Abstract
Background
Inflammation and oxidative stress contribute to the development of diabetic nephropathy (DN). Baicalin (BA) shows renal protection against DN through its anti-inflammatory and anti-oxidant properties. However, the molecular mechanism by which BA exerts the therapeutic effects on DN remains to be investigated.
Methods
The db/db mice and high glucose (HG)-induced HK-2 cells were used as the in vivo and in vitro model of DN, respectively. The effects of BA were assessed by detecting the related blood and urine biochemical parameters, kidney histopathology, inflammatory cytokine production, oxidative stress indicators, and apoptosis. Cell viability and apoptosis were detected by CCK-8 assay and TUNEL assay, respectively. Related protein levels were measured by an immunoblotting method.
Results
In db/db model mice, BA reduced serum glucose concentration, decreased blood lipid levels, ameliorated kidney functions, and decreased histopathological changes in kidney tissues. BA also alleviated oxidative stress and inflammation in db/db mice. In addition, BA blocked the activation of sphingosine kinases type 1/sphingosine 1-phosphate (SphK1/S1P)/NF-κB pathway in db/db mice. In HK-2 cells, BA hindered HG-induced apoptosis, oxidative stress and inflammation, while overexpression of SphK1 or S1P could reverse these effects. BA alleviated HG-induced apoptosis, oxidative stress and inflammation in HK-2 cells through the S1P/NF-κB pathway. Furthermore, BA blocked the NF-κB signaling by diminishing p65 nuclear translocation via the SphK1/S1P pathway.
Conclusion
Our study strongly suggests that BA protects against DN via ameliorating inflammation, oxidative stress and apoptosis through the SphK1/S1P/NF-κB pathway. This study provides a novel insight into the therapeutic effects of BA in DN.
Keywords: diabetic nephropathy, baicalin, SphK1/S1P/NF-κB pathway, inflammation, oxidative stress
Introduction
Diabetic nephropathy (DN), the most frequent complication of diabetes mellitus, is the leading cause of end-stage renal disease and is responsible for significantly increased cardiovascular morbidity and mortality worldwide.1 The pathological mechanisms behind DN progression are multifactorial, including the interactions between oxidation, inflammation, apoptosis, and ultimately fibrosis in the kidney.2 There is strong evidence that inflammation and oxidative stress are vital risk factors in the occurrence and progression of DN.3,4 Current treatments can only slow the progression of DN but cannot cure it.5 Therefore, it is necessary to develop safe and effective drugs to treat DN.6
Baicalin (BA), a natural flavone glycoside extracted from Scutellaria root, displays multiple pharmacological effects, such as antineoplastic, antimicrobial, antioxidant, and anti-inflammatory activities.7 BA is reported to exert the protective effects in various human diseases. For instance, BA has been reported as a potent anti-tumor agent for many cancer cell lines by targeting different pathways, such as PI3K/Akt and NF-κB signaling.8 BA attenuates myocardial ischaemia/reperfusion injury through repressing the JAK/STAT pathway to exert anti-inflammatory activity.9 BA protects against fluoxetine-induced hepatic injury by repressing inflammation and oxidative stress.10 BA has also been found to alleviate the progression of DN by relieving podocyte injury,11 diminishing renal fibrosis,12 and improving renal function.13 Moreover, BA exerts a renoprotective role in DN by suppressing inflammation and oxidative stress.14 However, the molecular basis underlying BA-mediated inhibitory effects on DN remains to be further observed.
Sphingosine kinase 1 (SphK1), a lipid kinase responsible for phosphorylating sphingosine into sphingosine-1-phosphate (S1P), can regulate various cellular processes in a variety of pathophysiological conditions, such as cancer, chronic inflammation, and metabolic diseases.15 The SphK1/S1P signaling pathway has been reported to be dysregulated in numerous pathological and disease states.16 The SphK1/S1P pathway, activated by hyperglycemia, advanced glycation end products, and many proinflammatory cytokines, facilitates glomerular mesangial cell proliferation and extracellular matrix accumulation in DN.17 The SphK1/S1P pathway has also been found to be associated with the protective effects of several natural compounds on DN. For instance, polydatin alleviates the pathological progression of DN by inhibiting AGEs-induced fibronectin (FN) and intercellular adhesion molecule-1 (ICAM-1) expression by the inactivation of the SphK1-S1P signaling pathway.18 Resveratrol exerts a renoprotective effect in the cell model of DN through suppressing the SphK1/S1P/NF-κB signaling pathway.19 However, there is no study deciphering the correlation between the SphK1/S1P/NF-κB pathway and the protective effect of BA in DN.
By using db/db mice and HG-induced HK-2 cells, we found that BA suppressed inflammation, oxidative stress, and apoptosis in DN by inactivating the SphK1/S1P/NF-κB pathway.
Materials and Methods
Animal Experiments
Eight-week-old male db/db mice and db/m mice were purchased from the Model Animal Research Center of Nanjing University. All mice were housed in a specific pathogen-free standard environment (temperature, 24 ± 2°C; humidity, 55 ± 5%) with a 12 h light/dark cycle and were given access to food and water ad libitum. After one week of acclimatization, diabetic db/db mice were randomly grouped into four designated groups (model group, 25 mg/kg treatment group, 50 mg/kg treatment group, and 100 mg/kg treatment group), and with 10 mice in each group. Ten db/m mice were used as the control group. In the BA treatment groups, mice were administered with the indicated dose of BA (MedChemExpress, Shanghai, China) once a day via gavage. Meantime, db/m mice in the control group and db/db mice in the model group were gavaged with the same volume of normal saline daily. The drug intervention lasted for 12 weeks. Body weight and fasting blood glucose were periodically measured. Animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University and performed according to the National Institutes of Health guide for the care and use of Laboratory animals.
Measurement of Fasting Blood Glucose
During the experiment, the blood sample was harvested from mice via the tail vein. Fasting blood glucose was monitored by an ACCU-CHEK Performa glucose meter (Roche, Shanghai).
Blood Collection
At the end of the dosing cycle, the mice were fasted for 12 h and anesthetized with 10% chloral hydrate. Then, the blood was obtained from the eyeball. After being kept at room temperature for 30 min, the blood samples were separated by centrifugation at 3000 rpm for 15 min at 4°C to separate the serum. The serum samples were frozen at −80°C for further biochemical assays.
Urine Collection
At the end of the dosing cycle, all mice were individually fed in metabolic cages and 24 h urine samples were collected. The urine samples were centrifuged at 1000 g for 10 min at 4°C to obtain the supernatant. Urine samples were stored at −80°C for further analysis.
Serum Biochemical Assay
The levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum creatinine (Scr), and blood urea nitrogen (BUN) were measured by an automatic biochemical analyzer (Beckman Coulter, Brea, CA, USA).
Urine Parameter Assay
Urinary albumin levels were determined using an ELISA kit (Abcam, Cambridge, MA, USA). The creatinine levels were measured with an automatic biochemical analyzer (Beckman). Urinary albumin to creatinine ratio (UACR) was calculated.
Histopathological Observation
At the end of the experiment, the mice were euthanized and the kidneys were harvested for further analysis. Kidney tissues were fixed in 4% paraformaldehyde for 24 h at 4°C, dehydrated with gradient ethanol, embedded in paraffin, sectioned (5 µm thickness), and then stained with hematoxylin and eosin (H&E). A light microscope was used to observe the histological phenotypes of H&E-stained sections.
Cell Line and Cell Treatment
Human kidney proximal tubular cells (HK-2) (ATCC, Rockville, MD, USA) were cultured in DMEM/F12 medium (Gibco, Waltham, MA, USA) with 10% FBS and 1% penicillin-streptomycin at 37°C and 5% CO2. For high glucose (HG) stimulation, HK-2 cells were exposed to a complete culture medium containing 30 mM glucose (Sigma-Aldrich, St. Louis, MO, USA) for 48 h. Cells cultured with 5.5 mM glucose were used as a control. For BA administration, HK-2 cells were maintained in growth media for 48 h in the presence of 10 µM of BA. To inactivate the NF-κB signaling, HK-2 cells were pretreated with 7.5 µM BAY11-7085 (Sigma-Aldrich) for 1 h prior to BA treatment.
Plasmid, siRNA, and Transient Transfection
Human SphK1 expression plasmid pECMV-SPHK1-3×FLAG (ov-SphK1) and S1P expression construct pEnCMV-S1P-3×FLAG (ov-S1P) were acquired from Sangon Biotech (Shanghai, China). To inhibit S1P expression, siRNA targeting S1P (si-S1P) was synthesized from GenePharma Co., Ltd (Shanghai, China). For transfection, HK-2 cells were plated at 1×105 cells per well in 6-well culture dishes. The next day, 10 nM of si-S1P or 3 μg of plasmids were transfected into cells using Lipofectamine 3000 (Invitrogen), as described by the manufacturer. Cells were harvested after 24 h and then subjected to co-treatment of HG+BA or HG+BA+BAY11-7085 as described above.
Determination of Oxidative Stress Markers
The frozen kidney tissues were homogenized with pre-cooled PBS and then centrifuged at 10,000 g for 10 min. Then, the supernatant was collected to evaluate the oxidative stress indicators. The superoxide dismutase (SOD) activity, catalase (CAT) activity, and malondialdehyde (MDA) levels in kidney tissues and HK-2 cells were measured by commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as per the instruction.
Detection of Pro-Inflammatory Cytokines
Following the manufacturer’s guidance, the levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) in mice serum samples and HK-2 cell culture supernatant were detected by corresponding ELISA kits.
Immunoblotting
Kidney tissues and HK-2 cells were homogenized in RIPA buffer (Beyotime, Shanghai, China) containing 1% PMSF and protease inhibitor cocktail. The protein concentration was analyzed with a BCA kit (Beyotime). The protein lysate (30 µg per lane) was loaded on 10% SDS-PAGE and then transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The membranes were incubated with primary antibodies including anti-SphK1, anti-S1P, anti-p65, anti-p-p65, anti-GAPDH, and anti-Lamin B at 4°C overnight. After 3 washes with TBST, the membranes were probed with the secondary antibody at 37°C for 1 h. The protein bands were visualized using the enhanced chemiluminescence Kit (Millipore) and quantified with the Image J software.
CCK-8 Viability Assay
HK-2 cell viability was analyzed using Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). Cells (4 × 103/well) were seeded in 96-well culture dishes and stimulated with HG, HG+BA, or HG+BA+BAY11-7085 as described above. At the indicated time points, CCK-8 reagent (10 µL) was added for 1.5 h of incubation. The optical density at 450 nm was determined with a Bio-Rad microplate reader.
Lactate Dehydrogenase (LDH) Activity Assay
The LDH activity in HK-2 cells culture supernatant was analyzed with LDH Cytotoxicity Assay Kit (Beyotime).
TUNEL Apoptosis Assay
HK-2 cell apoptosis was examined using TUNEL Apoptosis Assay Kit (Solarbio, Beijing, China). Briefly, treated cells were fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100 and incubated with a TUNEL reaction mixture for 1 h at 37°C. After the nucleus staining with DAPI for 10 min, TUNEL-positive cells were observed under a fluorescence microscope.
Statistical Analysis
All values are expressed as means ± SD. Statistical analysis was performed using ANOVA with Dunnett’s post-hoc test on GraphPad Prism 8. The statistical significance was defined at P < 0.05.
Results
BA Ameliorates Hyperglycemia and Hyperlipidemia Symptoms in db/db Mice
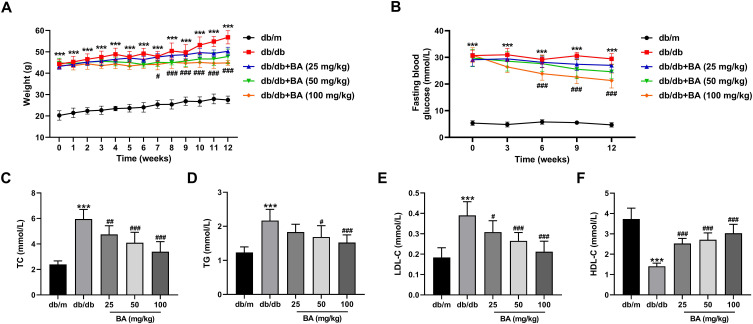
db/db mice were used as the DN model in vivo to validate the relieving effect of BA on DN. Compared with the db/m mice, the body weight and fasting blood glucose levels were significantly higher in db/db mice (Figure 1A and B). After 7 weeks of BA administration, db/db mice displayed lower weight gain (Figure 1A). From the sixth week, BA treatment significantly reduced the fasting blood glucose levels in db/db mice (Figure 1B). We also determined the impact of BA on blood lipid levels, including TC, TG, LDL-C, and HDL-C, at the end of the experiment. Compared with the db/m mice, we observed increased TC (Figure 1C), TG (Figure 1D), and LDL-C (Figure 1E), as well as decreased HDL-C (Figure 1F) in db/db mice. However, BA administration resulted in dose-dependent decrease in TC, TG, and LDL-C and increase in HDL-C in db/db mice (Figure 1C–F). These data indicated that BA reduced blood glucose and lipid levels in db/db mice.
Figure 1.
BA reduces blood glucose concentration and relieves the symptom of hyperlipidemia in db/db mice. (A–F) Diabetic db/db mice were administrated with various doses of BA (25, 50, and 100 mg/kg) or the same volume of normal saline (model control) for 12 weeks. The db/m mice were used as the negative controls. (A) Body weight was measured weekly during the experiment. (B) Fasting blood glucose levels of the mice were detected every 3 weeks during the experiment. (C–F) The levels of TC (C), TG (D), LDL-C (E), and HDL-C (F) in the serum samples of the mice at the end of the experiment. ***P<0.001 vs db/m mice; #P<0.05, ##P<0.01, and ###P<0.001 vs db/db model mice.
Abbreviations: TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
BA Improves Kidney Functions and Histopathological Injury in db/db Mice
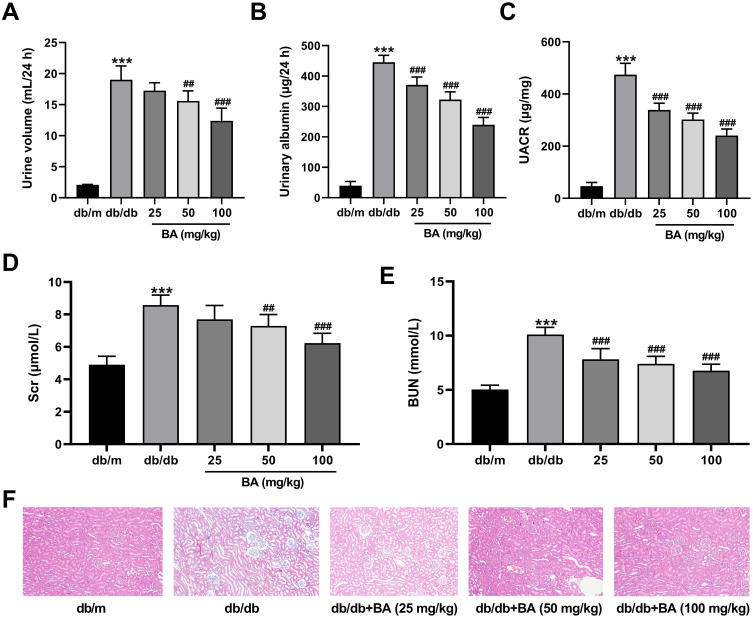
Next, we confirmed the protective effect of BA on kidney functions in db/db mice. Compared with control db/m mice, db/db mice had higher levels of 24-h urine volume (Figure 2A), urinary albumin (Figure 2B), and UACR (Figure 2C). However, these parameters were dose-dependently reduced by BA (Figure 2A–C). We also examined the serum levels of SCr and BUN. Compared with the db/m mice, both Scr (Figure 2D) and BUN (Figure 2E) levels were increased in db/db mice. After BA treatment, Scr and BUN levels in db/db mice were suppressed (Figure 2D and E). H&E staining of kidney tissues showed thickened glomerular basement membrane, dilated mesangial matrix, and increased inflammatory infiltration in db/db mice, but these pathological changes were attenuated by BA treatment (Figure 2F). These data demonstrated that BA improved kidney functions and abated histopathologic changes in db/db mice.
Figure 2.
BA ameliorates renal microstructure injury and kidney functions in db/db mice. (A–E) Effects of BA on renal function indexes, including 24-h urine volume (A), urinary albumin (B), UACR (C), Scr (D), and BUN (E). (F) Representative images for H&E staining of kidney tissues in the mice. ***P<0.001 vs db/m mice; ##P<0.01 and ###P<0.001 vs db/db model mice.
Abbreviations: UACR, urine albumin/creatinine ratio; Scr, serum creatinine; BUN, blood urea nitrogen.
BA Weakens Oxidative Stress and Inflammation in db/db Mice
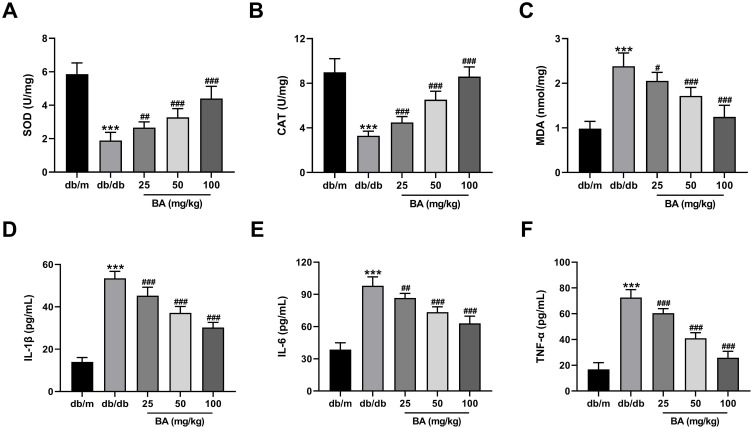
We further determined the influence of BA on oxidative stress and inflammatory response in db/db mice. Administration of db/db mice with BA dose-dependently increased SOD (Figure 3A) and CAT (Figure 3B) activities and reduced MDA levels (Figure 3C) in the kidney tissues of db/db mice. Moreover, BA administration also lowered the levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in serum samples of db/db mice (Figure 3D–F). Together, BA attenuated oxidative stress and inflammation in db/db mice.
Figure 3.
BA diminishes oxidative stress and inflammation in db/db mice. (A–C) SOD activity (A), CAT activity (B), and MDA level (C) in the kidneys of mice treated with various doses of BA (25, 50 and 100 mg/kg) or the same volume of normal saline for 12 weeks. (D–F) IL-1β secretion (D), IL-6 production (E), and TNF-α level (F) in the serum samples of mice in all groups at the end of the experiment. ***P<0.001 vs db/m mice; #P<0.05, ##P<0.01, and ###P<0.001 vs db/db model mice.
Abbreviations: SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
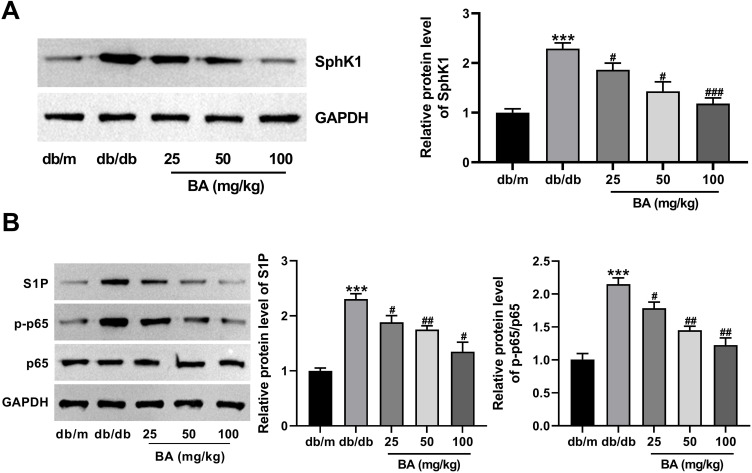
BA Inactivates the SphK1/S1P/NF-κB Signaling Pathway in db/db Mice
The SphK1/S1P-mediated NF-κB signaling pathway is involved in LPS-induced inflammation and fibrosis in rat glomerular mesangial cells, suggesting its significance in the pathogenesis of DN.19 Thus, we attempted to elucidate whether this pathway is associated with the ameliorative effect of BA on DN. The results showed that db/db model mice exhibited higher protein expressions of SphK1, S1P, and p-p65 in the kidneys than those in the db/m control group (Figure 4A and B). Intriguingly, BA administration downregulated the expression of these proteins dose-dependently (Figure 4A and B). In conclusion, BA treatment repressed the activation of SphK1/S1P/NF-κB signaling in db/db mice.
Figure 4.
BA suppresses the activation of SphK1/S1P/NF-κB signaling in db/db mice. (A and B) SphK1, S1P, p65, and p-p65 protein expression in the kidney tissues from different groups was analyzed using immunoblotting. ***P<0.001 vs db/m mice; #P<0.05, ##P<0.01, and ###P<0.001 vs db/db model mice.
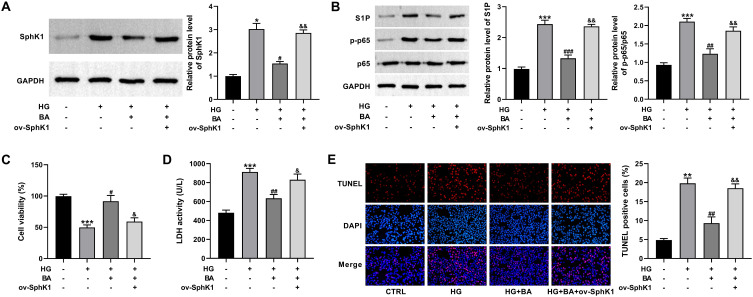
Overexpression of SphK1 Reverses the Inhibition of BA on Apoptosis in HG-Induced HK-2 Cells
Then, we further investigated whether the protective effects of BA on DN were associated with the downregulation of SphK1 in HG-induced HK-2 cells. To address this, an SphK1 cDNA plasmid (ov-SphK1) was introduced into HK-2 cells prior to HG and BA stimulation. Treatment of BA significantly decreased SphK1, S1P, and p-p65 protein expressions in HG-treated HK-2 cells, whereas the introduction of ov-SphK1 greatly eliminated these effects (Figure 5A and B). Additionally, BA treatment promoted cell viability, reduced LDH activity, and hindered apoptosis in HG-treated HK-2 cells, while these influences were effectively reversed by the overexpression of SphK1 (Figure 5C–E). Collectively, BA inhibited HG-induced cell injury by blocking the SphK1 signaling.
Figure 5.
Upregulation of SphK1 abolishes the effects of BA on cell viability and apoptosis in HG-treated HK-2 cells. (A–E) SphK1 protein expression (A), S1P, p65, and p-p65 protein levels (B), CCK-8 assay of cell viability (C), LDH activity (D), and TUNEL assay of apoptosis (E) in HK-2 cells transfected with or without ov-SphK1 before treatment with HG (30 mM) or HG+BA (10 µM). *P<0.05, **P<0.01, and ***P<0.001 vs control; #P<0.05, ##P<0.01, and ###P<0.001 vs HG treatment; &P<0.05 and &&P<0.01 vs HG+BA treatment.
Abbreviation: LDH, lactate dehydrogenase.
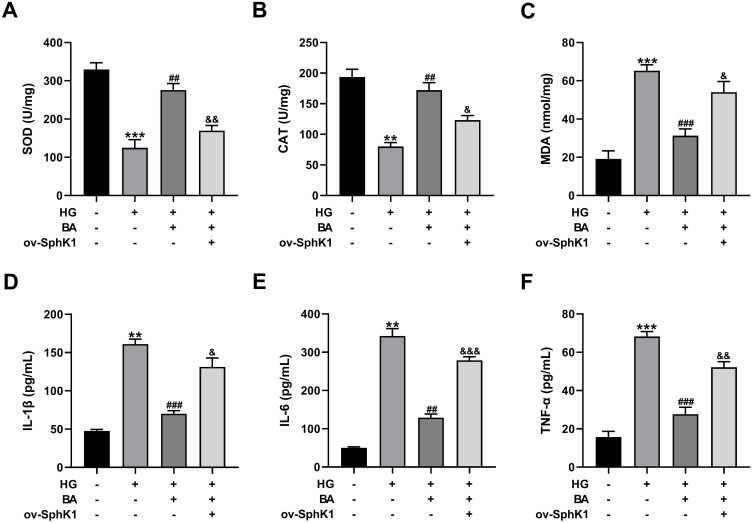
Overexpression of SphK1 Abates the Inhibitory Effects of BA on Oxidative Stress and Inflammation in HG-Induced HK-2 Cells
Treatment with BA resulted in the increase of SOD and CAT activities and the reduction of MDA levels in HG-treated HK-2 cells. However, these effects were weakened by SphK1 upregulation (Figure 6A–C). In addition, the inhibitory effects of BA on the production of inflammatory factors (IL-1β, IL-6, and TNF-α) were effectively alleviated by SphK1 overexpression (Figure 6D–F). These results supported our hypothesis that BA relieved HG-induced oxidative stress and inflammation in HK-2 cells by suppressing SphK1 expression.
Figure 6.
Overexpression of SphK1 reverses the effects of BA on oxidative stress and inflammation in HG-treated HK-2 cells. (A–C) SOD activity (A), CAT activity (B), and MDA level (C) in HK-2 cells transfected with or without ov-SphK1 before treatment with HG (30 mM) or HG+BA (10 µM). (D–F) IL-1β secretion (D), IL-6 production (E), and TNF-α level (F) in the culture supernatant of HK-2 cells treated as indicated. **P<0.01 and ***P<0.001 vs control; ##P<0.01 and ###P<0.001 vs HG treatment; &P<0.05, &&P<0.01, and &&&P<0.001 vs HG+BA treatment.
Abbreviations: SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
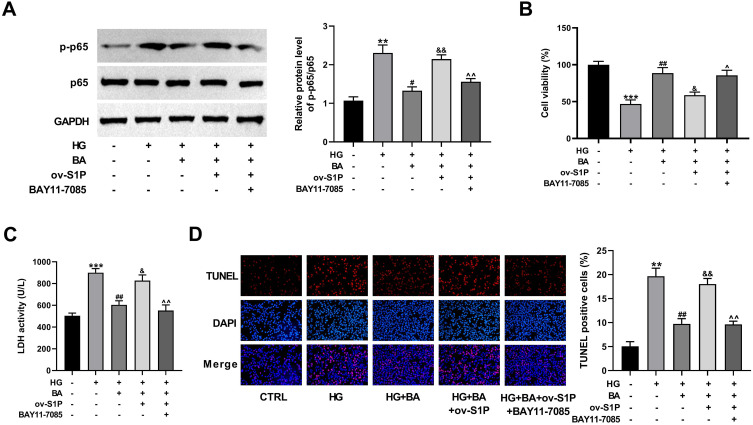
BA Attenuates HG-Induced Apoptosis in HK-2 Cells by Inactivating the S1P/NF-κB Pathway
To elucidate whether the S1P/NF-κB signaling is responsible for the protective effect of BA on DN, NF-κB inhibitor BAY11-7085 was used to treat ov-S1P-transfected HK-2 cells under HG and BA stimulation. As validated by immunoblotting, upregulation of S1P abolished the BA-driven reduction of p-p65 protein expression in HG-treated HK-2 cells, while co-treatment of BAY11-7085 significantly abated the activation of the NF-κB signaling (Figure 7A). These data suggested that BA inactivated NF-κB signaling by inhibiting S1P expression. The ameliorative effect of BA on cell viability in HG-treated HK-2 was attenuated due to S1P overexpression, while cell viability was increased after BAY11-7085 treatment (Figure 7B). The inhibitory effect of BA on HG-induced cytotoxicity and apoptosis was weakened by S1P overexpression, while LDH activity and apoptosis were decreased after further treatment with BAY11-7085 (Figure 7C and D). Based on the above findings, BA inhibited HG-induced cytotoxicity and apoptosis in HK-2 cells by blocking the NF-κB signaling pathway via down-regulating S1P expression.
Figure 7.
BA alleviates HG-induced cell apoptosis via the S1P/NF-κB pathway. (A–D) HK-2 cells transfected with or without ov-S1P were stimulated with HG, HG+BA, or HG+BA+BAY11-7085. (A–D) p65 and p-p65 protein levels (A), cell viability (B), LDH activity (C), and cell apoptosis (D) in HK-2 cells treated as indicated. **P<0.01 and ***P<0.001 vs control; #P<0.05 and ##P<0.01 vs HG treatment; &P<0.05 and &&P<0.01 vs HG+BA treatment; ^P<0.05 and ^^P<0.01 vs ov-S1P+HG+BA treatment.
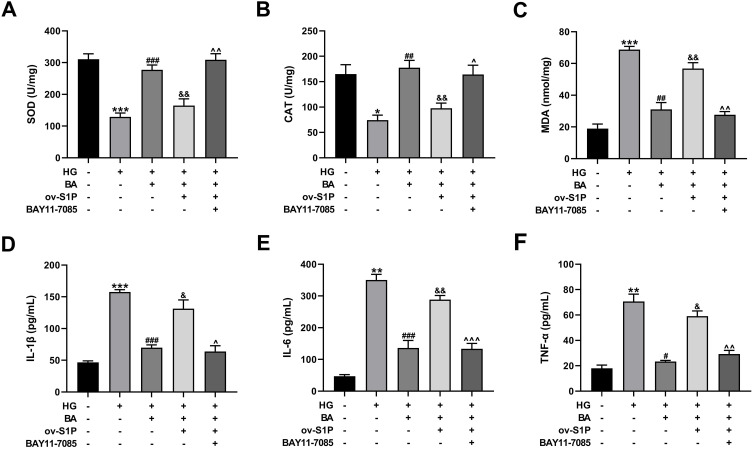
BA Attenuates HG-Induced Oxidative Stress and Inflammation in HK-2 Cells by Inactivating the S1P/NF-κB Pathway
After transfection with ov-S1P overexpression plasmid, the alleviating effect of BA on HG-induced oxidative stress in HK-2 cells was reversed, while SOD and CAT activities were increased and MDA levels were reduced after further treatment with BAY11-7085 (Figure 8A–C). The alleviating effect of BA on HG-induced inflammation in HK-2 cells was reversed by S1P overexpression; however, the levels of IL-1β, IL-6, and TNF-α in the culture supernatant were decreased after further treatment with BAY11-7085 (Figure 8D–F). These results indicated that BA alleviated HG-induced oxidative stress and inflammation in HK-2 cells by inhibiting S1P expression to inactivate the NF-κB signaling pathway.
Figure 8.
BA alleviates HG-induced oxidative stress and inflammation via the S1P/NF-κB pathway. (A–F) HK-2 cells transfected with or without ov-S1P were stimulated with HG, HG+BA, or HG+BA+BAY11-7085. SOD activity (A), CAT activity (B), and MDA level (C) in kidney tissues. IL-1β secretion (D), IL-6 production (E), and TNF-α expression (F) in serum samples. *P<0.05, **P<0.01 and ***P<0.001 vs control; #P<0.05, ##P<0.01, and ###P<0.001 vs HG treatment; &P<0.05 and &&P<0.01 vs HG+BA treatment; ^P<0.05, ^^P<0.01, and ^^^P<0.001 vs ov-S1P+HG+BA treatment.
Abbreviations: SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
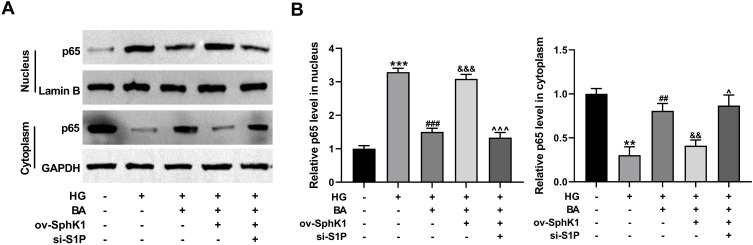
BA Suppresses the Nuclear Translocation of NF-κB in HG-Treated HK-2 Cells Through the SphK1/S1P Pathway
Nuclear translocation of NF-κB is an indicator of the activation of NF-κB signaling.20 In HK-2 cells, HG significantly increased the protein level of NF-κB p65 in the nucleus and reduced p65 level in the cytoplasm (Figure 9A and B), suggesting that HG can promote nuclear translocation of p65 in HK-2 cells. Interestingly, BA treatment repressed p65 nuclear translocation in HG-treated HK-2 cells, while enforced expression of S1P reversed this effect (Figure 9A and B). However, S1P overexpression-mediated promotion of p65 nuclear translocation was abolished by the silencing of S1P in HG-induced HK-2 cells with BA treatment (Figure 9A and B). All these findings suggest that BA inactivates the NF-κB signaling in HG-treated HK-2 cells by suppressing p65 nuclear translocation by the SphK1/S1P pathway.
Figure 9.
BA suppresses HG-induced p65 nuclear translocation in HK-2 cells by the SphK1/S1P pathway. (A and B) HK-2 cells transfected with ov-SphK1 or ov-SphK1+si-S1P were treated with HG or HG+BA. (A) NF-κB p65 protein levels in the nucleus and cytoplasm were detected by immunoblotting assay, with Lamin B and GAPDH as the nuclear and cytoplasmic controls, respectively. (B) Quantitative analysis of NF-κB p65 protein levels in the nucleus and cytoplasm. **P<0.01 and ***P<0.001 vs control; ##P<0.01 and ###P<0.001 vs HG treatment; &&P<0.01 and &&&P<0.001 vs HG+BA treatment; ^P<0.05 and ^^^P<0.001 vs ov-SphK1+HG+BA treatment.
Discussion
Diabetic nephropathy (DN), a microvascular complication in diabetics, occupies 40% of patients requiring renal replacement therapy.21,22 Inflammation, oxidative stress, and apoptosis play important roles in the occurrence and progression of DN.23 New drugs targeting inflammation and oxidative stress display the potential value in preventing, delaying, and treating DN.24,25 Natural products flavonoids, including BA, are reported to alleviate DN primarily by regulating oxidative stress and inflammation.26 Our experiments confirmed that BA exerted protective effects in DN by reducing inflammation, oxidative stress, and apoptosis. Furthermore, SphK1/S1P/NF-κB signaling pathway was found to be responsible for the protective effects of BA in DN.
Poor glycemic control and production of advanced glycation end products (AGEs) are associated with the development of DN.27 Dyslipidemia is also demonstrated as a significant contributor to the pathogenesis of DN.28 Thus, controlling serum glucose levels and lipid metabolism is essential to prevent or delay diabetes complications.29 Diabetic db/db mice have been widely used as an in vivo model of DN to perform investigations in DN pathogenesis.30,31 By using db/db mice as the mouse model of DN, we found that BA reduced fasting blood glucose levels and weight gain in diabetic mice after 12 weeks of treatment. In addition, the serum levels of TC, TG, and LDL-C were decreased and HDL-C level was increased in BA-treated db/db mice. These results suggested that BAprotected against DN by exerting hypoglycemic and hypolipidemic effects.
During the progression of DN, glomerulosclerosis, renal dysfunction, and proteinuria are usually observed. Clinically, albuminuria is the most sensitive biomarker for diagnosing the onset of DN and is generally evaluated by calculating the UACR.32 Scr and BUN are also reported as typical indicators of renal function.33 In the current study, BA treatment abated the kidney damage in db/db mice, as evidenced by the decrease of 24-h urine volume, urinary albumin, UACR, Scr, and BUN. Consistently, H&E staining also confirmed that BA attenuated the structural alterations, such as glomerular basement membrane thickening, mesangial matrix expansion, and inflammatory infiltration, in kidney tissues. These data indicated the renoprotective effect of BA in DN.
Oxidative stress and inflammation are major drivers in the pathogenesis of DN.34 Inflammatory cytokines, including IL-1β, IL-6, and TNF-α, are closely involved in the development and progression of DN.35 Excessive ROS production can activate the NF-κB signaling to induce the release of cytokines, and the generated pro-inflammatory factors further promote ROS production in DN.36 Here, we found that BA repressed oxidative stress in DN mice and HG-stimulated HK-2 cells by increasing SOD and CAT activities and decreasing MDA levels. Moreover, BA inhibited inflammation in DN mice and HG-stimulated HK-2 cells by reducing the levels of IL-1β, IL-6, and TNF-α. These findings demonstrated that BA exerted a beneficial effect on DN by alleviating oxidative stress and inflammation. Consistent with our findings, Ou et al revealed that BA attenuated podocyte damage in DN rats partially via the suppression of inflammatory responses and oxidative stress by inactivating the PI3K/Akt/mTOR pathway.11 As demonstrated by Yang et al, BA might improve renal function and delay disease progression in DN through its anti-inflammatory and anti-oxidative properties.13 Ma et al reported that BA treatment suppressed inflammation and oxidative stress in DN by inhibiting the MAPK and Nrf2 signaling.14 Additionally, previous reports showed that BA treatment repressed the renal fibrosis in streptozotocin (STZ)-induced DN mouse model and HG-treated HK-2 cells.12,37 Together with our current results, these data suggest that BA may relieve renal injury and improve renal function in DN through its anti-inflammatory and anti-oxidative activities.
The SphK1/S1P/NF-κB signaling pathway is implicated in the regulation of inflammation in different diseases, such as spinal cord injury, acute liver failure, and haemorrhagic cystitis.38–40 Inactivation of SphK1/S1P/NF-κB pathway by diacerein leads to suppression of apoptosis and oxidative stress in an LPS-induced rat model of acute lung injury.41 It is worth noting that resveratrol inhibited LPS-mediated inflammation and fibronectin expression in rat glomerular mesangial cells by blocking the SphK1/S1P/NF-κB pathway.19 These findings prompt us to examine whether SphK1/S1P/NF-κB pathway is a downstream mediator of BA in protecting against DN. Expectedly, we uncovered, for the first time, that BA suppressed the activation of SphK1/S1P/NF-κB pathway in db/db model mice and HG-stimulated HK-2 cells. Using a series of restore experiments, we demonstrated that the SphK1/S1P/NF-κB pathway is responsible for the repressive effects of BA on apoptosis, inflammation, and oxidative stress in HG-treated HK-2 cells. More importantly, we proved that BA blocked the NF-κB signaling in HG-treated HK-2 cells by suppressing p65 nuclear translocation through the SphK1/S1P pathway. Similarly, resveratrol suppresses p65 nuclear translocation to inactivate the NF-κB signaling in LPS-induced rat glomerular mesangial cells through the SphK1/S1P pathway.19 Based on these findings, BA appears to be a promising new anti-DN drug that can suppress inflammation and oxidative stress. Extensive studies are required to elucidate the safety and long-term efficacy of BA in various DN experimental models. However, owing to the low bioavailability, BA has a huge limitation in clinical application.26 The field of improving its pharmacokinetic parameters in the form of liposomes and nanoparticles is warranted.
In summary, these data highlight that BA exerts a renoprotective effect in DN through suppression of inflammation and oxidative stress by inactivating the SphK1/S1P/NF-κB pathway. These findings provide a theoretical basis for developing BA as a potential therapeutic drug against DN.
Funding Statement
The present study was supported by Henan Province Key Research and Development and Promotion Special Project (212102310804), Henan Province University Key Project (223320008), and Henan Province Medical Science and Technology Key Project (SBGJ202002069).
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(S1):3–15. doi: 10.1111/dom.14007 [DOI] [PubMed] [Google Scholar]
- 2.Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi: 10.1155/2021/1497449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno JA, Gomez-Guerrero C, Mas S, et al. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs. 2018;27(11):917–930. doi: 10.1080/13543784.2018.1538352 [DOI] [PubMed] [Google Scholar]
- 4.Hernandez LF, Eguchi N, Whaley D, Alexander M, Tantisattamo E, Anti-Oxidative IH. Therapy in diabetic nephropathy. Front Biosci. 2022;14(2):14. doi: 10.31083/j.fbs1402014 [DOI] [PubMed] [Google Scholar]
- 5.Zoja C, Xinaris C, Macconi D. Diabetic nephropathy: novel molecular mechanisms and therapeutic targets. Front Pharmacol. 2020;11:586892. doi: 10.3389/fphar.2020.586892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palabıyık E, Sulumer AN, Uguz H, et al. Assessment of hypolipidemic and anti-inflammatory properties of walnut (Juglans regia) seed coat extract and modulates some metabolic enzymes activity in triton WR-1339-induced hyperlipidemia in rat kidney, liver, and heart. J Mol Recognit. 2023;36(3):e3004. doi: 10.1002/jmr.3004 [DOI] [PubMed] [Google Scholar]
- 7.Huang T, Liu Y, Zhang C. Pharmacokinetics and bioavailability enhancement of baicalin: a review. Eur J Drug Metab Pharmacokinet. 2019;44(2):159–168. doi: 10.1007/s13318-018-0509-3 [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Meena A, Luqman S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol Res. 2021;164:105387. doi: 10.1016/j.phrs.2020.105387 [DOI] [PubMed] [Google Scholar]
- 9.Xu M, Li X, Song L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm Biol. 2020;58(1):655–663. doi: 10.1080/13880209.2020.1779318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganguly R, Kumar R, Pandey AK. Baicalin provides protection against fluoxetine-induced hepatotoxicity by modulation of oxidative stress and inflammation. World J Hepatol. 2022;14(4):729–743. doi: 10.4254/wjh.v14.i4.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou Y, Zhang W, Chen S, Deng H. Baicalin improves podocyte injury in rats with diabetic nephropathy by inhibiting PI3K/Akt/mTOR signaling pathway. Open Med. 2021;16(1):1286–1298. doi: 10.1515/med-2021-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Xu L, Liang R, Yang C, Wang P. Baicalin suppresses renal fibrosis through microRNA-124/TLR4/NF-κB axis in streptozotocin-induced diabetic nephropathy mice and high glucose-treated human proximal tubule epithelial cells. J Physiol Biochem. 2020;76(3):407–416. doi: 10.1007/s13105-020-00747-z [DOI] [PubMed] [Google Scholar]
- 13.Yang M, Kan L, Wu L, Zhu Y, Wang Q. Effect of baicalin on renal function in patients with diabetic nephropathy and its therapeutic mechanism. Exp Ther Med. 2019;17(3):2071–2076. doi: 10.3892/etm.2019.7181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma L, Wu F, Shao Q, Chen G, Xu L, Lu F. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Devel Ther. 2021;15:3207–3221. doi: 10.2147/DDDT.S319260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulkoski-Gross MJ, Obeid LM. Molecular mechanisms of regulation of sphingosine kinase 1. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(11):1413–1422. doi: 10.1016/j.bbalip.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr Gandy KA, Obeid LM. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim Biophys Acta Mol Cell Biol Lipids. 2013;1831(1):157–166. doi: 10.1016/j.bbalip.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Y, Lan T, Huang J, Huang H. Sphingosine Kinase-1/sphingosine 1-phosphate pathway in diabetic nephropathy. Chin Med J. 2014;127(16):3004–3010. [PubMed] [Google Scholar]
- 18.Chen C, Huang K, Hao J, et al. Polydatin attenuates AGEs-induced upregulation of fibronectin and ICAM-1 in rat glomerular mesangial cells and db/db diabetic mice kidneys by inhibiting the activation of the SphK1-S1P signaling pathway. Mol Cell Endocrinol. 2016;427:45–56. doi: 10.1016/j.mce.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Gong W, Li J, Chen W, Feng F, Deng Y. Resveratrol inhibits lipopolysaccharide-induced extracellular matrix accumulation and inflammation in rat glomerular mesangial cells by SphK1/S1P2/NF-κB pathway. Diabetes Metab Syndr Obes. 2020;13:4495–4505. doi: 10.2147/DMSO.S278267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T, Iino M. Nuclear translocation of p65 is controlled by Sec6 via the degradation of IκBα. J Cell Physiol. 2016;231(3):719–730. doi: 10.1002/jcp.25122 [DOI] [PubMed] [Google Scholar]
- 21.John S. Complication in diabetic nephropathy. Diabetes Metab Syndr. 2016;10(4):247–249. doi: 10.1016/j.dsx.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 22.Demir Y, Köksal Z. Some sulfonamides as aldose reductase inhibitors: therapeutic approach in diabetes. Arch Physiol Biochem. 2022;128(4):979–984. doi: 10.1080/13813455.2020.1742166 [DOI] [PubMed] [Google Scholar]
- 23.Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the four horsemen of the apocalypse. Int Urol Nephrol. 2017;49(5):837–844. doi: 10.1007/s11255-016-1488-4 [DOI] [PubMed] [Google Scholar]
- 24.Winiarska A, Knysak M, Nabrdalik K, Gumprecht J, Stompór T. Inflammation and oxidative stress in diabetic kidney disease: the targets for SGLT2 Inhibitors and GLP-1 receptor agonists. Int J Mol Sci. 2021;22(19):10822. doi: 10.3390/ijms221910822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sever B, Altıntop MD, Demir Y, et al. Identification of a new class of potent aldose reductase inhibitors: design, microwave-assisted synthesis, in vitro and in silico evaluation of 2-pyrazolines. Chem Biol Interact. 2021;345:109576. doi: 10.1016/j.cbi.2021.109576 [DOI] [PubMed] [Google Scholar]
- 26.Hu Q, Qu C, Xiao X, et al. Flavonoids on diabetic nephropathy: advances and therapeutic opportunities. Chin Med. 2021;16(1):74. doi: 10.1186/s13020-021-00485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su W, Cao R, He YC, Guan YF, Ruan XZ. Crosstalk of hyperglycemia and dyslipidemia in diabetic kidney disease. Kidney Dis. 2017;3(4):171–180. doi: 10.1159/000479874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14(4):257–267. doi: 10.1111/j.1529-8027.2009.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demir Y, Özaslan MS, Duran HE, Küfrevioğlu Öİ, Beydemir Ş. Inhibition effects of quinones on aldose reductase: antidiabetic properties. Environ Toxicol Pharmacol. 2019;70:103195. doi: 10.1016/j.etap.2019.103195 [DOI] [PubMed] [Google Scholar]
- 30.Yasuda I, Hasegawa K, Sakamaki Y, et al. Pre-emptive short-term nicotinamide mononucleotide treatment in a mouse model of diabetic nephropathy. J Am Soc Nephrol. 2021;32(6):1355–1370. doi: 10.1681/ASN.2020081188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng W, Qian C, Xu F, et al. Fuxin Granules ameliorate diabetic nephropathy in db/db mice through TGF-β1/Smad and VEGF/VEGFR2 signaling pathways. Bio Pharmac. 2021;141:111806. doi: 10.1016/j.biopha.2021.111806 [DOI] [PubMed] [Google Scholar]
- 32.Lee SY, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol. 2015;30(7):1063–1075. doi: 10.1007/s00467-014-2888-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Y, Wang S, Liu Y, et al. The effects of salvianolate combined with western medicine on diabetic nephropathy: a systematic review and meta-analysis. Front Pharmacol. 2020;11:851. doi: 10.3389/fphar.2020.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charlton A, Garzarella J, Jandeleit-Dahm KAM, Jha JC. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology. 2021;10(1):18. doi: 10.3390/biology10010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015;2015:948417. doi: 10.1155/2015/948417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mima A. Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J Diabetes Res. 2013;2013:248563. doi: 10.1155/2013/248563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam JE, Jo SY, Ahn CW, Kim YS. Baicalin attenuates fibrogenic process in human renal proximal tubular cells (HK-2) exposed to diabetic milieu. Life Sci. 2020;254:117742. doi: 10.1016/j.lfs.2020.117742 [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Xu T, Lachance BB, et al. Critical roles of sphingosine kinase 1 in the regulation of neuroinflammation and neuronal injury after spinal cord injury. J Neuroinflammation. 2021;18(1):50. doi: 10.1186/s12974-021-02092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crespo I, San-Miguel B, Sánchez DI, et al. Melatonin inhibits the sphingosine kinase 1/sphingosine-1-phosphate signaling pathway in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2016;61(2):168–176. doi: 10.1111/jpi.12335 [DOI] [PubMed] [Google Scholar]
- 40.Abdel Baky NA, Al-Najjar AH, Elariny HA, Sallam AS, Mohammed AA. Pramipexole and Lactoferrin ameliorate Cyclophosphamide-Induced haemorrhagic cystitis via targeting Sphk1/S1P/MAPK, TLR-4/NF-κB, and NLRP3/caspase-1/IL-1β signalling pathways and modulating the Nrf2/HO-1 pathway. Int Immunopharmacol. 2022;112:109282. doi: 10.1016/j.intimp.2022.109282 [DOI] [PubMed] [Google Scholar]
- 41.Youssef NS, Elzaitony AS, Abdel Baky NA. Diacerein attenuate LPS-induced acute lung injury via inhibiting ER stress and apoptosis: impact on the crosstalk between SphK1/S1P, TLR4/NFκB/STAT3, and NLRP3/IL-1β signaling pathways. Life Sci. 2022;308:120915. doi: 10.1016/j.lfs.2022.120915 [DOI] [PubMed] [Google Scholar]