Abstract
Background:
Double-stranded fragmented extracellular DNA is a participant, inducer, and indicator of various processes occurring in the organism. When investigating the properties of extracellular DNA, the question regarding the specificity of exposure to DNA from different sources has always been raised. The aim of this study was to perform comparative assessment of biological properties of double-stranded DNA obtained from the human placenta, porcine placenta and salmon sperm.
Methods:
The intensity of leukocyte-stimulating effect of different dsDNA was assessed in mice after cyclophosphamide-induced cytoreduction. The stimulatory effect of different dsDNA on maturation and functions of human dendritic cells and the intensity of cytokine production by human whole blood cells was analyzed ex vivo. The oxidation level of the dsDNA was also compared.
Results:
Human placental DNA exhibited the strongest leukocyte-stimulating effect. DNA extracted from human and porcine placenta exhibited similar stimulatory action on maturation of dendritic cells, allostimulatory capacity, and ability of dendritic cells to induce generation of cytotoxic CD8+CD107a+ T cells in the mixed leukocyte reaction. DNA extracted from salmon sperm stimulated the maturation of dendritic cells, while having no effect on their allostimulatory capacity. DNA extracted from human and porcine placenta was shown to exhibit a stimulatory effect on cytokine secretion by human whole blood cells. The observed differences between the DNA preparations can be caused by the total methylation level and are not related to differences in oxidation level of DNA molecules.
Conclusions
Human placental DNA exhibited the maximum combination of all biological effects.
Key Words: Cytokines, Cytotoxic T cells, Dendritic cells, Double-stranded DNA
Introduction
Double-stranded fragmented extracellular exogenous and endogenous DNA is a participant, inducer, and indicator of various processes occurring in the organism. Exogenous nucleic acids are the pathogen-associated molecular patterns activating various components of the immune system aiming at pathogen elimination. Double-stranded extracellular endogenous DNA (dsDNA) can also activate the adaptive immune response (1–4) Furthermore, the amount of endogenous extracellular dsDNA contained in blood plasma and interstitial fluids shows the functional status of the organism and may act as an indicator of pathological processes (5) It is known that dsDNA induces autoimmune processes (6) and is one of the signals of the bystander effect (7) It has also been demonstrated that the systemic inflammatory response and sepsis are accompanied by massive increase in plasma DNA concentration (8)
At the cellular level, dsDNA fragments delivered to the internal cellular compartments are involved in various cellular processes. First of all, the entry of extracellular DNA fragments into the cell activates cell cycle arrest and induces repair processes (9) Under certain conditions, dsDNA fragments start to get involved in the repair process and interfere with it (10,11). The dsDNA fragments delivered into the somatic cell cytoplasm activates the cytosolic sensors and induces the adaptive immune response (2,12,13) It has been reported that extracellular dsDNA can be involved in transfer of genetic material from the destroyed cancer cells to other cells in the organism, thus causing malignization of these cells. This process is known as "genometastasis" (14,15). It was found that nucleic acids, including dsDNA, are found in exosomes and might act as a "tuning fork" of the organism's functional status used by certain cell populations for "fine tuning" their physiological and molecular processes (16,17)
According to the current viewpoint, extracellular nucleic acids are a novel type of the regulatory system of the organism involving complex mechanisms of regulation of cellular processes (16,18,19). Understanding of the potential involvement of dsDNA molecule in functioning of the entire organism as the fourth known biological mediator along with hormones, cytokines, and microvesicles (exosomes) has started to be gained only recently.
It was demonstrated in our studies that extracellular dsDNA activates antigen-presenting properties of dendritic cells (DCs), blood mononuclear cells and the mucosal immune system to produce specific cytokines (20–23). When investigating the properties of extracellular DNA, the question regarding the specificity of exposure to DNA from different sources has always been raised. Therefore, the aim of this study was to perform comparative assessment of biological properties of dsDNA obtained from different sources: DNA extracted from human (hDNA) or porcine (pDNA) placenta and salmon sperm (ssDNA). The following effects of dsDNA were assessed by comparison: (i) the intensity of leukocyte-stimulating effect in mice after cyclophosphamide-induced cytoreduction; (ii) the stimulatory effect on maturation and functions of human DCs; and (iii) the intensity of cytokine production by human whole blood cells ex vivo. Finally, the oxidation level of the dsDNA and the intensity of the revealed stimulatory effects as one of the potential impact factors were also compared.
Materials and Methods
DNA preparations
The hDNA was extracted from placenta delivered by healthy women; pDNA, from porcine placenta; and ssDNA, from salmon milt with the commonly used purification procedure. Ultrasonic DNA fragmentation was performed using an ultrasonic disintegrator at a frequency of 22 kHz; the mixture of DNA fragments sized 200–6000 bp was obtained. The DNA preparations were stored at –20 °C.
Assessment of the leukocyte stimulating activity of the DNA
We used 4-month-old CBA/Lac female mice bred at the Common Use Center Vivarium for Conventional Animals of the Institute of Cytology and Genetics, SB RAS. The animals were grown in groups of 6–10 mice per cage, with free access to food and water. All animal experiments were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes and the Protocol approved by the Interinstitutional Bioethics Commission of the Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences.
The mice received an intraperitoneal injection of cyclophosphamide (300 mg/kg). Mice in the study groups were receiving intraperitoneal injections of 0.2 mg of the DNA (hDNA, pDNA, or ssDNA according to the group they were allocated to) the same day and within three consecutive days. Mice in the control group received injections of saline solution of identical volume (0.2 mL). Blood for WBC count test was sampled one day before the cyclophosphamide injection (the baseline level) and on days 3, 5, and 7 after the mice had received cyclophosphamide. Blood samples were fixed in 3% acetic acid; the cells were stained with 0.01% trypan blue dye, and WBC count was determined under a microscope.
Generation of DCs
Mononuclear cells (MNCs) were obtained from peripheral blood of healthy donors by Ficoll®400 (Sigma-Aldrich, Saint Louis, MO, USA) density-gradient centrifugation. All the studies were performed after receiving a written informed consent. DCs were obtained using the protocol elaborated by Della Bella et al (24) In brief, DCs were generated by culturing plastic-adherent MNCs in RPMI-1640 medium (Sigma-Aldrich) supplemented with 0.3 mg/mL L-glutamine, 5 mM HEPES buffer, 100 mg/mL gentamicin, and 2.5% fetal calf serum (FCS, Sigma-Aldrich) with rhGM-CSF (40 ng/mL, Sigma-Aldrich) and rhIFN-α (Roferon-A, 1000 U/mL, Roche, Switzerland) for 4 days at 37 °C and 5% CO2 atmosphere. The maturation of DCs was induced by further exposure to 10 μg/mL lipopolysaccharide (LPS E. colli O114:B4, Sigma-Aldrich) or to 5 μg/mL of various DNA for additional 24 hrs.
DC phenotyping
DC phenotyping was performed by flow cytometry (FACS Calibur, Becton-Dickinson, San Jose, CA, USA) in the large granular lymphocyte gate using the CellQuest software (BD Becton-Dickinson). Phenotypes were determined following direct single-color staining of DCs with FITC-labeled monoclonal antibodies against CD14, CD25, CD86, HLA-DR, and PE-labeled anti-CD83 monoclonal antibodies (BD PharMingen, NJ, USA). In each experiment, isotype-matched control monoclonal antibodies were included to determine non-specific background staining. A minimum of 10,000 events was measured for each DC preparation.
Assessment of the allostimulatory capacity of DCs
The allostimulatory capacity of DCs was evaluated in mixed leukocyte reactions (MLRs) using IFN-DCs as stimulatory cells and allogeneic MNCs (105/well) as responder cells (DCs/MNCs = 1:10). Cells were grown in round-bottomed 96-well plates in RPMI-1640 supplemented with 10% inactivated AB (IV) serum at 37 °C in a CO2 incubator for 5 days. The level of proliferation was assessed by [3H] thymidine incorporation (1 μCi per well) added for the last 18 hrs of culturing. The stimulation index was counted as the ratio between proliferation in allo-MLR and response from MNCs cultured with unstimulated immature DCs.
CD107a degranulation assay
Degranulating cytotoxic CD8+ T cells are identified by their surface expression of CD107a, which is a lysosomal associated membrane protein (LAMP-1) residing in cytolytic granule membranes located within the cytoplasm. The marker is mobilized to the cell surface following activation-induced granule exocytosis. Unstimulated immature DCs (negative control), or DCs stimulated with LPS (positive control), hDNA or pDNA were incubated with allogeneic MNCs (105/well; DC/MNC ratio being 1:10) for 5 days. As a result of degranulation, CD107a was transiently expressed on the cell surface and was rapidly re-internalized via the endocytic pathway. Therefore, in order to prevent acidification followed by degradation of CD107a/antibody complexes, CD107a detection was performed in the presence of APC-conjugated anti-CD107a antibodies and monensin A (10 μM; all BD Pharmingen) added for the last 18 hrs of culturing. DCs/MNCs were then washed, stained with PE-labeled monoclonal anti-CD8 antibodies and analyzed by flow cytometry (FACS Calibur, Becton-Dickinson, San Jose, CA, USA). The frequency of degranulating cytotoxic T cells was measured as CD107a+ cells gated within CD8+ cells. A minimum of 10,000 events within the gate region were collected for each sample.
Assessment of cytokine-stimulating effect of DNA
Peripheral blood samples from conditionally healthy volunteers (n=6) were used to assess the cytokine-stimulating activity of DNA. All the volunteers provided a written informed consent for having their blood sampled and using these samples for research purposes.
The freshly collected samples of peripheral venous blood (1 mL each) were mixed with 4 mL of DMEM supplemented with heparin (2.5 U/mL), gentamicin (100 µg/mL), and L-glutamine (0.6 mg/mL) while maintaining sterility. In order to assess cytokine production, 1 mL of the diluted blood samples was transferred into vials for spontaneous and mitogen-induced cytokine production under sterile conditions. The 1 mL diluted blood samples were mixed with hDNA or pDNA under sterile conditions to achieve the final concentration of 10 µg/mL, and stirred thoroughly.
All the vials were incubated at 37 °C for 24 hrs. The blood cells were then precipitated at 10,000 G for 3 min. The supernatant fluid was transferred into a new vial and frozen at –20 °C to be stored for several days before the quantitative enzyme-linked immunosorbent assay of the cytokines was performed. Cytokine concentrations in the analyzed samples were measured using suitable test kits (Vector-Best CJSC, Novosibirsk, Russia).
Quantification of 8-oxoguanine in DNA samples
The amount of 8-oxydG per 1 million of DNA bases was determined by dot blotting. DNA samples at a certain concentration were applied onto a nitrocellulose membrane, and the amount of 8-oxydG was evaluated using anti-8-oxydG antibodies. The integral intensity and spot area were determined using the software, and the average density of the spot for the respective amount of applied DNA was calculated. The 8-oxydG contents in the samples under study were determined with respect to the control sample.
Statistical analysis
Statistical analysis was performed using the Statistica 6.0 analytics software portfolio for Windows (StatSoft). The Wilcoxon matched pairs test and the Mann-Whitney U test were used to compare nonparametric values. P< 0.05 was considered statistically significant.
Results
Leukocyte stimulation
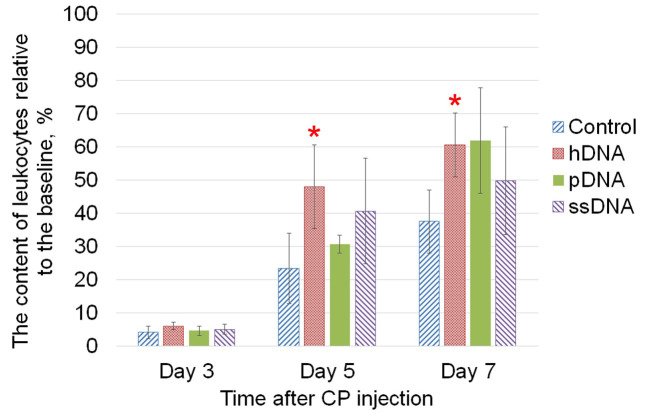
The leukocyte stimulating effect of three DNA types (hDNA, pDNA, and ssDNA) after the exposure to cyclophosphamide cytostatic agent was evaluated. In the group of mice receiving injections of hDNA, the leukocyte count was restored to 50% of the baseline as soon as on day 5 after cyclophosphamide injection (Fig. 1). The leukocyte stimulating effect of DNA was also observed in the groups that had received injections of pDNA and ssDNA, but differences with the control group were not statistically significant.
Fig. 1.
Leukocyte stimulating activity of the three types of DNA (hDNA, pDNA, and ssDNA) in the murine model of cyclophosphamide-induced cytopenia. CBA mice received intraperitoneal injections of cyclophosphamide (300 mg/kg). Mice in the study groups were receiving intraperitoneal injections of 0.2 mg of the respective DNA the same day and within three consecutive days. Animals in the control group were given injections of saline solution. The content of leukocytes relative to the baseline before the exposure to cyclophosphamide is shown (mean ± SD), n=5. * – statistically significant differences compared to the control group, P<0.05, Mann-Whitney U test.
The effect of DNA on DCs
The effects of various DNA on the phenotype of DCs, their stimulatory activity, and ability of DCs to induce generation of cytotoxic T cells in the allo-MLR as compared to those of conventional LPS activator were evaluated.
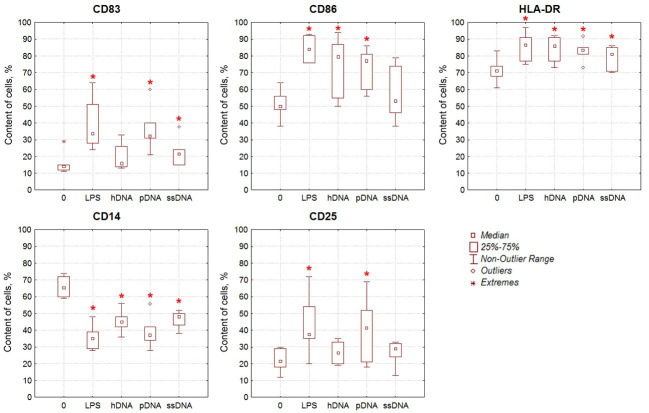
The effect of DNA on DC maturation
DCs were generated from plastic-adherent MNCs of healthy donors supplemented with either LPS or DNA as a maturation-stimulating agent. The phenotype of the DCs was evaluated on a flow cytometer using monoclonal antibodies. According to the literature data, mature DCs differ from immature ones by higher levels of expression of CD83, the costimulatory molecule CD86, and class II histocompatibility molecules HLA-DR, while simultaneously having a lower expression of CD14. Furthermore, the content of CD25+ DCs carrying the activation marker of mature DCs was analyzed (Fig. 2). The percentage of cells expressing CD83, CD86, and HLA-DR was statistically significantly higher, while CD14 expression parameters were lower in LPS-stimulated cells compared to intact (immature) DCs. The percentage of CD25+ DCs was also significantly increased in the LPS-stimulated cells. Stimulation of DCs with DNA caused similar changes in the phenotype. Meanwhile, stimulation with pDNA also significantly increased the percentage of cells expressing CD83, CD86, HLA-DR, and CD25, while reducing the expression of CD14 marker. Increased expression levels of CD83, CD86, HLA-DR, and CD25 and reduced expression of marker CD14 were also observed for hDNA and ssDNA; however, the differences with the control group were not always significant.
Fig. 2.
The effect of DNA on the phenotype of DCs. The content of cells expressing the respective markers among intact immature DCs (0, negative control) and in DC populations stimulated with either LPS (positive control) or dsDNA (hDNA, pDNA, and ssDNA) is shown, n=6. * –statistically significant differences compared to the negative control, P<0.05, Wilcoxon matched pairs test.
Therefore, pDNA exhibited the strongest effect on DC maturation, which was comparable to the action of LPS.
The effect of DNA on the allostimulatory capacity of DCs
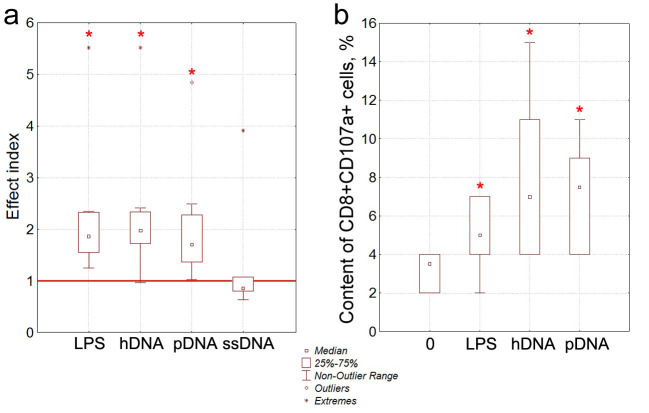
According to the literature data, mature DCs exhibit an increased ability to stimulate proliferation of allogeneic T cells in the MLR. The allostimulatory capacity of DCs whose maturation had been induced by DNA was studied. For this purpose, the proliferative response in the MLR (where allogeneic mononuclear cells acted as responder cells, while DCs activated with LPS or various dsDNA for 24 hrs were the stimulating agents) was evaluated (Fig. 3a).
Fig. 3.
The effect of DNA on the allostimulatory capacity of DCs and the ability of DCs to induce cytotoxic CD8+ T cells. a) The effect of DNA on the allostimulatory capacity of DCs. The stimulation indices calculated as the ratio between the proliferation intensity in allo-MLR, induced DCs stimulated by LPS or dsDNA (hDNA, pDNA, or ssDNA) and proliferation intensity in the allo-MLR induced by immature DCs (bold line) harvested from the respective donor (pairwise comparisons) are presented, n=8. b) Generation of cytotoxic CD8+CD107a+ T cells induced by immature and stimulated DCs in the allo-MLR. Intact immature DCs (0) and DCs stimulated by LPS, hDNA or pDNA, which were cultured for 5 days in the presence of responder MNCs from an allogeneic donor, n=6. The percentage of CD8+CD107a+ T cells was then evaluated by flow cytometry. * – statistical significance of the differences compared to intact DCs, P<0.05, Wilcoxon matched pairs test.
According to our findings, the proliferative response of mononuclear cells in the allo-MLR induced by LPS-activated DCs was on average twice stronger than that in the MLR induced by intact immature DCs. The allostimulatory capacity of DCs whose maturation was induced by hDNA or pDNA was also on average twice higher than that for the intact DCs. ssDNA had no effect on the allostimulatory capacity of DCs, so it was excluded from analysis in the subsequent experiments.
Hence, hDNA and pDNA are characterized by a comparable ability to enhance the allostimulatory capacity of DCs. The stimulatory effect of hDNA and pDNA was as strong as that of LPS.
The effect of DNA on the ability of DCs to induce cytotoxic CD8+ T cells
Comparative analysis of the ability of hDNA and pDNA to induce generation of cytotoxic CD8+ T cells expressing CD107a in the allo-MLR was conducted in a separate series of experiments (Fig. 3b). CD107a molecule is a component of the membrane of cytotoxic granules and is not found on the surface of naive CD8+ T cells. During degranulation of CD8+ T cells, the membrane of the granules gets fused with the cell membrane; as a result, CD107a appears on the surface of CD8+ T cells. Therefore, the number of CD8+CD107a+ cells shows the percentage of functionally active cytotoxic T cells.
Comparison of the effects of LPS, hDNA, and pDNA on the ability of DCs to induce functionally active cytotoxic CD8+ T cells in the allo-MLR demonstrated that DCs activated by hDNA or pDNA on average induced a twice greater amount of CD8+CD107a+ cytotoxic T cells compared to intact DCs (Fig. 3b).
Therefore, the stimulatory effect of pDNA on ability of DCs to induce generation of cytotoxic T cells was similar to that exhibited by hDNA. The effect of stimulating DCs with DNA was stronger than that observed upon stimulation with LPS.
Overall, these findings confirm that hDNA stimulates maturation of DCs, enhances their allostimulatory capacity, and ability of DCs to induce generation of cytotoxic T cells. It has been demonstrated that pDNA is not inferior to hDNA in terms of the intensity of stimulatory effects; i.e., it exhibits a similar stimulatory action on maturation of DCs, their allostimulatory capacity, and ability to induce generation of cytotoxic T cells. ssDNA stimulated DC maturation but had no effect on their allostimulatory capacity.
Cytokine-stimulating activity of DNA
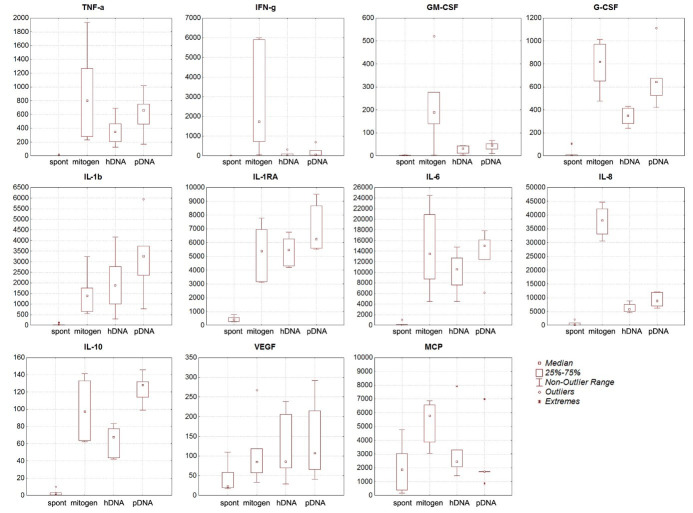
The cytokine-stimulating activities of hDNA and pDNA were evaluated (Fig. 4). It was revealed that both hDNA and pDNA stimulate production of TNF-α, G-CSF, IL-1β, IL-1RA, IL-6, IL-10, and VEGF comparable to that achieved by mitogenic stimulation. hDNA and pDNA also increased production of such cytokines as IFN-γ, GM-CSF, IL-8, and MCP, while the increase was not as significant as that achieved by mitogenic stimulation.
Fig. 4.
The cytokine-stimulating activity of hDNA and pDNA compared to spontaneous and mitogen-induced production of cytokines. On Y axis there is cytokine content (pg/mL), n=6.
The correlation between cytokine production after stimulation with hDNA and pDNA was assessed. No significant correlation between the induction of cytokine production by porcine and human DNA was revealed for IL-6, IL-8, IL-10, IL-1RA, and G-CSF cytokines. For TNF-α, IFN-γ, IL-1β, VEGF, MCP, and GM-CSF cytokines, the fitting coefficient was close to 1. pDNA was more efficient in stimulating cytokine production compared to hDNA.
The content of 8-oxoguanine in DNA
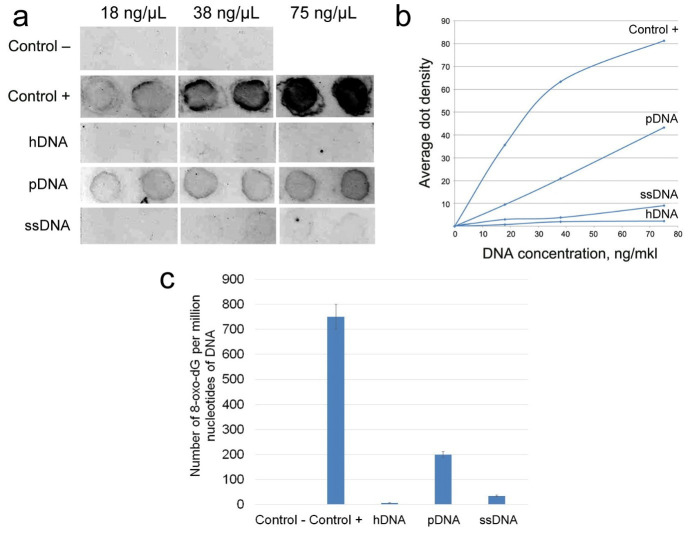
The content of 8-oxoguanine as a parameter characterizing the oxidation level of the DNA was analyzed. The hDNA, pDNA and ssDNA samples were assessed. The amount of 8-oxydG per 1 million of DNA nucleotides was determined by dot blotting. DNA samples with certain concentration were applied onto a nitrocellulose membrane, and the amount of 8-oxydG was determined using anti-8-oxydG antibodies (Fig. 5). The highest oxidation level was revealed for the pDNA sample, while the hDNA was characterized by the lowest oxidation level.
Fig. 5.
Measuring the amount of 8-oxydG for the hDNA, pDNA, and ssDNA. DNA samples containing 0.1 8-oxydG per 1 million of nucleotides (Control–) and 750 8-oxydG per 1 million nucleotides (Control+) were used as control samples. a) Dot-blot hybridization of DNA samples with anti-8-oxydG antibodies. b) The average dot density (integral intensity divided by the dot area) according to the dot-blot hybridization data. c) The amount of 8-oxydG per 1 million DNA nucleotides for DNA samples.
Discussion
The study revealed that only the hDNA exhibited a statistically significant leukocyte-stimulating effect in the murine model of cyclophosphamide-induced cytopenia. An analysis of the effect on maturation and functions of human DCs in the ex vivo culture showed that the efficiencies of hDNA and pDNA were almost identical, comparable to that of the standard activating agent, LPS. pDNA was somewhat more efficient in activating DC maturation. The ssDNA stimulated DC maturation, while having no effect on the allostimulatory capacity of DCs. Assessment of the cytokine-stimulating activity showed that the effects of the hDNA and pDNA were similar, although being stronger to some extent for pDNA. Hence,only the preparation based on human placental DNA exhibited the maximum combination of all biological effects.
The main question in this study concerned the mechanisms responsible for the differences in biological effects of DNA extracted from different taxonomic species, both evolutionarily related and distant ones.
The results obtained suggest that the main differences in the biological properties of the three types of DNA are associated with epigenetic modifications, namely, cytosine methylation at C5 and the level of DNA oxidation. The preparations used in this study were deproteinized, so the nucleosome code, which is also one of the determinants of epigenetic modifications of DNA, cannot affect the revealed biological properties in this case.
According to the literature data, hDNA, pDNA, and ssDNAs have almost identical GC contents (41–42%) (25–27). Approximately 3% of human genomic DNA is methylated (28–31). The total methylation degree of porcine genomic DNA is 2–2.5% (32) Salmon sperm genomic DNA contains ∼ 7.5% 5mdC (25,33,34) In other words, the methylation degree of hDNA and pDNA is two- to fourfold lower than that for ssDNA.
Within the DNA molecule, guanine is the most sensitive target to reactive oxygen species (35). 8-Oxoguanine is the product of guanine damage and is considered to be one of the key biomarkers of oxidative DNA damage (36) This study revealed that all three types of DNA are characterized by different oxidation levels, which were determined according to the 8-oxydG content. The average 8-oxydG content in hDNA, pDNA, and ssDNA is 6, 200, and 35 8-oxydG per 1 million DNA nucleotides, respectively. In our study, the preparation based on DNA extracted from the species evolutionarily closest to humans is characterized by a 33-fold higher level of DNA oxidation. Meanwhile, the level of DNA oxidation for the evolutionarily remote species (salmon) was only six times higher than that for hDNA.
We have interpreted the revealed features of the biological effects of different DNA types in the following way.
Only hDNA was shown to exhibit a reliable leukocyte-stimulating effect. However, the pDNA showed a trend in the leukocyte-stimulating activity similar to that for hDNA, while the level of pDNA oxidation is 33 times higher than that of hDNA. Furthermore, both preparations efficiently stimulate cytokine production by human whole blood cells. There can be two mechanisms of activating leukocyte stimulation in experimental animals. The first one is obvious and is related to stimulating cytokine production by whole blood cells, which has been described in our study. It is known that IL-1, IL-3, TNF-α, and colony-stimulating factors activate proliferation of hematopoietic stem/progenitor cells (37,38) IL-1, TNF-α, and G-CSF are produced by treating blood mononuclear cells with hDNA and pDNA and can induce proliferation of resting hematopoietic stem cells.
Another mechanism of inducing proliferation of hematopoietic progenitor cells was described in our previous studies (39,40) and is related to the ability of hematopoietic stem cells to naturally internalize extracellular dsDNA fragments. After being internalized into a cell, dsDNA fragments become involved in various molecular processes depending on cell type and state. When being internalized into hematopoietic stem cells, free ends of double-stranded DNA induce cell cycle arrest (10,39). This inevitably results in hematopoietic stem cell division followed by proliferation manifesting itself as hematopoiesis stimulation (39,41) According to the findings obtained in this study, the leukocyte-stimulating effect is not related to the oxidized state of DNA. The degree of cytosine methylation correlates with the leukocyte stimulation phenomenon; however, it has not been reliably demonstrated in our experiments.
Bacterial DNA is known to mainly contain unmethylated CpG. These very sequences are identified by TLR9, resulting in activation of the immune cells (first of all, antigen-presenting DCs) (1–3,18) It was demonstrated in different studies that the extracellular genomic self-dsDNA is also a stress-inducing factor (42) The GC content and oxidation level of DNA bases are the key characteristics of deproteinized extracellular dsDNA exposing it to stress.
It is fair to assume that the differences in efficiency of DC activation between hDNA/pDNA versus ssDNA are caused by the 2–4-fold difference in the methylation degree of GC loci.
The comparison of the oxidation levels of three DNA types and the intensity of DC activation revealed no correlation between the factors being compared. The least and the most oxidized hDNA and pDNA exhibit similar efficiencies of DC activation. Meanwhile, ssDNA characterized by oxidation level close to that of hDNA cannot induce the allostimulatory capacity of DCs. In a similar way, despite the significant difference in 8-oxoguanine content, hDNA and pDNA exhibit similar levels of inducing cytokine production by whole blood cells.
Our analysis demonstrates that only one of the parameters of DNA under study (namely, the methylation degree of molecules) can be the reason why DNA preparations exhibit different biological activity in activating human DCs and whole blood cells in ex vivo cultures.
Hence, fragmented genomic dsDNA can trigger biological signaling cascades and is a functionally active component of the internal environment of the body.
Funding
This study was funded by the Institute of Cytology and Genetics (State Budget Project No. FWNR-2022-0016).
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgements
The work was supported by the Russian Ministry of Science and High Education.
References
- 1.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisetsky DS. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol. 2012;144(1):32–40. doi: 10.1016/j.clim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann G. Nucleic Acid Immunity. Adv Immunol. 2017;133:121–69. doi: 10.1016/bs.ai.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torabi A, Tahmoorespour M, Vahedi F, Mosavari N, Nassiri M. Construction of eukaryotic expression vectors encoding CFP-10 and ESAT-6 genes and their potential in lymphocyte proliferation. Rep Biochem Mol Biol. 2013;2(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- 5.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61(4):1659–65. [PubMed] [Google Scholar]
- 6.Choubey D. DNA-responsive inflammasomes and their regulators in autoimmunity. Clin Immunol. 2012;142(3):223–31. doi: 10.1016/j.clim.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermakov AV, Konkova MS, Kostyuk SV, Egolina NA, Efremova LV, Veiko NN, et al. Oxidative stress as a significant factor for development of an adaptive response in irradiated and nonirradiated human lymphocytes after inducing the bystander effect by low-dose X-radiation. Mutat Res. 2009;669(1-2):155–61. doi: 10.1016/j.mrfmmm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Pugin J. How tissue injury alarms the immune system and causes a systemic inflammatory response syndrome. Ann Intensive Care. 2012;2(1):27. doi: 10.1186/2110-5820-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou L. Single- and double-stranded DNA: building a trigger of ATR-mediated DNA damage response. Genes Dev. 2007;21(8):879–85. doi: 10.1101/gad.1550307. [DOI] [PubMed] [Google Scholar]
- 10.Dolgova EV, Proskurina AS, Nikolin VP, Popova NA, Alyamkina EA, Orishchenko KE, et al. “Delayed death” phenomenon: A synergistic action of cyclophosphamide and exogenous DNA. Gene. 2012;495(2):134–45. doi: 10.1016/j.gene.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Ritter GS, Nikolin VP, Popova NA, Proskurina AS, Kisaretova PE, Taranov OS, et al. Characterization of biological peculiarities of the radioprotective activity of double-stranded RNA isolated from Saccharomyces cerevisiae. Int J Radiat Biol. 2020 Sep;96(9):1173–1191. doi: 10.1080/09553002.2020.1793020. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey A, Bowie AG. Innate immune recognition of DNA: A recent history. Virology. 2015;479-480:146–52. doi: 10.1016/j.virol.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahedi F, Ghorbani E, Falsafi T. Construction of an expression plasmid (vector) encoding Brucella melitensis outer membrane protein, a candidate for DNA vaccine. Rep Biochem Mol Biol. 2013;1(2):82–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Yakubov LA, Rogachev VA, Likhacheva AC, Bogachev SS, Sebeleva TE, Shilov AG, et al. Natural human gene correction by small extracellular genomic DNA fragments. Cell Cycle. 2007;6(18):2293–301. doi: 10.4161/cc.6.18.4729. [DOI] [PubMed] [Google Scholar]
- 15.García-Olmo DC, Picazo MG, García-Olmo D. Transformation of non-tumor host cells during tumor progression: Theories and evidence. Expert Opin Biol Ther. 2012;12(Suppl 1):S199–207. doi: 10.1517/14712598.2012.681370. [DOI] [PubMed] [Google Scholar]
- 16.Ronquist G. Prostasomes are mediators of intercellular communication: from basic research to clinical implications. J Intern Med. 2012;271(4):400–13. doi: 10.1111/j.1365-2796.2011.02487.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernando MR, Jiang C, Krzyzanowski GD, Ryan WL. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS One. 2017;12(8):e0183915. doi: 10.1371/journal.pone.0183915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvvuri B, Lood C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front Immunol. 2019;10:502. doi: 10.3389/fimmu.2019.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44(1):11–5. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Alyamkina EA, Leplina OY, Ostanin AA, Chernykh ER, Nikolin VP, Popova NA, et al. Effects of human exogenous DNA on production of perforin-containing CD8+ cytotoxic lymphocytes in laboratory setting and clinical practice. Cell Immunol. 2012;276(1-2):59–66. doi: 10.1016/j.cellimm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Alyamkina EA, Leplina OY, Sakhno L V, Chernykh ER, Ostanin AA, Efremov YR, et al. Effect of double-stranded DNA on maturation of dendritic cells in vitro. Cellular Immunology. 2010;266:46–51. doi: 10.1016/j.cellimm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Alyamkina EA, Dolgova EV, Likhacheva AS, Rogachev VA, Sebeleva TE, Nikolin VP, et al. Exogenous allogenic fragmented double-stranded DNA is internalized into human dendritic cells and enhances their allostimulatory activity. Cell Immunol. 2010;262(2):120–6. doi: 10.1016/j.cellimm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Orishchenko KE, Ryzhikova SL, Druzhinina YG, Ryabicheva TG, Varaksin NA, Alyamkina EA, et al. Effect of human double-stranded DNA preparation on the production of cytokines by dendritic cells and peripheral blood cells from relatively healthy donors. Cancer Therapy. 2011;8:191–205. [Google Scholar]
- 24.Della Bella S, Nicola S, Riva A, Biasin M, Clerici M, Villa ML. Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha. J Leukoc Biol. 2004;75(1):106–16. doi: 10.1189/jlb.0403154. [DOI] [PubMed] [Google Scholar]
- 25.Wrobel K, Landero Figueroa JA, Zaina S, Lund G, Wrobel K. Phosphorus and osmium as elemental tags for the determination of global DNA methylation--a novel application of high performance liquid chromatography inductively coupled plasma mass spectrometry in epigenetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(5-6):609–14. doi: 10.1016/j.jchromb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Marmur J, Doty P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. Journal of Molecular Biology. 1962;5:109–18. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- 27.Choi M, Lee J, Le MT, Nguyen DT, Park S, Soundrarajan N, et al. Genome-wide analysis of DNA methylation in pigs using reduced representation bisulfite sequencing. DNA Res. 2015;22(5):343–55. doi: 10.1093/dnares/dsv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breiling A, Lyko F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics Chromatin. 2015;8:24. doi: 10.1186/s13072-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrlich M, Gama-Sosa MA, Huang LH, Midgett RM, Kuo KC, McCune RA, Gehrke C. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. 1982;10(8):2709–21. doi: 10.1093/nar/10.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuke C, Shimabukuro M, Petronis A, Sugimoto J, Oda T, Miura K, Miyazaki T, et al. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet. 2004;68(Pt 3):196–204. doi: 10.1046/j.1529-8817.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 31.Sandhu J, Kaur B, Armstrong C, Talbot CJ, Steward WP, Farmer PB, Singh R. Determination of 5-methyl-2'-deoxycytidine in genomic DNA using high performance liquid chromatography-ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(20-21):1957–61. doi: 10.1016/j.jchromb.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Jin L, Jiang Z, Xia Y, Lou P, Chen L, Wang H, et al. Genome-wide DNA methylation changes in skeletal muscle between young and middle-aged pigs. BMC Genomics. 2014;15(1):653. doi: 10.1186/1471-2164-15-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcazar Magana A, Wrobel K, Corrales Escobosa AR, Wrobel K. Application of liquid chromatography/electrospray ionization ion trap tandem mass spectrometry for the evaluation of global nucleic acids: Methylation in garden cress under exposure to CuO nanoparticles. Rapid Commun Mass Spectrom. 2016;30(1):209–20. doi: 10.1002/rcm.7440. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Kadarmideen HN. An Epigenome-Wide DNA Methylation Map of Testis in Pigs for Study of Complex Traits. Front Genet. 2019;10:405. doi: 10.3389/fgene.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H, et al. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7(11):1849–51. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths HR, Møller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, et al. Biomarkers. Mol Aspects Med. 2002;23(1-3):101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 37.Slørdal L, Warren DJ, Moore MA. Effect of recombinant murine tumor necrosis factor on hemopoietic reconstitution in sublethally irradiated mice. J Immunol. 1989;142(3):833–5. [PubMed] [Google Scholar]
- 38.Nakai S, Aihara K, Hirai Y. Interleukin-1 potentiates granulopoiesis and thrombopoiesis by producing hematopoietic factors in vivo. Life Sci. 1989;45(7):585–91. doi: 10.1016/0024-3205(89)90043-x. [DOI] [PubMed] [Google Scholar]
- 39.Dolgova EV, Efremov YR, Orishchenko KE, Andrushkevich OM, Alyamkina EA, Proskurina AS. Delivery and processing of exogenous double-stranded DNA in mouse CD34+ hematopoietic progenitor cells and their cell cycle changes upon combined treatment with cyclophosphamide and double-stranded DNA. Gene. 2013;528(2):74–83. doi: 10.1016/j.gene.2013.06.058. [DOI] [PubMed] [Google Scholar]
- 40.Dolgova EV, Alyamkina EA, Efremov YR, Nikolin VP, Popova NA, Tyrinova TV, et al. Identification of cancer stem cells and a strategy for their elimination. Cancer Biol Ther. 2014;15(10):1378–94. doi: 10.4161/cbt.29854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Likhacheva AS, Nikolin VP, Popova NA, Rogachev VA, Prokhorovich MA, Sebeleva TE, et al. Exogenous DNA can be captured by stem cells and be involved in their rescue from death after lethal-dose γ-radiation. Gene Ther Mol Biol. 2007;11(2):305–14. [Google Scholar]
- 42.Gravina S, Sedivy JM, Vijg J. The dark side of circulating nucleic acids. Aging Cell. 2016;15(3):398–9. doi: 10.1111/acel.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]