Summary
Background
Adult T-cell leukemia/lymphoma (ATL), one of the most aggressive cancers in the world, occurs in 5% of the 10 million people living with HTLV-1 worldwide. French Guiana, a French overseas territory in South America, is one of the highest endemic areas of HTLV-1 worldwide. Here, we describe the demographic and clinical characteristics and outcome of ATL in this area.
Methods
We retrospectively collected data from all patients diagnosed between 2009 and 2019. Patients were distributed according to Shimoyama's classification. Prognostic factors were explored through univariate analysis.
Findings
Over the 10-year study period, 41 patients with a median age of 54 years at diagnosis were identified, among whom 56% were women. Sixteen (39%) patients were Maroons, a cultural group descendant of the runaway enslaved Africans from former Dutch Guiana. Among the study population, 23 (56%) had an acute type, 14 (34%) a lymphoma type, and one and one chronic and primary cutaneous tumour, respectively. First-lines of treatment included either chemotherapy or Zidovudine combined with pegylated interferon alpha. The 4-year overall survival was 11.4% for the entire population with 0% and 11% for lymphoma and acute forms, respectively. The median progression-free survival was 93 and 115 days for the acute and lymphoma groups (p = 0.37), respectively. Among the twenty-nine patients who died, 8 (28%) died of toxicity, 7 (24%) died of disease progression and the cause of death remained unknown in 14 (48%) patients. Due to the overall poor prognosis, no significant prognostic factors could be identified.
Interpretation
This study provides real-life data from ATL patients in French Guiana, a remote territory in a middle-income region. Patients, mostly Maroons, presented with a younger age and the prognosis was worse than expected compared to Japanese patients.
Funding
None.
Keywords: ATL, HTLV-1, French Guiana, Prognosis, Retrospective cohort
Research in context.
Evidence before this study
Recent international efforts, coordinated by the WHO, shed light on the HTLV-1 retrovirus and associated pathologies (WHO report on HTLV-1, March 2021). Adult T-cell leukaemia/lymphoma (ATL) is one of the most aggressive cancers worldwide and is associated with HTLV-1. This disease concerns 5% of the 10 million people living with HTLV-1 around the world. The report published in February 2023 by Satsuki Owatari et al., presented a decrease in newly diagnosed patients with ATL in Japan, one of the most HTLV-1 endemic region. Meanwhile, recent studies in Latin America have highlighted some particularities of ATL in the region: younger age at diagnostic and worse survival rates compared with Japanese patients. French Guiana is one of the highest HTLV-1 endemic areas, yet data concerning ATL are lacking.
Added value of this study
In this multicentre, retrospective study, we collected data from 41 patients that developed ATL between 2009 and 2019 in French Guiana. Overall, our results are in line with recent publications from the American subcontinent (Malpica, 2021) with, however, some specificities. We demonstrated that the predominant form was the acute one. Most patients were women and Maroons, a cultural group descendant of enslaved Africans. We also demonstrated that the prognosis was extremely poor with overall survival of only 11% at 4 years. Our analysis is the first to evaluate data from ATLs in the region, it will aim to be the benchmark for future studies.
Implications of all the available evidence
Patients suffering from ATL in French Guiana unfortunately have an appalling prognosis. Definite efforts in terms of public health must be put in place to determine the carriers of HTLV-1 and to detect ATL early. A specific course of care must be established for these pathologies with, for example, debulking chemotherapy and rapid referral to the expert centre (Hospital Necker, Paris).
Introduction
The human T-cell leukaemia virus type-1 (HTLV-1) is the first reported human oncogenic retrovirus.1 HTLV-1 is the causative agent of a severe form of leukaemia/lymphoma designated Adult T-cell Leukemia/Lymphoma (ATL) and characterized by the malignant transformation of CD4+ T-cells.2 It may also cause a severe neurological disorder designated HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP), a progressive and chronic neuro-myelopathy characterized by spastic paraparesis, sensory dysfunction and sphincter function defects.3
Worldwide, HTLV-1 infects at least approximately 5–10 million people who are mostly asymptomatic.4 However, 2–7% of people living with HTLV-1 will develop HAM/TSP or ATL.2,3 ATL usually arises after decades of asymptomatic and chronic HTLV-1 infection.5 Risk factors to develop ATL have been identified that include contamination early in life through breastfeeding, co-infection with Strongyloides stercoralis, high-proviral load (>4%, proportion of infected peripheral blood mononuclear cells, PBMC) and possibly somatic genetic mutations of infected cells.6, 7, 8, 9 ATL prognosis is generally poor. Prognostic factors are classically age >70 years, visceral involvement, poor performance status, hypercalcemia and high serum lactic dehydrogenase (LDH) level.10
Standards of care include chemotherapy and/or the combination of Zidovudine and interferon-alpha including its pegylated form.11 Allogeneic stem cell transplantation is the only curative treatment.12 But it is only accessible in high income countries, which are often distinct from the HTLV-1 endemic areas.13
The high HTLV-1 infection endemic regions are Southwestern Japan, Australo-Melanesia, the Middle East, South America, sub-Saharan Africa and the Caribbean area.14 In South America, the highest prevalence is found in the Dominican Republic, north-eastern Brazil, Haiti, Peru and French Guiana.15 French Guiana is the largest French overseas territory with an area of 83,846 km2. Its inhabitants are composed of different cultural populations including Aboriginal populations (Amerindians and Maroons), Creoles (many coming from French West Indies), Europeans, Chinese, Hmong, Haitians, Brazilians. In addition, many Surinamese (often of Maroon culture) live in the western part of French Guiana (Saint-Laurent du Maroni).
Recently, a study from the Grupo de Estudio Latino-americano de Linfoproliferativos reported real-world data on ATL in South America, outside the Guiana shield, on 256 patients during a 25 years period.16 This study highlighted features of ATL in Latin America such as a younger age, a high incidence of lymphomatous type, a low incidence of indolent subtypes and worse survival rates in comparison of Japanese patients. These distinctive features in Latin America may reflect differences in environmental and socioeconomic factors, access to care and treatment, virological factors, or host immunogenetics. French Guiana has singular features that may have a significant impact on ATL characteristics. Its population often has African ancestry and the health system is French (it has the highest health expenditure per capita in Latin America), hence diagnostic and treatment costs are less a limiting factor. Yet, over half of the population lives under the poverty threshold. In addition, parasitic diseases, notably strongyloidiasis, are still highly prevalent within some communities.17,18 The purpose of the present study was thus to describe the demographic, clinical and outcome characteristics of ATL in French Guiana. Thus, this study will provide the first description, the benchmark for further studies of the ATLs in French Guiana and will allow a better prioritization of the targets for action.
Methods
Study design
We conducted a retrospective, multicentre, observational, hospital-based study of a cohort of newly diagnosed ATL patients managed between 2009 and 2019 in the hospitals of Cayenne, Kourou, and Saint-Laurent du Maroni in French Guiana.
Patients’ selection
Cases identification was based on International Classification of Disease and were cross-checked with the Pathology Department and specific hospital data bases. The diagnosis of ATL was based on serologic evidence of HTLV-1 by enzyme-linked immunosorbent assay (ELISA) confirmed by Western Blot. Identification of clonal CD4+ CD7− CD25+ (−) T-cells was determined in peripheral blood and/or in tissues.
Data base collection
All data were collected by the first author (KA) by the end of July 2021 and the data cleaning process was completed by the end of October 2021. Patients’ charts were anonymized. Demographic data collected were date of birth, age, gender, birth and living place, language (allowing to indirectly define cultural group), date of first symptoms and diagnosis, date of last follow-up and status. General medical informations were medical background, comorbidities, weight, Human Immunodeficiency Virus (HIV) serology results. ATL characteristics data included, initial symptoms (“B” symptoms and weight loss), liver, spleen, superficial and deep lymph nodes enlargements, visceral involvement on CT scan, serum creatinine level, calcemia, LDH, β2-microglobulin level, gut invasion by Strongyloides stercoralis, HTLV-1 ELISA and Western-Blot, Complete blood count (CBC), blood cytology (search of atypical lymphocytes), marrow blood smear, blood immuno-phenotype, lymph node pathological pattern. ATL was classified according to the Shimoyama classification as previously described (smouldering, chronic, lymphoma and acute).19 Recently, it has been proposed to add Primary Cutaneous Tumor of ATL (PCT-ATL) as a specific clinical entity to this classification in case of exclusive cutaneous manifestation.20 Data on treatment included: supportive care (deworming, hypercalcemia management), specific treatment (chemotherapy protocol, Interferon, allogenic stem-cell transplantation), number of cycles of chemotherapy, lines of treatment, Central Nervous System (CNS) prophylaxis. Six patients were missing data for outcome. These patients were treated as censored in the survival analysis. Outcomes data included treatment response, treatment complications (particularly haematological toxicities), date of last follow-up and status (death of disease, of toxicities, of other cause, alive with disease and alive with no evidence of disease).21,22
Statistical analysis
The study population number allowed to stratify into “acute” and “lymphoma” subgroups. Continuous variables were summarized as medians, interquartile ranges and were compared using Mann Whitney tests. Differences in categorical variable frequencies were compared using Fisher's exact test. Follow-up duration was calculated from the date of treatment initiation to the date of death or the last follow-up information. Progression-free survival was estimated using the Kaplan–Meier method and comparison between groups was performed using the Logrank test. To evaluate prognostic factors, we used a Cox model. Graphical and statistical analyses were performed using GraphPad Prism 9.0™ (GraphPad Software). The two-sided significance level of the p-value was chosen as 0.05.
Regulatory and ethical aspects
The study protocol was approved by the ethics committee and institutional review board of French Guiana (F20211125135043). The ethical committee waived the need for written informed consent because of the retrospective nature of the study and anonymous data collection. The hospitals databases were declared according to the reference methodology MR-004 and the European General Data Protection (GRPD) guidelines.
Role of the funding source
This study received no funds.
Results
Patients’ characteristics
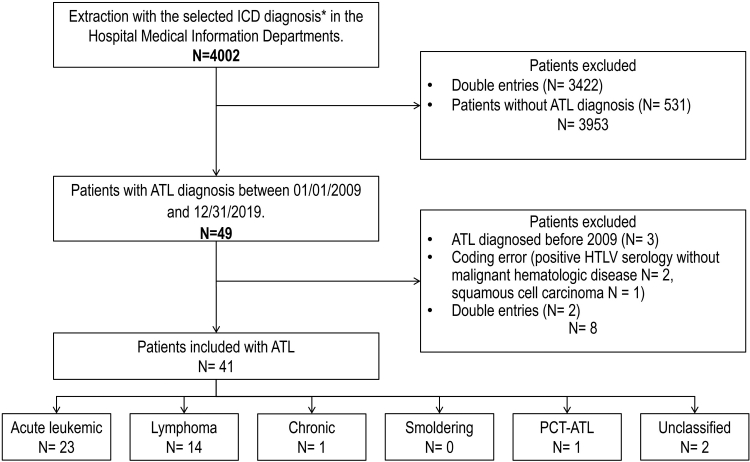
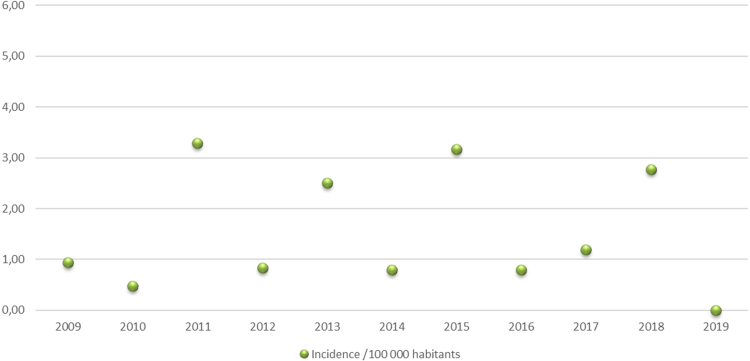
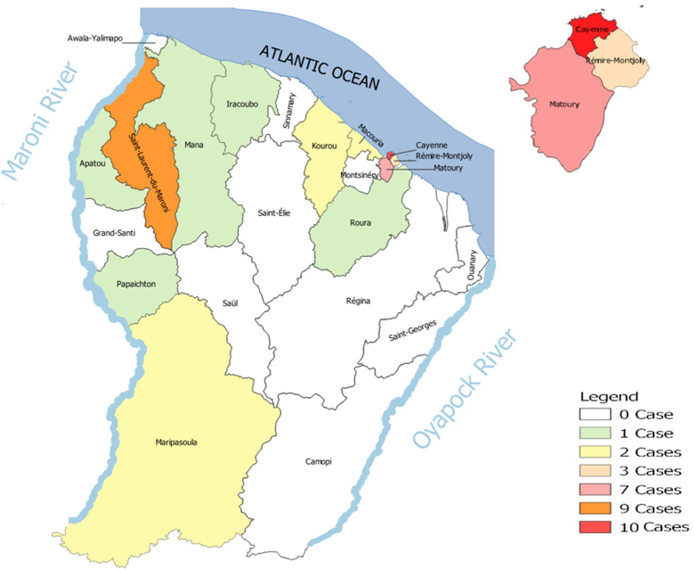
Between January 2009 and December 2019 in French Guiana hospitals, out of 4022 cases analysed, forty-one patients were newly diagnosed as having ATL (Fig. 1). Retrospectively, this represented an estimated incidence of 1.52 per 100,000 inhabitants. The incidence per year of ATL, varied between 1 and 6 per year in French Guiana and is represented in Fig. 2. When focusing on different population the incidence was estimated at 1.83 along the Maroni River (for a population of approximately 93,000 inhabitants) and at 1.6 in the Creole population (for a population estimated at between 80,000 and 110,000) (Fig. 3 represents the place of patients’ residence at diagnosis).
Fig. 1.
Flow chart of the study. ∗International Classification of Diseases (ICD) selected: C77 Secondary and unspecified malignant neoplasm of lymph nodes, C81 Hodgkin lymphoma, C82 Follicular lymphoma, C83 Non-follicular lymphoma, C84 Mature T/NK-cell lymphomas, C85 Other specified and unspecified types of non-Hodgkin lymphoma, C86 Other specified types of T/NK-cell lymphoma, C88 Malignant immunoproliferative diseases and certain other B-cell lymphomas, C91 Lymphoid leukaemia, C92 Myeloid leukaemia, C93 Monocytic leukaemia, C94 Other leukaemia's of specified cell type, C95 Leukaemia of unspecified cell type, C96 Other and unspecified malignant neoplasms of lymphoid, hematopoietic and related tissue.
Fig. 2.
Incidence of ATL in French Guiana from 2009 to 2019.
Fig. 3.
Place of patients' residence at diagnosis.
Patients’ characteristics are described in Table 1. The majority (n = 38; (93%)) of patients presented an aggressive form of ATL with acute-type (n = 23; (56%)), and lymphoma-type (n = 14 (34%)), and one presented a Primary Cutaneous Tumor of ATL (PCT-ATL). One patient was diagnosed with an indolent chronic form of ATL. Two cases remained unclassified because clinical and laboratory data were missing. No case of smoldering-ATL was diagnosed during this period. The median age at diagnosis was 54 years (range 17–88; IQR 42–64). Among the overall study population, 56% were female patients (Male/Female ratio of 0.78), and there was a female predominance in acute-type and a male predominance in lymphoma-type. Data on cultural group were available in 25 (61%) patients of whom the majority were Maroons (n = 16 (64%)). When looking at the place of birth, the ATL population was consistent with the great diversity of the French Guianese population including 14 (34%) born in French Guiana, 14 (34%) in Suriname, 3 (7%) in Haiti, 2 (5%) in Guyana, 2 (5%) in Saint Lucia, 1 (2%) in Dominican Republic, 2 (5%) in French West Indies and 1 (2%) in Ivory Coast.
Table 1.
Categorical outcomes of ATL patients at diagnosis and sanitary evacuation status.
| Characteristic | Unit | All N = 41 | ATL subtypes according to Shimoyama's |
|||
|---|---|---|---|---|---|---|
| Acute N = 23 | Lymphoma N = 14 | Chronic N = 1 | PCT-ATL N = 1 | |||
| Female gender | N (%) | 23 (56%) | 16 (70%) | 5 (36%) | 1 (100%) | 1 (100%) |
| Male gender | N (%) | 16 (39%) | 7 (30%) | 9 (64%) | 0 | 0 |
| Extranodal involvement | ||||||
| Bone marrow | N (%) | 7 (17%) | 3 (13%) | 4 (29%) | – | – |
| Skin | N (%) | 16 (39%) | 11 (48%) | 4 (29%) | Crusted scabies | 1 (100%) |
| CNS | N (%) | 1 (2%) | – | 1 (7%) | 0 | 0 |
| Hepatic | N (%) | 9 (22%) | 7 (30%) | 2 (14%) | 0 | 0 |
| Bone | N (%) | 7 (17%) | 2 (9%) | 5 (36%) | 0 | 0 |
| Lung | N (%) | 5 (12%) | 3 (13%) | 2 (14%) | 0 | 0 |
| GI tractus | N (%) | 3 (7%) | 2 (9%) | 1 (7%) | 0 | 0 |
| Co-infections and co-morbidities | ||||||
| HIV | N (%) | 3 (7%) | 1 (4%) | 2 (14%) | 0 | 0 |
| HBV | N (%) | 3 (7%) | 1 (4%) | 2 (14%) | 0 | 0 |
| HCV | N (%) | 1 (2%) | – | 1 (7%) | 0 | 0 |
| Cancer | N (%) | 2 (5%) | 2 (9%) | – | 0 | 0 |
| Strongyloides stercolaris | N (%) | 4 (10%) | 2 (9%) | 2 (14%) | 0 | 0 |
| Dys-immnune | N (%) | 1 (2%) | – | 1 (7%) | 0 | 0 |
| Thrombosis | N (%) | 4 (10%) | 4 (17%) | – | 0 | 0 |
| Pancreatitis | N (%) | 4 (10%) | 3 (13%) | 1 (7%) | 0 | 0 |
| Sanitary evacuation | ||||||
| La Martinique | Paris | Total | ||||
| N (%) | 1 (9%) | 9 (81%) | 10 (100%) | |||
Abbreviations: PCT-ATL: Primary Cutaneous Tumor-Adult T-cell Leukemia; CNS: Central Nervous System; GI: Gastro Intestinal; HIV: Human Immunodeficiency virus; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus.
Extranodal involvement is detailed in Table 1. Skin lesions attributable to the disease were described in 16 (39%) patients. Among them, 48% presented the acute-type of ATL. Hypercalcemia was associated with acute-type (69.5% vs. 42.8% in the lymphoma-type; p = 0.015) with a median calcemia level of 3.66 mmol/L and 2.50 mmol/L in the acute and the lymphoma types, respectively (Table 2).
Table 2.
Quantitative outcomes of ATL patients at diagnosis and sanitary evacuation status.
| Characteristic | Unit | All N = 41 | ATL subtypes according to Shimoyama's |
p | |||
|---|---|---|---|---|---|---|---|
| Acute N = 23 | Lymphoma N = 14 | Chronic N = 1 | PCT-ATL N = 1 | ||||
| Age at diagnosis | Median (IQR) years | 54.5 (17–88) | 54 (30–88) | 61 (17–78) | 59 | 42 | 0.637 |
| Leukocytes | G/L (range) | 12 (1.6–120) | 44.4 (3.9–120) | 7 (1.6–15) | 6.8 | 2.4 | 0.0002 |
| Abnormal lymphocytes | N/mm 3 (% of Leukocytes) | – | 35,000 (48.9%) | 0 | 0 | NA | 0.0001 |
| Creatinine | μmol/L (range) | 76 (42–403) | 89 (44–403) | 73.5 (42–137) | 55 | 61 | 0.2844 |
| LDH | UI/L (range) | 790 (221–12,739) | 820 (221–3351) | 703.5 (286–12739) | 367 | 683 | 0.675 |
| Calcemia | mmol/L (range) | 3.1 (2.09–6.5) | 3.66 (2.3–6.5) | 2.5 (2.09–4.8) | 2.51 | 2.48 | 0.0155 |
| Hypercalcemia at diagnosis | % | 55% | 69.5% | 42.8% | 0 | 0 | 0.0155 |
| Time interval from diagnosis to initiation of treatment | Median (IQR) days | 13 (7–29) | 12 (7–25) | 15.5 (7–30) | 0 | 0 | 0.8 |
| Median survival time from the initiation of treatment | Median (IQR) days | 107 (51–257) | 92 (51–259) | 108 (51–124) | 0 | 0 | 0.42 |
Quantitative outcomes and sanitary evacuation of 41 ATL patients diagnosed between 2009 and 2019.
Abbreviations: PCT-ATL: Primary Cutaneous Tumor-Adult T-cell Leukemia; IQR: Interquartile; LDH: Lactate Dehydrogenase.
Co-infections and co-morbidities are summarized in Table 1. Viral co-infections were seen in 6 (14%) patients including three (7%) with HIV of whom two were diagnosed lymphoma-type and one acute-type of ATL. One had an undetectable viral load while receiving antiretroviral therapy (ART) (CD4 status unknown), one had a CD4 count of 344/mm3 and a high blood viral load and one had a CD4 count of 364/mm3 and a detectable blood viral load while treated by ART. One patient was co-infected with both HCV (Hepatitis C Virus) and HIV and was diagnosed lymphoma-type ATL. Three (7%) other patients were co-infected with HBV (Hepatitis B Virus) of whom two were diagnosed lymphoma-type and one acute-type.
Four out of 16 (25%) patients tested for Strongyloides stercoralis were carriers (2 lymphoma-type and 2 acute-type ATL). Overall, 16 patients received Ivermectin. Interestingly, among Strongyloides stercoralis carriers, 3 had no blood hypereosinophilia. Four other parasites were present in the stools of patients of the cohort including 3 non-pathogenic amoeba (Entamoeba hartmanni, Entamoeba coli, Endolimax nana) and one nematode (Ankylostomatidae).
Two patients presented a cancer before the onset of ATL, one with breast cancer and one with endometrial adenocarcinoma. All of them were diagnosed as having acute-type ATL. One patient presented a Rheumatoid Arthritis and Sjögren's Syndrome, diagnosed 3 years before the onset of a lymphoma-type ATL and was treated with Methotrexate and Rituximab.
Treatment
The overall median interval from diagnosis to initiation of treatment was 13 days and did not differ significantly between acute and lymphoma types of ATL (Table 2). Among the 41 patients, 32 (78%) had complete data on the treatment (30 patients with an aggressive form and 2 patients with a chronic form). They received at least one line of treatment. Table 3 shows the details of the first three treatment lines of both chronic (n = 2) and aggressive forms (n = 30). First line chemotherapy treatments were CHOP-like regimens (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone), AZT/IFN (Zidovudine, Interferon alpha) alone or in combination. Only 11 patients received a second line treatment out of 32 who received a first line. Finally, 4 patients were treated in the third line. The patient with chronic-type ATL was treated since 2012 with AZT/IFN and was still well at last check-up. The patient with PCT-ATL received AZT/IFN before switching to CHOP one year later. CNS prophylaxis consisted exclusively in intra-thecal injection of methotrexate. CNS prophylaxis was administered in 7 (30%) patients with acute type and 4 (29%) patients with lymphoma type. Patients who were unstable or with rapid disease progression in the setting of vital emergency received no specific treatment.
Table 4.
Treatment responses to first line chemotherapy of the 32 patients of the study that received systemic therapy.
| Treatment Response | ATL subtypes |
|||
|---|---|---|---|---|
| Acute | Lymphoma | Chronic | PCT-ATL | |
| CR | 1 (5%) | 0 | 1 (100%) | 0 |
| PR | 5 (26%) | 2 (17%) | 0 | 1 (100%) |
| ORR | 6 (31%) | 2 (17%) | 1 (100%) | 1 (100%) |
| PD | 11 (58%) | 9 (75%) | 0 | 0 |
| SD | 2 (11%) | 1 (8%) | 0 | 0 |
Abbreviations: HR: Hazard Ratio; LDH: Lactate Dehydrogenase; IQR: Interquartile; CI: Confidence Interval.
Abbreviations: CR: Complete Remission; PR: Partial Remission; PD: Progressive Disease; SD: Stable Disease; ORR: Overall Response Rate (CR and PR); PCT-ATL: Primary Cutaneous Tumor-Adult T-cell Leukemia/Lymphoma.
Health transfer was proposed to 11 (27%) patients: to Martinique (n = 1) and to Paris (n = 9) in three different hospitals (Necker, Saint-Louis and Saint-Antoine hospitals). No allogenic stem-cell transplantation was performed due to uncontrolled diseases.
Treatment responses
Table 4 summarizes responses to initial treatment for patients with aggressive types of ATL. Of 18 patients with acute-type ATL receiving any treatment, 1 achieved a complete response (CR), 5 (26%) achieved a partial response (PR) but a majority (n = 11 (58%)) had disease progression despite treatment. Of 12 patients with lymphoma-type ATL receiving any treatment, none achieved CR status, and 2 (17%) achieved PR but 9 (75%) experienced a progression.
Table 5.
Prognosis factors of survival of ATL patients.
| Variable | Unit | Deceased median (IQR) | Survivors median (IQR) | HR | 95% CI | p value |
|---|---|---|---|---|---|---|
| LDH | UI/L | 798 (465.3–1517) | 899 (169.5–2314) | 1 | 1 to 1 | 0.423 |
| Creatinin | 79 (60–120) | 85.5 (58.75–145) | 1.007 | 1–1.013 | 0.041 | |
| Age at diagnosis | Years | 54 (42–64) | 62 (53–72.5) | 0.998 | 0.969–1.029 | 0.916 |
| Gender | Male gender | – | – | 1.401 | 0.551–3.560 | 0.479 |
| Weight | Kg | 71 (57–82) | 63.5 (53.75–90.5) | 0.999 | 0.971–1.028 | 0.955 |
| Natremia | mmol/L | 136 (134–140) | 138 (133–140) | 1.065 | 0.985–1.151 | 0.112 |
| Kaliemia | mmol/L | 3.9 (3.4–4.5) | 4 (3.2–4.8) | 1.041 | 0.573–1.889 | 0.896 |
| Uric acid | mmol/L | 550 (407–679) | 578 (444–725) | 1.006 | 1–1.012 | 0.071 |
| Calcemia | mmol/L | 2.7 (2.4–4.1) | 2.5 (2.37–3.54) | 1.086 | 0.773–1.524 | 0.635 |
| Calcemia maxima | mmol/L | 3.55 (2.7–4.85) | 3.2 (2.51–3.61) | 1.275 | 0.879–1.849 | 0.201 |
| Hemoglobin | g/dL | 11.4 (10.4–13.2) | 11.5 (9.7–12.8) | 0.907 | 0.742–1.109 | 0.343 |
| Platelet | G/L | 243 (175–329) | 198 (113–250) | 1 | 1 to 1 | 0.443 |
| Deworming | Presence | – | – | 0.680 | 0.280–1.652 | 0.394 |
| « B » signes | Presence | – | – | 1.602 | 0.556–4.617 | 0.383 |
| Skin involvement | Presence | – | – | 1.3 | (0.43–5.5) | 0.91 |
Abbreviations: HR: Hazard Ratio; LDH: Lactate Dehydrogenase; IQR: Interquartile; CI: Confidence Interval.
Abbreviations: CR: Complete Remission; PR: Partial Remission; PD: Progressive Disease; SD: Stable Disease; ORR: Overall Response Rate (CR and PR); PCT-ATL: Primary Cutaneous Tumor-Adult T-cell Leukemia/Lymphoma.
Outcomes and causes of death
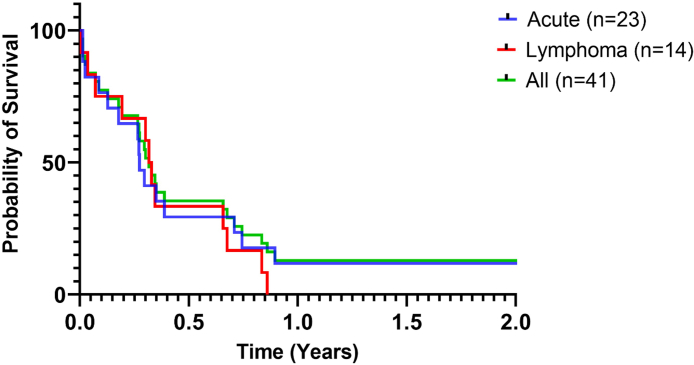
Progression-free survival (PFS) time from the initiation of treatment to death were 93 and 115 days for the acute and lymphoma types, respectively. At the time of last follow-up, 29 (71%) of the 41 patients had died and 6 (15%) were still alive. Six patients were missing data for outcome. At 28 days, PFS was 77%, 76%, 100% and 100% for the acute, lymphoma, chronic and PCT-ATL, respectively. The 1-year survival was 11, 4% for all patients with 0% and 11% in the lymphoma and acute type, respectively. Among the twenty-nine patients who died, 8 (28%) died of toxicity with infections in the foreground and 7 (24%) died from disease progression—mostly from hypercalcemia. However, the cause of death remained unknown for 14 (48%) patients. For patients with lymphoma-type and acute-type the most common cause of death was infection (n = 4 (29%)) and disease progression (n = 6 (30%)), respectively. The patient with PCT-ATL died of unknown cause. The patient with chronic-ATL was still alive with no evidence of disease, under treatment, 10 years after the diagnosis. Clinical outcomes of patients and PFS curves are shown in Table 4 and Fig. 4.
Fig. 4.
Progression-free survival (Kaplan–Meier) by adult T-cell leukemia/lymphoma (ATL) subtype among 41 patients on the period 2009–2019.
Prognostic factors
In univariate analysis no prognostic factor reached statistical significance. However, a trend of a poor prognosis was associated with higher serum creatinine and uric acid levels. Given the absence of significant univariate factors and the lack of sample size, no multivariate analysis was performed (Table 5).
Table 3.
First three therapy approaches in acute and lymphoma types of ATL patients.
| First therapy approaches in ATL patients | ||||
|---|---|---|---|---|
| Subtypes of ATL | Patients treated, n (% of patients) | CHOP-like alone n (% of treated patients) | AZT/IFN alone n (% of treated patients) | AZT/IFN plus chemotherapy n (% of treated patients) |
| Acute (n = 23) | 18 (78%) | 5 (27%) | 9 (50%) | 4 (22%) |
| Lymphoma (n = 14) | 12 (85%) | 6 (50%) | 1 (8%) | 5 (42%) |
| Chronic (n = 1) | 1 (100%) | 0 | 1 (100%) | 0 |
| PCT-ATL (n = 1) | 1 (100%) | 0 | 1 (100%) | 0 |
| Second line therapy approaches | |||||
|---|---|---|---|---|---|
| CHOP-like alone n (% of treated patients) | DHAOx n (% of treated patients) | Bendamustine and idelalisib n (% of treated patients) | Ritonavir and interferon n (% of treated patients) | ||
| Acute (n = 23) | 6 (26%) | 6 (100%) | 0 | 0 | 0 |
| Lymphoma (n = 14) | 5 (36%) | 2 (40%) | 1 (20%) | 1 (20%) | 1 (20%) |
| Chronic (n = 1) | 0 | 0 | 0 | 0 | 0 |
| PCT-ATL (n = 1) | 1 (100%) | 1 (100%) | 0 | 0 | 0 |
| Third line therapy approaches | ||||
|---|---|---|---|---|
| DHAP n (% of treated patients) | Lamivudine-zidovudine-Interferon-Etoposide n (% of treated patients) | Bendamustine and idelalisib n (% of treated patients) | ||
| Acute (n = 23) | 2 (9%) | 1 (50%) | 0 | 1 (50%) |
| Lymphoma (n = 14) | 2 (14%) | 1 (50%) | 1 (50%) | 0 |
| Chronic (n = 1) | 0 | 0 | 0 | 0 |
| PCT-ATL (n = 1) | 0 | 0 | 0 | 0 |
Abbreviations: CHOP: Cyclophosphamide, Doxorubicin, Vincristine, Prednisone; AZT/IFN: Zidovudine combined with PEGylated Interferon alpha; ATL: Adult T-cell Leukemia/Lymphoma; DHAOx: Dexamethasone, Cytarabin, Oxaliplatin; DHAP: Dexamethasone, Cytarabin and Cisplatin.
Discussion
This study is the first to provide a comprehensive clinical picture of ATL patients in French Guiana. Although ATL is probably underdiagnosed in French Guiana,23 its incidence of 1.52 per 100,000 inhabitants over the whole French Guianese population is high compared to other endemic countries. It is important to note that this is an estimate, biased by population movements within the territory and by access to care for the populations studied. In addition, our data may provide an estimation of ATL incidence in other Latin America population where HTLV-1 serology is not systematically evaluated. Some countries have 100% screening coverage, while some others, such as Ecuador, Bolivia, El Salvador and Guatemala have less than 10%.24 Remarkably, ATL's prognosis in French Guiana is one of the poorest in the world when compared to Japan and other Latin American countries (see Supplementary Table S1). Early mortality, at 28 days, did not differ between the two aggressive groups but mortality at 6 months and 1 year was catastrophic (Fig. 4). The severity of ATL and its acute onset with hypercalcemia and organs' involvement, combined with the distance from the hospital centres of the populations most affected by HTLV-1, may contribute to this under-diagnosis and delay to start treatment that may also result in poor prognosis. Indeed, many of these patients live in remote areas, in the countryside, Amazonian Forest, and have limited access to secondary and tertiary health services. Furthermore, undocumented persons–a very common vulnerability in the border areasaccumulate difficulties in accessing care.25 It has been suggested that Maroons experienced the greatest difficulty in accessing healthcare.26,27 It may also explain why patients with smouldering and chronic forms are underdiagnosed, because no systematic screening of HTLV-1 infection and ATL is performed in this area. In agreement with this hypothesis, in a previous and first prospective study in French Guiana, with a 16.7 years follow-up duration, ATL incidence rate was 2.03 per 1000 HTLV-1 carrier-years in women (95% confidence interval, 0.93–3.85 per 1000 HTLV-1 carrier-years) with more indolent forms of ATL.28 In French Guiana, the median age at diagnosis was 54 years similar to the one in Latin America (57 years)16 but lower than in Japan (68 years).29 Among environmental factors, high rate of HIV co-infection may contribute to accelerate the onset of disease and worsen its prognosis.30 In French Guiana it has been reported a high incidence of HTLV-1 infection in HIV infected patients, particularly in Surinamese women (5–10%),31, 32, 33 who are representing a significant proportion of our study. Strongyloidiasis might be another factor impacting the presentation and prognosis, since it increases proviral load compared to HTLV-1 infection alone, and may also accelerate proliferation and induce resistance to chemotherapy.34 In our study, not fully following international recommendations, only 39% of ATL patients received anthelmintic drugs (ivermectin), but only one case of disseminated strongyloidiasis was observed. It will be interesting in the future to evaluate the effect of deworming treatment in HTLV-1 infected population of French Guiana on the incidence and prognosis of ATL.
Genetic diversity studies showed a major African contribution (up to 95.7%) in Maroons.35 This highly preserved African gene pool is believed to be unique of Maroons compared to other African American populations of Latin America, including creoles in French Guiana.36 The Maroons, taken as a whole, are larger in number than any other component of the Guianese population, including the Creoles. They now number some 100,000 individuals or 36% of French Guiana's population.37 Clustering of ATL followed by several epidemiological studies revealed a high prevalence of HTLV-1 in the Maroon ethnic groups.32,38, 39, 40 Indeed, some villages present a HTLV-1 seroprevalence of 8%, reaching 40% in women older than 50 years.39 Maroons are therefore a population at risk of developing ATL and should be more carefully followed in this respect. Viral factors might be also involved in French Guiana. Thus, evaluation of viral strains could be explored as it may partly explain the clinical differences observed, particularly compared to Japan.
Median PFS from the initiation of treatment was poor with 93 and 115 days for the acute and lymphoma types, respectively. Our study population showed great heterogeneity in the type of treatment received according to Shimoyama's subtypes. This is certainly due to the absence of management procedures according to the on-going guidelines. One of the reason might be linked to the health professional turn-over in French Guiana hospitals and the absence during this period of a board-certified hematologist on-site.41 This shows the importance of defining the type of ATL and its extension and establishing clear management following the recommendations, particularly at diagnosis, to prevent lethal acute complications including severe hypercalcemia, organs' failure and infections.42 Several studies have shown that responses to intensive chemotherapy do not last, particularly in lymphoma groups. Thus, it is now recommended to perform early allo-HSCT, ideally in the first 100 days, in responding patients, even if CR is not reached.22 In our cohort, because of the lack of response, only half of our patients should have been potential candidates to allo-HSCT, suggesting that other chemotherapy or treatment, including monoclonal antibodies, not too toxic in this population of immune compromised patients, should be used to increase overall response. The response in acute ATL was also lower than previously reported with AZT/IFN. This might be due to the dose of interferon used with pegylated form that might not be sufficient when compared with the ones administrated with regular INF (3MUX3/w vs 9M/d). Interestingly, the patient with chronic ATL under this treatment exhibited a response superior to 10 years. These findings suggest that HTLV-1 carriers should be screened and treated earlier with AZT and interferon.
A specific care pathway must be organized in French Guiana as allo-HSCT requires sanitary medical evacuation to mainland France and to search for a donor as early as possible, especially in at-risk ethnic minorities. The increase number of haplo-identical donors now render possible with new protocols, to perform allo-HSCT in the majority of cases. These informations should be considered in the future for the management of ATL patients in French Guiana.
The limitations of this study relate to its retrospective nature and related missing data, as well as the relatively small size of the study population.
In conclusion, ATL disease in French Guiana has similar characteristics to other Latin America countries but has worse prognosis. This may be explained by its occurrence in specific minorities, specific genetic characteristics and access to specialized health care limitations. Thus, the future objectives include implementation of early diagnosis facilities and management procedures based on recent recommendations as well as participation on basic and clinical research on the disease in this particular population. To do so, strong social support and efficient health network are essential to early diagnosis and treatment of people living with HTLV-1 at risk of developing ATL in French Guiana.
Contributors
Karim Abdelmoumen: literature search, study design, writing.
Kinan Drak Alsibai: data collection.
Sebastien Rabier: figure generation.
Mathieu Nacher: data analysis.
N'detodji-Bill Wankpo: data collection.
Antoine Gessain: data interpretation.
Florin Santa: data collection.
Olivier Hermine: Data interpretation, review & editing.
Ambroise Marçais: literature search.
Pierre Couppié; data curation.
Jean-Pierre Droz: data curation, literature search, data interpretation, review.
Loïc Epelboin: study design, data interpretation, writing.
Editor's note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
None.
Acknowledgements
The authors would like to thank Jean-Michel Cauvin for assistance with data collection; Pauline Naudion, Béatrice Cenciu, Pierre Fenaux, Caroline Misslin-Tritsch, and Felix Djossou for their care of patients.
Funding: None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100492.
Appendix A. Supplementary data
References
- 1.Poiesz B.J., Ruscetti F.W., Gazdar A.F., Bunn P.A., Minna J.D., Gallo R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips A.A., Harewood J.C.K. Adult T cell leukemia-lymphoma (ATL): state of the art. Curr Hematol Malig Rep. 2018;13:300–307. doi: 10.1007/s11899-018-0458-6. [DOI] [PubMed] [Google Scholar]
- 3.Bangham C.R.M., Araujo A., Yamano Y., Taylor G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.12. [DOI] [PubMed] [Google Scholar]
- 4.Gessain A., Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangham C.R., Ratner L. How does HTLV-1 cause adult T-cell leukaemia/lymphoma (ATL)? Curr Opin Virol. 2015;14:93–100. doi: 10.1016/j.coviro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carneiro-Proietti A.B.F., Amaranto-Damasio M.S., Leal-Horiguchi C.F., et al. Mother-to-Child transmission of human T-cell lymphotropic viruses-1/2: what we know, and what are the gaps in understanding and preventing this route of infection. J Pediatric Infect Dis Soc. 2014;3:S24–S29. doi: 10.1093/jpids/piu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Percher F., Jeannin P., Martin-Latil S., et al. Mother-to-Child transmission of HTLV-1 epidemiological aspects, mechanisms and determinants of mother-to-child transmission. Viruses. 2016;8:40. doi: 10.3390/v8020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demontis M.A., Hilburn S., Taylor G.P. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retroviruses. 2013;29:359–364. doi: 10.1089/AID.2012.0132. [DOI] [PubMed] [Google Scholar]
- 9.Rowan A.G., Dillon R., Witkover A., et al. Evolution of retrovirus-infected premalignant T-cell clones prior to adult T-cell leukemia/lymphoma diagnosis. Blood. 2020;135:2023–2032. doi: 10.1182/blood.2019002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukasaki K., Hermine O., Bazarbachi A., et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazarbachi A., Hermine O. Les leucémies/lymphomes T de l’adulte liées au rétrovirus T-lymphotropique humain 1. Hématologie. 2018;24:104–110. [Google Scholar]
- 12.Ito A., Nakano N., Tanaka T., et al. Improved survival of patients with aggressive ATL by increased use of allo-HCT: a prospective observational study. Blood Adv. 2021;5:4156–4166. doi: 10.1182/bloodadvances.2021004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gratwohl A., Baldomero H., Aljurf M., et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afonso P.V., Cassar O., Gessain A. Molecular epidemiology, genetic variability and evolution of HTLV-1 with special emphasis on African genotypes. Retrovirology. 2019;16:39. doi: 10.1186/s12977-019-0504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malpica L., Pimentel A., Reis I.M., et al. Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2:607–620. doi: 10.1182/bloodadvances.2017011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malpica L., Enriquez D.J., Castro D.A., et al. Real-world data on adult T-cell leukemia/lymphoma in Latin America: a study from the Grupo de Estudio Latinoamericano de Linfoproliferativos. JCO Glob Oncol. 2021;7:1151–1166. doi: 10.1200/GO.21.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Melle A., Cropet C., Parriault M.-C., et al. Renouncing care in French Guiana: the national health barometer survey. BMC Health Serv Res. 2019;19:99. doi: 10.1186/s12913-019-3895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaizot R., Simon S., Brottier J., et al. Utility of PCR in patients with Strongyloides stercoralis and HTLV-1 coinfection in French Guiana. Am J Trop Med Hyg. 2019;101:848–850. doi: 10.4269/ajtmh.19-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsukasaki K., Imaizumi Y., Tokura Y., et al. Meeting report on the possible proposal of an extranodal primary cutaneous variant in the lymphoma type of adult T-cell leukemia-lymphoma. J Dermatol. 2014;41:26–28. doi: 10.1111/1346-8138.12374. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P., Arbuck S.G., Eisenhauer E.A., et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Cook L.B., Fuji S., Hermine O., et al. Revised adult T-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol. 2019;37:677–687. doi: 10.1200/JCO.18.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosadas C., Puccioni-Sohler M., Oliveira A.C.P., Casseb J., Sousa M., Taylor G.P. Adult T-cell leukaemia/lymphoma in Brazil: a rare disease or rarely diagnosed? Br J Haematol. 2020;188:e46–e49. doi: 10.1111/bjh.16318. [DOI] [PubMed] [Google Scholar]
- 24.Eusebio-Ponce E., Candel F.J., Anguita E. Human T-cell lymphotropic virus type 1 and associated diseases in Latin America. Trop Med Int Health. 2019;24:934–953. doi: 10.1111/tmi.13278. [DOI] [PubMed] [Google Scholar]
- 25.Jolivet A. 2014. Migrations, health and care in French Guiana. published online July 7. [Google Scholar]
- 26.Diallo F.Y. 2021. Estelle Carde, Discrimination and access to care in French Guiana. Lectures. published online June 3. [Google Scholar]
- 27.Carde E. Accompagner Soigner En Contexte Pluriethnique Pluriculturel; 2021. Maroon black users in the eyes of metropolitan professionals: ethnicization and access to care in French Guiana. published online Jan 1. [Google Scholar]
- 28.Ramassamy J.-L., Tortevoye P., Ntab B., et al. Adult T-cell leukemia/lymphoma incidence rate in French Guiana: a prospective cohort of women infected with HTLV-1. Blood Adv. 2020;4:2044–2048. doi: 10.1182/bloodadvances.2020001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nosaka K., Iwanaga M., Imaizumi Y., et al. Epidemiological and clinical features of adult T-cell leukemia–lymphoma in Japan, 2010–2011: a nationwide survey. Cancer Sci. 2017;108:2478–2486. doi: 10.1111/cas.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosadas C., Taylor G.P. HTLV-1 and Co-infections. Front Med. 2022;9 doi: 10.3389/fmed.2022.812016. https://www.frontiersin.org/article/10.3389/fmed.2022.812016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobesky M., Couppie P., Pradinaud R., et al. [Coinfection with HIV and HTLV-I infection and survival in AIDS stage. French Guiana Study. GECVIG (Clinical HIV Study Group in Guiana)] Presse Med. 2000;29:413–416. [PubMed] [Google Scholar]
- 32.Tortevoye P., Tuppin P., Carles G., Peneau C., Gessain A. Comparative trends of seroprevalence and seroincidence rates of human T cell lymphotropic virus type I and human immunodeficiency virus 1 in pregnant women of various ethnic groups sharing the same environment in French Guiana. Am J Trop Med Hyg. 2005;73:560–565. [PubMed] [Google Scholar]
- 33.Gouhier E., Gaubert-Maréchal E., Abboud P., Couppié P., Nacher M. Predictive factors of HTLV1-HIV coinfections in French Guiana. Am J Trop Med Hyg. 2013;89:549–553. doi: 10.4269/ajtmh.12-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabet A.S., Mortreux F., Talarmin A., et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954–4960. doi: 10.1038/sj.onc.1203870. [DOI] [PubMed] [Google Scholar]
- 35.Brucato N., Tortevoye P., Plancoulaine S., et al. The genetic diversity of three peculiar populations descending from the slave trade: gm study of Noir Marron from French Guiana. C R Biol. 2009;332:917–926. doi: 10.1016/j.crvi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Fortes-Lima C., Gessain A., Ruiz-Linares A., et al. Genome-wide ancestry and demographic history of African-descendant Maroon communities from French Guiana and Suriname. Am J Hum Genet. 2017;101:725–736. doi: 10.1016/j.ajhg.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price R., Price S. Châteauneuf-le-Rouge: vents d’ailleurs; 2003. Les marrons. [Google Scholar]
- 38.Gérard Y., Lepere J.F., Pradinaud R., et al. Clustering and clinical diversity of adult T-cell leukemia/lymphoma associated with HTLV-I in a remote black population of French Guiana. Int J Cancer. 1995;60:773–776. doi: 10.1002/ijc.2910600607. [DOI] [PubMed] [Google Scholar]
- 39.Plancoulaine S., Buigues R.P., Murphy E.L., et al. Demographic and familial characteristics of HTLV-1 infection among an isolated, highly endemic population of African origin in French Guiana. Int J Cancer. 1998;76:331–336. doi: 10.1002/(sici)1097-0215(19980504)76:3<331::aid-ijc8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 40.Tuppin P., Lepère J.F., Carles G., et al. Risk factors for maternal HTLV-I infection in French Guiana: high HTLV-I prevalence in the Noir Marron population. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:420–425. [PubMed] [Google Scholar]
- 41.Wakerman J., Humphreys J., Russell D., et al. Remote health workforce turnover and retention: what are the policy and practice priorities? Hum Resour Health. 2019;17:99. doi: 10.1186/s12960-019-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guery R., Suarez F., Lanternier F., et al. Poor outcome and high prevalence of invasive fungal infections in patients with adult T-cell leukemia/lymphoma exposed to zidovudine and interferon alfa. Ann Hematol. 2021;100 doi: 10.1007/s00277-021-04622-9. published online Aug 13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.