Abstract
The reconceptualization of Alzheimer’s disease (AD) as a clinical and biological construct has facilitated the development of biomarker-guided, pathway-based targeted therapies, many of which have reached late-stage development with the near-term potential to enter global clinical practice. These medical advances mark an unprecedented paradigm shift and requires an optimized global framework for clinical care pathways for AD. In this Perspective, we describe the blueprint for transitioning from the current, clinical symptom-focused and inherently late-stage diagnosis and management of AD to the next-generation pathway that incorporates biomarker-guided and digitally facilitated decision-making algorithms for risk stratification, early detection, timely diagnosis, and preventative or therapeutic interventions. We address critical and high-priority challenges, propose evidence-based strategic solutions, and emphasize that the perspectives of affected individuals and care partners need to be considered and integrated.
AD is a chronic, nonlinearly progressive, multifactorial neurodegenerative disease that affects multiple domains of an affected individual’s life during advanced stage of progression, such as cognition, behavior, functional abilities and social interactions. AD is the most common cause of dementia, accounting for around 60–80% of cases1. In 2019, over 50 million people were living with dementia worldwide, and the number is expected to rise to 152 million by 2050, largely driven by the projected increases in low-income and middle-income countries2. With population growth and aging, AD is becoming one of the most burdensome and costly diseases facing global society today3.
Historically, the diagnosis and treatment of AD focused on clinical symptoms. In the past three decades, in vivo biomarker studies identified core pathophysiological alterations—including amyloid and tau—that characterize and underly AD across its decades-long preclinical and prodromal phases. Such evidence has transformed the disease concept from clinically defined to biologically defined (Box 1)4,5. This transformation opened the gate to biomarker-guided, molecular pathway-based targeted therapies6,7. To date, a new wave of pharmacological compounds targeting AD pathophysiological hallmarks have reached late-stage clinical development with one agent recently approved by the US Food and Drug Administration (FDA) for clinical use in treating AD8–12.
Box 1 |. The conceptual transformation of Alzheimer’s disease from a clinical–pathological and primarily symptom-based entity to a clinical–biological construct.
- In 1984, diagnostic criteria for AD were first defined by the US NINCDS-ADRDA137.
- Definitive AD: diagnosis requires autopsy.
- In 2007, the IWG defined AD as a clinical–biological entity.
- AD defined by specific clinical phenotypes and in vivo fluid and neuroimaging biomarkers.
- Definition broadened and included the pre-dementia stages141.
In 2010, the NIA-AA working groups formulated three research diagnostic criteria based on cognitive changes and pathophysiological evidence using biomarkers. These included the dementia phase of AD, the symptomatic pre-dementia phase (MCI) of AD, and the asymptomatic, preclinical phase of AD144–146.
- In 2016, the AT(N) research framework was developed (Table 1)147.
- ‘A’ = Aβ biomarker (amyloid PET or CSF Aβ42 or Aβ42/40 ratio)
- ‘T’ = tau pathology biomarker (CSF p-tau or tau PET)
- ‘N’ = neurodegeneration or neuronal injury (CSF total tau, 18F-FDG-PET, or structural MRI)
- ATX(N): ‘X’ = additional novel pathophysiological markers9.
IWG argued AD diagnosis should include positive biomarkers (that is, amyloid-positive and tau-positive) and specific AD clinical phenotypes, whereas cognitively unimpaired individuals with positive biomarkers should be considered ‘at risk for progression to AD’4.
- In 2018, the FDA staging system for AD was developed to facilitate treatment development in the early stages148.
- Stage 1: normal cognition and biomarker evidence of AD.
- Stage 2: cognitive symptoms detectable with very sensitive assessments and biomarker evidence of AD.
- Stage 3: easily demonstrable cognitive abnormalities; functional deficits detectable only with sensitive measures and biomarker evidence of AD.
- Stages 4–6: mild, moderate and severe dementia148.
Evolution of the diagnostic criteria for Alzheimer’s disease.
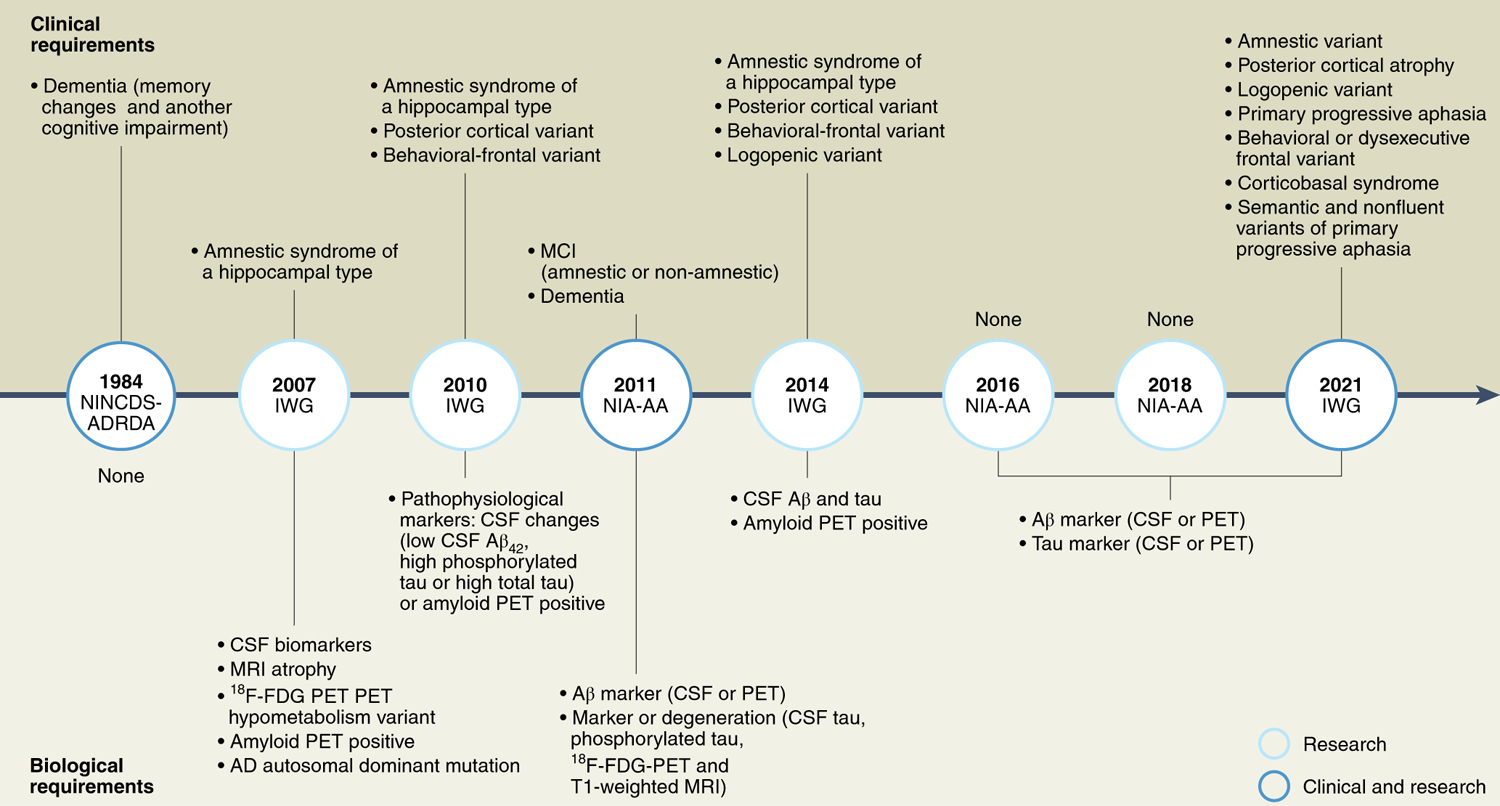
This timeline highlights key milestones in the development and updates to the diagnostic criteria for AD, the biological and clinical requirements that accompany their use, and their applicability in research and clinical settings. ADRDA, Alzheimer’s Disease and Related Disorders Association (now the Alzheimer’s Association) Work Group. IWG, International Working Group; NIA-AA, US National Institute on Aging and Alzheimer’s Association; NINCDS, National Institute of Neurological and Communicative Disorders and Stroke. Cognitively unimpaired individuals are considered at risk for AD. Schematic is based on the information in ref.4.
The possibility of detecting AD in its preclinical or prodromal stages and the opportunity of therapeutic intervention to alter clinical progression call for a next-generation global framework of clinical care pathways for individuals with AD. Under this framework, new clinical pathways—which may differ by country and clinical context—must enable timely, accurate and effective detection, diagnosis and treatment of AD at the early stages of mild cognitive impairment (MCI) due to AD and mild AD dementia (collectively defined as early AD hereafter). To this end, in vivo assessment of AD biological continuum (that is, through fluid testing and imaging biomarkers) and ecologically valid/low-threshold assessment of clinical symptomatology through digital health technologies will inevitably play a critical and guiding role in early detection, diagnosis, prognosis and therapeutic decision-making.
It is important to highlight the substantial inequalities in AD and dementia care currently. For example, in the United States, older Black and Hispanic individuals are disproportionally more likely to be affected by AD and other primary dementia disorders, and are more likely to have missed diagnoses, than older white individuals13. Various social determinants of health, such as socioeconomic status and educational attainment, also influence cognition and risk of AD14. Globally, more than two-thirds of people with dementia live in low-income and middle-income countries; however, the condition is often underdiagnosed and undertreated due to various factors such as lack of awareness, stigma and limited health care resources15. One key priority is to achieve access and equity of care for the growing number of people living with the disease16.
In this Perspective, we describe a blueprint for transitioning from the current clinical symptom-focused and inherently late-stage management of AD to a biomarker-guided and digitally facilitated clinical care pathway that focuses on detection and intervention at early stages of the disease. We will address critical hurdles to the practical implementation of such a paradigm shift. We emphasize that patient and care partner perspectives must be considered and become central when developing and implementing a new clinical care pathway for AD.
Defining the next-generation clinical care pathway for Alzheimer’s disease
At present in a routine clinical setting, AD is often detected at the mild-to-moderate dementia stage. Diagnosis is usually based on clinical symptoms without biomarker confirmation, and pharmacological treatment options are largely limited to those addressing the symptoms of AD dementia, such as cholinergic and glutamatergic modulators approved two decades ago to mitigate cognitive and behavioral/psychological symptoms. Non-pharmacological treatments have shown promise in preventing cognitive decline; for example, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) demonstrated that a multidomain intervention aimed at reducing lifestyle-related and vascular-related risk factors was effective at preventing cognitive decline among older individuals at risk for dementia17. This landmark study has led to the development of the World-Wide FINGERS network aiming to adapt, test and optimize the FINGER model for risk reduction and prevention of cognitive decline across different countries and settings18.
Emerging treatments targeting AD-associated pathophysiology are directed at earlier stages of the disease and aim at maintaining cognition and function11. Early intervention may allow the affected individual to function at the highest level longer. The availability of such emerging treatments necessitates the identification of individuals with early-stage AD in routine clinical settings beyond academic and/or trial centers. Early detection of AD could empower affected individuals and their care partners to make decisions about future treatment and care proactively, and to anticipate and adapt to the cognitive and behavioral changes associated with disease progression19.
However, multiple hurdles to early detection and early intervention exist in routine clinical practice. Affected persons and family may not understand the early signs of AD and how they differ from normal aging, and may avoid seeking medical attention due to the stigma associated with dementia diagnosis20. Currently across the globe, the rate of undetected dementia is as high as ~60%21,22, with diagnosis of MCI being a rare exception rather than the norm. It is crucial for clinicians as well as patients and families to recognize the importance of early detection and diagnosis, and not overlook the early symptoms of AD or mislabel these as normal aging23. With these barriers in mind, we describe the next-generation clinical care pathway for AD and its implementation in daily clinical practice requiring innovation in health care system infrastructures and workflow and bridging of the general public into a participatory framework of medicine.
Summary of key steps in future clinical care pathway for Alzheimer’s disease.
First-line diagnostic workup: primary care.
The first step of the next-generation clinical care pathway for AD (Fig. 1) involves a potentially affected individual or family members noticing subtle changes in cognition and/or behavior and proactively seeking medical and/or psychological consultation in a primary care setting. It may also be possible to detect changes during a routine visit.
Fig. 1 |. The next-generation clinical care pathway for Alzheimer’s disease.
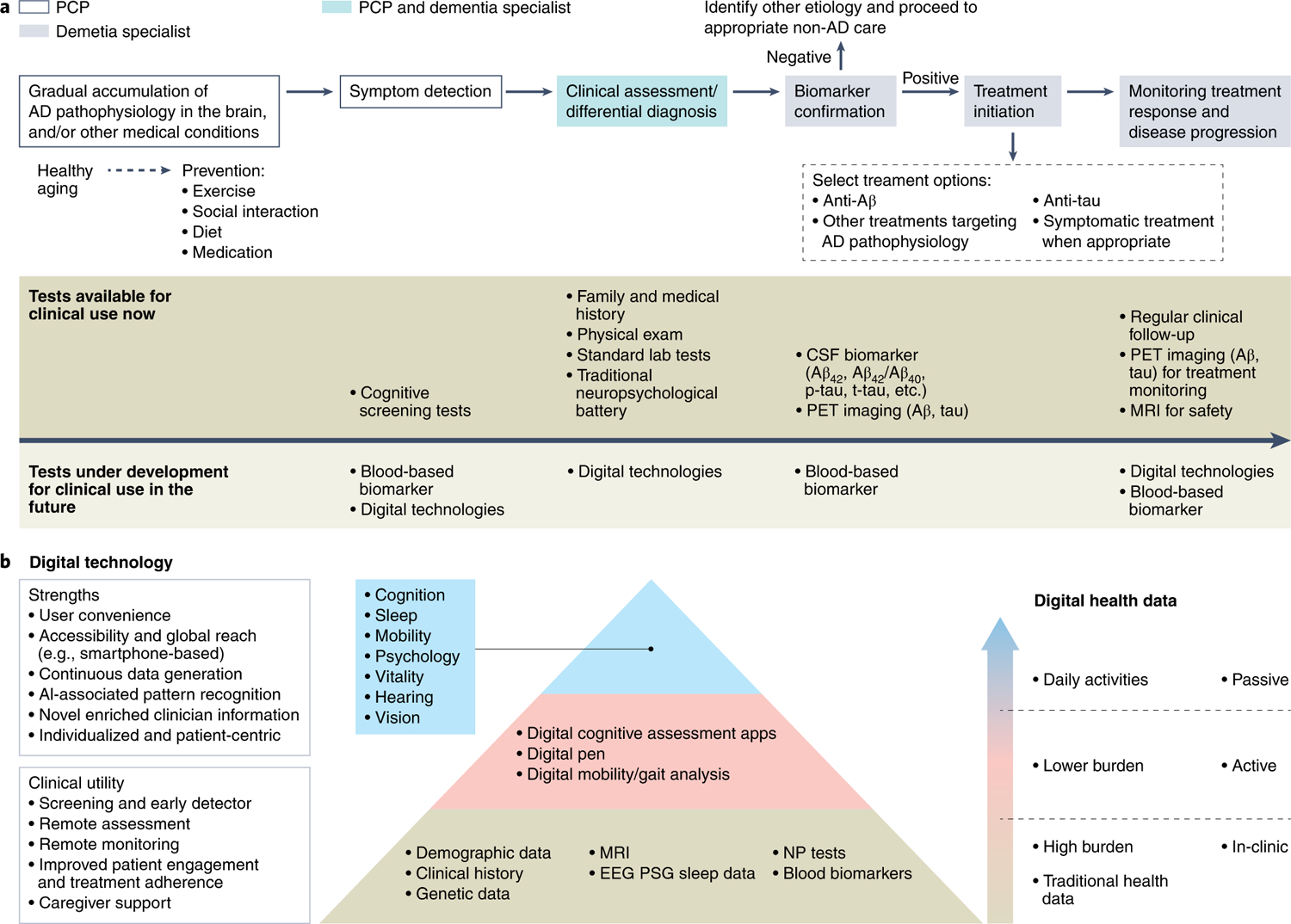
a, An overarching illustration. The next-generation clinical care pathway begins with healthy aging and participation in preventive lifestyle measures to slow or prevent accumulation of AD pathophysiology, with the goal of extending healthspan across populations. Symptom detection, triggered by concerned individuals or family members, or detected during a routine wellness visit, may involve cognitive testing and, in the future, blood-based biomarkers and digitally based assessments. This will be accompanied by clinical assessments involving standard laboratory tests and physical examination. Any recorded cognitive impairment will be confirmed with standardized biomarker tests. Individuals with confirmed disease will proceed to treatment initiation with relevant AD therapy followed by long-term monitoring, of which digital technologies and blood-based biomarkers will play a key role in the future. b, Digital health technologies in future AD clinical care and the path toward a precision monitoring and detection platform. A precision monitoring and detection platform will require a transformation from the traditional data collection methods to the inclusion of digital technologies. This will include active engagement technologies that require individual interaction and engagement to passive engagement technologies that collect data in the background while the individuals keep to their daily routine. AI, artificial intelligence; EEG, electroencephalogram; NP, neuropsychiatric; PSG, polysomnography.
The first-line medical assessment consists of recording family and medical history to assess for risk factors for AD (family history for neurodegenerative diseases, diabetes, history of traumatic brain injury, and so on) or other causes of reversible/irreversible cognitive impairment (for example, cerebrovascular and cardiocerebrovascular diseases, psychiatric disorders, metabolic/endocrinological diseases with neurological manifestations, cancer and its treatments, and potentially, neurological consequences of corona-virus disease 2019)5,24–26. Assessments may be collected digitally to facilitate both patient experience and clinician workflow.
Physical examination (general and neurological) can identify signs of central and autonomous nervous system impairment that may suggest non-AD diagnoses (for example, early psychosis, bradykinesia, postural reflexes, involuntary movements, severe orthostatic hypotension and others)5,24. Digital assessments of bradykinesia, tremor and blood pressure may augment clinical evaluations. First-line medical evaluation would continue to include laboratory tests to identify other potential causes of reversible/irreversible cognitive impairment, such as routine blood tests for vitamin B12 deficiency and hypothyroidism, electrolyte imbalance, severe anemia, hepatic and renal diseases, and, when appropriate, screening tests for infectious diseases such as syphilis and human immunodeficiency virus.
Quick, easy-to-use and validated clinical assessment tools can be used to identify impairment in cognition, function and behavior. Such tools already exist, including the Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA) or Mini Cognitive Assessment Instrument (Mini-Cog) for assessing cognition, the Instrumental Activities of Daily Living (IADL) or Functional Activities Questionnaire (FAQ) for assessing daily function, and the Neuropsychiatric Inventory Questionnaire (NPI-Q) for assessing behavior27. However, primary care physicians (PCPs) often have substantial time constraints and may require training and support to evaluate individuals with such methods. Moreover, many of the standard instruments are impacted by linguistic, cultural, educational and demographic factors, and referral to specialists for more in-depth neuropsychological evaluation may be required for accurate diagnosis in certain situations28. In the future, low-threshold digital assessment tools for measuring cognitive performance will be needed27,29,30. In addition, blood-based biomarkers of AD pathophysiology—currently under clinical validation and/or qualification—may also be used in the primary care setting in the future to better inform referral to AD specialists who would be in charge of the second-line diagnostic workup and therapeutic decision-making29,31–34.
Second-line diagnostic workup and therapeutic decision-making: Alzheimer’s disease specialist.
In the AD specialist setting with a neurologist, geriatrician or geriatric psychiatrist, a more comprehensive clinical evaluation will determine if the clinical presentation is consistent with AD. Specialist assessment is particularly important in complex cases with atypical presentation, early-onset or rapid progression35. Brain computerized tomography (CT) and magnetic resonance imaging (MRI) are recommended to identify structural explanations for cognitive impairment, such as neoplasm, past stroke or hydrocephalus, to name a few36. Atrophy patterns of the brain can provide first signs for the presence of a neurodegenerative disease35.
The second-line diagnostic workup is characterized by in vivo demonstration of AD hallmark pathophysiological changes reflected by the amyloid/tau/neurodegeneration (AT(N)) classification system, that is, proteinopathies involving amyloid-β (Aβ) and tau pathways, axonal damage and neuronal loss (Table 1)5. In the medium to long term, blood-based biomarkers may evolve from triage tools to confirmatory biomarkers comparable to the current standard of amyloid positron emission tomography (PET) or cerebrospinal fluid (CSF) biomarkers. It is important to exclude any amnestic cognitive syndromes without Aβ and tau pathology such as limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC)37,38. Currently, this is only possible by excluding AD specific pathology; in the future, positive biomarkers for TDP-43 in the CSF may become available39.
Table 1 |.
| AT(N) | Imaging | CSF | Blood | FDA Class |
|---|---|---|---|---|
| A/amyloid | Amyloid PET | Aβ42, Aβ42/Aβ40 | Aβ42/Aβ40 | Diagnostic monitoring |
| T/tau | Tau PET | p-tau181, p-tau217 | p-tau181, p-tau217 | Prognostic monitoring |
| N/neurodegeneration | MRI, FDG PET | NfL, tau | NfL, tau, GFAP | Pharmacodynamic monitoring |
| ATX(N) examples | SV2A PET, microglial PET, astrocytosis PET | Synaptic analytes, inflammatory measures | Synaptic analytes, inflammatory measures | Pharmacodynamic monitoring |
The various biomarkers under the AT(N) system can be measured by neuroimaging or by detection in blood and CSF. ATX(N) demonstrates the dynamic and evolving nature of the AT(N) classification system where the X component represents additional biomarkers, for example, inflammatory biomarkers, that improve classification, based on the pathophysiology of disease.
In situations where AD is excluded as the cause for cognitive impairment, the path for the individual would be redirected toward non-AD conditions. A diagnosis of AD would require discussions between the clinician and the individual/family for prognosis. The most critical prognostic outcomes to individuals and their care partners are related to cognitive decline, dependency and physical health40.
Therapeutic interventions will consist of agents targeting AD-associated pathophysiology, although at present patients must be aware that these treatments are unlikely to stop or reverse cognitive decline. These therapies will likely be given in combination with existing symptomatic treatments (cholinesterase inhibitors or memantine), and likely guided by the profile of biomarker and behavioral or functional changes11,41. Treatment continuation, cessation or dose adjustment will be determined based on clinical and biological factors42,43.
In the future when treatments for preclinical (presymptomatic) stages of AD become available, identifying such populations will become critical. At such time, a personalized, multidimensional approach to the diagnosis of preclinical AD should be considered for the best chance of diagnosis and progression prediction. This will likely include identifying genetic risk factors associated with AD and abnormalities in fluid and neuroimaging AD biomarkers; in particular, periodic screening with blood-based biomarkers of AD pathophysiology (Aβ42/40 and phosphorylated tau (p-tau) species) in appropriate populations should be considered44. Such a complex approach has several outstanding issues such as diagnostic accuracy, cost of diagnosis and treatment relative to benefit) yet without consensus on when a preclinical diagnosis of AD is indicated and how it should be performed22,45,46. Consensus process in the future should involve clinical and biomarker experts, but also patient advocacy groups and representatives of regulatory agencies and payers. In addition to pharmacological interventions, multidomain lifestyle interventions could prove beneficial over the long term in such populations, which may include modifications of diet, exercise, sleep and social and cognitive stimulation47.
Communication with patients and their care partners.
Patient and care partner perspectives must be considered when developing and implementing the next-generation clinical care pathway for AD48–51. Their collective involvement is essential to provide insight into possible gaps in existing health services48–50. Qualitative studies have revealed the need for: (1) early diagnosis through a well-organized process, (2) a notably shorter pathway to accessing support services for their current needs and care goals, (3) easily accessible, adequate and clear information about cognitive testing, medications, disease progression, finances and behavior, (4) effective disease management by highly knowledgeable and experienced clinicians, and (5) good communication skills of clinicians48. Individuals at risk for AD or with early AD and their families will need substantial health literacy to understand and apply the increasingly complex and nuanced information such as risk prediction, early detection and prognosis for decision-making on health-related issues. Overall health literacy can vary substantially, and effective and clear communication from clinicians with empathy and sensitivity to individual needs and preferences is critical52. In the context of diagnosis and therapeutic workup, among a number of informative topics, clinicians must effectively communicate the rationale for biomarker testing, the results and implications for treatment and to set appropriate expectations, as treatments that target AD-associated pathophysiology are likely to slow clinical decline without noticeable improvement in symptoms53,54. Involving the patient and care partner to understand what matters to them regarding health, cognitive, behavioral and functional status as a measure of treatment success and developing a tool for this purpose is equally important55,56. In addition, patient and care partner preference in how they would like to be informed must be taken into consideration.
Use of biomarkers in clinical care of Alzheimer’s disease
The incorporation of biomarkers represents a major innovation in the next-generation clinical care pathway for AD, supporting screening, diagnosis and disease staging as well as predicting the rate of progression, determining prognosis and assisting therapeutic decision-making9. Established Aβ biomarkers such as those measured by PET imaging or CSF analysis should be used to assess the presence of amyloid pathology and is mostly used today in specialized and tertiary care for diagnostic confirmation and therapeutic decision-making. Three radioligands for amyloid PET have been approved by the US FDA and European Medicines Agency (EMA) for amyloid plaque imaging in cognitively impaired individuals being clinically evaluated for AD and other causes of cognitive decline. These include 18F-florbetapir57, 18F-flutemetamol58 and 18F-florbetaben59. Amyloid PET was validated against the gold standard of neuropathology, has undergone extensive standardization and has been widely used in AD clinical trials34. The appropriate use criteria for amyloid PET are available, providing guidance to clinicians on the types of patients and clinical circumstances in which amyloid PET should be used60,61. Interestingly, an applied study showed relevant clinical benefit of amyloid PET imaging even for individuals who did not meet the appropriate use criteria62. The Imaging Dementia-Evidence For Amyloid Scanning (IDEAS) study provided evidence that amyloid PET positively impacts diagnostic accuracy and certainty as well as patient management63. As such, amyloid PET is likely to be the first choice for clinical use in the context of anti-Aβ agents, especially in the United States and Europe. However, the limited availability of PET scanners, radioligand manufacturing centers and nuclear medicine teams, as well as the high cost and lack of reimbursement are all factors that constrain its global use in routine clinical practice34,64.
CSF biomarker analysis for Aβ42 has been developed and standardized using certified reference materials and methods65. The CSF Aβ42/40 ratio is highly concordant with amyloid PET, and evidence suggests that Aβ abnormalities may be detected in CSF earlier than by amyloid PET. Both the FDA and the EMA have encouraged further study of CSF biomarkers in the context of clinical AD diagnostics34,65. The appropriate use criteria for lumbar puncture and CSF testing during the diagnostic workup of AD have been established66, and further recommendations to optimize the safety profile of lumbar puncture are available67. Recommendations and protocols to standardize the pre-analytical aspects of CSF biomarker testing for AD are also established68,69.
Besides Aβ, development of tau biomarkers has also advanced markedly. Various tau PET radioligands could chart the spatial spreading of tau pathophysiology in vivo, which tightly correlates with cognitive and functional outcomes across AD clinical stages34,70. Flortaucipir F18 was recently approved in the United States for imaging tau pathology in individuals with cognitive impairment who are being evaluated for AD, and several other investigational tau tracers are being actively studied34,70. Among several contexts of use of tau biomarkers71–73, monitoring downstream biological effects on tau pathways following anti-Aβ treatment and guiding future anti-Aβ and tau combination therapies represent two unique opportunities34.
As PET imaging is expensive and of limited availability, and CSF sampling may be considered invasive, the rapidly advancing blood-based biomarkers for AD are particularly promising, given the broad availability, scalability and cost-effectiveness of blood tests globally (Box 2). Plasma Aβ42/40 shows great promise in accurately reflecting amyloid PET and CSF Aβ42/40 results74–76. A mass spectrometry-based plasma Aβ42/40 test achieved an accuracy of 0.81 (area under the receiver operating characteristic (ROC) curve) in predicting brain amyloid status, and has recently received Clinical Laboratory Improvement Amendments certification77. Besides Aβ, plasma p-tau181, p-tau217 and p-tau231 are emerging as accurate, specific and accessible biomarkers for detecting early AD-related pathophysiology78–84. In the near term, blood-based biomarkers reflecting core AD pathophysiology have the potential to serve as screening and triage tools to identify those who should be tested with more resource-demanding techniques such as PET imaging and/or CSF biomarker analysis. It will be important to define standard diagnostic pathways following a positive blood biomarker test, including the development of an evidence base for predictive accuracy within a primary care setting, provision of access to specialized care, and determination of thresholds of positivity that will guide the use of new treatments targeting AD pathophysiology9,32,85. In the future, blood-based biomarkers may be developed for other contexts of use such as to predict disease risk, track disease progression and monitor treatment response9,32.
Box 2 |. Strengths and limitations of each biomarker modality.
| Modality | Strengths | Limitations |
|---|---|---|
| PET |
|
|
| CSF |
|
|
| Blood |
|
|
Biomarkers reflecting other components of AD-related pathophysiology, such as neuronal injury/neurodegeneration (CSF total tau and neurofilament light chain (NfL), volumetric MRI), synaptic dysfunction (CSF neurogranin, 18F-fluorodeoxyglucose (FDG)-PET and synaptic vesicle glycoprotein 2A (SV2A) PET) and inflammation (CSF chitinase-3-like protein (YKL-40), glial fibrillary acidic protein (GFAP) and soluble triggering receptor expressed on myeloid cells 2 (TREM2), translocator protein (TSPO) PET and monoamine oxidase B (MAO-B) PET) are emerging, but are not yet ready for clinical implementation (Table 1)34.
It is worth noting that a sizable portion of individuals with AD exhibit comorbid pathologies such as vascular lesions or intracellular inclusions of TDP-43 (refs. 86,87). There is an urgent need for research to develop imaging and fluid biomarkers for common co-pathologies such as TDP-43, α-synuclein and other misfolded proteins. Further studies are needed to understand how comorbid pathologies contribute to the biological and clinical progression of AD, and how to factor these in during the clinical management of patients. In addition, biomarker data on individuals aged 85 and older (oldest old) are scarce; given that age is the greatest risk factor for late-onset AD and considering the pace of population aging worldwide, more research is needed to map the biomarker landscape in this population13. Similarly, more biomarker research on middle-age, at-risk populations is needed given the importance for prevention efforts in such populations (Box 3).
Box 3 |. Glossary.
Biomarker
Usually refers to a group of broad medical characteristics that can be objectively measured as an indicator of the body’s normal biological processes, or as an indicator of pathogenic processes and response to therapy. To qualify as a biomarker, a characteristic must be measurable, quantifiable, accurate and reproducible.
Context of use
In relation to biomarkers, this usually refers to the description of the biomarker’s specified and appropriate use and how the biomarker is applied in drug development and clinical care.
Digital biomarker
Clinically meaningful and objective physiological and behavioral data that can be measured using digital and sensor-based technologies, including mobile and wearable devices such as smartphones and smart watches.
Digital health technologies
These refer to technologies that require the use of computers, connectivity, software and sensors for health care and all its related uses.
Functional Activities Questionnaire (FAQ)
A screening tool for evaluating difficulties in activities of daily living that allow an individual to care for themselves. The main distinguishing feature of the FAQ is that, unlike the IADL, it measures more basic tasks such as eating and bathing.
Instrumental Activities of Daily Living (IADL)
This assesses the ability or need for assistance to perform activities that allow an individual to live independently in a community and to improve quality of life. Domains assessed include cooking, cleaning, transportation, laundry and managing finances.
Mini Cognitive Assessment Instrument (Mini-Cog)
Compared to other cognitive screening tools, this is a relatively quicker 3-min test to screen for cognitive impairment in older adults in both the community and health care settings. The test uses a three-item recall test for memory and a clock-drawing test.
Mini-Mental State Examination (MMSE)
An 11-question measure widely used to test cognitive function among the older population; it includes a systematic and thorough test of five areas of cognitive function: orientation, registration, attention and calculation, recall, and language. Of a maximum score of 30, 23 or lower is indicative of cognitive impairment.
Montreal Cognitive Assessment (MoCA)
A rapid screening tool for mild cognitive dysfunction that assesses cognitive domains including attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations and orientation. Of a maximum score of 30, below 26 is indicative of cognitive impairment. This is considered a good screening tool for persons who score above the cutoff for MMSE.
Neuropsychiatric Inventory Questionnaire (NPI-Q)
An informant-based test for assessing behavior. It assesses neuropsychiatric symptoms including delusions, hallucinations, aggression, dysphoria/depression, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviors, night-time behavioral disturbances and appetite/eating disturbances.
Patient journey
A term usually used to refer to a patient’s experience throughout an episode of care. This may entail the entire scope of events of patient experiences within a health care ecosystem, including undergoing regular checkups and receiving treatment.
Polygenic risk score
A score related to the risk of developing a disease, estimated based on the total number of changes or variations in an individual’s genes that are related to the disease. This has potential to improve personalized disease risk prediction using genetic data.
Sensor technologies
Sensor technologies require the use of sensors to detect physical, chemical or biological properties of an individual and convert them into readable and meaningful information.
Although biomarkers form the cornerstone for the next-generation clinical care pathway for AD, their use is currently limited in clinical practice. Clinicians may be reluctant to discuss biomarkers to avoid burdening their patients, perhaps in part because there are still uncertainties regarding the clinical utility of a biomarker-based diagnosis. These uncertainties may result in some clinicians steering their patients away from further biomarker testing88. Moreover, clinicians vary in their approach to informing a patient they have early disease, with about one-half of clinicians preferring not to use the term MCI88. Web-based tools are emerging to support clinicians and patients with decisions on diagnostic testing, interpretation of individually tailored biomarker test results, and the communication of test results to individuals and their families89. Clinicians should receive appropriate education and practical training on the use of new tools and assessments5,67,90.
Use of digital health technologies in clinical care pathway
The rise of digital health technologies represents another major opportunity to improve the AD clinical care pathway (Fig. 1). Such technologies are particularly poised for early detection/case finding and tracking longitudinal disease progression and/or treatment response.
The transition from traditional cognitive testing to nonintrusive digital assessment offers several advantages. First, nonintrusive testing offers ecological validity owing to the patient assessment in their normal environment and outside the hospital setting. Second, thanks to its convenience, digital assessments can be more frequent as compared to traditional in-clinic assessments, thus allowing documentation of, and control for, day-to-day variations in cognitive function. Third, assessment of everyday activities as a surrogate indicator of abstract cognitive functions increases the functional relevance of the patient assessment. And finally, zero-effort technologies provide access to patients outside standard cognitive testing, such as individuals with advanced stages of dementia, those with reduced hearing, or those who are illiterate42. Digital cognitive testing also has the potential to overcome the socioeconomic and cultural biases embedded in some traditional neuropsychological tests.
In the near term, digital health technologies that require active input from the user to assess changes in cognition, function and quality of life are under development29,42,91. In the long term, the rapid progress in sensor-based technologies, including mobile and wearable devices (smartphones and smart watches) may support increasingly early detection of subtle changes associated with AD onset (for example, speech/language, oculomotor skills and movement) in a continuous, passive and unobtrusive manner (that is, digital biomarkers). This will enable risk assessment, screening and disease prediction with little or no active engagement by the participants42,91–98. As an example, the Oregon Center for Aging & Technology (ORCATECH)/Collaborative Aging Research Using Technology (CART) platform utilizes ambient technologies and wearables to longitudinally monitor cognition, physical mobility, sleep and the level of social engagement in the homes of older adults99. Such data can be integrated and analyzed to find meaningful behaviors that identify people at different stages of cognitive decline100. Digital health technologies will play a central role in an integrated care model (for example, the Integrated Care for Older People (ICOPE) recommended by the World Health Organization) that not only focuses on cognition but also addresses other functions that maintain brain research, such as mobility, psychology, vitality, hearing and vision101–103.
Important considerations for the use of digital technologies include privacy issues, specificity and sensitivity to AD versus other causes of cognitive impairment or dementia, user friendliness, reliability, costs and access, among others42,104. Once in clinical practice, the ultimate goal of digital health technologies is to facilitate patient self-management and maintain people living independently for as long as possible42,105,106.
Challenges and potential solutions: a holistic perspective
With biomarker-guided, pathway-based targeted therapies emerging, modeling studies predict that health care systems in many regions/countries will not be able to meet the demand of patients for diagnosis and treatment of AD107–110. In the United States, ~15 million individuals with MCI would need to be evaluated by specialists, undergo diagnostic testing and pursue treatment107. Current estimates show that the expected caseload of patients will result in long average wait times for specialist visits (~50 months) with many patients developing AD dementia while on waiting lists107,111. The strained capacity of memory specialists will limit access to diagnosis and treatment107,111.
There are additional obstacles to meet the demand of people with AD. Although some countries have dementia plans in place, the emphasis is often on the management of patients with descriptive dementia syndromes and does not adequately address prodromal symptomatic MCI, and etiology in general or early-stage disease112–114. Coverage of services is also limited, especially for routine use of confirmatory biomarker tests. For countries with the capacity to absorb increases in service demand, there may not be an incentive to scale up patient volume due to budgetary considerations112. Moreover, there will be challenges keeping pace with the necessary infrastructure to accommodate recommended procedures, such as adding PET tracer manufacturing capacity and installing PET scanners, increasing the volume of biomarker testing, ensuring sufficient availability and accessibility to infusion centers and having the necessary infrastructure for monitoring treatment safety and efficacy112,115,116. Other potential issues also include clinician capacity and capabilities; recent reports show a limited number of dementia specialists in the United States, Canada, Europe and Japan, and clinicians may be reluctant to evaluate a patient for a decline in cognitive function if they don’t feel adequately trained or believe that there are no therapeutic advantages to identifying a decline in cognitive function112,113.
With these obstacles and challenges, there is a pressing need to determine the essential steps toward system preparedness for the next-generation AD clinical care pathway. Primary care is a critical entry point into health care systems with a larger number of general providers compared with specialist services107,112. Better tools are being developed to identify and triage patients in the primary care setting29. Digital health technologies, including digital cognitive assessments, have the potential to detect early cognitive decline and monitor progression, while the emerging blood-based biomarkers—following analytical and clinical validation—could be used to enhance the likelihood of AD as the etiology of the observed cognitive decline31,32,42,97,117. To this end, a study showed that a brief cognitive test in combination with a blood-based biomarker test of AD pathophysiology at the primary care level can substantially improve triaging in primary care and lead to reduced waiting times for a specialist visit during the diagnostic process31. Besides diagnostic evaluation, prognostic information including the risk of disease progression is important to guide treatment decisions. For example, for cardiovascular diseases, the American Heart Association and the American College of Cardiology have issued predictive equations to guide treatment decisions based on projected risk of cardiovascular events; similar tools for AD could give clinicians a holistic view of a patient’s risk of progression118. To this end, the Interceptor Project in Italy is monitoring a group of patients with early-stage cognitive decline to determine factors from the initial evaluation of the patient that could predict progression119.
Telehealth will be an essential component of the next-generation pathway by providing coordinated care between a patient, care partners and clinicians (that is, nurse, PCP and specialist), and will allow access to memory care and remote monitoring in individuals who cannot leave home, or in those without adequate transportation or living in a rural area120. More defined hub and spoke arrangements linking PCPs to specialists through telehealth and related technologies may facilitate care by coordinated teams.
Better care models and incentives are needed to increase a PCP’s involvement in AD care. For example, cognitive screening is a mandatory requirement of the driver’s license renewal process for older people in Japan, while walk-in clinics are available for screening and consultation in memory centers in Korea110. The emergence of telecare-enabled specialist support has helped to empower PCP sites in the United States107. In addition, accountability schemes have emerged as incentives, such as use of age-adjusted dementia diagnosis rates as a quality measure for general practitioners in the United Kingdom112.
Specialty care for AD will need to evolve to accommodate a shift from the current focus on diagnosis often at late stages of the disease and counseling to more emphasis on diagnosing the disease at early stages and offering new treatments that target the underlying pathophysiology. For example, memory clinics have been logistically located near general hospitals in the United Kingdom to provide one-stop, large-scale practices that can handle all aspects of care with biomarker testing, differential diagnosis and infusion therapy112. Agile learning health care systems will be required to adjust continuously to new and emerging therapies for AD and related disorders.
Ethnic, socioeconomic and racial disparities have been identified in people with AD121–123. Differences in risk factors (for example, genetics, comorbid cardiovascular disease or metabolic syndrome) between races may play a role in the incidence and prevalence of AD, while cultural factors (for example, lack of access to medical care, trust issues between marginalized groups and the health care system) may influence diagnosis and treatment121–123. Inherent biases may exist in cognitive screening tools that complicate the diagnosis of AD in less educated groups121 and there is an underrepresentation of marginalized groups in clinical research and clinical trials122,124,125. Improving diverse participation in clinical research and clinical trials is paramount to understanding how factors like race, ethnicity, socioeconomic status, gender, sexual orientation, education and culture interact with biological factors associated with AD122,125–129. The next-generation clinical care pathway will need to address the issue of diversity as well as social determinants of health to optimize equity of care for all individuals with AD121–125.
Conclusions and perspectives
The conceptualization of AD as a clinical–biological construct and the emerging biomarker-guided pathway-based treatments targeting AD-associated pathophysiology highlight the importance and urgency of developing and implementing a global framework for the next-generation AD clinical care pathway. Detecting the disease at its initial and early stages will be crucial, and primary care will have an important role in case finding. Utilizing a ‘memory care enabled’ workforce including nurse practitioners, community health workers and geriatric care managers may reduce the burden and complete reliance on the PCPs as the gateway to diagnosis and care130. Diagnosis will include biomarker assessments, which will also guide the initiation of treatment as well as monitoring of treatment response, dose adjustments, and treatment continuation or cessation. Including patients and care partners early in the development process will ensure acceptance and accessibility of novel pathways and technology for those most affected. Although patient and public involvement has been utilized in other medical specialties such as oncology and pediatrics, a pragmatic approach needs to be adapted and transformed for AD clinical care.
The successful development and implementation of the next-generation AD clinical care pathway outlined above depends on close interaction and cross-functional collaboration with stakeholders including regulators, pharmaceutical and biotechnology industry, policymakers, and payors. While the current paper centered on the clinician and patient as well as the care partner perspective, optimization of the clinical care pathway needs to be complemented by the health system and cost viewpoints. Both perspectives need to be integrated in the near future once a clinical care pathway addressing the most urgent needs of patients and family has been agreed on by health care system stakeholders.
The next-generation clinical care pathway for AD must address the critical issues of diversity and inclusion to ensure health equity for the enormous and rapidly growing number of AD patients across the globe. A starting point is to ensure more inclusive participation in observational biomarker research and in clinical trials so that therapeutic approaches will be broadly applicable and available. The new pathway needs to be adapted to local resources and capabilities to maximize the health benefit for patients. From there it will become clear that the next step is to devise the roadmap toward a transformation to precision neurology—a holistic and synergistic approach to AD care that encompasses genetic, biological (that is, biomarker), clinical and environmental profiling of individual patients to guide the development of individualized treatment schemes and ultimately, prevention and extension of brain healthspan131–135.
Acknowledgements
R.A.’s grant support includes National Institutes of Health (NIH) grants (AG062109, AG068753, AG072654 and AG063635). Additional support was provided by the American Heart Association (20SFRN35360180 and 20SFRN35490098), the Alzheimer’s Drug Discovery Foundation (201902-2017835) and Gates Ventures. W.M.v.d.F received support from the Research of Alzheimer center Amsterdam, which is part of the neurodegeneration research program of Amsterdam Neuroscience. The chair of W.v.f.F is supported by the Pasman stichting. W.F. is a recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP allowance, LSHM20106). More than 30 partners participate in ABOARD. H.W. receives research grant from the National Brain Project funded by the Ministry of Science and Technology, China (2021ZD0201805). C.C. is supported by the National Medical Research Council of Singapore (MOH-000707-00, NMRC/OFLCG/2019, NMRC/CIRG/1485/2018 and NMRC/CSA-SI/0007/2016). The Gérontopôle (chair B.V.) has received research grant support from the European Commission as well as industries including Biogen, Green Valley Pharmaceuticals, Novo Nordisk, Pfizer, Pierre-Fabre, Roche, Lily and Eisai. J.C. is supported by NIGMS grant P20GM109025, NINDS grant U01NS093334, NIA grants R01AG053798, P20AG068053 and R35AG71476 and the Alzheimer’s Disease Drug Discovery Foundation. A.S. receives support from multiple NIH grants (P30 AG010133, P30 AG072976, R01 AG019771, R01 AG057739, U01 AG024904, R01 LM013463, R01 AG068193, T32 AG071444, U01 AG068057 and U01 AG072177). The authors thank D. Henley for his contribution to the critical revision of the Perspective. Medical writing support was provided by L. O’Brien of CMC AFFINITY, McCann Health Medical Communications and was funded by Eisai.
Competing interests
H.H. is an employee of Eisai and serves as senior associate editor for the Journal Alzheimer’s & Dementia and has not received any fees or honoraria since May 2019. H.H. is inventor of 11 patents and has received no royalties for: In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders patent no. 8916388; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases patent no. 8298784; Neurodegenerative Markers for Psychiatric Conditions publication no. 20120196300; In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders publication no. 20100062463; In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders publication no. 20100035286; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases publication no. 20090263822; In Vitro Method for The Diagnosis of Neurodegenerative Diseases patent no. 7547553; CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases publication no. 20080206797; In Vitro Method for The Diagnosis of Neurodegenerative Diseases publication no. 20080199966; Neurodegenerative Markers for Psychiatric Conditions publication no. 20080131921; Method for diagnosis of dementias and neuroinflammatory diseases based on an increased level of procalcitonin in cerebrospinal fluid: US patent no. 10921330. R.A. is a scientific advisor to Signant Health and consultant to Biogen. S.M. serves on the board of directors of Senscio Systems and the scientific advisory board of AiCure Technologies, and Boston Millennia Partners, and has received consulting fees from AARP, Biogen, Biotronik, Bristol-Myers Squibb, C2N, Eisai and Roche. Research programs of W.M.v.d.F. have been funded by ZonMW, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Biogen MA, Boehringer Ingelheim, Life-MI, AVID, Roche BV, Fujifilm and Combinostics. W.F. holds the Pasman chair. W.F. is a recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP allowance, LSHM20106). W.F. has performed contract research for Biogen MA and Boehringer Ingelheim. W.F. has been an invited speaker at Boehringer Ingelheim, Biogen MA, Danone, Eisai, WebMD Neurology (Medscape) and Springer Healthcare. W.F. is consultant to Oxford Health Policy Forum CIC, Roche and Biogen MA. W.F. participated in advisory boards of Biogen MA and Roche. All funding is paid to the institution of W.F. W.F. was associate editor of Alzheimer, Research & Therapy in 2020/2021. W.F. is associate editor at Brain. P.A. reports research agreements with Janssen, Lilly and Eisai, grants from NIA, the Alzheimer’s Association and FNIH and consulting fees from Biogen, Roche, Merck, Abbvie, Immunobrain Checkpoint, Rainbow Medical and Shionogi. L.A. has provided consultation to Eli Lilly, Biogen, Eisai, GE Healthcare and Two Labs. L.G.A. receives research support from NIA U01 AG057195, NIA R01 AG057739, NIA P30 AG010133, Alzheimer Association LEADS GENETICS 19–639372, Roche Diagnostics RD005665, AVID Pharmaceuticals and Life Molecular Imaging. L.G.A. received honoraria for participating in independent data safety monitoring boards and providing educational CME lectures and programs. L.G.A. has stock in Cassava Sciences and Semiring. C.C. receives research grants from the National Medical Research Council of Singapore. C.C. also receives research support from Moleac, Roche, Eisai and Lundbeck; and has participated in advisory boards for Cerecin and Eisai in the past 3 years. A.I. receives research grant from AMED (Japanese Agency for Medical Research), JSPS (Japan Society for Promotion of Science), Eisai, Daiichi Sankyo, Shionogi, Chugai-Roche and Kyowa Kirin. A.I. also receives consultant fees from Eisai, Biogen and Janssen Pharmaceuticals. A.I. also receives lecture fees from Eisai, Daiichi Sankyo, Otsuka, Ono Pharmaceutical and Fujirebio. A.S. received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor), Bayer Oncology (Scientific Advisory Board), Eisai (Scientific Advisory Board), Siemens Medical Solutions USA (Dementia Advisory Board) and Springer Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior). S.T. has served on the advisory boards of Roche, Biogen, Eisai and Grifols within the last 3 years. B.V. has served as consultant/advisor to Eisai, Biogen, Lilly, Longeveron, Novo Nordisk, TauRx P and Roche in the past 3 years. A.V. declares no competing interests related to the present paper and the contribution of A.V. to this paper reflects entirely and only A.V.’s own academic expertise on the matter. A.V. was an employee of Eisai (November 2019–June 2021). A.V. did not receive any fees or honoraria since November 2019. Before November 2019, A.V. received lecture honoraria from Roche, MagQu and Servier. H.W. has provided consultation to Eisai, Lundbeck, Roche and Signant Health pharmaceutical and assessment companies. H.W. owns the copyright of the individualized management system of neuropsychiatric symptoms (NPSIMS) and the smartphone-based application of brief cognitive screening kit (shairenzhi). J.C. provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPathFinder, Annovis, AriBio, Artery, Avanir, Biogen, Biosplice, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro pharmaceutical, assessment and investment companies. M.C., S.D.S., P.G. and R.K. are employees of Eisai.
References
- 1.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 14, 367–429 (2018). [Google Scholar]
- 2.GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheltens P et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 20, 484–496 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR Jr. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aisen PS, Vellas B & Hampel H Moving towards early clinical trials for amyloid-targeted therapy in Alzheimer’s disease. Nat. Rev. Drug Discov 12, 324 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Hampel H et al. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 26, 5481–5503 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings J The National Institute on Aging-Alzheimer’s Association Framework on Alzheimer’s disease: application to clinical trials. Alzheimers Dement. 15, 172–178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampel H et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurology 17, 580–589 (2021). [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. FDA’s decision to approve new treatment for Alzheimer’s Disease (2021).
- 11.Cummings J, Lee G, Zhong K, Fonseca J & Taghva K Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement. 7, e12179 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biogen. Aduhelm (aducanumab-avwa) [package insert]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761178s000lbl.pdf (2021). [Google Scholar]
- 13.Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 17, 327–406 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Majoka MA & Schimming C Effect of social determinants of health on cognition and risk of Alzheimer disease and related dementias. Clin. Ther 43, 922–929 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Ferri CP & Jacob KS Dementia in low-income and middle-income countries: different realities mandate tailored solutions. PLoS Med. 14, e1002271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampel H & Lista S Dementia: the rising global tide of cognitive impairment. Nat. Rev. Neurol 12, 131–132 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Ngandu T et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Kivipelto M et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 16, 1078–1094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 15, 321–387 (2019). [Google Scholar]
- 20.Dubois B, Padovani A, Scheltens P, Rossi A & Dell’Agnello G Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J. Alzheimers Dis 49, 617–631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichler T et al. Rates of formal diagnosis in people screened positive for dementia in primary care: results of the DelpHi-Trial. J. Alzheimers Dis 42, 451–458 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Lang L et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open 7, e011146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toepper M Dissociating normal aging from Alzheimer’s disease: a view from cognitive neuroscience. J. Alzheimers Dis 57, 331–352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois B et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Mandelblatt JS et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin. Oncol 40, 709–725 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spudich S & Nath A Nervous system consequences of COVID-19. Science 375, 267–269 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN & Rubino I Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J. Prev. Alzheimers Dis 8, 371–386 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Ng KP et al. The influence of language and culture on cognitive assessment tools in the diagnosis of early cognitive impairment and dementia. Expert Rev. Neurother 18, 859–869 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Sabbagh MN et al. Early detection of mild cognitive impairment in primary care. J. Prev. Alzheimers Dis 7, 165–170 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Rhodius-Meester HFM et al. cCOG: a web-based cognitive test tool for detecting neurodegenerative disorders. Alzheimer’s Dement. 12, e12083 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattke S, Cho SK, Bittner T, Hlávka J & Hanson M Blood-based biomarkers for Alzheimer’s pathology and the diagnostic process for a disease-modifying treatment: projecting the impact on the cost and wait times. Alzheimer’s Dement. 12, e12081 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hampel H et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat. Rev. Neurol 14, 639–652 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teunissen CE et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 21, 66–77 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Zetterberg H & Bendlin BB Biomarkers for Alzheimer’s disease—preparing for a new era of disease-modifying therapies. Mol. Psychiatry 26, 296–308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauthier S, Rosa-Neto P, Morais J & Webster C World Alzheimer Report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International https://www.alzint.org/resource/world-alzheimer-report-2021/ (2021). [Google Scholar]
- 36.Robinson L, Tang E & Taylor JP Dementia: timely diagnosis and early intervention. BMJ 350, h3029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson PT et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142, 1503–1527 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graus F et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 15, 391–404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scialo C et al. TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2, fcaa142 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mank A et al. Identifying relevant outcomes in the progression of Alzheimer’s disease; what do patients and care partners want to know about prognosis? Alzheimer’s Dement. 7, e12189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hane FT et al. Recent progress in Alzheimer’s disease research, part 3: diagnosis and treatment. J. Alzheimers Dis 57, 645–665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kourtis LC, Regele OB, Wright JM & Jones GB Digital biomarkers for Alzheimer’s disease: the mobile/wearable devices opportunity. NPJ Digit. Med 2, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie CW et al. The Edinburgh Consensus: preparing for the advent of disease-modifying therapies for Alzheimer’s disease. Alzheimer’s Res. Ther 9, 85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keshavan A et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain 144, 434–449 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frozza RL, Lourenco MV & De Felice FG Challenges for Alzheimer’s disease therapy: insights from novel mechanisms beyond memory defects. Front Neurosci. 12, 37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan TK An algorithm for preclinical diagnosis of Alzheimer’s disease. Front. Neurosci 12, 275 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kivipelto M, Mangialasche F & Ngandu T Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol 14, 653–666 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Prorok JC, Horgan S & Seitz DP Health care experiences of people with dementia and their caregivers: a meta-ethnographic analysis of qualitative studies. CMAJ 185, E669–E680 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank C & Forbes RF A patient’s experience in dementia care: using the ‘lived experience’ to improve care. Can. Fam. Physician 63, 22–26 (2017). [PMC free article] [PubMed] [Google Scholar]
- 50.Kowe A et al. Stakeholder involvement in dementia research: a qualitative approach with healthy senior citizens and providers of dementia care in Germany. Health Soc. Care Community 30, 908–917 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Kunneman M et al. Patients’ and caregivers’ views on conversations and shared decision making in diagnostic testing for Alzheimer’s disease: the ABIDE project. Alzheimer’s Dement. 3, 314–322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rostamzadeh A et al. Health literacy in individuals at risk for Alzheimer’s dementia: a systematic review. J. Prev. Alzheimers Dis 7, 47–55 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Fruijtier AD et al. What patients want to know, and what we actually tell them: the ABIDE project. Alzheimer’s Dement. 6, e12113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fruijtier AD et al. ABIDE Delphi study: topics to discuss in diagnostic consultations in memory clinics. Alzheimer’s Res. Ther 11, 77 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiBenedetti DB et al. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: a qualitative study evaluating symptoms, impacts, and outcomes. Alzheimers Res. Ther 12, 90 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tochel C et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? a systematic review. Alzheimers Dement. 11, 231–247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amyvid (florbetapir F18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202008s000lbl.pdf (2012). [Google Scholar]
- 58.Vizamyl (flutemetamol F18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203137s000lbl.pdf (2013). [Google Scholar]
- 59.Neuraceq (florbetaben F18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204677s000lbl.pdf (2014). [Google Scholar]
- 60.Johnson KA et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 9, e1–e16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson KA et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 9, e106–e109 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Altomare D et al. Quantitative appraisal of the Amyloid Imaging Taskforce appropriate use criteria for amyloid-PET. Alzheimers Dement. 14, 1088–1098 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Rabinovici GD et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321, 1286–1294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tousi B & Sabbagh MN Editorial: a time of transition of Alzheimer’s disease in the advent of anti-amyloid monoclonal antibodies. Neurol. Ther 10, 409–413 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zetterberg H & Blennow K Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol. Neurodegener 16, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaw LM et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 14, 1505–1521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hampel H et al. State-of-the-art of lumbar puncture and its place in the journey of patients with Alzheimer’s disease. Alzheimers Dement. 18, 159–177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansson O et al. Pre-analytical protocol for measuring Alzheimer’s disease biomarkers in fresh CSF. Alzheimers Dement 12, e12137 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanderstichele H et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 8, 65–73 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Tauvid (flortaucipir F 18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212123s000lbl.pdf (2020). [Google Scholar]
- 71.Moscoso A et al. Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 78, 396–406 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen SD et al. Longitudinal plasma phosphorylated tau 181 tracks disease progression in Alzheimer’s disease. Transl. Psychiatry 11, 356 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moscoso A et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain 144, 325–339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janelidze S et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep 6, 26801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schindler SE et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, e1647–e1659 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura A et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554, 249–254 (2018). [DOI] [PubMed] [Google Scholar]
- 77.West T et al. A blood-based diagnostic test incorporating plasma Abeta42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol. Neurodegener 16, 30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barthelemy NR, Horie K, Sato C & Bateman RJ Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med 217, e20200861 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janelidze S et al. Plasma p-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med 26, 379–386 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Karikari TK et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Mielke MM et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 14, 989–997 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palmqvist S et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324, 772–781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thijssen EH et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med 26, 387–397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ashton NJ et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 141, 709–724 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ashton NJ et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur. J. Nucl. Med. Mol. Imaging 48, 2140–2156 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langa KM, Foster NL & Larson EB Mixed dementia: emerging concepts and therapeutic implications. JAMA 292, 2901–2908 (2004). [DOI] [PubMed] [Google Scholar]
- 87.McAleese KE et al. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. 27, 472–479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Visser LNC et al. Clinicians’ communication with patients receiving a MCI diagnosis: the ABIDE project. PLoS ONE 15, e0227282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Maurik IS et al. Development and usability of ADappt: web-based tool to support clinicians, patients and caregivers in the diagnosis of mild cognitive impairment and Alzheimer disease. JMIR Form. Res 3, e13417 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiotis K et al. Clinical validity of increased cortical uptake of amyloid ligands on PET as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol. Aging 52, 214–227 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Sabbagh MN et al. Early detection of mild cognitive impairment in an at-home setting. J. Prev. Alzheimers Dis 7, 171–178 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Stroud C, Onnela JP & Manji H Harnessing digital technology to predict, diagnose, monitor, and develop treatments for brain disorders. NPJ Digit. Med 2, 44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Au R, Ritchie M, Hardy S, Ang TFA & Lin H Aging well: using precision to drive down costs and increase health quality. Adv. Geriatr. Med. Res 1, e190003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seelye A et al. Embedded online questionnaire measures are sensitive to identifying mild cognitive impairment. Alzheimer Dis. Assoc. Disord 30, 152–159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirste T et al. Detecting the effect of Alzheimer’s disease on everyday motion behavior. J. Alzheimers Dis 38, 121–132 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Lüdtke S, Hermann W, Kirste T, Beneš H & Teipel S An algorithm for actigraphy-based sleep/wake scoring: comparison with polysomnography. Clin. Neurophysiol 132, 137–145 (2021). [DOI] [PubMed] [Google Scholar]
- 97.Sabbagh MN et al. Rationale for early diagnosis of mild cognitive impairment supported by emerging digital technologies. J. Prev. Alzheimers Dis 7, 158–164 (2020). [DOI] [PubMed] [Google Scholar]
- 98.Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. in Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining 2145–2155 (2019). [Google Scholar]
- 99.Beattie Z et al. The Collaborative Aging Research Using Technology Initiative: an open, sharable, technology-agnostic platform for the research community. Digit. Biomark 4, 100–118 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu CY et al. Unobtrusive sensing technology detects ecologically valid spatiotemporal patterns of daily routines distinctive to persons with mild cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci glab293 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Piau A et al. Intrinsic capacitiy monitoring by digital biomarkers in integrated care for older people (ICOPE). J. Frailty Aging 10, 132–138 (2021). [DOI] [PubMed] [Google Scholar]
- 102.Takeda C, Guyonnet S, Sumi Y, Vellas B & Araujo de Carvalho I Integrated care for older people and the implementation in the INSPIRE Care Cohort. J. Prev. Alzheimers Dis 7, 70–74 (2020). [DOI] [PubMed] [Google Scholar]
- 103.Tavassoli N et al. Implementation of the WHO integrated care for older people (ICOPE) programme in clinical practice: a prospective study. Lancet Healthy Longev. 3, 394–404 (2022). [DOI] [PubMed] [Google Scholar]
- 104.van Gils AM et al. Assessing the views of professionals, patients and care partners concerning the use of computer tools in memory clinics: International Survey Study. JMIR Form. Res 5, e31053 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McAlearney AS et al. High Touch and High Tech (HT2) proposal: transforming patient engagement throughout the continuum of care by engaging patients with portal technology at the bedside. JMIR Res. Protoc 5, e221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hampel H & Vergallo A The Sars-CoV-2 pandemic and the brave new digital world of environmental enrichment to prevent brain aging and cognitive decline. J. Prev. Alzheimers Dis 7, 294–298 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu JL, Hlavka JP, Hillestad R & Mattke S Assessing the Preparedness of the US Health Care System Infrastructure for an Alzheimer’s Treatment (RAND Corporation, 2017). [PMC free article] [PubMed] [Google Scholar]
- 108.Hlavka JP, Mattke S & Liu JL Assessing the preparedness of the health care system infrastructure in six European countries for an Alzheimer’s treatment. Rand Health Q. 8, 2 (2019). [PMC free article] [PubMed] [Google Scholar]
- 109.Mattke S, Hlavka JP, Yoong J, Wang M & Goto R Assessing the preparedness of the Japanese health care system infrastructure for an Alzheimer’s treatment. (USC Dornsife: Center for Economic and Social Research, 2019). [Google Scholar]
- 110.Jun H, Cho SK, Yoong J & Mattke S Assessing the preparedness of the Korean health care system infrastructure for an Alzheimer’s treatment. (USC Dornsife: Center for Economic and Social Research, 2020). [Google Scholar]
- 111.Mattke S & Hanson M Expected wait times for access to a disease-modifying Alzheimer’s treatment in the United States. Alzheimers Dement. 18, 1071–1074 (2021). [DOI] [PubMed] [Google Scholar]
- 112.Mattke S, Ullrich A & Wang M Implications of Alzheimer’s treatment for organization and payment of medical practices in the EU-5 countries. (USC Dornsife: Center for Economic and Social Research, 2020). [Google Scholar]
- 113.Mattke S & Wang M Implications of Alzheimer’s treatment for organization and payment of medical practices in the United States. (USC Dornsife: Center for Economic and Social Research, 2020). [Google Scholar]
- 114.Prince M, Comas-Herrera A, Knapp M, Guerchet M & Karagiannidou M World Alzheimer Report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. (Alzheimer’s Disease International, 2016). [Google Scholar]
- 115.Sperling RA et al. Amyloid-related imaging abnormalities (ARIA) in amyloid modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 7, 367–385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu JL, et al. Assessing the preparedness of the Canadian health care system infrastructure for an Alzheimer’s treatment (RAND Corporation, 2019). [PMC free article] [PubMed] [Google Scholar]
- 117.Au RK, Kolachalama VB, Paschalidis ICH Redefining and validating digital biomarkers as fluid, dynamic multi-dimensional digital signal patterns. Front. Digit. Health 3, 751629 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lloyd-Jones DM et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation 139, e1162–e1177 (2019). [DOI] [PubMed] [Google Scholar]
- 119.Rossini PM et al. The Italian INTERCEPTOR Project: from the early identification of patients eligible for prescription of antidementia drugs to a nationwide organizational model for early Alzheimer’s disease diagnosis. J. Alzheimers Dis 72, 373–388 (2019). [DOI] [PubMed] [Google Scholar]
- 120.National Institute on Aging. Telehealth: improving dementia care. https://www.nia.nih.gov/news/telehealth-improving-dementia-care (2020).
- 121.Chin AL, Negash S & Hamilton R Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis. Assoc. Disord 25, 187–195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.US Department of Health and Human Services. Racial and ethnic disparities in Alzheimer’s Disease: a literature review. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//138596/RacEthDis.pdf (2014).
- 123.Vega IE, Cabrera LY, Wygant CM, Velez-Ortiz D & Counts SE Alzheimer’s disease in the Latino community: intersection of genetics and social determinants of health. J. Alzheimers Dis 58, 979–992 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKindra L Dementia researchers issue urgent call for more diversity in clinical trial participation. (University of Kansas Medical Center, 2021). [Google Scholar]
- 125.Wilkins CH, Schindler SE & Morris JC Addressing health disparities among minority populations: why clinical trial recruitment is not enough. JAMA Neurol. 77, 1063–1064 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anderson JG, Flatt JD, Jabson Tree JM, Gross AL & Rose KM Characteristics of sexual and gender minority caregivers of people with dementia. J. Aging Health 33, 838–851 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ferretti MT et al. Optimal Alzheimer’s disease detection and diagnosis under the sex and gender lens: a crucial step towards precision neurology. in World Alzheimer Report 2021: Journey through the diagnosis of dementia. Alzheimer’s Disease International, 238–241 (2021). [Google Scholar]
- 128.Ferretti MT et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat. Rev. Neurol 14, 457–469 (2018). [DOI] [PubMed] [Google Scholar]
- 129.Fredriksen-Goldsen KI, Jen S, Bryan AEB & Goldsen J Cognitive impairment, Alzheimer’s disease and other dementias in the lives of lesbian, gay, bisexual and transgender (LGBT) older adults and their caregivers: needs and competencies. J. Appl Gerontol 37, 545–569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galvin JE et al. Early stages of Alzheimer’s disease: evolving the care team for optimal patient management. Front. Neurol 11, 592302 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hampel H, Lista S & Khachaturian ZS Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 8, 312–336 (2012). [DOI] [PubMed] [Google Scholar]
- 132.Hampel H et al. PRECISION MEDICINE—the golden gate for detection, treatment and prevention of Alzheimer’s disease. J. Prev. Alzheimers Dis 3, 243–259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hampel H et al. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: analysis of the blarcamesine (ANAVEX2–73) phase 2a clinical study. Alzheimer’s Dement. 6, e12013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hampel H, Vergallo A, Perry G, Lista S & Alzheimer Precision Medicine Inititaive. The Alzheimer Precision Medicine Initiative. J. Alzheimers Dis 68, 1–24 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Altomare D et al. Brain Health Services: organization, structure, and challenges for implementation. a user manual for Brain Health Services—part 1 of 6. Alzheimers Res. Ther 13, 168 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cummings J The role of biomarkers in Alzheimer’s disease drug development. Adv. Exp. Med. Biol 1118, 29–61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McKhann G et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 (1984). [DOI] [PubMed] [Google Scholar]
- 138.Beach TG, Monsell SE, Phillips LE & Kukull W Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol 71, 266–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kovacs GG et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 126, 365–384 (2013). [DOI] [PubMed] [Google Scholar]
- 140.Graff-Radford J et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20, 222–234 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dubois B et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Dubois B et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 9, 1118–1127 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Dubois B et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Albert MS et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McKhann GM et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sperling RA et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jack CR et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Food & Drug Administration. Early Alzheimer’s Disease: Developing Drugs for Treatment: Guidance for Industry. https://www.fda.gov/media/110903/download (2018).


