Abstract
Objectives:
Portal hypertension (PH) is associated with complications such as ascites and esophageal varices, and is typically diagnosed through invasive hepatic venous pressure gradient (HVPG) measurement, which is not widely available. In this study, we aim to assess the diagnostic performance of 2D/3D MR elastography (MRE) and shear wave elastography (SWE) measures of liver and spleen stiffness (LS and SS) and spleen volume, to noninvasively diagnose clinically significant portal hypertension (CSPH) using HVPG measurement as the reference.
Methods:
In this prospective study, patients with liver disease underwent 2D/3D MRE and SWE of the liver and spleen, as well as HVPG measurement. The correlation between MRE/SWE measures of LS/SS and spleen volume with HVPG was assessed. ROC analysis was used to determine the utility of MRE, SWE and spleen volume for diagnosing CSPH.
Results:
36 patients (M/F 22/14, mean age 55±14y) were included. Of the evaluated parameters, 3D MRE SS had the strongest correlation with HVPG (r=0.686, p<0.001), followed by 2D MRE SS (r=0.476, p=0.004). 3D MRE SS displayed the best performance for diagnosis of CSPH (AUC=0.911) followed by 2D MRE SS (AUC=0.845) and 3D MRE LS (AUC=0.804). SWE SS showed poor performance for diagnosis of CSPH (AUC=0.583) while spleen volume was a fair predictor (AUC=0.738). 3D MRE SS was significantly superior to SWE LS/SS (p≤0.021) for diagnosis of CSPH.
Conclusion:
SS measured with 3D MRE outperforms SWE for the diagnosis of CSPH. SS appears to be a useful biomarker for assessing PH severity. These results need further validation.
Keywords: Elasticity Imaging Techniques, Liver, Spleen, Hypertension, Portal
Introduction
Portal hypertension (PH) is defined as an elevated portal venous pressure gradient across the liver due to increased intrahepatic vascular resistance and is a serious complication of advanced liver disease. The reference standard for diagnosis of PH is the invasive measurement of hepatic venous pressure gradient (HVPG); with HVPG >5 mmHg indicating PH and HVPG ≥10 mmHg considered clinically significant (CS)PH [1]. Despite its utility in diagnosing PH, HVPG measurement is generally limited to tertiary referral centers due to the technical expertise and facilities required. Patients with CSPH are at greater risk of developing portosystemic collaterals, bleeding from esophageal varices and experiencing liver decompensation [2; 3] therefore noninvasive tools for early diagnosis of CSPH would be beneficial for effective clinical management such as beta-blocker therapy [4].
Architectural distortion due to liver fibrosis is a frequent cause of PH and so liver stiffness (LS), an indirect measure of fibrosis, offers a useful biomarker to assess PH severity [5] with most of the literature being based on transient elastography (TE) [6–8]. Collagen proportionate area (CPA) obtained from liver biopsy provides a quantitative measure of liver fibrosis, thus comparison of both LS and CPA with HVPG may provide useful information regarding the influence of fibrosis on portal pressure and the utility of LS as a noninvasive surrogate of CPA in the setting of PH. In addition to liver changes in the presence of PH, the spleen undergoes remodeling due to passive congestion, increased arterial inflow, increased hyperactive splenic lymphoid tissue and enhanced angiogenesis and fibrogenesis [9; 10]. This has generated interest in the evaluation of spleen stiffness (SS) as a potential predictor of PH severity [11]. TE, the most widely used elastography technique, has some drawbacks for measurement of SS including lack of integrated imaging complicating localization and high failure rate [12]. 2D magnetic resonance elastography (MRE) and shear wave elastography (SWE) overcome this drawback by offering concomitant imaging capabilities enabling high success rates for SS measurement [12; 13]. More recently 3D MRE, in which the full 3D wave field is acquired [14], has shown promising results for assessing PH based on spleen measurements [15] but no comparison was made with 2D MRE or SWE.
The aim of our study was to assess the diagnostic performance of 2D/3D MRE and SWE measures of liver and spleen stiffness (LS and SS) and spleen volume, to noninvasively diagnose CSPH in patients with liver disease using HVPG measurement as the reference.
Materials and Methods
Patients
This HIPAA‐compliant prospective single centre study was approved by our local Institutional Review Board. Between March 2018 and January 2020, signed informed consent was obtained from 37 patients. Inclusion criteria were: adult patients with liver disease scheduled for clinically indicated HVPG measurement and transjugular liver biopsy or transjugular intrahepatic portosystemic shunt (TIPS) placement within 6 months of research MRI and ultrasound (US) elastography. Six months is a common timeframe used in studies assessing liver fibrosis/cirrhosis which is a key driver of PH [16; 17]. Exclusion criteria were: history of liver transplant; initiation of beta‐blocker therapy within 6 months of MRI (which may cause hemodynamic variation owing to fluctuating dosage); portal vein thrombosis; contraindications to MRI. One patient was excluded from the study due to an interval >6 months between HVPG measurement and imaging. The study protocol can be found at https://clinicaltrials.gov/ct2/show/NCT03436550. The indications for HVPG and transjugular liver biopsy were: characterization of abnormal liver function tests (n=23), confirmation of PH (n=10), TIPS placement (n=2, due to refractory ascites) and ascites (n=1).
HVPG measurement
HVPG measurements were acquired in all patients as previously described using a wedged catheter approach [18]. In the case of TIPS procedures, HVPG was recorded prior to TIPS placement. A transjugular liver biopsy was obtained following HVPG measurement in all cases. The interval between imaging and HVPG measurement was 24.7 ± 22.8 days (range 0–102 days).
MRI acquisition
All patients underwent MRI using a 1.5T system (Magnetom Aera, Siemens Healthineers) with 3 patients receiving additional imaging at 3T (Discovery 750W, GE Healthcare). All patients were imaged following a 6-hour fast to avoid potential postprandial effects on LS [19]. 2D MRE data were acquired axially at mid-liver using a prototype spin-echo echo planar imaging (SE-EPI) sequence with imaging parameters and reconstruction as previously described [20]. Two 19 cm plastic paddles were used to simultaneously generate 60Hz shear waves in the liver and spleen [21]. 2D MRE image acquisition took approximately 15s. Inline elastography image reconstruction was completed in approximately 1 minute. Tissue stiffness was reported in terms of the magnitude of the complex shear modulus |G*|.
3D MRE data were acquired in 30 patients at 1.5T (n=27) or 3.0T (n=3) using prototype SE-EPI sequences. Acquisition time was approximately 1 minute for all three directions of motion. Simultaneous liver and spleen data were acquired over 32 axial slices on both systems. 3D MRE data were reconstructed inline using a prototype 3D multimodal direct inversion (MMDI) reconstruction algorithm at 1.5T and using a previously described inversion algorithm at 3.0T [22]. Inline reconstruction of elastography images was completed in approximately 3 minutes. In addition to MRE, research sequences including 4D flow imaging, dynamic contrast-enhanced MRI, T1, T2 and T1ρ mapping were also acquired and are/will be reported elsewhere. Further details related to the MRI acquisition can be found in the supplementary materials.
SWE acquisition
Immediately before/after the MRI, patients underwent an SWE exam using an Acuson S3000 (Siemens Healthineers, 6C1 HD transducer, n=25) or Acuson Sequoia (Siemens Healthineers, DAX transducer, n=11) US systems with SWE capability (Virtual Touch Imaging Quantification, Siemens Healthineers). SWE measurements were acquired by one of three radiologists (--, -- and -- with 6, 7 and 8 years of experience, respectively), each of whom were blinded to patient’s clinical, pathological and MRE data. Further details of the SWE acquisition are included in the supplementary materials. Briefly, 5 SWE measurements were acquired from both the liver and spleen during neutral breath-hold. Median stiffness (expressed as Young’s Modulus, E, with units of kPa) and interquartile range (IQR)/median ratio were recorded. A measurement with IQR/median ratio of ≤30% was considered reliable. SWE acquisition time varied according to the number of unsuccessful measurements acquired, with an approximate range of 5–15 minutes per patient.
MRE analysis
MRE data were analyzed using ImageJ software [23] by an MR physicist with 5 years of MRE experience (--) who was blinded to HVPG results. Free-hand ROIs were prescribed on the MRE magnitude images in the liver and spleen in areas of reliable measurement while avoiding blood vessels and other structures, and staying 1 cm away from the organ boundaries. ROIs were drawn on each 2D MRE slice, with 3 slices on each end of the 3D MRE slice packet discarded to avoid boundary effects. Mean stiffness values were weighted by ROI size in units of kPa. MRE analysis of 2D and 3D datasets took approximately 20 minutes in total per patient.
SWE analysis
SWE data were analyzed by the same observer (--) who performed the MRE analysis, who remained blinded to the HVPG results. The median value from 5 reliable measurements in both the liver and spleen of each patient was recorded. SWE analysis took approximately 10 minutes per patient.
Spleen volume
Spleen volume was measured on volumetric T1-weighted imaging post-contrast by a radiologist (--) with 1 year experience in abdominal imaging, using Vitrea software 6.7.1 (Canon Medical). Spleen volume was measured as the summation of the product of spleen area and slice thickness on all slices containing the spleen.
Histopathological analysis
Transjugular liver biopsy samples were examined by a liver pathologist (--) with >20 years’ experience who was blinded to the initial clinical read. Fibrosis stage and inflammation grade were evaluated based on METAVIR and Brunt (for NASH) scoring systems. Liver CPA analysis using picrosirius red staining was also performed to quantify fibrosis through direct measurement of collagen in the sample [24]. CPA was performed in order to correlate LS/SS with degree of collagen providing an indication of the impact of fibrosis on LS in the setting of PH.
Statistical Analysis
In order to have 80% power to detect a significant difference (p<0.05) between CSPH and non-CSPH patients based on SS of both groups from a previously published large series [25] we required 28 enrolled patients, with additional patients enrolled in case of unsuccessful data acquisition. The relationship between elastography parameters and HVPG as well as pathological measures were assessed using Spearman correlations. Differences in elastography parameters between patients with and without CSPH were tested using Mann-Whitney U test. The diagnostic performance of elastography parameters and spleen volume for assessment of CSPH and presence of liver cirrhosis was evaluated using paired ROC analysis. AUC values from ROC analysis were considered as follows: 0.90–1.00 – excellent, 0.80–0.89 – good, 0.70–0.79 – fair, 0.50–0.69 – poor [26]. Delong tests were used to assess differences in diagnostic performance for diagnosing CSPH and cirrhosis. Statistical analysis was performed in SPSS (version 27, IBM) with a p-value <0.05 indicating significance. Power analysis was performed using G*Power software (version 3.1.9.2) [27].
Results
Patient characteristics
The final cohort consisted of 36 patients (mean age 54.8±13.8 y, M/F 22/14). Patient characteristics are summarized in Table 1. Mean HVPG was 7.2±5.0 mmHg (range 0 – 18 mmHg). Normal HVPG (no PH) was observed in 14 patients (39%), while 22 patients (61%) were classified as having PH (HVPG >5 mmHg), including 10 with CSPH (28% of total cohort). CPA quantification was performed in 32 patients, with mean CPA of 14.7±9.4% (range 1.8–40.0%).
Table 1:
Clinical and laboratory characteristics of our cohort distributed as patients without CSPH (clinically significant portal hypertension, HVPG <10 mmHg) vs those with CSPH (HVPG ≥10 mmHg). Data are presented as mean±SD (range).
| Parameter | No CSPH (n=26) | CSPH (n=10) | p |
|---|---|---|---|
| Age (y) | 54.1±13.9 (26–78) | 55.0±14.3 (21–67) | 0.794a |
| Gender (M/F) | 15/11 | 7/3 | 0.436b |
| HVPG (mmHg) | 4.7±2.9 (0–9) | 13.7±2.6 (10–18) | <0.001a |
| HVPG-imaging interval (days) | 22.1±19.1 (0–78) | 31.5±30.7 (6–102) | 0.286a |
| Aetiology | |||
| NASH | 6 | 3 | |
| AIH | 6 | 0 | |
| EtOH | 2 | 3 | |
| PSC | 2 | 2 | |
| HCV | 3 | 0 | |
| NSRH | 3 | 0 | |
| Cryptogenic cirrhosis | 0 | 2 | |
| Other | 4 | 0 | |
| Fibrosis stage F0 F1 F2 F3 F4 |
6 4 4 4 8 |
0 0 1 4 5 |
|
| Inflammation grade A0 A1 A2 A3 |
12 2 10 2 |
2 5 3 0 |
|
| AST (IU/L) | 63.8±58.9 (8–244) | 80.2±49.0 (32–172) | 0.135a |
| ALT (IU/L) | 83.6±116.6 (8–557) | 64.4±50.8 9 (17–188) | 0.768a |
| GGT (IU/L) | 108.5±123.5 (13–478) | 403.0±778.7 (15–1973) | 0.494a |
| Alkaline phosphatase (IU/L) | 156.7±145.6 (48–72) | 253.5±185.2 (75–671) | 0.023 a |
| Bilirubin (mg/dl) | 1.75±3.5 (0.4–18) | 2.8±3.5 (0.4–10) | 0.137a |
| Albumin (g/dl) | 3.9±0.5 (3–5) | 3.6±0.6 (3–5) | 0.137a |
| Platelets (103/μL) | 174.8±89.3 (40–416) | 133.8±67.8 (52–252) | 0.303a |
AIH – autoimmune hepatitis, ALT – alanine transaminase, AST- aspartate transaminase, EtOH – alcohol induced liver disease, GGT- gamma-glutamyl transpeptidase, HCV: hepatitis C virus, HVPG – hepatic venous pressure gradient, NSRH – non-specific reactive hepatitis, PSC – primary sclerosing cholangitis.
: p-value derived from Mann-Whitney U-test.
: p-value derived from Fisher’s Exact test.
Bolded p-valuesindicate significance.
Technical success
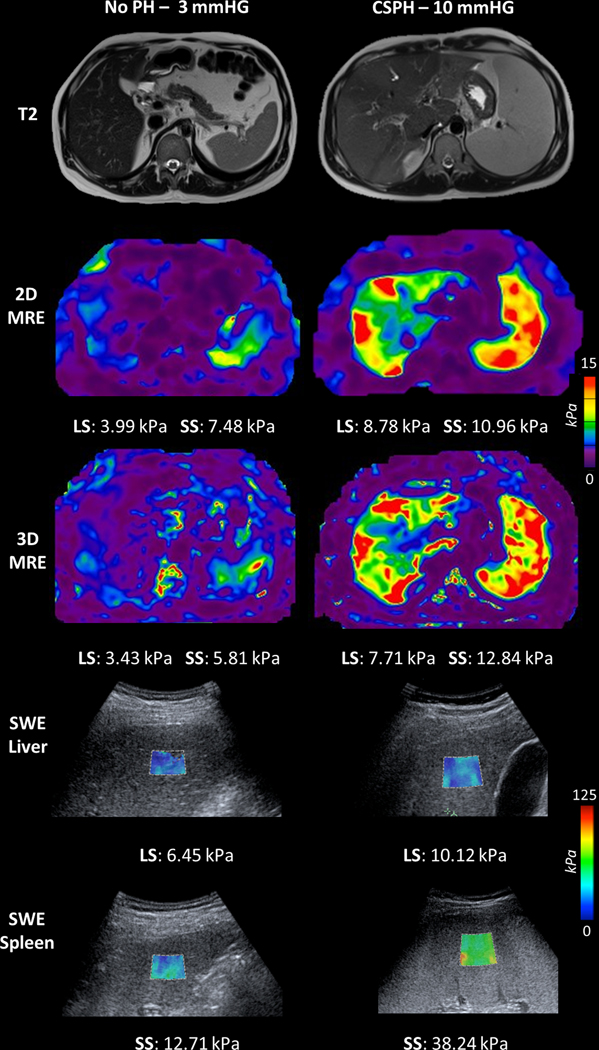
Due to massive ascites and large body habitus, MRE failed in both the liver and spleen in one patient and in the spleen only in another patient. MRE failure rate was 1/36 (2.8%) for the liver and 2/36 (5.6%) for the spleen. SWE measurements did not meet the reliability criteria of IQR/median ≤30% in the liver of 4 patients (11.1%) and the spleen of 3 patients (8.3%). Example images from a patient without PH and a patient with CSPH are shown in Figure 1.
Figure 1:
Illustrative examples showing anatomical images (top row), 2D magnetic resonance elastography (MRE, second row), 3D MRE (third row), liver shear wave elastography (SWE, fourth row) and spleen SWE (bottom row) in a 45-year-old male with stage 2 fibrosis in the setting of primary sclerosing cholangitis (PSC) and normal hepatic venous pressure gradient (HVPG, 3 mmHg, left), and a 22-year-old male with cirrhosis secondary to PSC and elevated HVPG of 10 mmHg indicating clinically significant portal hypertension (CSPH, right). HVPG, liver stiffness (LS) and spleen stiffness (SS) are also shown. LS and SS are increased in patient with CSPH compared to patient with no PH. MRE image scaling has been modified to better illustrate SS (0–15 kPa).
Correlation between imaging parameters and HVPG
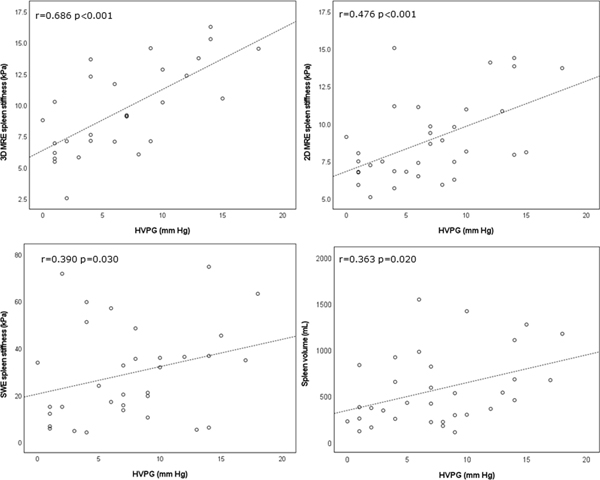
Of the evaluated spleen parameters, SS measured with 3D MRE (r=0.686, p<0.001), 2D MRE (r=0.476, p=0.004), SWE (r=0.390, p=0.030), and spleen volume (r=0.363, p=0.020) were all significantly correlated with HVPG, with 3D MRE SS demonstrating the strongest correlation. Of the evaluated liver parameters, 2D MRE and SWE significantly correlated with HVPG (r=0.407, p=0.015, r=0.355, p<0.05 respectively), while 3D MRE LS did not (r=0.344, p=0.063).
Diagnosis of CSPH
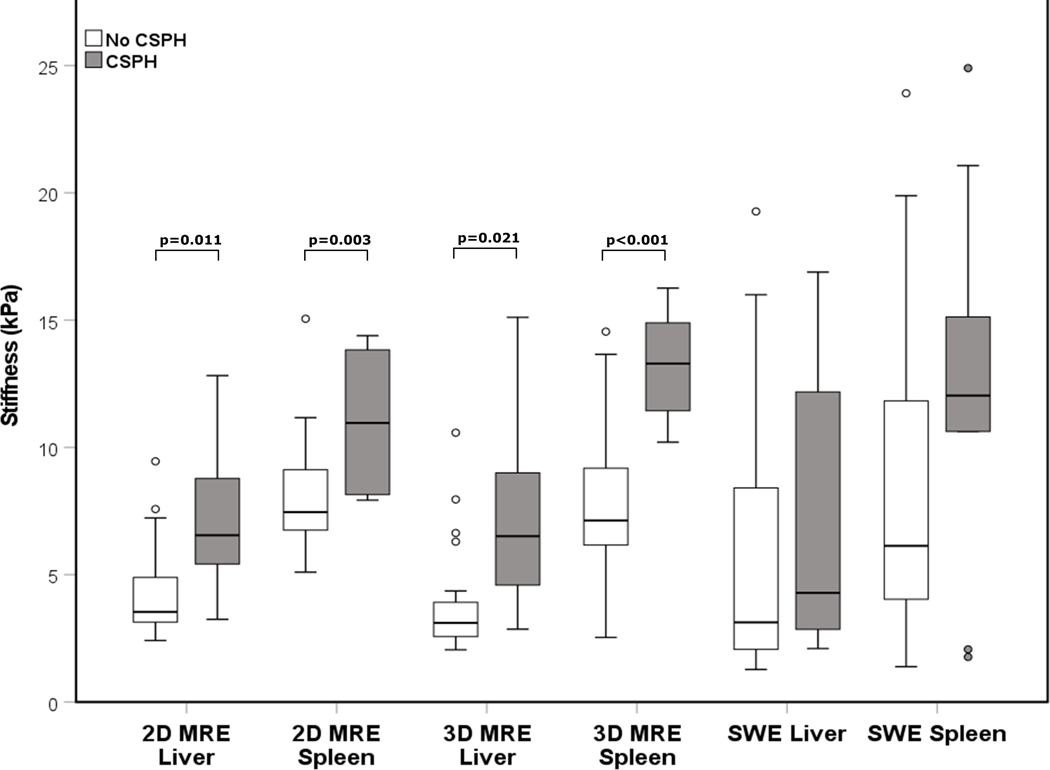
Data showing measurement differences between patients with and without CSPH are shown in Table 2. Patients with CSPH exhibited significantly elevated LS and SS using 3D MRE (p=0.021 and p<0.001 respectively) and 2D MRE (p=0.011 and p=0.003 respectively) but not using SWE (p=0.334 and p=0.094 respectively) (Figures 3, 4). Spleen volume was significantly higher in CSPH compared to non-CSPH patients (p=0.010).
Table 2:
Imaging and histopathologic measures (mean ± SD) in patients without CSPH (clinically significant portal hypertension, HVPG <10 mmHg) vs. those with CSPH (HVPG ≥10 mmHg).
| Parameter | No CSPH | CSPH | p* |
|---|---|---|---|
| LS-2D MRE (kPa) | 4.7±3.0 | 7.1±2.9 | 0.011 |
| LS-3D MRE (kPa) | 4.7±4.1 | 7.3±3.9 | 0.021 |
| LS-SWE (kPa) | 17.4±17.5 | 22.9±18.1 | 0.187 |
| SS-2D MRE (kPa) | 8.0±2.2 | 11.3±2.8 | 0.003 |
| SS-3D MRE (kPa) | 8.3±2.9 | 13.2±2.2 | <0.001 |
| SS-SWE (kPa) | 23.1±17.1 | 41.7±20.6 | 0.043 |
| Spleen volume (mL) | 452.6±333.7 | 794.8±408.4 | 0.010 |
| CPA (%)** | 15.5±14.5 | 17.7±7.31 | 0.207 |
LS: liver stiffness, MRE: magnetic resonance elastography, SS: spleen stiffness, SWE: shear wave elastography, CPA: collagen proportionate area
p-values derived from Mann-Whitney U-test
n=32
Bolded p-values indicate significance.
Figure 3:
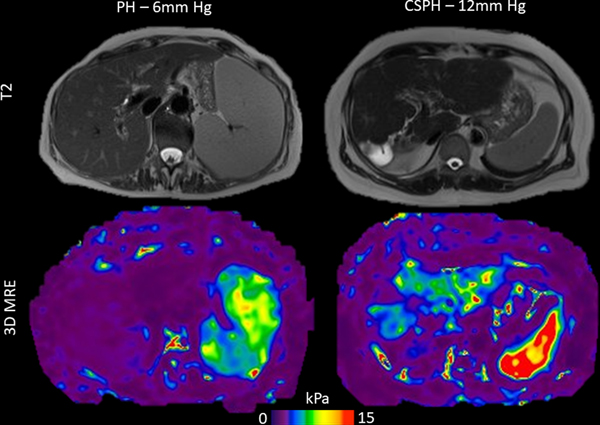
Illustration of the discordance between spleen volume and hepatic venous pressure gradient (HVPG). Left: 79-year-old female patient with drug induced liver injury and mild portal hypertension (HVPG=6 mmHg) and splenomegaly (volume 1,541 mL) with normal spleen stiffness on 3D MRE (7.6 kPa, below threshold of 9.6 kPa). Right: 58-year-old female patient with cryptogenic cirrhosis and clinically significant portal hypertension (CSPH, HVPG=12 mmHg) and normal spleen size (volume 358 mL), however with increased spleen stiffness on 3D MRE (14.2 kPa, above threshold of 9.6 kPa). MRE image scaling has been modified to better illustrate spleen stiffness (0–15 kPa scale).
Figure 4:
Boxplot distributions of liver and spleen stiffness measured with magnetic resonance elastography (MRE) and shear wave elastography (SWE) in patients with clinically significant portal hypertension (CSPH) vs those without CSPH. No significant difference was observed for SWE.
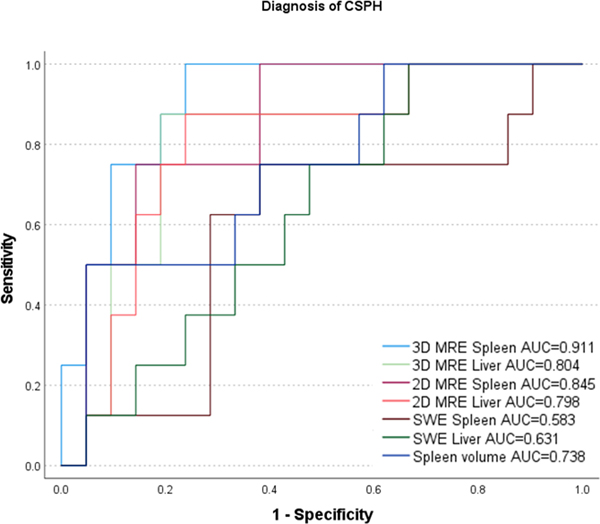
Results from ROC analysis are shown in Table 3 and Figure 5. MRE SS had the best diagnostic performance for diagnosis of CSPH, with an AUC of 0.911 (p<0.001) for 3D MRE and 0.845 (p<0.001) for 2D MRE, with no significant difference between 2D and 3D MRE (p=0.202). LS measured with 3D and 2D MRE (AUC=0.804, p=0.001 and AUC=0.798, p=0.001, respectively) and spleen volume (AUC=0.738, p=0.024) showed good/fair performance. SWE LS (AUC=0.631, p=0.228), SWE SS (AUC=0.583, p=0.128) showed poor, non-significant, performance for diagnosis of CSPH. SS measured with 3D MRE was significantly superior to SWE LS (p=0.015) and SWE SS (p=0.021), while 2D/3D MRE LS and 2D MRE SS were not significantly superior to SWE measures (p>0.065, Table 4).
Table 3:
ROC analysis of imaging parameters for the diagnosis of CSPH (clinically significant portal hypertension).
| Parameter | AUC | CI | p | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| LS-2D MRE (kPa) | 0.798 | 0.614–0.981 | 0.001 | 4.4 | 85.7 | 76.2 |
| LS-3D MRE (kPa) | 0.804 | 0.620–0.987 | 0.001 | 4.4 | 85.7 | 81.0 |
| LS-SWE (kPa) | 0.631 | 0.418–0.844 | 0.228 | 6.2 | 100.0 | 33.3 |
| SS-2D MRE (kPa) | 0.845 | 0.696–0.994 | <0.001 | 8.0 | 100.0 | 61.9 |
| SS-3D MRE (kPa) | 0.911 | 0.807–1.000 | <0.001 | 9.6 | 100 | 76.2 |
| SS-SWE (kPa) | 0.583 | 0.332–0.834 | 0.128 | 26.1 | 75.0 | 61.9 |
| Spleen volume (mL) | 0.738 | 0.532–0.945 | 0.024 | 1039.2 | 50.0 | 95.2 |
AUC: area under the curve, CI: confidence intervals, LS: liver stiffness, SS: spleen stiffness, MRE: magnetic resonance elastography, SWE: shear wave elastography
Bolded p-values indicate significance
Figure 5:
ROC plots illustrating diagnostic performance of elastography parameters and spleen volume in diagnosing CSPH.
Table 4:
Multiple ROC comparison of imaging parameters for the diagnosis of CSPH (clinically significant portal hypertension), using Delong test.
| Parameter | LS-2D MRE | LS-3D MRE | LS-SWE | SS-2D MRE | SS-3D MRE | SS-SWE | Spleen volume |
|---|---|---|---|---|---|---|---|
| LS-2D MRE | 0.731 | 0.114 | 0.64 | 0.209 | 0.197 | 0.685 | |
| LS-3D MRE | 0.109 | 0.686 | 0.231 | 0.188 | 0.657 | ||
| LS-SWE | 0.083 | 0.015 | 0.639 | 0.412 | |||
| SS-2D MRE | 0.202 | 0.065 | 0.353 | ||||
| SS-3D MRE | 0.021 | 0.093 | |||||
| SS-SWE | 0.297 |
LS: liver stiffness, SS: spleen stiffness, MRE: magnetic resonance elastography, SWE: shear wave elastography
Bolded p-values indicate significance
Correlation between imaging parameters and CPA
LS measured with 3D MRE (r=0.551, p=0.003), 2D MRE (r=0.472, p=0.006), and SWE (r=0.498, p=0.006) were all correlated with quantitative CPA measurement, while MRE/SWE SS and spleen volume did not (p=0.074/0.251/0.833). HVPG did not correlate with CPA (r=0.288, p=0.105).
Diagnosis of cirrhosis
The performance of MRE and SWE for diagnosing cirrhosis is described in the supplementary materials.
Discussion
In this prospective study, we investigated the ability of 2D/3D MRE and SWE measures of LS and SS for diagnosing CSPH. We found that SS measured with MRE, showed utility in identifying patients with CSPH from those with HVPG <10 mmHg. 3D MRE SS outperformed SWE for diagnosis of CSPH in our cohort.
The utility of SS for PH severity assessment has shown promise in previous meta-analyses [11; 28; 29], however few studies investigated the relationship between spleen MRE measures and HVPG [15; 30]. In our study we found 3D MRE SS had the strongest correlation with HVPG. Ronot et al report similar results, with 3D MRE spleen parameters providing the strongest correlation with HVPG however no 2D MRE or SWE data were acquired [15]. In contrast to our results, Wagner et al reported no correlation between 2D MRE SS and HVPG [30]. This could be explained by lack of 3D MRE spleen acquisition, however in the current study we found both 2D and 3D MRE SS to be significantly correlated with HVPG. The differing intervals between HVPG and MRE may be one cause for the discordant results, with a mean interval of 24 days in the current study and 78 days in the study by Wagner et al. Other potential causes for discordance include differing MRE paddle placement and the use of GRE MRE sequences at 3T. Similarly to an earlier study by Gharib et al [31], Wagner et al reported a correlation between 2D MRE LS and HVPG, a result replicated in the current study.
SWE SS and LS were found to significantly correlate with HVPG, however both were not significant predictors of CSPH in this cohort. Numerous previous studies have found SWE LS and SS to correlate with HVPG [32–34]. We found SWE of the liver and spleen to have an AUC in the range 0.58–0.63 for diagnosis of CSPH. These values are slightly below the published AUC range of 0.61–0.84 for SWE SS and 0.72–0.86 for SWE LS, with the small sample size of our study and varying liver disease etiology potentially a cause for the lack of significant results.
Our results showed that 3D MRE did not significantly outperform 2D MRE for diagnosis of CSPH in this initial cohort, however 3D MRE SS showed significantly superior diagnostic performance compared to SWE LS/SS, a result not found with 2D MRE SS. 2D and 3D MRE LS showed similar performance for diagnosis of CSPH. From these results, it appears that 3D MRE does not provide additional benefit when examining LS, but may be clinically useful for measuring SS. The improved performance of SS over LS for diagnosing CSPH may be related to changes associated with increasing PH severity. As liver fibrosis progresses in chronic liver disease, the subsequent morphological changes cause elevated intrahepatic resistance and concomitant portal pressure increase [35]. However, as PH progresses extrahepatic factors such as hyperdynamic circulation begin to play a role in maintaining elevated portal pressure which are not reflected in LS measurement [36]. We see evidence of this in our study with quantitative collagen quantification not correlating significantly with HVPG, suggesting liver fibrosis is not the only factor affecting portal pressure. As expected, LS performs well for diagnosing cirrhosis when measured with MRE and SWE.
The results from this study highlight the diagnostic potential of SS measurements over LS measurements for assessing severity of PH. A single MRI exam incorporating liver and spleen MRE may be an effective and comprehensive solution for cirrhotic patients, incorporating both liver cancer screening and assessment of PH severity.
Our study has several limitations. The sample size was small as this reflects our initial experience comparing MRE and SWE for diagnosis of CSPH. In 3/30 patients with 3D MRE measures, data were acquired at 3T rather than 1.5T. Mean 3D MRE LS variation between 1.5 T and 3 T is low (~ 5%) [37], however the difference in spleen measures has not been evaluated. The etiology of liver disease was varied and although all patients had biopsy, the majority were noncirrhotic. Finally, HVPG measurement was not performed on the same day as the research exams.
In conclusion, our initial results demonstrate that MRE SS, particularly 3D MRE SS, has excellent diagnostic performance for diagnosis of CSPH and outperforms SWE. These promising results require validation in a larger cohort.
Supplementary Material
Figure 2:
Scatter plots displaying significant correlations between each of spleen stiffness measured with 3D MRE (top left), 2D MRE (top right), shear wave elastography (SWE, bottom right) as well as spleen volume (bottom left) and hepatic venous pressure gradient (HVPG). The strongest correlation was observed for SS measured with 3D MRE.
Key points:
Spleen stiffness measured with 2D and 3D MR elastography correlates significantly with hepatic venous pressure gradient measurement.
Spleen stiffness measured with 3D MR elastography demonstrates excellent performance for diagnosis of clinically significant portal hypertension (AUC 0.911).
Spleen stiffness measured with 3D MR elastography outperforms liver and spleen stiffness measured with shear wave elastography for diagnosis of clinically significant portal hypertension.
Abbreviations:
- CPA
collagen proportionate area
- CSPH
clinically significant portal hypertension
- HVPG
hepatic venous pressure gradient
- LS
liver stiffness
- MRE
magnetic resonance elastography
- PH
portal hypertension
- SS
spleen stiffness
- SWE
shear wave elastography
- TE
transient elastography
- US
ultrasound
References
- 1.de Franchis R (2015) Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 63:743–752 [DOI] [PubMed] [Google Scholar]
- 2.Ripoll C, Groszmann R, Garcia-Tsao G et al. (2007) Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 133:481–488 [DOI] [PubMed] [Google Scholar]
- 3.Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC (2009) The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 6:573–582 [DOI] [PubMed] [Google Scholar]
- 4.Leithead JA, Rajoriya N, Tehami N et al. (2015) Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut 64:1111–1119 [DOI] [PubMed] [Google Scholar]
- 5.Kennedy P, Bane O, Hectors SJ et al. (2020) Noninvasive imaging assessment of portal hypertension. Abdominal Radiology. 10.1007/s00261-020-02729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vizzutti F, Arena U, Romanelli RG et al. (2007) Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 45:1290–1297 [DOI] [PubMed] [Google Scholar]
- 7.Bureau C, Metivier S, Peron JM et al. (2008) Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Alimentary Pharmacology & Therapeutics 27:1261–1268 [DOI] [PubMed] [Google Scholar]
- 8.Lemoine M, Katsahian S, Ziol M et al. (2008) Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Alimentary Pharmacology & Therapeutics 28:1102–1110 [DOI] [PubMed] [Google Scholar]
- 9.Bolognesi M, Merkel C, Sacerdoti D, Nava V, Gatta A (2002) Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis 34:144–150 [DOI] [PubMed] [Google Scholar]
- 10.Mejias M, Garcia-Pras E, Gallego J, Mendez R, Bosch J, Fernandez M (2010) Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J Hepatol 52:529–539 [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Wilson MP, Katlariwala P, Murad MH, McInnes MDF, Low G (2020) Accuracy of liver and spleen stiffness on magnetic resonance elastography for detecting portal hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 10.1097/MEG.0000000000001724 [DOI] [PubMed] [Google Scholar]
- 12.Elkrief L, Rautou P-E, Ronot M et al. (2014) Prospective Comparison of Spleen and Liver Stiffness by Using Shear-Wave and Transient Elastography for Detection of Portal Hypertension in Cirrhosis. Radiology 275:589–598 [DOI] [PubMed] [Google Scholar]
- 13.Talwalkar JA, Yin M, Venkatesh S et al. (2009) Feasibility and Significance of in vivo Mean Spleen Stiffness Measurement by Magnetic Resonance Elastography for Assessing Portal Hypertension. AJR American journal of roentgenology 193:122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy P, Wagner M, Castera L et al. (2018) Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology 286:738–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronot M, Lambert S, Elkrief L et al. (2014) Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. European Radiology 24:1394–1402 [DOI] [PubMed] [Google Scholar]
- 16.Sethasine S, Jain D, Groszmann RJ, Garcia-Tsao G (2012) Quantitative histological-hemodynamic correlations in cirrhosis. Hepatology 55:1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G (2006) Histological-hemodynamic correlation in cirrhosis—a histological classification of the severity of cirrhosis. Journal of Hepatology 44:111–117 [DOI] [PubMed] [Google Scholar]
- 18.Hectors SJ, Bane O, Stocker D et al. (2020) Splenic T1rho as a noninvasive biomarker for portal hypertension. J Magn Reson Imaging. 10.1002/jmri.27087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B (2014) Quantitative Liver MRI Combining Phase Contrast Imaging, Elastography, and DWI: Assessment of Reproducibility and Postprandial Effect at 3.0 T. PLoS One 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy P, Bane O, Hectors SJ et al. (2020) Magnetic resonance elastography vs. point shear wave ultrasound elastography for the assessment of renal allograft dysfunction. Eur J Radiol 126:108949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyvorne HA, Jajamovich GH, Besa C, Cooper N, Taouli B (2015) Simultaneous measurement of hepatic and splenic stiffness using MR elastography: preliminary experience. Abdominal Imaging 40:803–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL (2015) Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J Magn Reson Imaging 41:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvaruso V, Burroughs AK, Standish R et al. (2009) Computer-assisted image analysis of liver collagen: relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology 49:1236–1244 [DOI] [PubMed] [Google Scholar]
- 25.Jansen C, Bogs C, Verlinden W et al. (2017) Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: A prospective multicentre study. Liver International 37:396–405 [DOI] [PubMed] [Google Scholar]
- 26.Pines JM, Carpenter CR, Raja AS, Schuur JD (2013) Evidence-Based Emergency Care: Diagnostic Testing and Clinical Decision Rules. John Wiley & Sons [Google Scholar]
- 27.Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160 [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Wang L, Wu H et al. (2016) Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS One 11:e0165786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manatsathit W, Samant H, Kapur S et al. (2018) Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J Gastroenterol Hepatol 33:1696–1706 [DOI] [PubMed] [Google Scholar]
- 30.Wagner M, Hectors S, Bane O et al. (2018) Noninvasive prediction of portal pressure with MR elastography and DCE-MRI of the liver and spleen: Preliminary results. J Magn Reson Imaging 48:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gharib AM, Han MAT, Meissner EG et al. (2017) Magnetic Resonance Elastography Shear Wave Velocity Correlates with Liver Fibrosis and Hepatic Venous Pressure Gradient in Adults with Advanced Liver Disease. BioMed Research International 2017:2067479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Procopet B, Berzigotti A, Abraldes JG et al. (2015) Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol 62:1068–1075 [DOI] [PubMed] [Google Scholar]
- 33.Jansen C, Bogs C, Verlinden W et al. (2017) Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: A prospective multicentre study. Liver Int 37:396–405 [DOI] [PubMed] [Google Scholar]
- 34.Zhu YL, Ding H, Fu TT et al. (2019) Portal hypertension in hepatitis B-related cirrhosis: Diagnostic accuracy of liver and spleen stiffness by 2-D shear-wave elastography. Hepatol Res 49:540–549 [DOI] [PubMed] [Google Scholar]
- 35.Lim JK, Groszmann RJ (2007) Transient elastography for diagnosis of portal hypertension in liver cirrhosis: Is there still a role for hepatic venous pressure gradient measurement? Hepatology 45:1087–1090 [DOI] [PubMed] [Google Scholar]
- 36.Colecchia A, Montrone L, Scaioli E et al. (2012) Measurement of Spleen Stiffness to Evaluate Portal Hypertension and the Presence of Esophageal Varices in Patients With HCV-Related Cirrhosis. Gastroenterology 143:646–654 [DOI] [PubMed] [Google Scholar]
- 37.Trout AT, Serai S, Mahley AD et al. (2016) Liver Stiffness Measurements with MR Elastography: Agreement and Repeatability across Imaging Systems, Field Strengths, and Pulse Sequences. Radiology 281:793–804 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.