy adults consuming antioxidant-rich juice (n=28) (Díaz-Rubio et al., 2015) or consuming orange juice (n=30) (Rangel-Huerta et al., 2017) showed a distinct metabolite profile compared to the baseline. Similarly, Santiago et al. reported a change in urinary and fecal metabolites in Wistar rats consuming oral resveratrol compared to the water group (Torres Santiago et al., 2019). Thus, our data showing a distinct metabolite profile in mice on an isoflavone diet is in accordance with previous studies reporting that dietary supplements can have a global effect on the host metabolome.
Using the Wilcoxon Signed Rank Test, we identified 132 significantly different metabolites between these two groups. Specifically, there were 109 metabolites significantly increased, and 23 metabolites significantly decreased on the isoflavone diet compared to the phyto-free diet. Of note, equol sulfate was significantly increased, and this is expected as isoflavone is broken down into equol which is then converted into equol sulfate. This finding validates that our diets did in fact differ in the presence of isoflavones, which were then metabolized into equol by gut bacteria (Díaz-Rubio et al., 2015). Our pathway analysis showed that several pathways were different between the two groups. Interestingly, there were modulations in phenylalanine metabolism, several pathways involved in lipid metabolism, vitamin metabolism, and a number of metabolites involved in nucleic acid metabolism as well.
Numerous significantly altered metabolites were linked to phenylalanine (Phe) metabolism. Phe is an aromatic amino acid, which is essential for the host and obtained from the diet as many mammals, including humans and mice, do not naturally produce it. Dietary Phe is broken down into Tyrosine (Tyr) or 4-hydroxyphenylpyruvic acid (Gryp et al., 2017). These metabolites can further be broken down into 4-ethylphenyl sulfate, p-cresol sulfate, and p-cresol glucuronide (Gryp et al., 2017). Phenylalanine was decreased in the isoflavone diet along with its secondary metabolites p-cresol sulfate (PCS) and p-cresol glucuronide (PCG). PCS and PCG are broken down from Phe and Tyr through anaerobic intestinal bacteria (Wishart et al., 2022). Interestingly, pwMS have higher levels of PCS in their plasma as well as their cerebrospinal fluid, and PCS can induce axonal damage in cultured neurons (Ntranos et al., 2022). Additionally, pwMS treated with dimethyl fumarate showed decreased levels of PCS compared to the untreated group (Ntranos et al., 2022). PCS has also been proposed as a biomarker for autism spectrum disorder (ASD), as its levels are elevated in the urine and feces of children with ASD (el Aidy et al., 2021). The current understanding is that PCS passes the blood-brain barrier and affects microglia and neurons to induce neuroinflammation and axonal damage in neurological diseases such as MS and ASD. Additionally, isoflavone consumption may reduce the levels of phenylalanine and its associated by-products due to the types of bacteria that flourish in an environment rich in isoflavones. For example, many bacteria that breakdown isoflavones also break down phenylalanine, such as Clostridium species and Lactobacillus species (Malashree et al., 2012; Mayo et al., 2019; Pinheiro de Oliveira et al., 2016). Thus, isoflavone may reduce neuroinflammation by modulation of phenylalanine metabolism, specifically by the reduction in the level of pro-inflammatory PCS.
Further investigation of the metabolome revealed that the isoflavone diet had altered fatty acid metabolism. Firstly, we found palmitoleate, oleate, and 10-heptadecenoate were all significantly increased on the isoflavone diet. Palmitoleate, oleate, and 10-heptadecenoate are the salt form of their corresponding monounsaturated fatty acid (MUFAs). MUFAs are known to have anti-inflammatory effects (Ravaut et al., 2020). In fact, palmitoleate is modulated in several inflammatory diseases, but depending on the disease, palmitoleate levels are either increased or decreased (Akazawa et al., 2021). Oleate has also been shown to possess anti-inflammatory activity. Specifically, fatty tissue from pwMS lacks oleic acid, and this absence is suggested to be responsible for regulatory T cell (Treg) dysfunction (Pompura et al., 2021). The exposure of Tregs from pwMS and healthy controls (HC) with oleic acid in culture restored the suppressive function of these Tregs (Pompura et al., 2021). Additionally, the presence of both palmitoleate and oleate had been linked with reduced inflammation in skeletal muscle cells (Coll et al., 2008). Thus, isoflavones may also mediate their anti-inflammatory effect through induction of these MUFAs, which have been linked with the healthy state of the host.
Additionally, linoleate and linolenate, both polyunsaturated fatty acids (PUFAs) and the salt form of linoleic acid and linolenic acid, respectively, were increased in the mice on an isoflavone diet. Metabolism of linoleic acid and alpha-linolenic acid (ALA) were also found to be pathways significantly modulated based on our pathway analysis. PUFAs have been linked to protection from many inflammatory diseases, including MS. A study evaluating the effects of dietary intake of PUFAs and MS risk showed that among all PUFA tested, only ALA showed an inverse association with MS risk (Bjørnevik et al., 2017). In a follow-up study, the group reported that higher serum levels of ALA were associated with lower disease activity in pwMS (Bjørnevik et al., 2019). Additionally, a recent study showed that pwMS with high ALA levels showed a reduced risk of relapse and a longer time to convert from clinically isolated syndrome to clinically definite multiple sclerosis (Munger et al., 2022). Utilizing an animal model of MS, we have also identified metabolites linked with PUFAs, such as ALA and linoleic acid, as significant biomarkers of disease (Mangalam et al., 2013; Poisson, Suhail, Singh, Datta, Deni, et al., 2015). Additionally, prior studies have revealed that resolvin D1 (RvD1), a downstream metabolite of ALA, ameliorated disease in a chronic EAE model (Poisson, Suhail, Singh, Datta, Deni, et al., 2015; Zahoor et al., 2021). Thus, increased levels of beneficial PUFAs in mice on a diet with isoflavones suggest that PUFAs may have a role in creating an anti-inflammatory environment due to the consumption of isoflavone. Alongside the increases in levels of MUFAs and PUFAs, we also observed increased levels of oleoylcholine, palmitoylcholine, and linoleoylcholine in the sera from mice on the isoflavone diet compared to the phyto-free diet. All three of these metabolites are acylated derivatives of choline and are involved in fatty acid metabolism (Gao et al., 2021; Wishart et al., 2022). A study on the chronic immune inflammatory disease, asthma, investigated the effects of choline therapy. They discovered that choline therapy modulated immune inflammation and suppressed oxidative stress in asthma patients, suggesting it could be used as a therapy option for asthma (Mehta et al., 2010). In total, these acyl cholines involved in fatty acid metabolism, as well as the previously mentioned MUFAs and PUFAs, can be linked to the creation of an anti-inflammatory environment. There is evidence that suggests isoflavones may interact with nuclear receptors that regulate cellular lipid metabolism, specifically the peroxisome proliferator activator receptors (Mezei et al., 2006). Linoleic acids, mentioned previously, have also shown to interact with these receptors, and thus isoflavone may be altering lipid metabolism through direct or indirect interactions with these receptors.
As for fatty acids that were depleted on an isoflavone diet compared to phyto-free, glycerophospholipids and sphingolipids from Phosphatidylcholine (PC) metabolism and ceramide metabolism were significantly decreased. Specifically, the glycerophospholipids involved in PC metabolism that were depleted in the isoflavone group include 1-stearoyl-2-docosahexaenoyl-GPE, 1-oleoyl-2-docosahexaenoyl-GPE, linoleoyl-docosahexaenoyl-glycerol (18:2/22:6), and 1-(1-enyl-palmitoyl)-2-oleoyl-GPC (P-16:0/18:1)* (Wishart et al., 2022). A study on the cerebral spinal fluid of MS and non-MS patients found that the majority of the glycerophospholipids that they analyzed were significantly increased in MS patients (Nogueras et al., n.d.), suggesting that alteration of glycerophospholipid composition contributes to disease state and thus the suppression of these metabolites on a diet with isoflavones supports an anti-inflammatory role for isoflavones.
PC also contributes to the production of sphingolipids with the help of ceramides (Li et al., 2008). Ceramides are lipid molecules that are metabolized into sphingolipids, namely sphingomyelin and sphingosine, which can be further broken down into sphingosine-1-phosphate (Hannun et al., 2010). Sphingomyelin and stearoyl sphingomyelin were found to be significantly decreased on the isoflavone diet. Sphingomyelins are found in animal cell membranes, and most abundantly, in the myelin sheath protecting nerve cell axons (Jana & Pahan, 2010). Sphingosine-1-phosphate is a signaling molecule also found in animal cell membranes which is present during inflammation (Spiegel et al., 2011). In fact, sphingosine 1-phosphate is activated by pro-inflammatory cytokines and is involved in T-cell trafficking. One MS treatment involves using sphingosine 1-phosphate receptor modulators to mediate its beneficial effects in pwMS (Mcginley et al., 2021). Due to the reduction in PC metabolism and the aforementioned ceramide derivatives, our results suggest isoflavone promotes an anti-inflammatory environment by reducing the metabolism of glycerophospholipids and sphingolipids. Interestingly, gut bacteria, specifically Bacteroides species, have been linked to the production of sphingolipids as their genomes encode serine palmitoyl transferase, a key enzyme in sphingolipid metabolization (Johnson et al., 2020).
Another lipid alteration found in the plasma of mice on the isoflavone diet was a reduction in the arachidonic acid derivatives 1-stearoyl-2-arachidonoyl-GPE (18:0/20:4), 1-oleoyl-2-arachidonoyl-GPE (18:1/20:4)*, and 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4). Arachidonic acid was also identified as a significant pathway in our pathway analysis. Arachidonic acid metabolism produces prostaglandins (Jewell et al., 2022), which are lipids produced in damaged and infected tissues to control inflammation, clotting, and overall blood flow (Ricciotti et al., 2011). The reduction of arachidonic acid metabolism in mice on an isoflavone diet suggests that an isoflavone diet can reduce inflammation through the suppression of pro-inflammatory arachidonic metabolism.
Prior studies have highlighted that isoflavones may regulate lipid homeostasis by increasing SCFAs that maintain gut barrier integrity, decreasing lipid accumulation in the blood, suppressing cholesterol absorption, up-regulating hepatic LDL receptor activity, and increasing serum thyroxine levels (Luo et al., 2019; Tan et al., 2019; Xiao, 2008). Our results also reflect many of these lipid findings: MUFAs and PUFAs were increased in the plasma of mice on an isoflavone diet while glycerophospholipids, sphingolipids, and fatty acids derived from arachidonic acid were all decreased. Thus, the shifts in lipid metabolism caused by isoflavone are complex and warrant further investigation to determine their role in maintaining a healthy state.
On the isoflavone diet, we also observed an increase in alpha-tocopherol, an active form of Vitamin E and an active antioxidant (Wishart et al., 2022). Vitamin E supplementation has been associated with a decreased risk of breast cancer in women, and the possible mechanism may be through its suppression of cell growth (Chamras et al., 2005). In addition to vitamin E, pyridoxate (vitamin B6) was increased on the isoflavone diet, along with modulations of Thiamin (B1 vitamin) and B6 vitamin metabolism. Vitamin B deficiencies result in severe demyelination and inflammation (Nemazannikova et al., 2018). Specifically, vitamin B1 is associated with healthy nerve function and prevention of myelin damage, and vitamin B6 is associated with neurotransmitter formation and proper nerve function (Nemazannikova et al., 2018). Additionally, gut bacteria have been linked to the generation of many vitamins, specifically, colonic bacteria can generate B vitamins (Oliphant et al., 2019). Thus, the modulations in vitamin metabolism on the isoflavone diet may be related to the gut bacteria that thrive in an isoflavone rich environment.
In addition to phenylalanine, lipid, and vitamin metabolism alterations seen on an isoflavone diet, we also identified a significant decrease in adenine, adenosine, and adenosine monophosphate (AMP) along with alterations in purine metabolism and Aminoacyl-tRNA biosynthesis on the isoflavone diet. These changes suggest modulation in nucleic acid metabolism on an isoflavone diet. Interestingly gut microbiota-derived purines had been shown to play an important role in maintaining gut barrier integrity by supporting proliferation of intestinal epithelial cells (Lee et al., 2020). Thus, our findings suggest a relationship between nucleic acid metabolism and isoflavone consumption.
In conclusion, our work reveals that dietary isoflavones alter the host metabolome by promoting anti-inflammatory metabolites and suppressing pro-inflammatory metabolites. Specifically, we found major alterations in phenylalanine metabolism and lipid metabolism. Several MUFAs, beneficial PUFAs, and vitamins were increased in sera of mice kept on an isoflavone diet compared to a phyto-free diet. At the same time, several pro-inflammatory metabolites, including glycerophospholipids, sphingolipids, and fatty acids derived from arachidonic acid, were all depleted in the isoflavone diet compared to the phyto-free diet. The pathways that were important in distinguishing the two diets directly relate to these findings, as the metabolism of phenylalanine, linoleic acid, ALA, and arachidonic acid were significantly altered based on our pathway analysis. These pathways, and many of their metabolites, have been associated with inflammatory diseases as aforementioned. Overall, these results indicate that dietary isoflavones induce changes in the systemic metabolome, potentially by alteration of the gut microbiome and/or host response. Our study provides a rationale for future studies investigating the mechanism through which dietary isoflavones modulate the host metabolic network, specifically phenylalanine and lipid metabolism.
Supplementary Material
Acknowledgements and Funding:
We acknowledge funding from the National Institutes of Health/NIAID 1RO1AI137075 (A.K.M), Veteran Affairs Merit Award 1I01CX002212 (A.K.M), University of Iowa Environmental Health Sciences Research Center, NIEHS/NIH P30 ES005605 (A.K.M), Gift from P. Heppelmann and M. Wacek to A.K.M and Carver Trust Pilot Grant (A.K.M). R.L.S. was supported by the Informatics Fellowship from the University of Iowa. S.N.J was supported by an institutional training grant (T32AI007485 to G. Bishop) and a diversity supplement award to A.K.M on parent 1RO1AI137075. We also thank Leeann Aguilar (University of Iowa) and Stephanie Peterson (University of Iowa) for their editorial assistance on this manuscript.
Footnotes
Conflicts of Interest: A.K.M. is one of the inventors of a technology claiming the use of Prevotella histicola to treat autoimmune diseases. A.K.M. received royalties from Mayo Clinic (paid by Evelo Biosciences). However, no funds or products from the patent were used in the present study. All other authors declare no commercial or financial relationships that could be a potential conflict of interest.
Institutional Review Board Statement: All procedures were done according to the Institutional Animal Care and Use Committee (IACUC) guidelines at the University of Iowa.
Data Availability Statement:
Metabolomic data utilized for analysis is in Table S1.
References
- Aboushanab SA, Khedr SM, Gette IF, Danilova IG, Kolberg NA, Ravishankar GA, Ambati RR, & Kovaleva EG (2021). Isoflavones derived from plant raw materials: bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. In Critical Reviews in Food Science and Nutrition. Bellwether Publishing, Ltd. 10.1080/10408398.2021.1946006 [DOI] [PubMed] [Google Scholar]
- Akazawa Y, Morisaki T, Fukuda H, Norimatsu K, Shiota J, Hashiguchi K, Tabuchi M, Kitayama M, Matsushima K, Yamaguchi N, Kondo H, Fujita F, Takeshita H, Nakao K, & Takeshima F (2021). Significance of serum palmitoleic acid levels in inflammatory bowel disease. Scientific Reports, 11(1). 10.1038/s41598-021-95923-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnevik K, Chitnis T, Ascherio A, & Munger KL (2017). Polyunsaturated fatty acids and the risk of multiple sclerosis. Multiple Sclerosis, 23(14), 1830–1838. 10.1177/1352458517691150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornevik K, Myhr KM, Beiske A, Bjerve KS, Holmøy T, Hovdal H, Midgard R, Riise T, Wergeland S, & Torkildsen Ø (2019). α-Linolenic acid is associated with MRI activity in a prospective cohort of multiple sclerosis patients. Multiple Sclerosis Journal, 25(7), 987–993. 10.1177/1352458518779925 [DOI] [PubMed] [Google Scholar]
- Chamras H, Barsky SH, Ardashian A, Navasartian D, Heber D, & Glaspy JA (2005). Novel Interactions of Vitamin E and Estrogen in Breast Cancer. Nutrition and Cancer, 52(1), 43–48. 10.1207/s15327914nc5201_6 [DOI] [PubMed] [Google Scholar]
- Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP, Luckey DH, Marietta E. v., Jeraldo PR, Chen X, Weinshenker BG, Rodriguez M, Kantarci OH, Nelson H, Murray JA, & Mangalam AK (2016). Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Scientific Reports, 6. 10.1038/srep28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, & Vázquez-Carrera M (2008). Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. Journal of Biological Chemistry, 283(17), 11107–11116. 10.1074/jbc.M708700200 [DOI] [PubMed] [Google Scholar]
- Díaz-Rubio ME, Pérez-Jiménez J, Martínez-Bartolomé MÁ, Álvarez I, & Saura-Calixto F (2015). Regular Consumption of an Antioxidant-rich Juice Improves Oxidative Status and Causes Metabolome Changes in Healthy Adults. Plant Foods for Human Nutrition, 70(1), 9–14. 10.1007/s11130-014-0455-4 [DOI] [PubMed] [Google Scholar]
- el Aidy S, Burokas A, Cristiano C, Marzal PL, Pardo PP, Zheng Y, Bek MK, Prince NZ, Peralta Marzal LN, Garssen J, Perez Pardo P, & Kraneveld AD (2021). The Role of Bacterial-Derived Aromatic Amino Acids Metabolites Relevant in Autism Spectrum Disorders: A Comprehensive Review. 10.3389/fnins.2021.738220 [DOI] [PMC free article] [PubMed]
- François M, Karpe A, Liu J-W, Beale D, Hor M, Hecker J, Faunt J, Maddison J, Johns S, Doecke J, Rose S, & Leifert WR (2021). Salivaomics as a Potential Tool for Predicting Alzheimer’s Disease During the Early Stages of Neurodegeneration. Journal of Alzheimer’s Disease, 82, 1301–1313. 10.3233/JAD-210283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Quick C, Guasch-Ferre M, Zhuo Z, Hutchinson JM, Su L, Hu F, Lin X, & Christiani D (2021). The Association Between Inflammatory and Oxidative Stress Biomarkers and Plasma Metabolites in a Longitudinal Study of Healthy Male Welders. Journal of Inflammation Research, Volume 14, 2825–2839. 10.2147/JIR.S316262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimire S, Cady NM, Lehman P, Peterson SR, Shahi SK, Rashid F, Giri S, & Mangalam AK (n.d.). Dietary Isoflavones Alter Gut Microbiota and Lipopolysaccharide Biosynthesis to Reduce Inflammation. Gut Microbes, 14(1), 2127446. 10.1080/19490976.2022.2127446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryp T, Vanholder R, Vaneechoutte M, & Glorieux G (2017). p-cresyl sulfate. Toxins, 9(2). 10.3390/TOXINS9020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Gault CR, & Obeid LM (2010). Sphingolipids as Signaling and Regulatory Molecules An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. [DOI] [PMC free article] [PubMed]
- Jana A, & Pahan K (2010). Sphingolipids in Multiple Sclerosis. Neuromolecular Medicine. 10.1007/s12017-010-8128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SN, Cady NM, Shahi SK, Peterson SR, Gupta A, Gibson-Corley KN, & Mangalam AK (2021). Isoflavone diet ameliorates experimental autoimmune encephalomyelitis through modulation of gut bacteria depleted in patients with multiple sclerosis. In Sci. Adv (Vol. 7, Issue 9). https://www.science.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell DE, & Jackson MI (2022). Dietary Betaine and Fatty Acids Change Circulating Single-Carbon Metabolites and Fatty Acids in the Dog. Animals, 12(6). 10.3390/ani12060768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL, Heaver SL, Waters JL, Kim BI, Bretin A, Goodman AL, Gewirtz AT, Worgall TS, & Ley RE (2020). Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nature Communications, 11(1), 2471. 10.1038/s41467-020-16274-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A (2020). ggpubr: “ggplot2” Based Publication Ready Plots. R package version 0.4.0. [Google Scholar]
- Lee JS, Wang RX, Goldberg MS, Clifford GP, Kao DJ, & Colgan SP (2020). Microbiota-Sourced Purines Support Wound Healing and Mucous Barrier Function. IScience, 23(6), 101226. 10.1016/j.isci.2020.101226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, & Vance DE (2008). Phosphatidylcholine and choline homeostasis. In Journal of Lipid Research (Vol. 49, Issue 6, pp. 1187–1194). 10.1194/jlr.R700019-JLR200 [DOI] [PubMed] [Google Scholar]
- Luo Q, Cheng D, Huang C, Li Y, Lao C, Xia Y, Liu W, Gong X, Hu D, Li B, He X, & Chen Z (2019). Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules, 24(6), 1139. 10.3390/molecules24061139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashree L, Mudgil P, Dagar SS, Kumar S, & Puniya AK (2012). β-Glucosidase Activity of Lactobacilli for Biotransformation of Soy Isoflavones. Food Biotechnology, 26(2), 154–163. 10.1080/08905436.2012.670832 [DOI] [Google Scholar]
- Mangalam A, Poisson L, Nemutlu E, Datta I, Denic A, Dzeja P, Rodriguez M, Rattan R, & Giri S (2013). Profile of Circulatory Metabolites in an Animal Model of Multiple Sclerosis using Global Metabolomics. Journal of Clinical & Cellular Immunology, 04(03). 10.4172/2155-9899.1000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam A, Yadav M, & Yadav R (2021). The emerging world of microbiome in autoimmune disorders: Opportunities and challenges. In Indian Journal of Rheumatology (Vol. 16, Issue 1, pp. 57–72). Wolters Kluwer Medknow Publications. 10.4103/injr.injr_210_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo B, Vázquez L, & Flórez AB (2019a). Equol: A bacterial metabolite from the Daidzein isoflavone and its presumed beneficial health effects. Nutrients, 11(9). 10.3390/NU11092231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo B, Vázquez L, & Flórez AB (2019b). Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients, 11(9), 2231. 10.3390/nu11092231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcginley MP, & Cohen JA (2021). Therapeutics Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. The Lancet, 398, 1184–1194. 10.1016/S0140-6736(21)00244-0 [DOI] [PubMed] [Google Scholar]
- Mehta AK, Singh BP, Arora N, & Gaur SN (2010). Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology, 215(7), 527–534. 10.1016/j.imbio.2009.09.004 [DOI] [PubMed] [Google Scholar]
- Metabolon Inc. (2022). Experimental Procedures. Metabolon Help Center. https://help.metabolon.com/docs/microbiome-smartpanel/knowledge-base/experimental-procedures/. [Google Scholar]
- Mezei O, Li Y, Mullen E, Ross-Viola JS, & Shay NF (2006). Dietary isoflavone supplementation modulates lipid metabolism via PPARalpha-dependent and -independent mechanisms. Physiological Genomics, 26(1), 8–14. 10.1152/physiolgenomics.00155.2005 [DOI] [PubMed] [Google Scholar]
- Munger K, Bjornevik K, Cortese M, Edan G, Freedman M, Hartung H-P, Montalban X, Sandbrink R, Radue E-W, Barkhof F, Wicklein E-M, Kappos L, & Ascherio A (2022). A Prospective Study of Serum Levels of Polyunsaturated Fatty Acids and Effects on Multiple Sclerosis Disease Activity and Progression (S40.006). In Supplement) THURSDAY (Vol. 98, Issue 7). [Google Scholar]
- Nemazannikova N, Mikkelsen K, Stojanovska L, Blatch GL, & Apostolopoulos V (2018). Is there a Link between Vitamin B and Multiple Sclerosis? Medicinal Chemistry, 14(2). 10.2174/1573406413666170906123857 [DOI] [PubMed] [Google Scholar]
- Nogueras L, Gonzalo H, Jové M, Sol J, Gil-Sanchez A, Hervás J. v, Valcheva P, Solana MJ, peralta S, pamplona R, & Brieva L (n.d.). Lipid profile of cerebrospinal fluid in multiple sclerosis patients: a potential tool for diagnosis. 10.1038/s41598-019-47906-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntranos A, Park HJ, Wentling M, Tolstikov V, Amatruda M, Inbar B, Kim-Schulze S, Frazier C, Button J, Kiebish MA, Lublin F, Edwards K, & Casaccia P (2022). Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain, 145(2), 569–583. 10.1093/brain/awab320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant K, & Allen-Vercoe E (2019). Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome, 7(1), 91. 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabich M, & Materska M (2019). Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients, 11(7). 10.3390/nu11071660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques PÉ, Li S, & Xia J (2021). MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Research, 49(W1), W388–W396. 10.1093/nar/gkab382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro de Oliveira F, Mendes RH, Dobbler PT, Mai V, Pylro VS, Waugh SG, Vairo F, Refosco LF, Roesch LFW, & Schwartz IVD (2016). Phenylketonuria and Gut Microbiota: A Controlled Study Based on Next-Generation Sequencing. PLOS ONE, 11(6), e0157513. 10.1371/journal.pone.0157513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisson LM, Suhail H, Singh J, Datta I, Deni A, Labuzek K, Hoda N, Shankar A, Kumar A, Cerghet M, Elias S, Mohney RP, Rodriguez M, Rattan R, Mangalam AK, & Giri S (2015). Untargeted plasma metabolomics identifies endogenous metabolite with drug-like properties in chronic animal model of multiple sclerosis. Journal of Biological Chemistry, 290(52), 30697–30712. 10.1074/jbc.M115.679068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompura SL, Wagner A, Kitz A, LaPerche J, Yosef N, Dominguez-Villar M, & Hafler DA (2021). Oleic acid restores suppressive defects in tissue-resident FOXP3 Tregs from patients with multiple sclerosis. Journal of Clinical Investigation, 131(2). 10.1172/JCI138519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A Language and Environment for Statistical Computing (4.1.1).
- Rangel-Huerta OD, Aguilera CM, Perez-de-la-Cruz A, Vallejo F, Tomas-Barberan F, Gil A, & Mesa MD (2017). A serum metabolomics-driven approach predicts orange juice consumption and its impact on oxidative stress and inflammation in subjects from the BIONAOS study. Molecular Nutrition and Food Research, 61(2). 10.1002/mnfr.201600120 [DOI] [PubMed] [Google Scholar]
- Ravaut G, Légiot A, Bergeron K-F, & Mounier C (2020). Monounsaturated Fatty Acids in Obesity-Related Inflammation. International Journal of Molecular Sciences, 22(1), 330. 10.3390/ijms22010330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, & Fitzgerald GA (2011). Prostaglandins and Inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyapalan T, Aye M, Rigby AS, Thatcher NJ, Dargham SR, Kilpatrick ES, & Atkin SL (2018). Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutrition, Metabolism and Cardiovascular Diseases, 28(7), 691–697. 10.1016/j.numecd.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Spiegel S, & Milstien S (2011). The outs and the ins of sphingosine-1-phosphate in immunity. In Nature Reviews Immunology (Vol. 11, Issue 6, pp. 403–415). 10.1038/nri2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm-Larsen C, Myhr K-M, Farbu E, Midgard R, Nyquist K, Broch L, Berg-Hansen P, Buness A, Holm K, Ueland T, Fallang L-E, Burum-Auensen E, Hov JR, & Holmøy T (2019). Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis-a pilot trial. 10.1177/2055217319888767 [DOI] [PMC free article] [PubMed]
- Sun Y, Tao W, Huang H, Ye X, & Sun P (2019). Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food Chemistry, 279, 321–327. 10.1016/j.foodchem.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Tan J, Huang C, Luo Q, & Chen Z (2019). Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO). Molecules, 24(15), 2809. 10.3390/molecules24152809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres Santiago G, Serrano Contreras JI, Meléndez Camargo ME, & Zepeda Vallejo LG (2019). NMR-based metabonomic approach reveals changes in the urinary and fecal metabolome caused by resveratrol. Journal of Pharmaceutical and Biomedical Analysis, 162, 234–241. 10.1016/j.jpba.2018.09.025 [DOI] [PubMed] [Google Scholar]
- Wang S, Li H, Song M, Tao Z, Wu T, He Z, Zhao X, Wu K, & Liu X-S (2021). Copy number signature analysis tool and its application in prostate cancer reveals distinct mutational processes and clinical outcomes. PLOS Genetics, 17(5), e1009557. 10.1371/journal.pgen.1009557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Huang B, & Gustafsson JA (2017). Estrogen Receptor β as a Pharmaceutical Target. In Trends in Pharmacological Sciences (Vol. 38, Issue 1, pp. 92–99). Elsevier Ltd. 10.1016/j.tips.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis. In ggplot2: Elegant Graphics for Data Analysis (978th-3rd-319th-24277th–4th ed.). [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen T, Miller E, Bache S, Müller K, Ooms J, Robinson D, Seidel D, Spinu V, … Yutani H (2019). Welcome to the Tidyverse. Journal of Open Source Software, 4(43), 1686. 10.21105/joss.01686 [DOI] [Google Scholar]
- Wickham H, Francois R, Henry L, & Muller K (2022). dplyr: A Grammar of Data Manipulation. R package version 1.0.9. [Google Scholar]
- Wishart DS, Guo A, Oler E, Wang F, Anjum A, Peters H, Dizon R, Sayeeda Z, Tian S, Lee BL, Berjanskii M, Mah R, Yamamoto M, Jovel J, Torres-Calzada C, Hiebert-Giesbrecht M, Lui VW, Varshavi D, Varshavi D, … Gautam V (2022). HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Research, 50. 10.1093/nar/gkab1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao CW (2008). Effect of soy proteins and isoflavones on lipid metabolism and involved gene expression. Frontiers in Bioscience, 13(13), 2660. 10.2741/2873 [DOI] [PubMed] [Google Scholar]
- Yu H, Bai S, Hao Y, & Guan Y (2022). Fatty acids role in multiple sclerosis as “metabokines.” Journal of Neuroinflammation, 19(1), 157. 10.1186/s12974-022-02502-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahoor I, Rui B, Khan J, Datta I, & Giri S (2021). An emerging potential of metabolomics in multiple sclerosis: a comprehensive overview. In Cellular and Molecular Life Sciences (Vol. 78, Issue 7, pp. 3181–3203). Springer Science and Business Media Deutschland GmbH. 10.1007/s00018-020-03733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahoor I, Suhail H, Datta I, Ahmed ME, Poisson LM, Waters J, Rashid F, Bin R, Singh J, Cerghet M, Kumar A, Hoda MN, Rattan R, Mangalam AK, & Giri S (2022). Blood-based untargeted metabolomics in relapsing-remitting multiple sclerosis revealed the testable therapeutic target. Proceedings of the National Academy of Sciences of the United States of America, 119(25), e2123265119. 10.1073/pnas.2123265119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Sun H, Yan G, Wang P, & Wang X (2015). Review Article Metabolomics for Biomarker Discovery: Moving to the Clinic. 10.1155/2015/354671 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metabolomic data utilized for analysis is in Table S1.


