Abstract
Background
Parthenocarpy, or fruit development in the absence of fertilization, has been genetically engineered in eggplant and in other horticultural species by using the DefH9-iaaM gene. The iaaM gene codes for tryptophan monoxygenase and confers auxin synthesis, while the DefH9 controlling regions drive expression of the gene specifically in the ovules and placenta. A previous greenhouse trial for winter production of genetically engineered (GM) parthenocarpic eggplants demonstrated a significant increase (an average of 33% increase) in fruit production concomitant with a reduction in cultivation costs.
Results
GM parthenocarpic eggplants have been evaluated in three field trials. Two greenhouse spring trials have shown that these plants outyielded the corresponding untransformed genotypes, while a summer trial has shown that improved fruit productivity in GM eggplants can also be achieved in open field cultivation. Since the fruits were always seedless, the quality of GM eggplant fruits was improved as well. RT-PCR analysis demonstrated that the DefH9-iaaM gene is expressed during late stages of fruit development.
Conclusions
The DefH9-iaaM parthenocarpic gene is a biotechnological tool that enhances the agronomic value of all eggplant genotypes tested. The main advantages of DefH9-iaaM eggplants are: i) improved fruit productivity (at least 30–35%) under both greenhouse and open field cultivation; ii) production of good quality (marketable) fruits during different types of cultivation; iii) seedless fruit with improved quality. Such advantages have been achieved without the use of either male or female sterility genes.
Background
Fruit-set and growth of several horticultural plants are negatively affected by adverse environmental conditions. In general, sub and/or supra-optimal temperatures negatively affect reproductive processes and therefore curtail fruit production [1,2]. Under greenhouse cultivation, low temperature, insufficient light intensity, and/or high humidity drastically reduce fruit productivity and quality in eggplant and other species. Moreover, environmental conditions often met in open field cultivation such as drought and high temperatures have a negative effect on fruit productivity and quality in eggplant and other species (e.g. tomato).
Parthenocarpic fruit development (i.e. fruit-set and growth without fertilization) can significantly aid in the resolution of the aforementioned problems. Parthenocarpy can be triggered by exogenous factors, such as plant growth regulators, or it can be achieved by genetic factors. Genes causing parthenocarpic development have been identified in several plant species [3-5], and parthenocarpic eggplant varieties (e.g. Talina, Galine) have been introduced in the production process (e.g. protected cultivation). However, during winter cultivation of eggplant varieties in unheated greenhouses in the Mediterranean area, the negative effect of suboptimal environmental conditions on fruit production is usually counteracted by treating flower buds with plant growth regulators. Phytohormonal treatments make the production process more expensive due to the cost of both chemicals and labor.
In principle, the genetic engineering of plants allows one to confer a trait of interest to different species and within a species to all the varieties of interest. To confer parthenocarpic fruit development, a chimeric gene has been constructed [6]. Specifically, the DefH9-iaaM gene contains the coding region of the iaaM gene from Pseudomonas syringae pv. savastanoi under the control of the placenta and ovule-specific promoter from the DefH9 gene from Anthirrinum majus[6]. The iaaM gene codes for a tryptophan monoxygenase, which produces indolacetamide that in turn, is either chemically or enzymatically converted to the auxin indole-3-acetic acid. To date, the DefH9-iaaM chimeric gene has been shown to cause parthenocarpic fruit development in tobacco [6], eggplant [6], tomato [7,8], strawberry and raspberry [9].
We have previously shown that DefH9-iaaM eggplants outperform control plants during protected winter cultivation by an average of 33% [10]. The present manuscript presents data on the agronomic performance of DefH9-iaaM eggplant hybrids during spring and summer cultivation. Seed-derived F1 plants perfectly reflects the real agronomical situation of eggplant production. In addition, they have allowed to demonstrate that the transgene is active after meiosis and give satisfactory results in the hemizygous state. Different types of eggplant hybrids have been evaluated during springtime in unheated greenhouses in two different locations. To evaluate GM parthenocarpic eggplants under optimal environmental conditions, a single trial has been carried out using two different genotypes under standard open field cultivation during summertime. Furthermore, we demonstrate that the DefH9-iaaM gene is expressed during late stages of fruit development.
Results and discussion
Greenhouse spring production
Spring production was evaluated in trials performed in two different locations: Monsampolo and Pontecagnano. Spring production was divided into early production, consisting of the first four harvests, and total production, consisting of sixteen harvests. Early spring production corresponds to the cultivation period from March to the first half of May, with temperatures somewhat low for fruit-set and growth. During this period, the average minimum and maximum temperatures ranged from 7° to 17°C in southern Italy (Pontecagnano) and from 14° to 17°C in central Italy (Monsampolo). The transgenic parthenocarpic hybrids gave a significantly higher yield, on the average a six-fold increase during early production, in comparison to their controls (Table 1). The increment in the number of fruits per plant and the higher average weight of the fruits were the main causes of the increased early spring production of the parthenocarpic hybrids. The suboptimal/adverse environmental conditions did not affect the growth of GM parthenocarpic fruits and the average fruit weight was significantly higher in the GM eggplants in comparison to untransformed controls. The traditional parthenocarpic cultivar Talina produced fruits with an average weight similar to those of GM eggplant fruits (Table 1). However, due to the higher number of fruits per plant, the productivity of the transgenic parthenocarpic hybrids was also increased, by an average of five-fold, when compared to cv Talina (Table 1). During the whole harvesting period, the GM fruits were always seedless (Fig. 1) and were normal in both size and shape.
Table 1.
Eggplant production of parthenocarpic hybrids and their respective controls at springtime.
| Genotype | Early production | Total production | ||||
| Yield/plant (g) | Fruits/plant (n) | Fruit weight (g) | Yield/plant (g) | Fruits/plant (n) | Fruit weight (g) | |
| P1 | 488 b | 1.9 a | 268.3 a | 2241 a | 8.4 a | 253.9 a |
| P2 | 695 a | 2.6 a | 290.8 a | 2288 a | 8.6 a | 260.2 a |
| C1 | 75 c | 0.4 b | 227.0 b | 1547 b | 7.8 a | 187.5 b |
| P5 | 522 b | 2.0 a | 281.8 a | 2163 a | 9.2 a | 230.5 a |
| C2 | 116c | 0.7 b | 170.6 b | 1574 b | 9.3 a | 167.3 b |
| Talina | 114c | 0.3 b | 270.5 a | 2360 a | 9.4 a | 229.9 a |
For each trait at least one common letter indicates no significant difference according to the Duncan test (P = 0.05). Mean values of yields per plant, number of fruits per plant, and fruit weight of three transgenic parthenocarpic hybrids (P1, P2 and P5), two controls (C1 and C2) and the commercial cultivar Talina. C1 hybrid plants represent the controls of P1 and P2 transgenic hybrid plants. C2 hybrid plants are the controls of the P5 transgenic hybrids. Data are the average of the Monsampolo and Pontecagnano locations. The experiments were carried out in greenhouse at springtime.
Figure 1.

Eggplant fruits of the transgenic parthenocarpic hybrid P1 from the greenhouse spring trial. Left: fruit at the stage of commercial ripeness; middle: an overripe eggplant fruit from a border plant; right: a longitudinally cut fruit showing the complete absence of seeds.
The increased productivity of GM hybrids characterised both the early spring production (i.e. the first four harvests) and the whole spring production cycle (i.e. sixteen harvests). During the whole spring production cycle, the hybrids P1 and P2 gave an average yield that was 46% higher with respect to the corresponding control C1 (Table 1). The hybrid P5 gave a 37% higher yield with respect to its control C2. The average total number of fruits produced per plant in both locations was similar in all the hybrids (8–9 fruits/plant). However, the higher average weight of the GM fruits led to a higher total yield of transgenic hybrids with respect to their controls. When considering the whole spring cultivation cycle, the parthenocarpic cultivar Talina gave a total production that was not significantly different from either of the three GM hybrids (Table 1).
Open field (summer) production
Summer production was evaluated in an open field trial carried out during the optimal period of eggplant cultivation. Plants were transplanted on May 20th and the last harvest took place on September 11th. The early production of the transgenic hybrids, consisting of the first three harvests, was significantly higher than that of the untransformed hybrids (Table 2): P1 and P10 hybrids yielded, respectively, 36% and 76% more than their corresponding controls, C1 and C10. The difference in productivity between P1 and C1 hybrids, which have long-shaped fruits, was caused by the higher average weight of GM fruits. When comparing P10 and C10 hybrids, which have sub-oval fruits, the higher yield obtained with GM plants during the early harvesting period was due to the increased number of fruits per plant.
Table 2.
Eggplant production of parthenocarpic hybrids and their respective controls at summertime.
| Genotypes | Early production | Total production | ||||
| Yield/plant (g) | Fruits/plant (n) | Fruit weight (g) | Yield/plant (g) | Fruits/plant (n) | Fruit weight (g) | |
| P1 | 1158a | 2.7 a | 433 b | 3039 a | 9.1 a | 335 b |
| C1 | 846 b | 2.4 a | 344 c | 2211 b | 7.7 ab | 288 b |
| P10 | 1287 a | 2.4 a | 553 a | 2791 ab | 6.8 bc | 410 a |
| C10 | 731 b | 1.3 b | 541 a | 2386 ab | 5.7 c | 415 a |
For each trait, at least one common letter indicates no significant difference according to the Duncan test (P = 0.05). Mean value of yields per plant, number of fruits and fruit weight of two transgenic hybrids (P1 and P10) and their corresponding untransformed hybrids (C1 and C10), cultivated during summertime under open field conditions.
Consisting of ten harvests, the total production of P1 hybrids was 37% higher than control C1 eggplants (Table 2). The difference in total yield between P1 and control C1 hybrids was statistically significant and due both to the higher number of fruits/plant and to the increased weight of GM fruits. It is noteworthy to point out that when considered individually, neither trait (number of fruits/plant or fruit weight) showed statistically significant differences between the GM and untransformed plants (Table 2). Although higher in P10 hybrids in comparison to its control C10, the total yield (the number and average weight of the fruits) was not significantly different between the two. During the whole harvesting period, fruits from both P1 and P10 parthenocarpic hybrids were always seedless (Fig. 2), whilst control fruits always had seeds. Therefore, under open field cultivation, the DefH9-iaaM gene had a positive influence on fruit quality, as GM DefH9-iaaM fruits were always seedless. Fruit quality affects the economic value of eggplant production.
Figure 2.
Eggplant fruits from the open field summer trial. Left, uncut and cut fruit of the transgenic hybrid P10; Right, cut and uncut fruit of the C10 control hybrid.
Although the environmental conditions were optimal and consequently did not affect negatively fruit-set, the DefH9-iaaM gene caused both faster development and growth of the fruits as indicated by the increased early-summer production (the first three harvests). In this regard, it is worthwhile to stress that expression of the DefH9-iaaM gene takes place in the placenta and ovules before pollen development. As a consequence, in GM parthenocarpic plants fruits are seedless and fruit development initiates well before non-GM controls [11].
In all trials we have never used homozygous lines because growers mostly employ F1 hybrids. The use of hemizygous primary transformants as pollinator plants allowed us to obtain in rather short time, by in vivo selection for kanamycin resistance, F1 plants transgenic for the DefH9-iaaM gene. Young, healthy and vigorously growing plants did not produce seeds. However, it is possible to obtain seeds from aged DefH9-iaaM transgenic plants both by selfing and crossing. By exploiting the delayed female fertility we have produced the homozygous plants needed as male parents for rapid seed multiplication of F1 eggplant hybrids. Therefore, the female sterility of young and mature plants is not an insuperable hindrance for mass propagation and commercial fruit production.
Expression of the DefH9-iaaM gene takes place during both flower and fruit development in transgenic parthenocarpic eggplants
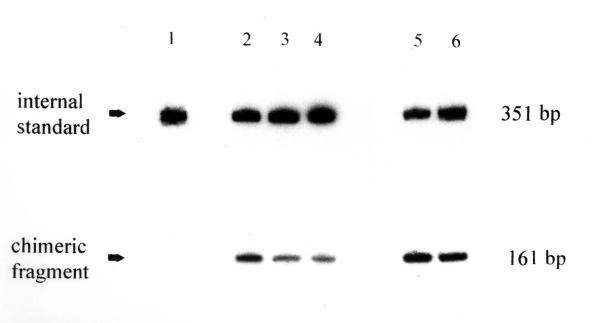
The DefH9 gene is expressed specifically in the placenta and ovules during early phases of flower development [6]. To determine whether expression of the DefH9-iaaM gene also occurs during later stages of fruit growth and whether it is influenced by pollen fertilization, mRNA from transgenic flowers and fruits obtained either from emasculated or hand pollinated flowers was analyzed by RT-PCR at different stages of development, until the fruit attained a length of 28 cm. An amplicon of 161 bp corresponding to the spliced DefH9-iaaM mRNA was detected in all stages analyzed (Fig. 3, lanes 2–6). Thus, the presence of the mRNA of the DefH9-iaaM gene and consequently its action is most likely not limited to early stages of flower and fruit development. Pollination did not affect the steady state level of DefH9-iaaM mRNA (Fig. 3, compare lane 5 and lane 6).
Figure 3.
Expression analysis of the DefH9-iaaM gene by competitive RT-PCR during flower and fruit development. Untransformed plant (lane 1); 5, 8, 12 mm long flower buds (lanes 2, 3, 4, respectively); 40 mm long hand-pollinated fruit (lane 5); 280 mm long emasculated fruit (lane 6). An internal standard of 351 bp is present in all lanes.
Treatment with auxin often causes parthenocarpic development in several plant species [for review, see: [12]]. However, in some species and/or varieties, to efficiently sustain fruit growth, the hormonal treatment of the flowers must be repeated [13]. The finding that DefH9-iaaM mRNA is also present during later stages of fruit development is consistent with the interpretation that in DefH9-iaaM parthenocarpic plants, the placenta, the ovules, and the tissues derived therefrom are a source of auxin during the whole growth of the fruit. As a consequence, they efficiently sustain fruit growth.
Conclusions
The data hereby presented show the positive influence of the DefH9-iaaM parthenocarpic gene on eggplant productivity under both greenhouse (spring) and open field (summer) cultivation. Taking into account the data previously obtained under winter greenhouse cultivation [10], we conservatively estimate that the overall increase in productivity is at least 30–35%. The increase in productivity of DefH9-iaaM eggplants is mainly due to a drastically improved fruit-set under sub-optimal temperatures and to an enhanced fruit growth and weight. Fruit quality is also improved because the fruits are seedless and do not show a placental cavity. The qualitative improvement of DefH9-iaaM eggplant fruits is interesting both for the fresh market and for the processing industry. During early spring greenhouse production, DefH9-iaaM parthenocarpic hybrids always gave fruits with an average weight suitable for fresh market commercialization, while untransformed hybrids, under sub-optimal conditions, rarely produced commercial fruits. Thus, the DefH9-iaaM gene quantitatively and qualitatively improves eggplant production under both greenhouse and open field cultivation. In all genotypes tested the DefH9-iaaM gene had a very positive effect on production and quality parameters. Such findings are of paramount importance as the hybrids tested have the same genetic background that the relative controls, except for the presence of the DefH9-iaaM gene. The DefH9-iaaM gene, already known to be expressed in the placenta and ovules during early phases of flower development, is expressed also in mature fruits, most likely in tissues derived from the ovules.
From an economical standpoint, the main advantages conferred to eggplant by the DefH9-iaaM gene are: i) production of marketable fruits under environmental conditions adverse for fruit-set and growth; ii) reduction of cultivation costs (energy, phytohormones and labor) necessary for off-season and open field eggplant cultivation; and iii) enhancement of fruit quality. Last but not least, contrary to conventional wisdom, these advantages have been achieved without the use of either male or female sterility genes.
Materials and methods
Greenhouse spring cultivation
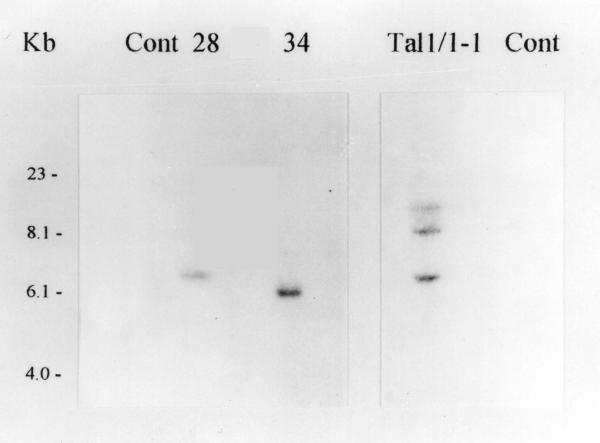
Trials were carried out in central Italy (Monsampolo del Tronto-AP) and in southern Italy (Pontecagnano-SA) (approval of the Italian Ministry of Health N° B/IT/97-29). The greenhouses were rather similar and made of galvanized steel and covered with plastic polyethylene (0.12 mm thick). An apparatus for drip-irrigation was used and the soil was completely mulched. A complete randomized block design with three replicate hybrid genotypes was adopted. Each experimental plot measured 3.12 m2 and contained eight plants in a double row. Transplanting was performed on March 3rd in southern Italy and on March 27th in central Italy. The P1, P2, P5, C1, C2 and the commercial Talina hybrids were employed. Transgenic parthenocarpic hybrids P1, P2 and P5 were obtained by crossing (as male parent) the primary transgenic plant DR2 iaaM #34-1 with the line Tal 1/1 (P1), the primary transgenic plant DR2 iaaM #28-1 with Tal 1/1 (P2) and the transgenic plant Tal 1/1 iaaM #1-1 with the line Tina (P5). The hybrids P1 and P2 are homologous to C1 (DR2 × Tal1/1), except for the presence of the DefH9-iaaM gene integrated in their genome. The transgenic hybrid P5 is homologous to its untransformed control C2 (Tal1/1 × Tina). DR2 and Tina are parental lines obtained through classical breeding, Tal1/1 is a double haploid line derived from anther culture of the F1 commercial cultivar Talina. The segregation of the marker gene nptII was checked by spraying the plants with kanamycin [14] and allowed for the conclusion that the transgenes segregate as a single locus in the backcrossed progenies of the three independent events analyzed (Tal iaaM 1-1: χ2 = 0.01065, P = 0.917; DR2 iaaM 34-1: χ2 = 0.0496 P = 0.824; DR2 iaaM 28-1 χ2 = 0.06467 P = 0.799). Southern blot analysis showed that DR2 iaaM 28-1 and 34-1 had a single copy of the transgene, while Tal iaaM 1-1 had three copies of the transgene (Fig 4). Since the interaction genotype/location was not significant for the yield, the data were computed as average of the two locations and subjected to analysis of variance according to a randomized complete block design. Duncan's Multiple Range Test (P = 0.05) was used for means separations.
Figure 4.
Southern blot analysis of transgenic eggplants. Numbers above the lanes indicate the independent transgenic plant DR2iaaM#28-1 (28), DR2iaaM#34-1 (34) and Tal1/1iaaM#1-1 (Tal1/1-1). Cont indicates untransformed plants, i.e. DR2 and Tal1/1, respectively. The probe used corresponds to the DefH9 regulatory region.
Open field (summer) cultivation
The open field trial was carried out under open field conditions at Monsampolo del Tronto (approval of the Italian Ministry of Health B/IT/99/21). Two transgenic parthenocarpic hybrids were tested: the hybrid P1 (Tal1/1 × DR2 iaaM #34-1) with elongated fruits and the hybrid P10 (UGA × Tal1/1 iaaM #1-1) with sub-oval fruits were compared to their homologous non-transgenic controls C1 (DR2 × Tal1/1) and C10 (UGA × Tal1/1), respectively. The UGA line has oval dark purplish fruits and it has been provided by Dr. S.C. Phatak. A complete randomized block design with the hybrids replicated four times was adopted. Each experimental plot measured 11.7 m2 and contained 30 plants in a double row. Transplanting was performed on May 10th.
Early spring production consisted of the first four harvests (i.e. 4 out of 16 harvests during the whole production cycle), while early summer production, whose cultivation cycle consists of ten harvests, corresponds to the first three harvests. For all trials the number and weight of fruits were recorded. In addition, fruit sample for each harvest and replication was cut to check for the presence of seeds. Data were computed for the early harvesting time and for the whole harvesting season. Analysis of variance was performed according to a randomized complete block design. Duncan's Multiple Range Test (P = 0.05) was used for means separations.
Plant DNA isolation and Southern blot analysis
High-molecular-weight DNA was isolated from the young leaves of transgenic and untransformed eggplants by using Plant DNAzol (Invitrogen), according to the manufacturer's instructions. Ten μg of DNA was digested overnight with 80 units of KpnI (Promega) in a volume of 500 μl, separated on a 0.7% agarose gel and transferred to Hybond N (Amersham Pharmacia Biotech). A 1350 bp fragment of the DefH9 gene was used as a radiolabeled probe. The membrane was hybridized overnight in 5X SSC/50% formamide (Sigma) at 42°C and washed two times for 15 min. in 2 × SSC/0.1% SDS, and two times for 15 min. in 0.1 × SSC/0.1% SDS at 42°C. Signals were detected using Kodak X-OMAT AR5 film (Sigma).
RT-PCR analysis
Semiquantitative (competitive) PCR analysis was carried out for 38 cycles (annealing temperature 63°C) using as template cDNA (8 ng) obtained by priming poly(A)+ mRNA with an iaaM specific primer (5'-AATAGCTGCCTATGCCTCCCGTCAT-3'). The mRNA was extracted from either young flower buds (5,8,11 mm) or eggplant fruits (placental tissue from fruits either 40 or 280 mm long). As an internal standard, 0.5 fg of a 600 bp long DefH9 cDNA fragment was used in the PCR assay. To amplify the 161 bp long amplicon an iaaM specific primer (5'-GGGTGAATTAAAATGGTCATACAT-3') and a DefH9 specific primer (5'-CTTTGGAACTCGTGTTGAGCTCTCA-3') were used. For the internal standard, a 3' primer (5'-TGAGCATTGATCTCCTGAGTGGTGT-3') together with the DefH9 specific primer were used to produce the 351 bp long amplicon. The PCR assays were performed with a thermostable DNA polymerase mixture (Expand High Fidelity PCR system, Roche) in presence of 3 μCi of 32P dCTP. The intensity of the bands was quantified by using an Instant Imager (Packard, Meriden, CT).
Acknowledgments
Acknowledgements
This research was partially supported by the Consiglio Nazionale delle Ricerce (Progetto Finalizzato Biotecnologie II) and by the Ministero Politiche Agricole e Forestali Progetto "Biotecnologie vegetali". We thank Prof. Phatak, University of Georgia (USA) for the UGA line.
Contributor Information
Nazzareno Acciarri, Email: acciarri@libero.it.
Federico Restaino, Email: ist.orticoltura@tiscalinet.it.
Gabriele Vitelli, Email: orticolt@insinet.it.
Domenico Perrone, Email: perrone.do@libero.it.
Michela Zottini, Email: mzottini@civ.bio.unipd.it.
Tiziana Pandolfini, Email: pandolfini@sci.univr.it.
Angelo Spena, Email: spena@sci.univr.it.
Giuseppe Leonardo Rotino, Email: pinuzzu@libero.it.
References
- Nothman J, Koller D. Effects of growth regulators on fruit and seed development in eggplant (S. melongena L.). J Hort Sci. 1975;50:23–27. [Google Scholar]
- Romano D, Leonardi C. The responses of tomato and eggplant to different minimum air temperature. Acta Hort. 1994;366:57–66. [Google Scholar]
- De Ponti OMB. Breeding parthenocarpic pickling cucumbers (Cucumis sativus L.): necessity, genetical possibilities, environmental influences and selection criteria. Euphytica. 1976;25:29–40. doi: 10.1007/BF00041526. [DOI] [Google Scholar]
- Philouze J. Parthenocarpie naturelle chez tomate. II. Etude d'une collection varietale. Agronomie. 1985;5:47–54. [Google Scholar]
- Restaino F, Onofaro-Sanajà V, Mennella G. Facultivative parthenocarpic genotypes of eggplant obtained through induced mutations. XIII Eucarpia Congress, 6–11 July, Angers, France. 1992. pp. 297–298.
- Rotino GL, Perri E, Zottini M, Sommer H, Spena A. Genetic engineering of parthenocarpic plants. Nat Biotechnol. 1997;15:1398–1401. doi: 10.1038/nbt1297-1398. [DOI] [PubMed] [Google Scholar]
- Ficcadenti N, Sestili S, Pandolfini T, Cirillo C, Rotino GL, Spena A. Genetic enginnering of parthenocarpic fruit development in tomato. Mol Breed. 1999;5:463–470. doi: 10.1023/A:1009665409959. [DOI] [Google Scholar]
- Pandolfini T, Rotino GL, Camerini S, Defez R, Spena A. Optimisation of transgene action at the post-transcriptional level: high quality parthenocarpic fruits in industrial tomatoes. BMC Biotechnology. 2002;2:1. doi: 10.1186/1472-6750-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzetti B, Landi L, Scortichini L, Rebori A, Spena A, Pandolfini T. Genetic engineering of parthenocarpic fruit development in strawberry. Proc. 4th ISHS Strawberry symposium, Tampere (Finland) 9–14 July 2000. Acta Hortic. 2002;567:101–104. [Google Scholar]
- Donzella G, Spena A, Rotino GL. Transgenic parthenocarpic eggplants: superior germplasm for increased winter production. Mol Breed. 2000;6:79–86. doi: 10.1023/A:1009613529099. [DOI] [Google Scholar]
- Spena A, Rotino GL. Parthenocarpy: state of the art. In: Bhojwani SS, Soh WY, editor. Current trends in the embryology of angiosperm. Kluwers Academic Publishers; 2001. pp. 435–450. [Google Scholar]
- Schwabe WW, Mills JJ. Hormones and parthenocarpic fruit-set: a literature survey. Hort Abstracts. 1981;51:661–698. [Google Scholar]
- Saavedra E. Set and growth of Annona cherimolia Mill. Fruit obtained by hand-pollination and chemical treatments. J Am Hort Sci. 1979;104:668–673. [Google Scholar]
- Sunseri F, Fiore MC, Mastrovito F, Tramontano E, Rotino GL. In vivo selection and genetic analysis for kanamycin resistance in transgenic eggplant (Solanum melongena L.). J Genet Breed. 1993;47:299–306. [Google Scholar]