Abstract
Chronic Helicobacter pylori disease is reduced with Allium vegetable intake. This study was designed to assess the in vivo anti-H. pylori potential of a variety of garlic substances. The garlic materials all showed substantial but widely differing anti-H. pylori effects against all strains and isolates tested. The MICs (range, 8 to 32 μg/ml) and minimum bactericidal concentrations (MBCs) (range, 16 to 32 μg/ml) of undiluted garlic oil (GO) were smaller than those of garlic powder (GP) (MIC range, 250 to 500 μg/ml; MBC range, 250 to 500 μg/ml) but greater than the MIC of allicin (4.0 μg/ml) (Table 2) present in GP. Allicin (MIC, 6 μg/ml; MBC, 6 μg/ml) was more potent than diallyl disulfide (MIC range, 100 to 200 μg/ml; MBC range, 100 to 200 μg/ml), its corresponding sulfide, but of a strength similar to that of diallyl tetrasulfide (MIC range, 3 to 6 μg/ml; MBC range, 3 to 6 μg/ml). Antimicrobial activity of the diallyl sulfides increased with the number of sulfur atoms. Time course viability studies and microscopy showed dose-dependent anti-H. pylori effects with undiluted GO, GP, allicin, and diallyl trisulfide after a lag phase of ca. 1 to 2 h. Substantial in vitro anti-H. pylori effects of pure GO and GP and their diallyl sulfur components exist, suggesting their potential for in vivo clinical use against H. pylori infections.
Helicobacter pylori is now recognized as the causal agent of chronic gastritis (8) and gastric and duodenal ulcers (21). In addition, clinical evidence shows that eradication of H. pylori results in significant remission from these diseases (2, 14). In affluent societies H. pylori infection is increasingly treated with potent combination therapies (e.g., a proton pump inhibitor and two antibiotics). These have a success rate of 80 to 90% (24), but problems, including undesirable side effects (1, 3) and poor patient compliance, are associated with significant levels of treatment failure and contraindications for some patients. In addition, the cost of combination therapy is significant, and because prevalences of more than 90% have been reported in developing countries (9) and around half of the world's population is infected (25), a need for more widely available means of treating, suppressing, or preventing H. pylori infection exists. An additional reason for seeking new therapies is that widespread use of antibiotics in combination therapies is associated with increasing drug resistance problems, for example, metronidazole- and clarithromycin-resistant H. pylori strains have been reported (20).
Garlic materials might provide a suitable basis for new anti-H. pylori therapies because they possess well-established antimicrobial actions (5, 23) and the chemical complexity of garlic materials and their broad-spectrum effectiveness suggest that acquired antibiotic resistance would be unlikely. In addition, direct intragastric effects are feasible because garlic antimicrobials are unaffected by acid environments (16) and because gastric juice enhances the antimicrobial activity of garlic constituents (10).
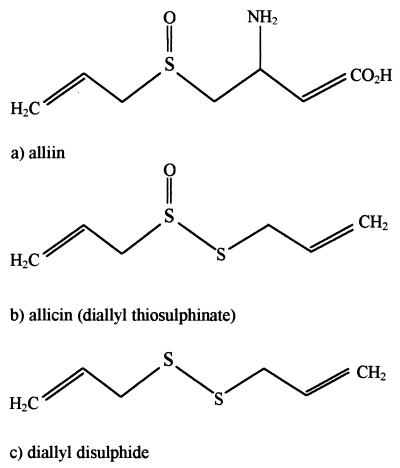
Key studies by Cavallito and Bailey (5) and Stoll and Seebeck (28) identified the compound allicin (allyl 2-propene thiosulfinate [Fig. 1b]) as the main active antimicrobial agent provided by garlic. Allicin is formed catalytically when garlic cloves are crushed and the enzyme alliinase of the bundle sheath cells mixes with its substrate, alliin (Fig. 1a), which is released from mesophyll cells (16).
FIG. 1.
Chemical structures of compounds found in garlic cloves (a), wet GP (b), and GO (c).
Many commercial garlic preparations are available, most of which contain either garlic powder (GP) or garlic oil (GO). GP is a preparation of sliced, dried, and then pulverized garlic cloves which forms allicin upon the addition of water (16). GO is produced commercially by heating crushed garlic cloves to 100°C and collecting the vapor as a distillate (17). During this process, which has effects similar to those of cooking crushed garlic, allicin converts to diallyl sulfide (DADS) (Fig. 1c) and other garlic sulfides (Table 1). Commercially available GO is normally diluted approximately 200:1 with a vegetable oil so that the final product reflects the level of allicin in freshly crushed garlic.
TABLE 1.
Analysis of undiluted GO
| GO component | Concn (mg/g)a |
|---|---|
| Diallyl monosulfide | 106 ± 7 (10.6) |
| Diallyl disulfide | 530 ± 7 (53.0) |
| Diallyl trisulfide | 115 ± 4 (11.5) |
| Diallyl tetrasulfide | 43 ± 2 (4.3) |
| Diallyl pentasulfide | 10.5 ± 0.4 (1.1) |
| Diallyl hexasulfide | 0.14 ± 0.01 (0.01) |
| Methyl allyl disulfide | 44.1 ± 2 (4.4) |
| Methyl allyl trisulfide | 69.9 ± 2.2 (7) |
| Methyl allyl tetrasulfide | 24.6 ± 2.0 (2.5) |
| Methyl allyl pentasulfide | 6.3 ± 0.6 (0.6) |
| Methyl allyl hexasulfide | 1.5 ± 0.1 (0.2) |
| Dimethyl trisulfide | 12.0 ± 1.3 (1.2) |
| Dimethyl tetrasulfide | 4.3 ± 0.6 (0.2) |
| Dimethyl pentasulfide | 2.0 ± 0.4 (0.2) |
Values in parentheses are percentages.
Epidemiological studies show a reciprocal relationship between stomach cancer, which is strongly correlated with H. pylori infection, and the consumption of Allium vegetables (4, 31). Also, recent reports provide in vitro evidence for anti-H. pylori effects of aqueous garlic extracts (6, 11, 27), commercial garlic tablets (11), allyl sulfide components (7), oil-macerated garlic constituents, and allicin (22). Previous studies may have underestimated anti-H. pylori activity because none of them describe precautions for preventing loss of garlic components during testing. Such losses were feasible because of the volatility of garlic thiosulfinates (16) and, especially, sulfides and because the slow growth of H. pylori meant that tests were extended over periods of 3 to 4 days.
The aim of the present study was to accurately determine the effects of GP and GO and their major diallyl sulfur constituents upon the viability of H. pylori. To achieve this objective a method was developed and implemented to minimize the loss of anti-H. pylori activity resulting from volatilization of garlic components. In addition, garlic components were accurately quantified by using established high-performance liquid chromatography methods for the allyl thiosulfinate (17) and sulfide (18) constituents of garlic. The present study includes the first in vitro investigation of the actions of GO, GP, and higher polysulfides against H. pylori.
H. pylori NCTC 11637, a type strain originally isolated in Australia in 1982 (29) and Roberts, a clinical isolate, were donated by the Department of Microbiology, University of Birmingham. Recent clinical isolates were provided by the Department of Microbiology, Gloucester Royal Infirmary. Long-term storage of H. pylori samples was at −70°C in Isosensitest broth (ISB; Oxoid) supplemented with 10% fetal calf serum (Sigma). For the duration of the study, bacteria were routinely subcultured every 2 to 3 days on Isosensitest agar (Oxoid) supplemented with 2% heat-inactivated newborn calf serum (NCS) (Sigma) in an anaerobic cabinet (model AW200SG; Electrotek, Ltd., West Yorkshire, United Kingdom) containing a microaerophilic gas mixture of 5% O2, 10% CO2, and 85% N2.
Undiluted GO (98% allyl and methyl sulfides) was supplied by R. P. Scherer, Ltd. (Swindon, Wiltshire, United Kingdom) and stored at 4°C in a tightly sealed vessel throughout the study. GP was supplied by Interprise, Ltd. (Port Talbot, Wales, United Kingdom) and was stored sealed in dry conditions at room temperature. DADS, diallyl trisulfide (DATS), and diallyl tetrasulfide (DATTS) were donated by Murdock Madaus Schwabe, Inc. (Springville, Utah) and stored at −20°C. Diallyl sulfide (DAS) was prepared from GO in our laboratory by fractional vacuum steam distillation and was also stored at −20°C. Reverse-phase high-performance liquid chromatography with a diode-array detector (Beckman, Ltd., High Wycombe, United Kingdom) set at 240 nm was used to quantify GO components (Table 1) and GP allicin content and to check their purity and stability throughout the study. GP and allicin were analyzed with a C18 Supelcosil column (Supelco, Ltd.) with a mobile phase of 50:50 (methanol to water) and a flow rate of 1 ml per min (19). GO sulfides were quantified with the same column but with a mobile phase of 70:27:3 (acetonitrile to water to tetrahydrofuran) and a flow rate of 1 ml per min (18).
Garlic components are volatile, so to minimize any loss during studies of antimicrobial effect, a broth method involving tightly sealed culture vessels was devised. This was experimentally validated to ensure its ability to support vigorous growth of H. pylori to high population densities (∼108 CFU/ml).
For MIC and minimum bactericidal concentration (MBC) determinations, serial twofold dilutions of garlic substances in 5 ml of ISB plus 2% NCS were prepared in cotton wool-plugged 25-ml conical flasks and inoculated with 50 μl of a 24-h H. pylori broth culture (inoculum, ∼106 CFU/ml). The flasks were pregassed for 10 min in the microaerophilic cabinet and subsequently sealed with Teflon-sleeved ground-glass bungs. All flasks were then incubated for 24 h in the cabinet, with shaking at ∼150 rpm. Viable counts were then determined for the first MIC flask in the dilution range showing visible growth and the next three broths with no visible growth by the drop plate technique, and measurements of the optical density at 600 nm (OD600) and phase-contrast microscopy examinations were also performed. The MICs recorded were the lowest concentrations showing complete inhibition of growth based on viable count results (checked by absorbances at OD600). The MBCs recorded were the lowest concentrations that killed all the bacteria detectable by the viable count procedure, i.e., viable count was >125 CFU/ml. All MICs and MBCs were confirmed by duplicate assays. Time course viability studies were set up in the same way as the MIC and MBC tests, but the broths were sampled hourly over the first 4 h and then again at 6 and 24 h. Experiments with GO and GP were performed in duplicate.
The effects of GO upon H. pylori cells were determined by phase-contrast microscopy (magnification, ×1,250). This involved using a higher inoculum (∼108 CFU/ml) than was used for the MIC and MBC procedures so that numerous cells were clearly visible under the microscope. Cells were harvested from Isosensitest agar plus 2% NCS plates incubated for 24 h and standardized to a concentration of ∼108 CFU/ml in ISB plus 2% NCS. A 2.5-ml volume of this inoculum was incubated in 25-ml ground-glass-stoppered conical flasks in the microaerophilic cabinet for 2 h to allow the bacteria to adjust to their new environment. On addition of 2.5 ml of an appropriate dilution of GO, flasks (containing GO at concentrations of 0, 6, 129, and 258 μg/ml) were immediately returned to the cabinet and then periodically sampled over a 24-h period by viable counting and semiquantitative microscopic assessment.
The MICs and MBCs of GO and GP (Table 2) and of their major diallyl sulfur components (Table 3) show that they have significant but varied activity against H. pylori. No MIC or MBC data for replicates encompassed more than two adjacent broth concentrations, and results for most duplicates were identical; thus, within the error of the method (i.e., ±1 doubling concentration), the MICs and MBCs of each material were consistently similar to one another irrespective of the strain or clinical isolate tested. Anti-H. pylori potency was considerably greater (8- to 62-fold-lower MICs and MBCs) with GO than with GP, and large differences were also detected among diallyl components. For DASs a wide range of MICs and MBCs was observed that was reduced with each additional sulfur atom (Table 3). These values encompass those of GO, although DADS, the main constituent (Table 1) of GO, exhibited considerably lower activity than GO itself. Allicin, the only diallyl compound and major thiosulfinate formed from GP (30), was more inhibitory (MIC range, 6 to 12 μg/ml) than any DAS except DATTS (MIC range, 3 to 6 μg/ml).
TABLE 2.
MICs and MBCs of undiluted GO and GP for eight strains of H. pyloria
| Helicobacter strain | GO
|
GP
|
||
|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |
| NCTC 11637 | 8–16 | 16–32 | 250 | 500 |
| Roberts | 32 | 32 | 500 | 500 |
| 106552B | 32 | 32 | 250–500 | 500 |
| 106556B | 16–32 | 16–32 | 500 | 500 |
| 107018B | 16 | 32 | 250 | 250–500 |
| 107061B | 32 | 32 | 500 | 500 |
| 107465B | 16 | 32 | 500 | 500 |
| 107467B | 32 | 32 | 500 | 500 |
Values are ranges derived from two determinations (except five determinations were done with strain NCTC 11637 and three determinations were done with strain 106556B). A 500-μg/ml concentration of GP is the equivalent of a 4-μg/ml concentration of allicin.
TABLE 3.
MICs and MBCs of the GO sulfides and allicin for three strains of H. pylori
| GO sulfide | Result for strain:
|
|||||
|---|---|---|---|---|---|---|
| NCTC 11637
|
Roberts
|
107018B
|
||||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| DAS | 2,074 | 2,074 | 2,074 | 4,148 | 4,148 | 4,148 |
| DADS | 100 | 100–200 | 100 | 100–200 | 100 | 100 |
| DATS | 13 | 13 | 13 | 25 | 25 | 25 |
| DATTS | 3–6 | 6 | 6 | 6 | 3 | 6 |
| Allicin | 6 | 6–12 | 6–12 | 6–12 | 6–12 | 12 |
Values are ranges (in micrograms per milliliter) derived from two determinations.
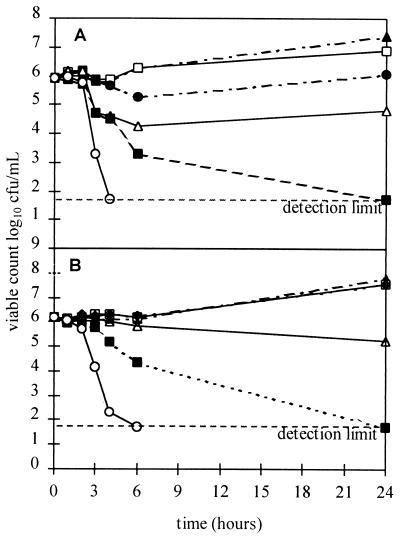
In time course studies (Fig. 2) with GO and GP, similar and consistently dose-dependent patterns of effect upon H. pylori NCTC 11637 were observed at all concentrations and the MICs and MBCs obtained corresponded well with those obtained in 24-h MIC and MBC studies. Lag phases of 1 to 2 h were observed. Similar results were obtained with other garlic components (allicin and DATS [results not shown]).
FIG. 2.
Typical time course viability studies of activities of GO and GP against H. pylori NCTC 11637 (each study was duplicated). (A) Bactericidal effect of GO: ▴, control; □, 4 μg/ml; ●, 8 μg/ml; ▵, 16 μg/ml; ■, 32 μg/ml; ○, 64 μg/ml. (B) Bactericidal effect of GP: ▴, control; □, 63 μg/ml; ●, 125 μg/ml; ▵, 250 μg/ml; ■, 500 μg/ml; ○, 1,000 μg/ml.
Phase-contrast microscopy revealed marked alterations in the behavior and morphology of H. pylori cells (isolate 107018) following exposure to GO (Table 4). Within 1 h of exposure to a GO concentration of 129 μg/ml, previously vigorous motility greatly declined, clumping substantially increased, between one-fourth and one-half of cells originally present appeared as coccoidal forms, and substantial reductions in the numbers of normal spiral rod isoforms occurred. Within 2 h, viability was below detectable levels; by 6 h, most cells were coccoidal; and after 24 h, debris had noticeably increased (Table 4). With increasing GO concentrations these detrimental effects to the bacterial cell occurred more rapidly (data not shown).
TABLE 4.
Visible effects of 129 μg of GO per ml on H. pylori 107018
| Time (h) | No. of spiral rodsa | Clumpingb | Cocci (%) | Motilityc | Viable count log10 | Debris (%) |
|---|---|---|---|---|---|---|
| 0 | >50 | 0 | 0 | +++++ | 8.21 | 0 |
| 1 | 20–50 | 2 | 26–50 | ++ | 3.64 | 0 |
| 2 | 1–5 | 3 | 51–75 | + | <2.1 | 1–25 |
| 3 | 1–5 | 3 | 51–75 | − | <2.1 | 1–25 |
| 4 | 1–5 | 3 | 51–75 | − | <2.1 | 1–25 |
| 5 | <1 | 3 | 51–75 | − | <2.1 | 1–25 |
| 6 | <1 | 3 | 76–100 | − | <2.1 | 1–25 |
| 24 | 0 | 3 | 100 | − | <2.1 | 25–50 |
Number of rods observed per field of view.
Clumping was rated on a scale of 1 to 4, as follows: 1, motile clumps containing ≤10 cells; 2, nonmotile clumps containing 5 to 20 cells; 3, nonmotile clumps containing 20 to 50 cells; 4, nonmotile clumps containing 50 cells.
−, no movement; +, slow; ++, moderate; +++++, very fast movement.
The traditional use of dietary garlic to fight infections and the medicinal use of GO to combat various diseases (26) suggest that the systemic distribution of garlic materials is efficient. GO sulfides are readily absorbed (12); some, such as DADS, have significant half-lives in blood, and the major metabolite of allicin derivatives, allyl methyl sulfide (15), is relatively persistent, as it is excreted on the breath. Thus, an antimicrobial effect of circulating garlic materials against H. pylori colonizing the gastric mucosa is feasible.
Earlier studies of the effects of aqueous garlic extracts showed MIC ranges of 5 to 17.5 mg/ml (equivalent to 2 to 7 mg of GP/ml), and from spectrophotometric estimation of total thiosulfinate, MICs of 28 μg/ml (27) and 123 μg/ml (11) are calculable. MICs reported here for GP and allicin were between several times less and an order of magnitude less than the MICs already reported or calculated. The considerably lower MICs reported in the present study most likely result from the precautions taken to prevent loss of garlic components by volatilization.
The MIC of GP (0.5 mg/ml) corresponds to an allicin concentration of 4 μg/ml and a thiosulfinate concentration of approximately 6 μg/ml (assuming allicin is 70% of total thiosulfinates present [19]) which corresponds well to the MIC range of 6 to 12 μg/ml obtained for pure allicin and also indicates that the low potency of GP is principally related to its high vegetable matter content (16). These data suggest that around 1.0 g of GP (equivalent to ca. 2.5 g of vegetable garlic with a 60% water content) could be sufficient to produce intragastric inhibition or killing of H. pylori when diluted (to ca. 1 g/liter) within the stomach. In practice, a major problem with the ingestion of GP in tablet or capsule form would be that alliinase is very substantially inactivated or totally destroyed at gastric pH, preventing the formation of allicin and therefore precluding either intragastric or systemic activities. However, initial mixing of GP with water or milk, or consumption of vegetable garlic after extensive cell disruption (and associated mixing of alliin and alliinase) produced by crushing or mastication prior to ingestion would provide significant amounts of allicin. Allicin remains stable when diluted and in an acidic milieu (16). However, there is evidence for a gastric irritating effect (12) and allicin should therefore be used with caution, especially by those with overt gastric mucosal damage or inflammation (13).
Synergistic effects of aqueous garlic extract with omeprazole (6, 11) in vitro suggest that exploration of a role for garlic components in combination with this H+,K+-ATPase inhibitor would be worthwhile.
GO was consistently more potent than GP in all studies but less so than allicin. The high levels of anti-H. pylori activity reported for the diallyl polysulfides DATS and DATTS (Table 2) and the steep increase in antimicrobial potency along with the numbers of sulfur atoms per molecule suggest that these and other higher polysulfides could have clinical anti-H. pylori potential.
The DASs tested (Table 3), which together compose 80% of GO (Table 1), partly explain the considerable anti-H. pylori activity of GO itself (Table 2). Additional activity could result from synergistic effects or, alternatively, high anti-H. pylori activities among untested GO constituents. Higher polysulfides (Table 3) and methyl-substituted sulfides (30) are possible sources of such activity.
In the study of visible effects upon cells, reduction in spiral rods and cell motility but increase in coccoidal forms, clumping, and debris with time were observed that paralleled the decline in viability. The increase in cell debris indicates progressive cellular destruction, suggesting that GO may interfere with the bacterial cell envelope, causing cell lysis, or with cell metabolism, triggering autolysis.
The MIC and MBC data obtained in the present study show that GO doses in the range of 2 to 32 mg could, in principle, produce significant loss of viability of H. pylori, irrespective of strain, when present in postprandial gastric volumes of 0.25 to 1.0 liter. Moreover, time course studies and microscopy indicate that significant anti-H. pylori effects of GO could occur sufficiently rapidly to precede substantial loss of activity resulting from secretory dilution and gastric emptying. These observations suggest that repeat dosing with GO has the potential to progressively reduce and possibly eradicate H. pylori infections by direct intragastric action.
In conclusion, the high levels of anti-H. pylori activity of garlic components reported in this study indicate that additional in vitro studies and clinical trials are justified to further evaluate the in vivo potential of garlic for the eradication or control of H. pylori.
Acknowledgments
We thank Charles Penn and David Reynolds of the School of Biological Sciences, University of Birmingham; Dean Chamberlain of the University of Wolverhampton for help and involvement in preliminary studies; and Bruce Stephenson of the University of Wolverhampton for providing DAS.
REFERENCES
- 1.Bardhan K D, Wurzer H, Marcelino M, Jahnsen J, Lotay N, Roberts P M. Ranitidine bismuth citrate with clarithromycin given twice daily effectively eradicates Helicobacter pylori and heals duodenal ulcers. Am J Gastroenterol. 1998;93:380–385. doi: 10.1111/j.1572-0241.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 2.Bayerdröffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 3.Bell G D, Powell K U, Burridge S M, Bowden A N, Rameh B, Bolten G, Purser K, Harrison G, Brown C, Gant P W, Jones P H, Trowell J E. Helicobacter pylori eradication: efficacy and side effect profile of a combination of omeprazole, amoxycillin and metronidazole compared with four alternative regimens. Q J Med J Assoc Phys. 1993;86:743–750. [PubMed] [Google Scholar]
- 4.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A, Marubini E, Puntoni R, Vinigni C, Fraumeni J, Blot W. A case control study of gastric cancer and diet in Italy. Int J Cancer. 1989;44:611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 5.Cavallito C J, Bailey J H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J Am Chem Soc. 1944;66:1950–1954. [Google Scholar]
- 6.Cellini L, Di Campli E, Masulli M, Di Bartolomeo S, Allocati N. Inhibition of Helicobacter pylori by garlic extract (Allium sativum) FEMS Immunol Med Microbiol. 1996;13:273–277. doi: 10.1111/j.1574-695X.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 7.Chung J G, Chen G W, Wu L T, Chang H L, Lin J G, Yeh C C, Wang T F. Effects of garlic compounds diallyl sulphide and diallyl disulphide on arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Am J Chin Med. 1998;26:353–364. doi: 10.1142/S0192415X98000397. [DOI] [PubMed] [Google Scholar]
- 8.Dixon M F. Helicobacter pylori and chronic gastritis. In: Rathbone B J, Heatley R V, editors. Helicobacter pylori and gastroduodenal disease. Oxford, United Kingdom: Blackwell Scientific Publications; 1992. pp. 124–139. [Google Scholar]
- 9.Feldman R A, Eccersley A J, Hardie J M. Epidemiology of Helicobacter pylori: acquisition, population prevalence and disease-to-infection ratio. Br Med Bull. 1998;54:39–53. doi: 10.1093/oxfordjournals.bmb.a011678. [DOI] [PubMed] [Google Scholar]
- 10.Fortunatov M N. On the activity of the phytoncides from garlic in the human organism upon peroral administration. Farmakol Toksikol. 1955;18:43–46. [PubMed] [Google Scholar]
- 11.Jonkers D, VandenBroek E, VanDooren I, Thijs C, Dorant E, Hageman G, Stobberingh E. Antibacterial effect of garlic and omeprazole on Helicobacter pylori. J Antimicrob Chemother. 1999;43:837–839. doi: 10.1093/jac/43.6.837. [DOI] [PubMed] [Google Scholar]
- 12.Koch H P. Biopharmaceutics of garlic's effective compounds. In: Koch H P, Lawson L D, editors. Garlic. The science and therapeutic application of Allium sativum L. and related species. Baltimore, Md: Williams & Wilkins; 1996. pp. 213–220. [Google Scholar]
- 13.Koch H P. Toxicity, side effects, and unwanted effects of garlic. In: Koch H P, Lawson L D, editors. Garlic. The science and therapeutic application of Allium sativum L. and related species. Baltimore, Md: Williams & Wilkins; 1996. pp. 221–229. [Google Scholar]
- 14.Labez J, Brösch G. Highly significant change of the clinical course of relapsing and complicated peptic ulcer disease after cure of Helicobacter pylori infection. Am J Gastroenterol. 1994;89:1785–1788. [PubMed] [Google Scholar]
- 15.Lawson L D. Garlic: a review of its medicinal effects and indicated active compounds. In: Lawson L D, Bauer R, editors. Phytomedicines of Europe: their chemistry and biological activity. ACS Symposium Series 691. Washington, D.C.: American Chemical Society; 1998. pp. 176–209. [Google Scholar]
- 16.Lawson L D. The composition and chemistry of garlic cloves and processed garlic. In: Koch H P, Lawson L D, editors. Garlic. The science and therapeutic application of Allium sativum L. and related species. Baltimore, Md: Williams & Wilkins; 1996. pp. 37–107. [Google Scholar]
- 17.Lawson L D, Hughes B G. Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med. 1992;58:345–350. doi: 10.1055/s-2006-961482. [DOI] [PubMed] [Google Scholar]
- 18.Lawson L D, Wang Z Y J, Hughes B G. Identification and HPLC quantification of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Med. 1991;57:363–370. doi: 10.1055/s-2006-960119. [DOI] [PubMed] [Google Scholar]
- 19.Lawson L D, Wood S G, Hughes B G. HPLC analysis of allicin and other thiosulfinates in garlic clove homogenates. Planta Med. 1991;57:263–270. doi: 10.1055/s-2006-960087. [DOI] [PubMed] [Google Scholar]
- 20.Mégraud F, Doermann H P. Clinical relevance of resistant strains of Helicobacter pylori: a review of current data. Gut. 1998;43(Suppl.):S61–S65. doi: 10.1136/gut.43.2008.s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomura A, Stemmerann G N, Po-Huang C, Péréz-Péréz G I, Blaser M J. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–981. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Ohta R. In vitro inhibition of the growth of Helicobacter pylori by oil-macerated garlic constituents. Antimicrob Agents Chemother. 1999;43:1811–1812. doi: 10.1128/aac.43.7.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasteur L. Memoire sur la fermentation appelle lactique. Ann Chim Phys Ser. 1858;52:404–418. [Google Scholar]
- 24.Peitz U, Menegatti M, Vaira D, Malfertheiner P. The European meeting on Helicobacter pylori: therapeutic news from Lisbon. Gut. 1998;43(Suppl):S66–S69. doi: 10.1136/gut.43.2008.s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pisani P, Parkin D M, Munoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomark Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- 26.Reuter H D, Koch H P, Lawson L D. Therapeutic effects and applications of garlic and its preparations. In: Koch H P, Lawson L D, editors. Garlic. The science and therapeutic application of Allium sativum L. and related species. Baltimore: Williams & Wilkins; 1996. pp. 135–212. [Google Scholar]
- 27.Sivam G P, Lampe J E, Ulness B, Swanzy S R, Potter J D. Helicobacter pylori—in vitro susceptibility to garlic (Allium sativum) extract. Nutr Cancer. 1997;27:118–121. doi: 10.1080/01635589709514512. [DOI] [PubMed] [Google Scholar]
- 28.Stoll A, Seebeck E. Chemical investigations of alliin, the specific principle of garlic. Adv Enzymol. 1951;11:377–400. doi: 10.1002/9780470122563.ch8. [DOI] [PubMed] [Google Scholar]
- 29.Warren J R, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 30.Yoshida H, Iwata N, Katsuzaki H, Naganawa R, Ishikawa K, Fukuda H, Fujino T, Suzuki A. Antimicrobial activity of a compound isolated from an oil-macerated garlic extract. Biosci Biotechnol Biochem. 1998;62:1014–1017. doi: 10.1271/bbb.62.1014. [DOI] [PubMed] [Google Scholar]
- 31.You W C, Blot W J, Chang Y S, Ershow A G, Yang Z T, An Q, Henderson B, Xu G W, Fraumeni J F, Wang T G. Diet and high risk of stomach cancer in Shandong, China. Cancer Res. 1988;48:3518–3523. [PubMed] [Google Scholar]