Abstract
Background
Idiopathic inflammatory myopathies (IIM) are a rare heterogeneous group of diseases characterised by chronic skeletal muscle inflammation, but other organs are also frequently involved. IMM represent a diagnostic challenge and a multidisciplinary approach is important to ensure successful diagnosis and adequate follow-up of these patients.
Objective
To describe the general functioning of our multidisciplinary myositis clinic, highlighting the benefits of multidisciplinary team management in patients with confirmed or suspected IIM and to characterise our clinical experience.
Methods
Description of the organization of a dedicated multidisciplinary myositis outpatient clinic, supported by IMM specific electronic assessment tools and protocols based on our Portuguese Register - Reuma.pt. In addition, an overview of our activity between 2017 and 2022 is provided.
Results
An IIM multidisciplinary care clinic, based on a close collaboration between Rheumatologists, Dermatologists and Physiatrist is detailed in this paper. One hundred and eighty-five patients were assessed in our myositis clinic; 138 (75%) of those were female, with a median age of 58 [45–70] years. At the last appointment, 130 patients had a confirmed IIM diagnosis, and the mean disease duration was 4 [2–6] years. The most frequent diagnosis was dermatomyositis (n = 34, 26.2%), followed by antisynthetase syndrome (n = 27, 20.8%) and clinically amyopathic/paucimyopathic dermatomyositis (n = 18, 13.8%). Twenty-four patients (18.5%) were on monotherapy and 94 (72.3%) were on combination therapy.
Conclusion
A multidisciplinary approach is important to ensure the correct diagnosis and follow-up of these patients. A myositis clinic, with a standardised practice at a tertiary hospital level, contributes to a standardization of care and opens research opportunities.
Keywords: inflammatory myopathies, rheumatology, dermatology, rehabilitation medicine, multidisciplinary clinic
Introduction
Idiopathic inflammatory myopathies (IIM) are heterogeneous, systemic inflammatory diseases that share muscle inflammation as a hallmark. Besides muscles, skin, joints, lungs and gastrointestinal tract are frequently involved. IIM are rare diseases with an estimated prevalence of 10 in 100 000 individuals.1,2 The diagnosis is often a challenge and usually requires clinical evaluation, testing for autoantibodies and other complementary diagnosis exams like electromyography (EMG), muscle magnetic resonance imaging (MRI) or muscle biopsy. Furthermore, IIM in adults might be associated with malignancies with an increased risk of 2- to 7- fold compared to the general population.3
Multidisciplinary teams are the core model for managing complex chronic conditions.4 Therefore, several specialities are usually required for optimal care of myositis patients, to provide the most differentiated care to patients, avoiding the delay of diagnosis and allowing the timely initiation of adequate treatment.5,6
In 2017, the Rheumatology Department of Centro Hospitalar Universitário Lisboa Norte (CHULN), Lisbon Academic Medical Center (CAML) established a dedicated multidisciplinary outpatient clinic for patients with suspected or confirmed IIM. The main goals of this IIM clinic are to standardise the diagnostic investigation and avoid delays in treatment onset. It can also improve patient satisfaction through fewer visits and consistent clinical communication and promote enrolment in research studies or clinical trials.
This experience-based manuscript describes the general functioning of our multidisciplinary IIM outpatient clinic. We describe the assessment tools embedded in the myositis module of Reuma.pt – the Portuguese Rheumatic Diseases Register (https://reuma.pt/en/), emphasising the benefits of multidisciplinary team management. In addition, the manuscript provides an overview of our activity with a brief description of the patients evaluated between July 2017 and May 2022.
Material and Methods
Multidisciplinary Myositis Outpatient Clinic
Description
The Rheumatology Department of CHULN developed a dedicated multidisciplinary myositis outpatient clinic in July 2017. Currently, the myositis outpatient clinic is carried out by 2 rheumatologists, supported at least by 1 resident, taking care weekly of 6 to 9 patients. Once a month, a physiatrist and a dermatologist participate in the appointments and jointly evaluate around 12 patients. Whenever necessary, a neurologist is also present to perform a more detailed neurologic examination and to discuss differential diagnosis of IIM. We perform ultrasound guided muscular biopsies at our department and there is a direct pathway with a neuropathologist for muscular biopsy analysis each time there is a procedure. A fresh muscle sample is immediately prepared for light microscopy (hematoxylin-eosin, Gomori trichrome and periodic acid Schiff stain), enzyme histochemistry (succinate dehydrogenase, cytochrome-c-oxidase, combined cytochrome-c-oxidase/succinate dehydrogenase, myosinic ATPase, myophosphorylase), and immunohistochemistry (CD3, CD20, CD68, MHC class I).
In addition, a monthly joint clinic with pulmonologists and radiologists is available if interstitial lung disease (ILD) is identified or suspected and otolaryngologists/gastroenterologists give support to patients with dysphonia/dysphagia.
Adult patients with confirmed/suspected IIM or with other connective tissue diseases having myositis as a significant feature are evaluated in our myositis clinic. The rheumatologist is at the core of the multidisciplinary team, coordinating it, integrating the various health professionals’ contributions, and being co-responsible for all treatment decisions.
The organised IIM clinic structure enables the establishment of clinical and scientific objectives, the implementation of a standardised intervention plan, and the assessment of its efficacy. In addition, individual patients’ particularities and preferences are incorporated into the decision process, influencing diagnostic and therapeutic plans whenever appropriate. The combination of this up-to-date specialised care and patient-centred medicine ensures a high-quality personalised healthcare service for patients with IIM. Besides, a standardised electronic clinical record promotes clinical rigor and ensures the precision and detail of data collection.
Development
An integrated care system was designed and implemented for this clinic and is revised every three years to ensure excellent and up-to-date clinical care. It contains a general description of the clinic, including the health professionals involved, associated infrastructures, and clinical guidelines. The clinical guidelines include referral and discharge criteria, the diagnostic criteria used, and treatment recommendations.
Focus areas recognised as crucial in the development of the myositis clinic are depicted in Table 1.
Table 1.
Focus Areas of the Myositis-Clinic Team
| Focus Area | |
|---|---|
| Principles for referral |
|
| Infrastructure and technology |
|
| Appointments |
|
| Meetings |
|
| Organisation of human resources |
|
Clinical Approach and Complementary Diagnostic Exams
IIM diagnosis is based on clinical symptoms and clinical examination in combination with blood tests, including creatine kinase (CK) and autoantibodies, EMG, MRI and muscle biopsy histopathology.7 Whenever necessary, a skin biopsy is also performed.
In a patient with a suspected IIM, a correct evaluation of muscle strength is essential. Muscle strength is measured by Manual Muscle Testing 8 (MMT-8), which has high practicality8 but should be performed by a trained examiner. The modified skin Disease Activity Score (skin DAS) is always performed and allows for evaluating the cutaneous disease evolution. The number of painful, swollen joints and joints with limited range of motion are also registered. Due to the IIM’s systemic involvement, a complete evaluation with cardiac and pulmonary auscultation is mandatory.
In our centre, we use the EUROLINE Autoimmune Inflammatory Myopathies 16 Ag (IgG) immunoblot for testing for myositis-specific [anti-complex nucleosome remodeling histone deacetylase (anti-Mi2a, anti-Mi2b), anti-melanoma differentiation-associated gene 5 (anti-MDA5), anti-transcription intermediary factor 1γ (anti-TIF1 γ), anti-histidyl transfer RNA synthetase (anti-Jo1), anti-threonyl-tRNA synthetase (anti-PL7), anti-alanyl-tRNA synthetase (anti-PL12), anti-isoleucyl-tRNA synthetase (anti-OJ), anti- glycyl -tRNA synthetase (anti-EJ), anti-small ubiquitin-like modifier-1 activating enzyme (anti-SAE), anti-signal recognition particle (anti-SRP), anti-nuclear matrix protein 2 (anti-NXP2)]; and myositis-associated [anti-Ro52, anti-Ku, anti-U1ribonucleoprotein (anti-U1RNP), anti-polymyositis/systemic scleroderma (PmScl100, anti-PmScl75)] antibodies. This is a fast and helpful method in terms of future monitoring and prognosis. A full blood count, inflammatory markers, and the serum concentrations of creatinine, aspartate transaminase (AST), alanine transaminase (ALT), CK, aldolase, myoglobin, lactate dehydrogenase (LDH) and urine sediment examination are requested in the first appointment and repeated in every appointment for follow-up of disease activity and treatment side effects. Tests for myositis differential diagnosis are also performed, including infectious diseases (like Human Immunodeficiency Virus, influenza, Coxsackieviruses, Echoviruses, Borrelia burgdorferi), endocrine disorders (especially thyroid function), electrolyte disturbances and vitamin D deficiency.
An ultrasound guided muscle biopsy is performed on every patient that consents the procedure. Samples are evaluated by dedicated pathologists and also stored at the institutional biobank (Biobanco-IMM, CAML) for future research. Biobanco-IMM, CAML was approved by the National Data Protection Authority and by the CAML ethics committee. In selected patients, EMG and muscle MRI are also performed in the diagnostic phase. EMG of affected muscles usually displays a myopathic pattern (the motor unit action potential is short, small and polyphasic), being useful to integrate with other clinical and laboratorial variables in the work out phase.7,9 Whenever symptoms are unclear, an MRI of the skeletal muscle is valuable for identifying the sites of major inflammation and can help select the anatomical structure for the muscle biopsy. In addition, it supports the differential diagnosis by depicting the pattern of affected muscles, helping in distinguishing between the different subtypes of myositis and ruling out other types of myopathy7 Whole-body MRI may add relevant information and evaluate all muscle groups simultaneously. However, whole-body MRI remains time-consuming, and consequently uncomfortable for patients10 and is not widely available in several centres. In our myositis clinic, MRI is usually performed on the thighs or other muscles possibly involved, according to the previous clinical evaluation.
A nailfold capillaroscopy is also requested at disease onset for every patient. Some studies suggest that changes in nailfold capillaries may have a role at diagnosis, with dermatomyositis (DM) and antisynthetase syndrome (ASS) patients showing more alterations than polymyositis (PM) patients.11 Nailfold capillaroscopy alterations may reflect disease activity in DM11,12 and some studies found an improvement of nailfold capillaroscopy alterations with immunosuppressive therapy in IIM.11,13 Once there are no definite recommendations, the decision to repeat the exam is decided on a case-by-case basis.
When the IIM diagnosis has been confirmed, other organ involvements should be searched for.1 Thus, a baseline assessment also includes chest radiography and lung function tests. If pulmonary involvement is suspected, high-resolution computed tomography (HRCT) is performed. An electrocardiogram (ECG) and Doppler echocardiography are part of the initial evaluation, and its repetition depends on clinical evolution, once the frequency of cardiopulmonary evaluation in the course of IIM is not well defined.14 A formal assessment by otolaryngologists/gastroenterologists is requested in case of dysphonia or dysphagia. Adult-onset IIM is associated with increased cancer risk, especially within three years of the myositis diagnosis.15 The most common cancer subtypes are lung, ovary, colorectal, lymphoma, breast, and nasopharyngeal and thus these organs and systems are carefully reviewed during history taking and physical examination.3,15 Particular care is applied for patients at the highest risk of cancer: age>40 years at IIM onset, persistent high disease activity, dysphagia, cutaneous necrosis, DM diagnosis and the presence of anti-TIF1-gamma and anti-NXP2. Male sex, anti-SAE1, anti-HMGCR, anti-Mi2, anti-MDA5 antibodies and the subtypes clinically amyopathic dermatomyositis (CADM), PM and necrotizing myopathy are also associated with an intermediate risk.15 If these risk factors are absent, we only perform a complete blood count, liver function tests, erythrocyte sedimentation rate, C-reactive protein, protein electrophoresis, urinalysis and plain chest x-ray radiograph. When the patient has one or more of those risk factors, we additionally perform a complete malignancy screening, including thoraco-abdomino-pelvic computed tomography (CT), endoscopy and colonoscopy, mammography, pelvic examination and prostate-specific antigen (PSA) measurement at the time of diagnosis. Positron emission tomography/CT (PET/CT) can also be an important screening test; however, it is not widely available.
Functional assessment questionnaires are performed in every appointment, as described in the next section.
The Added Value of Reuma.pt
In 2008, the Portuguese Society of Rheumatology launched the Rheumatic Diseases National Register (Reuma.pt), which is accessed through an electronic medical record application.16,17 Reuma.pt login is assured by username and password, which are unique to each rheumatologist, and users can only visualise data from their centre’s patients. Registered patients must sign an informed consent stating if their clinical data can be registered by the assisting physicians and whether it can be used for clinical research. Besides, patients have a dedicated area that can be accessed online to complete the patient-reported outcomes.
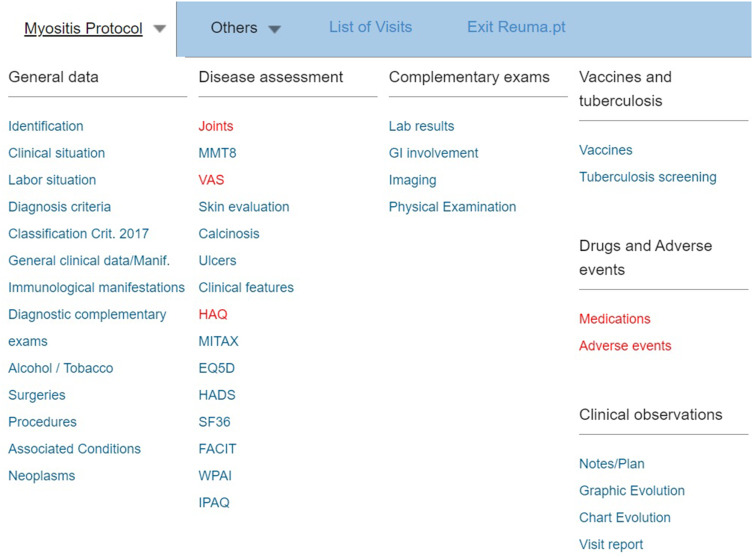
Reuma.pt has several modules organised according to the patient’s diagnosis, contributing to the standardisation of procedures among the Portuguese Rheumatology departments (Figure 1). There is a specific myositis module available since April 2019, developed by us and used in daily practice in our clinic. The Reuma.pt/myositis module displays a table of contents where standard items and disease-specific items are collected (Figure 2). These data also allow us to assess the prevalence of clinical and immunological manifestations in the Portuguese IIM population.
Figure 1.
Modules available on Reuma.pt.
Figure 2.
Myositis module on Reuma.pt.
Several specific disease activity scores are evaluated in this protocol: MMT-8 and Childhood Myositis Assessment Scale (CMAS) for children for muscle involvement, modified skin DAS and other skin features (like the presence of calcinosis, cutaneous ulcers, oedema, mechanic hands, etc.) for skin involvement, the Myositis Intention to Treat Activity Index (MITAX) for joint, gastrointestinal, pulmonary and cardiovascular involvements.
In this specific module, we can also include information on associated malignancies. In addition, questionnaires to assess the functional impact of the disease are also available: Health Assessment Questionnaire (HAQ), 36-Item Short-Form Survey (SF-36), EuroQol-5D (EQ5D), Hospital Anxiety and Depression Scale (HADS), Functional Assessment of Chronic Illness Therapy (FACIT), and Work Productivity and Activity Impairment (WPAI).
Reuma.pt can easily upload blood test results, enabling prospective laboratory data monitoring. In addition, it also has fields for the registry of other exams’ results, such as EMG, muscle MRI or muscle biopsy. Furthermore, we can add information about extra-articular manifestations and exam results (like endoscopy or lung function tests). Finally, it also allows the recording of the patient’s treatment and evaluation of efficacy and safety. These medical records are clinically very valuable and at the same time can be used for research.
This project was approved by the Ethics Committee of the Lisbon Academic Medical Center. Furthermore, Reuma.pt was approved by the National Data Protection Authority and the local ethics committees of the participating centres.
Results
Experience
A total of 185 patients (corresponding to 831 outpatient clinical appointments) were referred and assessed in the CHULN myositis clinic between July 2017 and May 2022. Of these, 138 (75%) were female, with a median age of 58 [45–70] years.
Patients’ referrals to the CHULN myositis clinic came from General Rheumatology clinics (n = 84; 45.4%), General Practitioner (n = 34; 18.4%), Dermatology (n = 17; 9.2%), other Rheumatology subspeciality clinics (n = 13, 7%), Pneumology (n = 11, 5.9%), Internal Medicine (n = 11; 5.9%), Neurology (n = 9; 4.9%), Oncology (n = 2; 1.1%), Emergency Department (n = 2; 1.1%), Immuno-allergology (n = 1; 0.5%) and Infectiology (n = 1; 0.5%).
At the time of the last evaluation, 130 (70.3%) patients had a confirmed inflammatory myopathy, and 55 had a non-confirmed previously suspected IIM. The median disease duration at the last appointment of patients with a confirmed IIM was 4 [2–6] years.
Among the 55 patients with previously suspected inflammatory myopathy, there was 1 case of mitochondrial myopathy, 1 case of glycogenosis type IX, 1 case of myopathy secondary to subclinical hypothyroidism, 1 case of myopathy secondary to glucocorticoids, 1 case of systemic lupus erythematosus, 1 case of primary Sjögren’s syndrome, and 4 cases of fibromyalgia. The other patients had idiopathic CK elevation or are still in etiologic investigation.
Demographic, clinical and immunological features of patients with confirmed inflammatory myopathies at the time of this assessment are described in Table 2. Six patients had myositis-associated neoplasia and in all of them the diagnosis was identified during cancer screening. There were 2 cases of breast cancer, 1 case of ovarian cancer, 1 case of multiple myeloma, 1 case of prostate cancer, and 1 case of rectal cancer. Most were females (83%, n = 5), but all of them had a myositis subtype that implied complete malignancy screening: 3 cases of DM and 3 cases of PM. Five had an age at diagnosis superior to 40 years (83%, n = 5). The sixth patient was a 35-year-old female with a DM diagnosis. No patient had persistent high disease activity or cutaneous necrosis, but dysphagia was documented in 2 cases. One of the patients with dysphagia had positivity to anti-Mi2. Anti-SAE1 was identified in another patient with DM diagnosis.
Table 2.
Demographic, Clinical and Immunological Profile of the Patients Followed at Our Myositis Outpatient Clinic
| DM (n=34, 26.2%) | Antisynthetase Syndrome (n=27, 20.8%) | CADM (n=18, 13.8%) | MCTD (n=17, 13.1%) | Overlap Syndromes* (n=14, 10.8%) | PM (n=10, 7.7%) | UCTD (n=8, 6.2%) | Necrotising Myopathy (n=2, 1.5%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Median age at onset, years [IQR] | 46 [38–62] | 50 [38–66] | 55 [47–62] | 33 [25–50] | 55 [34–69] | 61 [52–71] | 56 [45–69] | 57 (minimal 26, maximal 87) | |
| Female, N (%) | 20 (59%) | 22 (82%) | 13 (72%) | 14 (82%) | 12 (86%) | 8 (80%) | 6 (75%) | 2 (100%) | |
| Median disease duration [IQR] | 4 [3–14] | 4 [3–12] | 3 [2–4] | 5 [3–8] | 3 [2–5] | 5 [1–7] | 3 [2–5] | 7 (minimal 6, maximal 8) | |
| Clinical features | |||||||||
| Median modified DAS skin [IQR] of last appointment | 2 [0–2] | 0 [0–2] | 1 [0–2] | 0 [0–1] | 1 [0–2] | 0 | 0 [0–2] | 0 | |
| Median MMT-8 [IQR] of last appointment | 80 [75–80] | 80 [78–80] | 80 [76–80] | 80 [79–80] | 79 [74–80] | 80 [70–80] | 80 [80–80] | 66 (minimal 52; maximal 80) | |
| Arthritis (52; 40%) | 7 (21%) | 19 (70%) | 7 (39%) | 12 (71%) | 5 (36%) | 1 (10%) | 1 (13%) | 0 | |
| RF (52; 40%) | 4 (12%) | 13 (48%) | 5 (28%) | 13 (76%) | 10 (71%) | 0 | 7 (88%) | 0 | |
| Lung disease (n=38; 29%) | NSIP (29; 22%) | 3 (9%) | 16 (59%) | 2 (11%) | 1 (6%) | 3 (21%) | 1 (10%) | 3 (38%) | 0 |
| UIP (5; 4%) | 0 | 3 (11%) | 0 | 0 | 2 (14%) | 0 | 0 | 0 | |
| COP (2; 1.5%) | 1 (3%) | 0 | 1 (6%) | 0 | 0 | 0 | 0 | 0 | |
| LIP (1; 0.8%) | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| DIP (1; 0.8%) | 0 | 0 | 0 | 0 | 0 | 1 (10%) | 0 | 0 | |
| Mechanic hands (24, 18%) | 3 (9%) | 11 (41%) | 5 (28%) | 1 (6%) | 2 (14%) | 0 | 2 (25%) | 0 | |
| Dysphagia (19; 15%) | 3 (9%) | 4 (15%) | 2 (11%) | 2 (12%) | 3 (21%) | 2 (20%) | 1 (13%) | 2 (100%) | |
| Gastroesophageal reflux disease (11; 8%) | 1 (3%) | 1 (4%) | 1 (6%) | 3 (18%) | 2 (14%) | 1 (10%) | 2 (25%) | 0 | |
| Malignancy (8; 6%) | 3 (9%) | 0 | 0 | 0 | 0 | 3 (30%) | 0 | 0 | |
| Calcinosis (7; 5%) | 4 (12%) | 1 (4%) | 0 | 0 | 1 (7%) | 0 | 1 (13%) | 0 | |
| Heart involvement (n=4; 3%) | Myocarditis (2; 1.5%) | 0 | 0 | 1 (6%) | 0 | 0 | 1 (10%) | 0 | 0 |
| Atrioventricular block (1; 0.8%) | 1 (3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pericardial effusion (1; 0.8%) | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | |
| Death (2; 1.5%) | 0 | 0 | 0 | 0 | 1 (7%) | 1 (10%) | 0 | 0 | |
| Antibodies | |||||||||
| Antinuclear antibody (78; 60%) | 17 (50%) | 19 (70%) | 6 (33%) | 16 (94%) | 11 (79%) | 3 (30%) | 5 (63%) | 1 (50%) | |
| Anti-Ro52 (36; 28%) | 7 (21%) | 16 (59%) | 2 (11%) | 4 (24%) | 4 (29%) | 3 (30%) | 0 | 0 | |
| Anti-Jo1 (17; 13%) | 0 | 17 (63%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anti-U1RNP (17; 13%) | 0 | 0 | 0 | 16 (94%) | 1 (7%) | 0 | 0 | 0 | |
| Rheumatoid factor (11; 8%) | 2 (6%) | 1 (4%) | 1 (6%) | 5 (29%) | 1 (7%) | 0 | 0 | 1 (50%) | |
| Anti-Mi2b (11; 8%) | 4 (12%) | 1 (4%) | 5 (28%) | 0 | 0 | 1 (10%) | 0 | 0 | |
| Anti-MI2a (6; 5%) | 4 (12%) | 0 | 1 (6%) | 0 | 0 | 1 (10%) | 0 | 0 | |
| Anti-Ku (6; 5%) | 3 (9%) | 0 | 1 (6%) | 0 | 2 (14%) | 0 | 0 | 0 | |
| Anti-PmScl75 (6; 5%) | 0 | 0 | 0 | 0 | 5 (36%) | 0 | 1 (13%) | 0 | |
| Anti-MDA5 (5; 4%) | 2 (6%) | 0 | 2 (11%) | 0 | 1 (7%) | 0 | 0 | 0 | |
| Anti-PL7 (5; 4%) | 1 (3%) | 4 (15%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anti-PmScl100 (5; 4%) | 2 (6%) | 0 | 0 | 0 | 3 (21%) | 0 | 0 | 0 | |
| Anti-SAE (4; 3%) | 3 (9%) | 0 | 1 (6%) | 0 | 0 | 0 | 0 | 0 | |
| Anti-SRP (4; 3%) | 0 | 0 | 1 (6%) | 0 | 0 | 1 (10%) | 1 (13%) | 1 (50%) | |
| Anti-PL12 (3; 2%) | 0 | 2 (7%) | 0 | 0 | 0 | 0 | 1 (13%) | 0 | |
| Anti-NXP2 (3; 2%) | 3 (9%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anti-EJ (2; 1.5%) | 1 (3%) | 0 | 1 (6%) | 0 | 0 | 0 | 0 | 0 | |
| Anti-Tif1g (2; 1.5%) | 1 (3%) | 0 | 1 (6%) | 0 | 0 | 0 | 0 | 0 | |
| Anti-ThTo (2; 1.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (25%) | 0 | |
| Anti-Scl70 (1; 0.8%) | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | |
| Anti-RNA-polimerase 3 (1; 0.8%) | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | |
| Anti-NOR90 (1; 0.8%) | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | |
| Treatment | |||||||||
| Oral glucocorticoids (93; 72%) | 24 (71%) | 21 (78%) | 9 (50%) | 16 (94%) | 11 (79%) | 9 (90%) | 2 (25%) | 1 (50%) | |
| Hydroxychloroquine (63; 48%) | 15 (44%) | 11 (41%) | 10 (56%) | 12 (71%) | 9 (64%) | 0 | 6 (75%) | 0 | |
| Methotrexate (40; 31%) | 14 (41%) | 6 (22%) | 4 (22%) | 8 (47%) | 3 (21%) | 3 (30%) | 1 (13%) | 1 (50%) | |
| Mycophenolate mofetil (26; 20%) | 3 (9%) | 13 (48%) | 2 (11%) | 1 (6%) | 4 (29%) | 2 (20%) | 0 | 1 (50%) | |
| Azathioprine (16;12%) | 5 (15%) | 1 (4%) | 1 (6%) | 5 (29%) | 3 (21%) | 1 (10%) | 0 | 0 | |
| Rituximab (11; 8%) | 0 | 2 (7%) | 2 (11%) | 1 (6%) | 2 (14%) | 1 (10%) | 0 | 1 (50%) | |
| Immunoglobulin (11; 8%) | 3 (9%) | 1 (4%) | 1 (6%) | 2 (12%) | 1 (7%) | 2 (20%) | 0 | 1 (50%) | |
| Tacrolimus (5; 4%) | 2 (6%) | 1 (4%) | 1 (6%) | 1 (6%) | 0 | 0 | 0 | 0 | |
| Cyclosporine (2; 1.5%) | 0 | 1 (4%) | 0 | 1 (6%) | 0 | 0 | 0 | 0 | |
| Cyclophosphamide (1; 0.8%) | 0 | 0 | 1 (6%) | 0 | 0 | 0 | 0 | 0 | |
| Leflunomide (1; 0.8%) | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| TNFi (1; 0.8%) | 1 (3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Note: *Overlap syndrome included patients with DM/PM and systemic sclerosis or rheumatoid arthritis, or systemic erythematosus lupus.
Abbreviations: CADM, clinically amyopathic/paucimyopathic dermatomyositis; COP, cryptogenic-organising pneumonia; DAS, disease activity score; DIP-Desquamative interstitial pneumonia; DM, dermatomyositis; ILD, interstitial lung disease; LIP, lymphocytic interstitial pneumonitis; MCTD, mixed connective tissue disease; MMT, manual muscle test; NSIP, nonspecific interstitial pneumonia; PM, polymyositis; RP, Raynaud phenomenon; TNFi, tumor necrosis factor inhibitor; UCTD, undifferentiated connective tissue disease; UIP, usual interstitial pneumonia.
Glucocorticoids (GC) are still in use by 93 patients (71.5%). After GC, the most frequent current treatments are hydroxychloroquine (n = 63; 48.5%), methotrexate (n = 40; 30.8%) and mycophenolate mofetil (n = 26; 20%).
Internal Collaborations
All patients who are candidates for bDMARD therapy are discussed in a weekly biologics’ decision clinic. This joint meeting brings together all medical members of the Rheumatology department, benefitting from the group experience and knowledge. It allows detailed discussions of each case, focusing on the patient’s current disease activity, previous and current treatment, and prognosis to define the best treatment option.
Monthly, there is an ILD multidisciplinary meeting with pneumologists, radiologists and rheumatologists. Here, challenging cases of patients with lung involvement are discussed, and diagnostic and treatment choices are made in a multidisciplinary environment. Also, there is a monthly multidisciplinary Rheumatology/Radiology meeting where musculoskeletal imaging exams, like muscle MRI, can be discussed. Although there is no formal scheduled meeting with Neurology, there is direct contact with a Neuropathologist to perform urgent muscle biopsy histopathologic analysis and discuss their results.
External Collaborations and Scientific Activity
Given the large volume of patients at our myositis clinic and the close collaboration of our group with other centres, we integrate local and multicentric networks and studies. Since 2017 our centre has been included in the European Reference Network on Rare and Complex Connective Tissue and Musculoskeletal Diseases (ERN-ReCONNET). ERN-ReCONNET is a virtual network involving healthcare providers from reference centres across Europe and promotes optimal patient management and the development of common clinical practice guidelines.18 Besides, elements of our centre are part of The International Myositis Society (iMyoS) and The International Myositis Assessment and Clinical Studies Group (IMACS). Thereby, members of the myositis clinic can participate in digital conference discussions of clinical cases with international experts.
Scientific projects in the field of myositis are ongoing and one example of international collaboration is the participation of several members in EULAR-ACR Classification Criteria for Antisynthetase Syndrome (CLASS project).
The team has been scientifically active, contributing with their experience for the progress of knowledge in inflammatory myopathies.19–30
Discussion
IIM may pose significant clinical challenges and different health professionals play a vital role in providing adequate, evidence-based, cost-effective care.31 Multidisciplinary teamwork involves coordinated efforts from professionals with diverse backgrounds and expertise, coming together to manage patients, aiming for the best care. Maintaining the structure of a multidisciplinary clinic requires coordination and teamwork, facilitating communication between peers and increasing the involvement of patients in decisions.
Physical rehabilitation is essential for treating patients with IIM since several studies reported the benefits of exercise on disease activity and inflammation.32–34 Physiotherapy sessions should be prescribed, and several individually tailored physical exercises to enhance muscle strengthening and aerobic capacity are taught to patients.
Skin involvement is frequent in IIM, but cutaneous manifestations may be variable.35 The skin lesions may precede or follow myositis and could be the main manifestation of the disease. Discussions regarding the choice of therapy are adjusted to fit the needs and expectations of each patient.
The close contact with the Neurology Department allows for support in the differential diagnosis of noninflammatory myopathies.
Despite the significant variability of specialities that refer patients, most come from other rheumatologists, demonstrating the recognition of the benefit of the multidisciplinary clinic.
As has been reported in the literature,2 there is a female predominance in our cohort. The clinical features of our patients show multiorgan involvement. Almost 30% of patients had associated ILD, an important cause of morbidity and mortality. The most frequent lung manifestation was nonspecific interstitial pneumonia, as described in the literature.36
As expected, DM was the most frequent type of inflammatory myopathy. The most common manifestations in these patients were arthritis (n = 7, 21%), lung involvement (n = 4, 12%), Raynaud phenomenon (n = 4, 12%) and calcinosis (n = 4, 12%). The most prevalent myositis-specific antibody was anti-Mi2 (n = 8, 23.5%), which is in agreement with the estimated prevalence.35
CADM defines a subgroup of patients with predominant skin involvement and includes both patients with amyopathic dermatomyositis and patients with hypomyopathic dermatomyositis.1 Anti-Mi-2 e anti-MDA5 antibodies were the two most frequent myositis-specific antibodies in CADM patients.
ASS is characterised by multiple organ involvement. Myositis, ILD, arthritis, Raynaud’s phenomenon, mechanic’s hands and skin rashes are typical.27 The hallmark is the presence of anti-aminoacyl-tRNA synthetase (ARS) antibodies, anti-Jo1 being the most common. In our cohort, anti-Jo1 was identified in 17 (63%) of our patients. In addition, anti-Ro52 was identified in 16 (59%) patients, which supports the idea that most ASS patients also have anti-Ro52 autoantibodies.27 As expected, it was in this group of patients that arthritis, lung disease and mechanic’s hands were more common. In overlap myositis, autoantibodies typical of other connective tissue diseases were common, as expected.
A myositis-associated neoplasia was diagnosed in 6 patients. Five of the cancer subtypes were the most usually described: breast, ovary, prostate and colorectal.3,37 There was also one case of multiple myeloma. All patients had high-risk factors for neoplasia and the diagnosis emerged in the cancer screening, following the myositis diagnosis. All of them had a high-risk myositis subtype: 3 cases of DM and 3 cases of PM. Malignancy is described in 25% of patients with DM and 10–15% of PM patients in the literature.37 In our DM cohort, malignancy was only detected in 9% of cases, but more diagnoses may emerge in the coming years. On the other hand, malignancy was diagnosed in 30% of PM patients, the highest rate among all IIM subtypes. However, the age at diagnosis in this group of patients was also higher, and it is known that older patients have a higher risk of malignancy.36,38 Five patients had an age of diagnosis superior to 40 years. Dysphagia, one of the clinical risk factors, was documented in two cases. Regarding antibodies, one patient had positivity to anti-Mi2 and another to anti-SAE1. Although there are a small number of disease-related malignancies in our cohort, we are alert to the possibility of their appearance in the following years. Malignancy is one of the leading causes of death in patients with myositis,36 and our practice is to perform malignancy screening tests whenever justified, as explained previously.
Undifferentiated connective tissue disease (UCTD) patients in our cohort had a low median disease duration. Therefore, we believe some UCTD patients may still develop a well-defined connective tissue disease in the future. The two patients with immune-mediated necrotising myopathy had severe myopathy and dysphagia. They have no other extra-muscular manifestations, in agreement with what has been most commonly described in the literature for this disease subtype.36
In adults, we typically initiate treatment with high-dose glucocorticoids and a steroid-sparing agent at the time of IIM diagnosis. The DMARD choice depends on the main manifestations, but methotrexate, azathioprine, or mycophenolate mofetil are the most common choices.37 Those three agents are usually used in mild to moderate muscle disease.37,39 Methotrexate is often the first option as it is effective on arthritis, one of the most common manifestations in our patients, and has a quick onset on action on muscle disease, comparing to other agents like azathioprine.34 Mycophenolate mofetil is the preferred option when ILD is present, as it is generally effective, well tolerated and has fewer potential side effects than cyclophosphamide.34,40 It works well in myositis as well as the rash of DM.34 Azathioprine is an alternative to mycophenolate mofetil when ILD is present and has adequate efficacy on muscle disease.34,40 In our cohort, these three agents are the most frequently used DMARDs after hydroxychloroquine. The extensive use of hydroxychloroquine may be explained by the fact that DM was the most frequent IIM subtype in our cohort since we use hydroxychloroquine primarily for treating DM’s cutaneous manifestations.
As previously explained, in our myositis clinic, there is a standardised collection of data, namely demographic, clinical, laboratorial and therapeutic data. Every appointment is registered in Reuma.pt/Myositis and thus it is possible to monitor the clinical evolution of the patient, as well as laboratorial changes and response to therapy. This also allows multiple-network integrations and collaboration on national and international projects, once there is a constant updating and easy data collection.
Conclusions
Multidisciplinary clinics contribute to a better coordination of the medical care, consolidation of medical knowledge and clinical skills development. A formal standardised approach to IIM, including patient pathways for diagnosis and follow-up, improves the quality-of-care delivered and supports the organization of research. Collaboration between different departments leads to a quicker and more complete clinical evaluation and is essential to increase the chances of successful management of IIM patients. The continuous registry of patients in Reuma.pt/Myositis has been essential for standardising care and levering research.
Disclosure
Dr Inês Cordeiro reports being a BMS employee, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Lundberg IE, Miller FW, Tjärnlund A, Bottai M. Diagnosis and classification of idiopathic inflammatory myopathies. J Intern Med. 2016;280(1):39–51. doi: 10.1111/joim.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5(2):109–129. doi: 10.3233/JND-180308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208–215. doi: 10.1007/s11926-011-0169-7 [DOI] [PubMed] [Google Scholar]
- 4.Leal I, Romão VC, Mano S, et al. A non-infectious uveitis multidisciplinary clinic in a tertiary referral center: clinical impact and added value. J Multidiscip Healthc. 2021;14:695–704. doi: 10.2147/JMDH.S292981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobo-Ibáñez T, Villaverde V, Seoane-Mato D, et al. Multidisciplinary dermatology-rheumatology management for patients with moderate-to-severe psoriasis and psoriatic arthritis: a systematic review. Rheumatol Int. 2016;36(2):221–229. doi: 10.1007/s00296-015-3377-z [DOI] [PubMed] [Google Scholar]
- 6.Horvath LE, Yordan E, Malhotra D, et al. Multidisciplinary care in the oncology setting: historical perspective and data from lung and gynecology multidisciplinary clinics. J Oncol Pract. 2010;6(6):e21–6. doi: 10.1200/JOP.2010.000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carstens PO, Schmidt J. Diagnosis, pathogenesis and treatment of myositis: recent advances. Clin Exp Immunol. 2014;175(3):349–358. doi: 10.1111/cei.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rider LG, Aggarwal R, Machado PM, et al. Update on outcome assessment in myositis. Nat Rev Rheumatol. 2018;14(5):303–318. doi: 10.1038/nrrheum.2018.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paganoni S, Amato A. Electrodiagnostic evaluation of myopathies. Phys Med Rehabil Clin N Am. 2013;24(1):193–207. doi: 10.1016/j.pmr.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filli L, Maurer B, Manoliu A, Andreisek G, Guggenberger R. Whole-body MRI in adult inflammatory myopathies: do we need imaging of the trunk? Eur Radiol. 2015;25(12):3499–3507. doi: 10.1007/s00330-015-3783-3 [DOI] [PubMed] [Google Scholar]
- 11.Piette Y, Reynaert V, Vanhaecke A, et al. Standardised interpretation of capillaroscopy in autoimmune idiopathic inflammatory myopathies: a structured review on behalf of the EULAR study group on microcirculation in Rheumatic Diseases. Autoimmun Rev. 2022;21(6):103087. doi: 10.1016/j.autrev.2022.103087 [DOI] [PubMed] [Google Scholar]
- 12.Mugii N, Hasegawa M, Matsushita T, et al. Association between nail-fold capillary findings and disease activity in dermatomyositis. Rheumatology. 2011;50(6):1091–1098. doi: 10.1093/rheumatology/keq430 [DOI] [PubMed] [Google Scholar]
- 13.Kubo S, Todoroki Y, Nakayamada S, et al. Significance of nailfold videocapillaroscopy in patients with idiopathic inflammatory myopathies. Rheumatology. 2019;58(1):120–130. doi: 10.1093/rheumatology/key257 [DOI] [PubMed] [Google Scholar]
- 14.Opinc A, Brzezińska O, Makowska J. Underdiagnosis of cardiopulmonary involvement in patients with idiopathic inflammatory myopathies. Reumatologia. 2021;59(5):276–284. doi: 10.5114/reum.2021.110609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACR Meeting Abstracts. Cancer screening recommendations for patients with idiopathic inflammatory myopathy [Internet]; 2022. Available from: https://acrabstracts.org/abstract/cancer-screening-recommendations-for-patients-with-idiopathic-inflammatory-myopathy/. Accessed April 19, 2023.
- 16.Santos MJ, Canhão H, Mourão AF, et al. Reuma.pt contribution to the knowledge of immune-mediated systemic rheumatic diseases. Acta Reumatol Port. 2017;42(3):232–239. [PubMed] [Google Scholar]
- 17.Canhão H, Faustino A, Martins F, Fonseca JE. Rheumatic Diseases Portuguese Register Board Coordination, Portuguese Society of Rheumatology. Reuma.pt - the rheumatic diseases Portuguese register. Acta Reumatol Port. 2011;36(1):45–56. [PubMed] [Google Scholar]
- 18.Our Network | ERN ReCONNET. European reference network on rare and complex connective tissue and musculoskeletal diseases [Internet]; 2022. Available from: https://reconnet.ern-net.eu/our-network/. Accessed April 19, 2023.
- 19.Martins P, Dourado E, Melo AT, et al. Clinical characterisation of a multicentre nationwide cohort of patients with antisynthetase syndrome. ARP Rheumatol. 2022;1(ARP Rheumatology, n°3 2022):190–196. [PubMed] [Google Scholar]
- 20.Bandeira M, Di Cianni F, Marinello D, et al. An overlook on the current registries for rare and complex connective tissue diseases and the future scenario of TogethERN ReCONNET. Front Med. 2022;9:889997. doi: 10.3389/fmed.2022.889997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandeira M, Vieira A, Guimarães V, et al. Off-label use of mycophenolate mofetil in the treatment of rare and complex rheumatic connective tissue diseases. Clin Exp Rheumatol. 2022;40(5):32–39. doi: 10.55563/clinexprheumatol/v1e7s2 [DOI] [PubMed] [Google Scholar]
- 22.Nascimento J, Tenazinha C, Campanilho-Marques R, Cordeiro I, Salgado S. Rituximab in the treatment of anti-MDA5 dermatomyositis-associated interstitial lung disease: a case-based literature review. ARP Rheumatol. 2022;1(2):168–173. [PubMed] [Google Scholar]
- 23.Cavagna L, Meloni F, Meyer A, et al. Clinical spectrum time course in non-Asian patients positive for anti-MDA5 antibodies. Clin Exp Rheumatol. 2022;40(2):274–283. doi: 10.55563/clinexprheumatol/di1083 [DOI] [PubMed] [Google Scholar]
- 24.Abouyahya I, Liem SIE, Amoura Z, et al. Health-related quality of life in patients with mixed connective tissue disease: a comparison with matched systemic sclerosis patients. Clin Exp Rheumatol. 2022;40(5):66–70. doi: 10.55563/clinexprheumatol/x5aras [DOI] [PubMed] [Google Scholar]
- 25.Palermo BL, Bottazzi F, Dourado E, et al. Capillary leak syndrome in a patient with cancer-associated anti-transcriptional intermediary factor 1γ dermatomyositis treated with rituximab. Clin Exp Rheumatol. 2022;40(5):118–120. doi: 10.55563/clinexprheumatol/j26b6o [DOI] [PubMed] [Google Scholar]
- 26.Campanilho-Marques R, Deakin CT, Simou S, et al. Retrospective analysis of infliximab and Adalimumab treatment in a large cohort of juvenile dermatomyositis patients. Arthritis Res Ther. 2020;22(1):79. doi: 10.1186/s13075-020-02164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahler M, Miller FW, Fritzler MJ. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev. 2014;13(4–5):367–371. doi: 10.1016/j.autrev.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deakin CT, Campanilho-Marques R, Simou S, et al. Efficacy and safety of cyclophosphamide treatment in severe juvenile dermatomyositis shown by marginal structural modeling. Arthritis Rheumatol. 2018;70(5):785–793. doi: 10.1002/art.40418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campanilho-Marques R, Almeida B, Deakin C, et al. Comparison of the utility and validity of three scoring tools to measure skin involvement in patients with juvenile dermatomyositis. Arthritis Care Res. 2016;68(10):1514–1521. doi: 10.1002/acr.22867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida B, Campanilho-Marques R, Arnold K, et al. Analysis of published criteria for clinically inactive disease in a large juvenile dermatomyositis cohort shows that skin disease is underestimated. Arthritis Rheumatol. 2015;67(9):2495–2502. doi: 10.1002/art.39200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner EH. Effective teamwork and quality of care. Med Care. 2004;42(11):1037–1039. doi: 10.1097/01.mlr.0000145875.60036.ed [DOI] [PubMed] [Google Scholar]
- 32.Alexanderson H. Exercise in myositis. Curr Treat Options Rheumatol. 2018;4(4):289–298. doi: 10.1007/s40674-018-0113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alemo Munters L, Dastmalchi M, Katz A, et al. Improved exercise performance and increased aerobic capacity after endurance training of patients with stable polymyositis and dermatomyositis. Arthritis Res Ther. 2013;15(4):R83. doi: 10.1186/ar4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pipitone N, Salvarani C. Treatment of inflammatory myopathies. Expert Rev Clin Immunol. 2018;14(7):607–621. doi: 10.1080/1744666X.2018.1491307 [DOI] [PubMed] [Google Scholar]
- 35.DeWane ME, Waldman R, Lu J. Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol. 2020;82(2):267–281. doi: 10.1016/j.jaad.2019.06.1309 [DOI] [PubMed] [Google Scholar]
- 36.Meyer A, Sibilia J. Strategy for suspected myositis. Joint Bone Spine. 2019;86(5):568–575. doi: 10.1016/j.jbspin.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 37.Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88(1):83–105. doi: 10.1016/j.mayocp.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 38.Qiang JK, Kim WB, Baibergenova A, Alhusayen R. Risk of Malignancy in Dermatomyositis and Polymyositis. J Cutan Med Surg. 2017;21(2):131–136. doi: 10.1177/1203475416665601 [DOI] [PubMed] [Google Scholar]
- 39.Ashton C, Paramalingam S, Stevenson B, Brusch A, Needham M. Idiopathic inflammatory myopathies: a review. Intern Med J. 2021;51(6):845–852. doi: 10.1111/imj.15358 [DOI] [PubMed] [Google Scholar]
- 40.van den Bosch L, Luppi F, Ferrara G, Mura M. Immunomodulatory treatment of interstitial lung disease. Ther Adv Respir Dis. 2022;16:17534666221117002. doi: 10.1177/17534666221117002 [DOI] [PMC free article] [PubMed] [Google Scholar]