Abstract
Purpose
Women living with HIV (WLWH) and breast cancer (BC) have worse overall survival than HIV-negative women with BC, and poor adherence to prescribed tamoxifen is known to contribute to poor survival. We therefore investigated the association of HIV infection with adherence to adjuvant tamoxifen among women with localized hormone receptor (HR)-positive breast cancer in South Africa.
Methods
Among 4,097 women diagnosed with breast cancer at six hospitals in the prospective South African Breast Cancer and HIV Outcomes (SABCHO) cohort study between July 2015 and December 2020, we focused on black women with stages I-III HR-positive breast cancer who were prescribed 20 mg of adjuvant tamoxifen daily. We collected venous blood once from each participant during a routine clinic visit, and analyzed concentrations of tamoxifen and its metabolites using a triple quadruple mass spectrometer. We defined non-adherence as a tamoxifen level < 60 ng/mL after 3 months of daily tamoxifen use. We compared tamoxifen-related side effects, and concurrent medication use among women with and without HIV and developed multivariable logistic regression models of tamoxifen non-adherence.
Results
Among 369 subjects, 78 (21.1%) were WLWH and 291 (78.9%) were HIV-negative. After a median (interquartile range) time of 13.0 (6.2–25.2) months since tamoxifen initiation, the tamoxifen serum concentration ranged between 1.54 and 943.0 ng/mL and 208 (56.4%) women were non-adherent to tamoxifen. Women < 40 years of age were more likely to be non-adherent than women > 60 years (73.4% vs 52.6%, odds ratio (OR) = 2.49, 95% confidence interval (CI) = 1.26–4.94); likewise, WLWH (70.5% vs 52.6%, OR = 2.16, 95% CI = 1.26–3.70) than HIV-negative women. In an adjusted model WLWH had twice the odds of non-adherence to tamoxifen, compared to HIV-negative women (OR = 2.40, 95% CI = 1.11–5.20).
Conclusion
High rates of non-adherence to adjuvant tamoxifen may limit the overall survival of black South African women with HR-positive breast cancer, especially among WLWH.
Keywords: Breast cancer, HIV, Tamoxifen, Non-adherence
Introduction
Breast cancer remains the most common cancer among women worldwide, with an estimated 2.3 million new cases and 685,000 deaths in 2020 [1]. In high-income countries (HICs), breast cancer prognosis has significantly improved over time; however, in sub-Saharan Africa (SSA), the 3 year overall survival (OS) is estimated to be 50% [2], mainly due to late-stage at diagnosis and sub-optimal multi-modality treatment access and completion. Among women with stages I-III breast cancer, the OS is worse in women living with HIV (WLWH) than HIV-negative women in SSA (3 year OS 52% vs 63%) [3]. In South Africa where the prevalence of HIV infection among black women is 21.0% [4], the 2 year OS was 72.4% vs. 80.1%, p < 0.001 [5]. The reasons for worse survival among WLWH with breast cancer are complex but may include access to care issues, more treatment-related adverse events, or possible biological associations between HIV and tumour behaviour. Some studies have evaluated the association of malignancy and comorbid HIV with adherence to treatment of either condition. It is known that increasing the pill burden decreases adherence to antiretroviral therapy (ART) among people living with HIV (PLWH) in SSA [6]; a study from the Republic of Korea found that a cancer diagnosis was a risk factor for low ART adherence [7]. Another study found that only 54% of adult PLWH and cancer in Uganda adhered to both ART and chemotherapy [8].
Molecular subtypes are a major determinant of treatment options and disease outcomes; the hormone receptor (HR)-positive breast cancers have a better outcome than others [9, 10]. Hormonal therapies, such as tamoxifen, are among the most effective systemic treatments for HR-positive breast cancer. They lower the risk of breast cancer recurrence by 41% and cancer-specific mortality by 31% [11–13].
Treatment adherence is defined as the extent to which patients take their medication as prescribed [14]. There are multiple ways to identify non-adherence. Indirect methods, such as patient-administered questionnaires [15, 16] and pharmacy prescription refills [17, 18], are most frequently used; however, these methods do not capture the actual medication intake. It is known that patients’ self-reports tend to overestimate adherence rates and pharmacy reports do not adequately reflect medication intake, especially if out-of-pocket costs are low [19]. Direct methods, such as measurement of the level of the drug or its metabolites in the blood, are much more precise, but more expensive, less well studied, and not commonly used in clinical practice [20–22].
Despite the survival benefit of adjuvant tamoxifen in women with HR-positive breast cancer [12], 31 to 73% of these women have been found not to take the full dosage or to discontinue their treatment early [23–25], thereby reducing its therapeutic benefits [26]. Previous studies have suggested that non-adherence to tamoxifen in women with HR-positive breast cancer is common and is associated with shorter time to recurrence, higher cost of treatment, poorer quality of life, and ultimately poorer survival [20, 26]. Differences in tamoxifen adherence may contribute to the differences in survival seen in South African stage I-III HRpositive breast cancer patients with and without HIV [5].
Cytochrome P450 2D6 (CYP2D6) is involved in tamoxifen metabolism predicting the formation of endoxifen, but its association with therapeutic efficacy is still debated [27–30]. Side effects, such as hot flushes, are a frequent reason for non-adherence to tamoxifen therapy [31–33]. The severity of tamoxifen-related adverse effects may be increased among WLWH due to drug-drug interactions with protease inhibitors (PIs) or non-nucleoside reverse transcriptase inhibitors (NNRTIs) [34]. The risks of discontinuing tamoxifen therapy in women with HR-positive breast cancer in SSA are not well understood.
Improving adherence is increasingly important, as tamoxifen is now often prescribed for up to ten years [35]. In this paper, we examined non-adherence among HR-positive breast cancer patients participating in the South African Breast Cancer and HIV Outcomes (SABCHO) study by analysing the serum concentrations of tamoxifen using a triple quadruple mass spectrometer, and we evaluated factors associated with non-adherence to tamoxifen, including HIV status.
Methods
Study setting and population
Between July 2015 and December 2020, the SABCHO study recruited 4097 women with newly diagnosed breast cancer from six government hospitals. On each participant, sociodemographic and clinical information were recorded and patients were subsequently followed for treatment and outcomes [36]. Participants in this sub-study were recruited consecutively from active SABCHO enrolees who attended routine breast clinic visits between March 2019 and April 2021. To be eligible for this sub-study, participants had to be black women with stages I-III HR-positive breast cancer followed at two hospitals in Gauteng province (Chris Hani Baragwanath Academic Hospital (CHBAH) and Charlotte Maxeke Johannesburg Academic Hospital (CMJAH). We included women who initiated tamoxifen between July 2015 and December 2020 and at least 3 months before enrolment (to ensure that the levels of tamoxifen and metabolites had reached a steady state), and all who reported on their adherence to tamoxifen. We telephoned potentially eligible patients due for clinic follow-up during the enrolment period and asked them to bring all medications they were currently taking to their upcoming appointment.
Data collection and processing
Socio-demographic information (such as age, marital status, the highest level of education, and employment status) and lifestyle factors (alcohol consumption, smoking), self-reported comorbidities (hypertension, diabetes, cerebrovascular disease, asthma/chronic obstructive pulmonary disease (COPD), and tuberculosis) were collected upon SABCHO enrolment. Participants who did not report known HIV infection at enrolment were tested for HIV (after providing informed consent), using the National Health Laboratory Services’ enzyme-linked immunosorbent assay; SABCHO enrolees living with HIV who were not already on treatment were immediately referred for initiation of ART. Participants were clinically staged using the 7th edition of the American Joint Committee on Cancer [37], and stage, receptor status including oestrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), Ki67 percentage, and treatment information were collected directly from the medical record. For this sub-study, we also collected information on the treatment of comorbidities and the side effects of tamoxifen. Patients were asked to answer yes or no if they were having hot flushes, dry vagina or discharge or bleeding, nausea, fluid retention, blood clots, or bone or joint pains, and if they had ever been diagnosed with cancer of the womb while taking tamoxifen.
Bioanalysis of tamoxifen and genotypes
Non-adherence to tamoxifen was determined using an objective and direct method, tamoxifen serum assessment. At study enrollment, we collected venous blood from each participant for plasma extraction, from which tamoxifen, and its metabolite concentrations, were analysed using liquid chromatography-mass spectrometry (LC-MSMS). The CYP2D6 genotype was determined using reverse transcription polymerase chain reaction (RT-PCR) on a Quanti-studio 12 platform. We classified CYP2D6 into ultra-rapid metabolizer (UM), normal metabolizer (NM), intermediate metabolizer (IM), and poor metabolizer (PM) according to the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines [38].
Our primary outcome was pharmacokinetic non-adherence to tamoxifen, defined as a random plasma tamoxifen level of < 60 ng/mL, a threshold based on previous pharmacological studies [20–22, 39]. (More detailed information on the bioanalysis of tamoxifen and CYP2D6 genotype determination is presented as supplementary material.) This study was approved by the University of the Witwatersrand Human Research Ethics Committee, Johannesburg, South Africa, and the Institutional Review Board of Columbia University in New York, NY. Written informed consent was obtained from all individual participants in the study.
Statistical analysis
We compared the distribution of the categorical and continuous variables by HIV status and the serum-defined tamoxifen adherence levels (non-adherent (< 60 ng/mL) vs adherent (≥ 60 ng/mL)), using Pearson’s chi-squared test and Fisher’s exact tests for categorical variables. Means ± standard deviations (SDs), median and interquartile range (IQR) were computed for continuous variables using the Student’s t-test and the Wilcoxon Rank Sum test to report differences between groups. To examine associations with tamoxifen non-adherence, we used a multivariable logistic regression model. Variables for which Wald testing p-values were < 0.1 in univariate analysis were included in our multivariate model. Analysis was performed using Stata version 16 (StataCorp Ltd, College Station, TX).
Results
Among the consecutive 369 women who were enrolled, 78 (21.1%) were WLWH and 291 (78.9%) were HIV negative. Most of the women were not married (221/369, 59.9%), had secondary education (303/368, 82.3%), were unemployed (221/369, 59.9%), and had a body mass index (BMI) > 30 kg/m2 (224/369, 60.7%). Compared to HIV-negative women, WLWH were more likely to be < 40 years of age [26/78 (33.3%) vs 38/291 (13.1%), p < 0.001], educated beyond the primary level [71/78 (91.0%) vs. 232/290 (80.0%), p = 0.023], and with a BMI ≤ 30 kg/m2 [44/78 (56.4%) vs. 101/291 (34.7%), p < 0.001] (Table 1). Most of the women (268/351 (76.4%) mastectomy and 83/351 (23.6%) breast-conserving surgery); 290/369 (78.6%) received chemotherapy; and 226/369 (61.2%) received radiation therapy (Table 2).
Table 1.
Socio-demographic, lifestyle characteristics, and serum-defined tamoxifen and endoxifen levels of women with breast cancer by HIV status
| HIV-negative | HIV-positive | Total | P value | |
|---|---|---|---|---|
| Total number of patientsa | 291 (78.9%) | 78 (21.1%) | 369 (100.0%) | |
| Age at diagnosis in years | < 0.001 | |||
| < 40 | 38 (13.1) | 26 (33.3) | 64 (17.3) | |
| 40–49 | 78 (26.8) | 34 (43.6) | 112 (30.4) | |
| 50–59 | 81 (27.8) | 15 (19.2) | 96 (26.0) | |
| ≥ 60 | 94 (32.3) | 3 (3.9) | 97 (26.3) | |
| Marital status | 0.738 | |||
| Married/cohabiting | 118 (40.5) | 30 (38.5) | 148 (40.1) | |
| Unmarried | 173 (59.5) | 48 (61.5) | 221 (59.9) | |
| Highest level of education | 0.023 | |||
| Primary education and below | 58 (20.0) | 7 (9.0) | 65 (17.7) | |
| Secondary education and above | 232 (80.0) | 71 (91.0) | 303 (82.3) | |
| Employment status | 0.334 | |||
| Employed | 113 (38.8) | 35 (44.9) | 148 (40.1) | |
| Unemployed | 178 (61.2) | 43 (55.1) | 221 (59.9) | |
| Body mass index (BMI) | < 0.001 | |||
| < 30 kg/m2 | 101 (34.7) | 44 (56.4) | 145 (39.3) | |
| ≥ 30 kg/m2 | 190 (65.3) | 34 (43.6) | 224 (60.7) | |
| Smoking | 0.778 | |||
| No | 274 (94.2) | 75 (96.2) | 349 (94.6) | |
| Yes | 17 (5.8) | 3 (3.8) | 20 (5.4) | |
| Alcohol | 0.159 | |||
| No | 234 (80.4) | 57 (73.1) | 291 (78.9) | |
| Yes | 57 (19.6) | 21 (26.9) | 78 (21.1) | |
| b Serum-defined tamoxifen level in ng/mL median (IQR) | 56.9 (29.1–101.0) | 25.9 (15.7–74.5) | 52.3 (24.2–95.1) | < 0.001 |
| Serum-defined endoxifen level in ng/mL median (IQR) | 4.2 (2.1–7.0) | 2.8 (1.2–4.7) | 3.8 (1.9–6.3) | 0.001 |
Missing for variables: highest level of education (n = 1)
The percentages in the total number of patient row are row percentages, the other percentages are column percentages
interquartile range (IQR)
Table 2.
Socio-demographic, lifestyle factors, comorbidities, clinical, and treatment factors associated with serum-defined tamoxifen level among women with breast cancer
| Serum-defined tamoxifen adherence level |
||||
|---|---|---|---|---|
| Non-adherent (< 60 ng/mL) | Adherent (≥ 60 ng/mL) | Total | P value | |
| Total number of patientsa | N = 208 (56.4%) | N = 161 (43.6%) | N = 369 (100.0%) | |
| Tamoxifen serum concentration, ng/mL | ||||
| bMedian (IQR) | 27.3 (16.3–43.8) | 103.0 (80.0–146.0) | 52.3 (24.1–95.1) | |
| Range, min–max | 1.54–59.7 | 60.8–943.0 | 1.54–943 | |
| Age at diagnosis (years) | 0.027 | |||
| < 40 | 47 (22.6) | 17 (10.6) | 64 (17.3) | |
| 40–49 | 59 (28.4) | 53 (32.9) | 112 (30.4) | |
| 50–59 | 51 (24.5) | 45 (28.0) | 96 (26.0) | |
| ≥ 60 | 51 (24.5) | 46 (28.6) | 97 (26.3) | |
| Marital status | 0.071 | |||
| Married/cohabiting | 75 (36.1) | 73 (45.3) | 148 (40.1) | |
| Unmarried | 133 (63.9) | 88 (54.7) | 221 (59.9) | |
| Highest level of education | 0.667 | |||
| Primary education and below | 35 (16.9) | 30 (18.6) | 65 (17.7) | |
| Secondary education and above | 172 (83.1) | 131 (81.4) | 303 (82.3) | |
| Employment status | 0.233 | |||
| Employed | 89 (42.8) | 59 (36.6) | 148 (40.1) | |
| Unemployed | 119 (57.2) | 102 (63.4) | 221 (59.9) | |
| Body mass index (BMI) | 0.178 | |||
| < 30 kg/m2 | 88 (42.3) | 57 (35.4) | 145 (39.3) | |
| ≥ 30 kg/m2 | 120 (57.7) | 104 (64.6) | 224 (60.7) | |
| Smoking | 0.736 | |||
| No | 196(94.2) | 153 (95.0) | 349 (94.6) | |
| Yes | 12 (5.8) | 8 (5.0) | 20 (5.4) | |
| Alcohol | 0.121 | |||
| No | 158 (76.0) | 133 (82.6) | 291 (78.9) | |
| Yes | 50 (24.0) | 28 (17.4) | 78 (21.1) | |
| Hypertension | 0.087 | |||
| No | 137 (65.9) | 92 (57.1) | 229 (62.1) | |
| Yes | 71 (34.1) | 69 (42.9) | 140 (37.9) | |
| Diabetes | 0.540 | |||
| No | 196 (94.2) | 154 (95.7) | 350 (94.9) | |
| Yes | 12 (5.8) | 7 (4.3) | 19 (5.1) | |
| HIV | 0.005 | |||
| Negative | 153 (73.6) | 138 (85.7) | 291 (78.9) | |
| Positive | 55 (26.4) | 23 (14.3) | 78 (21.1) | |
| Tuberculosis | 0.960 | |||
| No | 203 (97.6) | 157 (97.5) | 360 (97.6) | |
| Yes | 5 (2.4) | 4 (2.5) | 9 (2.4) | |
| Cerebrovascular disease | 0.459 | |||
| No | 203 (97.6) | 155 (96.3) | 358 (97.0) | |
| Yes | 5 (2.4) | 6 (3.7) | 11 (3.0) | |
| cAsthma/COPD | 0.543 | |||
| No | 199 (95.7) | 156 (96.9) | 355 (96.2) | |
| Yes | 9 (4.3) | 5 (3.1) | 14 (3.8) | |
| Any comorbidity | 0.522 | |||
| No | 81 (38.9) | 68 (42.2) | 149 (40.4) | |
| Yes | 127 (61.1) | 93 (57.8) | 220 (59.6) | |
| Multimorbidity (≥ 2 comorbidities apart from breast cancer) | 0.988 | |||
| No | 173 (83.2) | 134 (83.2) | 307 (83.2) | |
| Yes | 35 (16.8) | 27 (16.8) | 62 (16.8) | |
| Time on tamoxifen in months | ||||
| dMedian (IQR) | 12.5 (6.3–25.0) | 13.4 (6.1–26.4) | 13.0 (6.2–25.2) | 0.591 |
| Range, min–max | 3.0–57.4 | 3.0–57.4 | 3.0–57.4 | |
| Tamoxifen side effect(s) | 0.729 | |||
| No | 97 (46.6) | 78 (48.4) | 175 (47.4) | |
| Yes | 111 (53.4) | 83 (51.6) | 194 (52.6) | |
| Number of medications apart from tamoxifen | 0.398 | |||
| 0 | 77 (37.0) | 72 (44.7) | 149 (40.4) | |
| 1 | 62 (29.8) | 34 (21.1) | 96 (26.0) | |
| 2 | 33 (15.9) | 26 (16.1) | 59 (16.0) | |
| 3 | 21 (10.1) | 16 (9.9) | 37 (10.0) | |
| ≥ 4 | 15 (7.2) | 13 (8.1) | 28 (7.6) | |
| eCYP2D6 predicted phenotype | 0.417 | |||
| UM & NM | 74 (56.9) | 67 (65.0) | 141 (60.5) | |
| NM/IM | 35 (26.9) | 24 (23.3) | 59 (25.3) | |
| IM & PM | 21 (16.2) | 12 (11.7) | 33 (14.2) | |
| Stage | 0.367 | |||
| 1 | 18 (8.7) | 14 (8.7) | 32 (8.7) | |
| 2 | 107 (51.4) | 94 (58.4) | 201 (54.5) | |
| 3 | 83 (39.9) | 53 (32.9) | 136 (36.9) | |
| Histology | 0.378 | |||
| Invasive ductal carcinoma | 204 (98.1) | 156 (96.9) | 360 (97.6) | |
| Other | 4 (1.9) | 5 (3.1) | 9 (2.5) | |
| Histology grade | 0.744 | |||
| Grade 1 | 19 (9.5) | 14 (8.9) | 33 (9.3) | |
| Grade 2 | 115 (57.8) | 97 (61.8) | 212 (59.6) | |
| Grade 3 | 65 (32.7) | 46 (29.3) | 111 (31.2) | |
| Immunohistochemistry-defined subtype | 0.872 | |||
| fHR + /HER2-− | 154 (74.0) | 118 (73.3) | 272 (73.7) | |
| HR + /HER2 + | 54 (26.0) | 43 (26.7) | 97 (26.3) | |
| Surgery | 0.164 | |||
| No | 13 (6.3) | 5 (3.1) | 18 (4.9) | |
| Yes | 195 (93.8) | 156 (96.9) | 351 (95.1) | |
| Type of surgery (N = 351) | 0.779 | |||
| Mastectomy | 150 (76.9) | 118 (75.6) | 268 (76.4) | |
| Breast conserving surgery | 45 (23.1) | 38 (24.4) | 83 (23.6) | |
| Chemotherapy | 0.375 | |||
| No | 48 (23.1) | 31 (19.3) | 79 (21.4) | |
| Yes | 160 (76.9) | 130 (80.7) | 290 (78.6) | |
| Radiotherapy | 0.933 | |||
| No | 81 (38.9) | 62 (38.5) | 143 (38.8) | |
| Yes | 127 (61.1) | 99 (61.5) | 226 (61.2) | |
The percentages in the total number of patient row are row percentages. The other percentages are column percentages
IQR Interquartile range
COPD Chronic obstructive pulmonary disease
IQR Interquartile range
CYP2D6 Cytochrome P450 2D6
UM Ultra-rapid metabolizer, NM Normal metabolizer, IM intermediate metabolizer PM Poor metabolizer UM (n = 10), PM (n = 4),
HR + Hormone receptor-positive, HER2 Human epidermal growth factor receptor 2 missing for variables: Highest level of education (n = 1), CYP2D6 (n = 135)
After at least 3 months of tamoxifen use with a median (IQR) time on tamoxifen of 13.0 (6.2–25.2) months, the serum concentrations of tamoxifen ranged between 1.54 and 943.0 ng/mL, with a median of 52.3 ng/mL (Table 2). The median (IQR) concentration of tamoxifen differed between WLWH and HIV-negative women [25.9 (15.7–74.5) ng/mL vs 56.9 (29.1–101.0) ng/mL, p < 0.001] (Table 1).
Overall, 208/369 (56.4%) women were non-adherent to tamoxifen and 161/369 (43.6%) women were adherent. Women who were non-adherent to tamoxifen were more likely to be < 40 years old [47/208 (22.6%) vs 17/161 (10.6%), p = 0.027] and living with HIV [55/208 (26.4%) vs. 23/161 (14.3%), p = 0.005] than women who were adherent. An exploratory sensitivity analyses with different thresholds of serum-tamoxifen level is shown in Supplementary Table 1 to better understand the relationship between adherence and HIV status/age. Using the tamoxifen cut-off level of 20 ng/mL, 40 ng/mL, and 80 ng/mL, women who were < 40 years old and WLWH were more likely to be non-adherent than adherent to tamoxifen (Supplementary Table 1). Women who were not married [133/208 (63.9%) vs 88/161 (54.7%), p = 0.07] and not hypertensive [137/308 (65.9%) vs 92/161(57.1%), p = 0.09] were marginally less adherent than others (See Table 2).
Most women (194/369 (52.6%)) reported tamoxifen side effects (Table 2). Figure 1 and Supplementary Table 2 show the distribution of reported side effects by serum-defined tamoxifen levels and by HIV status. Hot flushes were the most common side effect, and the prevalence did not differ between WLWH and HIV-negative women [36/78 (46.2%) vs 108/291 (37.1%), p = 0.146] (Supplementary Table 2), and among women who were non-adherent compared to those who were adherent [86/208 (41.3%) vs 58/161 (36.0%), p = 0.299] to tamoxifen (Fig. 1A and Supplementary Table 2). The prevalence of hot flushes also did not differ among women who were non-adherent and adherent to tamoxifen in both WLWH [28/55 (50.9%) vs 8/23 (34.8%), p = 0.193], and HIV-negative women [58/153 (37.9%) vs 50/138 (36.2%), p = 0.768] (Fig. 1B and C).
Fig. 1.
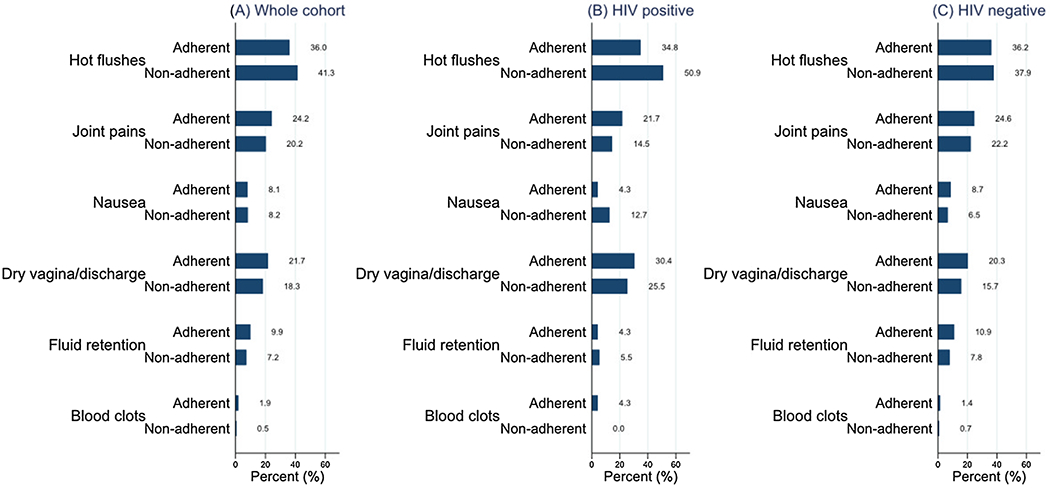
The reported side effects of tamoxifen use, overall and by HIV status among women with breast cancer
On univariate analysis, women < 40 years old were more than twice as likely to be non-adherent as women ≥ 60 years [Odds ratio (OR) = 2.46, 95% confidence interval (CI) = 1.26–4.94, p = 0.024], and WLWH were twice as likely as HIV negative women [OR = 2.16, 95% CI = 1.26–3.70, p = 0.005] to be non-adherent to tamoxifen (Table 3). On multivariable analysis, adjusting for the level of education, time on tamoxifen, combined tamoxifen side effects, and CYP2D6 predicted phenotype, HIV status was the only variable associated with tamoxifen adherence; the odds of non-adherence to tamoxifen use among WLWH was 2.40 times the odds of non-adherence among HIV-negative women [OR = 2.40, 95% CI = 1.11–5.20), p = 0.026) (Table 4].
Table 3.
Determinants of non-adherence to tamoxifen among women with breast cancer
| Serum-defined tamoxifen adherence level |
||||
|---|---|---|---|---|
| Non-adherent (< 60 ng/mL) | Adherent (≥ 60 ng/mL) | Univariate analysis | P value | |
| Total number of patients | N = 208 (56.4) | N = 161 (43.6) | Odds ratio (95% CI)a | |
| Age at diagnosis (years) | 0.024 | |||
| < 40 | 47 (73.4) | 17 (26.6) | 2.49 (1.26–4.94) | |
| 40–49 | 59 (52.7) | 53 (47.3) | 1.00 (0.58–1.73) | |
| 50–59 | 51 (53.1) | 45 (46.9) | 1.04 (0.59–1.83) | |
| ≥ 60 | 51 (52.6) | 46 (47.4) | 1.00 (Ref) | |
| Marital status | 0.072 | |||
| Married/cohabiting | 75 (50.7) | 73 (49.3) | 1.00 (Ref) | |
| Unmarried | 133 (60.2) | 88 (39.8) | 1.48 (0.97–2.56) | |
| Highest level of education | 0.652 | |||
| Primary education and below | 35 (53.8) | 30 (46.2) | 1.00 (Ref) | |
| Secondary education and above | 172 (56.8) | 131 (43.2) | 1.13 (0.66–1.94) | |
| Employment status | 0.233 | |||
| Employed | 89 (60.1) | 59 (39.9) | 1.29 (0.85–1.97) | |
| Unemployed | 119 (53.8) | 102 (46.2) | 1.00 (Ref) | |
| Body mass index (BMI) | 0.179 | |||
| < 30 kg/m2 | 88 (60.7) | 57 (39.3) | 1.34 (0.88–2.05) | |
| ≥ 30 kg/m2 | 120 (53.6) | 104 (46.4) | 1.00 (Ref) | |
| Smoking | 0.737 | |||
| No | 196 (56.2) | 153 (43.8) | 1.00 (Ref) | |
| Yes | 12 (60.0) | 8 (40.0) | 1.17 (0.47–2.94) | |
| Alcohol | 0.122 | |||
| No | 158 (54.3) | 133 (45.7) | 1.00 (Ref) | |
| Yes | 50 (64.1) | 28 (35.9) | 1.50(0.90–2.52) | |
| Hypertension | 0.087 | |||
| No | 137 (59.8) | 92 (40.2) | 1.00 (Ref) | |
| Yes | 71 (50.7) | 69 (49.3) | 0.69 (0.45–1.06) | |
| Diabetes | 0.541 | |||
| No | 196 (56.0) | 154 (44.0) | 1.00 (Ref) | |
| Yes | 12 (63.2) | 7 (36.8) | 1.35 (0.52–3.50) | |
| HIV | 0.005 | |||
| Negative | 153 (52.6) | 138 (47.4) | 1.00 (Ref) | |
| Positive | 55 (70.5) | 23 (29.5) | 2.16 (1.26–3.70) | |
| Tuberculosis | 0.960 | |||
| No | 203 (56.4) | 157 (43.6) | 1.00 (Ref) | |
| Yes | 5 (55.6) | 4 (44.4) | 0.97 (0.26–3.66) | |
| Cerebrovascular disease | 0.462 | |||
| No | 203 (56.7) | 155 (43.3) | 1.00 (Ref) | |
| Yes | 5 (45.5) | 6 (54.5) | 0.64 (0.19–2.12) | |
| bAsthma/COPD | 0.544 | |||
| No | 199 (56.1) | 156 (43.9) | 1.00 (Ref) | |
| Yes | 9 (64.3) | 5 (35.7) | 1.41 (0.46–4.30) | |
| Any comorbidity | 0.523 | |||
| No | 81 (54.4) | 68 (45.6) | 1.00 (Ref) | |
| Yes | 127 (57.7) | 93 (42.3) | 1.15 (0.75 -1.74) | |
| Multimorbidity (≥ 2 comorbidities apart from breast cancer) | 0.988 | |||
| No | 173 (56.4) | 134 (43.6) | 1.00 (Ref) | |
| Yes | 35 (56.5) | 27 (43.5) | 1.00 (0.58–1.74) | |
| cTime on tamoxifen in months, median (IQR) | 12.5 (6.3–24.9) | 13.4 (6.1–26.4) | 0.99 (0.97–1.01) | 0.591 |
| Tamoxifen side effect(s) | 0.730 | |||
| No | 97 (55.4) | 78 (44.6) | 1.00 (Ref) | |
| Yes | 111 (57.2) | 83 (42.8) | 1.08 (0.71–1.62) | |
| Number of medications apart from tamoxifen | 0.392 | |||
| 0 | 77 (51.7) | 72 (48.3) | 1.00 (Ref) | |
| 1 | 62(64.6) | 34 (35.4) | 1.71 (1.01–2.89) | |
| 2 | 33 (55.9) | 26 (44.1) | 1.19 (0.65–2.18) | |
| 3 | 21 (56.8) | 16 (43.2) | 1.23 (0.59–2.54) | |
| ≥ 4 | 15 (53.6) | 13 (46.4) | 1.08 (0.48–2.42) | |
| dCYP2D6 predicted phenotype | 0.415 | |||
| UM & NM | 74 (52.5) | 67 (47.5) | 1.00 (Ref) | |
| NM/IM (Normal/intermediate metabolizer) | 35 (59.3) | 24 (40.7) | 1.32 (0.71–2.44) | |
| IM & PM | 21 (63.6) | 12 (36.4) | 1.58 (0.72–3.47) | |
| Stage | 0.751 | |||
| 1 | 18 (56.3) | 14 (43.8) | 1.00 (Ref) | |
| 2 | 107 (53.2) | 94 (46.8) | 0.89 (0.42–1.86) | |
| 3 | 83 (61.0) | 53 (39.0) | 1.22 (0.56–2.65) | |
| Histology | 0.469 | |||
| Invasive ductal carcinoma | 204 (56.7) | 156 (43.3) | 1.00 (Ref) | |
| Others | 4 (44.4) | 5 (55.6) | 0.61 (0.16–2.32) | |
| Histologic grade | 0.496 | |||
| Grade 1 & 2 | 134 (54.7) | 111 (45.3) | 1.00 (Ref) | |
| Grade 3 | 65 (58.6) | 46 (41.4) | 1.17 (0.74–1.84) | |
| Immunohistochemistry-defined subtype | 0.872 | |||
| eHR + /HER2− | 154 (56.6) | 118 (43.4) | 1.04 (0.65–1.66) | |
| HR + /HER2 + | 54 (55.7) | 43 (44.3) | 1.00 (Ref) | |
| Surgery | 0.173 | |||
| No | 13 (72.2) | 5 (27.8) | 2.08 (0.72–5.96) | |
| Yes | 195 (55.6) | 156 (44.4) | 1.00 (Ref) | |
| Type of surgery (N = 348) | 0.779 | |||
| Mastectomy | 150 (56.0) | 118 (44.0) | 1.07 (0.65–1.76) | |
| Wide local excision | 45 (54.2) | 38 (45.8) | 1.00 (Ref) | |
| Chemotherapy | 0.375 | |||
| No | 48 (60.8) | 31 (39.2) | 1.26 (0.76–2.09) | |
| Yes | 160 (55.2) | 130 (44.8) | 1.00 (Ref) | |
| Radiotherapy | 0.933 | |||
| No | 81 (56.6) | 62 (43.4) | 1.02 (0.67–1.55) | |
| Yes | 127 (56.2) | 99 (43.8) | 1.00 (Ref) | |
All percentages shown are row percentages
CI Confidence interval
COPD Chronic obstructive pulmonary disease
IQR Interquartile range
CYP2D6 Cytochrome P450 2D6,
UM Ultra-rapid metabolizer, NM Normal metabolizer, IM intermediate metabolizer, PM Poor metabolizer, UM (n = 10), PM (n = 4)
HR + Hormone receptor-positive, HER2 Human epidermal growth factor receptor 2. Missing for variables: Highest level of education (n = 1), CYP2D6 (n = 135)
Table 4.
Multivariable analysis of factors associated with non-adherence to tamoxifen in women with breast cancer in the SABCHO cohort
| Multivariate analysis | P value | |
|---|---|---|
|
| ||
| OR (95% CI) | ||
| Age at diagnosis (years) | ||
| < 40 | 2.03 (0.63–6.52) | 0.235 |
| 40–49 | 0.72 (0.28–1.87) | 0.503 |
| 50–59 | 1.08 (0.49–2.37) | 0.846 |
| ≥ 60 | 1.00 (Ref) | |
| Marital status | 0.232 | |
| Married/cohabiting | 1.00 (Ref) | |
| Unmarried | 1.44 (0.79–2.63) | |
| HIV | 0.026 | |
| Negative | 1.00 (Ref) | |
| Positive | 2.40 (1.11–5.20) | |
| Hypertension | 0.118 | |
| No | 1.00 (Ref) | |
| Yes | 0.58 (0.29–1.15) | |
Multivariate model adjusted for level of education, time on tamoxifen, tamoxifen side effects, and CYP2D6 (Cytochrome P450 2D6) predicted phenotype
In an exploratory analysis among WLWH, tamoxifen adherent and non-adherent women did not differ in median (IQR) time on antiretroviral therapy [96.0 (60.0–144.0) months vs. 84.0 (48.0–120.0) months, p = 0.328] (Supplementary Fig. 1).
Discussion
In our sample of black women with stages I-III breast cancer, we found that 56.4% were non-adherent to tamoxifen use after a median (IQR) time on tamoxifen of 13.0 (6.2–25.2) months. Women < 40 years and WLWH were more likely than older women and uninfected women to be non-adherent to tamoxifen use. In the adjusted model, WLWH were 2.40 (95% CI = 1.11–5.20) times more likely to be non-adherent to tamoxifen than HIV-negative women.
Women receiving tamoxifen for breast cancer are generally expected to be highly motivated because they have a life-threatening disease against which hormone therapy is effective. Prior population studies on biochemical non-adherence have found a 16% non-adherence rate [20], and medical possession ratio (MPR) / tamoxifen prescription filled rates of 13–31% in HICs [15, 17, 40]. However, a smaller study in Ethiopia by Reibold et al. in 2021 found a 65% non-adherence rate [41], similar to those reported for chronic medications in a meta-analysis ranging from 76.5% for self-report, 69.4% for pharmacy data, and 44.1% for electronic monitoring [42] and much higher than the 21–36% reported in Soweto, South Africa among patients on treatment for chronic heart failure [43].
Our finding in univariate (but not in adjusted) analysis that women < 40 years were at least twice as likely as older women to be non-adherent to tamoxifen use (73.9% vs 52.6%, OR = 2.49, 95% CI = 1.26–4.94) confirmed earlier findings of non-adherence in younger women. It can be speculated that the observed higher non-adherence rate to tamoxifen among younger patients in our study is related to the adverse effects of tamoxifen on women’s sexuality, including menopausal symptoms and fertility issues. Other studies from low- and middle-income countries (LMICs) [44, 45] and HICs [46] have also found young age to be a predictor of tamoxifen non-adherence; in studies focused solely on tamoxifen adherence among young women with breast cancer, the range of reported rates was 18–51% [46–48]. The main reasons younger women reported non-adherence were side effects and not fully understanding the benefits of tamoxifen [44]. We found no significant association of adherence with reported side effects in women < 40 years of age (data not shown). High non-adherence rates in younger women with breast cancer are a special concern and far more complex given their long potential life expectancy and ongoing ovarian function. Most younger women are also on gonadotropin-releasing hormone (GnRH) agonists which can cause the same menopausal symptoms as tamoxifen [49]. Improving tamoxifen adherence, especially in younger women, maybe more important now that longer durations of tamoxifen use or further ovarian function suppression have become the standard of care [50]. Women who experience treatment-related adverse effects may be the ones who benefit most from the therapy, because the adverse effects could be a proxy for therapy response [51]. Women who experience worse treatment-related side effects have been shown to have a lower rate of breast cancer recurrence than those not reporting symptoms [51, 52].
In a previous analysis of the SABCHO cohort, we found that WLWH with breast cancer had worse 2 year overall survival than HIV-negative women with breast cancer (adjusted hazard ratio = 1.49, 95% CI = 1.22–1.83) [5]. A contributing factor could be non-adherence to tamoxifen. We found that WLWH were less-adherent to tamoxifen than HIV-negative women (OR = 2.40, 95% CI = 1.11–5.20). Poor adherence to oral medication is a common problem in hypertension, hyperlipidaemia, diabetes, and HIV management [51–54]. In South Africa, only 54% of all PLWH are virally suppressed, primarily due to suboptimal adherence [55]. For PLWH, as well as for those with other chronic diseases, adherence often decreases over time, due to ‘treatment fatigue’, development of complacency, or loss of motivation [56]. Among WLWH in this cohort, the time on antiretroviral therapy did not predict tamoxifen adherence (Supplementary Fig. 1). A possible reason for non-adherence to tamoxifen among WLWH in this cohort could be drug-drug interactions and the combined side effects of both tamoxifen and anti-retroviral medications.
The CYP2D6 enzyme is involved in the metabolic activation to endoxifen, the therapeutic metabolite [27], and is implicated in cancer formation and treatment. Being on multiple medications whose metabolism depends on the same set of drug metabolising CYP2D6 poses a risk for drug-drug interactions that could affect the safety and efficacy of tamoxifen [57, 58]. We however did not detect any correlation between tamoxifen exposure levels and metabolites and predicted enzyme activities of the involved CYPs. No association of co-medication with antidiabetics or antihypertensives with non-adherence was observed. Individually ART and tamoxifen have been associated with similar side effects, such as hot flushes, fluid retention/bloating, and nausea, which may be transient or may persist throughout treatment [59, 60]. Indeed, we observed trends towards increased hot flushes and vaginal symptoms in WLWH. The side effects from the combination of both medications may be overwhelming for patients, motivating non-adherence to either ART or tamoxifen or both.
Regarding the stage at diagnosis and adherence to tamoxifen, findings have been contradictory. The report by Brito et al. of lower adherence among patients at advanced stages (III and IV) [45] contradicts that of Wigertz et al., who found greater adherence among women with larger tumors [61]. Kimmick et al., like us, found no association between stage and adherence to tamoxifen [62]. Importantly, however, many studies on this topic were based only on cohorts of women with early-stage disease, mainly in HICs, where far more women are diagnosed with early-stage breast cancer than in LMICs.
Nonadherence to tamoxifen for breast cancer is often under-recognized partly because of the lack of a gold standard method for adherence detection and challenges in the measurement of compliance in routine clinical practice. Our study is the largest cohort to estimate non-adherence to tamoxifen biochemically among women with breast cancer with or without HIV in SSA; such measurement is likely to be more accurate than patient self-report, which tends to underestimate non-adherence.
Some limitations should be noted. The tamoxifen concentration threshold used to define biochemical non-adherence was not previously validated, but the approach used was consistent with previous studies [20, 22, 39]. Our sample size was relatively small compared to large studies from HICs. The serum-tamoxifen measurement is only from a single time point, and we do not have data on subsequent relapse; therefore, it is not possible to discriminate between short and longer-term non-adherence. Also, ours was a purely quantitative study; a mixed-method design with qualitative approaches would have enabled us to explore the findings in more depth. We did not consider drug stock-outs at the pharmacy which could have contributed to the prevalence of non-adherence to tamoxifen in our study, but we have no reason to believe that this cause of adherence would impact women differently based on their HIV status. In addition, the baseline HIV-uninfected cohort was not retested during the follow-up period; failure to retest could have underestimated the HIV prevalence in the cohort. The use of GnRH agonists also was not taken into consideration, although patients under 40 years of age might be on these agents, which may cause the same menopausal symptoms as tamoxifen, affecting adherence.
Conclusions
The proportion of non-adherence to tamoxifen use in this current study was higher than that in many other studies; we also reported a higher non-adherence rate in WLWH. Achieving optimal tamoxifen benefits in women with HR-positive breast cancer with or without HIV may require more aggressive screening for treatable side effects, such as hot flushes, and possibly treating women with breast cancer and HIV on ART regimens less likely to interact with tamoxifen. Also, continuous provision of tamoxifen adherence education, with emphasis on the value of the prescribed medication may improve adherence.
Supplementary Material
Acknowledgements
We thank the women who participated in this study. We also thank the dedicated team of study research assistants for their assistance with the study.
Funding
NIH/R01-CA19262701 and R01-CA250012, The South African Medical Research Council/ University of the Witwatersrand Common Epithelial Cancer Research Center (MRC/WITS CECRC), The South African Medical Research Council, Pharmacogenomics in Precision Medicine (MRC-RFA-SHIP 01–2019) award entitled “Understanding the pharmacogenetic and pharmacokinetic basis of tamoxifen (TAM) treatment-failure for breast cancer in black African women”, MRC UK, Cancer Research and Global Health: Pump-Priming Call entitled “Molecular Genetics of Lobular Breast Cancer in a South African cohort and effect of HIV infection” DSO is a K12 Scholar supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA226330. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The Wits Health Consortium (PTY) Ltd provided support in the form of payroll administration of salaries from grant funds for authors OA and MJ but did not have any additional role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Competing interests
AIN has consulted for Otsuka, Eisai, GlaxoSmith-Kline, United biosource Corp, Hospira. He has support from Otsuka. He is on the medical advisory board of EHE Intl. All other authors declare that they have no conflict of interest.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10549-022-06835-6.
Ethics approval This study was approved by the University of the Witwatersrand Human Research Ethics Committee, and the Institutional Review Board of Columbia University in New York, NY.
Consent to participate All women provided written or fingerprint-confirmed informed consent. The study was performed in accordance with the Declaration of Helsinki.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J clin 71(3):209–249 [DOI] [PubMed] [Google Scholar]
- 2.McCormack V, McKenzie F, Foerster M, Zietsman A, Galukande M, Adisa C et al. (2020) Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. Lancet Glob Health 8(9):e1203–e1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chasimpha S, McCormack V, Cubasch H, Joffe M, Zietsman A, Galukande M et al. (2022) Disparities in breast cancer survival between women with and without HIV across sub-Saharan Africa (ABC-DO): a prospective, cohort study. Lancet HIV 9(3):e160–e171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The fifth South African National HIV prevalence, incidence, behavior and communication survey, 2017. (SABSSM V1; ). http://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_Summary_ZA_ADS_cleared_PDFA4.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayeni OA, O’Neil DS, Pumpalova YS, Chen WC, Nietz S, Phakathi B et al. (2022) Impact of HIV infection on survival among women with stage I-III breast cancer: results from the South African breast cancer and hiv outcomes study. Int J Cancer. 10.1002/ijc.33981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K (2016) Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-Saharan Africa: a systematic review. BMJ Glob Health 1(4):e000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Lee E, Park B-J, Bang JH, Lee JY (2018) Adherence to antiretroviral therapy and factors affecting low medication adherence among incident HIV-infected individuals during 2009–2016: a nationwide study. Sci Rep 8(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achieng C, Bunani N, Kagaayi J, Nuwaha F. (2021) Adherence to Antiretroviral and Cancer Chemotherapy, and Associated Factors Among Patients with HIV–Cancer Co-Morbidity at the Uganda Cancer Institute: A Cross Sectional Study. https://www.assets.researchsquare.com/files/rs-770283/v1/bdc7bd46-6a50-4a4f-a13d-cef853661654.pdf?c=1631888864 [DOI] [PMC free article] [PubMed]
- 9.Hennigs A, Riedel F, Gondos A, Sinn P, Schirmacher P, Marmé F et al. (2016) Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer 16(1):734–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L et al. (2015) Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 24:S26–S35 [DOI] [PubMed] [Google Scholar]
- 11.Fowble B, Fein DA, Hanlon AL, Eisenberg BL, Hoffman JP, Sigurdson ER et al. (1996) The impact of tamoxifen on breast recurrence, cosmesis, complications, and survival in estrogen receptorpositive early-stage breast cancer. Int J Radiat Oncol Biol Phys 35(4):669–677 [DOI] [PubMed] [Google Scholar]
- 12.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V et al. (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group EBCTC (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717 [DOI] [PubMed] [Google Scholar]
- 14.Sabaté E, Sabaté E (2003) Adherence to long-term therapies evidence for action. World Health Organization, USA [Google Scholar]
- 15.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99(2):215–220 [DOI] [PubMed] [Google Scholar]
- 16.Moon Z, Moss-Morris R, Hunter MS, Hughes LD (2017) More than just side-effects: the role of clinical and psychosocial factors in non-adherence to tamoxifen. Br J Health Psychol 22(4):998–1018 [DOI] [PubMed] [Google Scholar]
- 17.Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606 [DOI] [PubMed] [Google Scholar]
- 18.Wulaningsih W, Garmo H, Ahlgren J, Holmberg L, Folkvaljon Y, Wigertz A et al. (2018) Determinants of non-adherence to adjuvant endocrine treatment in women with breast cancer: the role of comorbidity. Breast Cancer Res Treat 172(1):167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu CY, Zhang F, Wagner AK, Nekhlyudov L, Earle CC, Callahan M et al. (2018) Impact of high-deductible insurance on adjuvant hormonal therapy use in breast cancer. Breast Cancer Res Treat 171(1):235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pistilli B, Paci A, Ferreira A, Di Meglio A, Poinsignon V, Bardet A et al. (2020) Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. J Clin Oncol 38(24):2762–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C et al. (2004) Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res 10(7):2336–2343 [DOI] [PubMed] [Google Scholar]
- 22.MacCallum J, Cummings J, Dixon J, Miller W (2000) Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br J Cancer 82(10):1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134(2):459–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L et al. (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126(2):529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Herk-Sukel MP, van de Poll-Franse LV, Voogd AC, Nieuwenhuijzen GA, Coebergh JWW, Herings R (2010) Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat 122(3):843–851 [DOI] [PubMed] [Google Scholar]
- 26.McCowan C, Wang S, Thompson A, Makubate B, Petrie D (2013) The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer 109(5):1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karle J, Bolbrinker J, Vogl S, Kreutz R, Denkert C, Eucker J et al. (2013) Influence of CYP2D6-genotype on tamoxifen efficacy in advanced breast cancer. Breast Cancer Res Treat 139(2):553–560 [DOI] [PubMed] [Google Scholar]
- 28.Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW et al. (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23(36):9312–9318 [DOI] [PubMed] [Google Scholar]
- 29.Dezentjé VO, Gelderblom H, Van Schaik RH, Vletter-Bogaartz JM, Van der Straaten T, Wessels JA et al. (2014) CYP2D6 genotype in relation to hot flashes as tamoxifen side effect in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trial. Breast Cancer Res Treat 143(1):171–179 [DOI] [PubMed] [Google Scholar]
- 30.Binkhorst L, Mathijssen RH, Jager A, van Gelder T (2015) Individualization of tamoxifen therapy: much more than just CYP2D6 genotyping. Cancer Treat Rev 41(3):289–299 [DOI] [PubMed] [Google Scholar]
- 31.Tinari N, Fanizza C, Romero M, Gambale E, Moscetti L, Vaccaro A et al. (2015) Identification of subgroups of early breast cancer patients at high risk of nonadherence to adjuvant hormone therapy: results of an Italian survey. Clin Breast Cancer 15(2):e131–e137 [DOI] [PubMed] [Google Scholar]
- 32.Paranjpe R, John G, Trivedi M, Abughosh S (2019) Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res Treat 174(2):297–305 [DOI] [PubMed] [Google Scholar]
- 33.Rangel-Méndez JA, Rubi-Castellanos R, Sánchez-Cruz JF, Moo-Puc RE (2019) Tamoxifen side effects: pharmacogenetic and clinical approach in Mexican mestizos. Transl Cancer Res 8(1):23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berretta M, Caraglia M, Martellotta F, Zappavigna S, Lombardi A, Fierro C et al. (2016) Drug-drug interactions based on pharmacogenetic profile between highly active antiretroviral therapy and antiblastic chemotherapy in cancer patients with HIV infection. Front Pharmacol 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE et al. (2014) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol 32(21):2255–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cubasch H, Ruff P, Joffe M, Norris S, Chirwa T, Nietz S et al. (2017) South African breast cancer and HIV outcomes study: methods and baseline assessment. J Glob Oncol 3(2):114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edge SB, Compton CC (2010) The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474 [DOI] [PubMed] [Google Scholar]
- 38.Caudle KE, Sangkuhl K, Whirl-Carrillo M, Swen JJ, Haidar CE, Klein TE et al. (2020) Standardizing CYP 2D6 genotype to phenotype translation: consensus recommendations from the clinical pharmacogenetics implementation consortium and dutch pharmacogenetics working group. Clin Transl Sci 13(1):116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saladores P, Mürdter T, Eccles D, Chowbay B, Zgheib N, Winter S et al. (2015) Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogen J 15(1):84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neugut AI, Zhong X, Wright JD, Accordino M, Yang J, Hershman DL (2016) Nonadherence to medications for chronic conditions and nonadherence to adjuvant hormonal therapy in women with breast cancer. JAMA Oncol 2(10):1326–1332 [DOI] [PubMed] [Google Scholar]
- 41.Reibold CF, Tariku W, Eber-Schulz P, Getachew S, Addisie A, Unverzagt S et al. (2021) Adherence to newly implemented tamoxifen therapy for breast cancer patients in rural Western Ethiopia. Breast Care 16(5):484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foley L, Larkin J, Lombard-Vance R, Murphy AW, Hynes L, Galvin E et al. (2021) Prevalence and predictors of medication non-adherence among people living with multimorbidity: a systematic review and meta-analysis. BMJ Open 11(9):e044987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruf V, Stewart S, Pretorius S, Kubheka M, Lautenschläger C, Presek P et al. (2010) Medication adherence, self-care behaviour and knowledge on heart failure in urban South Africa: the heart of soweto study. Cardiovasc J Afr 21(2):86–92 [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Cannon BA, Castro-Sanchez A, Barragan-Carrillo R, de la Rosa PS, Platas A, Fonseca A et al. (2021) Adherence to adjuvant tamoxifen in Mexican young women with breast cancer. Patient Prefer Adher 15:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brito C, Portela MC, de Vasconcellos MTL (2014) Adherence to hormone therapy among women with breast cancer. BMC Cancer 14(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huiart L, Bouhnik A-D, Rey D, Tarpin C, Cluze C, Bendiane MK et al. (2012) Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer 48(13):1939–1946 [DOI] [PubMed] [Google Scholar]
- 47.Cluze C, Rey D, Huiart L, BenDiane M, Bouhnik A, Berenger C et al. (2012) Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol 23(4):882–890 [DOI] [PubMed] [Google Scholar]
- 48.Wassermann J, Gelber SI, Rosenberg SM, Ruddy KJ, Tamimi RM, Schapira L et al. (2019) Nonadherent behaviors among young women on adjuvant endocrine therapy for breast cancer. Cancer 125(18):3266–3274 [DOI] [PubMed] [Google Scholar]
- 49.Christinat A, Di Lascio S, Pagani O (2013) Hormonal therapies in young breast cancer patients: when, what and for how long? J Thorac Dis 5(Suppl 1):S36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA Jr, Bianchi-Micheli G et al. (2020) ESO–ESMO 4th International consensus guidelines for breast cancer in young women (BCY4). Ann Oncol 31(6):674–696 [DOI] [PubMed] [Google Scholar]
- 51.Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA (2017) Nonadherence to antihypertensive drugs a systematic review and meta-analysis. Medicine 96(4):e5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rwegerera GM (2014) Adherence to anti-diabetic drugs among patients with type 2 diabetes mellitus at muhimbili national hospital, dar es salaam, Tanzania-a cross-sectional study. Pan Afr Med J 17:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kardas P (2013) Prevalence and reasons for non-adherence to hyperlipidemia treatment. Cent European JMed 8(5):539–547 [Google Scholar]
- 54.Bhat V, Ramburuth M, Singh M, Titi O, Antony A, Chiya L et al. (2010) Factors associated with poor adherence to anti-retroviral therapy in patients attending a rural health centre in South Africa. Eur J Clin Microbiol Infect Dis 29(8):947–953 [DOI] [PubMed] [Google Scholar]
- 55.Bessong PO, Matume ND, Tebit DM (2021) Potential challenges to sustained viral load suppression in the HIV treatment programme in South Africa: a narrative overview. AIDS Res Ther 18(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayer KH, Stone VE (2001) Strategies for optimizing adherence to highly active antiretroviral therapy: lessons from research and clinical practice. Clin Infect Dis 33(6):865–872 [DOI] [PubMed] [Google Scholar]
- 57.Miller CD, El-Kholi R, Faragon JJ, Lodise TP (2007) Prevalence and risk factors for clinically significant drug interactions with antiretroviral therapy. Pharmacotherapy 27(10):1379–1386 [DOI] [PubMed] [Google Scholar]
- 58.Badowski M, Burton B, Shaeer K, Dicristofano J (2019) Oral oncolytic and antiretroviral therapy administration: Dose adjustments, drug interactions, and other considerations for clinical use. Drugs Context 8:1–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorizio W, Wu AHB, Beattie MS, Rugo H, Tchu S, Kerlikowske K et al. (2012) Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat 132(3):1107–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Looby SE, Shifren J, Corless I, Rope A, Pedersen MC, Joffe H et al. (2014) Increased hot flash severity and related interference in perimenopausal human immunodeficiency virus-infected women. Menopause 21(4):403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wigertz A, Ahlgren J, Holmqvist M, Fornander T, Adolfsson J, Lindman H et al. (2012) Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res Treat 133(1):367–373 [DOI] [PubMed] [Google Scholar]
- 62.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R (2009) Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol 27(21):3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


