Abstract
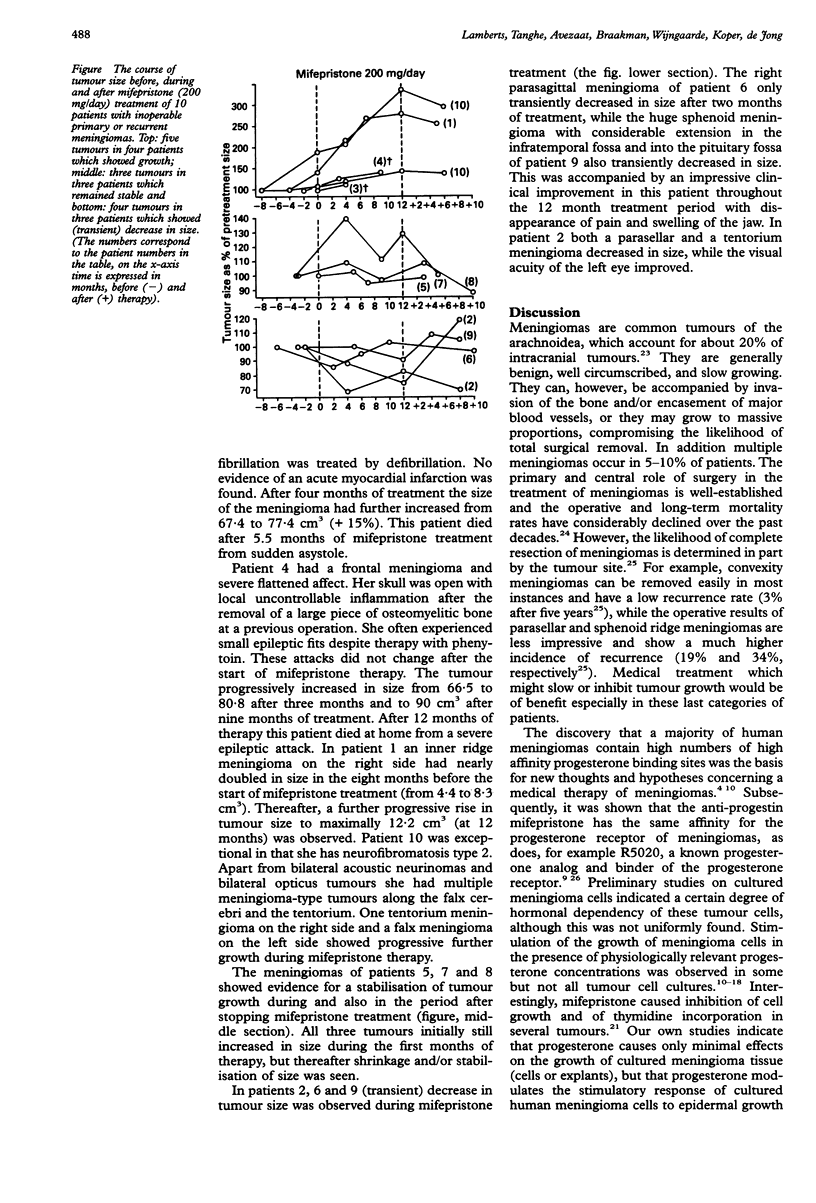
Meningiomas are common brain tumours which are generally benign, well circumscribed and slow growing. In a minority of patients complete surgical removal is not possible and re-growth of tumour tissue is a major clinical problem. Most meningiomas contain progesterone receptors. The anti-progestational drug mifepristone (RU 486) binds to these receptors. Ten patients were treated with 12 recurrent or primary "inoperable" meningiomas, all of whom had shown recent neuroradiological and/or ophthalmological evidence of tumour growth. They received 200 mg mifepristone daily for 12 months. Most patients initially had complaints of nausea, vomiting and/or tiredness. In four patients prednisone (7.5 mg/day) was given after which these side-effects subsided. CT scan analysis of tumour size, showed progression of growth of five meningiomas in four patients, stable disease in three patients with three tumours and regression of four tumours in three patients. A decrease in the complaints of headache and an improved general well being was observed in five patients. Two patients died during the treatment period from unrelated causes. Mifepristone treatment resulted in control of tumour growth (= stable disease) in six of 10 patients who had shown recent evidence of tumour growth. In three of these six patients consistent tumour shrinkage was observed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BICKERSTAFF E. R., SMALL J. M., GUEST I. A. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958 May;21(2):89–91. doi: 10.1136/jnnp.21.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker G. H., Setyono-Han B., Henkelman M. S., de Jong F. H., Lamberts S. W., van der Schoot P., Klijn J. G. Comparison of the actions of the antiprogestin mifepristone (RU486), the progestin megestrol acetate, the LHRH analog buserelin, and ovariectomy in treatment of rat mammary tumors. Cancer Treat Rep. 1987 Nov;71(11):1021–1027. [PubMed] [Google Scholar]
- Bardon S., Vignon F., Chalbos D., Rochefort H. RU486, a progestin and glucocorticoid antagonist, inhibits the growth of breast cancer cells via the progesterone receptor. J Clin Endocrinol Metab. 1985 Apr;60(4):692–697. doi: 10.1210/jcem-60-4-692. [DOI] [PubMed] [Google Scholar]
- Blaauw G., Blankenstein M. A., Lamberts S. W. Sex steroid receptors in human meningiomas. Acta Neurochir (Wien) 1986;79(1):42–47. doi: 10.1007/BF01403464. [DOI] [PubMed] [Google Scholar]
- Blankenstein M. A., Blaauw G., Lamberts S. W., Mulder E. Presence of progesterone receptors and absence of oestrogen receptors in human intracranial meningioma cytosols. Eur J Cancer Clin Oncol. 1983 Mar;19(3):365–370. doi: 10.1016/0277-5379(83)90134-7. [DOI] [PubMed] [Google Scholar]
- Blankenstein M. A., Blaauw G., Lamberts S. W. Progestin and estrogen receptors in human meningioma. Clin Neuropharmacol. 1984;7(4):363–367. doi: 10.1097/00002826-198412000-00016. [DOI] [PubMed] [Google Scholar]
- HOESSLY G. F., OLIVECRONA H. Report on 280 cases of verified parasagittal meningioma. J Neurosurg. 1955 Nov;12(6):614–626. doi: 10.3171/jns.1955.12.6.0614. [DOI] [PubMed] [Google Scholar]
- Jay J. R., MacLaughlin D. T., Riley K. R., Martuza R. L. Modulation of meningioma cell growth by sex steroid hormones in vitro. J Neurosurg. 1985 May;62(5):757–762. doi: 10.3171/jns.1985.62.5.0757. [DOI] [PubMed] [Google Scholar]
- Jäskeläinen J., Laasonen E., Kärkkäinen J., Haltia M., Troupp H. Hormone treatment of meningiomas: lack of response to medroxyprogesterone acetate (MPA). A pilot study of five cases. Acta Neurochir (Wien) 1986;80(1-2):35–41. doi: 10.1007/BF01809555. [DOI] [PubMed] [Google Scholar]
- Klijn J. G., de Jong F. H., Bakker G. H., Lamberts S. W., Rodenburg C. J., Alexieva-Figusch J. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res. 1989 Jun 1;49(11):2851–2856. [PubMed] [Google Scholar]
- Koper J. W., Foekens J. A., Braakman R., Lamberts S. W. Effects of progesterone on the response to epidermal growth factor and other growth factors in cultured human meningioma cells. Cancer Res. 1990 May 1;50(9):2604–2607. [PubMed] [Google Scholar]
- Lamberts S. W., Uitterlinden P., Bons E. G., Verleun T. Comparison of the actions of RU 38486 and megestrol acetate in the model of a transplantable adrenocorticotropin- and prolactin-secreting rat pituitary tumor. Cancer Res. 1985 Mar;45(3):1015–1019. [PubMed] [Google Scholar]
- Lamberts S. W., van Koetsveld P., Verleun T. Prolactin release-inhibitory effects of progesterone, megestrol acetate, and mifepristone (RU 38486) by cultured rat pituitary tumor cells. Cancer Res. 1987 Jul 15;47(14):3667–3671. [PubMed] [Google Scholar]
- Magdelenat H., Pertuiset B. F., Poisson M., Martin P. M., Philippon J., Pertuiset B., Buge A. Progestin and oestrogen receptors in meningiomas. Biochemical characterization, clinical and pathological correlations in 42 cases. Acta Neurochir (Wien) 1982;64(3-4):199–213. doi: 10.1007/BF01406053. [DOI] [PubMed] [Google Scholar]
- Markwalder T. M., Gerber H. A., Waelti E., Schaffner T., Markwalder R. V. Hormonotherapy of meningiomas with medroxyprogesterone acetate. Immunohistochemical demonstration of the effect of medroxyprogesterone acetate on growth fractions of meningioma cells using the monoclonal antibody Ki-67. Surg Neurol. 1988 Aug;30(2):97–101. doi: 10.1016/0090-3019(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Markwalder T. M., Waelti E., König M. P. Endocrine manipulation of meningiomas with medroxyprogesterone acetate. Effect of MPA on receptor status of meningioma cytosols. Surg Neurol. 1987 Jul;28(1):3–9. doi: 10.1016/0090-3019(87)90198-4. [DOI] [PubMed] [Google Scholar]
- Markwalder T. M., Waelti E., König M. P. Endocrine manipulation of meningiomas with medroxyprogesterone acetate. Effect of MPA on receptor status of meningioma cytosols. Surg Neurol. 1987 Jul;28(1):3–9. doi: 10.1016/0090-3019(87)90198-4. [DOI] [PubMed] [Google Scholar]
- Mirimanoff R. O., Dosoretz D. E., Linggood R. M., Ojemann R. G., Martuza R. L. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985 Jan;62(1):18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- Olson J. J., Beck D. W., Schlechte J. A., Loh P. M. Effect of the antiprogesterone RU-38486 on meningioma implanted into nude mice. J Neurosurg. 1987 Apr;66(4):584–587. doi: 10.3171/jns.1987.66.4.0584. [DOI] [PubMed] [Google Scholar]
- Olson J. J., Beck D. W., Schlechte J., Loh P. M. Hormonal manipulation of meningiomas in vitro. J Neurosurg. 1986 Jul;65(1):99–107. doi: 10.3171/jns.1986.65.1.0099. [DOI] [PubMed] [Google Scholar]
- Poisson M., Pertuiset B. F., Hauw J. J., Philippon J., Buge A., Moguilewsky M., Philibert D. Steroid hormone receptors in human meningiomas, gliomas and brain metastases. J Neurooncol. 1983;1(3):179–189. doi: 10.1007/BF00165601. [DOI] [PubMed] [Google Scholar]
- Quest D. O. Meningiomas: an update. Neurosurgery. 1978 Sep-Oct;3(2):219–225. doi: 10.1227/00006123-197809000-00016. [DOI] [PubMed] [Google Scholar]
- Romieu G., Maudelonde T., Ulmann A., Pujol H., Grenier J., Cavalie G., Khalaf S., Rochefort H. The antiprogestin RU486 in advanced breast cancer: preliminary clinical trial. Bull Cancer. 1987;74(4):455–461. [PubMed] [Google Scholar]
- Schnegg J. F., Gomez F., LeMarchand-Beraud T., de Tribolet N. Presence of sex steroid hormone receptors in meningioma tissue. Surg Neurol. 1981 Jun;15(6):415–418. doi: 10.1016/s0090-3019(81)80024-9. [DOI] [PubMed] [Google Scholar]
- Schoenberg B. S., Christine B. W., Whisnant J. P. Nervous system neoplasms and primary malignancies of other sites. The unique association between meningiomas and breast cancer. Neurology. 1975 Aug;25(8):705–712. doi: 10.1212/wnl.25.8.705. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., de la Monte S., Atkins L., Gusella J. F., Martuza R. L. Molecular genetic approach to human meningioma: loss of genes on chromosome 22. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5419–5423. doi: 10.1073/pnas.84.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti E. R., Markwalder T. M. Endocrine manipulation of meningiomas with medroxyprogesterone acetate. Effect of MPA on growth of primary meningioma cells in monolayer tissue culture. Surg Neurol. 1989 Feb;31(2):96–100. doi: 10.1016/0090-3019(89)90318-2. [DOI] [PubMed] [Google Scholar]
- Weisman A. S., Villemure J. G., Kelly P. A. Regulation of DNA synthesis and growth of cells derived from primary human meningiomas. Cancer Res. 1986 May;46(5):2545–2550. [PubMed] [Google Scholar]
- Yu Z. Y., Wrange O., Haglund B., Granholm L., Gustafsson J. A. Estrogen and progestin receptors in intracranial meningiomas. J Steroid Biochem. 1982 Mar;16(3):451–456. doi: 10.1016/0022-4731(82)90059-0. [DOI] [PubMed] [Google Scholar]
- Zava D. T., Markwalder T. M., Markwalder R. V. Biological expression of steroid hormone receptors in primary meningioma cells in monolayer culture. Clin Neuropharmacol. 1984;7(4):382–388. doi: 10.1097/00002826-198412000-00019. [DOI] [PubMed] [Google Scholar]



