Abstract
Background:
Breast cancer (BC) plays a major public health in Egyptian woman. In Upper Egypt, there is an increase in incidence of BC compared to other Egyptian areas. Triple-negative BC, estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-neu-negative, is a high-risk BC that lacks the benefit of specific therapy that targets these proteins. Accurate determination of Caveolin-1(Cav-1), Caveolin-2 (Cav-2) and HER-2/neu status have become of major clinical significance in BC by focusing about its role as a tumor marker for response to different therapies
Methods:
The present study was performed on 73 female BC patients in the South Egypt Cancer Institute. Blood samples were used for Cav-1, Cav-2, and HER-2/neu genes amplification and expression. In addition, immunohistological analysis of mammaglobin, GATA3, ER, PR, and HER-2/neu was done.
Results:
There was a statistically significant association between Cav-1, 2 and HER-2/neu genes expression and the age of patients (P< 0.001). There are increase in the level of Cav-1, 2 and increase in HER-2/neu mRNA expression in groups treated with chemotherapy and group treated with both chemotherapy and radiotherapy compared to each group baseline level of genes mRNA expression before treatment. On the contrary, the group treated with chemotherapy, radiotherapy and hormonal therapy revealed increase on the level of Cav-1, 2 and HER-2/neu mRNA expression when compared with their baseline for the same patients before treatment.
Conclusions
Noninvasive molecular biomarkers such as Cav-1 and Cav-2 have been proposed for use in the diagnosis and prognosis for women with BC.
Key Words: Breast Cancer, Caveolin-1, ER, GATA-3, HER-2/neu, Mammaglobin, PR, 2
Introduction
Every year, over 1.7 million women throughout the globe are diagnosed with breast carcinoma, making it the most prevalent malignant tumors in women and the leading cause of cancer mortality (1, 2).
Breast cancer (BC) is the most prevalent malignancy in Egypt (2). Upper Egypt had the highest rate of new cases of breast cancer (38.7%), followed by Middle Egypt (33.8%), and finally Lower Egypt (26.8%). Breast cancer is a diverse illness with varying biological and clinical features due to distinct genetic change,which has been the focus of many investigations (2).
Proliferation, apoptosis, autophagy, invasion, migration, metastasis, and drug resistance are all steps in the multi-stage process known as tumorigenesis in breast cancer (3,4). Human breast cancer can be subdivided into many subtypes based on pathological characteristics (2). These include non-invasive breast cancer, such as Paget disease of the breast, (5), invasive breast carcinoma of special type, such as invasive apocrine carcinoma or invasive micropapillary carcinoma, and invasive breast carcinoma of no special type, such as invasive lobular carcinoma or invasive ductal carcinoma, inflammatory breast cancer (IBC), and so on. The most prevalent kind is invasive breast cancer (IBC), and patients with IBC tend to have a dismal outlook (5).
Human breast cancer is classified as luminal A, estrogen receptor (ER) positive, progesterone receptor (PR) positive, human epidermal growth factor receptor-2 (HER-2) negative and luminal B, ER positive, PR positive, HER -2 positive (5). Unlike basal-like breast cancers, which express ER, PR, and HER2, invasive breast tumors that lack ER, PR, and HER-2 expression are known as triple negative (TNBC) (2,5). This is a clinical challenge for targeted treatment because to its strong proliferation capability and recurrence rate, poor differentiation, and big tumors size (2). Existing therapies for BC are based on the tumors subtype and anatomic stage of malignancy. Radiation, chemotherapy, hormonal receptor modulators, immunotherapy, and endocrine therapy are all examples of such interventions (2,5).
Breast cancer has numerous critical actors that are involved in the cell cycle, proliferation, apoptosis, cell migration, and metastasis, such as Caveolin-1 (Cav-1), Caveolin-2 (Cav-2), Caveolin-3 (Cav-3), GATA-3, and mammoglobin.
Caveolins, such as Cav-1, Cav-2, and Cav-3, are scaffold proteins Cav-1 and Cav-2 are found in all human tissues, whereas Cav-3 is present in muscles. Caveolins are 50-100 nm shaped, 22 cholesterol-enriched, stiff membrane microdomains (6). The coding gene for Cav-1, a 21-22 kDa integral membrane protein, is found at the D7S522 locus on q31.1 of human chromosome 7.29 (7). Moreover, Cav-1 is essential for caveolae to function and plays a role in several biological processes, including as endocytosis, signal transduction, membrane trafficking, cholesterol homeostasis, lipid transport and storage, the cell cycle, proliferation, apoptosis, cell migration, and metastasis (8). Growing evidence from experimental research implicates Cav-1 in key steps of breast cancer (8). Additional evidence implicates Cav-1 in chemotherapeutics and radiation resistance, two of the most pressing issues in modern breast cancer treatments (8).
Members of the GATA family of zinc finger transcription factors (of which GATA binding protein 3 is one) recognize a particular nucleotide sequence in the promoter region of target genes (9). Genes linked to the GATA transcription factor are essential in mammary gland and thymocyte development, as well as other complicated regulatory networks (10). Specifically, GATA-3 has been shown to have a significant impact in tumor initiation (10) due to its critical function in regulating mammary-gland morphogenesis and luminal cell differentiation. Recently, GATA-3 was shown to be an immunohistochemical marker for both breast and urothelial differentiation (10), indicating that it may be used in both contexts. A small percentage of squamous cell carcinomas, tumors of the salivary glands, mesotheliomas, gynecologic carcinomas, and cutaneous adnexal tumors also express it (10).
It has shown that GATA-3 has a high positive predictive value (96.2%), despite being a multi-specific gene (11). This may be used to identify breast origin in serous malignant effusion. Since GATA-3 and ER are part of the same cross-regulatory loop, it is not surprising that early reports linked GATA-3 expression to ER expression. However, new evidence suggests that GATA-3 is expressed in triple-negative breast cancer, making it a more sensitive marker than gross cystic disease fluid protein 15 (GCDFP-15) and mammaglobin (MAM) (10, 11). Despite advances in morphologic analysis, more sensitive and specific indicators are still needed to detect the type of malignant effusions.
Materials and Methods
Subjects
A total of 93 people participated in the research between 2015 and 2017; they included 73 women with confirmed cases of breast cancer and 20 healthy volunteers to serve as controls. Patients and healthy volunteers' ages varied from 27 to 56, with a mean difference of 1.53 years. They either had a modified radical mastectomy or a conservative extensive local excision with axillary lymph node dissection.
Ethics approval and consent to participate
The South Egypt cancer Institute Hospital's Ethic Committee authorized the research (Ethical approval no. 15473) in accordance with the 1964 Declaration of Helsinki, and all healthy and patients signed an informed written consent was acquired.
Tissues sampling
In this study tissue samples were surgical collected from all patients. Cav-1, Cav-2, and HER-2/neu mRNA expressions were measured before and post 6 months following therapy. Hematoxylin and eosin staining were used on paraffin-embedded tissue blocks for a morphological analysis (12). Immunohistochemistry (IHC) were used two probes for mammaglobin, GATA3-binding protein3, ER, PR, and HER-2/neu. Tumor grade was determined using the Elston and Ellis grading system (13) and tumor stage was determined using TNM (14) according to the WHO 2003 classification of breast tumors. The type of tumors recognized by the WHO/ISUP 2004 classification system (Luminal A, Luminal B, Basal-Like, and HER2/neu).
Immunohistochemistry
The streptoavidin-biotin immunoperoxidase technique was used for immunohistochemical staining (Dakocytomation, Glostrup, Denmark). Monoclonal antibodies against human mammaglobin, GATA3, ER, PR, and HER2 receptors (Santa Cruz Biotechnology, CA) and 2nd antibodies were used to assay hormone receptors.
Gene expression quantifications Cav-1, 2, and Her2/neu
The tissue samples were processed using the extraction kit (Fermentus, Thermo Fisher Scientific Inc, UK) procedures, and the RNA concentrations were measured using a NanoDrop.
Synthesis of cDNA was performed using a commercially available cDNA synthesis kit (Fermentus, Thermo Fisher Scientific Inc, UK).
The cDNAs for Cav-1, 2, and Her2/neu were amplified using polymerase chain reaction (PCR) in a Rotor Gene 2000 real-time fluorescence thermal cycler (Corbett Ltd., Australia). All the genes, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were amplified twice from cDNA. Primers were constructed in accordance with the guidelines laid forth in and were acquired from (Jena Bioscience, Germany). Forty cycles of 30 seconds at 95 °C, one minute at 60 °C, and three minutes at 72 °C were applied to the reactions for each of them. A rise in fluorescence was tracked in real time as the process progressed into the extension phase. Gene expression levels were calculated using the ΔΔCt manner for relative quantification of gene expression. The formula 2−ΔΔCt was used to get the fold change (15).
Statistical analysis
To examine the data, IBM SPSS Advanced Statistics Version 22 was used (SPSS Inc., Chicago, IL). Depending on the data set, numerical information was shown using a median and range or a mean and standard deviation. Statistical measures of frequency and percentage were used to describe qualitative information. The Mann-Whitney test was used to compare two groups when the underlying quantitative data did not follow a normal distribution (non-parametric t-test). Kruskal-Wallis test (non-parametric ANOVA) was used to compare more than two groups, and the post-Hoc "Schefe test" was used to compare pairs of groups using Kruskal-distribution. Wallis's correlation between quantitative variables was analyzed using the Spearman-rho technique. Two numerical variables were measured at regular intervals and compared using the Wilcoxon-signed ranks test (a non-parametric paired t-test). There were no one-tailed tests. In this study, significance was defined as a P-value <0.05.
Results
A total of 93 people with 73 BC and 20 healthy females (aged 27 to 56) were included in the research. There was a total of 73 diagnoses, with 21 (30.1%) being Luminal A, 15 (20.6%) being Luminal B, 15 (20.6%) being Her-2/neu,and 21 (28.7%) being Basal-like. In this analysis, 54 (74%) of the cases were classified as Stage II, 6 (8.2%) as Stage I, and 13 (17.8%) as Stage III (Table 1).
Table 1.
Clinicopathologic features of 73 breast cancer female patients treated in the oncology outpatient clinic, South Egypt cancer Institute.
| Clinicopathologic features | Number of patients | |||
|---|---|---|---|---|
| ≤ 45 years | > 45 years | Total | % | |
| (31) | (42) | |||
| Subtypes | ||||
| Luminal A | 1 | 21 | 22 | 30.1 |
| Luminal B | 1 | 14 | 15 | 20.6 |
| Her-2/neu | 9 | 6 | 15 | 20.6 |
| Basal Like | 20 | 1 | 21 | 28.7 |
| Tumor size (T) (cm) | ||||
| T1 > 2 | 1 | 5 | 6 | 8.2 |
| T2 = 2- 5 | 18 | 36 | 54 | 74 |
| T3 > 5 cm | 12 | 1 | 13 | 17.8 |
| Lymph node number (N) | ||||
| N0 = 0 | 1 | 5 | 6 | 8.2 |
| N1 = 1 | 18 | 36 | 54 | 74 |
| N2 = 2 | 6 | 1 | 7 | 9.6 |
| N3 = 3 | 6 | 0 | 6 | 8.2 |
| Metastasis (M0) | 31 | 42 | 73 | 100 |
| *TNM stage | ||||
| I | 1 | 5 | 6 | 8.2 |
| II | 18 | 36 | 54 | 74 |
| III | 12 | 1 | 13 | 17.8 |
T: refers to tumor size, N: node status, M: metastasis.
There was a total of 73 diagnoses; 29 (39.7%) were ER-negative, while 44 (60.3%) were ER-positive; likewise, 31 (42.5%) were PR-negative, while 42 (57.5%) were PR-positive; and finally, 46 (63%) were categorized as HER-2/neu-negative, while 27 (37%) were HER-2/neu-positive (Fig. 1).
Fig. 1.
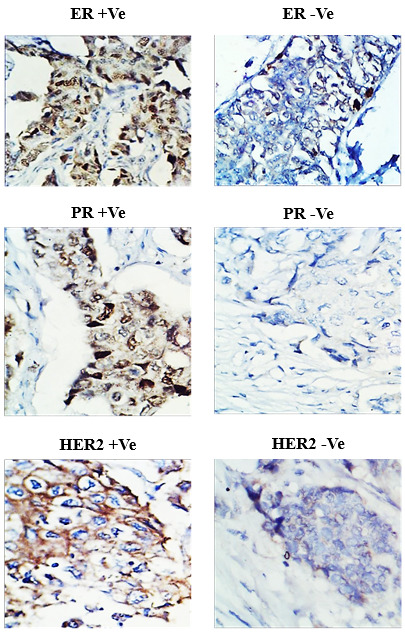
Immunohistochemistry for hormonal receptors in female breast cancer patients A) Positive estrogen receptor (ER +Ve). B) Negative estrogen receptor (ER -Ve). C) Positive progesterone receptor (PR +Ve). D) Negative progesterone receptor (PR -Ve). E) Positive HER-2/neu (HER2 +Ve). F) Negative HER-2/neu (HER2 -Ve).
Out of the 73 cases analyzed, 64 were found to have positive GATA-3 and 38 had positive MAM ( Fig. 2 and Table 2). GATA-3's predicted 87.6% sensitivity for breast cancer was statistically substantially (P< 0.001) greater than that of MAM's 52.6% sensitivity. 60.3%, 57.5%, and 37.5% of breast cancer cases showed positive expression of ER, PR,and Her-2/neu, respectively. In the group of ductal carcinomas, 54/62 instances tested positive for GATA-3, 27/62 for MAM, 39/62 for ER, 37/62 for PR, and 24/62 for Her-2/neu. Lobular carcinomas accounted for all but one of the GATA-3, MAM, ER, PR, and Her-2/neu-positive patients, 6/6, 6/6, 3/6, 3/6 and 3/6, respectively. All three GATA-3 positives, all three MAM positives, none of the ER positives, none of the PR positives, and none of the Her-2/neu positives were not squamous cell (mixed) carcinomas. Mucinous carcinomas accounted for 100% of the patients that tested positive for GATA-3, 100% for MAM, 100% for ER, 100% for PR, and 0% for Her-2/neu.
Fig. 2.
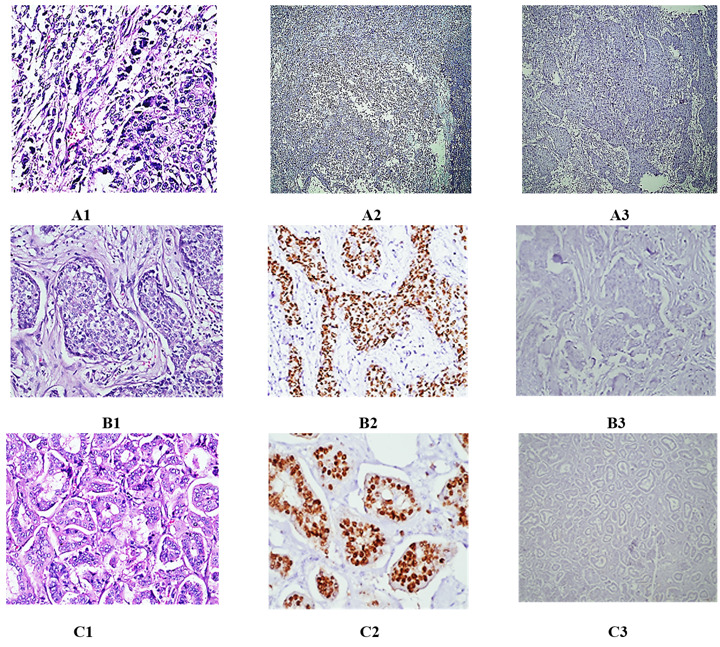
(A1-A3) A case of poorly differentiated mammary carcinoma (Ductal type). A1) H&E (400X). A2) Nuclear negatively for GATA-3 (100X). A3) Nuclear negatively for mammaglobin (100X). (B1-B3) A case of moderately differentiated mammary carcinoma. B1) H&E (400X). B2) Nuclear positively for GATA-3 (400X). B3) Cytoplasmic negatively for mammaglobin (400X). (C1-C3) A case of well differentiated mammary carcinoma. C1) H&E (400X). C2) Nuclear positively for GATA-3 (400X). C3) Cytoplasmic negatively for mammaglobin (400X).
Table 2.
Breast cancer: an immunohistochemical study.
| GATA-3 | Mammaglobin | ER | PR | Her-2/neu | |
|---|---|---|---|---|---|
| Positive cases | 64/73 | 38/73 | 44/73 | 42/73 | 27/73 |
| Ductal | 54/62 | 27/62 | 39/62 | 37/62 | 24/62 |
| Lobular | 6/6 | 6/6 | 3/6 | 3/6 | 3/6 |
| Mixed | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 |
| Mucinous | 2/2 | 2/2 | 2/2 | 2/2 | 0/3 |
| Percentage | 87.6 | 52 | 60.3 | 57.5 | 37 |
ER, estrogen receptor; PR, progesterone receptor, Her-2/neu, human epidermal growth factor receptor-2, n=73.
Her-2/neu gene expression is significantly reduced (P< 0.001) in BC patients as compared to healthy people. According to the BC group, after undergoing Chemo-Radiotherapy, Chemo-Hormonal therapy, or Chemo-Radio-Hormonal therapy, the expression of this gene was up-regulated by a factor of 1.46, 1.84, and 3.53, respectively (P< 0.001). Her-2/neu gene expression after chemotherapy, however the difference was not statistically significant (P<0.001). Her-2/neu gene expression after chemotherapy, however the difference was not statistically significant (P< 0.001) (Fig. 3).
Fig. 3.
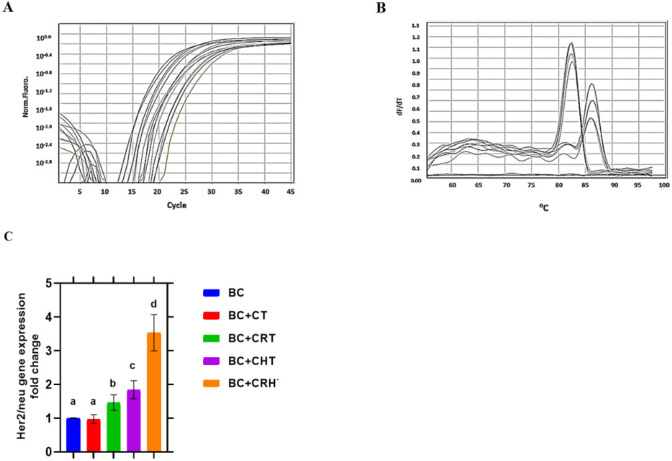
Quantitative real time PCR for Her2/neu gene expression and ± S.D. in breast cancer patiens. A) CT curve. B) Melting curve. C) Gene expreression fold change histogram. Breast Cancer (BC), Chemotherapy (CT), Chemo-Radiotherapy (CRT), Chemo-Hormonal therapy (CHT), and Chemo-Radio-Hormonal therapy (CRHT).
A statistically significant (P< 0.001) decrease in Cav-1 gene expression between BC patients and healthy individuals was found (Fig. 4). The BC group found that the expression of this gene was increased by a factor of 1.37, 2.28, 2.31, and 3.64 following CT, CRT, CHT, and CRHT, respectively (P< 0.001). After CRT, Her-2/neu gene expression was not changed than it was after CHT, although the difference was not statistically significant (P< 0.001). The efficacy of CRHT (which consists of chemotherapy, radiation, and hormone therapy) much exceeds that of any of these therapies used alone.
Fig. 4.
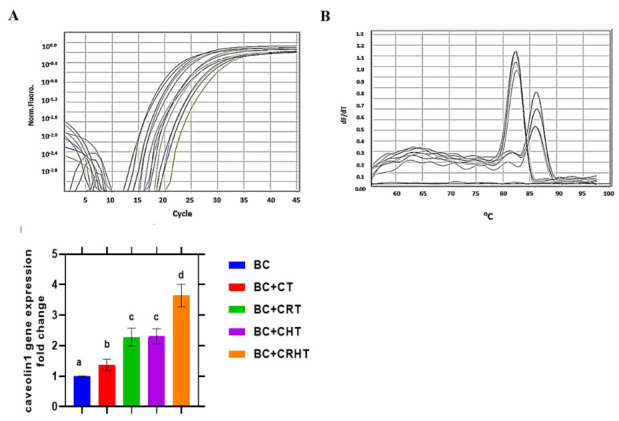
Quantitative real time PCR for Cav-1 gene expression and ± S.D. in breast cancer patiens. Breast Cancer (BC), Chemotherapy (CT), Chemo-Radiotherapy (CRT), Chemo-Hormonal therapy (CHT), and Chemo-Radio-Hormonal therapy (CRHT).
Cav-2 gene expression is much lower in BC patients compared to healthy people (Fig. 5). This difference is statistically significant (P< 0.001). After CT, CRT, CHT, and CRHT, found that the expression of this gene rose by 2.34, 2.91, 3.67, and 7.11 folds, respectively (P< 0.001). When combined, chemotherapy, radiation, and hormone therapy (CRHT) are much more effective than any of these treatments alone.
Fig. 5.
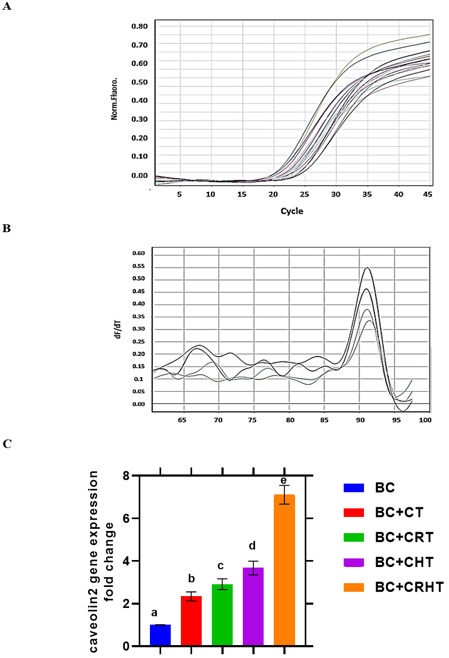
Quantitative real time PCR for Cav-2 gene expression and ± S.D. in breast cancer patients. Breast Cancer (BC), Chemotherapy (CT), Chemo-Radiotherapy (CRT), Chemo-Hormonal therapy (CHT), and Chemo-Radio-Hormonal therapy (CRHT).
Discussion
Out of 73 patients, we found that 27 were positive using IHC, or 37 %, and that 66 were positive using PCR. Overexpression of HER2/neu was found in 9–40% of human breast carcinoma, which is consistent with the bulk of prior research suggesting an incidence of 20–25% of tumors (16). On the other hand, a study by Fabi et al. (17) found that only 18.24% of cases had detectable HER-2/neu expression. Overexpression of Her-2 or amplification of the Her-2 gene has been found in 10-30% of primary mammary carcinomas, as shown by research by Rydén et al. (18) . Previous research has linked HER2/neu positive malignancies to an increased likelihood of being ER and PR negative (19).
Patients with ER and PR positive breast cancers saw a reduction in their tumors after receiving hormone treatment, with the number ranging from 50-70% (20). It is essential to conduct hormone receptors and HER2/neu in all situations, since one cannot forecast expression in any given patient, even though ER and PR expression was dramatically lowered in HER2/neu positive malignancies. Furthermore, there are many instances that did not express ER, PR, or HER2/neu, whereas the percentage of ER+/PR+/HER2/neu+ patients is quite low. According to these findings, there is no connection between HER2/neu and hormone receptors.
Using IHC and real-time PCR on paraffin-embedded tissue samples, we found that 37 % (27/73) and 39.7 % (29/73) of samples, respectively, were positive for HER2/neu. Thus, fewer positive instances were found by analysing protein overexpression using IHC than were found using real-time PCR to analyse DNA. It's possible that this means the amplified area doesn't include the promoter, or that HER2/neu amplification is a rather common occurrence, leading to persistent, if less noticeable, overexpression of the protein (21). Real-time polymerase chain reaction identified all instances with allelic uneven characteristics, indicating protein overexpression. This finding agrees with those of previous research efforts (22).
Seventy percent of Luminal breast cancers have GATA-3 as a marker. Direct regulation of ER- by GATA-3 is required for estradiol-induced cell cycle progression in breast cancer. To activate the transcription factor, GATA-3 forms a positive cross-regulatory loop with ER. As a result, GATA-3 and ER may cooperate to regulate key genes in hormone-responsive breast cancer (23). It has been shown that GATA-3 is an immunohistochemical marker for urothelial and breast cancers (11,24). In high-grade triple-negative metastatic breast cancer, MAM's insensitivity is especially problematic since ER and PR are prognostic indicators with little supplemental diagnostic significance. Our research using CMA reveals that GATA-3 is more sensitive than MAM (87.6% vs. 52.0%). Comparable results were observed by Shield et al. (25), who examined serous effusions and showed GATA-3 and MAM sensitivity of 90% and 57%, respectively. When comparing GATA-3 and MAM sensitivity, Braxton et al. (26) discovered that the former was 86% while the latter was 26%. Their study, like Shield's, found that ER-negative carcinomas were more common. GATA-3 and MAM sensitivity were shown to be 93.5 and 22.4 percent in another research of cell blocks (26). Similarly, the sensitivity of MAM in tissue specimens for detecting ductal cancer was 54%, for lobular carcinoma it was 69%, for apocrine mammary carcinomas it was 36.4%, for ER-negative carcinoma it was 35%, and for triple-negative carcinoma it was 32%. Breast carcinomas have a sensitivity of over 90% when examined in GATA-3 tissue. Sixty-nine percent of ER-negative and forty-four percent of triple-negative breast cancer patients were found to have GATA-3 (27). Our research, along with those of others, suggests that GATA-3 is a sensitive and superior marker for breast differentiation in situations when just a few samples can be analysed and a sensitive, straightforward marker is needed. Both ER and PR are useless as prognostic markers in ER-negative and triple-negative breast carcinomas (11,28).
The proportion of HER2/neu overexpression by IHC was also lower than that discovered by real time PCR, as was the case for Bánkfalvi et al. (21). This suggests that even in individuals known to be HER2 negative in tissue testing, real-time PCR may be able to identify HER2/neu. One possible explanation for their findings is that real-time PCR is able to distinguish and measure small amounts of specific nucleic acid sequences in the most specific, sensitive, and reproducible manner, which may explain why amplification of HER2/neu is an early event and therefore, may go before protein expression (21).
The findings showed that after 6 months of treatment, the level of HER.2/neu mRNA expression was significantly lower than the pre-treatment level. Consistent with these findings, Lee et al. (29) found that patients who had surgery with chemotherapy and radiation therapy had substantially (P 0.05) lower blood HER.2/neu levels, indicating a positive response to treatment. In addition, McArdle et al. (30) discovered that disease-specific survival was mostly dependent on the number of positive nodes, and that the combination of radiation and chemotherapy was associated with decreased recurrence and a trend toward greater malignancy specific survival. Patients who had radiation in addition to chemotherapy and/or hormonal treatment had a reduced risk of developing secondary malignancies, as reported by Zhang et al. (31).
Our findings indicate that patients treated with hormone therapy in conjunction to chemotherapy and radiation therapy had higher levels of HER2/neu mRNA expression than those who received just those two forms of treatment. The findings corroborated those of Lee et al. (29), who found that patients who had endocrine therapy in addition to the standard surgical excision, chemotherapy, and radiotherapy showed no significant decrease in blood HER2/neu levels, indicating treatment resistance. Dimerization of HER2 with other members of the HER family leads to kinase phosphorylation and the activation of many downstream pathways that promote cell survival, proliferation, and migration under overexpression of HER2 (29). These responses may be activated by PI3K/Akt or MAPK, but these same pathways may also be involved in the activation of ER or its coregulators. This paves the way for ER to develop its biological effects, which include a greater promotion of tumor growth and resistance to endocrine treatment in HER2 and ER-high cells (29).
In ER-positive, primary, and metastatic breast cancer, tamoxifen resistance is associated with overexpression of HER2/neu. People who are HR+/HER2/neu+ have a poor response to endocrine therapy. Endocrine treatment resistance in breast cancer patients may be due to crosstalk between the ER/HER family of receptors. Increased tumor stimulation and resistance to endocrine treatment are two of the cellular consequences that may be established thanks to HER2+ cells' (29) capacity to produce its cellular consequences (29). In ER-positive, primary, and metastatic breast cancer, tamoxifen resistance is associated with overexpression of HER2/neu. People who are HR+/HER2/neu+ have a poor response to endocrine therapy. Endocrine treatment resistance in breast cancer patients is associated with cross-talk between the ER/HER family of receptors (29).
Mammary cancer may be classified as luminal A, luminal B, normal breast-like, HER-2 over-expressing, or basal-like depending on the expression of ER, PR, and HER-2 in the plasma membrane of cancer cells. Transverse-shaped nuclear breast cancer (TNBC) is a subtype of basal-like breast cancer with a dismal survival rate. Cav-1 is the primary caveolae component and is involved in a wide range of biological processes. Cav-1 operates as both a tumor suppressor and an oncogene, and plays a crucial part in the carcinogenesis of breast cancer, and it has been shown that genetic variations in Cav-1 could alter the risk for breast cancer (32).
Cav-1 is being studied as a possible therapeutic biomarker for use in breast cancer therapy(32). 40 Epithelial Cav-1 and stromal Cav-1 levels in patients may help predict how well a treatment will work and guide the development of individualized treatment plans(33). Cav-1 has been identified in both the epithelial and stromal components of breast cancer, providing further information for prognosis prediction. Negative Cav-1 expression in CAFs is associated with a poor prognosis and a low survival rate in affected patients (32). In cases when lymph node metastases are present and the patient's prognosis is poor, CAFs may be administered. A poor prognosis and decreased survival time are associated with low levels of Cav-1 expression in the stroma (34). As shown by the high expression level of Cav-1 in invasive breast cancer cells, it is correlated with tumor aggressiveness and poor prognosis (32). In addition, Qian et al. found that high Cav-1 expression in tumors but not in stroma was strongly correlated with poor prognosis in individuals with primary human breast cancer (32).
Cav-1 regulates proteins in the intrinsic and extrinsic apoptotic pathways, including Bcl-2, Bax, survivin, caspase-8, and caspase-3; Cav-1-induced autophagy alternation affects cell apoptosis; chemotherapeutic drugs alter the transcriptional level of CAV-1 and promote apoptosis; and the expression and activation of apoptosis-related molecules, including TIGAR, ROS (35). Through overexpression of p21, p27, and cyclin B1 and downregulation of cyclin D2, Cav-1 works as an antiproliferative factor in MDA-MB-231 and MCF-7 cells, and this antiproliferative action is amplified in the presence of docetaxel (DTX) (35). Several lines of evidence show that DTX administration triggers apoptosis in MCF-7 and MDA-MB-231 cells by phosphorylating (inactivating) Bcl-2 and upregulating the expression of p53, Bax, and cleaved poly-ADP ribose polymerase in TNBC cells (36).
The tumor-suppressing role of Cav-1 in MCF-7 cells is mediated in part by its downregulation, which in turn promotes cell proliferation via an increase in the expression and activity of large conductance membrane channels. The malignancy-inducing gene that codes for the Ca2+-activated potassium (BKCa) channel also speeds up carcinogenesis (37). Accelerated breast cancer cell proliferation may be attributed to the increased expression and secretion of stromal cell-derived factor-1, epidermal growth factor (EGF), and fibroblast-specific protein-1 (FSP-1) triggered by decreased Cav-1 mRNA and Cav-1 in CAFs (38). Modifying autophagy in response to Cav-1 may affect cell death. Cav-1 is upregulated by 17-estradiol (E2), leading to the creation of autophagosomes and the suppression of apoptosis in BT474 cells. This is accomplished via increasing the expression of autophagy-related proteins (Beclin-1, light chain 3-II, and Atg12/5) in these cells (39). Shi et al.(40) Discovered that blocking Cav-1 and disrupting lipid rafts led to increased autophagy and blocked apoptosis via activating lysosomal activity and the autophagosome through V-ATPase assembly joining of lysosomes (40). By blocking the FAK/SRC/PI3K/AKT signaling pathway, DHA from Antarctic krill suppressed MMP-2 synthesis in MCF-7 cells. To do this, we boosted CD95 (also known as Fas) and Cav-1 interactions (40).
Witkiewicz et al (41) showed that reduced CAV-1 expression in breast cancer tumor stroma is linked to increased expression of the glycolytic enzymes PKM2 and LDH (41). This phenomenon, called the "reverse Warburg effect," describes how the absence of CAV-1 triggers a metabolic shift from oxidative phosphorylation to glycolysis, creating a microenvironment that promotes the development of the neighboring tumor. Cav-1 impairment and lipid raft disruption were discovered to increase autophagy and decrease apoptosis by activating lysosomal activity and autophagosome-lysosome fusion by boosting V-ATPase assembly (40).
It is well accepted that drug resistance is a major contributor to the poor outcomes seen by cancer patients. 30% of patients with early breast cancer will go on to acquire metastatic illness, and the vast majority of those patients will have developed resistance to standard chemotherapies by the time they do (42). Cav-1 was a target for improving clinical outcomes and decreasing radiation and medication resistance in cancer. U.S. regulators have green-lighted the use of trastuzumab emtansine (T-DM1), an antibody drug combination (ADC), to treat HER-2 positive, metastatic breast cancer. T-DM1 contains trastuzumab, which binds to HER-2 receptors and then causes cell toxicity by internalizing within cells and releasing emtansine (42). Cav-1 has been shown in recent investigations to co-localize with trastuzumab, which increases drug toxicity via mediating T-DM1 internalization (43). Raised levels of Cav-1 expression may facilitate endocytosis and the internalization of T-DM1 in HER-2-positive cancer cells (44). Researchers, Chung et al. (44) found that Cav-1 in BT-474 cells increased drug sensitivity by encouraging T-DM1 internalization (45). Cav-1, a tumor suppressor thought to contribute to resistance to chemotherapeutics and radiation, were phosphorylated by the mitogen-activated protein kinase (MAPK) pathway and imparted acquired resistance to lapatinib in ER(+) breast cancer cells (43).
The apoptotic rate of cancer cells has been shown to decrease when cav-1 and cav-2 levels are lowered. Some research has shown a decline in the expression of cav-1 and cav-2 in breast cancer tumor tissues, while other research indicates that these genes are expressed in triple- negative and basal-like breast cancers (34). This study confirms and extends the findings of prior research in this area. When comparing tumor tissues to breast tissue from the periphery, researchers found a downward trend in the transcription levels of cav-1 and cav-2 (transcript I, II). When comparing the breast tissue on either side, the same downward tendency was also seen in the periphery. Both cav-1 and cav-2 levels declined at the same rate, which was correlated. So, it looks like the co-expression of these caveolins helps prevent cancer cells from growing, especially in breast tissues (43).
Cav-1 and Cav-2 may be considered as non-invasive molecular biomarkers in diagnosing BC and in evaluating female patients with breast cancer's reaction to different treatment.
Funding
This research did not receive any specific grant from funding agencies.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose. Authors declared that the publishing of this article does not constitute a conflict of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
Authors are thankful to the support of Radiation Biology Department, National Center for Radiation Research & Technology (NCRRT), and Egyptian Atomic-Energy Authority (EAEA) for helping in conducting the RT-qPCR experiments. As well as authors are thankful to the support of The South Egypt cancer Institute National, and Cancer Institute for follow up the patients.
References
- 1.Sabitha K, Kodous A, Rajkumar T. Computational analysis of mutations in really interesting new gene finger domain and BRCA1 c terminus domain of breast cancer susceptibility gene. Asian J Pharm Clin Res. 2016;9(3):96–102. [Google Scholar]
- 2.Shaban NZ, Ibrahim NK, Saada HN, El-Rashidy FH, Shaaban HM, Farrag MA, et al. miR-34a and miR-21 as biomarkers in evaluating the response of chemo-radiotherapy in Egyptian breast cancer patients. J Radiat Res Appl Sci. 2022;15(3):285–92. [Google Scholar]
- 3.Omran MH, Fotouh BE, Shosha WG, Ismail A, Ramadan SS. Gene-Gene Interaction Study Between Genetic Polymorphisms of Folate Metabolism and MTR SNPs on Prognostic Features Impact for Breast Cancer. Rep Biochem Mol Biol. 2022;11(1):89–101. doi: 10.52547/rbmb.11.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aglan SA, Zaki AM, EL Sedfy AS, El-Sheredy HG, Elgaddar OH. O6-Methylguanine-DNA Methyltransferase and ATP-Binding Cassette Membrane Transporter G2 Promotor Methylation: Can Predict the Response to Chemotherapy in Advanced Breast Cancer? Rep Biochem Mol Biol. 2022;11(1):20–9. doi: 10.52547/rbmb.11.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verret B, Bottosso M, Hervais S, Pistilli B. The Molecular Predictive and Prognostic Biomarkers in Metastatic Breast Cancer: The Contribution of Molecular Profiling. Cancers (Basel). 2022;14(17):4203. doi: 10.3390/cancers14174203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Almeida CJG. Caveolin-1 and Caveolin-2 Can Be Antagonistic Partners in Inflammation and Beyond. Front Immunol. 2017;8:1530. doi: 10.3389/fimmu.2017.01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian XL, Pan YH, Huang QY, Shi YB, Huang QY, Hu ZZ, Xiong LX. Caveolin-1: a multifaceted driver of breast cancer progression and its application in clinical treatment. Onco Targets Ther. 2019;12:1539–1552. doi: 10.2147/OTT.S191317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simón L, Campos A, Leyton L, Quest AFG. Caveolin-1 function at the plasma membrane and in intracellular compartments in cancer. Cancer Metastasis Rev. 2020;39(2):435–53. doi: 10.1007/s10555-020-09890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassandri M, Smirnov A, Novelli F, Pitolli C, Agostini M, Malewicz M, et al. Zinc-finger proteins in health and disease. Cell Death Discov. 2017;3:17071. doi: 10.1038/cddiscovery.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Querzoli P, Pedriali M, Rinaldi R, Secchiero P, Rossi PG, Kuhn E. GATA3 as an Adjunct Prognostic Factor in Breast Cancer Patients with Less Aggressive Disease: A Study with a Review of the Literature. Diagnostics (Basel). 2021;11(4):604. doi: 10.3390/diagnostics11040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38(1):13–22. doi: 10.1097/PAS.0b013e3182a0218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catto JWF, Rosario DJ. The road to cystectomy: Who, when and why? EAU Updat Ser. 2005;3:118–28. [Google Scholar]
- 13.Elston CW, Ellis IO, Pinder SE. Pathological prognostic factors in breast cancer. Crit Rev Oncol. 1999;31(3):209–23. doi: 10.1016/s1040-8428(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 14.Vajpeyi R. WHO classification of tumours: pathology and genetics of tumours of the breast and female genital organs. World Heal Organ. 2005;58(6):671–72. [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Arafah M. Correlation of hormone receptors with Her-2 Neu protein expression and the histological grade in invasive breast cancers in a cohort of Saudi Arabia. Turk Patoloji Derg. 2012;28(1):38–43. doi: 10.5146/tjpath.2012.01095. [DOI] [PubMed] [Google Scholar]
- 17.Fabi A, Di Benedetto A, Metro G, Perracchio L, Nisticò C, Di Filippo F, et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res. 2011;17(7):2055–64. doi: 10.1158/1078-0432.CCR-10-1920. [DOI] [PubMed] [Google Scholar]
- 18.Rydén L, Landberg G, Stål O, Nordenskjöld B, Fernö M, Bendahl PO. HER2 status in hormone receptor positive premenopausal primary breast cancer adds prognostic, but not tamoxifen treatment predictive, information. Breast Cancer Res Treat. 2008;109(2):351–7. doi: 10.1007/s10549-007-9660-2. [DOI] [PubMed] [Google Scholar]
- 19.Ansquer Y, Mandelbrot L, Lehy T, Salomon L, Dhainaut C, Madelenat P, et al. Expression of BRCA1, HER-1 (EGFR) and HER-2 in sporadic breast cancer and relationships to other clinicopathological prognostic features. Anticancer Res. 2005;25(6C):4535–41. [PubMed] [Google Scholar]
- 20.Margalit DN, Sreedhara M, Chen YH, Catalano PJ, Nguyen PL, Golshan M, et al. Microinvasive breast cancer: ER, PR, and HER-2/neu status and clinical outcomes after breast-conserving therapy or mastectomy. Ann Surg Oncol. 2013;20(3):811–8. doi: 10.1245/s10434-012-2640-8. [DOI] [PubMed] [Google Scholar]
- 21.Bánkfalvi A, Simon R, Brandt B, Bürger H, Vollmer I, Dockhorn-Dworniczak B, et al. Comparative methodological analysis of erbB-2/HER-2 gene dosage, chromosomal copy number and protein overexpression in breast carcinoma tissues for diagnostic use. Histopathology. 2000;37(5):411–9. doi: 10.1046/j.1365-2559.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 22.Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods. 2010;50(4):289–97. doi: 10.1016/j.ymeth.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 23.El Hag MI, Hag AM, Ha JP, Michael CW. Comparison of GATA-3, mammaglobin, GCDFP-15 expression in breast carcinoma in serous effusions: A cell-block micro-array study. Pleura Peritoneum. 2017;2(3):143–8. doi: 10.1515/pp-2017-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9(2):201–9. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 25.Shield PW, Papadimos DJ, Walsh MD. GATA3: a promising marker for metastatic breast carcinoma in serous effusion specimens. Cancer Cytopathol. 2014;122(4):307–12. doi: 10.1002/cncy.21393. [DOI] [PubMed] [Google Scholar]
- 26.Braxton DR, Cohen C, Siddiqui MT. Utility of GATA3 immunohistochemistry for diagnosis of metastatic breast carcinoma in cytology specimens. Diagn Cytopathol. 2015;43(4):271–7. doi: 10.1002/dc.23206. [DOI] [PubMed] [Google Scholar]
- 27.Huo L, Gong Y, Guo M, Gilcrease MZ, Wu Y, Zhang H, et al. GATA-binding protein 3 enhances the utility of gross cystic disease fluid protein-15 and mammaglobin A in triple-negative breast cancer by immunohistochemistry. Histopathology. 2015;67(2):245–54. doi: 10.1111/his.12645. [DOI] [PubMed] [Google Scholar]
- 28.Sangoi AR, Shrestha B, Yang G, Mego O, Beck AH. The Novel Marker GATA3 is Significantly More Sensitive Than Traditional Markers Mammaglobin and GCDFP15 for Identifying Breast Cancer in Surgical and Cytology Specimens of Metastatic and Matched Primary Tumors. Appl Immunohistochem Mol Morphol. 2016;24(4):229–37. doi: 10.1097/PAI.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Min W, Kim S, Son B. Comparison of serum HER-2/neu between trastuzumab-based regimen and anthyracycline-based regimen during neoadjuvant chemotherapy in advanced primary breast cancer. J Clin Oncol. 2009;27(15 suppl):e11582. [Google Scholar]
- 30.McArdle CS, McMillan DC, Greenlaw N, Morrison DS. Adjuvant radiotherapy and chemotherapy in breast cancer: 30 year follow-up of survival. BMC Cancer. 2010;10:398. doi: 10.1186/1471-2407-10-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Becciolini A, Biggeri A, Pacini P, Muirhead CR. Second malignancies in breast cancer patients following radiotherapy: a study in Florence, Italy. Breast Cancer Res. 2011;13(2):R38. doi: 10.1186/bcr2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fard ZT, Nafisi N. The Relationship Between 6 Polymorphisms of Caveolin-1 Gene and the Risk of Breast Cancer. Clin Breast Cancer. 2018;18(5):e893–e898. doi: 10.1016/j.clbc.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Pucci M, Bravatà V, Forte GI, Cammarata FP, Messa C, Gilardi MC, Minafra L. Caveolin-1, breast cancer and ionizing radiation. Cancer Genomics Proteomics. 2015;12(3):143–52. [PubMed] [Google Scholar]
- 34.Yeong J, Thike AA, Ikeda M, Lim JCT, Lee B, Nakamura S, et al. Caveolin-1 expression as a prognostic marker in triple negative breast cancers of Asian women. J Clin Pathol. 2018;71(2):161–7. doi: 10.1136/jclinpath-2017-204495. [DOI] [PubMed] [Google Scholar]
- 35.Kang J, Park JH, Lee HJ, Jo U, Park JK, Seo JH, et al. Caveolin-1 Modulates Docetaxel-Induced Cell Death in Breast Cancer Cell Subtypes through Different Mechanisms. Cancer Res Treat. 2016;48(2):715–26. doi: 10.4143/crt.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badana AK, Chintala M, Gavara MM, Naik S, Kumari S, Kappala VR, et al. Lipid rafts disruption induces apoptosis by attenuating expression of LRP6 and survivin in triple negative breast cancer. Biomed Pharmacother. 2018;97:359–368. doi: 10.1016/j.biopha.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Du C, Chen L, Zhang H, Wang Z, Liu W, Xie X, Xie M. Caveolin-1 limits the contribution of BKCa channel to MCF-7 breast cancer cell proliferation and invasion. Int J Mol Sci. 2014;15(11):20706–22. doi: 10.3390/ijms151120706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi XY, Xiong LX, Xiao L, Meng C, Qi GY, Li WL. Downregulation of caveolin 1 upregulates the expression of growth factors and regulators in co culture of fibroblasts with cancer cells. Mol Med Rep. 2016;13(1):744–52. doi: 10.3892/mmr.2015.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R, He W, Li Z, Chang W, Xin Y, Huang T. Caveolin-1 functions as a key regulator of 17β-estradiol-mediated autophagy and apoptosis in BT474 breast cancer cells. Int J Mol Med. 2014;34(3):822–7. doi: 10.3892/ijmm.2014.1836. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, et al. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy. 2015;11(5):769–84. doi: 10.1080/15548627.2015.1034411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witkiewicz AK, Kline J, Queenan M, Brody JR, Tsirigos A, Bilal E, et al. Molecular profiling of a lethal tumor microenvironment, as defined by stromal caveolin-1 status in breast cancers. Cell Cycle. 2011;10(11):1794–809. doi: 10.4161/cc.10.11.15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev. 2014;40(3):341–8. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Ariana M, Arabi N, Pornour M, Vaseghi H, Ganji SM, Alivand MR, et al. The diversity in the expression profile of caveolin II transcripts, considering its new transcript in breast cancer. J Cell Biochem. 2018;119(2):2168–2178. doi: 10.1002/jcb.26378. [DOI] [PubMed] [Google Scholar]
- 44.Chung YC, Kuo JF, Wei WC, Chang KJ, Chao WT. Caveolin-1 Dependent Endocytosis Enhances the Chemosensitivity of HER-2 Positive Breast Cancer Cells to Trastuzumab Emtansine (T-DM1). PLoS One. 2015;10(7):e0133072. doi: 10.1371/journal.pone.0133072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SR. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid Med Cell Longev. 2018;2018:9156285. doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]


