Introduction
Paraneoplastic pemphigus (PNP) is a rare autoimmune mucocutaneous blistering disease. When associated with underlying malignancies, such as myeloid leukemia and lymphoma, PNP generally has a poor prognosis.1,2 When associated with benign conditions, such as Castleman disease and thymoma, it generally has much better prognosis.3 Here, we describe what we believe, to our knowledge, is the first case of PNP associated with a locally invasive, unresectable, lymphocyte-predominant type B2 thymoma complicated by an intestinal perforation. Although the patient had an initial response to a combination of high-dose systemic corticosteroids, intravenous immunoglobulin (IVIg), and rituximab, he subsequently succumbed to his disease.
Case report
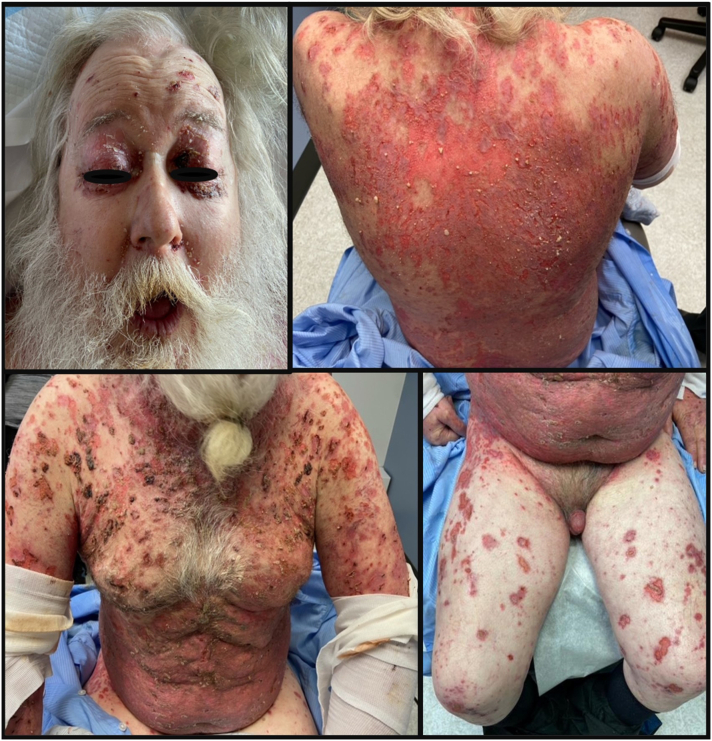
A 60-year-old Caucasian man with no known medical history presented with progressive weight loss and a 1-month history of worsening erythematous, eroded, and crusted patches on the trunk, extremities, lips, and genitalia (Fig 1). An autoimmune blistering disease was suspected, and cutaneous biopsy analyses revealed both intraepidermal acantholysis and subepidermal blistering (Fig 2). Direct immunofluorescence was positive for intraepidermal and subepidermal IgG deposition. Enzyme-linked immunosorbent assay results of the patient’s serum was positive for desmoglein-1 antibodies at 166.6 U/mL (negative < 18; positive > 36 U/mL), desmoglein-3 antibodies at 119.0 U/mL (negative < 19; positive > 37 U/mL), and negative for anti-envoplakin antibodies. Indirect immunofluorescence on rat bladder epithelium was negative for IgG antibodies.
Fig 1.
The patient’s cutaneous presentation involving >50% body surface area; on the trunk, upper extremities, lower extremities, eyelids, oral mucosa, and genitalia, with bright red eroded and crusted patches and plaques without apparent bullae.
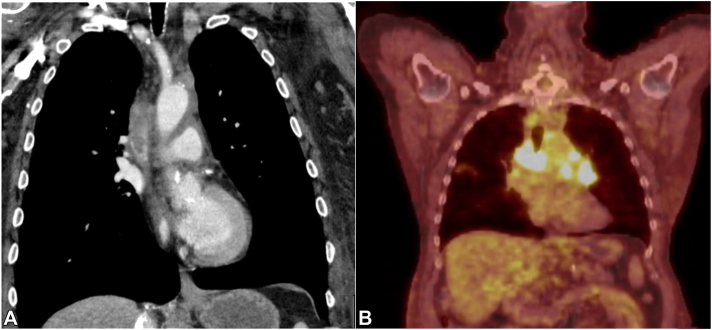
Fig 2.
A, Chest computed tomography image showing a heterogenous mass suggestive of thrombosis and partial occlusion of the superior vena cava. Several enlarged pericardial and hilar lymph nodes are noted. B, Positron emission tomography computed tomography scan image showing several foci of increased uptake in the mediastinum, which is consistent with an underlying malignancy.
One week before the onset of the cutaneous eruption, the patient presented to the emergency room with dyspnea. Extensive evaluation revealed a thrombus in the superior vena cava and mediastinal infiltration (Fig 3), and mediastinal biopsy result revealed a type B2 thymoma. Clinical and histopathologic presentations, supportive laboratory test results, and the presence of an underlying thymoma led to a diagnosis of PNP.
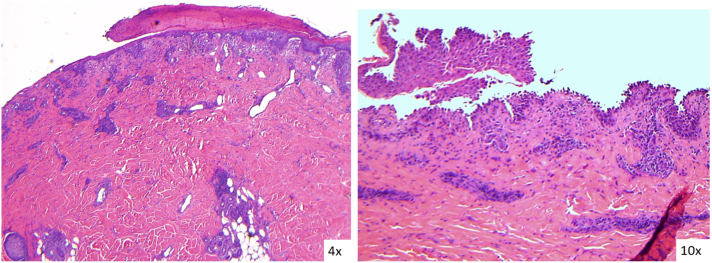
Fig 3.
Hematoxylin-eosin stained images of the left side of the upper abdomen show acantholysis with areas of possible subepidermal blisters. There is a superficial lymphocytic infiltrate with eosinophils. (Original magnification: A, ×4; B, ×100).
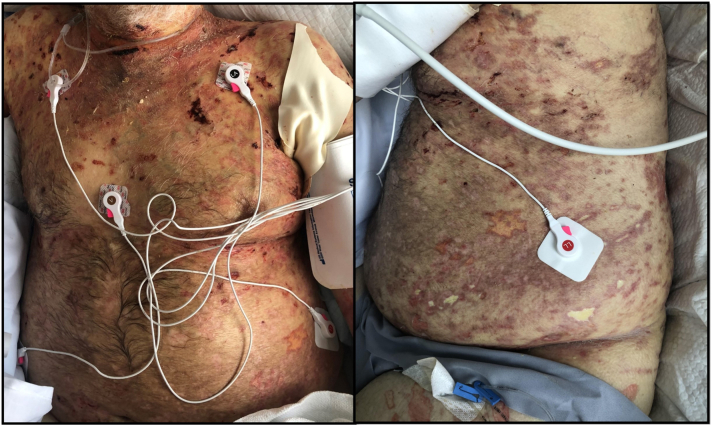
The patient was started on intravenous methylprednisolone 1 mg/kg daily and wet wraps with topical corticosteroids. Subsequently, IVIg 2 mg/kg every 2 weeks and rituximab 375 mg/m2 weekly was added. Due to the locally invasive nature of the thymoma, which infiltrates the lungs and adjacent vasculature, surgical resection was not feasible. However, with the combination therapy, the patient’s cutaneous lesions responded well, with evidence of reepithelization within 1 week of treatment initiation (Fig 4). Notably, on subsequent follow-up computed tomography of the chest, a decrease in the size of the thymoma was also observed. Despite apparent positive response to therapies, the patient unfortunately deteriorated and died from complications of an intestinal perforation.
Fig 4.
Clinical photos showing reepithelialization of the patient’s skin that was noted 1 week after treatment with high-dose systemic corticosteroids, rituximab, and intravenous immunoglobulin.
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that the patient gave consent for photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Discussion
Paraneoplastic pemphigus is a rare autoimmune blistering disease associated with an underlying neoplasm that was first described in 1990.1 Since then, several revised criteria have been proposed with different variations, but they all share the following 4 consistent features: (1) severe and intractable stomatitis, (2) acantholysis and/or interface dermatitis on histology, (3) positive anti-plakin autoantibodies, and (4) the presence of an underlying associated condition.3 Although our patient did not have any detectable anti-plakin antibodies, the diagnosis of PNP was made on the basis of the modified diagnostic criteria described by Svoboda et al.4
The prognosis of PNP is generally poor with up to 90% reported mortality.1,2 The high mortality of PNP is typically associated with hematologic malignancy, whereas association with benign neoplasms, such as Castleman disease or thymoma, typically has better clinical outcomes due to curative surgical resections.5,6 To date, to our knowledge, there has not been any reported case of PNP associated with a unresectable type B2 thymoma. Type B2 thymoma is a rare type of thymoma that consists of neoplastic epithelial cells scattered among a dense population of lymphocytes. It has a prevalence of 20% of all thymomas.5 Although type B2 thymoma is a benign neoplasm, it is biologically aggressive in nature, with the potential to invade the surrounding organs and metastasize to distant sites at later stages.7 Surgical management can be indicative in early stages, whereas radiation therapy and chemotherapy are necessary for advanced disease.5 For our patient, chemotherapy and radiation therapy were to be initiated following discharge from hospitalization.
The pathophysiology of PNP is not well understood. Both humoral and cell-mediated immunity are believed to contribute to the development of PNP. The presence of a thymoma affects the negative selection process of central tolerance, which enhances autoreactivity in peripheral T cells. For this reason, thymoma is known to induce autoimmunity in conditions, such as in myasthenia gravis. It has been proposed that thymoma induces PNP via a similar mechanism of enhanced autoimmunity.3 The antibodies against desmosomal proteins play an important role in the development of acantholytic blisters in PNP. Additionally, the presence of CD8+ T cells infiltrating keratinocytes suggests their role in the formation of lichenoid dermatitis in PNP.3,8
Gastrointestinal complications, such as intestinal perforations, are rarely associated with PNP (Table I).9, 10, 11 Odani et al10 have shown that PNP damages the gastrointestinal epithelium through deposition of IgG autoantibodies. Nutan et al11 reported a fatal outcome for a patient with sarcoma who developed PNP complicated by a colonic perforation. This patient died despite treatments with colonic resection and systemic therapy consisting of methylprednisolone, IVIg, and rituximab. Similarly, despite initial response to systemic treatment, our patient died because of complications of an intestinal perforation.
Table I.
Gastrointestinal findings in patients with paraneoplastic pemphigus
| Reference | Patient | Gastrointestinal and histopathologic findings |
|---|---|---|
| Miida et al (2006)9 | 57-year-old woman with PNP |
|
| ||
| Odani et al (2020)10 | 70-year-old man with PNP associated with lymphoma |
|
| ||
| ||
| ||
| Nutan et al (2021)11 | 68-year-old woman with PNP associated with poorly differentiated sarcoma |
|
| ||
| Current case | 60-year-old man with PNP associated with type B2 thymoma |
|
| ||
| ||
|
CMV, Cytomegalovirus.
Bowel perforations have also been reported in previous studies as a rare adverse effect of high-dose corticosteroid therapy.12,13 It is possible that the weakened intestinal mucosae in patients with PNP might be more susceptible to this rare adverse effect from the high-dose corticosteroid therapy. Weiner et al13 suggest that the antiinflammatory properties of corticosteroids might mask the initial presenting symptoms for bowel perforations. Therefore, bowel perforation should be considered when patients being treated with high-dose corticosteroids develop abdominal discomfort, fever of unknown origin, or unexplained leukocytosis.13
There is no standard treatment for PNP. Several combinations of systemic therapy have been used to decrease autoimmunity by targeting both humoral and cell-mediated pathways, which include combinations of corticosteroids with other steroid-sparing immunosuppressive agents, such as azathioprine, cyclosporine, mycophenolate mofetil, and cyclophosphamide, as well as rituximab, IVIg, and plasmapheresis. Notably, mucosal lesions, especially stomatitis, which is an important feature of PNP, tend to be refractory to treatment.8
In our patient, the combination therapy of high-dose corticosteroids, rituximab, and IVIg resulted in considerable improvement of the mucocutaneous symptoms as well as reduction in the size of the underlying thymoma. Although PNP associated with thymoma is typically treated with surgical resection of the underlying neoplasm,3 this was not possible in our patient with nonresectable type B2 thymoma. In addition, our case suggests that early surveillance for possible gastrointestinal complications is important in treating patients with PNP, especially since these patients are typically managed with high-dose corticosteroids. To the best of our knowledge, this is the first report of nonsurgical management of PNP-associated thymoma complicated by an intestinal perforation.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
This patient case has been presented at the American Academy of Dermatology annual meeting, March 26, 2022.
IRB approval status: Not applicable.
References
- 1.Anhalt G.J., Kim S.C., Stanley J.R., et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323(25):1729–1735. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 2.Mutasim D.F., Pelc N.J., Anhalt G.J. Paraneoplastic pemphigus. Dermatol Clin. 1993;11(3):473–481. doi: 10.1016/S0733-8635(18)30244-4. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.H., Kim S.C. Paraneoplastic pemphigus: paraneoplastic autoimmune disease of the skin and mucosa. Front Immunol. 2019;10:1259. doi: 10.3389/fimmu.2019.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svoboda S.A., Huang S., Liu X., Hsu S., Motaparthi K. Paraneoplastic pemphigus: revised diagnostic criteria based on literature analysis. J Cutan Pathol. 2021;48(9):1133–1138. doi: 10.1111/cup.14004. [DOI] [PubMed] [Google Scholar]
- 5.Song Z., Jin X., Zhang Y. Treatment and prognosis of type B2 thymoma. World J Surg Oncol. 2014;12:291. doi: 10.1186/1477-7819-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Qiao Q.L., Chen X.X., et al. Improved outcomes after complete resection of underlying tumors for patients with paraneoplastic pemphigus: a single-center experience of 22 cases. J Cancer Res Clin Oncol. 2011;137(2):229–234. doi: 10.1007/s00432-010-0874-z. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.K., Choi Y.S., Kim J., Shim Y.M., Han J., Kim K. Type B thymoma: is prognosis predicted only by World Health Organization classification? J Thorac Cardiovasc Surg. 2010;139(6):1431–1435.e1. doi: 10.1016/j.jtcvs.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Paolino G., Didona D., Magliulo G., et al. Paraneoplastic pemphigus: insight into the autoimmune pathogenesis, clinical features and therapy. Int J Mol Sci. 2017;18(12):2532. doi: 10.3390/ijms18122532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miida H., Kazama T., Inomata N., et al. Severe gastrointestinal involvement in paraneoplastic pemphigus. Eur J Dermatol. 2006;16(4):420–422. [PubMed] [Google Scholar]
- 10.Odani K., Itoh A., Yanagita S., et al. Paraneoplastic pemphigus involving the respiratory and gastrointestinal mucosae. Case Rep Pathol. 2020;2020 doi: 10.1155/2020/7350759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nutan F., De Carvalho H., Ngo H., Danielides S. Sarcoma-associated lichen planus-like paraneoplastic autoimmune multiorgan syndrome with colonic perforation. JAAD Case Rep. 2021;17:107–110. doi: 10.1016/j.jdcr.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauber J.S., Abrams H.L., Ray M.C. Silent bowel perforation occurring during corticosteroid treatment for pemphigus vulgaris. Cutis. 1989;43(1):27–28. [PubMed] [Google Scholar]
- 13.Weiner H.L., Rezai A.R., Cooper P.R. Sigmoid diverticular perforation in neurosurgical patients receiving high-dose corticosteroids. Neurosurgery. 1993;33(1):40–43. doi: 10.1227/00006123-199307000-00006. [DOI] [PubMed] [Google Scholar]