Abstract
Objective
Dietary intake of fruit is associated with lower incidence of hypertension and cardiovascular risk. Papaya is a kind of delicious fruit and reported has dietary therapeutic effects, such as digestive stimulation and hypotensive efficacy. However, the mechanism of pawpaw involved have not been elucidated. Here, we illustrate that the effect of pawpaw on the gut microbiota and the prevention of cardiac remodeling.
Methods
Gut microbiome, cardiac structure/function, and blood pressure were examined in SHR and WKY groups. The intestinal barrier was tested with histopathologic; immunostaining and Western blot were used to measure the tight junction protein level; Gpr41 was tested by RT-PCR, and inflammatory factors were detected with ELISA.
Results
We observed a significant decrease in microbial richness, diversity, and evenness is the spontaneously hypertensive rat (SHR), in addition to an increased Firmicutes/Bacteroidetes (F/B) ratio. These changes were accompanied by decreased in acetate and butyrate-producing bacteria. Compared with SHR, treatment with pawpaw at the dosage of 10 g/kg for 12 weeks significantly reduced the blood pressure, cardiac fibrosis and cardiac hypertrophy, while the ratio of F/B decreased. We also found that the concentration of short-chain fatty acids (SCFAs) was increased in SHR fed with pawpaw compared with that in control group, while the gut barrier was restored and level of proinflammatory cytokines in the serum were decreased.
Conclusions
Pawpaw, rich of high fiber, led to changes in the gut microbiota that played a protective role in the development of cardiac remodeling. The potential mechanism of pawpaw may explained by the generation of one of the main metabolites of the gut microbiota, the short-chain fatty acid acetate, increasing tight junction protein level occluding to enhance the gut barrier for less releasing the inflammation cytokines, and upregulating G-protein-coupled receptor 41 (GPR41) to reduce blood pressure.
Keywords: Pawpaw fruit, Hypertension, Gut microbiota, Acetate
1. Introduction
Cardiac remodeling, primarily due to stress stimuli such as pressure overload (hypertension) and inflammation, has adverse effects on cardiac function and predisposes the heart to failure if left unresolved. Cardiac remodeling not only severely impairs cardiac function in patients with myocardial infarction, increases complication neuropathy, but also increases mortality [1,2]. Therefore, preventing cardiac remodeling would be beneficial for patients with chronic hypertension. Although dietary sodium content significantly contributes to hypertension [3], other dietary components, such as fiber, have been found to regulate cardiovascular risk factors [4]. Epidemiological studies have shown that a high dietary intake of fruits and vegetables is accompanied by a lower incidence of cardiac remodeling and hypertension. Such dietary factors have been reported to be responsible for specific changes in the gut microbiota [5]. Modifications in the pattern of gut microbiota have been related to high BP levles that exhibit certain models of hypertension, such as those observed in spontaneously hypertensive rats (SHR) [6]. The favorable effects of fiber may be explained by the generation and distribution of one of the primary metabolites of the gut microbiota, the short-chain fatty acid acetate. Short-chain fatty acids (SCFAs), microbial end-products from dietary fiber, including acetic, propionic, and butyric acids can affect the structure of the intestinal flora, promote the proliferation of beneficial bacteria, and inhibit the proliferation of pathogens from maintaining intestinal health [7,8].
Pawpaw (Carica papaya), a tree species belonging to Brassicales: Caricaceae, is widely cultivated in eastern Asia for its delicious fruit [3]. Pawpaw fruit contains two rows of almond-size seeds surrounded by yellow to orange-colored flesh, with skin ranging from green to yellow when ripe. The fruit has high dietary fiber, protein, carbohydrates, vitamins, and minerals. Pawpaw is endowed with the meaning of reproductive worship in the Classic of Poetry, the oldest collection of poems in China. For hundreds of years, pawpaw has been used as a Chinese medicinal herb treating digestive system diseases, including indigestion and gastric ulcer [9]. Because of the increase in its commercial importance, several studies into various aspects of pawpaw fruit's pharmacological activity have been carried out [10,11]. In late years, it was reported that the intake of pawpaw fruit had been associated with reducing of blood pressure, but the precise mechanism is still unknown [12].
Accordingly, in the present study, we aimed to investigate whether pawpaw, through changes in the gut microbiota prevents the development of cardiac remodeling and hypertension in spontaneous hypertensive rats (SHR). Our findings may provide new insights into the protective actions of pawpaw on cardiac remodeling induced by hypertension.
2. Materials and methods
2.1. Chemicals and reagents
Fresh pawpaw fruit (Carica papaya L.) was provided by Guangdong Pharmaceutical Co., Ltd. (Guangdong, China). Captopril tablets (12.5 mg) were purchased from Bristol-Myers Squibb Co., Ltd. (Shanghai, China).
Biochemical assays of lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase-MB (CK-MB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), Creatinine (Cr), blood urea nitrogen (BUN), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and macrophage chemoattractant protein-1 (MCP-1) were obtained from Nanjing Jian Cheng Biotech Co., Ltd. (Nanjing, China). A PowerFecal™ DNA Isolation Kit was obtained from Shenzhen Anbisheng Technology Co., Ltd. (Shenzhen, China).
2.2. Analysis of pawpaw composition
Fresh pawpaw fruit was squeezed by juicer without residue filtering and stored at −20 °C. The following nutritive components were analyzed from pawpaw fruit: crude protein (GB/T 5009.5–2016), carbohydrate (GB 5009.8–2016), dietary fiber (GB/T 5009.10–2003), crude fat (GB/T 5009.6–2016), ash (GB 5009.4–2016), and moisture content (GB 5009.3–2016).
2.3. Animal grouping and administration
Here, 15 male SHRs at the age of 12 weeks and 5 age- and sex-matched Wistar-Kyoto (WKY) rats (Vital River Laboratory Animal Technology Co., Ltd., Beijing, China; certificate number: SCXK2016-0011) were maintained in a temperature and humidity controlled room with a 12 h light-dark cycle. Rats were fed a standard diet with free access to drinking water. After adaptive feeding for 5 days, 15 SHRs were randomly allocated to 3 groups (n = 5), including SHR group, pawpaw fruit (PAW) group and captopril (CAP) group, with WKY groups used as a control. PAW (10 g/kg, body surface area normalization method was used to convert the dose of human to rat, processed with fresh pawpaw juice) and CAP (13.5 mg/kg, dissolved in distilled water) groups were administered intragastrically with corresponding administration for 12 consecutive weeks, while those in WKY and SHR groups were given distilled water with the same volume. Doses of medication were adjusted according to the changes in body weight monitored weekly.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured weekly with the tail-cuff method (ALC-NIBP, Shanghai Alcott Biotechnology Co., Ltd., Shanghai, China). Ejection fraction (EF) and fractional shortening (FS) were measured in M-Mode with the Color Doppler Ultrasonography Diagnosis with a 10-MHz probe (S40 Exp, Shenzhen City Science and Technology Co., Ltd., Shenzhen, China).
Studies were performed by the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 85–23, revised 1996) and with approval from the Animal Care Committee of Southern Medical University.
2.4. Blood sample analysis
After administration for 12 consecutive weeks, blood samples were collected. Serum was obtained by centrifugation of the blood samples at 3000 r/min for 10 min at 4 °C. The supernatant was collected and stored at −80 °C. Myocardial damage was evaluated by measuring the plasma concentration of lactate dehydrogenase (LDH), creatine kinase (CK), and creatine kinase isoenzyme (CK-MB). At the same time, liver and renal function were examined using alanine aminotransferase (ALT), glutamic-oxalacetic transaminase (AST), creatinine (Cr), and creatinine (BUN), respectively. Additionally, the level of vascular inflammation was measured by tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1).
2.5. Cardiac histological examination
After administration for 12 consecutive weeks, rats were euthanized and their hearts were removed and weighed for HW/BW index (ratio of heart weight to body weight). The remaining hearts in each group were excised, fixed in buffered 4% paraformaldehyde for 48 h. Heart tissues were then embedded in paraffin and sectioned into 5 μm slices for Masson staining to observe the distribution of collagen in heart. The levels of myocardial fibrosis were quantified in 10 random fields of view per section as collagen volume fraction (CVF) with an Olympus CX23 microscope ( × 40 magnification) and ImagePro software (Adept Electronic Solutions Pty Ltd., Moorabbin, Australia). Collagen levels were expressed as a percentage of the area of the region of interest.
2.6. Determination of wet weights, dry weights, water content and pH in feces
After 12 weeks, the excreted feces of individual rats were collected and went through metage for their wet and dry weight. Based on this, the water content of the fecal samples was calculated. Besides, pH of colonic content was measured using accurate pH test paper (value from 5.5 to 9.0) to examine the survival circumstance of gut microbiota. The contents were placed in a sterile tube, immediately frozen in liquid N2, and stored at −80 °C for microbiota analysis.
2.7. Gut microbiota analysis
Fecal samples from individual rats were collected sterilely from the colon during euthanasia, stored in liquid nitrogen, and then transferred to an −80 °C freezer until further analysis. According to the manufacturer's instructions, DNA was isolated from fecal samples with a PowerFecal™ DNA Isolation Kit. The V3–V4 region of the bacterial 16 S rRNA was sequenced with the 341 F and 806 R primers in an Illumina MiSeq sequencer (300 bp paired-end reads). The raw mate-paired fastq files were merged and quality-filtered using Geneious 8.0.5 (Biomatters, Auckland, New Zealand) with an error probability limit of 0.01. Reads were assembled with PEAR (version 0.9.5). Lastly, sequences were analyzed with Quantitative Insights Into Microbial Ecology (1.9.1).
2.8. Quantification of SCFAs in cecal content
500 mg of the cecal content sample was mixed with 1000 μL of distilled water and adjusted to pH 2.0 using 50% sulfuric acid. The sample was centrifuged at 5000 g for 10 min. A total of 2 mL of ether was added to the supernatant to extract SCFAs and then analyzed by HPLC (FL220-2, DL39-FL220-2, Beijing, China) with a diode array detector set at 210 nm. Chromatographic separation of each acid was carried out on a C18 column (4.6 × 250 mm, 5 μm) at 30 °C. The mobile phase consisting of phosphate buffer solution (A) and acetonitrile solution (B) was as follows: 0–2 min, 100% A; 2–5 min, 90% A; 5–20 min, 50% A; and 20–25 min, 0% A. Acetic was obtained from Solarbio Science Technology Co.
2.9. Intestinal histopathologic analysis
The ileum 5 cm above the cecum was separated directly after the rats were sacrificed and fixed with 4% formaldehyde. Paraffin-embedded samples were sliced and stained to examine intestinal morphologic changes using a hematoxylin-eosin (H&E) stain.
For a detailed histological analysis, colonic sections of each animal were scored in a blinded fashion as follows: (a) Epithelial damage: 0 = none; 1 = minimal loss of goblet cells; 2 = extensive loss of goblet cells; 3 = minimal loss of crypts; 4 = extensive loss of crypts; (b) Infiltration: 0 = none; 1 = infiltrate around the crypt basis; 2 = infiltrate reaching the muscularis mucosae; 3 = extensive infiltration reaching the muscularis mucosae and thickening of the mucosa with edema; 4 = infiltration of the submucosa. The total histological score was the sum of the epithelium damage and infiltration score.
2.10. Immunostaining for tight junction protein
Immunohistochemical (IHC) reaction was used to visualize occludin as an intracellular tight junction protein. The deparaffinized sections were irradiated in a microwave oven in citrate buffer (pH 6.0) twice and at 700 W for epitope retrieval. After cooling and washing in PBS, the endogenous peroxidase was blocked, and sections were incubated with primary rabbit polyclonal anti-occludin antibody (Santa Cruz Biotechnology Inc., sc-5562). Then, tissue sections were stained using an avid inbiotin-peroxidase system with diaminobenzidine as the chromogen (HRP; Rabbit/Mouse/Goat-DAB+) in conformity with staining procedure instructions included in Dako LSAB + System-HRP. The sections were washed in distilled water and counterstained with hematoxylin. Positive staining was examined by visual identification of brown pigmentation under a microscope and measured by Image J software. The primary rabbit polyclonal anti-occludin antibody was bought from Abcam (ab31721).
2.11. Western blot analysis
The cardiac tissues were homogenized on ice in modified radioimmunoprecipitation assay lysis buffer containing 25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% NP-40, 0.1% sodium dodecyl sulfate, 1 mM phenylmethylsulfonyl fluoride and Protease Inhibitor Cocktail (Sigma-Aldrich, St louis, MO). Protein concentration in the tissue homogenates was determined by a bovine serum albumin assay kit (Pierce Rockford, IL), and 50 μg of total protein from each sample was fractionated on 4%–12% Bis-Tris gradient gel (Invitrogen, Carlsbad, CA) at 150 V for 2 h and transferred to a nitrocellulose membrane. The membrane was then incubated with rabbit anti-occludin at 1:500 dilutions (Abcam) and anti-GAPDH antibodies (Cell Signaling Technology) at 1:4000 dilution overnight. The appropriate horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich) were used at a 1:4000 dilution. The membrane was visualized with the FluorChem R system (ProteinSimple) and measured by Image J software.
2.12. RNA isolation and real-time quantitative RT-PCR
Total RNA was extracted from major artery using Trizolreagent according to the manufacturer's instructions. Theconcentration of RNA was quantified by a NanoDrop® ND-2000c UV–vis spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Total RNA from each sample was reverse transcribed with the Superscript First-Stand cDNA Synthesis Kit (Invitrogen CA, USA). The quantitative RT-PCR was performed with SYBR Green I on an ABI Prism 7300 sequence detection system (Applied Biosystem, Foster City, CA, USA). GAPDH was used as the internal control. The relative mRNA level of each sample was analyzed and calculated using the 2−ΔΔCt method. The specific primer sequences were as follows:
GPR41:
Forward primer: 5′-CCACCCCCATCTGACTATGC-3′,
Reverse primer: 5′-TCGCTTGCCATTCACTTTGC-3’.
GAPDH:
Forward primer: 5′-GATGGTGATGGGTTTCCCGT-3′,
Reverse primer: 5′-CGGACATTCCTTGGATGGCT-3’.
2.13. Statistical analysis
Results were presented as mean ± standard deviation (SD). Statistical analysis was carried out using one-way analysis of variance (ANOVA) and Dunnett's test. Statistical significance was considered to be achieved when the P values were smaller than 0.05. Statistical analysis was performed using the GraphPad Prism software (Version 5.01) for Windows.
3. Results
3.1. Effects of pawpaw on blood pressure and cardiac function
Time-related changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) for the four groups are shown in Fig. 1A and B. After administration for 12 consecutive weeks, SBP (186.24 ± 6.56 mmHg) and DBP (139.5 ± 4.91 mmHg) in the SHR group were significantly higher than that in the WKY group (119.93 ± 7.16, P < 0.001; 96.96 ± 6.44 mmHg, P < 0.001, respectively) (Fig. 1A–B). With the treatment of pawpaw, SBP (150.08 ± 15.73 mmHg, P < 0.001) and DBP (116.33 ± 7.03 mmHg, P < 0.001) were decreased at the end of experiment, but the effects were not as pronounced as in the CAP group (128.07 ± 5.23 and 101.61 ± 7.33 mmHg, respectively).
Fig. 1.
Effects of pawpaw treatment on blood pressure and cardiac function during administration for 12 consecutive weeks. (A) SBP, (B) DBP, (C) EF%, (D) FS%, and (E) Image of echocardiogram. N = 5 per group. ###, P < 0.001 vs. WKY. ##, P < 0.01 vs. WKY. #, P < 0.05 vs. WKY. ***, P < 0.001 vs. SHR. **, P < 0.01 vs. SHR. *, P < 0.05 vs. SHR.
Ejection fraction (EF) and fractional shortening (FS) were used to evaluate the damage to cardiac function, while the decrease of EF and FS suggests cardiac insufficiency [13]. As shown in Fig. 1C–D, EF (64 ± 1.87%, P < 0.001) and FS (36 ± 2.35%, P < 0.001) in SHR became significantly lower than those in WKY (80.2 ± 2.05% and 55.4 ± 1.14%, respectively), while pawpaw and captopril treatment increased EF and FS obviously (PAW: 71 ± 2.74% and 42.8 ± 1.48, P < 0.001; CAP: 75 ± 2% and 48.8 ± 1.3%, P < 0.001). Pictures of the M-mode echocardiogram are shown in Fig. 1E.
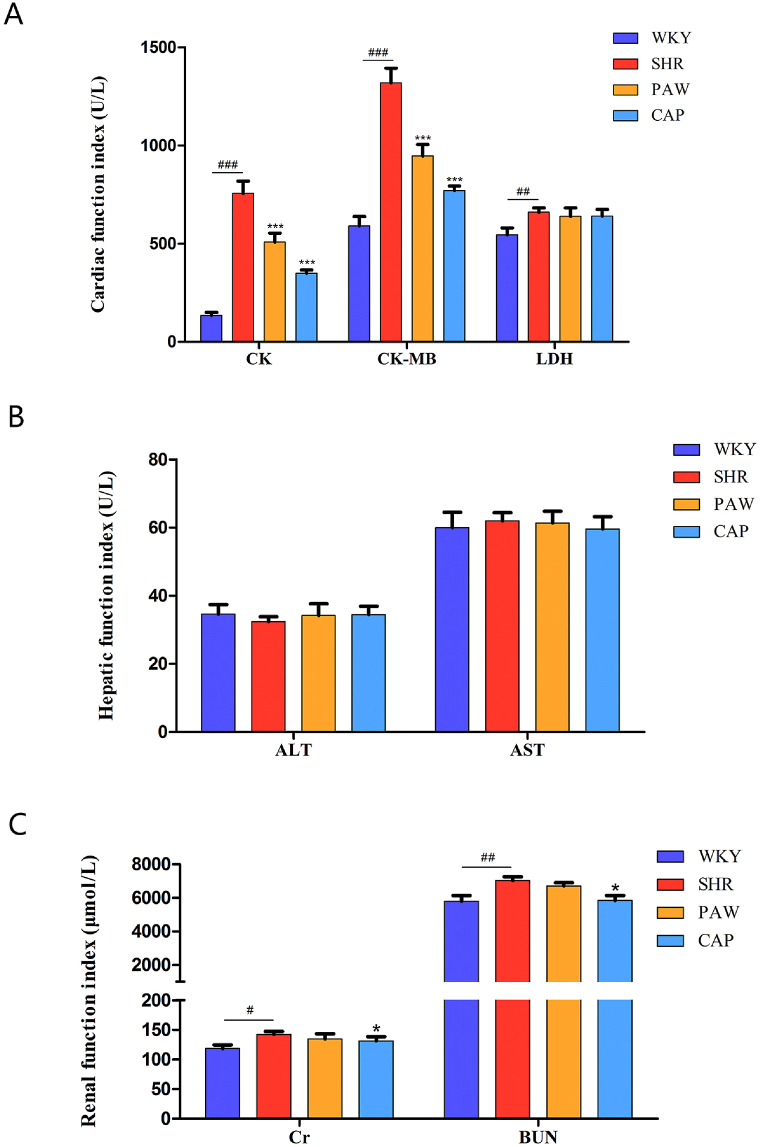
3.2. Effects of pawpaw treatment on cardiac, hepatic and renal function
In order to evaluate the protection of pawpaw on the heart and its safety for the liver and kidneys, myocardial zymogram (CK, CK-MB, and LDH), hepatic function indexes (ALT, AST, and ALP), and renal function indexes (Cr and BUN) were tested in the study. As shown in Fig. 2A–C, the serum concentration of CK (0.69 ± 0.06 U/mL, P < 0.001), CK-MB (35.04 ± 2.5 U/mL, P < 0.001) increased obviously in SHR compared with WKY (0.49 ± 0.07 and 24.89 ± 2.79 U/mL, respectively), indicating that hypertension led to cardiac structural damage. In contrast, pawpaw improved cardiac function compared with the SHR group, as evidenced by the reduction of serum CK (0.58 ± 0.06 U/mL, P < 0.001) and CK-MB (29.29 ± 2.82 U/mL, P < 0.001), while the indexes of hepatic adrenal function had no significant changes.
Fig. 2.
Effect of pawpaw treatment on serum biochemical index at 12 weeks. (A) Cardiac function index including CK, CK-MB and LDH; (B) Hepatic function index including AST and ALT; (C) Renal function index including Cr and BUN. ###, P < 0.001 vs. WKY. ##, P < 0.01 vs. WKY. #, P < 0.05 vs. WKY. ***, P < 0.001 vs. SHR. **, P < 0.01 vs. SHR. *, P < 0.05 vs. SHR.
3.3. Effects of pawpaw treatment on cardiac remodeling
Consistent with the changes in blood pressure, significant aggravation of cardiac remodeling on histological analysis was observed in SHR compared with WKY (Fig. 3A), as evidenced by the increase in the ratio of HW/BW (SHR versus WKY, 4.83 ± 0.32 versus 3.11 ± 0.27 g/kg, P < 0.001) (Fig. 3C). The treatment of pawpaw prevented cardiac remodeling and decreased HW/BW compared with SHR (4.13 ± 0.34 g/kg, P < 0.05).
Fig. 3.
Effects of pawpaw treatment on cardiac remodeling. (A) Masson staining images without magnification; (B) Masson staining images under 40 × magnification and the areas of collagen deposition were indicated by the black arrow; (C) HW/BW; (D) CVF. ###, P < 0.001 vs. WKY. ##, P < 0.01 vs. WKY. #, P < 0.05 vs. WKY. ***, P < 0.001 vs. SHR. **, P < 0.01 vs. SHR. *, P < 0.05 vs. SHR.
Based on Masson's trichrome stained sections (Fig. 3B), we found that the degree of cardiac fibrosis (collagen staining in blue color) in the PAW group to be significantly alleviated when compared with the SHR group (40 × magnification), which was further supported by the decreased levels of CVF (PAW versus SHR, 4.42 ± 0.86 versus 8.92 ± 0.3%, P < 0.001) (Fig. 3D).
3.4. Nutritional component of pawpaw and property of fecal
As shown in Table 1, the nutritional components of pawpaw were analyzed. Generally, pawpaw fruit mainly comprises a carbohydrate, crude protein, and crude fat. Notability, dietary fiber in pawpaw is more prosperous than grains like rice and wheat [14], which may lead to a high concentration of SCFAs in feces.
Table 1.
Nutritive components of pawpaw fruit.
| Nutritional component | Content (g/100 g) |
|---|---|
| Crude protein | 14.72 ± 0.86 |
| Dietary fiber | 4.52 ± 0.51 |
| Carbohydrate | 62.15 ± 1.16 |
| Ash | 2.81 ± 0.19 |
| Crude fat | 10.12 ± 0.62 |
| Moisture | 4.83 ± 0.24 |
Data are presented as the mean ± SD (n = 3).
The wet and dry weights and water content of feces are shown in Table 2. Our results showed that the pawpaw significantly increased the wet weight of feces than the SHR group (PAW versus SHR, 1.9 ± 0.16 versus 1.24 ± 0.15 g, P < 0.001). The water content of feces in the SHR group (0.28 ± 0.04%) was less than that of the WKY group (0.16 ± 0.03%, P < 0.05), whereas that was higher in the PAW group (0.28 ± 0.04%, P < 0.001) than in the SHR group. In general, the low water content of feces may induce constipation and affect intestinal absorption. In addition, no significant difference in pH value was found between PAW and other groups (Table 2).
Table 2.
Effects of pawpaw on water content and pH value in feces after 12 weeks.
| Group | Wet weights of feces (g) | Dry weights of feces (g) | Water content in feces (%) | pH |
|---|---|---|---|---|
| WKY | 1.58 ± 0.16 | 1.33 ± 0.12 | 0.16 ± 0.03 | 6.68 ± 0.46 |
| SHR | 1.24 ± 0.15## | 1.10 ± 0.15# | 0.11 ± 0.02# | 6.76 ± 0.28 |
| PAW | 1.9 ± 0.16*** | 1.36 ± 0.11* | 0.28 ± 0.04*** | 6.38 ± 0.28 |
| CAP | 1.35 ± 0.14 | 1.10 ± 0.13 | 0.18 ± 0.03** | 6.54 ± 0.26 |
Data are presented as the mean ± SD (n = 10). ###, P < 0.001 vs. WKY. ##, P < 0.01 vs. WKY. #, P < 0.05 vs. WKY. ***, P < 0.001 vs. SHR. **, P < 0.01 vs. SHR. *, P < 0.05 vs. SHR.
3.5. Overall structural changes in cecal microbiota composition
We investigated whether the treatment of pawpaw can change the gut microbiota composition compared with SHR by sequencing the 16 S bacterial gene. First, we examine the changes in the composition of the gut microbiota community between groups using beta-diversity and alpha-diversity metrics. Beta diversity evaluates the diversity in the microbial community between samples, while alpha diversity reflects species richness in given samples. As shown in Fig. 4A, WKY, PAW, and CAP groups had different gut microbiota compositions from SHR to beta diversity. We also determined alpha diversity which in PAW was higher than SHR group (Fig. 4B), indicating that PAW treatment increased phylogenetic diversity of gut bacteria. Linear discriminant analysis effect Size (LEfSe) revealed that 4 features, including Bacteroides, Bacteroidaceae, Terrisporobacter, and Peptostreptococcaceae with significant increases were found after administration of pawpaw after 12 weeks (Fig. 4C–D).
Fig. 4.
Comparison of gut microbiota structure in rats (A) Weighted UniFrac-based PCoA analysis of gut microbiota (beta diversity) via 16 S rRNA sequencing; (B) The phylogenetic diversity evaluated by PD whole tree (alpha diversity); (C) Cladogram of LDA Effect Size on gut microbiota; (D) Bar diagram of LDA Effect Size on gut microbiota; (E) Percentage of total bacteria presented at the phyla level; (F) Ratio of Firmicutes bacteria to Bacteroidetes bacteria. ###, P < 0.001 vs. WKY. ##, P < 0.01 vs. WKY. #, P < 0.05 vs. WKY. ***, P < 0.001 vs. SHR. **, P < 0.01 vs. SHR. *, P < 0.05 vs. SHR.
With a percentage of total bacteria presented at the phyla level (Fig. 4E), we found more Firmicutes bacteria and fewer Bacteroidetes bacteria in SHR. At the same time, pawpaw treatment reduced the number of Firmicutes bacteria and increased the number of Bacteroidetes bacteria, which was proved by the absolute quantity of bacteria. We then calculated the ratio between Firmicutes and Bacteroidetes, which is a widely used marker of gut dysbiosis and significantly lower in the PAW group (1.71 ± 0.01) compared with the SHR group (37.79 ± 2.88, P < 0.001) (Fig. 4F). Using PICRUSt predictions [15], the KEGG pathways including tight junction and butanoate metabolism showed significant differences between PAW and SHR group, which indicated that the influence of pawpaw on gut microbiota might be related to the intestinal epithelial tight junction and butanoate metabolism (Fig. 5A–E).
Fig. 5.
The effect of pawpaw treatment on microflora function and dominant microbiota. PICRUSt predictions to show the functional composition of gut microbiome between PAW and SHR groups (A). Abundance of (B) Bacteroides, (C) Bacteroidaceae, (D) Peptostreptococcaceae and (E) Terrisporobacter in gut microbiota.
3.6. Colon permeability evaluation
Due to the analysis of PICRUSt predictions, we further evaluate the gut barrier alterations. Histological measurement of ileal morphology revealed significant effects of pawpaw on the intestinal compared with the SHR group (Fig. 6A). The microvilli became rough, dull, and irregularly arranged. The epithelial cells of the intestinal villus were partly shed in the SHR group compared to the PAW group. Conducted by immunohistochemistry, the expression of occludin (tight junction protein as the intestinal permeability marker) in the gut barrier was also decreased in the SHR group (1.83 ± 0.48) and returned in the PAW group (2.99 ± 0.38, P < 0.01) (Fig. 6B–D), which was further confirmed by Western Blot (Fig. 6E–F).
Fig. 6.
The effect of pawpaw treatment on intestinal barrier and proinflammatory cytokines. (A) Histopathological examination of rats' distal ileum (H&E stain × 100). (B) Immunohistochemical stain of tight junction protein occludin ( × 100). (C) Histological scores of colon sections in rats. (D) The mean density of occludin immunostaining in rats. (E), (F) The relative expression of occludin protein in rat colon. And the level of (G) IL-6; (H) TNF-α and (G) MCP-1 in serum. ###, P < 0.001 vs. WKY. ##, P < 0.01 vs. WKY. #, P < 0.05 vs. WKY. ***, P < 0.001 vs. SHR. **, P < 0.01 vs. SHR. *, P < 0.05 vs. SHR.
3.7. Effects of pawpaw treatment on vascular inflammation
The integrity of the gut barrier is closely linked to the release of proinflammatory cytokines into circulation. The concentration of proinflammatory cytokines in serum was measured by biochemical kits after 12 weeks of treatment with pawpaw. As a result, there were significantly increased expressions of IL-6, TNF-α, and MCP-1 in the SHR group compared with the WKY group, but the upregulated expressions were less pronounced in the PAW group (Fig. 6G–I).
3.8. Effects of pawpaw treatment on concentrations of SCFAs and expression of GPR41
Fermentation of undigested proteinaceous material can produce putrefactive metabolites such as SCFAs, including acetate, propionate, and butyrate (butanoate) [16]. The concentrations of acetate, propionate, and butyrate in cecal content were all significantly decreased in the SHR group compared with WKY group, which was recovered in the PAW and the CAP groups (Fig. 7A–C). With SCFAs changes, GPR41 mRNA expression in significant arteries was dramatically increased in the PAW group (13.46 ± 2.57) compared with that in the SHR group (1.24 ± 0.23, P < 0.001) (Fig. 7D).
Fig. 7.
The effect of pawpaw treatment on SCFAs and it's receptor, GPR41. The concentrations of (A) acetate, (B) propionate, (C)butyrate in cecal contents. (D) mRNA expression of GPR41 in major artery. ###, P < 0.001 vs. WKY. ##, P < 0.01 vs. WKY. #, P < 0.05 vs. WKY. ***, P < 0.001 vs. SHR. **, P < 0.01 vs. SHR. *, P < 0.05 vs. SHR.
4. Discussion
In this study, we determined for the first time that as a dietary fiber intake, pawpaw could prevent adverse ventricular remodeling in SHR. Furthermore, this potential cardioprotective effect could be associated with changing the gut microbiota. The pawpaw fruit is highly nutritious, containing carbohydrates, crude protein, and considerable dietary fiber (Table 1). Since a low-fiber diet is associated with inflammation and high risk of cardiovascular diseases (CVD), we detected inflammatory factors in SHR. In results, our data demonstrated that oral administration of pawpaw could attenuate pressure overload-induced cardiac remodeling and decreased inflammation factors serum level, and also decreased the ratio between bacteria of the Firmicutes and Bacteroidetes, thus improving gut dysbiosis, and decreased the prevalence of SCFAs producing bacteria such as Bacteroides and Bacteroidaceae. A marked improvement in the gut barrier accompanied these changes.
Cardiac remodeling, characterized by cardiac hypertrophy and fibrosis, is one of the most common complications of hypertension, and has been found to be an independent determinant of prognosis in hypertension [17]. Clinical studies showed that hypertension is a lower-grade chronic inflammatory disease, while inflammtion is also a critical pathologic feature in cardiac remodeling induced by hypertension. In the present study, the spontaneously hypertensive rat model (SHR) was used as an animal model of hypertension-induced cardiac remodeling. SHR develops hypertension around 12–16 weeks of age, reaching systolic pressures between 180 and 200 mmHg. Starting around 25 weeks, SHR develops notable characteristics of cardiovascular disease, such as cardiac remodeling [18]. SHR also showed the existence of gut dysbiosis in hypertension supported by a decrease in microbial richness and a marked increase in the ratio of Firmicutes and Bacteroidetes bacteria (ratio of F/B) [6], which is appropriate for this study. Besides, due to the effective treatment of cardiac remodeling induced by hypertension in the clinic, which are the expected effects of pawpaw, captopril was chosen as a positive control in this study [19].
In the clinical examination of cardiac remodeling, ejection fraction (EF), and fractional shortening (FS) are sensitive indexes used to evaluate the impairment of cardiac function. The decreases of EF and FS suggest cardiac insufficiency [13]. As the results are shown in Fig. 1, pawpaw could lower blood pressure and maintain cardiac function with increased EF and FS, which were impaired in the SHR group. Furthermore, the treatment of pawpaw reversed histopathological heart damage accompanied by a decrease in collagen volume fraction and cardiac function indexes (Fig. 3), supporting the prevention of cardiac remodeling without renal and hepatic injuries (Fig. 2).
A previous study shows that a high dietary fruit intake is accompanied by a lower incidence of cardiac remodeling and hypertension [20,21]. Such dietary factors have been reported to be responsible for specific changes in the gut microbiota. The gut microbiota is a diverse bacterial ecosystem consisting of trillions of microorganisms, and 80% of the identified gut microbiota can be classified into three dominant phyla: Bacteroidetes, Firmicutes, and Actinobacteria [14]. An imbalance in gut microbiota is characterized by the changes in the ratio of Firmicutes (F) to Bacteroidetes (B), known as the F/B ratio, which can be used as a biomarker for pathological conditions [16,22]. Previous studies have shown that the ratio of F/B is relatively higher in hypertensive individuals than in healthy individuals, and gut microbial dysbiosis has been connected to cardiac inflammation, myocardial fibrosis, and systolic dysfunction as the pathological characteristics of cardiac remodeling [23]. In the present study, the ratio of F/B in the colonic digesta and blood pressure of the PAW group was markedly lower than that of the SHR group (Fig. 4F). Thus, the blood pressure regulation mechanism involved in taking pawpaw may be related to the adjustment in gut microbiota.
Linear discriminant analysis effect Size (LEfSe) was used to identify the specific phylotypes responding to different administrations (Fig. 4D). This analysis revealed that 4 features, including Bacteroides, Bacteroidaceae, Terrisporobacter, and Peptostreptococcaceae were found markedly increasing with the administration of pawpaw after 12 weeks (Fig. 4C–D). Among these microbiotas, Bacteroides, and Bacteroidaceae are associated with intestinal permeability and tight junction expression [24,25]. Treatment with Bacteroides can restore the intestinal permeability defects in a mouse model of Autism spectrum disorder [26]. Moreover, a study outlined the characteristics of intra-abdominal hypertension-induced barrier changes, including the decrease of Peptostreptococcaceae, which indicates that intestinal barriers might be treated to alleviate intra-abdominal hypertension, and the microbiota may be a relevant therapeutic target [27].
Accompanied by the analysis of PICRUSt predictions, the influence of pawpaw on gut microbiota may be related to the intestinal epithelial tight junction. The gut barrier limits intestinal bacteria, and toxic mediators escaping from the gut, thus avoiding a systemic inflammatory response [28,29]. Gut barrier breakdown increases intestinal permeability, bacteria and endotoxin translocation into the systemic circulation, and immune-inflammatory system activation, including IL-6, TNA-α, and MCP-1, are the main participants in hypertension and cardiac remodeling [[30], [31], [32]]. However, when in inflammatory conditions such as hypertension, inflammatory bowel disease, diabetes, and chronic kidney disease, the intestinal tight junction barrier is impaired allowing endotoxin and other pro-inflammatory products into the underlying intestinal tissue and even into the blood and affecting disease inflammation [[33], [34], [35]]. Our study showed that the intestinal barrier of the SHR group was destroyed. Increased dysplasia and loose cellular arrangements and thickening of the mucosa with edema were found in colon epithelial cells in the SHR group compared to the WKY group, while high-level IL-6, TNA-α, and MCP-1 were tested in serum. After treatment of pawpaw and catropil, the intestinal barrier was restored, which wascharacterized by less abscission of epithelial cells and regularly arranged microvilli (Fig. 6B). At the same time, the level of IL-6, TNA-α, and MCP-1 decreased. Moreover, we detected the intestinal barrier marker, tight junction protein occluding, which increased expression in the pawpaw and captopril groups compared with the SHR group. These suggested that pawpaw could reduce inflammation cytokine release by increasing tight junction protein occluding level and restoring the intestinal barrier, attributed to the rich of Bacteroides, Bacteroidaceae, and Peptostreptococcaceae in the gut with pawpaw diet.
High fiber and SCFA supplementation exerted favorable actions on the heart of SHR. Specifically, there was a significant reduction in heart weight, cardiac hypertrophy and cardiac fibrosis. Our study supports that gut microbiota dysbiosis is associated with the development of cardiac remodeling. Bacteroides and Bacteroidanceae can primarily produce SCFAs from the fermentation of dietary fiber in the gut [36,37]. SCFAs were confirmed to affect the structure of the gut microbiota, promote the proliferation of beneficial bacteria, and inhibit the proliferation of pathogens from maintaining intestinal health [38]. Our data have shown that pawpaw intake significantly increased the abundance of the bacteria Bacteroides and Bacteroidaceae. A previous study found that acetate and propionate significantly affect hypotension, due to the stimulation of G-protein-coupled receptor 41 (GPR41). GPR41 can decrease blood pressure when activated by acetate and propionate [39]. Our findings showed that pawpaw led to an increase of SCFAs including acetate and propionic in cecal contents. The mRNA expression of GPR41 in the major artery was significantly elevated in the PAW group than in the SHR group, which indicated that the hypotensive effect of pawpaw might be partially related to the stimulation of GPR41 induced by acetate and propionate. It was recommended that pawpaw intake could decrease BP and cardiac remodeling through increasing SCFAs’ level and the expression of GPR41 partly due to enriching Bacteroides and Bacteroidaceae in the intestine.
Besides, butyrate was reported to protect and strengthen intestinal barrier integrity [40]. The mechanisms by which butyrate protects against intestinal barrier dysfunction are complex and not fully clarified, which may involved restoring of intestinal tight junction protein and surpressing inflammation [41]. Accompanied by the analysis of PICRUSt predictions, butyrate metabolism was enhanced in the PAW group. As validation, in this study, butyrate was increased in the treatment of pawpaw for its considerable dietary fiber content. Accordingly, as described previously, the expression of tight junction protein occludin, gut barrier, and vascular inflammation was restored.
Pawpaw is generally employed as a dietary intervention in the form of herbal tea, juice, or even eaten raw, which is cost-effective, practical and convenient for people [42]. Such dietary interventions involving fiber supplementation could be used to prevent hypertension, and cardiac remodeling, and future research will excavate the ability of dietary intervention not only to prevent but also to treat illness [43,44]. It was evident that constructing a credible and public health point of view, such a study is promising because of implications for human diets and the relatively mild, economic, and nonpharmacological property of such treatments.
In conclusion, the results of our study showed that pawpaw attenuates the development of cardiac remodeling induced by hypertension in SHR through changes in the gut microbiota. The potential mechanism linked to improving intestinal permeability and increase of SCFAs and reducing proinflammatory cytokines. Thus, dietary strategies involving fiber supplementation, such as pawpaw, could prevent hypertension and ameliorate cardiac remodeling.
Author contribution statement
Lin LV, Guohua Zhang: Conceived and designed the experiments.
Kai Chen: Performed the experiments; Wrote the paper.
Yiqing Guan: Analyzed and interpreted the data.
Shaoyu Wu, Yu Huang, Dongling Quan: Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
Funding
This work was supported by the Natural Science Foundation of China (Project NO 82274292) and the Natural Science Foundation of Guangdong Province, China (Project NO 2021A1515011674, and G821291026).
Acknowledgement
We would like to thank all the authors of this study for their exceptional cooperation and valuable contributions. Moreover, we thank the School of Pharmaceutical Science, Southern Medical University, Guangzhou, Guangdong Province, China, for providing facilities for the present work.
Contributor Information
Lin Lv, Email: lynnlv@smu.edu.cn.
Guohua Zhang, Email: zghgz@163.com, zghlynn@smu.edu.cn.
References
- 1.Tomek J., Bub G. Hypertension-induced remodeling: on the interactions of cardiac risk factors. J. Physiol. 2017;595(12):4027–4036. doi: 10.1113/JP273043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drazner M.H. The progression of hypertensive heart disease. Circulation. 2011;123(3):327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 3.O Donnell M., Mente A., Yusuf S. Sodium intake and cardiovascular health. Circ. Res. 2015;116(6):1046–1057. doi: 10.1161/CIRCRESAHA.116.303771. [DOI] [PubMed] [Google Scholar]
- 4.Shen T.D. Diet and gut microbiota in health and disease. Nestle Nutr Inst Workshop Ser. 2017;88:117–126. doi: 10.1159/000455220. [DOI] [PubMed] [Google Scholar]
- 5.Aleixandre A., Miguel M. Dietary fiber and blood pressure control. Food Funct. 2016;7(4):1864–1871. doi: 10.1039/c5fo00950b. [DOI] [PubMed] [Google Scholar]
- 6.Yang T., Santisteban M.M., Rodriguez V., et al. Gut dysbiosis is linked to hypertension novelty and significance. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyamoto J., Kasubuchi M., Nakajima A., et al. The role of short-chain fatty acid on blood pressure regulation. Curr. Opin. Nephrol. Hypertens. 2016;25(5):379–383. doi: 10.1097/MNH.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 8.Tan J., Mckenzie C., Potamitis M., et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 9.Peterson K.P.D.L. The pawpaw regional variety trial. HortTechnology. 1999;3(13):412–417. [Google Scholar]
- 10.Pandey S., Cabot P.J., Shaw P.N., et al. Anti-inflammatory and immunomodulatory properties of Carica papaya. J. Immunot. 2016;13(4):590–602. doi: 10.3109/1547691X.2016.1149528. [DOI] [PubMed] [Google Scholar]
- 11.Ezike A.C., Akah P.A., Okoli C.O., et al. Carica papaya (Paw-Paw) unripe fruit may benefit ulcer. J. Med. Food. 2009;12(6):1268–1273. doi: 10.1089/jmf.2008.0197. [DOI] [PubMed] [Google Scholar]
- 12.Eno A.E., Owo O.I., Itam E.H., et al. Blood pressure depression by the fruit juice of Carica papaya (L.) in renal and DOCA-induced hypertension in the rat. Phytother Res. 2000;14(4):235–239. doi: 10.1002/1099-1573(200006)14:4<235::aid-ptr574>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Yancy Cw, J M B B ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J. Card. Fail. 2017;8(Aug 23):628–651. doi: 10.1016/j.cardfail.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Lay C., Sutren M., Rochet V., et al. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ. Microbiol. 2005;7(7):933–946. doi: 10.1111/j.1462-2920.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson T.J., Huws S.A., Edwards J.E., et al. CowPI: a rumen microbiome focussed version of the PICRUSt functional inference software. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koliada A., Syzenko G., Moseiko V., et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1) doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medvedev N.V., Gorshunova N.K. Age-related sarcopenia as the risk factor of development of myocardial dysfunction and chronic heart failure in elderly patients with arterial hypertension. Adv. Gerontol. 2012;25(3):456–460. [PubMed] [Google Scholar]
- 18.Conrad C.H., Brooks W.W., Hayes J.A., et al. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995;91(1):161–170. doi: 10.1161/01.cir.91.1.161. [DOI] [PubMed] [Google Scholar]
- 19.Hansson L., Lindholm L.H., Niskanen L., et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353(9153):611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 20.Marques F.Z., Nelson E., Chu P.Y., et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 21.Zeng H., Huang C., Lin S., et al. Lotus seed resistant starch regulates gut microbiota and increases short-chain fatty acids production and mineral absorption in mice. J. Agric. Food Chem. 2017;65(42):9217–9225. doi: 10.1021/acs.jafc.7b02860. [DOI] [PubMed] [Google Scholar]
- 22.Mariat D., Firmesse O., Levenez F., et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang T., Santisteban M.M., Rodriguez V., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cani P.D., Bibiloni R., Knauf C., et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 25.Mokkala K., Röytiö H., Munukka E., et al. Gut microbiota richness and composition and dietary intake of overweight pregnant women are related to serum zonulin concentration, a marker for intestinal permeability. J. Nutr. 2016;146(9):1694–1700. doi: 10.3945/jn.116.235358. [DOI] [PubMed] [Google Scholar]
- 26.Coretti L., Cristiano C., Florio E., et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep. 2017;7(1) doi: 10.1038/srep45356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leng Y., Yi M., Fan J., et al. Effects of acute intra-abdominal hypertension on multiple intestinal barrier functions in rats. Sci. Rep. 2016;6(1) doi: 10.1038/srep22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastorelli L., De Salvo C., Mercado J.R., et al. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiljar M., Merkler D., Trajkovski M. The immune system bridges the gut microbiota with systemic energy homeostasis: focus on TLRs, mucosal barrier, and SCFAs. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuda S., Umemoto S., Yoshimura K., et al. Angiotensin activates MCP-1 and induces cardiac hypertrophy and dysfunction via toll-like receptor 4. J. Atherosclerosis Thromb. 2015;22(8):833–844. doi: 10.5551/jat.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L., Cheng G., Jin R., et al. Deletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunction. Circ. Res. 2016;118(12):1918–1929. doi: 10.1161/CIRCRESAHA.116.308688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S.J., Kim W.J., Moon S.K. TNF-alpha regulates vascular smooth muscle cell responses in genetic hypertension. Int. Immunopharm. 2009;9(7–8):837–843. doi: 10.1016/j.intimp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Kuwahara F., Kai H., Tokuda K., et al. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43(4):739–745. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 34.Mcmaster W.G., Kirabo A., Madhur M.S., et al. inflammation, immunity, and hypertensive end-organ damage. Circ. Res. 2015;116(6):1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Iturbe B., Pons H., Johnson R.J. Role of the immune system in hypertension. Physiol. Rev. 2017;97(3):1127–1164. doi: 10.1152/physrev.00031.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Navarro T., Salazar N., Gutiérrez-Díaz I., et al. Bioactive compounds from regular diet and faecal microbial metabolites. Eur. J. Nutr. 2018;57(2):487–497. doi: 10.1007/s00394-016-1332-8. [DOI] [PubMed] [Google Scholar]
- 37.Liu B., Wang W., Zhu X., et al. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priyadarshini M., Kotlo K.U., Dudeja P.K., et al. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr. Physiol. 2018;8(3):1091–1115. doi: 10.1002/cphy.c170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microb. 2014;5(2):202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geirnaert A., Calatayud M., Grootaert C., et al. Butyrate-producing bacteria supplemented in vitro to Crohn's disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng Y., Wang Y., Wang P., et al. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell. Physiol. Biochem. 2018;49(1):190–205. doi: 10.1159/000492853. [DOI] [PubMed] [Google Scholar]
- 42.Templeton S.B., Marlette M., Pomper K.W., et al. Favorable taste ratings for several pawpaw products. HortTechnology. 2003;13(3):445–448. [Google Scholar]
- 43.Han F., Wang Y., Han Y., et al. Effects of whole-grain rice and wheat on composition of gut microbiota and short-chain fatty acids in rats. J. Agric. Food Chem. 2018;66(25):6326–6335. doi: 10.1021/acs.jafc.8b01891. [DOI] [PubMed] [Google Scholar]
- 44.Siva N., Johnson C.R., Richard V., et al. Lentil (lens culinaris medikus) diet affects the gut microbiome and obesity markers in rat. J. Agric. Food Chem. 2018;66(33):8805–8813. doi: 10.1021/acs.jafc.8b03254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.