Abstract
Introduction:
IgG4-related disease (IgG4-RD) is a systemic autoimmune fibroinflammatory disease that can affect multiple organ systems. Although large-vessel vasculitis is a well-recognized manifestation of IgG4-RD, this condition is generally not regarded as a vasculitis. We aimed to describe coronary artery involvement (CAI), a vascular distribution about which little is known in IgG4-RD.
Material and Methods:
Patients with IgG4-related CAI were identified from a large, prospective IgG4-RD cohort. CAI was confirmed by imaging evidence of arterial or periarterial inflammation in any coronary artery. We extracted details regarding demographics, features of IgG4-RD, and manifestations of CAI.
Results:
Of 361 cases in the cohort, 13 (4%) patients had IgG4-related CAI. All were male and all had highly-elevated serum IgG4 concentrations, with a median value of 955 mg/dL (interquartile range [IQR]: 510–1568 mg/dL; reference: 4–86 mg/dL). Median disease duration at the time of CAI diagnosis was 11 years (IQR: 8.23–15.5 years). Extensive disease in the coronary arteries was the rule: all three major coronary arteries were involved in 11 patients (85%). The coronary artery manifestations included wall thickening or periarterial soft tissue encasement (85%), stenosis (69%), calcification (69%), and aneurysms or ectasia (62%). Five patients (38%) had myocardial infarctions, 2 (15%) required coronary artery bypass grafting, and 2 (15%) developed ischemic cardiomyopathy.
Discussion:
Coronary arteritis and periarteritis are important manifestations of IgG4-RD, which should be regarded as a variable-vessel vasculitis that is among the most diverse forms of vasculitis known. Potential complications of CAI include coronary artery aneurysms, myocardial infarction, and ischemic cardiomyopathy.
Keywords: IgG4-related disease, Vasculitis, Variable-vessel vasculitis, Coronary arteritis
1. Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a systemic immune-mediated disease characterized by inflammation, fibrosis, and mass-forming lesions that can occur in nearly any organ system. Common manifestations include enlargement of the lacrimal and major salivary glands, various forms of pulmonary disease, autoimmune pancreatitis, sclerosing cholangitis, tubulointerstitial nephritis, and retroperitoneal fibrosis. The diagnosis of IgG4-RD requires clinicians to recognize typical sites of IgG4-RD involvement and to synthesize data from the disease’s clinical, radiological, laboratory, and pathological manifestations [1,2].
Vascular inflammation is common in IgG4-RD and can contribute to substantial morbidity and even mortality. The most commonly described histopathologic manifestation of the vascular disease in IgG4-RD is obliterative phlebitis, a microscopic finding that is recognized widely as a pathologic hallmark of this condition [3]. Although obliterative phlebitis is observed frequently in organs involved by IgG4-RD, clinical features of small-vessel vasculitis—which typically present as cutaneous leukocytoclastic vasculitis—are less common but reported [4–6]. The ability of IgG4-RD to affect the aorta is also well-known [7–10].
Between these extremes—the involvement of small vessels in the form of obliterative phlebitis that is detected only microscopically and the involvement of the aorta—IgG4-RD affecting medium-sized arteries is poorly described. A medium-vessel vasculitis is defined as inflammatory vascular disease affecting and damaging the walls of blood vessels that have penetrated a viscus [11].
Coronary artery involvement (CAI), a form of medium-vessel arteritis with potentially lethal consequences in IgG4-RD, has been described in case reports and small case series [7,8,12–17]. We identified 13 patients with IgG4-RD involving the coronary arteries from our single-center cohort and report data on the patients’ demographics, disease characteristics, radiologic patterns of disease, and clinical consequences here.
2. Material and Methods
The Massachusetts General Hospital (MGH) has a longitudinal cohort of IgG4-RD patients that is among the largest in the world. Patients from this cohort with CAI were identified using three strategies: 1) review of our database containing provider-completed surveys that detail the sites of IgG4-RD involvement; 2) review of the electronic medical records; and 3) provider referral for this study. We reviewed the charts of all patients in the MGH IgG4-RD cohort who fulfilled the 2019 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) Classification Criteria IgG4-RD Classification Criteria or had probable IgG4-RD [2]. Probable IgG4-RD was defined as having typical organ involvement and meeting no exclusion criteria but not achieving the requisite 20 points to fulfill the Classification Criteria. The typical scenario in which a patient would be classified as having probable IgG4-RD is when a biopsy of affected tissue was not obtained or was non-diagnostic [18]. Patients were included in this study if they had radiologic evidence of arterial or periarterial inflammatory changes of the coronary arteries. Manifestations of CAI consistent with this case definition included arterial wall thickening or enhancement; the presence of periarterial soft tissue; and vascular beading, ectasia, or aneurysms. Patients in whom the coronary artery abnormalities were typical of atherosclerosis alone, such as calcification or stenosis in the absence of other inflammatory features, were excluded.
The electronic medical records of all patients identified as having potential CAI were reviewed. For patients whose cardiac imaging was available, a cardiac imaging specialist reviewed all relevant studies, detailed the territories of CAI, and characterized the specific radiologic abnormalities observed in each case. We recorded the patients’ complications of coronary disease, including the need for invasive therapeutic procedures and the development of myocardial infarctions or ischemic cardiomyopathy. We also reviewed the details of patients’ overall IgG4-RD, including the spectrum of organ involvement, duration of disease, and vascular involvement in territories beyond the coronary vessels. Demographics including age, sex, and self-reported race were extracted by review of the medical records. Relevant laboratory findings were recorded, including peak serum concentrations of IgG4, IgG1, absolute eosinophils, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) and trough levels of C3 and C4 complement.
Twelve patients either met the 2019 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) Classification Criteria or met histologic criteria for the disease [2]. One patient (Patient 6) had typical organ involvement compatible with IgG4-RD but did not have a biopsy of affected tissue, and thus did not reach the 20 points required to meet the Classification Criteria. This patient was included because his clinical diagnosis was IgG4-RD.
2.1. Statistical analysis
Medians, ranges, and interquartile ranges (IQR) were reported for demographic variables as appropriate. The Fisher exact test was used to compare the sexes of patients with and without CAI.
3. Results
3.1. Patient demographics
Demographics and disease characteristics for all patients are summarized in Table 1. Nine patients (69%) with IgG4-RD involving the coronary arteries were identified directly by treating providers. Four (31%) additional cases were identified by medical records review of the 361 patients in the MGH IgG4-RD registry who fulfill the 2019 ACR/EULAR Classification Criteria or have probable IgG4-RD. This yielded a prevalence of CAI of 3.6% in our cohort. All 13 patients were male; in comparison, of the 361 patients in the MGH IgG4-RD cohort who fulfill 2019 ACR/EULAR Classification Criteria or have probable IgG4-RD, 234 (65%) are male (p<0.01). Eight (62%) were white, 2 (15%) Asian, 1 (8%) Black, and 2 (15%) other races. Ages of the patients at the time of discovery of CAI ranged from 46 to 75 years, with a median age of 61 years (IQR 55–69 years).
Table 1.
Patient Characteristics.
| Demographics | |
| Male sex | 13 (100) |
| Race | |
| White | 8 (62) |
| Asian | 2 (15) |
| Black | 1 (8) |
| Other | 2 (15) |
| Age at discovery of CAI, years | 61 (55–69) |
| Disease duration at time of CAI discovery, years | 11 (8.23–15.5)* |
| Disease characteristics | |
| IgG4-RD Classification Criteria points, mean (SD) | 39 (21) |
| Number of organs involved, mean (SD) | 4.8 (1.9) |
| Non-coronary large vessel involvement | 9 (69) |
| Atopy present | 8 (62) |
| Tissue diagnosis | 10 (77) |
| Laboratory values | |
| Peak IgG4 (ref: 4–86 mg/dL) | 955 (510–1568) |
| Peak IgG1 (ref: 382.4–928.6 mg/dL) | 868 (634–1280) |
| Peak IgE (ref: 0–100 IU/mL) | 638 (208–2277) |
| Eosinophilia present | 7 (54) |
| C3 or C4 hypocomplementemia present | 3 (23) |
| Erythrocyte sedimentation rate elevated | 11 (85) |
| C-reactive protein elevated | 9 (69) |
| Coronary arteries involved | |
| Left main coronary artery | 8 (62) |
| Left anterior descending artery | 13 (100) |
| Left circumflex artery | 12 (92) |
| Right coronary artery | 12 (92) |
| Branch vessels | 7 (54) |
| Coronary artery abnormalities | |
| Aneurysm/ectasia | 8 (62) |
| Wall thickening or periarterial soft tissue encasement | 11 (85) |
| Stenosis | 9 (69) |
| Calcification | 9 (69) |
| Complications of CAI | |
| Myocardial infarction | 5 (38) |
| Mural thrombus | 5 (38) |
| Ischemic cardiomyopathy | 2 (15) |
| Revascularization procedures | |
| Percutaneous coronary intervention | 2 (15) |
| Coronary artery bypass graft | 2 (15) |
Values reported as median (IQR) for continuous variables and n (%) for categorical variables unless otherwise noted. CAI: coronary artery involvement; IgG4-RD: IgG4-related disease.
Disease duration could not be determined in 3 patients.
3.2. IgG4-RD characteristics
The patients’ median number of ACR/EULAR Classification Criteria points was 39 (range: 11–78, IQR 25–45). Ten patients (77%) had biopsies that demonstrated histology and immunostaining suggestive of a diagnosis of IgG4-RD [3]. The tissue or organ biopsied was usually not the coronary arteries or large blood vessels. Rather, the organs biopsied were the submandibular glands (n=1), lacrimal glands (n=3), kidneys (n=4), lungs (n=1), common bile duct (n=1), or lymph nodes (n=1). Vascular tissue from large blood vessels was available in 2 patients and suggestive of IgG4-related vascular disease in 1 (Patient 11).
3.3. Laboratory features
Results of laboratory markers of IgG4-RD activity and features of organ involvement are summarized in Table 1. Laboratory markers of interest were available for all patients, with the exception C3 and C4 complement in 1 patient. All patients had striking serum IgG4 concentrations, with a median value of 955 mg/dL (IQR 510–1568 mg/dL; reference: 4–86 mg/dL). The lowest peak serum IgG4 concentration value in this cohort was 277.9 mg/dL, more than three times the upper limit of the reference range. Six patients (46%) had elevated IgG1 concentrations (median 867.8 mg/dL, IQR 634–1280 mg/dL; reference: 382–929 mg/dL) and 10 (77%) had elevated serum IgE concentrations. Eosinophilia (>500 cells/mL) was observed in 7 patients (54%). Of the 12 patients in whom C3 and C4 complement were checked, C3 hypocomplementemia was observed in 3 patients (23%) and C4 hypocomplementemia in only 1 patient (8%).
Elevations in ESR and CRP were observed in 11 (85%) and 9 (69%) patients, respectively. As is commonly the case in IgG4-RD, ESR was frequently elevated out of proportion to CRP: elevations beyond 3 times the upper limit of the reference range were observed in 7/11 (64%) of patients with elevated ESR, compared with 2/9 (22%) of those with elevated CRP [19,20].
3.4. Organ involvement beyond the coronary arteries
All patients had multisystem IgG4-RD. The most common extravascular organs involved were the lacrimal and major salivary glands (10, 77%); lymph nodes (7, 54%); lungs, airways, and pleura (7, 54%); pancreas (7, 54%); kidneys (6, 46%); and retroperitoneum (3, 23%). In addition, 8 (62%) had histories of atopy.
IgG4-related vascular disease beyond the coronary arteries was also common.
Nine patients (69%) had the simultaneous involvement of large blood vessels such as the aorta, pulmonary arteries, and iliac arteries.
3.5. Diagnosis of coronary artery involvement
Seven patients (54%) had their IgG4-related CAI detected through imaging performed for the evaluation of other symptoms attributed to IgG4-RD or to exclude chest disease in patients known to have IgG4-RD affecting other regions: 5 of these were detected via standard chest CT study, 1 via chest CTA, and 1 via coronary CTA. Four other patients (31%) had symptoms attributable to potential CAI (e.g., angina, syncope, heart failure), which was confirmed on further evaluation. Finally, 2 patients (15%) were found to have IgG4-RD as a result of incidental imaging findings of CAI. One of these underwent a coronary artery calcium score as part of routine cardiac risk assessment. The radiologic abnormalities detected on the coronary calcium score study—periarteritis—were then confirmed by a coronary computed tomography angiography (CTA). Further assessment then led to the diagnosis of IgG4-RD. The other patient with incidental discovery of IgG4-related CAI through a cardiac evaluation for other indications had a cardiac CTA as part of the pre-operative assessment for aortic valvuloplasty. In that patient, the diagnosis of IgG4-RD stemmed directly from further evaluation of the coronary artery abnormalities.
3.6. Duration of disease at CAI diagnosis
There was wide variation in the patients’ duration of known IgG4-RD at the time their CAI was recognized. The duration of IgG4-RD could not be determined in 3 patients (23%) because the coronary abnormalities were discovered simultaneously with other IgG4-RD features. In those patients, the symptomatology—both of IgG4-RD and of CAI—was sufficiently low that it was not possible to gauge when the underlying disease had begun. The remaining 10 patients had durations of known IgG4-RD prior to the recognition of coronary involvement that ranged from 1 to 17 years (median 11 years, IQR 8.23–15.5 years).
3.7. Imaging Studies
Both cardiac CTA and cardiac magnetic resonance imaging (CMR) demonstrated IgG4-related CAI in every case in which they were obtained (9/9 cases and 2/2 cases, respectively). Other imaging studies through which CAI was diagnosed included standard CT studies or CTA of the chest. These studies, performed in a total of 7 cases, identified CAI in 5 cases. Two of the 4 positron electron tomography CT (PET CT) studies obtained detected CAI (2/4). Transthoracic echocardiography (TTE) and magnetic resonance angiography (MRA) of the chest performed to evaluate large vessel involvement did not identify coronary lesions in any cases. Left heart catheterization was performed in 8 cases. Although this modality identified aneurysmal disease in 6 cases, catheterization was unable to demonstrate periarterial soft tissue or to differentiate between IgG4-RD and atherosclerosis as the cause of luminal irregularities or stenoses.
3.8. Patterns of coronary involvement
Various radiologic manifestations of CAI were observed in our cohort, including aneurysms, ectasia, stenosis, periarterial soft tissue proliferation, wall thickening, and beading (Figure 1). CAI in IgG4-RD followed three major patterns: arterial wall thickening or periarterial soft tissue encasement (11, 85%); stenosis (9, 69%); and aneurysmal dilatation or ectasia (8, 62%) (Figure 2). These patterns often overlapped. For example, 3 patients each had wall thickening/soft tissue and stenosis; aneurysm and wall thickening/soft tissue; or wall thickening/soft tissue, stenosis, and aneurysm. Calcification of coronary arterial walls was observed in 9 patients (69%). PET CT scans demonstrated fluorine-18 fluorodeoxyglucose (FDG) avidity in the coronary arteries during active disease that improved during remission (Figure 3).
Figure 1.
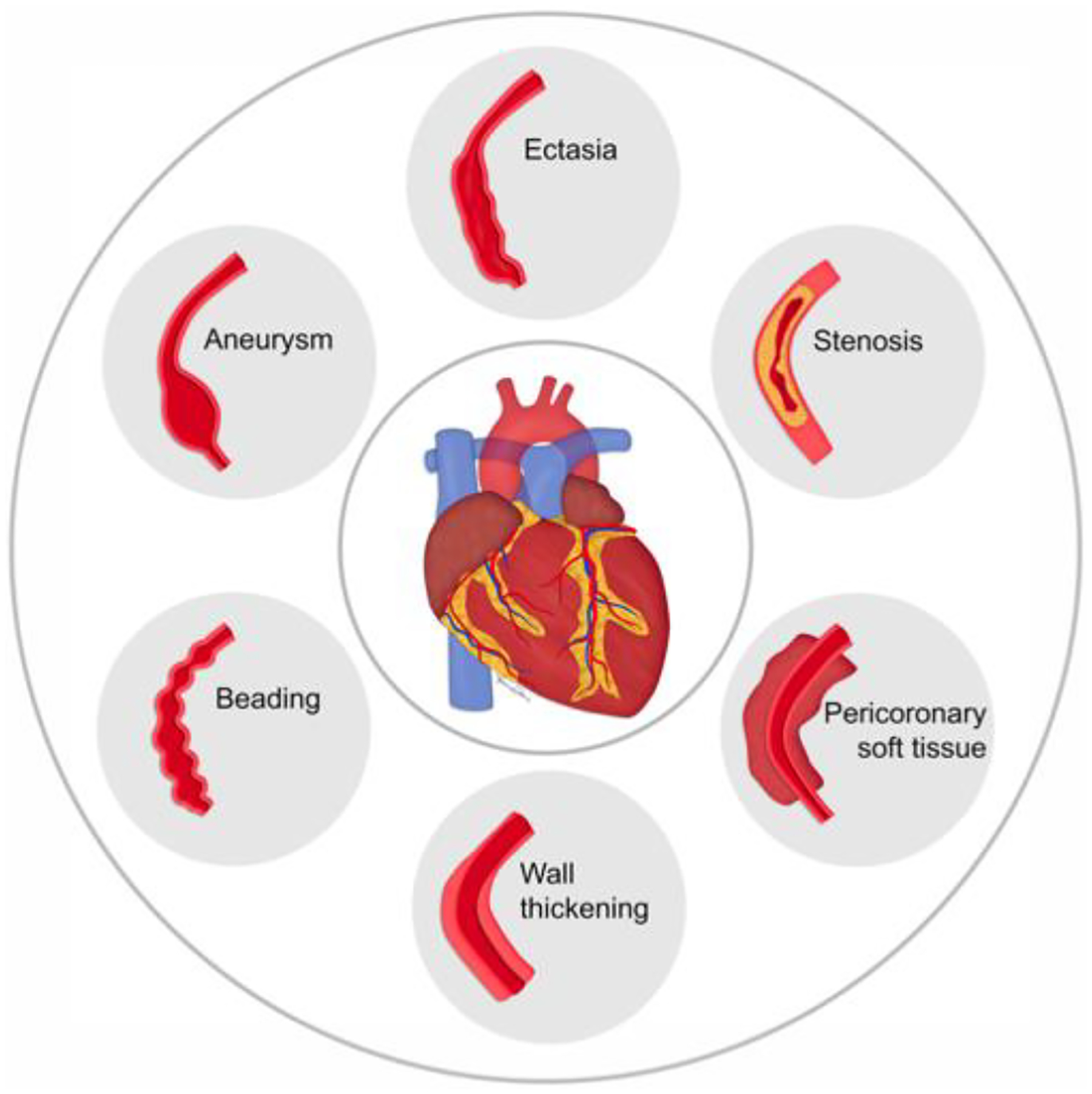
Spectrum of coronary artery involvement in IgG4-related disease.
Figure 2.
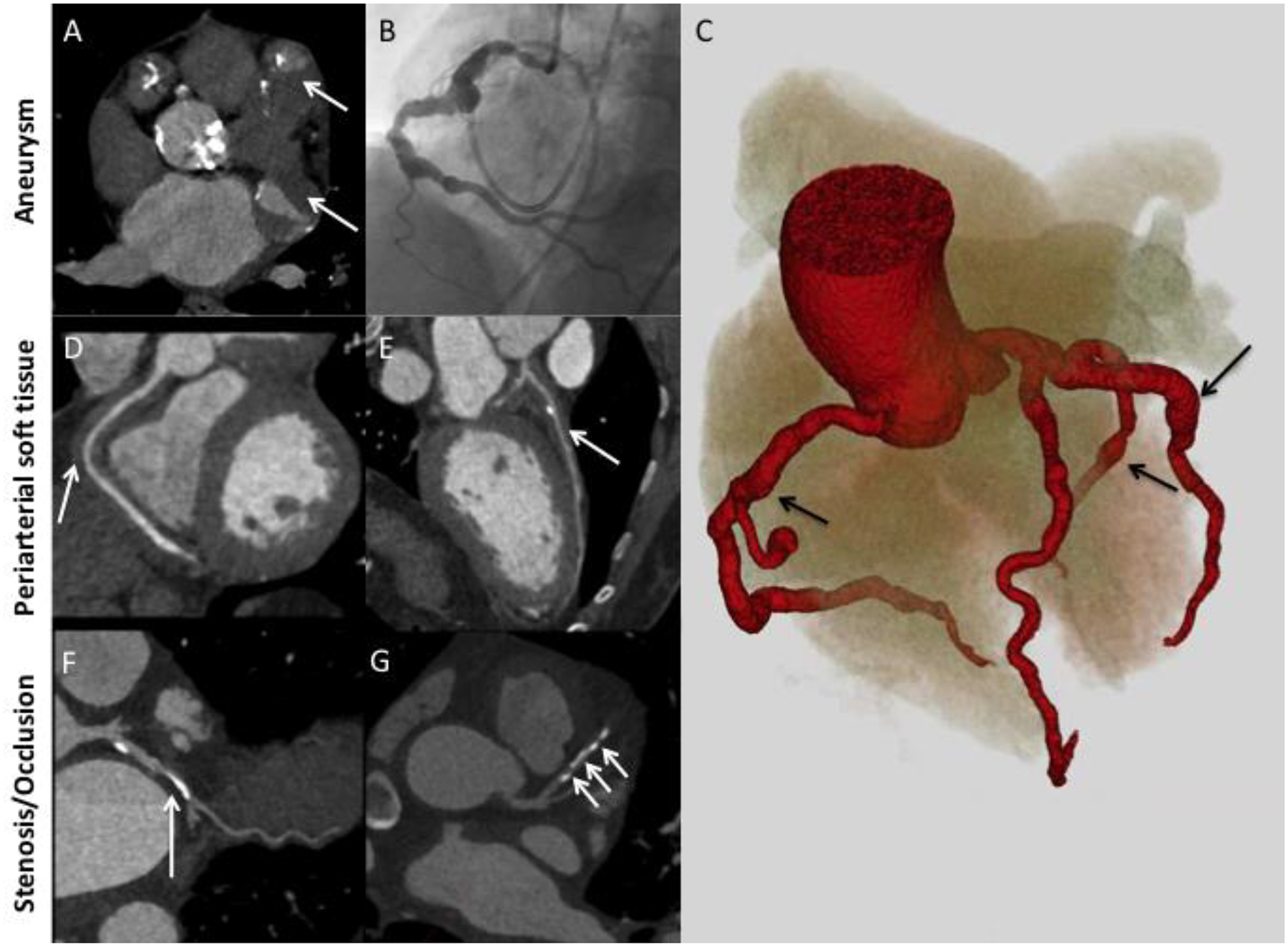
Major patterns of coronary artery involvement in IgG4-related disease. Images include axial CT (A), left heart catheterization (B), 3D volume-gated reconstruction of CTA (C), and curved planar reformatting of coronary CTA (D-G). Findings include fusiform aneurysmal dilatation (A-C) with mural thrombi (A, arrows), ectasia (C) periarterial soft tissue (D, E; arrows), and stenosis (F, G; arrows). Panel C demonstrates ectasia of the entire coronary tree with focal fusiform aneurysmal dilatations (arrows).
Figure 3.
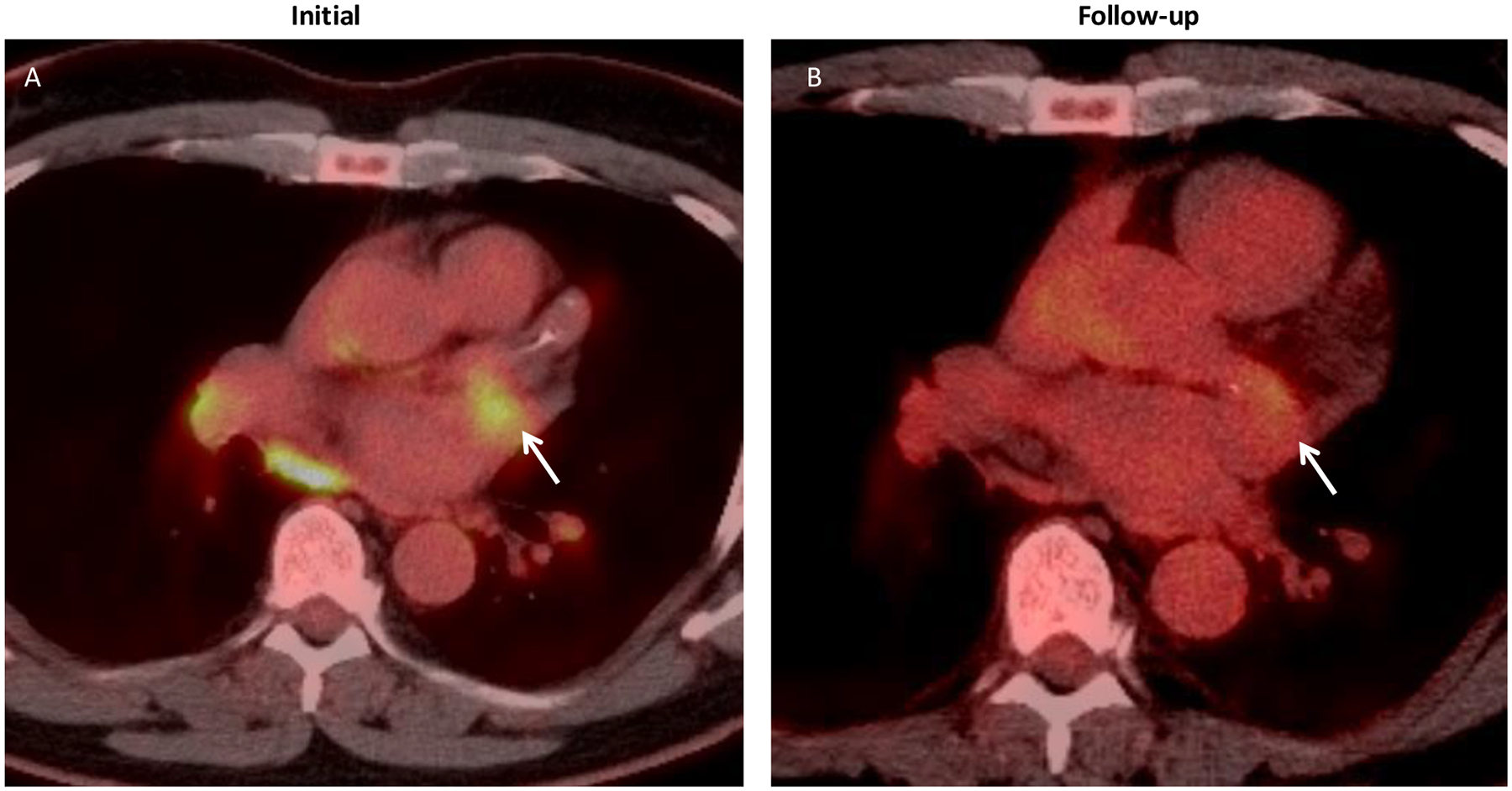
Left circumflex involvement in IgG4-related disease. A) Axial PET CT showing FDG avidity (arrow) centered around the left circumflex coronary during clinically active disease. B) Follow-up PET CT during clinical remission showing interval decrease in FDG uptake (arrow).
Coronary artery dilatation in these patients could present as either fusiform aneurysms (i.e., those that are longer than they are wide; Figure 2B) or ectasia (i.e., a diffuse, long-segment dilatation; Figure 2C) [21]. Many of the coronary artery aneurysms observed in these patients were large, with the maximal diameter in each patient ranging from 9 mm to 5 cm. Four patients had coronary arterial lesions measuring 2 cm or larger.
Differentiating between coronary arteritis (i.e., arterial wall thickening and enhancement) and periarteritis (i.e., periarterial soft tissue) was not possible in every case. This is partly because not all of the types of imaging employed had resolution sufficient to make this distinction. Periarteritis was frequently described as a confluent rind of soft tissue encasing the coronary arteries. It is important to note that none of the patients with this periarteritis also had accompanying pericardial inflammation. Thus, the periarterial inflammation that occurs in IgG4-RD (and is so distinctive of this condition) was not secondary to pericarditis. In some patients, imaging findings consistent with both arteritis and periarteritis were observed concurrently (Figure 4).
Figure 4.
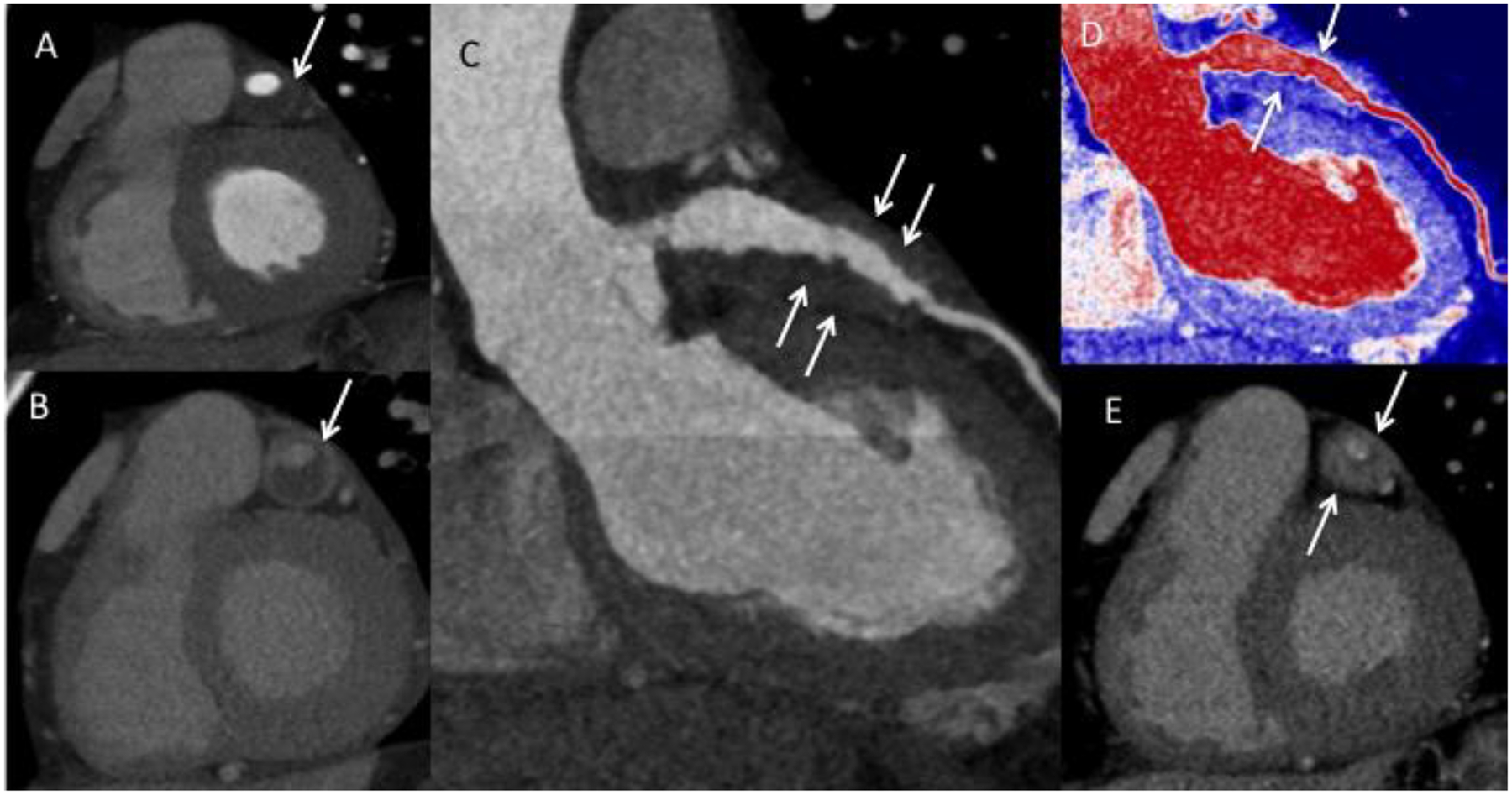
Arteritis and periarteritis. A) Coronary computed tomography (CTA) in the short-axis view demonstrating the left anterior descending artery (LAD) aneurysm (arrow). B) Delayed contrast-enhanced CT showing enhancement of the aneurysmal LAD wall consistent with arteritis (arrow). C) LAD curved planar reformatted (CPR) and D) Pseudo colorized image of the LAD CPR image showing the soft tissue thickening around the LAD (arrows). E) Delayed phase image in short axis showing enhancement of the soft tissue thickening consistent with periarteritis (arrows).
3.9. Distribution of coronary involvement
Eleven of the patients (85%) had involvement of all three major coronary arteries: the left anterior descending artery (LAD), the left circumflex artery (LCX), and the right coronary artery (RCA). The remaining 2 patients (15%) had only two of these arteries involved. The left main coronary artery was involved in 8 patients (62%), and 7 (54%) had involvement of smaller branches such as the ramus intermedius, the posterior descending artery, diagonal branches, and marginal branches. Involvement could be observed in the proximal, middle, or distal arterial segments. Although most patients had diffuse involvement throughout affected vessels, focal disease involving small segments of arteries was also observed (Figure 5B–C).
Figure 5.
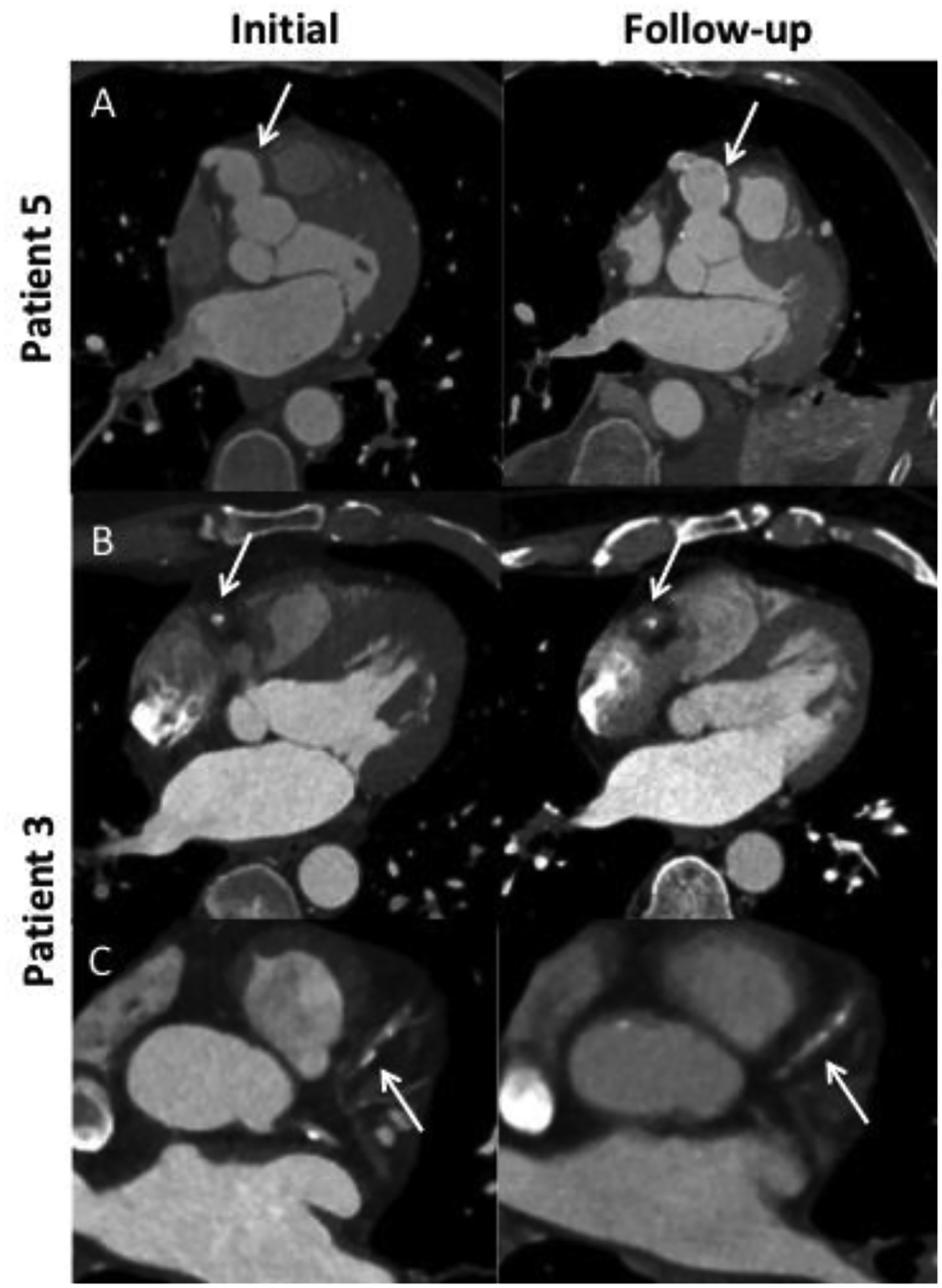
Evolution of IgG4-related coronary arteritis over time. A) Saccular right coronary artery (RCA) aneurysm with initial wall thickening which in follow-up is replaced by arterial wall calcification (arrows). B) Interval progression of focal RCA periarterial soft tissue during disease flare. C) New development of focal periarterial soft tissue surrounding the left anterior descending artery during disease flare.
3.10. Complications of coronary arteritis or periarteritis
Multiple cardiac manifestations and complications attributable to CAI of IgG4-RD were observed in this cohort. Five patients (38%) had mural thrombi within aneurysmal or ectatic vessels. Myocardial infarctions occurred in 5 patients (38%), including 1 clinically silent event in a patient who was entirely asymptomatic. Coronary revascularization procedures were performed for symptomatic, ischemic disease in 4 patients (31%). These included balloon angioplasty, percutaneous stent placement, and coronary artery bypass grafting (CABG). CABG was performed in 2 patients (15%). Ischemic cardiomyopathy developed in 2 patients (15%). The only death in this cohort during follow-up was in a patient who died from metastatic cancer years after his IgG4-RD diagnosis.
3.11. Evolution of coronary involvement over time
All patients were treated with B-cell depleting agents or systemic glucocorticoids. Available data on the response to various treatments are limited, as dedicated coronary artery imaging was not consistently obtained soon enough after treatment to assess response. In addition, long-term follow-up was not available for all patients. The follow-up studies available on multiple patients, however, documented the evolution of disease in an indolent but worrisome manner.
In Patient 5 (Figure 5A), periarterial soft tissue surrounding an aneurysmal portion of the RCA resolved after treatment, which led to a clinical remission. The involved portion of the artery became calcified. Patient 3 (Figure 5B–C) had an initial CT of the chest that demonstrated a focal RCA periarteritis. After monotherapy with a glucocorticoid taper, the patient had a disease flare 6 months after the initial scan, and a follow-up scan at that time demonstrated progression of the RCA lesion and new soft tissue surrounding the LAD. In patient 7, a left heart catheterization initially showed luminal irregularities but no aneurysms or ectasia; he had been treated with rituximab for active IgG4-RD one month prior. Two years later, while off treatment, he had a disease flare, and a coronary CTA revealed new aneurysms, ectasia, and wall thickening. Patient 13 suffered a myocardial infarction 21 years prior to being diagnosed as having IgG4-RD. He was found to have multivessel coronary artery stenoses at the time of his myocardial infarction and underwent a 4-vessel coronary artery bypass graft. As part of the workup of IgG4-RD, he had a coronary CTA that showed periarteritis of the native RCA.
3.12. Vascular Pathology
Vascular histology was available in two patients. Patient 5 had aortic wall tissue available for review from an aortic aneurysm repair that was performed after the initiation of treatment for IgG4-RD, but that tissue did not demonstrate the histologic features of IgG4-RD. Patient 11 had a resection of a right carotid artery aneurysm, the histopathology of which is shown in Figure 6. The carotid artery wall contained a dense IgG4-positive lymphoplasmacytic infiltrate. The patient had been diagnosed with IgG4-RD by a lacrimal gland biopsy in 2012 but had elected at that time not to begin treatment [22]. Seven years after his IgG4-RD diagnosis, he was recognized to have a right carotid artery aneurysm. CAI as well as tubulointerstitial nephritis accompanied by a left renal mass were diagnosed two years after that—9 years after the original IgG4-RD diagnosis and 14 years after the onset of IgG4-RD symptoms.
Figure 6.
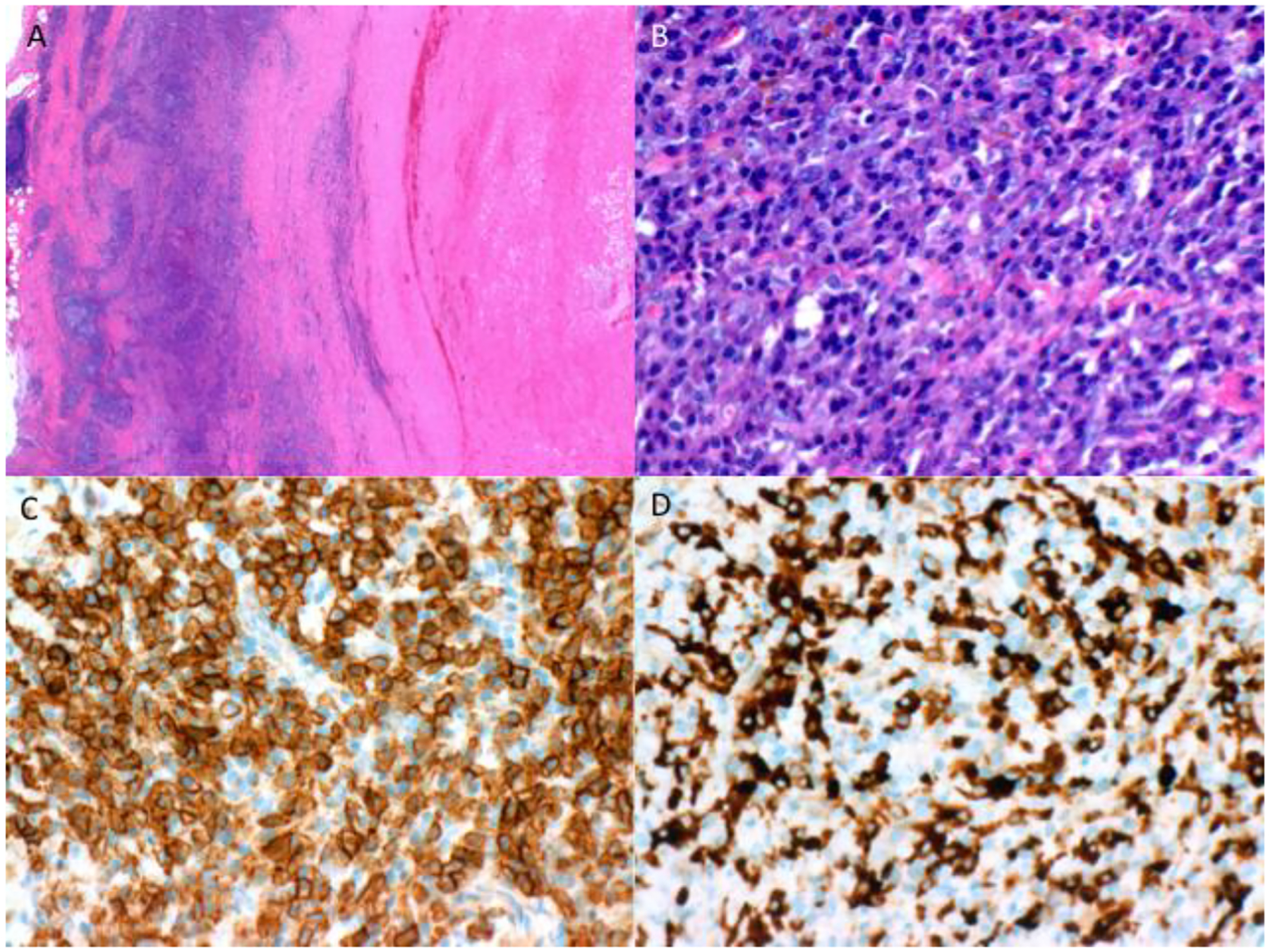
Histopathology. Carotid artery biopsy at A) 20x magnification and B) 400x magnification showing dense lymphoplasmacytic infiltrate with >200 IgG4+ cells/high-powered field. C) CD138 stain and D) IgG4 stain demonstrate that 56% of CD138+ cells are IgG4+.
4. Discussion
Beginning decades before the diagnosis of IgG4-RD was even recognized as a distinct condition, the vasculitides have long been classified according to whether they affect primarily the large-, medium-, or small-sized blood vessels [23]. Although IgG4-RD was in the mentioned in the Revised Chapel Hill Consensus Conference Nomenclature of Vasculitides for its ability to affect the aorta, this condition is seldom regarded as a primary form of vasculitis [11]. Data from this series of patients with IgG4-RD affecting the coronary arteries, however, suggest that IgG4-RD is in fact among the most versatile forms of vasculitis known. Not only does IgG4-RD affect the aorta in a subset of patients, it also affects small-vessels in the form of obliterative phlebitis across its full spectrum of organ involvement [3,7–10,15]. This series of CAI illustrates that IgG4-RD also affects medium-sized arteries in a substantial subset of patients, and that IgG4-RD often involves small-, medium-, and large-sized vessels in the same patient. These findings suggest that IgG4-RD should be regarded as a “variable-vessel” vasculitis. The two types of systemic vasculitis included in the variable-vessel vasculitis category in the Revised Chapel Hill Consensus Conference Nomenclature are Behcet disease and Cogan syndrome [11]. Neither Behçet disease nor Cogan syndrome involves the coronary arteries in the manner of IgG4-RD.
IgG4-RD affects the coronaries in a highly distinctive manner. Giant cell arteritis, Takayasu arteritis, polyarteritis nodosa, Kawasaki disease, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, Behçet disease, and the vasculitis associated with systemic lupus erythematosus are all known to cause coronary arteritis in a small percentage of cases in each disease [24–26]. Our cases demonstrate that unlike these other causes of coronary arteritis, which preferentially affect the proximal coronary arteries, IgG4-RD is capable of affecting smaller branches of the coronary vessels, as well [24,25,27]. The form of coronary arteritis that most closely resembles IgG4-related CAI, in fact, is Kawasaki disease, a pediatric disease of young children that seldom afflicts patients above the age of 5 years [28].
Coronary artery aneurysms are a common complication in patients with IgG4-related CAI. Moreover, the size of the resulting aneurysms is remarkable, with the maximal diameter of aneurysms in each patient from our cohort ranging from 9 mm to 5 cm. The aneurysms themselves varied widely in appearance in our patients: both focal fusiform aneurysms and diffuse vascular ectasia were observed [21]. Before the recognition of IgG4-RD as a unique, discrete disease entity, the identification of such aneurysms in adult patients would have led to speculation that the patient had had Kawasaki disease as a child. No other type of vasculitis that affects adults shows this type of expression.
The demographic features of the 13 patients in this cohort are remarkable. First, all 13 were male, offering a striking contrast to the demographics of our overall IgG4-RD cohort. Among the 285 patients in the IgG4-RD cohort who fulfill the ACR/EULAR Classification Criteria for IgG4-RD, only 195 (68%) of the patients are male. This finding is consistent with reports of IgG4-RD involvement of certain other organs. A male predilection has been reported in the kidneys, retroperitoneum, pancreas, and—most pertinently, perhaps—the large blood vessels [7,15,29–33]. Although cases of CAI have also been described in women [15,16], this occurrence appears to be substantially more common among male patients.
It is possible that this perceived difference between the sexes in the occurrence of CAI simply reflects the modest tendency of the disease to occur more commonly among men than women [20,34]. However, the degree of sex discrepancy in these patients may also reflect a biological tendency for this particular manifestation to affect males, as is the case for other vascular involvement in IgG4-RD [7,15]. It is also possible that, as is true for atherosclerotic coronary artery disease (CAD), IgG4-related CAI in females is recognized less commonly than in men because of the decreased likelihood that women will be evaluated for CAD [35]. Some of the patients in our series had their CAI detected in the context of screening for atherosclerosis—screening to which many female patients may not be sent.
Consistent with findings by Akiyama et al., our series demonstrates that coronary arteritis and periarteritis in IgG4-RD may present as luminal stenosis, aneurysmal dilatation, and arterial wall thickening and enhancement (Figure 1). The presence of periarterial soft tissue (i.e., periarteritis) may also be a clue to the presence of this condition. Moreover, these data indicate that IgG4-related CAI does not occur in isolation: every patient in this series had multisystem involvement of IgG4-RD. Patients were also highly serologically active, as demonstrated by their extremely high serum IgG4 concentrations. This finding typically sorts with disease affecting multiple organs simultaneously. Many patients also had elevated serum IgG1 and IgE concentrations. Frequent extravascular sites of involvement included the lacrimal and major salivary glands, lungs, kidneys, and pancreas, and atopy was also common in this cohort. Zhang and Stone have previously proposed two subtypes of IgG4-RD: “Proliferative,” characterized by multiorgan involvement, serologic activity, and involvement of organs with more inflammatory features (e.g., glands, lungs, kidneys, pancreas, and blood vessels); and “Fibrotic,” characterized by less extensive organ involvement, lower serologic activity, and involvement of organs characterized by prominent fibrosis (e.g., retroperitoneal fibrosis, pachymeningitis, Riedel thyroiditis, fibrosing mediastinitis, sclerosing mesenteritis)[36]. This series therefore demonstrates that coronary arteritis and periarteritis are more commonly a manifestation of the proliferative, rather than fibrotic, clinical subtype of IgG4-RD [36]. Patients in this disease subset have a high likelihood of achieving good treatment responses, underscoring the importance of diagnosing the complication of IgG4-related CAI before the occurrence of myocardial injury.
Another distinguishing feature of the CAI in IgG4-RD is the finding of periarterial soft tissue, which was shown to encase the coronary arteries in several of our patients. This type of periarteritis is highly atypical of any of the other systemic vasculitides known to affect the coronary arteries, including Kawasaki disease. In contrast, such soft tissue proliferation is known to occur in other blood vessels and organs affected by IgG4-RD. In IgG4-related retroperitoneal fibrosis, for example, the disease typically has a peri-aortic distribution, often leading to the designation of “chronic peri-aortitis” [15]. In our series, the finding of periarterial soft tissue surrounding the coronary arteries was frequently associated with other features such as aneurysm, stenosis, or wall thickening and enhancement.
The median duration of disease at the time of recognition of CAI in these patients was 11 years, emphasizing the indolent nature of IgG4-RD and the long periods of time over which its complications can arise. Despite the slow progression and the asymptomatic presentations of many patients in our cohort, serious cardiac complications ensued in many patients, exemplified by the myocardial infarctions, ischemic cardiomyopathy, and need for invasive procedures for cardiac revascularization. Moreover, the CAI of IgG4-RD is not always slow in onset: new areas of periarteritis were observed to occur in as little as 6 months in our series, and new aneurysms formed in as little as 2 years. Therefore, once IgG4-RD affecting the coronary arteries is identified, prompt and aggressive treatment is indicated with the intention of suppressing disease completely and preventing irreversible changes to the vasculature. Arteries affected by IgG4-RD are at risk for the development of giant aneurysms, arterial dissection, rupture, extrinsic compression of surrounding structures, and mural thrombosis with distal embolization[27,37,38]. Given the typical phenotype identified for such patients to date—male patients with longstanding, multi-organ disease and highly-elevated serum IgG4 concentrations—clinicians evaluating such patients should have a low threshold for screening them with coronary CTA studies [27].
A notable finding from our study is that, similar to other vasculitides, the vessel walls of affected vessels may become calcified over time [39]. This is of particular importance because calcification is frequently seen in atherosclerotic disease, and the demographics of patients who develop coronary arteritis or periarteritis from IgG4-RD overlap with those who develop atherosclerotic CAD. Both clinicians and radiologists must recognize that the presence or absence of calcification alone cannot distinguish between atherosclerotic and IgG4-related CAI. Rather, dedicated coronary imaging should be obtained with the goal of identifying other features that help distinguish between the two.
There are several limitations of this study. This was a retrospective review, and there was no standardized screening or monitoring protocol for the evaluation of coronary involvement. Inevitably, therefore, there were some missing data. In addition, although more than 300 charts were reviewed for indications of CAI, most of the patients in our IgG4-RD registry have not undergone dedicated coronary imaging. Furthermore, among the patients who did have imaging evidence of coronary artery calcification or stenosis, not all patients had more comprehensive evaluations for the express purpose of excluding IgG4-related CAI. It is likely, therefore, that our estimate of the prevalence of CAI in this cohort is low. Finally, because each patient in the series was treated and monitored differently, no inferences can be made regarding the effect of treatment on coronary arteritis or periarteritis.
Our study also has significant strengths. The patient cohort from which these patients with IgG4-related CAI were derived represents one of the world’s largest and most carefully studied cohorts. We were therefore able to provide a level of detail about the nature and radiologic appearance of CAI in IgG4-RD that is unique.
Our results have important implications for clinicians and for future studies. First, clinicians should be aware of this manifestation of IgG4-RD and include IgG4-RD in the differential diagnosis of coronary aneurysms, arteritis, and periarteritis. Clinicians should also consider screening for CAI in patients with known IgG4-RD, particularly those who fit a patient profile similar to that of the subjects in this study. Further research should evaluate patients with IgG4-RD involving the coronary arteries prospectively in order to understand the optimal approach to screening, treatment, and monitoring of coronary arteritis and periarteritis in this disease.
Funding:
This work was supported by the National Institutes of Health (grants T32 AR007258, K08 AR079615, UM1 AI144295) and the National Scleroderma Foundation (New Investigator Award. The authors have no relevant financial conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Katz G, Stone JH. Clinical Perspectives on IgG4-Related Disease and Its Classification. Annu Rev Med 2022;73:545–62. 10.1146/annurev-med-050219-034449. [DOI] [PubMed] [Google Scholar]
- [2].Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire J-F, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis 2020;79:77–87. 10.1136/annrheumdis-2019-216561. [DOI] [PubMed] [Google Scholar]
- [3].Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–92. 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- [4].Sato M, Honda A, Yamamoto T. Hypocomplementemic cutaneous small vessel vasculitis in a patient with IgG4-related disease. Dermatol Online J 2020;26:13030/qt4dg0x6rk. [PubMed] [Google Scholar]
- [5].Nakagawa S, Nakamura Y, Yasui S, Yokosuka O, Matsue H. A case of leucocytoclastic vasculitis as a complication of IgG4-related skin disease. Clin Exp Dermatol 2017;42:235–6. 10.1111/ced.13020. [DOI] [PubMed] [Google Scholar]
- [6].Katz G, Harvey L, Stone JH. Granulomatous uveitis secondary to IgG4-related disease. Rheumatol Adv Pract 2021;5:rkab084. 10.1093/rap/rkab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Perugino CA, Wallace ZS, Meyersohn N, Oliveira G, Stone JR, Stone JH. Large vessel involvement by IgG4-related disease. Medicine (Baltimore) 2016;95:e3344. 10.1097/MD.0000000000003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Urabe Y, Fujii T, Kurushima S, Tsujiyama S, Kihara Y. Pigs-in-a-blanket coronary arteries: a case of immunoglobulin G4-related coronary periarteritis assessed by computed tomography coronary angiography, intravascular ultrasound, and positron emission tomography. Circ Cardiovasc Imaging 2012;5:685–7. 10.1161/CIRCIMAGING.112.975946. [DOI] [PubMed] [Google Scholar]
- [9].Kasashima S, Kawashima A, Kasashima F, Endo M, Matsumoto Y, Kawakami K, et al. Immunoglobulin G4-related periaortitis complicated by aortic rupture and aortoduodenal fistula after endovascular AAA repair. J Endovasc Ther 2014;21:589–97. 10.1583/14-4670R.1. [DOI] [PubMed] [Google Scholar]
- [10].Hasegawa M, Sakurai Y, Nakata S, Horiuchi K, Komoda S, Mizutani S, et al. A Case of Ruptured Immunoglobulin G4-Related Periaortitis. Ann Vasc Dis 2019;12:545–7. 10.3400/avd.cr.19-00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- [12].Ansari-Gilani K, Gilkeson RC. Multimodality imaging of IgG4 related coronary artery aneurysm. Echocardiography 2020;37:979–81. 10.1111/echo.14746. [DOI] [PubMed] [Google Scholar]
- [13].Matsuyama S, Kishigami T, Sakamoto M. A case of giant right coronary artery aneurysm due to IgG4-related disease. Gen Thorac Cardiovasc Surg 2020;68:1453–6. 10.1007/s11748-019-01272-7. [DOI] [PubMed] [Google Scholar]
- [14].Ruggio A, Iaconelli A, Panaioli E, Bernardini F, Tinelli G, Savino G, et al. Coronary Artery Aneurysms Presenting as Acute Coronary Syndrome: An Unusual Case of IgG4-Related Disease Vascular Involvement. Can J Cardiol 2018;34:1088.e7–1088.e10. 10.1016/j.cjca.2018.04.026. [DOI] [PubMed] [Google Scholar]
- [15].Akiyama M, Kaneko Y, Takeuchi T. Characteristics and prognosis of IgG4-related periaortitis/periarteritis: A systematic literature review. Autoimmunity Reviews 2019;18:102354. 10.1016/j.autrev.2019.102354. [DOI] [PubMed] [Google Scholar]
- [16].Yardimci GK, Ardali Duzgun S, Bolek EC, Kilic L, Canpolat U, Hazirolan T, et al. Coronary (peri)-arteritis in patients with IgG4-related disease: A case series from the Central Anatolia Region of Turkey. Int J Rheum Dis 2021;24:1004–13. 10.1111/1756-185X.14153. [DOI] [PubMed] [Google Scholar]
- [17].Liu X, Zhao Y, Wu N, Zhang W. Occult myocardial infarction due to an unusual cause: a case report of periarteritis involving the left coronary artery. Eur Heart J Case Rep 2022;6:ytac182. 10.1093/ehjcr/ytac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14–8. 10.1136/annrheumdis-2013-204907. [DOI] [PubMed] [Google Scholar]
- [19].Tang J, Cai S, Ye C, Dong L. Biomarkers in IgG4-related disease: A systematic review. Semin Arthritis Rheum 2020;50:354–9. 10.1016/j.semarthrit.2019.06.018. [DOI] [PubMed] [Google Scholar]
- [20].Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, et al. IgG4-Related Disease: Clinical and Laboratory Features in One Hundred Twenty-Five Patients. Arthritis Rheumatol 2015;67:2466–75. 10.1002/art.39205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kawsara A, Núñez Gil IJ, Alqahtani F, Moreland J, Rihal CS, Alkhouli M. Management of Coronary Artery Aneurysms. JACC: Cardiovascular Interventions 2018;11:1211–23. 10.1016/j.jcin.2018.02.041. [DOI] [PubMed] [Google Scholar]
- [22].Ricketti AJ, Cleri DJ, Moser RL, Bilyk JR, Vernaleo JR, Unkle DW. A 44-year-old man with bilateral eyelid swelling. Allergy Asthma Proc 2012;33:205–11. 10.2500/aap.2012.33.3523. [DOI] [PubMed] [Google Scholar]
- [23].Zeek PM. Periarteritis nodosa; a critical review. Am J Clin Pathol 1952;22:777–90. 10.1093/ajcp/22.8.777. [DOI] [PubMed] [Google Scholar]
- [24].Miloslavsky E, Unizony S. The heart in vasculitis. Rheum Dis Clin North Am 2014;40:11–26. 10.1016/j.rdc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- [25].Pazzola G, Pipitone N, Salvarani C. Cardiac involvement in the adult primary vasculitides. Expert Rev Clin Immunol 2020;16:985–91. 10.1080/1744666X.2021.1823219. [DOI] [PubMed] [Google Scholar]
- [26].Drenkard C, Villa AR, Reyes E, Abello M, Alarcón-Segovia D. Vasculitis in systemic lupus erythematosus. Lupus 1997;6:235–42. 10.1177/096120339700600304. [DOI] [PubMed] [Google Scholar]
- [27].Pham V, de Hemptinne Q, Grinda J-M, Duboc D, Varenne O, Picard F. Giant coronary aneurysms, from diagnosis to treatment: A literature review. Arch Cardiovasc Dis 2020;113:59–69. 10.1016/j.acvd.2019.10.008. [DOI] [PubMed] [Google Scholar]
- [28].Sundel RP. Kawasaki disease. Rheum Dis Clin North Am 2015;41:63–73, viii. 10.1016/j.rdc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- [29].Zeng Q, Gao J, Zhang X, Liu A, Wang Z, Wang Z, et al. Disparities between IgG4-related kidney disease and extrarenal IgG4-related disease in a case-control study based on 450 patients. Sci Rep 2021;11:10397. 10.1038/s41598-021-89844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu Y, Zhu L, Wang Z, Zeng Q, Yang F, Gao J, et al. Clinical features of IgG4-related retroperitoneal fibrosis among 407 patients with IgG4-related disease: a retrospective study. Rheumatology (Oxford) 2021;60:767–72. 10.1093/rheumatology/keaa411. [DOI] [PubMed] [Google Scholar]
- [31].Martín-Nares E, Hernandez-Molina G, Rodríguez-Ramírez S, Rivera-Fuentes L, Niño-Cruz JA, Núñez-Abreu A, et al. IgG4-related kidney disease: experience from a Mexican cohort. Clin Rheumatol 2020;39:3401–8. 10.1007/s10067-020-05135-y. [DOI] [PubMed] [Google Scholar]
- [32].Naitoh I, Kamisawa T, Tanaka A, Nakazawa T, Kubota K, Takikawa H, et al. Clinical characteristics of immunoglobulin IgG4-related sclerosing cholangitis: Comparison of cases with and without autoimmune pancreatitis in a large cohort. Dig Liver Dis 2021;53:1308–14. 10.1016/j.dld.2021.02.009. [DOI] [PubMed] [Google Scholar]
- [33].Pan Y-Y, Zhou S-C, Wang Y-J, Zhu T-T, Peng D, Guan H-X. IgG4-Related Disease: A Retrospective Chinese Study of Features and Treatment Response of 98 Patients Including 4 Rare Cases. Curr Med Sci 2021;41:390–7. 10.1007/s11596-021-2359-5. [DOI] [PubMed] [Google Scholar]
- [34].Inoue D, Yoshida K, Yoneda N, Ozaki K, Matsubara T, Nagai K, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 2015;94:e680. 10.1097/MD.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mehta PK, Wei J, Shufelt C, Quesada O, Shaw L, Bairey Merz CN. Gender-Related Differences in Chest Pain Syndromes in the Frontiers in CV Medicine Special Issue: Sex & Gender in CV Medicine. Front Cardiovasc Med 2021;8:744788. 10.3389/fcvm.2021.744788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang W, Stone JH. Management of IgG4-related disease. The Lancet Rheumatology 2019;1:e55–65. 10.1016/S2665-9913(19)30017-7. [DOI] [PubMed] [Google Scholar]
- [37].Manzo-Silberman S, Aelion H, Leprince P. Spontaneous rupture of a coronary artery. Arch Cardiovasc Dis 2014;107:704–5. 10.1016/j.acvd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- [38].Daneshvar DA, Czak S, Patil A, Wasserman PG, Coplan NL, Garratt KN. Spontaneous Rupture of a Left Main Coronary Artery Aneurysm. Circ: Cardiovascular Interventions 2012;5. 10.1161/CIRCINTERVENTIONS.112.971929. [DOI] [PubMed] [Google Scholar]
- [39].Banerjee S, Bagheri M, Sandfort V, Ahlman MA, Malayeri AA, Bluemke DA, et al. Vascular calcification in patients with large-vessel vasculitis compared to patients with hyperlipidemia. Semin Arthritis Rheum 2019;48:1068–73. 10.1016/j.semarthrit.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


