Abstract
Objectives
In a large U.S. cohort free of CVD evaluated by coronary computed CT angiography, we aimed to assess the association between established / high risk of Obstructive Sleep Apnea (OSA) and coronary plaque.
Background
There are limited data available depicting the association between established / high risk of OSA and the presence of coronary plaque in a population-based sample free from CVD.
Methods
Cross-sectional data from 2359 participants enrolled in the Miami Heart Study (MiHeart) who underwent coronary CT angiography was used for this study. The Berlin questionnaire was used to stratify patients as having high or low risk of OSA. Multiple multivariable logistic regression analyses were conducted to investigate the association between the risk of developing OSA with the presence, volume, and composition of plaque.
Results
According to the Berlin questionnaire, 1559 participants were (66.1%) at low risk of OSA and 800 patients (33.9%) with established / high risk of OSA. Plaque characterization on CCTA revealed a greater incidence of any possible plaque composition in the established / high risk of OSA category (59.6% vs. 43.5%) compared to the low risk of OSA cohort. In logistic regression models, after adjusting for demographics and cardiovascular risk factors, a significant association could still be noted between established / high risk of OSA and any coronary plaque on CCTA (OR=1.31, CI 1.05, 1.63, p = 0.016). Subgroup analysis in the Hispanic population also portrayed a significant association between established / high risk of OSA and the presence of coronary plaque on CCTA (OR = 1.55 CI 1.13, 2.12, p = 0.007).
Conclusion
After accounting for CVD risk factors, individuals at established / high risk of OSA have a higher likelihood of the presence of coronary plaque. Future studies should focus on OSA presence or risk, OSA severity, and the longitudinal consequences of coronary atherosclerosis.
1. Introduction
Obstructive sleep apnea (OSA) affects nearly 22% of men and 17% of women [1,2]. This condition is characterized by repeated episodes of partial or complete obstruction of the upper airway [2], [3], [4], [5]. Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide and incurs considerable healthcare expenditure [6].
The incidence of atherosclerotic CVD (ASCVD) is higher among people with OSA [7], [8], [9], [10], [11], [12]. Some plausible mechanisms linking this association include OSA-induced chronic intermittent hypoxia, changes in intrathoracic pressure, and elevated sympathetic nervous system resulting from both intermittent hypoxia and sleep fragmentation [13], [14], [15].
Predicting the future risk of CVD assumes paramount importance, and its approach has shifted to assessing the disease process rather than estimating surrogate risk factors alone [16,17]. In accordance, identification of atherosclerosis via measurement of plaque burden performs better than the evaluation of surrogate biomarkers alone [18].
Coronary Computed Tomographic Angiography (CCTA) is a non-invasive imaging modality that quantifies total plaque burden and identifies plaque subtypes, including those with high-risk features [19]. There is currently no known published data regarding the association between OSA or those at high risk of OSA and coronary plaque in a population-based sample free of CVD. Given the earlier identified relationship between OSA and ASCVD, exploring the relationship between OSA and atherosclerotic plaque will improve our understanding of the relationship between OSA and ASCVD. Specifically, there is an unmet need to understand this association in the Hispanic population, who are particularly vulnerable to CVD [20].
The Miami Heart Study (MiHeart) at Baptist Health South Florida (BHSF) is an ongoing prospective cohort study initiated in 2015 and enrolled 2359 participants from the Greater Miami Area, free of clinically overt CVD. We leveraged the MiHeart study data to assess the association of at risk OSA with subclinical coronary plaque burden measured by CCTA in a population-based sample enriched with Hispanics in South Florida. We hypothesize that there would be a significant association of high OSA risk with the presence and burden of ASCVD.
2. Methods
2.1. The Miami heart study
The MiHeart study is an observational, community-based, longitudinal, prospective cohort study and enrolled 2359 middle-aged participants from May 2015 to September 2018 (NCT02508454). It aims to provide essential information about the pathophysiology of early subclinical atherosclerotic disease development and its role in clinical CVD. It included a baseline examination consisting of an assessment of demographics, medical history, psychosocial characteristics, comprehensive physical exam, and assessment of subclinical coronary atherosclerotic plaque and vascular function.
Inclusion criteria included participants aged 40 to 65 years residing in the greater Miami area, in addition to employees of Baptist Health South Florida (BHSF). The study recruitment was based on volunteer participation, and patients included were individuals free of known CVD who could comprehend and sign the informed consent. Exclusion criteria included participants with any history of CVD, weight greater than 350 pounds, undergoing active treatment for cancer, pregnant, breastfeeding, having any contraindication to undergo a CT scan, or unable to provide informed consent. The study was approved by the BHSF institutional review board. All participants provided written informed consent before enrollment. Additional details of the study methods are provided below, and a report describing the study design and rationale has been published [21].
The primary aim of our study was to examine the association between high-risk OSA ascertained by the Berlin questionnaire and self-reported OSA and the presence and severity of coronary atherosclerotic disease among participants from the Miami Heart Study (MiHeart) free of CVD. Self-reported OSA was determined by participants answering the question, “Have You Ever Been Diagnosed With Any Of The Following?”. Among a list of options, it had OSA as one of the answers.
2.2. The Berlin questionnaire
The Berlin questionnaire consists of 10 items divided into three categories, with the results distinguishing patients having a low or high risk of OSA. These items are related to the presence of snoring, frequency of apneic events during sleep, nonrestorative sleep, sleepiness while driving, concomitant hypertension, and the patient's body mass index [22]. A high risk of OSA classification is denoted by the presence of 2 out of 3 categories with a positive score. When OSA is defined as an apnea-hypopnea index (AHI) ≥15 events per hour, a high-risk category on the Berlin questionnaire is associated with a sensitivity and specificity of 91% and 37%, respectively [23]. In our study, a high risk of OSA was defined as having either an OSA diagnosis (self-reported; n = 248) and/or a high risk of OSA assessed using the Berlin questionnaire. The participants without a known self-reported history of OSA based on the MiHeart study questionnaire and who did not meet the high risk of OSA by the Berlin questionnaire were classified as low risk of OSA / no OSA.
2.3. Coronary CT assessment
Cardiac computed tomography angiography (CCTA) scan was done to determine the presence of coronary plaque. A non-contrast cardiac-gated CT scan for CAC (coronary artery calcium) testing was performed in all participants prior to conducting a contrast-enhanced, cardiac-gated CCTA. The contrast and non-contrast imaging scans were performed using GE Revolution (GE Healthcare) scans. Before the test, study participants received oral and/or intravenous β-blocker (metoprolol) as needed to achieve a heart rate of < 75 beats per minute. Additionally, 0.4 mg of sublingual nitroglycerine was given immediately before angiographic image acquisition. The radiation dose was estimated from the dose-length product multiplied by the conversion factor (0.014 mSv/mGy • cm). The assessment of 17 segment plaque presence/burden and classification was performed at a central core imaging lab.
2.4. Statistical methods
We conducted cross-sectional analyses using data from the baseline cohort of the MiHeart study.
We categorized ethnicity as either non-Hispanic or Hispanic, and amongst non-Hispanic participants, further categorized as White (NHW), Black, or Other. Categorical variables such as sex, race, education, etc., were reported as frequency and percentage. Continuous variables such as age were reported as mean and standard deviation. Differences in proportions were analyzed using the Chi-square test, and differences in means were calculated using the analysis of variance test. Multiple multivariable logistic regression analyses were conducted to calculate odds ratios and 95% confidence intervals. Dependent variables or outcomes of interest were the presence of any coronary plaque, at least one lesion with a vulnerable plaque feature, calcified or predominantly calcified plaque, and noncalcified or predominantly noncalcified plaque. Patients were classified as “High risk of OSA” (yes or no) by categorizing the total score from the Berlin Questionnaire, i.e., 2 out of 3 categories with a positive score on Berlin. Participants were categorized as established OSA if they had a pre-existing diagnosis of OSA, based on the MiHeart study questionnaire. Together we refer to them as established / high risk of OSA. The low risk of OSA cohort comprised participants without a known self-reported history of OSA based on the MiHeart study questionnaire and those who did not meet the high risk of OSA by the Berlin questionnaire. In logistic regression analysis, three models were analyzed. Model 1 was unadjusted; Model 2 was adjusted for Model 1 with age, sex, and race/ethnicity; Model 3 was adjusted for Model 2 with diabetes, hypertension, high cholesterol, current smoking, and overweight/obesity variables. Vulnerable plaque features included: positive remodeling, low attenuation plaque, spotty calcification, and/or napkin ring. We performed an analysis using the Berlin questionnaire and an additional analysis among the subgroup of patients with self-reported OSA. All statistical analyses were performed using STATA software, version 13. A p-value <0.05 was considered statistically significant. (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) [24].
3. Results
3.1. Study participants
Overall, 2359 participants were included in the MiHeart study, with 1559 participants (66.1%) at low risk of OSA and 800 patients (33.9%) with established / high risk of OSA according to the Berlin questionnaire. A total of 248 patients who self-reported a past history of OSA as part of the questionnaire form were included in the established OSA cohort. The mean age of the participants enrolled was 53.4 ± 6.8 years among the low risk of OSA cohort and 53.5 ± 6.7 years among the established / high risk of OSA category. Overall, the study population had 79% of participants with bachelor's degrees or higher.
A total of 63.5% of the participants in the established / high risk of OSA category were male, compared to 43.7% in the low risk of OSA cohort. With regards to race/ethnicity, in the established / high risk of OSA cohort, 37.5% of the population was Non-Hispanic White, 4.8% was Non-Hispanic Black, and a majority, 51.6% were Hispanic, compared to 46.0%, 2.8%, 44.6% in the low risk of OSA group, respectively. (Table 1). Among those with established OSA, 159 participants (64%) also had features consistent with a high risk of OSA based on the Berlin Questionnaire, which is hence the sensitivity of the Berlin questionnaire as per our study. (Supplementary Tables 1 and 2).
Table 1.
Baseline characteristics of high risk OSA/OSA vs. low risk OSA.
| (N = 2359) | |||
|---|---|---|---|
| Demographics | Low risk | High risk of OSA/OSA | p-value |
| N | 1559 (66.1%) | 800 (33.9%) | |
| Age, mean (SD) | 53.4 (6.8) | 53.5 (6.7) | 0.71 |
| Male | 681 (43.7%) | 508 (63.5%) | 0.001 |
| Race/Ethnicity | 0.002 | ||
| Non-Hispanic White | 717 (46.0%) | 300 (37.5%) | |
| Non-Hispanic Black | 44 (2.8%) | 38 (4.8%) | |
| Hispanic/Latino | 696 (44.6%) | 413 (51.6%) | |
| Other race/Unknown/not disclosed | 102 (6.5%) | 49 (6.1%) | |
| Education | 0.016 | ||
| High School or less/ unknown | 127 (8.1%) | 86 (10.8%) | |
| Some College, no degree | 174 (11.2%) | 101 (12.6%) | |
| Bachelors | 755 (48.4%) | 405 (50.6%) | |
| Post Graduate Studies | 503 (32.3%) | 208 (26.0%) | |
On average, 29% of participants with established / high risk of OSA were overweight, compared to 48% with low risk of OSA, although a significant majority of those with established / high risk of OSA were obese (64.0%, p<0.001). Statistically significant differences were noted in the prevalence of comorbidities among the two categories, with patients at established / high risk of OSA with a higher percentage of hypertension (77% vs. 45%), diabetes (14% vs. 5%), hyperlipidemia (42% vs. 39%) and hypertriglyceridemia (29% vs. 14%) (p<0.001). Furthermore, patients in the established / high risk of OSA cohort had lower HDL levels (30.1% vs. 14.6%), higher mean glucose levels (100.1 mg/dl vs. 94.7 mg/dl), and were more likely to be compliant with their lipid-lowering therapy (p<0.001) (Table 2).
Table 2.
Baseline characteristics of high risk OSA/OSA vs. low risk OSA in CVD categories.
| N = 2359 | |||
|---|---|---|---|
| Clinical Features and Family Hx | Low risk of OSA | High risk of OSA/OSA | p-value |
| N | 1559 (66.08%) | 800 (33.9%) | |
| BMI Categories | <0.001 | ||
| Underweight | 10 (0.6%) | 0 (0.0%) | |
| Normal Weight | 543 (34.8%) | 56 (7.0%) | |
| Overweight | 748 (48.0%) | 232 (29.0%) | |
| Obese | 258 (16.5%) | 512 (64.0%) | |
| Current Cig Smoker | 40 (2.6%) | 31 (3.9%) | 0.078 |
| Mean Systolic Pressure (mmHg), mean (SD) | 121.0 (14.4) | 128.2 (13.8) | <0.001 |
| Mean Diastolic Pressure (mmHg), mean (SD) | 76.7 (8.1) | 81.3 (8.2) | <0.001 |
| Hypertension | 707 (45.3%) | 618 (77.2%) | <0.001 |
| Glucose (mg/dL), mean (SD) | 94.7 (16.9) | 100.1 (26.0) | <0.001 |
| HbA1c (%), median (IQR) | 5.5 (5.3, 5.7) | 5.6 (5.4, 5.9) | <0.001 |
| Diabetes | 84 (5.4%) | 111 (13.9%) | <0.001 |
| Total cholesterol (mg/dL), mean (SD) | 207.2 (39.4) | 202.0 (42.2) | 0.003 |
| High cholesterol, n (%) | 906 (58.1%) | 546 (68.2%) | <0.001 |
| LDL-C (mg/dL), median (IQR) | 120.0 (98.0, 144.0) | 122.0 (98.0, 148.5) | 0.40 |
| HDL-C (mg/dL), mean (SD) | 64.0 (20.3) | 51.7 (15.8) | <0.001 |
| Low HDL-C, n (%) | 228 (14.6%) | 241 (30.1%) | <0.001 |
| Triglycerides (mg/dL), median (IQR) | 87.0 (65.0, 122.0) | 117.0 (84.0, 160.0) | <0.001 |
| Hypertriglyceridemia | 220 (14.1%) | 234 (29.2%) | <0.001 |
| No Lipid Lowering Therapy | 1258 (80.7%) | 548 (68.5%) | <0.001 |
| Self-reported OSA | 0 | 248 (100%) | |
Abbreviations: LDL-C: Low-density lipoprotein cholesterol, HDL-C: High-density lipoprotein cholesterol.
3.2. CCTA-Based plaque analysis
Plaque characterization on CCTA revealed a greater incidence of any possible plaque composition in the established / high risk of OSA category (59.6% vs. 43.5%), with all results being statistically significant. The most frequent plaques found were calcified in 320 participants (40.0%) among participants with established / high risk of OSA, compared to 454 patients (29.1%) among the low risk of OSA cohort. The other plaque features observed in decreasing order of frequency on CCTA among patients with an established / high risk of OSA cohort compared to the low risk of OSA group were noncalcified plaque (34.8% vs. 22.8%), predominantly calcified (17.9% vs. 12.1%) and predominantly noncalcified (13.1% vs. 8.7%) (p<0.001).
The extent of plaque stenosis was found to be greater amongst all subgroups in patients with established / high risk of OSA. Most of the plaques observed in this cohort occluded 1–49% of the cross-sectional area (51.5% vs. 38.8%), followed by plaques leading to 50–69% stenosis (5.7% vs. 3.4%). An extent of ≥ 70% stenoses was observed in 21 (2.6%) participants in the established / high risk of OSA cohort, compared to 22 (1.4%) participants in the low risk of OSA cohort (p<0.001).
The presence of specific vulnerable plaque features, including but not limited to low attenuation, spotty calcification, napkin ring shape, and positive remodeling, were greater frequency among the established / high risk of OSA cohort. The most frequently encountered vulnerable plaque feature was positive remodeling, both in the established / high risk of OSA (6.4%) and low risk of OSA cohort (4.1%) (p = 0.015). Other vulnerable plaque features in the established / high risk of OSA cohort, in decreasing order of frequency, included spotty calcification (2.6%)(p = 0.37), low attenuation (2.1%) (p = 0.013), and napkin ring sign (0.8%) (p = 0.15) respectively. Finally, ≥ 2 high-risk features in a single vessel were noted in 28 (3.5%) patients in the established / high risk of OSA cohort and 27 (1.7%) participants in the low risk of OSA group (p = 0.007) (Table 3).
Table 3.
Baseline characteristics of high risk OSA/OSA vs. low risk OSA in CVD categories.
| N = 2359 | |||
|---|---|---|---|
| Plaque features | Low risk | High risk for OSA/OSA | p-value |
| N | 1559 | 800 | |
| Plaque characteristics by CCTA | |||
| Any Plaque | 678 (43.5%) | 477 (59.6%) | <0.001 |
| Noncalcified plaque | 356 (22.8%) | 278 (34.8%) | <0.001 |
| Calcified plaque | 454 (29.1%) | 320 (40.0%) | <0.001 |
| Predominantly noncalcified plaque | 136 (8.7%) | 105 (13.1%) | <0.001 |
| Predominantly calcified plaque | 189 (12.1%) | 143 (17.9%) | <0.001 |
| Maximum Stenosis | <0.001 | ||
| No stenosis/plaque | 874 (56.4%) | 320 (40.2%) | |
| 1–49% stenosis | 602 (38.8%) | 410 (51.5%) | |
| 50–69% stenosis | 52 (3.4%) | 45 (5.7%) | |
| ≥ 70% stenosis | 22 (1.4%) | 21 (2.6%) | |
| Positive remodeling | 64 (4.1%) | 51 (6.4%) | 0.015 |
| Low attenuation plaque | 36 (2.3%) | 33 (4.1%) | 0.013 |
| Spotty calcification | 32 (2.1%) | 21 (2.6%) | 0.37 |
| Napkin ring | 5 (0.3%) | 6 (0.8%) | 0.15 |
| Presence of high-risk features | 95 (6.1%) | 70 (8.8%) | 0.017 |
| Number of high-risk features | 0.087 | ||
| No high-risk features | 1464 (93.9%) | 730 (91.2%) | |
| 1 | 66 (4.2%) | 41 (5.1%) | |
| 2 | 18 (1.2%) | 19 (2.4%) | |
| 3 | 9 (0.6%) | 8 (1.0%) | |
| 4 | 2 (0.1%) | 2 (0.2%) | |
| Presence of ≥ 2 high-risk features in a vessel | 27 (1.7%) | 28 (3.5%) | 0.007 |
3.3. Association between OSA and CCTA findings
Associations between established / high risk of OSA have been summarized in Table 4. When not adjusted for any confounders (Model 1), a significant positive association was observed between established / high risk of OSA and the presence of coronary plaque (OR=1.92, 95% CI 1.61, 2.28, p<0.001), at least one coronary artery stenosis ≥ 50% (OR=1.80, 95% CI 1.28, 2.54, p = 0.001) and with at least one lesion with a vulnerable plaque feature on CCTA (OR=1.48, 95% CI 1.07, 2.04, p = 0.017). After adjusting for demographic variables such as age, sex, and race/ethnicity (Model 2), a significant association persisted between established / high risk of OSA and the presence of any coronary plaque (OR=1.58, 95% CI 1.31, 1.91, p<0.001), and with at least one coronary artery stenosis ≥ 50% on CCTA (OR=1.54, 95% CI 1.08, 2.20, p = 0.017.
Table 4.
Association between high risk OSA/OSA and CCTA plaque features.
| High Risk for OSA (ref: Low Risk) | Hispanic Population: High Risk for OSA | |||
|---|---|---|---|---|
| OR⁎(95% CI) | p-value | OR* (95% CI) | p-value | |
| Any coronary plaque | ||||
| Model 1 | 1.92 (1.61, 2.28) | <0.001 | 2.17 (1.70, 2.79) | <0.001 |
| Model 2 | 1.58 (1.31, 1.91) | <0.001 | 1.83 (1.40, 2.38) | <0.001 |
| Model 3 | 1.31 (1.05, 1.63) | 0.016 | 1.55 (1.13, 2.12) | 0.007 |
| At least one coronary stenosis ≥ 50% | ||||
| Model 1 | 1.80 (1.28, 2.54) | 0.001 | 1.63 (0.97, 2.71) | 0.064 |
| Model 2 | 1.54 (1.08, 2.20) | 0.017 | 1.46 (0.87, 2.47) | 0.153 |
| Model 3 | 1.07 (0.72, 1.59) | 0.75 | 1.01 (0.56, 1.84) | 0.97 |
| At least one lesion with a vulnerable plaque feature | ||||
| Model 1 | 1.48 (1.07, 2.04) | 0.017 | 1.40 (0.87, 2.23) | 0.163 |
| Model 2 | 1.18 (0.85, 1.64) | 0.32 | 1.13 (0.70, 1.83) | 0.162 |
| Model 3 | 1.003 (0.69, 1.45) | 0.98 | 1.04 (0.60, 1.81) | 0.898 |
| Calcified or predominantly calcified plaque | ||||
| Model 1 | 1.65 (1.39, 1.97) | <0.0001 | 1.76 (1.36, 2.27) | <0.001 |
| Model 2 | 1.36 (1.12, 1.66) | 0.002 | 1.44 (1.10, 1.89) | 0.01 |
| Model 3 | 1.15 (0.92, 1.44) | 0.223 | 1.27 (0.92, 1.76) | 0.15 |
| Non calcified or predominantly non calcified plaque | ||||
| Model 1 | 1.83 (1.53, 2.19) | <0.0001 | 1.98 (1.53, 2.57) | <0.001 |
| Model 2 | 1.52 (1.26, 1.84) | <0.001 | 1.71 (1.31, 2.24) | <0.001 |
| Model 3 | 1.17 (0.94, 1.46) | 0.155 | 1.42 (1.04, 1.94) | 0.029 |
Model 1=Unadjusted,.
Model 2=Adjusted for age, sex and race/ethnicity,.
Model 3=Model 2 + diabetes, high cholesterol, hypertension, current smoking, overweight/obesity.
Vulnerable plaque features include positive remodeling, low attenuation plaque, spotty calcification and/or napkin ring.
OR: Odds Ratio.
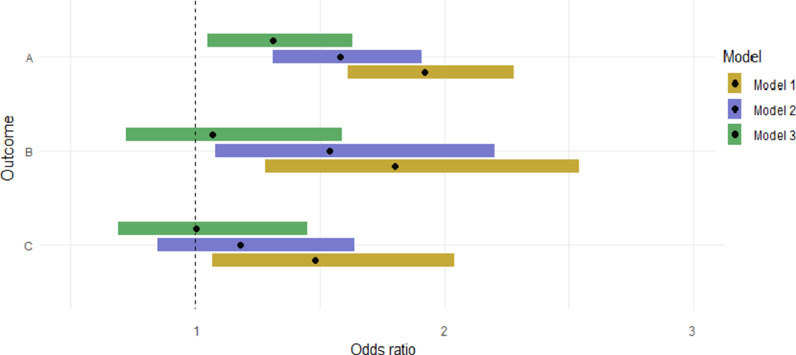
In model three, we adjusted for demographics and cardiovascular risk factors, including diabetes, high cholesterol, current smoking, and high BMI in the range of overweight/obese range. A significant association was noted between established / high risk of OSA and the presence of any coronary plaque on CCTA (OR=1.31, CI 1.05, 1.63, p = 0.016] (Fig. 1). In subgroup analysis that was restricted to the Hispanic population, a strong, independent, statistically significant association was observed between established / high risk of OSA and the presence of coronary plaque on CCTA (OR = 1.55 CI 1.13, 2.12, p = 0.007).
Fig. 1.
Association between high risk OSA/OSA and presence of CCTA/CAC findings
A: Any coronary plaque
B: At least one coronary stenosis ≥ 50%
C: At least one lesion with a vulnerable plaque feature
Model 1: unadjusted;
Model 2: adjusted for Model 1 with age, sex, and race/ethnicity;
Model 3: adjusted for Model 2 with diabetes, hypertension, high cholesterol, current smoking, overweight/obesity variables.
4. Discussion
In this large population-based study, persons with established / high risk of OSA had a > 50% increase in the odds of coronary plaque presence and >50% increased odds of plaque-causing significant stenosis even after accounting for demographic confounders. In addition, participants with an established / high risk of OSA were more likely to have vulnerable plaques compared to those without OSA.
Current literature suggests an established association between OSA and cardiovascular events. [25] Various studies have investigated this relationship between OSA and coronary plaque using CCTA and have shown similar results. For instance, Sharma et al. demonstrated a strong association between patients with OSA (mean AHI =42/hour) and the presence of noncalcified coronary plaques [26]. Several other studies have examined this association using CCTA and have reported similar results. [27], [28], [29], [30]. However, most of these studies were limited in their scope because of small sample sizes, retrospective design, and had sleep studies performed after being worked up due to a need for plaque characterization for a known history of CVD.
In a more recent study, Lu et al. showed an association between objectively determined obstructive sleep apnea and the presence and burden of coronary plaques in a population of approximately seven hundred participants. They showed a linear association between the AHI and plaque presence and burden. However, the study was done in an Asian population, with the patients having been recruited for sleep studies, including polysomnography and respiratory polygraphy [31]. Other small-scale studies conducted in patients with a formal diagnosis of OSA showed a similar association with the presence of coronary plaque [32], [33], [34], [35]. To our knowledge, the present study with over 2000 participants is the largest to investigate the association between OSA and coronary plaque presence and characteristics.
We also observed a higher prevalence of high-risk vulnerable plaque features, which have been observed to be associated with the future risk of acute coronary syndrome. The four major plaque characteristics that comprise these high-risk features include positive remodeling, low attenuation plaque (<30 Hounsfield units), spotty calcification, and napkin-ring sign. [36] The pathogenesis of atherosclerosis has shown that it is a lipoprotein-driven inflammatory disease with associated intimal inflammation, arterial remodeling, and calcification. [37] Plaque calcifications develop over time, and the necrotic core has been demonstrated to be fully calcified with time. [38] The mean age in our study cohort was 53 years, suggesting that the participants included were young and most likely in the early stages of plaque development, accounting for the low calcified plaque burden.
Emerging data suggest that persons of Hispanic ethnicity have an increased incidence of OSA and sleep apnea symptoms compared to non-Hispanic whites [39,40]. In a clinical study, ∼ 76% of the Hispanic population in a South Florida cohort were at increased risk of OSA, based on the STOP-Bang questionnaire [41]. Data from the CDC also shows a large burden of CVD risk factors among persons of Hispanic ethnicity [20]. Studies suggest that Hispanic individuals have a very high prevalence of OSA (nearly 40% in one series)[42], but most of them are undiagnosed. In addition, among persons of Hispanic ethnicity, OSA is associated with CVD risk factors, including hypertension and diabetes. The present study, which is the first to show the relationship between OSA and atherosclerotic plaque presence and burden in a largely Hispanic population, further highlights the urgent need for population-based screening of OSA and its associated CV risk factors in the Hispanic population.
The strengths of our study include the utilization of a large sample size and a population-based sample. To our knowledge, this is the largest cross-sectional analysis involving a population free from CVD to study the association between OSA severity and quantitative plaque analysis. Moreover, our sample is rich in the Hispanic population and females, comprising nearly half the population. We believe that the inclusion of a multiethnic composition is the most important strength of this study, which reports unique perspectives about risk factors pertaining to ethnic differences and provides the ability to tailor specific prevention efforts. Limitations include the unavailability of objective sleep study data for OSA risk assessment. Furthermore, established OSA was determined by self-reported sleep apnea, and sleep studies or treatment and treatment compliance could not be determined. The Berlin questionnaire has a high sensitivity but low specificity, and thus not a good tool to rule in cases of OSA. This may have led to a misclassification bias, by the inclusion of a greater number of participants in the high-risk OSA cohort, despite being at low risk for the disease. Our study type is cross-sectional; hence, causality could not be determined. Non-Hispanic Whites were underrepresented, in addition to non-Hispanic Blacks and Asians. We plan to follow up with the included participants longitudinally.
5. Conclusions
Individuals at established / high risk of OSA comprised one-third of the participants free from CVD included in the study. After accounting for CVD risk factors, individuals at established / high risk of OSA have a higher likelihood of the presence of coronary plaques. This finding persisted even when the analysis was restricted to only those who were Hispanic. Screening for OSA with simple tools like the STOP-Bang or Berlin Questionnaire may serve the dual role of identifying those who will derive benefit from a sleep test as well as CVD risk assessment and aggressive CVD risk prevention. Future studies should focus on the potential longitudinal progression of coronary atherosclerosis among patients with varying degrees of formally diagnosed OSA, particularly among Hispanics.
Funding
The Miami Heart study was funded by Baptist Health South Florida.
Disclosures
Dr Aneni is supported by a CTSA Grant Number KL2 TR001862, from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health. All other authors have no relevant disclosures.
CRediT authorship contribution statement
Harneet K Walia: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Supervision. Atulya Aman Khosla: Investigation, Writing – original draft, Writing – review & editing. Anshul Saxena: Investigation, Writing – original draft, Writing – review & editing. Ehimen Aneni: Investigation, Writing – original draft, Writing – review & editing, Supervision. Shozab S. Ali: Writing – review & editing. Javier Valero-Elizondo: Writing – review & editing. Miguel Cainzos-Achirica: Writing – review & editing. Theodore Feldman: Conceptualization, Writing – review & editing. Jonathan Fialkow: Conceptualization, Writing – review & editing. Khurram Nasir: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100497.
Appendix. Supplementary materials
References
- 1.Franklin K.A., Lindberg E. Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottlieb D.J., Punjabi N.M. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 3.Tuleta I., Pabst S., Juergens U.R., Nickenig G., Skowasch D. Obstructive sleep apnoea as a risk factor for atherosclerosis–implication for preventive and personalised treatment. EPMA J. 2011;2:39–47. doi: 10.1007/s13167-011-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T., Peppard P.E., Gottlieb D.J. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Punjabi N.M. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M., Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 7.Bradley T.D., Floras J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet North Am Ed. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 8.Kohli P., Sarmiento K., Malhotra A. Update in sleep medicine 2012. Am J Respir Crit Care Med. 2013;187:1056–1060. doi: 10.1164/rccm.201302-0315UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet North Am Ed. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 10.McNicholas W., Bonsignore M., B26 MCoECA Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 11.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 12.Shpilsky D., Erqou S., Patel S.R., Kip K.E., Ajala O., Aiyer A., Strollo P.J., Reis S.E., Olafiranye O. Association of obstructive sleep apnea with microvascular endothelial dysfunction and subclinical coronary artery disease in a community-based population. Vasc Med. 2018;23:331–339. doi: 10.1177/1358863X18755003. [DOI] [PubMed] [Google Scholar]
- 13.Kasai T., Floras J.S., Bradley T.D. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126:1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 14.Wu J., Stefaniak J., Hafner C., Schramel J.P., Kaun C., Wojta J., Ullrich R., Tretter V.E., Markstaller K., Klein K.U. Intermittent hypoxia causes inflammation and injury to human adult cardiac myocytes. Anesth Analg. 2016;122:373–380. doi: 10.1213/ANE.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 15.Pennings N., Golden L., Yashi K., Tondt J., Bays H.E. Sleep-disordered breathing, sleep apnea, and other obesity-related sleep disorders: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., Michos E.D., Miedema M.D., Muñoz D., Smith S.C., Jr., Virani S.S., Williams K.A., Sr., Yeboah J., Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland P., LaBree L., Azen S.P., Doherty T.M., Detrano R.C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 18.Nasir K., Bittencourt M.S., Blaha M.J., Blankstein R., Agatson A.S., Rivera J.J., Miedema M.D., Sibley C.T., Shaw L.J., Blumenthal R.S., Budoff M.J., Krumholz H.M. Implications of coronary artery calcium testing among statin candidates according to American college of cardiology/American heart association cholesterol management guidelines: mesa (Multiethnic study of atherosclerosis) J Am Coll Cardiol. 2015;66:1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 19.Abdelrahman K.M., Chen M.Y., Dey A.K., Virmani R., Finn A.V., Khamis R.Y., Choi A.D., Min J.K., Williams M.C., Buckler A.J., Taylor C.A., Rogers C., Samady H., Antoniades C., Shaw L.J., Budoff M.J., Hoffmann U., Blankstein R., Narula J., Mehta N.N. Coronary computed tomography angiography from clinical uses to emerging technologies: jacc state-of-the-art review. J Am Coll Cardiol. 2020;76:1226–1243. doi: 10.1016/j.jacc.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC . 2022. Health of Hispanic or Latino population. [Google Scholar]
- 21.Nasir K., Ziffer J.A., Cainzos-Achirica M., Ali S.S., Feldman D.I., Arias L., Saxena A., Feldman T., Cury R., Budoff M.J., Fialkow J. The miami heart study (MiHeart) at Baptist health South Florida, a prospective study of subclinical cardiovascular disease and emerging cardiovascular risk factors in asymptomatic young and middle-aged adults: the miami heart study: rationale and design. Am J Prev Cardiol. 2021;7 doi: 10.1016/j.ajpc.2021.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netzer N.C., Stoohs R.A., Netzer C.M., Clark K., Strohl K.P. Using the berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Chiu H.Y., Chen P.Y., Chuang L.P., Chen N.H., Tu Y.K., Hsieh Y.J., Wang Y.C., Guilleminault C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp . StataCorp LP; College Station, TX: 2013. Stata statistical software: release 13. [Google Scholar]
- 25.Lee C.H., Sethi R., Li R., Ho H.H., Hein T., Jim M.H., Loo G., Koo C.Y., Gao X.F., Chandra S., Yang X.X., Furlan S.F., Ge Z., Mundhekar A., Zhang W.W., Uchôa C.H., Kharwar R.B., Chan P.F., Chen S.L., Chan M.Y., Richards A.M., Tan H.C., Ong T.H., Roldan G., Tai B.C., Drager L.F., Zhang J.J. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. CirculationCirculation. 2016;133:2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S., Gebregziabher M., Parker A.T., Abro J.A., Armstrong A.M., Schoepf U.J. Independent association between obstructive sleep apnea and noncalcified coronary plaque demonstrated by non-invasive coronary computed tomography angiography. Clin Cardiol. 2012;35:641–645. doi: 10.1002/clc.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuvaraj J., Cameron W., Andrews J., Lin A., Nerlekar N., Nicholls S.J., Hamilton G.S., Wong D.T.L. Coronary computed tomography angiography-based assessment of vascular inflammation in patients with obstructive sleep apnoea and coronary artery disease. Cardiovasc Diagn Ther. 2022;12:123–134. doi: 10.21037/cdt-21-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo L., Gupta V., Modi R., Munnur K., Cameron J.D., Seneviratne S., Edwards B.A., Landry S.A., Joosten S.A., Hamilton G.S., Wong D.T.L. Severe obstructive sleep apnea is associated with significant coronary artery plaque burden independent of traditional cardiovascular risk factors. Int J Cardiovasc Imaging. 2020;36:347–355. doi: 10.1007/s10554-019-01710-w. [DOI] [PubMed] [Google Scholar]
- 29.Umut Somuncu M., Bulut U., Karakurt H., Utkusavas A., Akbay E., Kartal Kilinc F. The relationship between obstructive sleep apnea and coronary plaque: a coronary computed tomographic angiography study. Acta Cardiol Sin. 2019;35:325–334. doi: 10.6515/ACS.201905_35(3).20181029A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bikov A., Kolossváry M., Jermendy A.L., Drobni Z.D., Tarnoki A.D., Tarnoki D.L., Forgó B., Kovacs D.T., Losonczy G., Kunos L., Voros S., Merkely B., Maurovich-Horvat P. Comprehensive coronary plaque assessment in patients with obstructive sleep apnea. J Sleep Res. 2019;28:e12828. doi: 10.1111/jsr.12828. [DOI] [PubMed] [Google Scholar]
- 31.Lu M., Fang F., Wang Z., Xu L., Sanderson J.E., Zhan X., He L., Wu C., Wei Y. Association between OSA and quantitative atherosclerotic plaque burden: a coronary ct angiography study. Chest. 2021;160:1864–1874. doi: 10.1016/j.chest.2021.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Kent B.D., Garvey J.F., Ryan S., Nolan G., Dodd J.D., McNicholas W.T. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study. Eur Respir J. 2013;42:1263–1270. doi: 10.1183/09031936.00094812. [DOI] [PubMed] [Google Scholar]
- 33.Murphy D.J., Crinion S.J., Redmond C.E., Healy G.M., McNicholas W.T., Ryan S., Dodd J.D. Diagnostic accuracy of carotid intima media thickness in predicting coronary plaque burden on coronary computed tomography angiography in patients with obstructive sleep apnoea. J Cardiovasc Comput Tomogr. 2017;11:227–233. doi: 10.1016/j.jcct.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Turmel J., Sériès F., Boulet L.P., Poirier P., Tardif J.C., Rodés-Cabeau J., Larose E., Bertrand O.F. Relationship between atherosclerosis and the sleep apnea syndrome: an intravascular ultrasound study. Int J Cardiol. 2009;132:203–209. doi: 10.1016/j.ijcard.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 35.Konishi T., Kashiwagi Y., Funayama N., Yamamoto T., Murakami H., Hotta D., Tanaka S. Obstructive sleep apnea is associated with increased coronary plaque instability: an optical frequency domain imaging study. Heart Vessels. 2019;34:1266–1279. doi: 10.1007/s00380-019-01363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al'Aref S.J., Peña J.M., Min J.K. High-risk atherosclerotic plaque features for cardiovascular risk assessment in the prospective multicenter imaging study for evaluation of chest pain trial. Cardiovasc Diagn Ther. 2019;9:89–93. doi: 10.21037/cdt.2018.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 38.Stary H.C., Chandler A.B., Dinsmore R.E., Fuster V., Glagov S., Insull W., Jr., Rosenfeld M.E., Schwartz C.J., Wagner W.D., Wissler R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. a report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor G.T., Lind B.K., Lee E.T., Nieto F.J., Redline S., Samet J.M., Boland L.L., Walsleben J.A., Foster G.L. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the sleep heart health study. Sleep. 2003;26:74–79. [PubMed] [Google Scholar]
- 40.Ramos A.R., Wohlgemuth W.K., Dong C., Gardener H., Wright C.B., Boden-Albala B., Elkind M.S., Sacco R.L., Rundek T. Race-ethnic differences of sleep symptoms in an elderly multiethnic cohort: the Northern Manhattan Study. Neuroepidemiology. 2011;37:210–215. doi: 10.1159/000334315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafazand S., Wallace D.M., Vargas S.S., Del Toro Y., Dib S., Abreu A.R., Ramos A., Nolan B., Baldwin C.M., Fleming L. Sleep disordered breathing, insomnia symptoms, and sleep quality in a clinical cohort of U.S. Hispanics in south Florida. J Clin Sleep Med. 2012;8:507–514. doi: 10.5664/jcsm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redline S., Sotres-Alvarez D., Loredo J., Hall M., Patel S.R., Ramos A., Shah N., Ries A., Arens R., Barnhart J., Youngblood M., Zee P., Daviglus M.L. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The hispanic community health study/study of Latinos. Am J Respir Crit Care Med. 2014;189:335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.