Abstract
The impact of household processes on fenugreek leaves and seeds has been analyzed for total phenolic (TP) and total flavonoid content (TF), and in-vitro biological activities such as antioxidant, antimicrobial, and anti-inflammatory properties. Processes included air-drying for leaves and germinating, soaking, and boiling for seeds. Air-dried fenugreek leaves (ADFL) had high TP (15.27 mg GAE g−1 D.W.) and TF (7.71 mg QE g−1 D.W.) (milligram quercetin equivalents per gram dry weight). The TP contents of unprocessed, germinated, soaked, and boiled seeds were 6.54, 5.60, 4.59, and 3.84 mg gallic acid equivalents per gram of dry weight (mg GAE g−1 D.W.), respectively. The TF contents in unprocessed fenugreek seeds, germinated fenugreek seeds, soaked fenugreek seeds, and boiled fenugreek seeds (BFS) were 4.23, 2.11, 2.10, and 2.33 mg QE g−1 D.W., respectively. Sixteen phenolic and nineteen flavonoid compounds has been identified using high-performance liquid chromatography. Antioxidant activity using 2,2-diphenyl-1-picrylhydrazil (DPPH·), 2,2-azinobis (3-ethylbenothiazoline-6-sulfonic acid (ABTS+·), and ferric reducing antioxidant power (FRAP·) assays indicated that ADFL had the highest activity. Antimicrobial activity has been evaluated against each of the eight pathogenic bacterial and fungal strains. ADFL showed the strongest activity with minimum inhibitory concentrations values ranging from 0.03 to 1.06 and 0.04 to 1.18 mg ml·1 against bacterial and fungal strains, respectively. Anti-inflammatory activity was evaluated in-vitro against RAW 264.7 macrophage cells using the nitric oxide (NO) assay. Results revealed that ADFL had the highest cytotoxicity and anti-inflammatory activity according to the NO assay. Household processes significantly reduced the in-vitro biological properties of processed seeds.
Subject terms: Biochemistry, Biological techniques
Introduction
Phytochemical screening is a method of investigating the existence or absence of potential phytochemical components in the plant. This assay can help scientists to diagnose the bioactive components in plants. It advises not only on the existence of therapeutic agents but also gives information about the presence of economically active ingredients, such as vitamins, phenolics, alkaloids, saponins, tannins, oils, gums, and precursors for the synthesis of complex compounds, etc. Phenolic constituents in plants have potential health activities principally due to their antioxidant activities, such as metal chelation, electrophile scavenging, and reactive oxygen species (ROS) scavenging and inhibition1.
Fenugreek (Trigonella foenum-graecum) is an annual plant belonging to the family Fabaceae. The green leaves and seeds of fenugreek are widely used in food and medicinal applications dating back to ancient times. However, the seeds are sour in taste due to the presence of bitter saponins, which limit their acceptability in foods2. It has been possible to decrease the bitter taste of fenugreek seeds by using diverse household processes, such as soaking, germination, boiling, fermentation, etc.2,3. Many extracts of each seed or leaf and its active components have been studied for their pharmacological effects and have been reported to have hypocholestrolaemic, antidiabetic, anti-inflammatory, antiulcer, analgesic, antipyretic, CNS-stimulant, antioxidant, wound healing and immune modulatory activity as well as gastro-protective and chemo-preventive activities4.
Newly, various actions and behaviours of macrophage cells play an important role in surviving homeostasis and normal physiological conditions by manufacturing a diverse range of biological impacts5. Macrophages (RAW 264.7) have been chosen because they are accepted as targeted single cells for assessing immune reactivity6. Macrophages can be induced by lipopolysaccharide (LPS) to generate pro-inflammation molecules (e.g., NO and PGE2) through enhancing intracellular signaling pathways including NF-κB and MAPK7.
Nitric oxide (NO) is a signaling molecule that plays a crucial role in the way inflammation develops. It has anti-inflammatory activity in normal physiological situations, but it is regarded as a pro-inflammatory mediator in unusual conditions due to overproduction. NO has been generated and released to the endothelial cells. It is an active neurotransmitter at the neuronal synapses, and it also helps to regulate programmed cell death regulation. NO participates in the way the inflammatory disorder in joints, intestines, and in the upper and lower respiratory systems. Hence, NO inhibitors represent significant therapeutic advances in the control of inflammatory diseases8. It also has an important property as a bio-regulation molecule in the nervous, immune, and cardiovascular systems. The permanent release of NO is associated with several diseases, including; inflammation, cancers, and arthritis9.
RAW 264.7 macrophages induced by LPS are used to assess the in-vitro potential inhibitory effects of anti-inflammatory compounds. LPS is considered one of the most important stimuli used to upgrade pro-inflammatory protein arranging, such as inducible nitric oxide synthase (i–NOS) and cyclooxygenase-2 (COX-2), which are in charge of the high standards of prostaglandin spotted in various inflammatory confuses. Furthermore, i-NOS develop huge quantities of NO, which is considered to be important for showing a necessary role in the inflammation10. Beside, the highly reactive nitric oxide becomes more sensitive when it is linked with oxygen and thus produces highly reactive compounds which can cause some provisions cellular damage such as; cellular DNA fragmentation and lipid peroxidation11.
This work aimed to investigate the influences of household treatments, including air-drying for fresh fenugreek leaves and each soaking, germination, and boiling for edible fenugreek seeds, on both phenolic, flavonoid and isoflavonoid components, as well as on their in-vitro antioxidant, antimicrobial and anti-inflammatory activities of the selected samples.
Results and discussion
Total phenolic (TP) and total flavonoid (TF) content
Extraction with alcohol of 80% for all studied samples produced the following contents of crude extracts, 23.66% for ADFL, 22.64 for UFS, 19.08% for SFS, 20.15% for GFS and 18.91% for BFS.
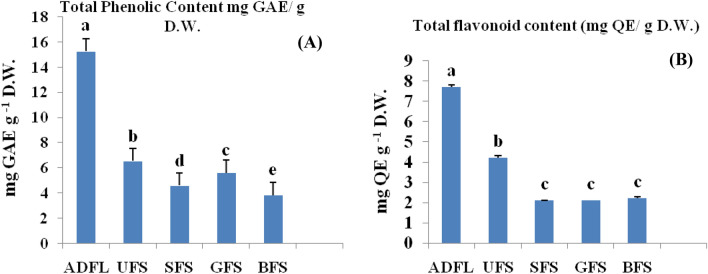
The values of total phenolic content (TP) and flavonoid content (TF) of alcoholic extracts of fenugreek leaves and household processed seeds have shown in Fig. 1. Among all studied samples, ADFL exhibited the highest significant value of TP (15.27 mg GAE g−1 D.W.) and TF (7.71 mg QE g−1 D.W). Hussain et al.12 found that TP and TF in dried fenugreek leaves were 425.4 and 205.1 mg 100 g−1, respectively.
Figure 1.
(A) Total phenolic content (TP), (B) Total flavonoid content (TF) of crude extracts of air-dried fenugreek leaves (ADFL), unprocessed fenugreek seeds (UFS), soaked fenugreek seeds (SFS), germinated fenugreek seeds (GFS) and boiled fenugreek seeds (BFS).
On the other hand, UFS had 6.54 mg GAEg−1 D.W. of TP and 4.23 mg QE g−1 D.W of TF. These amounts were significantly decreased after the household treatments as the following; soaked, germinated, and boiled fenugreek seeds contained 4.59, 5.60, and 3.84 mg GAEg−1 D.W. for TP and, 2.11, 2.10 and 2.33 mg QE g−1 D.W, respectively, for TF.
These results are in agreement with the earlier findings observed by Hooda and Jood13 and Shakuntala et al.14 who found that polyphenolic content and antioxidant activity declined after germination treatment. In contrast, another trend, an increase in TP after germination, had been investigated by Pająk et al.15,16. Pandey and Awasthi17 also approved that soaking and germination enhanced total phenolic content and the antioxidant activity of fenugreek seed flour as compared to raw fenugreek seed flour.
The profile of phenolic, flavonoid and isoflavone components
Vegetables and cereals (including seed and sprouts) are excellent sources of phenolic components. Legumes contain a high concentration of isoflavonoids18.
The phytochemical screening of each phenolic, flavonoid and isoflavonoid component evaluated using of HPLC has shown in the Table 1.The highest concentrations have obtained by the alcoholic crude extract of ADFL, UFS and GFS.
Table 1.
Phenolic, flavonoids and isoflavonoids screening of crude alcoholic extracts of air-dried fenugreek leaves (ADFL), unprocessed fenugreek seeds (UFS), soaked fenugreek seeds (SFS), germinated fenugreek seeds (GFS) and boiled fenugreek seeds (BFS) by HPLC (mg 100 g−1 D.W.).
| Compound | Rt* (min) | ADFL | Seeds | |||
|---|---|---|---|---|---|---|
| UFS | SFS | GFS | BFS | |||
| Phenolic compounds | ||||||
| Pyrogallol | 4.823 | 55.83 | 48.11 | 51.71 | 46.25 | 69.88 |
| Gallic acid | 5.347 | 0.73 | 0.42 | 0.66 | 1.13 | 0.29 |
| 3-hydroxy tyrosol | 6.227 | 5.39 | 0.42 | 0.40 | 1.56 | 0.20 |
| Catechol | 6.727 | 1.91 | 0.29 | 0.23 | 0.50 | 0.05 |
| 4-amino benzoic acid | 6.801 | 5.59 | 0.23 | 0.08 | 1.21 | 0.09 |
| Chlorogenic acid | 7.701 | 81.78 | 5.26 | 7.84 | 7.22 | 3.82 |
| Benzoic acid | 7.767 | 10.56 | 2.51 | 3.78 | 3.17 | 1.83 |
| P-hydroxy benzoic | 7.770 | 14.69 | 3.50 | 5.26 | 4.41 | 2.54 |
| Vanillic acid | 8.303 | 4.80 | 18.26 | 21.40 | 6.84 | 6.95 |
| Caffeic acid | 8.372 | 1.64 | 4.01 | 3.83 | 4.14 | 1.47 |
| Caffeine | 8.482 | 6.46 | 32.86 | 40.85 | 66.34 | 12.32 |
| Ferulic acid | 10.026 | 20.07 | 7.48 | 8.26 | 18.91 | 5.27 |
| Salicylic acid | 10.917 | 14.99 | 5.04 | 5.31 | 14.85 | 0.00 |
| Oleoropine | 11.195 | 162.73 | 20.68 | 0.00 | 50.88 | 5.16 |
| Ellagic acid | 11.534 | 23.60 | 2.67 | 51.71 | 6.12 | 1.14 |
| Coumarin | 11.779 | 11.28 | 0.89 | 0.66 | 1.18 | 0.84 |
| Flavonoid compounds | ||||||
| Isorhamnetin | 2.352 | 1.57 | 1.25 | 1.76 | 2.69 | 1.56 |
| Catechin | 7.122 | 77.65 | 14.19 | 12.36 | 40.09 | 4.15 |
| Apigenin-6-O-arabinose-8-O-galactose | 10.040 | 71.19 | 38.65 | 40.20 | 80.55 | 28.17 |
| Hesperidin | 10.753 | 24.60 | 83.70 | 67.45 | 110.30 | 102.00 |
| Luteoline-7-O-glucoside | 10.795 | 2.73 | 2.34 | 1.34 | 2.55 | 2.40 |
| Rosmarinic | 10.825 | 4.28 | 1.01 | 0.64 | 2.20 | 1.57 |
| Rutin | 10.971 | 1.63 | 3.30 | 6.32 | 10.75 | 3.32 |
| Apigenin-7-O-glucoside | 11.527 | 20.06 | 1.59 | 1.95 | 3.64 | 0.55 |
| Quercitrin | 11.909 | 4.92 | 15.93 | 11.32 | 12.29 | 27.65 |
| Naringin | 13.096 | 1.77 | 0.86 | 1.05 | 1.61 | 0.20 |
| Naringenin | 13.112 | 5.26 | 0.32 | 0.25 | 0.44 | 0.75 |
| Quercetin | 13.253 | 9.84 | 1.58 | 1.48 | 2.39 | 0.71 |
| Kaempferol-3,2-p-coumaroyl glucose | 13.809 | 48.04 | 3.77 | 4.69 | 8.57 | 1.00 |
| Kaempferol | 14.599 | 3.15 | 0.51 | 0.36 | 0.84 | ND |
| Apigenin | 14.886 | 3.21 | 0.35 | 0.03 | 0.08 | 0.09 |
| Isoflavonoids(Phytoestrogens) | ||||||
| Daidazein | 3.102 | 3.14 | 0.48 | 0.13 | 0.79 | 0.14 |
| Genistein | 3.934 | 0.85 | 0.21 | 0.39 | 0.69 | 0.10 |
| Isoformononetin | 6.153 | 3.87 | 0.10 | 0.20 | 0.26 | 0.05 |
| Biochanin A | 9.272 | 0.46 | 0.01 | 0.02 | 0.03 | 0.01 |
Rt retention time, ND not detected.
The contents (mg 100 g−1 D.W.) of phenolic compounds in ADFL were declined in the following order; oleoropin > chlorogenic acid > pyrogallol > ellagic acid > ferulic acid > salicylic acid > p-hydroxy benzoic acid > coumarin > benzoic > caffeine > 4-amino benzoic > 3-OH tyrosol > vanillic > catechol > caffeic. This finding is compatible with Hussain et al.12 who found that, among the hydroxyl benzoic acid derivatives, the most abundant acids present in fenugreek leaves were m and p-hydroxy benzoic acid, β-resorcylic acid, gentisic acid, and gallic acid. In the case of hydroxyl cinnamic acid derivatives, the major acids present included; o and m-coumaric acid and sinapic acid. HPLC analysis showed the absence of α-resorcylic acid, vanillic acid, and p-coumaric acid in air-dried fenugreek leaves.
The dominant components in UFS were pyrogallol, caffeine, oleoropin, vanillic, ferulic, chlorogenic, salicylic, caffeic acid, p-hydroxybenzoic, ellagic, and benzoic. Soaked fenugreek seeds (SFS) were rich in pyrogallol > caffeine > vanillic > ferulic > chlorogenic > salicylic > p-hydroxybenzoic > caffeic acid > benzoic > ellagic. As well as, GFS contained the following order; caffeine > oleoropin > pyrogallol > ferulic > salicylic > chlorogenic > vanillic > ellagic > p-hydroxybenzoic > caffeic > benzoic > 3-OH tyrosol > 4-amino benzoic > gallic. Finally, BFS was rich in pyrogallol > caffeine > vanillic > ferulic > oleoropin > chlorogenic > p-hydroxybenzoic > benzoic > caffeic > ellagic.
ADFL is rich in flavonoid components according to the following order; catechin > apigenin-6-O-arabinose-8-O-galactose > kaempferol-3,2-p-coumaroyl glucose > hesperidin > apigenin-7-O-glucoside. On the other side, the major flavonoid compounds in UFS were hesperidin (83.70 mg 100 g−1 D.W.), apigenin-6-O-arabinose-8-O-galactose (38.65 mg 100 g−1 D.W.), quercitrin (15.93 mg 100 g−1 D.W.) and catechin (14.19 mg 100 g−1 D.W.). Variations in the contents of flavonoid components had resulted after the household treatments as the followings; SFS were rich in hesperidin (67.45 mg 100 g−1 D.W.), apigenin-6-O-arabinose-8-O-galactose (40.20 mg 100 g−1 D.W.), catechin (12.36 mg 100 g−1 D.W.) and quercitrin (11.32 mg 100 g−1 D.W.), GFS rich in hesperidin (110.30 mg 100 g−1 D.W.), apigenin-6-O-arabinose-8-O-galactose (80.55 mg 100 g−1 D.W.), catechin (40.09 mg 100 g−1 D.W.), quercitrin (12.29 mg 100 g−1 D.W.) and rutin (10.75 mg 100 g−1 D.W.), and finally, BFS rich in hesperidin (102.00 mg 100 g−1 D.W.), apigenin-6-O-arabinose-8-O-galactose (28.17 mg 100 g−1 D.W.) and quercitrin (27.65 mg 100 g−1 D.W.).
The plant family most abundant in phytoestrogens is Fabaceae. The hormone-like bisphenol phytoestrogens, the isoflavonoids including daidzein and genistein, are of great interest because of their estrogenic, anti-estrogenic, anti-carcinogenic, antiviral, antifungal, and antioxidant activities19. In our work, four isoflavonoids (phytoestrogens) have been screened using an HPLC instrument and included daidzein, genistein, biochanin A, and isoformononetin. The data revealed that ADFL had the highest contents of isoflavonids, mainly isoformononetin and daidzein (3.87 and 3.14 mg 100 g−1 D.W., respectively). In contrast, UFS was high in daidzein (0.48 mg 100 g−1 D.W.) and genistein (0.21 mg 100 g−1 D.W.), and the household treatments included soaking and germination increased all contents of isoflavonids.
Antioxidant activity of the selected samples
“The most common antioxidant methods are ABTS·+ and DPPH•. DPPH free radical (DPPH·) does not require any special preparation; in contrast, the ABTS radical cation (ABTS·+) has generated by enzymes or chemical reactions20”. “Another important difference is that ABTS·+ can dissolve in aqueous and organic media, due to their hydrophilic and lipophilic nature of the compounds in samples. On the other side, DPPH can only dissolve in organic media, especially in ethanol, this being an important limitation when interpreting the role of hydrophilic antioxidants. Both radicals show similar bi-phase kinetic reactions with many antioxidants. Although, the ferric reducing antioxidant power (FRAP) method is based on the reduction of a ferroin analogue, the Fe3+ complex of tripyridyltriazine Fe (TPTZ)3+ to the intensely blue-colored Fe2+ complex Fe (TPTZ)2+ by antioxidants in acidic medium. However, the reducing capacity does not necessarily reflect antioxidant activity, as has been suggested by Katalinic et al.21 and Wong et al.22”.
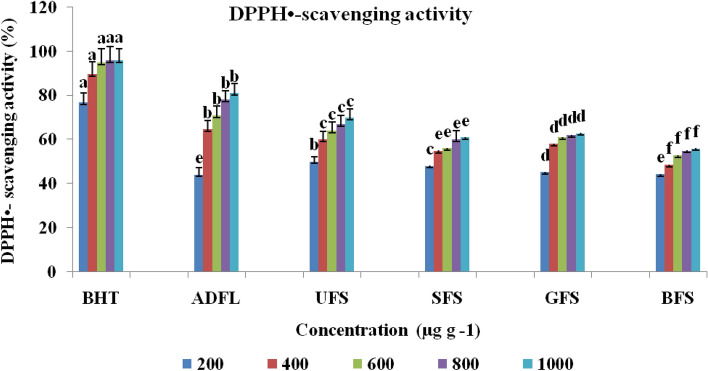
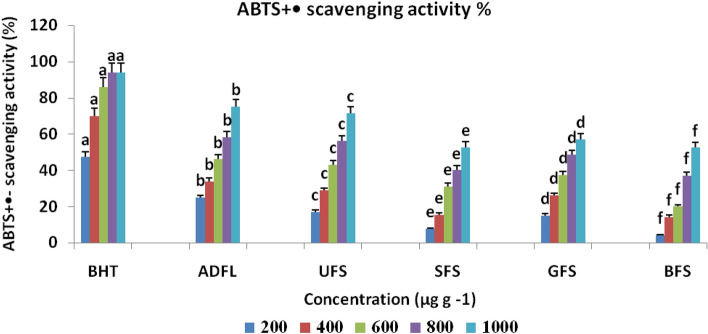
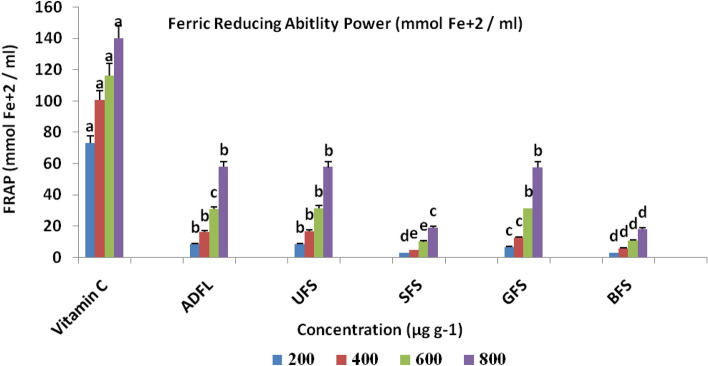
The results of the antioxidant activity of crude extracts of each studied samples have shown in Figs. 2, 3 and 4. These results indicated that at 1000 μg ml−1ADFL had the maximum antioxidant activity (81.11% and 75.01%) with IC50 = 330 μg ml−1, as shown in Table 2, according to both DPPH and ABTS methods, respectively. ADFL has the maximum potential (57.88 μM) for the reduction of ferric ions into ferrous ions at 800 μg ml−1 according to FRAP assay. Furthermore, UFS at 1000 μg ml−1 exhibited a higher radical scavenging activity against DPPH and ABTS free radicals(70.04% and 71.40%) than SFS (61.00% and 52.50%), GFS (62.91% and 57.00%) and BFS (56.00% and 52.41%), respectively. According to the FRAP assay, at 800 μg ml−1, UFS showed more potential (57.60 μM) for ferric ions reduction than SFS (19.00 μM), GFS (57.52 μM) and BFS (18.29 μM).
Figure 2.
DPPH radical scavenging activity (%) of air-dried fenugreek leaves (ADFL), unprocessed (UFS), soaked (SFS), germinated (GFS) and boiled (BFS) fenugreek seeds comparing to BHT (as standard compound).
Figure 3.
ABTS cation radical scavenging activity (%) of air-dried fenugreek leaves (ADFL), unprocessed (UFS), soaked (SFS), germinated (GFS) and boiled (BFS) fenugreek seeds comparing to BHT (as standard compound).
Figure 4.
Ferric reducing antioxidant power (FRAP) (μmol Fe2+ ml−1) of air-dried fenugreek leaves (ADFL), unprocessed (UFS), soaked (SFS), germinated (GFS) and boiled (BFS) fenugreek seeds comparing to vitamin C (as standard compound).
Table 2.
Antioxidant activity of crude extracts of air-dried fenugreek leaves (ADFL), unprocessed fenugreek seeds (UFS), soaked fenugreek seeds (SFS), germinated fenugreek seeds (GFS) and boiled fenugreek seeds (BFS) comparing to BHT (as standard components).
| DPPH· | ABTS+· | |
|---|---|---|
| IC50 (μg ml−1) | ||
| BHT | – | 240.00 |
| ADFL | 330.00 | 638.07 |
| UFS | 200.00 | 697.85 |
| SFS | 300.00 | 950.00 |
| GFS | 276.80 | 847.92 |
| BFS | 480.00 | 950.00 |
According to the previous data on TP and TF, there is a linear correlation between the TP and TF and each of the free radicals (DPPH and ABTS) scavenging activities and the reduction of ferric ions into ferrous ions.
Household treatments of the fenugreek seeds reduced the in-vitro antioxidant property. These results are compatible with the earlier findings of Hooda and Jood13 and Shakuntala et al.14. In contrast, Pandey and Awasthi15 approved that soaking and germination enhanced the total phenolic content and the antioxidant activity of fenugreek seed flour compared to raw seeds flour.
Antimicrobial activity
Antibacterial activity and MIC of the studied samples
The antibacterial activities of the alcoholic crude extracts of each studied samples were evaluated against four foodborne pathogenic Gram-positive and four foodborne pathogenic Gram-negative bacteria, as shown in Table 3. The data illustrated that crude extract of the ADFL exhibited the highest antibacterial activity against B. cereus, Staph. aureus, Staph. scuiri and S. typhi with inhibition zones 11.87, 11.61, 11.60 and 11.30 mm, respectively, compared to the positive control (tetracycline). On the other side, UFS had the maximum activity against both S. enterica (11.11 mm) and S. typhi (10.35 mm). Soaked and germinated fenugreek seeds (SFS and GFS) had the highest activity against S. typhi (9.52 and 9.71 mm), S. enterica (9.34 and 9.52 mm) and P. aeruginosa (9.00 and 9.15 mm), respectively. Finally, BFS had the greatest activity on E. coli O157 H7, S. enteric and P. aeruginosa with inhibition zones were 8.50, 8.18 and 8.12 mm, respectively.
Table 3.
Antibacterial activity of fenugreek leaves, unprocessed and processed seeds against foodborne pathogenic bacterial strains.
| Pathogenic bacteria | Inhibition zones (mm) (Mean ± STDEV*) | ||||||
|---|---|---|---|---|---|---|---|
| Negative control | Positive control | ADFL | Fenugreek seeds | ||||
| UFS | SFS | GFS | BFS | ||||
| (1) B. cereus | 0 | 25.91 ± 2.00b | 11.87 ± 1.01a | 8.99 ± 0.54cd | 7.15 ± 0.42c | 7.41 ± 0.49c | 7.00 ± 0.411b |
| (2) L. monocytogenes | 0 | 20.66 ± 1.46c | 10.00 ± 0.79bc | 9.42 ± 0.61c | 8.01 ± 0.50b | 8.72 ± 0.51b | 7.60 ± 0.51b |
| (3) S. sciuri | 0 | 28.97 ± 2.02a | 11.61 ± 0.10a | 9.72 ± 0.72c | 7.80 ± 0.51c | 7.83 ± 0.42c | 7.15 ± 0.42b |
| (4) S. aureus | 0 | 25.86 ± 1.94b | 11.30 ± 0.79a | 8.83 ± 0.56cd | 7.43 ± 0.44c | 7.61 ± 0.50c | 7.31 ± 0.42b |
| (5) E. coli O157 H7 | 0 | 10.88 ± 0.88e | 10.09 ± 0.89bc | 9.31 ± 0.50c | 8.33 ± 0.51b | 8.41 ± 0.54b | 8.50 ± 0.50a |
| (6) S. typhi | 0 | 25.06 ± 1.97b | 11.60 ± 0.79a | 10.35 ± 0.82b | 9.52 ± 0.60a | 9.71 ± 0.71a | 7.77 ± 0.40b |
| (7) S. enterica | 0 | 25.96 ± 2.02b | 10.71 ± 0.69a | 11.11 ± 0.79a | 9.34 ± 0.57a | 9.52 ± 0.63a | 8.18 ± 0.51a |
| (8) P. aeruginosa | 0 | 16.00 ± 1.94d | 9.11 ± 0.58c | 9.42 ± 0.75cc | 9.00 ± 0.52ab | 9.15 ± 0.60a | 8.12 ± 0.50a |
n = 3, *STDEV: Standard deviation, Different subscripts within column are significantly different at the 5% level, Negative control: DMSO, Positive control: Tetracycline.
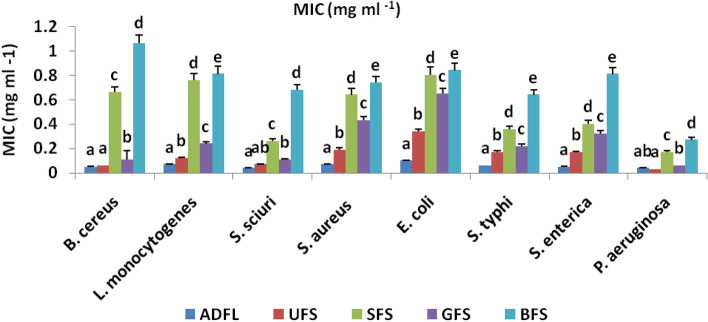
As illustrated in Fig. 5, ADFL had the highest MIC values (0.04 mg ml−1) against each of Staph. Scuiri and P. aeruginosa, but the lowest MIC value (0.10 mg ml−1) was observed against E. coli O157 H7.On the other side, UFS had the highest MIC value (0.03 mg ml−1) against P. aeruginosa and the lowest MIC value (0.19 mg ml−1) against Staph. aureus. The maximum MIC values for each SFS, GFS, and BFS were against P. aeruginosa with 0.17, 0.06, and 0.27 mg ml−1, respectively.
Figure 5.
Minimum inhibitory concentrations (MIC) (mg ml−1) of crude extracts of air-dried fenugreek leaves (ADFL), unprocessed (UFS), soaked (SFS), germinated (GFS) and boiled (BFS) fenugreek seeds against some foodborne pathogenic bacterial strains.
Antifungal activity and MIC of the studied samples
Antifungal activities of the crude extracts of selected samples against eight micotoxigenic fungal strains are shown in Table 4. Data showed that ADFL had the highest activity against A. ochraceus and F. proleferatum with zones of inhibition found to be 11.01, and 10.74 mm, respectively. Also, UFS and SFS had the highest activity against A. ochraceus (10.75, and 8.38 mm, respectively). Germinated seeds exhibited the greater property against both of A. ochraceus and A. purasiticus with inhibition zones found to be 9.18 and 9.02 mm, respectively. Finally, BFS showed the highest antifungal activity against A. carbonarius, A. flavus and P. verrucosum with inhibition zones 8.11, 8.00, and 8.00 mm, respectively.
Table 4.
Antifungal activity of fenugreek leaves, unprocessed and processed seeds against some mycotoxinogenic fungal strains.
| Mycotoxinogenic fungi | Inhibition zones (mm) (Mean ± STDEV*) | ||||||
|---|---|---|---|---|---|---|---|
| Negative control | Positive control | ADFL | Fenugreek seeds | ||||
| UFS | SFS | GFS | BFS | ||||
| (1) A. carbonarius | 0 | 18.78 ± 1.62c | 10.2 ± 0.86ab | 9.36 ± 0.52bc | 8.00 ± 0.51ab | 8.61 ± 0.49a | 8.11 ± 0.50a |
| (2) A. flavus | 0 | 23.33 ± 2.22b | 8.52 ± 0.52c | 8.50 ± 0.51cc | 8.30 ± 0.55a | 8.48 ± 0.51a | 8.00 ± 0.52ab |
| (3) A. niger | 0 | 23.12 ± 1.97b | 9.24 ± 0.66b | 8.76 ± 0.49bc | 7.63 ± 0.43b | 8.16 ± 0.55a | 7.15 ± 0.51b |
| (4) A. ochraceus | 0 | 19.42 ± 1.42cc | 11.01 ± 0.97a | 10.75 ± 0.87a | 8.38 ± 0.53 | 9.02 ± 0.71a | 8.15 ± 0.41a |
| (5) A. purasiticus | 0 | 27.01 ± 2.00a | 9.20 ± 0.56b | 9.00 ± 0.77bc | 8.16 ± 0.61ab | 8.34 ± 0.62a | 7.52 ± 0.43ab |
| (6) A. wasterdijikia | 0 | 23.92 ± 1.83ab | 10.22 ± 0.65ab | 9.46 ± 0.71bc | 7.37 ± 0.49b | 8.02 ± 0.72a | 7.46 ± 0.42ab |
| (7) F. proleferatum | 0 | 14.01 ± 1.04a | 10.74 ± 0.71a | 9.36 ± 0.63bc | 8.02 ± 0.62ab | 9.18 ± 0.72a | 7.55 ± 0.59ab |
| (8) P. verrucosum | 0 | 22.99 ± 1.99bc | 9.83 ± 0.62ab | 9.01 ± 0.58bc | 8.00 ± 0.66ab | 8.91 ± 0.70a | 8.00 ± 0.55ab |
n = 3, *STDEV: Standard deviation, Different subscripts within column are significantly different at the 5% level, Negative control: DMSO, Positive control: Nestatin.
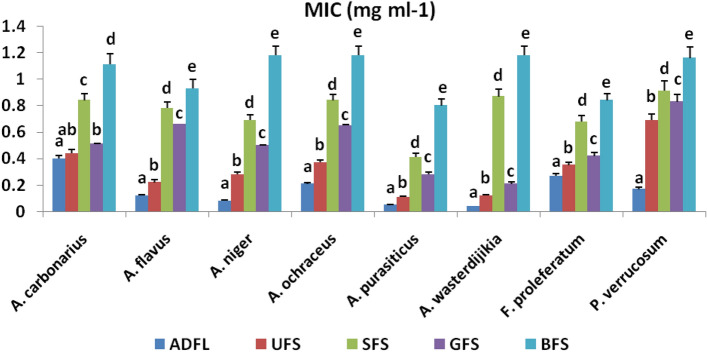
Figure 6 illustrates the MIC values of the tested samples against each studied fungal strain. The most effective levels of MIC values were recorded at 0.04 and 0.21 mg ml−1 with ADFL and GFS, respectively, against A. wasterdijikia. Whereas, UFS, SFS, and BFS crude extracts recorded the most significant levels of MIC values, which were 0.11, 0.41, and 0.80, respectively, against A. purasiticus.
Figure 6.
Minimum inhibitory concentrations (MIC) (mg ml−1) of crude extracts of air-dried fenugreek leaves (ADFL), unprocessed (UFS), soaked (SFS), germinated (GFS) and boiled (BFS) fenugreek seeds against some mycotoxinogenic fungal strains.
Al-Abdeen23 studied the antibacterial activity of aqueous and some organic compound extracts of stems, leaves, seeds, and roots of fenugreek against three Gram– negative and one Gram–positive bacteria by well-diffusion and colony-assay methods. The microorganisms were Staph.aureus, E.coli, P.aeruginosa and K. spp. All plant extracts did not exhibit any inhibitory activity against any of the microorganisms tested by the well-diffusion and colony-assay techniques. Abdalah24 found that the extract of fenugreek seeds at concentrations of 1000, 500, and 250 mg ml−1 inhibited the growth of Streptococcus pyogenes. The methanolic extract of fenugreek seeds with concentrations of 1000, 500, 250, and 125 mg ml−1 inhibited the growth Staphylococcus aureus. The aqueous extract for the fenugreek seeds was less active then methanol extract against the growth of pathogenic bacteria. Premanath25 studied the antimicrobial activity of various fenugreek extracts which were screened by disc diffusion method and ethanol extract was found to be more potent. The Minimum Inhibitory Concentration (MIC) of ethanol extract determined by broth dilution method showed a MIC value of 1 mg ml−1 for Staphylococcus aureus and Pseudomonas aeruginosa. Nandagopal et al.26 evaluated the phytochemical analysis for antibacterial activity of the seed extracts of Trigonella foenum-graecum L. against pathogenic bacteria like Gram positive (Staphylococcus aureus) and Gram negative (E. coli, Pseudomonas aeruginosa, and Klebsiella pneumonia) bacteria by in vitro agar well diffusion method. The seed extracts showed more inhibitory action on Klebsiellap neumonia and Pseudomonas aeruginosa than E. coli, Staphylococcus aureus. EI Nour et al.27 investigated the antimicrobial activity of petroleum ether extract of seeds and callus derived from hypocotyls and cotyledons explants of fenugreek. The petroleum ether extract showed highest antimicrobial activity. Antibacterial activity of petroleum ether extract was recorded 17 ± 0.33 mm and 15 ± 0.57 mm of inhibition zone against E. coli and Staphylococcus aureus, respectively, at concentration of 250 mg ml−1. It showed antifungal activity against Aspergillus niger and Candida albicans with maximum zone of inhibition (20 ± 0.88 mm) against A. niger by conc. 250 mg ml−1 and (17 ± 0.57 mm) of inhibition zone against Candida albicans by concentration of 250 mg ml−1.
In-vitro anti-inflammatory activity of the selected samples
Industrial steroidal and non-steroidal anti-inflammatory drugs have a wide range of side effects. So, diverse works are interested in finding anti-inflammatory agents from natural sources28.
Our work is the first study reports the impact of household techniques on the cytotoxic activity against RAW 264.7 macrophage cell line and the anti-inflammatory property by NO assay of both fenugreek leaves and seeds crude extracts.
Cell cytotoxicity assay
Results presented in Table 5 illustrated that ADFL crude extract exhibited the highest cytotoxic activity (89.03%) on the RAW 264.7 cell line at a concentration of 100 µg g−1. Furthermore, UFS at a concentration of 100 µg g−1 had higher activity (73.21%) than the processed samples, SFS, GFS and BFS, which showed 38.66%, 63.00%, and 18.19% cytotoxic activity, respectively, at the same concentration. So, the household treatments declined the cytotoxic property of the fenugreek seeds against the macrophage cell line but were not affected by the fenugreek leaves. These may be due to a decrease in TP, TF, and AOX activity after each soaking, germinating and boiling process.
Table 5.
Cytotoxicity of crude extracts of the studied samples against RAW 264.7 macrophage cell line.
| 10 | 20 | 40 | 80 | 100 | IC50 (µg g−1) | |
|---|---|---|---|---|---|---|
| Cytotoxicity of RAW cells (%) (Mean ± STDEV*) | ||||||
| Concentration (µg g−1) | ||||||
| ADFL | 15.21 ± 1.12c | 30.00 ± 2.00c | 52.23 ± 3.32a | 73.12 ± 4.23a | 89.03 ± 5.71a | 37.48 |
| UFS | 29.03 ± 2.00a | 38.11 ± 2.12a | 45.00 ± 3.01b | 65.02 ± 4.11b | 73.21 ± 4.61b | 60.16 |
| SFS | 10.01 ± 0.96d | 15.11 ± 1.21d | 20.03 ± 1.72d | 33.12 ± 2.16d | 38.66 ± 2.37d | – |
| GFS | 25.22 ± 1.79b | 31.21 ± 2.21b | 40.21 ± 2.66c | 52.93 ± 3.74c | 63.00 ± 4.21c | 71.05 |
| BFS | 8.37 ± 0.67e | 9.00 ± 0.86e | 13.21 ± 1.01e | 15.91 ± 1.03e | 18.19 ± 1.31e | – |
*Each value represents the mean ± standard deviation *The same letter of denoting over values proves that they are not at significantly different at (p ≤ 0.05), and comparison is done according to treatments. *(–) means unspecified IC 50(µg g−1).
Nitric oxide (NO) inhibition assay
Data shown in Table 6 demonstrated that ADFL at 100 µg g−1 exhibited the maximum inhibition (76.11%) of NO molecules. On the other side, at 100 µg g−1, UFS showed higher inhibition (62.11%) than each of SFS (39.91%), GFS (56.12%), and finally, BFS (33.11%).
Table 6.
Anti-inflammatory activity of crude extracts of selected samples against nitric oxide (NO).
| 10 | 20 | 40 | 80 | 100 | IC50 (µg g−1) | |
|---|---|---|---|---|---|---|
| Inhibition of NO (%) (Mean ± STDEV*) | ||||||
| Concentration (µg g−1) | ||||||
| ADFL | 20.47 ± 1.67a | 30.51 ± 1.78a | 38.26 ± 2.62a | 68.92 ± 4.16a | 76.11 ± 4.49a | 59.79 |
| UFS | 15.00 ± 1.13b | 26.12 ± 1.92b | 28.26 ± 2.01b | 59.21 ± 3.76b | 62.11 ± 3.91b | 72.55 |
| SFS | 8.02 ± 0.51d | 13.01 ± 1.01d | 21.00 ± 1.41d | 31.01 ± 2.11d | 39.91 ± 2.11d | – |
| GFS | 13.11 ± 1.00c | 20.21 ± 1.88c | 25.11 ± 1.93c | 50.31 ± 3.41c | 56.12 ± 3.16c | 82.77 |
| BFS | 6.12 ± 0.42e | 7.51 ± 0.52e | 15.73 ± 1.02e | 26.22 ± 1.96e | 33.11 ± 2.01e | – |
*Each value represents the mean ± Standard deviation **The same letter of denoting over values proves that they are not at significantly different at (p ≤ 0.05), and comparison is done according to treatments. *(–) means unspecified IC 50 (µg g−1).
Materials and methods
Chemicals
Ethanol (95%), methanol (96%), dimethyl sulfoxide (DMSO), sodium carbonate, aluminum chloride, sodium nitrate(III), acetic acids, potassium persulphate, sodium hydroxide, butylated hydroxytoluene (BHT), ascorbic acid were purchased from El Gomhoryia, El Nasr and Middle East Pharmaceutical Chemical companies, Egypt and the solvents were purified before using.
The Folin–Ciocalteau reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) (ABTS), 2,4,6-tri(2-pirydyl-s-triazine (TPTZ) were purchased from Sigma-Aldrich Chemie (Steinheim, Germany). Chemicals, solvents, all standards of phenolic acids, flavonoids and isoflavonoids used for fractionation and identification by HPLC were purchased from Sigma-Aldrich Chemie (Steinheim, Germany).
Plant materials
Our used of plant material and all methods in our research complies with all applicable local, regional, national, and international regulations.
Air-dried fenugreek leaves (ADFL)
Fresh and healthy leaves of fenugreek (Trigonella foenum-graecum Linn.) were purchased from the local market of Egypt in December 2019 and identified by the Faculty of Science, Cairo University. The leaves have washed thoroughly with tap water, and the surface water has removed by air-drying under shade for 15 days. The leaves have subsequently dried in a hot air-oven at 50 °C for 4 h, homogenized to a fine powder and then stored at 4 °C.
Fenugreek seeds
Two kilograms from fenugreek seeds were also purchased from the local market of Egypt, identified by the Faculty of Science, Cairo University, and divided into four groups, as the followings;
1st group (Untreated fenugreek seeds, UFS), 500 g of seeds were manually cleaned to remove dust and foreign particles, crushed into a fine powdered flour with the help of Moulinex blender LM 241 and were sieved in a 0.5 mm mesh size. The powdered flour has been kept at 4 °C to prevent changes till further analyses.
2nd group (Soaked fenugreek seeds, SFS), 500 g of raw seeds were soaked in tap water at the ratio of 1:5 (w/v) for 12 h at room temperature. After pouring off the soaking water, seeds were air-dried in shade for 5 days followed by hot air-oven drying at 50 °C for 4 h in a conventional oven, and stored at 4 °C.
3rd group (Germinated fenugreek seeds, GFS), 500 g of raw seeds were soaked in tap water at the ratio of 1:5 (w/v) for 12 h at room temperature. After pouring off the soaking water, seeds were kept in the dark for germination (tied in cotton cloth) at 20 °C for 60 h in darkness. After harvesting the sprouts, they were air-dried for 5 days followed by hot air-oven drying at 50 °C for 4 h in a conventional oven, and stored at 4 °C.
4thgroup (Boiled fenugreek seeds, BFS), 500 g of raw seeds were put in 2 L beaker containing 1250 ml tap H2O (1:5 w/v). The sample was boiled on a hot plate for 10 min and then, after water was discarded, the boiled seeds were air-dried in shade for 5 days followed by hot air-oven drying at 50 °C for 4 h in a conventional oven, and stored at 4 °C.
Extraction procedure of samples
One hundred grams of each sample were extracted with 1 L (1:10 w/v) 80% ethanol in distilled H2O by sonication for 60 min. Extraction had repeated three times. After filtration, each extract condensed to dryness (resulting in crude extracts) using a rotary evaporator at 40 °C. The obtained residue has collected to calculate the yield and finally stored in the freezer for further biochemical and in-vitro biological analyses.
A known weight of each crude extract obtained from rotary evaporation has dissolved in each ethanol 80% for determination of TP, TF, DPPH·, ABTS+·, and FRAB·, and in dimethyl sulphoxide (DMSO) for both in-vitro antimicrobial and anti-inflammatory activities.
Recovery of the extract was calculated as yield (%) using the following equation:
where Wf is the final weight of the crude extract and Wi is the initial weight of the tested sample.
Determination of total phenolic content (TP)
Total phenolic content of ethanol extracts was evaluated according to Singleton and Rossi29, using of Folin–Ciocalteau reagent. Total phenolic content was expressed as mg of gallic acid equivalents (GAE) per g dr weight (D.W.) of sample. All determinations were performed in triplicates.
Determination of total flavonoid content (TF)
Total flavonoid content was analyzed by a spectrophotometric method described by Boateng et al.30. Total flavonoid content was expressed as mg of quercetin equivalents (QE) per g D.W. of sample. All determinations were performed in triplicates.
Fractionation of phenolic and flavonoid components by HPLC
A high-performance liquid chromatography system equipped with a variable wave length detector (Agilant technologies, Germany) 1200 series. Also the HPLC was equipped with auto-sampler, Quaternary pump degasser and column compartment set at 35 °C. Analyses were performed on a C18 reverse phase (BDS 5 μm, Labio, Czech Republic) packed stainless-steel column (4 × 250 mm, i.d.).To determine phenolic and flavonoids compounds, samples were prepared according to the method described by31. All chromatograms were plotted at 280 nm to estimated phenolic compounds and at 330 nm for flavonoids and isoflavonoids. All components were identified and quantified by comparison of peak areas with external standards.
Antioxidant activity of alcoholic extracts of fenugreek samples
Determination of DPPH• scavenging activity
In order to determine DPPH radical-scavenging activity, a method described by Moure et al.32 was used with minor modification. Various concentrations of each sample (200, 400, 600, 800 and 1000 µg g−1) were prepared from the stock solution (10 mg ml−1). The DPPH radical-scavenging activity in the extracts was expressed as percentage inhibition activity.
The percentage inhibition activity was calculated from [(AC − AS)/AC] × 100.
AC is the absorbance of the control, and AS is the absorbance of the sample. The analyses were carried out in triplicate.
Determination of ABTS•+ scavenging activity
The free radical-scavenging activity has been determined by the ABTS radical cation decolorization assay described in33. The results were expressed as the percentage inhibition activity which calculated from [(Ac − As)/Ac] × 100. Ac is the absorbance of the control, and As is the absorbance of the sample. All determinations have been performed in triplicate.
Determination of ferric reducing antioxidant power (FRAP)
The activity of the extracts for reducing the ferric ion was assayed according to the method of Benzie and Strain34 method. Ferric reducing antioxidant power was expressed as μmol of Fe2+ per 100 g D.W. of each sample.
In-vitro antimicrobial activity
Tested microorganisms
The antimicrobial activity of alcoholic extracts of fenugreek leaves, unprocessed and processed seeds was evaluated against eight foodborne pathogenic bacterial strains and eight pathogenic fungal species. Four Gram-positive bacterial strains were Bacillus cereus (EMCC 1080), Listeria monocytogenes (ATCC 7644), Staphylococcus sciuri (2–6) and Staphylococcus aureus (ATCC 13565), and four Gram-negative bacterial strains Escherichia coli O157 H7 (ATCC 51659), Salmonella typhi (ATCC 25566), Salmonella entrica (SA 19992307) and Pseudomonas aeruginosa (NRRL B-272). All the studied bacterial strains were grown on nutrient agar slants at 37 °C for 24 h and then kept at 4 °C till use.
The tested fungal strains included Aspergillus carbonarius (ITAL 204), Aspergillus flavus (NRRL 3357), Aspergillus niger (IMI 288550), Aspergillus ochraceus (ITAL 14), Aspergillus parasiticus (SSWT 2999), Aspergillus westerdijikia (CCT 6795), Fusarium proliferation (MPVP 328) and Penicillium verrucosum (BFE 500) were grown on potato dextrose agar slants (PDA) at 25 °C for 5 days and then kept at 4 °C till use.
Agar disc-diffusion assay
Agar disc-diffusion assay was used for evaluation the antimicrobial activity of alcoholic extracts against some foodborne pathogenic bacteria and fungi according to Kavanagh35 method. The plates were incubated for 24 h at 37 °C and after the incubation period, the diameters of the cleared zones of inhibition (millimeter) were measured. Dimethyl sulphoxide (DMSO) was used as the negative control, tetracycline used as positive control. Mean and standard deviation (STDEV) values were tabulated.
Measurement of minimum inhibitory concentration (MIC)
MIC against fungi was studied by using the technique of Perrucci et al.36. The prepared plates were centrally inoculated with 3 μl of fungal suspension (108 CFU ml−1; 0.5 McFarland standards). The plates were incubated at 25 °C for 24–48 h. At the end of the incubation period, mycelial growth was noticed and MIC was determined.
In-vitro anti-inflammatory activity
Cell cultivation
RAW 264.7 macrophage cell line was acquired from the ATCC (American type culture collection). Cells were cultivated in RPMI, 1640 medium (Institute of Roswell Park Memorial), and subjoined with 1% pen/strep and 10% heat-inoperative fetal bovine serum. Cells were transferred in a moistened incubator, in an ambient of 5% CO2 at 37 °C, and they were subculture two times before the assay.
Proceedings
The following proceedings were obtained in a sterile area using a laminar bio safety flow cabinet class II level (Baker, SG403INT, Sanford, ME, USA). RAW 264.7 cells were suspended in RPMI medium. After 24 h of seeding 1 × 105 cells per well (in 96—well plates) and incubated for one day for the assay. Cells were then processed with the specimens at different concentrations of 10, 20, 40, 80 and 100 µg g−1 and incubated for 60 min. Cells were then enhanced with 10 μg ml−1 of LPS, as a negative control, for another one day. The supernatant was transferred wisely to a new 96-well plate and processed for NO determination, while the cells stayed in the old plate were used for the MTT protocol, to determine the percentage of the viable cells. Specimens (stock) were dissolute in DMSO, and the working specimens were prepared in the media. Viable cells were determined by the reduction of mitochondrial dependence of yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to purple formazan37. The percent of change in the number of viable cells was measured according to the following equation:
As R* is reading of the extract, and R0 reading of the control.
Nitric oxide (NO) protocol
The production of nitric oxide was processed by determining nitrite in the supernatants of cultivated RAW 264.7 macrophages. The protocol was processed with slight modification as previously described 38. After pre-incubation for one day of RAW 264.7 cells (1 μg ml−1) with LPS (10 μg ml−1), the quantity of nitrite, which is considered a stable NO—metabolite, and used as an indicator of NO—production in the culture medium, it was estimated using a reagent of (0.1% naphthyl ethylene diamine dihydrochloride + 1% sulfanil amide + 2.5% phosphoric acid), and this reagent is commonly known as Griess reagent. A 50 μl volume of the Griess reagent was mixed with 50 μl of the cell culture medium. Afterward, the mixture was incubated at ambient temperature for 15 min, and the absorbance was measured using a microplate multi-well reader (Model 3350, Hercules, California, USA, Bio-Rad Laboratories Inc.) at 540 nm. In every single experiment, a new culture medium was used as a blank. The amount of nitrite was estimated from a sodium nitrite standard curves phrased in the following equation:
Statistical analysis
All assays used in this work were evaluated triple times, and the data obtained were represented by the mean ± standard deviation (STDEV). Statistical Analysis Software (SAS 9.1) was applied for the statistical analysis of data, and IC50 was calculated by using of Graphed prisms. One-way analysis of variance (ANOVA) was used to analyze the difference between groups by applying the least significant difference (LSD) test with 1% and 5% levels of significance (p < 0.05).
Conclusions
Fenugreek seeds have a bitter taste due to saponins and specific smell due to alkaloids and volatile oils, which limit their useand acceptability in the food industry. It has been possible debittered by using various household treatments such as soaking, germinating, boiling, etc. Among all the studied samples, ADFL exhibited the highest in-vitro antioxidant, antibacterial, antifungal, and anti-inflammatory properties due to their high contents of phenolics, flavonoids, and isoflavonoids. Each soaking, germinating, and boiling treatments lowered the in-vitro biological activities because a decreasing occurred in TP, TF, and AOX activities. Finally, air-dried fenugreek leaves and unprocessed seeds must use in both industrial and pharmaceutical fields as an excellent natural antioxidant, antibacterial, antifungal and anti-inflammatory agents, as well as a natural source of potential phenolics, flavonoids, isoflavonoids, steroidal saponins, alkaloids, etc. in medicinal field.
Abbreviations
- ABTS
2,2-Azinobis (3-ethylbenothiazoline-6-sulfonic acid)
- AC
Absorbance of control
- ADFL
Air-dried fenugreek leaves
- As
Absorbance of sample
- ATCC
American type culture collection
- BFS
Boiled fenugreek seeds
- BHT
Butylated hydroxytoluene
- CFU
Colony forming unit
- CNS
Central nervous system
- COX-2
Cyclooxygenase-2
- D.W.
Dry weight
- DMSO
Dimethyl sulphoxide
- DPPH
2,2-Diphenyl-1-picrylhydrazil
- EMCC
Egypt microbial culture collection
- FRAP
Ferric reducing antioxidant power
- GAE
Gallic acid equivalents
- GFS
Germinated fenugreek seeds
- HPLC
High performance liquid chromatography
- i–NOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- LSD
Least significant difference
- MAPK
Mitogen-activated protein kinase
- MIC
Minimum inhibitory concentration
- NF-κB
Nuclear factor kappa light chain enhancer of activated B cells
- NO
Nitric oxide
- NRRL
Northern Regional Research Laboratory
- PDA
Potato dextrose agar
- PGE2
Prostaglandin E2
- QE
Quercetin equivalents
- ROS
Reactive oxygen species
- STDEV
Standard deviation
- SFS
Soaked fenugreek seeds
- TF
Total flavonoid
- TP
Total phenolic
- UFS
Unprocessed fenugreek seeds
Author contributions
Conceptualization, S.G.S. A; Data curation, M.M.R; Formal anal-ysis, N.A.I; Investigation, E.A.A; Supervision, T.A.A; Writing—original draft, A.A; Writing—review and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Data availability
Samples of the compounds and data used during the current study are available from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang, M. T., Ho, C. T. & Lee, C. Y. Phenolic Compounds in Food and Their Effects on Health II: Antioxidants and Cancer Prevention, American Chemical Society Symposium Series 507. 2–7 (American Chemical Society, Washington, 1992)
- 2.Sharma RD. Effects of fenugrek seeds and leaves on blood glucose and serum insulin response in human subjects. Nutr. Res. 1986;6:1353–1363. doi: 10.1016/S0271-5317(86)80020-3. [DOI] [Google Scholar]
- 3.Kala, S. Management of non-insulin dependent diabetes mellitus by using traditional medicinal plant products. Ph.D. Thesis. Haryana Agricultural University, Hisar, India (1997).
- 4.UrasGüngör SŞ, İlçim A, Kökdil G. A comparison of diosgenin, phenolics, fatty acid profiles and mineral contents with free radical scavenging activity of Trigonella l. Species from section Cylindricae. Rec. Nat. Prod. 2017;11(1):17–30. [Google Scholar]
- 5.Sugiyama Y, Hiraiwa Y, Hagiya Y, Nakajima M, Tanaka T, Ogura SI. 5 Amino levulinic acid regulates the immune response in LPS—Stimulated RAW 264.7 macrophages. BMC Immunol. 2018;19(1):41. doi: 10.1186/s12865-018-0277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Kwak CH, Ha SH, Kwon KM, Abekura F, Cho SH, Chang YC, Lee YC, Ha KT, Chung TW, Kim CH. Ganglioside GM3 suppresses lipopolysaccharide—Induced inflammatory responses in RAW 2647 macrophage cells through NF-κB, AP-1, and MAPKs signaling. J. Cell. Biochem. 2018;119(1):1173–1182. doi: 10.1002/jcb.26287. [DOI] [PubMed] [Google Scholar]
- 7.Harikrishnan H, Jantan I, Haque MA, Kumolosasi E. Anti-inflammatory effects of Phyllanthus amarus Schum. & Thonn. through inhibition of NF-κB, MAPK, and PI3K-Akt signaling pathways in LPS—Induced human macrophages. BMC Compl. Altern. Med. 2018;18(1):224. doi: 10.1186/s12906-018-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancion A, Tridetti J, Nguyen TM. A review of the role of Bradykinin and nitric oxide in the cardioprotective action of angiotensin-converting enzyme inhibitors: focus on perindopril. Cardiol. Therapeut. 2019;8:179–191. doi: 10.1007/s40119-019-00150-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindappa M, Hemashekhar B, Arthikala M-K, Rai VR, Ramachandra Y. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Res. Phys. 2018;9:400. [Google Scholar]
- 10.Lee SG, Brownmiller CR, Lee SO, Kang HW. Anti-inflammatory and antioxidant effects of anthocyanins of Trifolium pratense (red clover) in lipopolysaccharide—Stimulated RAW-267.4 macrophages. Nutrients. 2020;12(4):1089. doi: 10.3390/nu12041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock JT, Neill SJ. Nitric oxide: Its generation and interactions with other reactive signaling compounds. Plants. 2019;8(2):41. doi: 10.3390/plants8020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain PR, Suradkar P, Javaid S, Akram H, Parvez S. Influence of postharvest gamma irradiation treatment on the content of bioactive compounds and antioxidant activity of fenugreek (Trigonella foenum–graceum L.) and spinach (Spinacia oleracea L.) leaves. Innov. Food Sci. Emerg. Technol. 2016;33:268–281. doi: 10.1016/j.ifset.2015.11.017. [DOI] [Google Scholar]
- 13.Hooda S, Jood S. Effect of soaking and germination on nutrient and anti nutrient contents of fenugreek (Trigonella foenum-graecum L.) J. Food Biochem. 2003;27:165–176. doi: 10.1111/j.1745-4514.2003.tb00274.x. [DOI] [Google Scholar]
- 14.Shakuntala S, Naik JP, Jeyarani T, Naidu MM, Srinivas P. Characterization of germinated fenugreek (Trigonella foenum-graecum L.) seed fractions. Int. J. Food Sci. Technol. 2011;46:2337–2343. doi: 10.1111/j.1365-2621.2011.02754.x. [DOI] [Google Scholar]
- 15.Pająk P, Socha R, Gałkowska D, Rożnowski J, Fortuna T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014;143:300–306. doi: 10.1016/j.foodchem.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 16.Pająk P, Socha R, Broniek J, Królikowska K, Fortuna T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019;275:69–76. doi: 10.1016/j.foodchem.2018.09.081. [DOI] [PubMed] [Google Scholar]
- 17.Pandey H, Awasthi P. Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. J. Food Sci. Technol. 2015;52(2):1054–1060. doi: 10.1007/s13197-013-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin PY, Lai HM. Bioactive compounds in legumes and their germinated products. J. Agric. Food Chem. 2006;54(11):3807–3814. doi: 10.1021/jf060002o. [DOI] [PubMed] [Google Scholar]
- 19.Mazur WM, Duke JA, Wähälä K, Rasku S, Adlercreutz H. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. J. Nutr. Biochem. 1998;9:193–200. doi: 10.1016/S0955-2863(97)00184-8. [DOI] [Google Scholar]
- 20.Arnao MB. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000;11:419–421. doi: 10.1016/S0924-2244(01)00027-9. [DOI] [Google Scholar]
- 21.Katalinic V, Milos M, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. doi: 10.1016/j.foodchem.2004.12.004. [DOI] [Google Scholar]
- 22.Wong CC, Li HB, Cheng KW, Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. doi: 10.1016/j.foodchem.2005.05.049. [DOI] [Google Scholar]
- 23.Al-abdeen, S. Z., Faraj, B. M. & Nasrulla, O. J. Antibacterial effects of fenugreek. Department of Biology, Kerkuk, Iraq, 10(2), 133–138 (2010)
- 24.Abdalah M. The study of antibacterial activity of fenugreek (Trigonella foenum-graecum) seeds extract. Iraq J. Mark. Res. Consum. Prot. 2011;6(3):145–155. [Google Scholar]
- 25.Premanath R, Sudisha J, Aradhna SM. Antibacterial and antioxidant activities of fenugreek leaves. Res. J. Med. Plants. 2011;5:695–705. doi: 10.3923/rjmp.2011.695.705. [DOI] [Google Scholar]
- 26.Nandagopal S, Dhanalakshmi DP, Kumar AG, Sujitha D. Phytochemical and antibacterial studies of fenugreek (Trigonella foenum-graecum L.) J. Pharm. Res. 2012;5(1):413–415. [Google Scholar]
- 27.ElNour MEM, Ali AMA, Saeed BAE. Antimicrobial activities and phytochemical screening of callus and seeds extracts of Fenugreek (Trigonella foenum-graecum) Int. J. Curr. Microbiol. Appl. Sci. 2015;4(2):147–157. [Google Scholar]
- 28.Sharififara F, Khazaelia P, Alliba N. In vivo evaluation of anti-inflammatory activity of topical preparations from fenugreek (Trigonella foenum-graecum L.) seeds in a cream base. Iran. J. Pharm. Sci. 2009;5(3):157–162. [Google Scholar]
- 29.Singleton VL, Rossi JAJ. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol Viticult. 1965;16:144–158. doi: 10.5344/ajev.1965.16.3.144. [DOI] [Google Scholar]
- 30.Boateng J, Verghese M, Walker LT, Ogutu S. Effect of processing on antioxidant content in selected dry beans (Phaseolus spp. L.) LWT Food Sci. Technol. 2008;41:1541–1547. doi: 10.1016/j.lwt.2007.11.025. [DOI] [Google Scholar]
- 31.Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A. 2001;910:265–273. doi: 10.1016/S0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- 32.Moure A, Franco D, Sineiro J, Dominguez H, Nunez MJ, Lema JM. Antioxidant activity of extracts from Gevuinaavellana and Rosa rubiginosa defatted seeds. Food Res. Int. 2001;34:103–109. doi: 10.1016/S0963-9969(00)00136-8. [DOI] [Google Scholar]
- 33.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 34.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 35.Kavanagh F. Analytical Microbiology. Academic Press; 1972. pp. 737–741. [Google Scholar]
- 36.Perrucci A, Okmen A, Gulluce M, Akpulat H, Dafera D. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control. 2004;15:627–634. doi: 10.1016/j.foodcont.2003.10.005. [DOI] [Google Scholar]
- 37.Khor K, Joseph J, Shamsuddin F, Lim V, Moses EJ, Abdul Samad N. The cytotoxic effects of Moringa oleifera leaf extract and silver nanoparticles on human Kasumi—1 cells. Int. J. Nanomed. 2020;15:5661–5670. doi: 10.2147/IJN.S244834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abhaypratap V, Aakanksha W, Sonika P, Mallesham B, Kumari A, Kishorekumar R, Igamberdiev AU, Mur LAJ, Gupta KJ. Current approaches to measure nitric oxide in plants. J. Exp. Bot. 2019;70(17):4333–4343. doi: 10.1093/jxb/erz242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Samples of the compounds and data used during the current study are available from the corresponding author.