Summary
Background
Studies have shown that dengue virus transmission increases in association with ambient temperature. We performed a systematic review and meta-analysis to assess the effect of both high temperatures and heatwave events on dengue transmission in different climate zones globally.
Methods
A systematic literature search was conducted in PubMed, Scopus, Embase, and Web of Science from January 1990 to September 20, 2022. We included peer reviewed original observational studies using ecological time series, case crossover, or case series study designs reporting the association of high temperatures and heatwave with dengue and comparing risks over different exposures or time periods. Studies classified as case reports, clinical trials, non-human studies, conference abstracts, editorials, reviews, books, posters, commentaries; and studies that examined only seasonal effects were excluded. Effect estimates were extracted from published literature. A random effects meta-analysis was performed to pool the relative risks (RRs) of dengue infection per 1 °C increase in temperature, and further subgroup analyses were also conducted. The quality and strength of evidence were evaluated following the Navigation Guide systematic review methodology framework. The review protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO).
Findings
The study selection process yielded 6367 studies. A total of 106 studies covering more than four million dengue cases fulfilled the inclusion criteria; of these, 54 studies were eligible for meta-analysis. The overall pooled estimate showed a 13% increase in risk of dengue infection (RR = 1.13; 95% confidence interval (CI): 1.11–1.16, I2 = 98.0%) for each 1 °C increase in high temperatures. Subgroup analyses by climate zones suggested greater effects of temperature in tropical monsoon climate zone (RR = 1.29, 95% CI: 1.11–1.51) and humid subtropical climate zone (RR = 1.20, 95% CI: 1.15–1.25). Heatwave events showed association with an increased risk of dengue infection (RR = 1.08; 95% CI: 0.95–1.23, I2 = 88.9%), despite a wide confidence interval. The overall strength of evidence was found to be “sufficient” for high temperatures but “limited” for heatwaves. Our results showed that high temperatures increased the risk of dengue infection, albeit with varying risks across climate zones and different levels of national income.
Interpretation
High temperatures increased the relative risk of dengue infection. Future studies on the association between temperature and dengue infection should consider local and regional climate, socio-demographic and environmental characteristics to explore vulnerability at local and regional levels for tailored prevention.
Funding
Australian Research Council Discovery Program.
Keywords: High temperature, Heatwave, Dengue fever, Climate change, Emerging infectious disease, Arboviral infections
Research in context.
Evidence before this study
A comprehensive search was conducted in PubMed, Embase, Scopus and Web of Science databases to find available systematic reviews and meta-analysis of studies that reported the effect of temperature and heatwaves on dengue infection and published in English language. The databases were searched with outcome terms: (“dengue fever” or “dengue haemorrhagic fever” or “dengue infection” or “DF” or DHF) and combined with meteorological terms: (“temperature” or “heatwave” or “extreme weather” or “meteorological factor” or “climate” or “climatic factor” or “climate change” or “global warming”); and filtered using the term “review”. The search resulted five systematic review articles, of which two were only single country reviews. Among the five systematic reviews, two of them performed a meta-analysis of temperature and dengue infection. However, the association between temperatures and the risk of dengue infection was not explicitly differentiated across climate zones. In addition, the effect of heatwaves on dengue incidence was not yet addressed. Despite the inclusion of heterogeneous studies, the strength of evidence wasn't assessed and measured in previous reviews. To address these gaps, this systematic review and meta-analysis was conducted to assess the impact of high temperatures and heatwaves on dengue incidence across different climate zones, and to update the previous reviews regarding the quality and strength of included evidence.
Added value of this study
Our study has provided updated evidence and advanced the results of previous reviews by including heatwaves and assessing the quality and strength of available evidence. We estimated the effect of high temperature across different Köppen-Geiger climate zones. Our results showed that high temperatures increased the risk of dengue infection, albeit with varying risks across climate zones and different levels of national income.
Implications of all the available evidence
The current systematic review and meta-analysis provided sufficient epidemiological evidence that showed the risk of dengue infection increased with higher temperature. Further studies are required to ascertain the observed relationship between dengue incidence and heatwave events. These findings should provide much needed evidence for public health policy makers to establish localized adequate dengue early warning systems and develop preventive measures based on socio-economic conditions and climatic features.
Introduction
Dengue virus (DENV) infection is an acute systemic disease transmitted primarily by Aedes aegypti and the less effective Aedes albopictus mosquitoes.1 The World Health Organization (WHO) identified dengue fever as the fastest spreading mosquito-borne viral disease in the past 50 years, with global incidence increasing 30-fold since the 1960s.2, 3, 4 It is estimated there are 390 million dengue fever cases annually5 in 128 countries,2,4 with five to six billion people worldwide at risk by 2050.5,6 Most dengue cases are in Asia–Pacific regions (accounting for about 70% of total cases),7,8 followed by Africa (16%),1,3 North and South America (14%),5 and Oceania region (0.2%).5,8
Global warming is one of many significant risk factors of increased disease transmission.9, 10, 11 Climatic factors, notably temperature, have been identified as one of the determinants of dengue transmission.12, 13, 14, 15, 16, 17 Temperatures above regional threshold levels are conducive to the life cycle of dengue vectors, Aedes aegypti and Aedes albopictus mosquitoes. Temperature also increases the proliferation of the dengue virus, shortens the extrinsic incubation period (EIP) and increases mosquito biting frequency.18, 19, 20
Since the early 2000s, various studies have quantified the associations between temperature and dengue infection.21, 22, 23, 24, 25, 26, 27 Only two studies performed comprehensive systematic reviews and meta-analyses that quantified the association between high temperatures and dengue.28,29 A recent meta-analysis of 19 studies published until September 201929 estimated a 13% increase in dengue incidence per 1 °C temperature rise above threshold, which was higher compared to estimate from the previous meta-analysis of studies published until March 2014 indicating 7% increase in dengue incidence per 1 °C temperature rise above threshold.28
Although the previous studies estimated the overall effect, the association between high temperatures and the risk of dengue infection was not differentiated across climate zones. Moreover, the impact of heatwaves on dengue incidence was not yet addressed. To address these gaps, this systematic review and meta-analysis was conducted to assess the impact of high temperatures and heatwaves on dengue incidence across different climate zones, and to update the previous reviews regarding the quality and strength of included evidence. The review was formulated following the Population-Exposure-Comparisons-Outcome (PECO) framework30 to answer the question “In the general population, what is the change in risk of dengue fever infection per 1 unit change in exposure to ambient temperature and heatwaves, observed in human epidemiological studies?”
Methods
Overview
This systematic review and meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.31 A detailed description of the PRISMA guideline for this review is presented in the supplementary material (Table S1). The review protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42020211053). The review was formulated following the Population-Exposure-Comparisons-Outcome (PECO) framework30 to answer the question “In the general population (P), what is the increase in risk of dengue fever infection (O) per 1 unit change (C) in exposure to ambient temperature and heatwaves (E), observed in human epidemiological studies?”
Search strategy and selection criteria
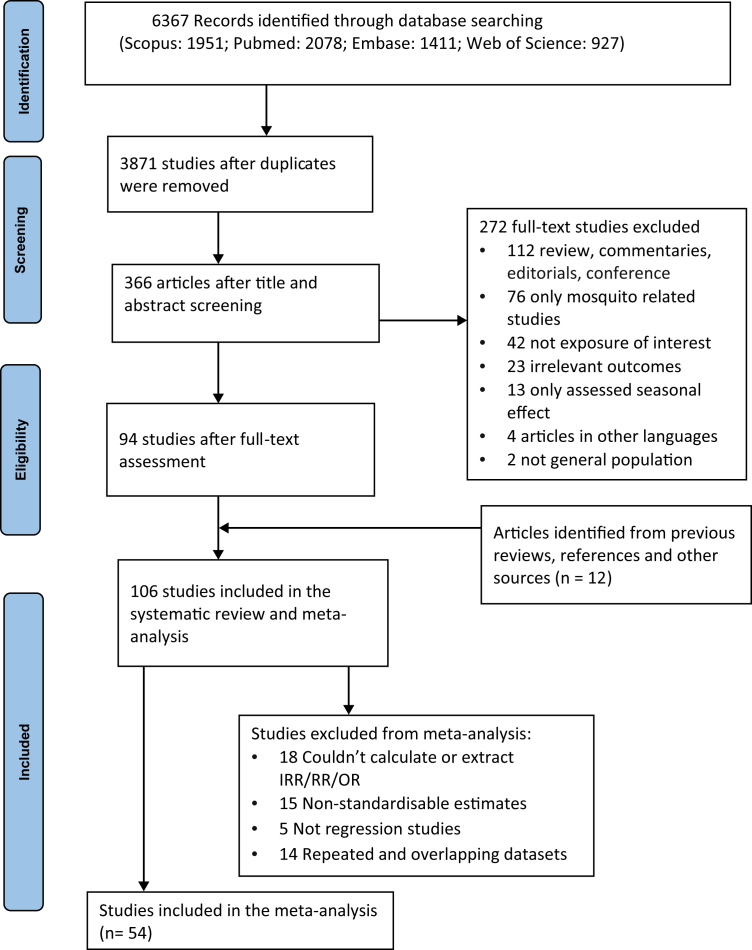
A comprehensive literature search strategy was developed (Appendix pp 3) to search in PubMed, Embase, Scopus and Web of Science databases from database inception to September 20, 2022. The databases were searched with the following keywords for the health outcome terms: (“dengue fever” or “dengue haemorrhagic fever” or “dengue infection”) and combined with meteorological terms: (“temperature” or “heatwave” or “extreme weather” or “meteorological factor” or “climate” or “climatic factor” or “climate change” or “global warming”). The articles obtained from all databases were imported into EndNote,32 and duplicates were removed. An additional manual search was also performed from previous review articles and all reference lists of included studies (Fig. 1).
Fig. 1.
PRISMA flow diagram for literature search and study selection process of the systematic review and meta-analysis.
The titles, abstracts and full text of the remaining articles were evaluated for inclusion by two of the reviewers (YTD and MT) in a double-blind independent screening using Rayyan, a web-tool developed for initial screening of abstracts and titles).33 Any disagreement between the two reviewers was resolved through discussion.
Studies were included if they met the eligibility criteria: (1) peer-reviewed original research articles, (2) quantitative observational time series, case-crossover or case series human studies in the general population with dengue infection cases or incidence as the outcome of interest and (3) the exposures of interest were high temperatures and heatwaves. The following study types were excluded from this review: (1) studies classified as case reports, clinical trials, non-human studies, conference abstracts, editorials, reviews, books, posters, commentaries; and (2) studies that examined only seasonal effects.
For studies based on the same study population and over the same study period (repeated datasets), we included more than one study if: (a) the study reported different location-specific estimates within a state or in different climate zones; (b) the study used different exposure time resolution of exposure (temporal resolution of exposure/outcome data) and outcome (daily, weekly or monthly); (c) the study used different temperature metrics (minimum, mean, maximum); or (d) the study used different lag period structures (single or cumulative lag) and different models. Otherwise, we included studies of longer duration periods and the most recent study.
Data extraction
Data extraction was performed using a customized Excel form prepared based on Cochrane Effective Practice and Organization of Care (EPOC) data collection resource guideline.34,35 Two of the Authors (YTD and MT) independently retrieved, extracted and cross-checked the following data: the last name of the first author, year of publication, study location (country, city, province, region, or state), study period, sample size and sources of outcome data, confounding and effect modifiers, study design and statistical models, temperature metrics, heatwave definitions, exposure time resolution, key findings, and effect estimates with corresponding 95% confidence interval (CI). Moreover, for studies with location-specific estimates, climate zones were extracted using Köppen–Geiger climate classification map36 and climate data website.37 For studies with multiple lags, exposure metrics, and climate zones, the effect estimates, and all other characteristics were recorded separately.
For meta-analysis effect estimates, including Incidence Rate Ratios (IRR) and Relative Risks (RRs) were extracted from the published literature. Regression coefficients (β), Odds Ratios (OR), and percent change (PC) were converted to RRs using standard methods.38,39 For studies that presented effect estimates only graphically, were extracted using the WebPlotDigitizer tool.40 For studies that reported only the effect size and p-value, a 95% CI was calculated according to the method of Altman and Bland.41
Data analysis
The study findings were synthesized quantitatively and narratively. Quantitative synthesis in the form of meta-analysis and forest plots was conducted for studies that fulfilled the eligibility criteria for inclusion in the meta-analysis. Otherwise, the key findings were summarized and synthesized narratively.
A random-effects meta-analysis was conducted to pool the effect estimates from eligible studies. For studies reporting estimates from different models, the final model estimates as specified by the authors were used. If a final model was not specified, the model with covariates (e.g. rainfall, humidity) were chosen.42 For a study that didn't favor effect estimate from a specific temperature metrics, we included all effect estimates using different temperature metrics (minimum, mean and maximum) separately. For multi-location studies, estimates from all locations were used to estimate the main pooled effect estimations. For studies that did not select or favor a specific lag, we included all RRs from either a single or a cumulative lag. For non-linear model estimates showing an effect at both ends of the temperature range, we chose the RRs for temperature effects above references temperature (temperature thresholds with no or minimum risk of dengue, or no heatwaves as defined by authors of the studies). Finally, all effect estimates were standardized for a 1 °C increase in temperature assuming a log–linear association of temperature with the incidence of dengue infections beyond reference temperature to allow pooling effect estimates.34,43 For studies that reported an increase in effect estimates per X degree Celsius rise in temperature (where X is different from 1), effect estimates were divided by X and standardized for a 1 °C increase in temperature.38 Random effects meta-analysis using the DerSimonian and Laird procedure44 was applied to pool the overall effect estimates from included studies.
The between-study heterogeneity was assessed using Higgins I2 statstics45 which is expressed as the proportion of observed variability not caused by sampling error. For Higgins I2 statistics, the heterogeneity was categorized as low (I2 ≤ 25%), moderate (25% < I2 < 75%), or high (I2 ≥ 75%). Moreover, Prediction intervals (PI) of 80% were adopted to quantify the range in which the effect estimate of a new observation from future studies would fall, based on the existing evidence.46,47 Severe heterogeneity was suspected when the 80% PI included the null effect and was more than twice the width of the 95% CI.48
After identifying the degree of heterogeneity, a subgroup analysis was performed to identify the possible source of heterogeneity when the total number of effect estimates were greater than or equal to two, i.e. k ≥2. This was based on temperature metrics (minimum, maximum, mean), national income levels,49 exposure time resolution (i.e. dengue case daily, weekly, or monthly), and Koppen Geiger climate zones.36,37 using location/city-specific effect estimates.
Sensitivity analyses were conducted to assess the robustness of the primary estimates by examining the extent to which the results are affected by changes in methods, models, values of unmeasured variables, the influence of individual studies, or assumptions through applying alternative analysis.
We carried out sensitivity analyses in the following steps. First, a leave-one-out analysis approach was performed by excluding one study at a time and examining the change in the pooled estimates. Second, a random effects analysis was performed after swapping multiple effect estimates stratified by temperature metrics and lag period in a single study, with a single pooled fixed effect estimate. Third, additional sensitivity analyses were conducted by analyzing different factors, including primary confounders and covariates adjustment, overall score in the risk of bias, modelling approach (linear vs. nonlinear), lag structure (single vs. cumulative), and lag periods. Lastly, we also performed analyses using studies with similar exposure time resolution and lag period only. Most of the studies used monthly temporal resolution and presented effect estimates with a lag period of up to three months. Therefore, we applied a sensitivity analysis using a restricted lag period of up to three months for a single lag structure and 0–3 months for a cumulative lag effect.43
Publication bias was visually evaluated using funnel plots and Egger regression tests of asymmetry.50 The ‘trim-and-fill’ method was applied to adjust the overall estimates after accounting for the number of studies that are missing owing to publication bias.51 Statistical analyses and figures were performed using R software version 4.0.2 (Team R, 2021) with the packages meta52 metafor,53 dmetar54 and forester.55 A p-value of <0.05 was considered statistically significant unless otherwise reported.
Quality and strength of evidence
The body of evidence was assessed following the Navigation Guide systematic review methodology framework.56,57
Risk of bias assessment was conducted using a modified version34,58 of the Office of Health Assessment and Translation (OHAT) Risk of Bias Rating Tool for Human and Animal Studies.59 Each study was evaluated against six domains of the risk of bias: selection, confounding, exposure assessment, outcome assessment, selective reporting, and appropriateness of statistical methods.58 Each domain is rated as “definitely low,” “probably low,” “probably high,” and “definitely high” (Appendix pp 4–7). A cautious approach was adopted to rate studies with insufficient information as “probably high” risk of bias. Two of the reviewers (YTD and MT) performed the risk of bias assessment. Three key domains (the assessment of exposure, the assessment of outcomes, and the assessment of confounding) were used (Table S4) to determine the overall rating of an individual study's bias.60 Although the Navigation Guide Methodology suggested to exclude studies with “probably high,” and “definitely high” risk of bias from the meta-analysis.56,57 In all included studies were rated “probably low,” and “definitely low”.
Following the Grading of Recommendations, Assessment, Development, and Evaluation methods (GRADE), the quality of the evidence was initially rated as “moderate”.61 Later, the overall quality was rated as “high”, “moderate” or “low” after considering upgrading or downgrading quality factors, including Risk of Bias across studies, indirectness of evidence, imprecision, inconsistency, publication bias, size of the effect, dose–response gradient, and whether confounding would minimize effects (Appendix pp 7). Using GRADE as a qualitative guide, the possible ratings were 0 (no change from the initial “moderate” quality), −1 (one level downgrade), −2 (two-level downgrade); +1 (one level upgrade), or +2 (two-level upgrade).56,57
The strength of evidence was assessed by combining the quality of the body of evidence with “direction of effect” (i.e. plausible consistency in findings across studies on whether dengue incidence gradient was positively associated with high temperature or heatwave exposure); “confidence in the effects” (the likelihood that a new study would change our conclusion); and any other characteristics of the data that may have an impact on certainty as described in the Navigation Guide.56,57 After full consideration of these factors, the overall strength or certainty of the evidence was categorized as “sufficient evidence,” “limited evidence,” or “inadequate evidence,” as outlined in the Navigation Guide guideline (Appendix pp 8). All body of evidence evaluation was carried out by two of the authors (YTD and MT).
Role of funders
The funder of the study had no role in study design, data collection, data analyses, data interpretation, or writing of the report.
Results
The study selection process yielded 6367 studies. After removing duplicates, title and abstract screening, a total of 378 studies underwent full text assessment. Finally, 106 studies published between 2005 and 2022 meeting our inclusion criteria were included, which covered over four million dengue cases from 32 countries (Fig. 1). Of the total, 101 studies investigated the association between high temperature and dengue infection, one study62 used both high temperature and heatwave as exposure, and the remaining four studies14,63, 64, 65 used heatwaves. Summarized key findings extracted from the included studies are presented in the supplementary material (Appendix pp 9–36).
Study context and characteristics
Most studies (n = 103) used surveillance data collected from 1974 to 2019. There are 98 studies published since 2010, particularly, 32 studies published since 2019. Two studies used hospital admissions data17,66 and one study67 used data obtained from published articles and grey literature. Temperature data were collected from weather stations monitored by national meteorological agencies in 87 studies.
For high temperature studies, the ambient temperatures had been used with different temporal exposure periods, including daily,14,23,63,64,68 bi-weekly,69 monthly and yearly.70 Mean temperature was frequently used as temperature metrics (n = 31).
Heatwave studies used two definitions including duration and intensity of high temperature14 and number of days exceeding certain percentile of daily and weekly temperature distribution.62, 63, 64, 65
A large number of the studies (n = 45) were from upper-middle-income countries, with only three coming from low-income countries.66 The study period ranged from one year71 to 37 years.72 The number of dengue cases included in each study ranged from 37673 to 469,171 cases.74 Fourteen studies were nationwide.17,25,62,72,75, 76, 77, 78, 79, 80, 81, 82, 83 The remaining studies were subdivided into various administrative divisions such as district, province, or state level.
Four climate zones (tropical savanna climate, humid subtropical climate, tropical rainforest climate and tropical monsoon climate) dominated the study locations, together comprising 77.4% of studies. The greatest proportion of studies (25.7%) was from tropical savanna climate.
Except for one study68 which applied a case-crossover design, the remaining 105 studies used a time-series study design. Regarding modelling approaches, the majority (n = 53) used generalized linear models and 11 studies applied distributed lag nonlinear models. The remaining studies used different modelling approaches such as generalised additive models, distributed lag model and autoregressive moving average models (Appendix pp 9–36).
Most studies (n = 75) adjusted for primary confounders (season and time trend). The other eleven studies adjusted for either one of these confounders. Rainfall (n = 96) and relative humidity (n = 76) were the most included covariates in our reviewed studies.
The reviewed studies reported effect estimates in terms of IRR, RR, OR, percent change, regression coefficients and correlation coefficients. A total of 54 articles (49 articles with 92 estimates for high temperatures and the other five articles with 13 estimates for heatwaves) met the inclusion criteria for the meta-analysis. The majority of the studies provided city-level estimates, whereas four studies provided country-level estimates.17,75,78,82
For studies that quantified the association between high temperature and dengue infection, the selected reference temperatures to estimate the increase in risk of dengue, varied across climate zones and on the utilisation of temperature metrics. Minimum temperature ranges were between 9.8 °C and 24.5 °C in the Tropical savanna climate zone; and between 20 °C and 25 °C, 22.8 °C–24 °C, 14.3–24.9 °C in the Humid subtropical climate, Tropical rainforest climate, and Tropical monsoon climate, respectively.
Different lagged effects were also employed in the studies. For studies that used monthly exposure time resolution, a lag period ranging from one month to 12 months was applied. For studies with weekly resolution, one to 52 weeks of lag period were used. For studies using daily resolution, one to 51 lag days were utilized.
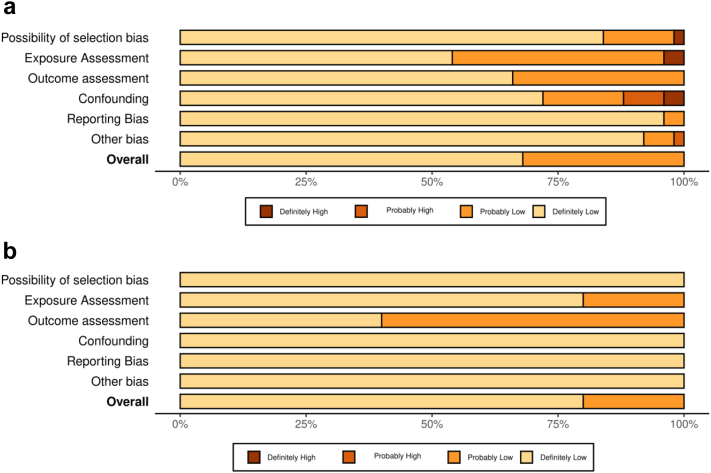
The overall risk of bias assessment was definitely low or probably low. For high-temperature studies, except four studies that were rated either “definitely high”67,84 or “probably high”,85,86 the remaining studies were rated “definitely low” or “probably low” in the overall risk of bias assessment. For heatwave studies, except for one study63 that rated “probably low”, the remaining heatwave studies were rated “definitely low” in the risk of bias assessment. Fig. 2 summary plot shows the percentage of the risk of bias judgement within each domain. The details of rating in each domain for individual studies were presented in the supplementary material (Appendix pp 37, 48–49).
Fig. 2.
Percentage distribution of Risk of Bias ratings within each bias domain for all included studies. (a) High temperatures studies (n = 102), (b) heatwaves studies (n = 5). The plots were created using the Risk of Bias Visualization Tool (ROBVIS).87
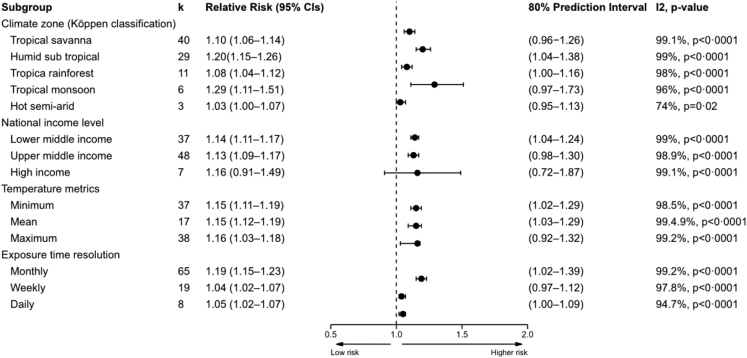
Random effects meta-analysis showed that the risk of dengue infection increased by 13% (95% CI: 1.11–1.16) for every 1 °C rise in high temperature (Appendix pp 4). The overall pooled estimate from high temperature resulted in a large heterogeneity (I2 = 98.0%) suggesting a subgroup analysis was needed when the total number of effect estimates were sufficient, i.e. k ≥2. Subgroup analysis was performed based on temperature metrics, climate zones, national income levels, and exposure time resolution (Fig. 3, Appendix pp 51–54). Subgroup analyses showed positive associations between higher temperatures and dengue incidence across all subgroups (Fig. 3).
Fig. 3.
Random-effects meta-analysis findings for high temperature studies by subgroup showing change in RR and 95% CIs per 1°C increase in temperature. k = the number of effects estimates; 95% CIs = 95% Confidence interval.
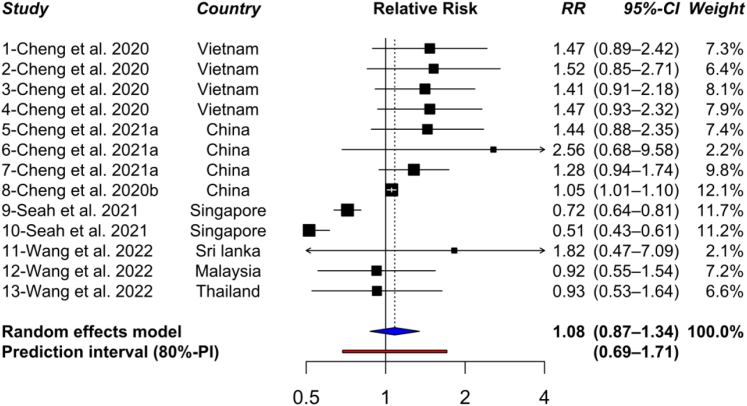
Heatwaves were also associated with an (non-significant) increased incidence of dengue (RR = 1.08; 95% CI: 0.87–1.34) (Fig. 4). A subgroup analysis by exposure time resolution did not show subgroup differences. In studies that used the weekly (n = 3; k = 9) and daily (n = 2; k = 4) resolutions, the overall pooled estimate was RR = 1.02 (95% CI: 0.76–1.37), and RR = 1.17 (95% CI: 0.96–1.42), respectively.
Fig. 4.
Forest plot with 80% prediction interval shows the effect of heatwave on dengue infection. RR = Relative Risk; 95% CI = 95% Confidence interval; 80%-PI = 80% confidence interval.
A qualitative synthesis was performed for the rest of the studies that did not meet the inclusion criteria for the meta-analysis (Appendix pp 9–36). Among studies that investigated the association between high temperature and dengue incidence, 51 studies were included in the narrative synthesis. Except for a few studies (n = 13) that found insignificant associations27,70,84,85,88, 89, 90 and negative associations67,76,86,91, 92, 93, 94 the rest of 90 studies reported a significant positive association between high temperature and dengue incidence. The observed associations were reported at different lag periods ranging from weeks to months. Most of the studies reported a positive association with 0–3 months lag time.
The summary of the overall quality of the evidence is presented in Table 1. Following the Navigation Guide guidelines for observational studies,56 the quality of the evidence was initially rated “moderate” and downgraded or upgraded considering the quality factors mentioned in Table 1. For both high temperatures and heatwave studies, the risk of bias was rated as “definitely low” and “probably low”. For high temperature studies, the quality of evidence was upgraded for the dose–response domain and there was no downgrade for any factor based on the results of the sensitivity analyses (Appendix pp 37). Meanwhile, for heatwave studies, the quality of the evidence was downgraded for “inconsistency” and “dose response pattern” but there was no upgrade for any factor. After full consideration of the above quality factors, the quality of evidence was upgraded from the initial “Moderate” rating to “High” in high-temperature studies but was downgraded to “Low” in heatwave studies.
Table 1.
Summary of the body of evidence for high temperatures and heatwaves as risk factors of dengue infection.
| High temperature studies (n = 102) |
Heatwave studies (n = 5) |
|||
|---|---|---|---|---|
| Quality of evidence assessment | ||||
| Down grading factors | ||||
| Rating | Rationale and summary of evidence | Rating | Rationale and summary of evidence | |
| Risk of bias | 0 | Only four studies were rated as “definitely high” or “probably high”. The remaining studies (98/103) were rated as “definitely low” and “probably low” risk of bias. | 0 | All five studies were rated as “definitely low” and “probably low” risk of bias. Thus, we judged there is no substantial risk of bias |
| Indirectness | 0 | Dengue cases included in most studies were either laboratory confirmed or clinically diagnosed according to WHO definition of dengue fever. Moreover, the majority of the studies employed direct measurements of exposure. | 0 | Dengue cases included in most studies were either laboratory confirmed or clinically diagnosed according to WHO definition of dengue fever. Moreover, the majority of the studies employed direct measurements of exposure. |
| Inconsistency | 0 | High heterogeneity was observed in the overall pooled estimate (I2 = 99%). However, 80% PI did not contain unity (Appendix pp 50–49). Moreover, sensitivity analysis based on the “leave-one-out” approach did not show considerable difference from overall pooled effect estimates (RR: 1.12–1.15), and there was no change in statistical significance. | −1 | The heterogeneity was high (I2 = 89%) and 95% prediction intervals (PIs) contain unity and were wider compared to confidence intervals (Fig. 4). Leave-one-out analysis also showed variation (RR: 1.06–1.16). Thus, the evidence of quality was downgraded for such inconsistencies. |
| Imprecision | 0 | Except four studies, the 95% CIs of the studies possessed notably narrow confidence intervals. Moreover, we judge most studies included representative of appropriate proportion of population of interest across different years of time. | 0 | We judge all five studies included representative of appropriate proportion of population of interest across different years of time. Only two effect (2/13) estimates possessed wider confidence intervals. |
| Publication bias | 0 | Despite the observed asymmetrical distribution of studies in the funnel plot and Egger's tests (Appendix pp 56), the pooled effect estimate adjusted after including imputed studies using the Trim and Fill method (Appendix pp 59) did not show difference in statistical significance. | 0 | Egger's test revealed statistically insignificant result that did not show publication bias (p = 0.156). However, the small number of studies (n <10), makes it uncertain and needs careful interpretation. |
| Upgrading factors | ||||
| Large magnitude of effect | 0 | Effect sizes were small in most studies, and the pooled effect estimate was below 2. | 0 | Effect sizes were small in most studies, and the pooled effect estimate was below 2. |
| Dose response pattern | +1 | The quality of the evidence was upgraded as majority of the studies indicated a statistically significant increase in the risk of dengue infection with an increase in temperatures above reference values. | −1 | Two of the five studies showed a decrease in effect estimate and the random effect contains unity and were statistically insignificant. |
| Confounding minimizes effect | 0 | One fourth (26/102) of the studies did not adjust for primary confounders. However, the sensitivity analysis did not show evidence to suggest that possible residual confounders would shift the effect to null. | 0 | Only one of the studies did not adjust for primary confounders. However, such did not suggest the possible residual confounders would shift the effect to null. |
| Summary of the quality assessment | ||||
| Quality of evidence | High | Moderate + (0) + (0) + (0) + (1) = 1. After one upgrade and no down grade in quality factors, the overall quality of evidence was rated as “High”. | Low | Moderate + (0) + (0) + (-1) + (−1) = −2. After down grading two quality factors, the overall quality of evidence was rated as “low” |
| Summary findings | n/a | Overall, the studies included in the meta-analysis and narrative synthesis resulted in “High” quality of the evidence. | n/a | Overall, the studies included in the meta-analysis resulted in “Low” quality of the evidence. |
| Strength of evidence assessment | ||||
| Quality of evidence | n/a | High | n/a | Low |
| Direction of effect estimates | n/a | The direction of effect estimates largely showed an increasing trend in dengue infection with increasing high temperatures beyond the reference values. | n/a | The direction of effect estimates largely showed an increasing trend in dengue infection associated with heatwave, but the inclusion of unity in the confidence interval makes the result uncertain. |
| Confidence in effect estimate | n/a | Majority of the studies showed a positive association between dengue infection and increased temperature. Moreover, the prediction interval did not include the null effect and supported the positive association between dengue incidence and temperature in the future studies. The number of studies included in the meta-analysis was also sufficient enough and provide certainty about the future. | n/a | Although an increasing trend was observed in the overall random effect the confidence and prediction intervals contain the null effect. Moreover, the number of studies included in the meta-analysis was small and made the result uncertainty about the future. |
| Other characteristics of the data that may influence certainty | n/a | Sensitivity analysis based on several factors did not show much variation from the main overall effect estimate and strengthen the certainty of the evidence. | n/a | The leave-one-out sensitivity analysis showed variation from the main overall effect estimates (Fig. S9). The small number of studies included hindered performing certain sensitivity analyses in addition to leave-one-out analysis and increase the uncertainty. |
| Overall strength of evidence | Sufficient | The quality of evidence was rated as “High”, the prediction interval suggested future studies may not affect the result and several sensitivity analyses showed similar overall estimates. Thus, we concluded that the evidence is sufficient enough to show the effect of high temperature on dengue infection. | Limited | Although a positive association is observed, the “Low” quality of evidence, inclusion of unity in confidence interval and the prediction interval, and the small number of studies included in the meta-analysis resulted a “Limited” evidence to conclude the association between dengue infection and heatwave. |
The strength of evidence was assessed based on overall quality of evidence, direction of the effect estimates, confidence in the effect estimate and other compelling characteristics (Table 1). In the case of high-temperature studies, the overall quality of evidence was “High”. Most studies showed a positive association suggesting an increasing trend in dengue infection with increasing high temperatures (Appendix pp 55), and confirmed by the results of the sensitivity analyses (Appendix pp 37). Based on these criteria, the strength of evidence for high temperature studies was graded as “Sufficient”.
For heatwave studies, the quality of evidence was rated as “Low”. Although the random effect estimate showed increasing risk of dengue infections with heatwaves, the confidence and prediction interval contained the null effect. Moreover, the number of studies included in the meta-analysis was small and future studies may change the direction and size of the effect estimate. Considering all these factors, we judged the strength of evidence for heatwave studies as “Limited”.
Discussion
The present study is an update of the previous two systematic reviews and meta-analyses28,43 that examined the relationship between high temperatures and dengue infection for studies published until 2019. Moreover, the current systematic review also assessed the effect of heatwave events on dengue infection. Overall, a total of 106 studies comprising over four million dengue cases were included with 32 studies published after 2019.
The overall effect estimates in our study showed similar direction, but higher in magnitude compared to the result reported from a previous meta-analysis conducted in 2014. Our result showed the risk of dengue infection increased by 13% for each 1 °C increase in high temperatures above the reference values, which is the same estimate as another meta-analysis conducted in 2019.
The impact of temperature on dengue transmission takes complex pathways and mechanisms,11,95 including impacts of temperature on the female mosquito's reproductive cycle, feeding activities and EIP.96, 97, 98 A study examining the effect of temperature on the EIP showed a reduction in the number of days from an average of 15 days to 6.5 days as temperature increased from 25 °C to 30 °C.99 The reference temperature values used to estimate effect estimates in the included studies matched with the temperature ranges associated with the biology of Aedes mosquito and dengue viruses, which could explain the observed temperature-related risk relationship in the current review.
Viral replication peaks at around 35 °C, which is higher than the temperature at which adult mosquito survival rates and feeding activities start to decline95 resulting in reduced dengue transmission potential. This may explain a higher relative risk associated with the minimum and mean temperatures than with the maximum temperature. Maximum temperature showed a lower relative risk of dengue incidence with a 10% increase per 1 °C compared to minimum and mean temperature, which were both associated with a 15% increase. Moreover, the observed higher relative risk related to a monthly time resolution could be related to life span of the mosquito, EIP, survivorship and feeding frequency and onset of clinical symptoms of dengue.100, 101, 102 For effective transmission, the vector needs to live long enough after initial blood meal to allow the virus replication in the vector to reach an infectious level (which on average takes 8–12 days) and then transmit the virus to a host by ingesting a blood meal. The observed time-lagged association also aligned with recent studies forecasting and providing temperature based early warning system for dengue outbreaks.103, 104, 105, 106
The subgroup analysis for high-temperature studies also revealed that higher relative risks were identified in tropical climate zones (tropical monsoon: RR = 1.29, 95% CI: 1.11–1.51; humid subtropical climate: RR = 1.20, 95% CI: 1.15–1.26). which could be due to high relative humidity that is conducive to mosquito breeding.11 A combination of high temperature and rainfall in tropical climate zones raises relative humidity, which in turn increases the feeding activity, survival, and egg development of Ae. aegypti.11,18,107,108 Moreover, the widespread presence of the vector Ae. aegypti in tropical and subtropical climate zones could contribute to the observed increased risk of dengue in these regions. Considering 40% of countries lies in the tropical climate zone, between 23.5° N and 23.5° S latitude, and inhabited by high number of population (40% of the world's population), human behaviour factors and population density could be another reason for the observed higher risk in the region.109, 110, 111 However, recent studies have shown that climate change with increasing temperatures may facilitate vector, particularly Ae. albopictus, spread to broader areas including higher altitudes area and temperate climate regions.107,108
Unlike high temperatures, the association of dengue incidence with heatwave events has not been extensively studied, as evidenced by the few studies that were included. A contrasting effect was reported among the included studies, reporting both negative and positive associations. The differences could be related to a wide variation in heatwave definition across countries, regions and climate zones among included studies. For example, a study in Singapore62 with a tropical rainforest climate zone defined the heatwave as three or more days exceeding the 90th percentile (33.2 °C). Meanwhile, a study in Vietnam63 with Humid subtropical climate zone defined a heatwave as mean temperature above the 95th percentile (24.3 °C) for more than 7 days.63 In this study, the temperature range (percentile) used to define heatwave was close to the optimal temperature for mosquito growth. These differences could cause the contrary associations between dengue and heatwaves and we were unable to rule out heatwave as a risk factor for dengue. Thus, more studies with consistent heatwave definition are needed to confirm the impact of heatwaves on dengue infections.
Significant heterogeneity was also found in high-temperature studies, even after subgroup analyses which is similar to the previous dengue reviews.28,43 The causes of the considerable heterogeneity are not clear but there are three plausible possibilities. First, underreporting of dengue cases could be an important factor. There may also be a mismatch between exposure and outcome measurement. Dengue cases may not be reported at the time of onset of infection, which would result in a temporal mismatch of exposure with the case. Second, primary confounders and covariates adjustment (particularly rainfall and relative humidity) could be another important source of heterogeneity. However, the sensitivity analysis showed a slight difference in the RRs for studies that did not control for primary confounders. Third, other factors such as exposure measurements, reference temperatures used, and unaccounted environmental, social and demographic factors may also contribute to the observed heterogeneity. In addition, other causes of heterogeneity may not be discernible from reviewed studies, which resulted in high heterogeneity in this review.
In this review, we found high-quality evidence on the associations between high temperature and dengue infection, the overall pooled estimate also showed an increase in dengue infection with higher temperatures, and sensitivity analyses confirmed the robustness of the result. Thus, the strength of evidence is sufficient to show the effect of high temperature on increased dengue infection. Meanwhile, for heatwave studies, the quality of evidence was “low”, and the strength of evidence was “limited” for the association between heatwave and dengue infection. The observed quality and strength reflect the uncertainty associated with the reasons described in the strength of evidence evaluation. However, the inclusion of studies with low and probably low risk of bias that adjusted for relevant confounding factors makes the observed estimate worth considering in the development of preventive measures to reduce the risk of dengue outbreaks in the context of climate change.
Although this review is not the first to assess the impact of temperature on dengue transmission, the extensive search approach, methodology used, and higher number of studies provided updated knowledge. We retrieved recent studies and performed a comprehensive systematic assessment of the quality and strength of existing evidence following the Navigation guide for systematic review and GRADE.61 guidelines, which were not used in previous reivew. We also used validated Risk of Bias assessment tools for our review. Further, unlike previous studies which chose to include only specific lag periods, temperature metrics and largest effect estimates, we included all effect estimates stratified based on factors, such as climatic zones and socioeconomic status. Considering the variations in weather patterns across regions, we have performed a subgroup analysis and showed the effect estimate in different climate zones. Despite high heterogeneity, the robustness of the overall pooled estimate was verified by sensitivity analyses. Moreover, heatwave was also included in this meta-analysis, that was not done in the previous reviews.
This study also has several limitations. First, only peer-reviewed journal articles published in English were included in this review. Second, there is currently a lack of relevant studies from regions such as Africa which accounts for 16% of the global dengue burden. These limitations may contribute to a selection bias and thus a cautious approach to generalizing the findings to unexplored regions is required. Third, we also found a significant publication bias and heterogeneity even after subgroup analysis. The observed substantial heterogeneity could be related to unaccounted socio-demographic and environmental factors that we were unable to investigate due to lack of such data in the included studies. Lastly, only five heatwave studies were included, which limited the strength of the evidence on association between heatwave and dengue infection.
Most of the reviewed studies were from the Asia–Pacific region. Despite varying reports on the incidence of dengue in the tropics, particularly in Africa and South America where we only found six studies that investigated the impact of high temperature and dengue association, more studies are needed to explore effects of high temperatures on dengue outside the Asia–Pacific region. Given the relatively small number of studies that have examined the effects of heatwaves, future epidemiological studies are warranted to investigate the association between dengue infection and heatwave events. Because of the role of socio-economic conditions and meteorological factors in vector-borne disease transmission, future studies may also need to account for socio-demographic, micro-climate, mosquito behaviour and other environmental factors that can explain vulnerability at the local and regional levels. Finally, global warming and increasing temperature posed tremendous challenges to dengue control and prevention in the context of climate change. This is particularly important in urban settings where city heat island effects and high density of population could make dengue more transmissible. There is a gap in knowledge on how climate change affect dengue transmission under 2 °C increase or even up to 4 °C increase scenarios across different regions.
The current systematic review and meta-analysis provided sufficient epidemiological evidence that showed the risk of dengue infection increased with higher temperature. We also believe further studies are required to ascertain the observed relationship between dengue incidence and heatwave events. These findings should provide much needed evidence for public health policy makers to establish localized adequate dengue early warning systems and develop preventive measures based on socio-economic conditions and climatic features.
Contributors
YTD was involved in the literature search, screening the studies, assessing the risk of bias, study design, data collection, data analysis, data interpretation, figure creation, and writing of the manuscript. MT was involved in the literature search, screening the studies, assessing the risk of bias, data collection, data interpretation, reviewing and revising the manuscript. BMV and OA oversaw the meta-analysis design, execution, statistical analysis, data interpretation, and was involved in reviewing and revising the manuscript. PB was involved in all stages of the project, including conception, design, data analysis, data presentation, interpretation, reviewing, and revising the manuscript. AH, KD, YZ, GM, TD, TC were involved in conception, design of the project, reviewing and revising the manuscript. All authors made substantial contributions to each part of the content. YTD and MT have accessed and approved the underlying data. All authors read and approved the final version of the manuscript and approved submission for publication. All authors confirm that they had full access to all the data in the study and accept responsibility for submission for publication.
Data sharing statement
This review used publicly available data; therefore, no original data are available for sharing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
YTD is a PhD candidate supported by the University of Adelaide under the Adelaide Scholarship International scholarship scheme and this project has been funded by the Australian Research Council Discovery Program (DP200102571). The sponsors have no role in this research.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104582.
Contributor Information
Yohannes Tefera Damtew, Email: yohannestefera.damtew@adelaide.edu.au.
Michael Tong, Email: Michael.Tong@anu.edu.au.
Blesson Mathew Varghese, Email: blesson.varghese@adelaide.edu.au.
Olga Anikeeva, Email: olga.anikeeva@adelaide.edu.au.
Alana Hansen, Email: alana.hansen@adelaide.edu.au.
Keith Dear, Email: keith.dear@adelaide.edu.au.
Ying Zhang, Email: ying.zhang@sydney.edu.au.
Geoffrey Morgan, Email: geoffrey.morgan@sydney.edu.au.
Tim Driscoll, Email: tim.driscoll@sydney.edu.au.
Tony Capon, Email: tony.capon@monash.edu.
Peng Bi, Email: peng.bi@adelaide.edu.au.
Appendix A. Supplementary data
References
- 1.Guzman M.G., Halstead S.B., Artsob H., et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messina J.P., Brady O.J., Scott T.W., et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 2014;22:138–146. doi: 10.1016/j.tim.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray N.E., Quam M.B., Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady O.J., Gething P.W., Bhatt S., et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6 doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S., Gething P.W., Brady O.J., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathers C.D., Lopez A.D., Murray C.J.L. In: Global burden of disease and risk factors. Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J.L., editors. The International Bank for Reconstruction and Development/The World Bank Oxford University Press Copyright ©; Washington (DC) New York: 2006. The burden of disease and mortality by condition: data, methods, and results for 2001. [PubMed] [Google Scholar]
- 7.Rigau-Pérez J.G., Clark G.G., Gubler D.J., Reiter P., Sanders E.J., Vorndam A.V. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 8.Kline K., McCarthy J.S., Pearson M., Loukas A., Hotez P.J. Neglected tropical diseases of Oceania: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebi K.L., Nealon J. Dengue in a changing climate. Environ Res. 2016;151:115–123. doi: 10.1016/j.envres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Campbell L.P., Luther C., Moo-Llanes D., Ramsey J.M., Danis-Lozano R., Peterson A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665) doi: 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morin Cory W., Comrie Andrew C., Ernst K. Climate and dengue transmission: evidence and implications. Environ Health Perspect. 2013;121:1264–1272. doi: 10.1289/ehp.1306556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunkard J.M., Cifuentes E., Rothenberg S.J. Assessing the roles of temperature, precipitation, and ENSO in dengue re-emergence on the Texas-Mexico border region. Salud Pública Méx. 2008;50:227–234. doi: 10.1590/s0036-36342008000300006. [DOI] [PubMed] [Google Scholar]
- 13.Horta M.A., Bruniera R., Ker F., Catita C., Ferreira A.P. Temporal relationship between environmental factors and the occurrence of dengue fever. Int J Environ Health Res. 2014;24:471–481. doi: 10.1080/09603123.2013.865713. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J., Bambrick H., Frentiu F.D., et al. Extreme weather events and dengue outbreaks in Guangzhou, China: a time-series quasi-binomial distributed lag non-linear model. Int J Biometeorol. 2021;65:1033–1042. doi: 10.1007/s00484-021-02085-1. [DOI] [PubMed] [Google Scholar]
- 15.Correia W.L.F. Influence of meteorological variables on dengue incidence in the municipality of Arapiraca, Alagoas, Brazil. Rev Soc Bras Med Trop. 2017;50:309–314. doi: 10.1590/0037-8682-0432-2016. [DOI] [PubMed] [Google Scholar]
- 16.Li C., Wu X., Sheridan S., et al. Interaction of climate and socio-ecological environment drives the dengue outbreak in epidemic region of China. PLoS Neglected Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limper M., Thai K.T.D., Gerstenbluth I., Osterhaus A., Duits A.J., van Gorp E.C.M. Climate factors as important determinants of dengue incidence in Curaçao. Zoonoses Public Health. 2016;63:129–137. doi: 10.1111/zph.12213. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer M.U.G., Sinka M.E., Duda K.A., et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butterworth M.K., Morin C.W., Comrie A.C. An analysis of the potential impact of climate change on dengue transmission in the Southeastern United States. Environ Health Perspect. 2017;125:579–585. doi: 10.1289/EHP218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y., Huang C. Climate change and vector-borne diseases in China: a review of evidence and implications for risk management. Biology. 2022;11(3):370. doi: 10.3390/biology11030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang K., Chen C.D., Shih C.M., et al. Time-lagging interplay effect and excess risk of meteorological/mosquito parameters and petrochemical gas explosion on dengue incidence. Sci Rep. 2016;6:1–10. doi: 10.1038/srep35028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S.C., Liao C.M., Chio C.P., Chou H.H., You S.H., Cheng Y.H. Lagged temperature effect with mosquito transmission potential explains dengue variability in southern Taiwan: insights from a statistical analysis. Sci Total Environ. 2010;408:4069–4075. doi: 10.1016/j.scitotenv.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Cheong Y.L., Burkart K., Leitão P.J., Lakes T. Assessing weather effects on dengue disease in Malaysia. Int J Environ Res Public Health. 2013;10:6319–6334. doi: 10.3390/ijerph10126319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowell G., Sanchez F. Climate-based descriptive models of dengue fever: the 2002 epidemic in Colima, Mexico. J Environ Health. 2006;68(10):40–44. [PubMed] [Google Scholar]
- 25.Chowell G., Torre C.A., Munayco-Escate C., et al. Spatial and temporal dynamics of dengue fever in Peru: 1994–2006. Epidemiol Infect. 2008;136:1667–1677. doi: 10.1017/S0950268808000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharbi M., Quenel P., Gustave J., et al. Time series analysis of dengue incidence in Guadeloupe, French West Indies: forecasting models using climate variables as predictors. BMC Infect Dis. 2011;11:1–13. doi: 10.1186/1471-2334-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goto K., Kumarendran B., Mettananda S., Gunasekara D., Fujii Y., Kaneko S. Analysis of effects of meteorological factors on dengue incidence in Sri Lanka using time series data. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan J., Wei W., Bai Z., et al. A systematic review and meta-analysis of dengue risk with temperature change. Int J Environ Res Public Health. 2014;12:1–15. doi: 10.3390/ijerph120100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Dou Q., Lu Y., Xiang H., Yu X., Liu S. Effects of ambient temperature and precipitation on the risk of dengue fever: a systematic review and updated meta-analysis. Environ Res. 2020;191 doi: 10.1016/j.envres.2020.110043. [DOI] [PubMed] [Google Scholar]
- 30.Morgan R.L., Whaley P., Thayer K.A., Schünemann H.J. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–1031. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The EndNote Team . 3 ed. Clarivate Analytics; Philadelphia, PA: 2013. EndNote. EndNote X9. [Google Scholar]
- 33.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damtew Y.T., Tong M., Varghese B.M., et al. Associations between temperature and Ross river virus infection: a systematic review and meta-analysis of epidemiological evidence. Acta Trop. 2022;231 doi: 10.1016/j.actatropica.2022.106454. [DOI] [PubMed] [Google Scholar]
- 35.Cochrane Effective Practice and Organisation of Care E EPOC resources for review authors. 2020. https://epoc.cochrane.org/resources/epoc-resources-reviewauthors Accessed November 2021.
- 36.Beck H.E., Zimmermann N.E., McVicar T.R., Vergopolan N., Berg A., Wood E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci Data. 2018;5 doi: 10.1038/sdata.2018.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Climate-Data.org . Climate data for cities worldwide. 2020. Climate-Data.org [Google Scholar]
- 38.Moghadamnia M.T., Ardalan A., Mesdaghinia A., Keshtkar A., Naddafi K., Yekaninejad M.S. Ambient temperature and cardiovascular mortality: a systematic review and meta-analysis. PeerJ. 2017;5 doi: 10.7717/peerj.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothman K.J., Greenland S., Lash T.L. 3rd ed. Lippincott Williams & Wilkins; 2008. Modern epidemiology. [Google Scholar]
- 40.Rohatgi A. Web based Tool to Extract Data from Plots, Images, and Maps. 4.2 ed. 2019. WebPlotDigitizer.https://arohatgi.info/WebPlotDigitizer Austin, TX. Available online at: [Google Scholar]
- 41.Altman D.G., Bland J.M. How to obtain the confidence interval from a p value. BMJ. 2011;343 doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo Q., Li S., Guo Y., Han X., Jaakkola J.J.K. A systematic review and meta-analysis of the association between daily mean temperature and mortality in China. Environ Res. 2019;173:281–299. doi: 10.1016/j.envres.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 44.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 45.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 46.Chiolero A., Santschi V., Burnand B., Platt R.W., Paradis G. Meta-analyses: with confidence or prediction intervals? Eur J Epidemiol. 2012;27:823–825. doi: 10.1007/s10654-012-9738-y. [DOI] [PubMed] [Google Scholar]
- 47.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. 2009. Prediction intervals, in introduction to meta-analysis; pp. 127–130. [Google Scholar]
- 48.IntHout J., Ioannidis J.P.A., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The World Bank Data World Bank country classifications by income level. 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 50.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duval S., Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 52.Schwarzer G. Meta: an R package for meta-analysis. RN. 2007;7:40–45. [Google Scholar]
- 53.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36:1–48. [Google Scholar]
- 54.Harrer M., Cuijpers P., Furukawa T.A., Ebert D.D. Chapman and Hall; CRC: 2021. Doing meta-analysis with R: a hands-on guide. [Google Scholar]
- 55.Boyes R. Forester: an R package for create publication-ready forest plots. R package version 0.3.0. 2021. https://github.com/rdboyes/forester
- 56.Johnson P.I., Sutton P., Atchley Dylan S., et al. The navigation guide—evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122:1028–1039. doi: 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson P.I., Koustas E., Vesterinen H.M., et al. Application of the navigation guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ Int. 2016;92–93:716–728. doi: 10.1016/j.envint.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimitrova A., Ingole V., Basagaña X., et al. Association between ambient temperature and heat waves with mortality in South Asia: systematic review and meta-analysis. Environ Int. 2021;146 doi: 10.1016/j.envint.2020.106170. [DOI] [PubMed] [Google Scholar]
- 59.Office of Health Assessment and Translation . National Institute of Environmental Health Sciences; 2019. Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. [Google Scholar]
- 60.Liu J., Varghese B.M., Hansen A., et al. Hot weather as a risk factor for kidney disease outcomes: a systematic review and meta-analysis of epidemiological evidence. Sci Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149806. [DOI] [PubMed] [Google Scholar]
- 61.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol. 2011;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Seah A., Aik J., Ng L.C., Tam C.C. The effects of maximum ambient temperature and heatwaves on dengue infections in the tropical city-state of Singapore–A time series analysis. Sci Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145117. [DOI] [PubMed] [Google Scholar]
- 63.Cheng J., Bambrick H., Yakob L., et al. Heatwaves and dengue outbreaks in Hanoi, Vietnam: new evidence on early warning. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng J., Bambrick H., Yakob L., et al. Extreme weather conditions and dengue outbreak in Guangdong, China: spatial heterogeneity based on climate variability. Environ Res. 2021;196 doi: 10.1016/j.envres.2021.110900. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Wei Y., Li K., et al. Impact of extreme weather on dengue fever infection in four Asian countries: a modelling analysis. Environ Int. 2022;169 doi: 10.1016/j.envint.2022.107518. [DOI] [PubMed] [Google Scholar]
- 66.Cabrera M., Taylor G. Modelling spatio-temporal data of dengue fever using generalized additive mixed models. Spat Spatiotemporal Epidemiol. 2019;28:1–13. doi: 10.1016/j.sste.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Shabbir W., Pilz J., Naeem A. A spatial-temporal study for the spread of dengue depending on climate factors in Pakistan (2006–2017) BMC Public Health. 2020;20:1–10. doi: 10.1186/s12889-020-08846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan J., Lin H., Wang C., et al. Identifying the high-risk areas and associated meteorological factors of dengue transmission in Guangdong Province, China from 2005 to 2011. Epidemiol Infect. 2014;142:634–643. doi: 10.1017/S0950268813001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eastin M.D., Delmelle E., Casas I., Wexler J., Self C. Intra-and interseasonal autoregressive prediction of dengue outbreaks using local weather and regional climate for a tropical environment in Colombia. Am J Trop Med Hyg. 2014;91(3):598. doi: 10.4269/ajtmh.13-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhewantara P.W., Marina R., Puspita T., et al. Spatial and temporal variation of dengue incidence in the island of Bali, Indonesia: an ecological study. Trav Med Infect Dis. 2019;32 doi: 10.1016/j.tmaid.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Faridah L., Fauziah N., Agustian D., et al. Temporal correlation between urban microclimate, vector mosquito abundance, and dengue cases. J Med Entomol. 2022;59:1008–1018. doi: 10.1093/jme/tjac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Struchiner C.J., Rockloev J., Wilder-Smith A., Massad E. Increasing dengue incidence in Singapore over the past 40 years: population growth, climate and mobility. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li T.G., Yang Z.C., Lei L.U.O., Biao D.I., Ming W. Dengue fever epidemiological status and relationship with meteorological variables in Guangzhou, Southern China, 2007–2012. Biomed Environ Sci. 2013;26:994–997. doi: 10.3967/bes2013.036. [DOI] [PubMed] [Google Scholar]
- 74.Campos N.B.D., Morais M.H.F., Ceolin A.P.R., et al. Twenty-two years of dengue fever (1996-2017): an epidemiological study in a Brazilian city. Int J Environ Health Res. 2021;31:315–324. doi: 10.1080/09603123.2019.1656801. [DOI] [PubMed] [Google Scholar]
- 75.Faruk M.O., Jannat S.N., Rahman M.S. Impact of environmental factors on the spread of dengue fever in Sri Lanka. Int J Environ Sci Technol. 2022;19(11):1–12. doi: 10.1007/s13762-021-03905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gui H., Gwee S., Koh J., Pang J. Weather factors associated with reduced risk of dengue transmission in an urbanized tropical city. Int J Environ Res Public Health. 2021;19:339. doi: 10.3390/ijerph19010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kakarla S.G., Caminade C., Mutheneni S.R., et al. Lag effect of climatic variables on dengue burden in India. Epidemiol Infect. 2019;147 doi: 10.1017/S0950268819000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowe R., Cazelles B., Paul R., Rodó X. Quantifying the added value of climate information in a spatio-temporal dengue model. Stoch Environ Res Risk Assess. 2016;30:2067–2078. [Google Scholar]
- 79.Lowe R., Gasparrini A., Van Meerbeeck C.J., et al. Nonlinear and delayed impacts of climate on dengue risk in Barbados: a modelling study. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lowe R., Lee S.A., O'Reilly K.M., et al. Combined effects of hydrometeorological hazards and urbanisation on dengue risk in Brazil: a spatiotemporal modelling study. Lancet Planet Health. 2021;5:e209–e219. doi: 10.1016/S2542-5196(20)30292-8. [DOI] [PubMed] [Google Scholar]
- 81.Phung D., Nguyen H.X., Nguyen H.L.T., et al. The effects of socioecological factors on variation of communicable diseases: a multiple-disease study at the national scale of Vietnam. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wangdi K., Clements A.C.A., Du T., Nery S.V. Spatial and temporal patterns of dengue infections in Timor-Leste, 2005–2013. Parasit Vectors. 2018;11:1–9. doi: 10.1186/s13071-017-2588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan H.Y., Liang J., Lin P.S., et al. The effects of seasonal climate variability on dengue annual incidence in Hong Kong: a modelling study. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-60309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nurdin N., Siregar Y.I., Mubarak M., Wijayantono W. Environmental factors linked to the presence of Aedes aegypti larvae and the prevalence of dengue hemorrhagic fever. Open Access Maced J Med Sci. 2022;10:475–480. [Google Scholar]
- 85.Su G.L.S. Correlation of climatic factors and dengue incidence in Metro Manila, Philippines. Ambio. 2008;37:292–294. doi: 10.1579/0044-7447(2008)37[292:cocfad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 86.Patil S., Pandya S. Forecasting dengue hotspots associated with variation in meteorological parameters using regression and time series models. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.798034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 88.Aswi A., Cramb S., Duncan E., Hu W., White G., Mengersen K. Climate variability and dengue fever in Makassar, Indonesia: bayesian spatio-temporal modelling. Spat Spatiotemporal Epidemiol. 2020;33 doi: 10.1016/j.sste.2020.100335. [DOI] [PubMed] [Google Scholar]
- 89.Silva F.D., dos Santos A.M., Corrêa Rda G., Caldas Ade J. Temporal relationship between rainfall, temperature and occurrence of dengue cases in São Luís, Maranhão, Brazil. Ciência Saúde Colet. 2016;21:641–646. doi: 10.1590/1413-81232015212.09592015. [DOI] [PubMed] [Google Scholar]
- 90.Xuan L.T.T., Van Hau P., Thu D.T., Toan D.T.T. Estimates of meteorological variability in association with dengue cases in a coastal city in northern Vietnam: an ecological study. Glob Health Action. 2014;7 doi: 10.3402/gha.v7.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polwiang S. The time series seasonal patterns of dengue fever and associated weather variables in Bangkok (2003-2017) BMC Infect Dis. 2020;20:1–10. doi: 10.1186/s12879-020-4902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thammapalo S., Chongsuwiwatwong V., McNeil D., Geater A. The climatic factors influencing the occurrence of dengue hemorrhagic fever in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:191–196. [PubMed] [Google Scholar]
- 93.Valson J.S., Soman B. Spatiotemporal clustering of dengue cases in Thiruvananthapuram district, Kerala. Indian J Public Health. 2017;61:74. doi: 10.4103/ijph.IJPH_26_16. [DOI] [PubMed] [Google Scholar]
- 94.Islam M.Z., Rutherford S., Phung D., et al. Correlates of climate variability and dengue fever in two metropolitan cities in Bangladesh. Cureus. 2018;10(10) doi: 10.7759/cureus.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Christophers S. Syndics of the Cambridge University Press; London: 1960. Aedes aegypti (L): the yellow fever mosquito. Its life history, bionomics and structure. [Google Scholar]
- 96.Brady O.J., Johansson M.A., Guerra C.A., et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors. 2013;6:351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang H.M., Macoris M.L., Galvani K.C., Andrighetti M.T., Wanderley D.M. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol Infect. 2009;137:1188–1202. doi: 10.1017/S0950268809002040. [DOI] [PubMed] [Google Scholar]
- 98.Yang H.M., Macoris M.L.G., Galvani K.C., Andrighetti M.T.M., Wanderley D.M.V. Assessing the effects of temperature on dengue transmission. Epidemiol Infect. 2009;137:1179–1187. doi: 10.1017/S0950268809002052. [DOI] [PubMed] [Google Scholar]
- 99.Chan M., Johansson M.A. The incubation periods of dengue viruses. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Platt K.B., Linthicum K.J., Myint K.S., Innis B.L., Lerdthusnee K., Vaughn D.W. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- 101.Kramer L.D. Complexity of virus-vector interactions. Curr Opin Virol. 2016;21:81–86. doi: 10.1016/j.coviro.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tjaden N.B., Thomas S.M., Fischer D., Beierkuhnlein C. Extrinsic incubation period of dengue: knowledge, backlog, and applications of temperature dependence. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Racloz V., Ramsey R., Tong S., Hu W. Surveillance of dengue fever virus: a review of epidemiological models and early warning systems. PLoS Negl Trop Dis. 2012;6(5) doi: 10.1371/journal.pntd.0001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lowe R., Bailey T.C., Stephenson D.B., et al. Spatio-temporal modelling of climate-sensitive disease risk: towards an early warning system for dengue in Brazil. Comput Geosci. 2011;37(3):371–381. [Google Scholar]
- 105.Lee J.S., Carabali M., Lim J.K., et al. Early warning signal for dengue outbreaks and identification of high risk areas for dengue fever in Colombia using climate and non-climate datasets. BMC Infect Dis. 2017;17(1):480. doi: 10.1186/s12879-017-2577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hii Y.L., Zhu H., Ng N., Ng L.C., Rocklöv J. Forecast of dengue incidence using temperature and rainfall. PLoS Negl Trop Dis. 2012;6(11) doi: 10.1371/journal.pntd.0001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kramer I.M., Kreb A., Klingelhöfer D., et al. Does winter cold really limit the dengue vector Aedes aegypti in Europe? Parasit Vectors. 2020;13:1–13. doi: 10.1186/s13071-020-04054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kramer I.M., Pfeiffer M., Steffens O., et al. The ecophysiological plasticity of Aedes aegypti and Aedes albopictus concerning overwintering in cooler ecoregions is driven by local climate and acclimation capacity. Sci Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146128. [DOI] [PubMed] [Google Scholar]
- 109.Romeo-Aznar V., Picinini Freitas L., Gonçalves Cruz O., King A.A., Pascual M. Fine-scale heterogeneity in population density predicts wave dynamics in dengue epidemics. Nat Commun. 2022;13(1):996. doi: 10.1038/s41467-022-28231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodrigues Mde M., Marques G.R.A.M., Serpa L.L.N., et al. Density of Aedes aegypti and Aedes albopictus and its association with number of residents and meteorological variables in the home environment of dengue endemic area, São Paulo, Brazil. Parasit Vectors. 2015;8(1):115. doi: 10.1186/s13071-015-0703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sirisena P., Noordeen F., Kurukulasuriya H., Romesh T.A., Fernando L. Effect of climatic factors and population density on the distribution of dengue in Sri Lanka: a gis based evaluation for prediction of outbreaks. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0166806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.