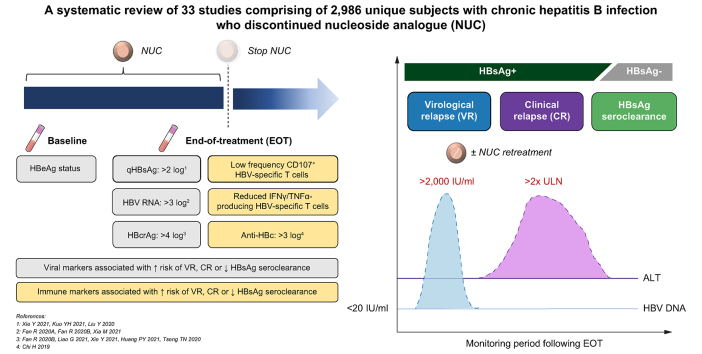
Abstract
Background & Aims
Antivirals represent the mainstay of chronic hepatitis B treatment given their efficacy and tolerability, but rates of functional cure remain low during long-term therapy. Treatment discontinuation has emerged as a strategy to maintain partial cure and achieve functional cure in select patient groups. We aimed to evaluate how data from treatment discontinuation studies exploring novel viral and/or immune markers could be applied to the functional cure program.
Methods
Treatment discontinuation studies evaluating novel viral and/or immune markers were identified by a systematic search of the PubMed database through to October 30, 2022. Data extraction focused on information regarding novel markers, including identified cut-off levels, timing of measurement, and associated effect on study outcomes of virological relapse, clinical relapse, and HBsAg seroclearance.
Results
From a search of 4,492 citations, 33 studies comprising a minimum of 2,986 unique patients met the inclusion criteria. Novel viral markers, HBcrAg and HBV RNA, were demonstrated across most studies to be helpful in predicting off-therapy partial cure, with emerging evidence to support a link with functional cure. From novel immune marker studies, we observed that treatment discontinuation has the potential to trigger immune restoration, which may be associated with a transient virological relapse. To this end, these studies support the combination of virus-directing agents with immunomodulator therapies to induce two key steps underlying functional cure: viral antigen load reduction and restoration of the host immune response.
Conclusions
Patients with a favourable profile of novel viral and immune markers stand to benefit from a trial of antiviral treatment discontinuation alongside novel virus-directing agents with the aim of achieving functional cure without excessive risk of severe clinical relapse.
Impact and implications
Select patients with chronic hepatitis B undergoing nucleoside analogue therapy may benefit from a trial of treatment discontinuation, aiming to maintain partial cure and/or achieve functional cure. We propose a profile of novel viral and immune markers to identify patients who are likely to achieve these goals without excessive risk of hepatic decompensation. Furthermore, treatment discontinuation may also be considered as a therapeutic strategy to trigger immune restoration, which may increase the chance of functional cure when used in conjunction with novel virus-directing agents.
Keywords: Hepatitis B virus, Antiviral agents, Biomarkers, Immune reconstitution
Graphical abstract
Highlights
-
•
There remains considerable interest in the potential for treatment discontinuation in select patients with CHB.
-
•
Novel viral markers, HBcrAg and HBV RNA, in conjunction with quantitative HBsAg, have demonstrated utility in predicting off-therapy cure.
-
•
Novel immune markers with the potential to predict immune restoration after treatment discontinuation are required.
-
•
Both virus-targeted and immunomodulatory agents should be used together to achieve functional cure.
Introduction
An estimated 296 million individuals are known to have chronic hepatitis B (CHB) worldwide, with 30% of the global population showing serological evidence of current or past infection.1,2 CHB resulted in an estimated 820,000 deaths in 2019 according to the World Health Organization (WHO), the vast majority of which are attributable to cirrhosis and hepatocellular carcinoma (HCC).3 Current CHB treatment aims primarily to prevent disease progression and the sequelae of chronic infection by providing continuous on-treatment viral suppression.
First-line antivirals, entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) represent the mainstay of treatment given their efficacy, tolerability, and favourable safety profile; moreover they are distinguished by their high barriers to resistance in addition to their ability to reverse liver fibrosis and reduce HCC incidence.4,5 Treatment with nucleoside analogues (NAs) is lifelong in the majority of patients. This is in contrast to treatment with interferon-alpha, the only recognised finite therapy in CHB, used in a small subset of patients only, because of its recognised systemic side effects. NAs lack the potential to achieve functional cure, defined as sustained off-treatment HBsAg loss, in the majority of CHB patients. The persistence of HBV infection is attributed to the cccDNA pool in infected hepatocytes; although it reduces naturally over the course of HBV infection,6 it persists even in patients with viral clearance.7
Novel therapeutic approaches for the management of CHB have been under evaluation to overcome these limitations. These comprise a number of promising agents or combination approaches currently being evaluated in pre-clinical or early-phase clinical trials, which target either the viral cycle directly or enhance host immunity. The former group includes viral entry inhibitors, RNA interference, capsid assembly modulators, nucleic acid polymers, strategies targeting cccDNA formation or degradation, amongst others. Examples of the latter group include therapeutic vaccines, toll-like receptor agonists, T cell redirection, checkpoint inhibitors, antibodies to HBV and indeed NA discontinuation. Recently, considerable focus has been given to NA discontinuation as a strategy to achieve functional cure. However, there is a lack of consensus between international guidelines[8], [9], [10] regarding the requirements for safe NA cessation in CHB patients (Table S1). Secondly, patients often experience viral relapse (VR), defined as a rebound of HBV DNA levels following treatment cessation; and clinical relapse (CR), defined by VR with an associated biochemical flare. Off-therapy rates of VR and CR vary largely between published studies, likely owing to heterogeneity in study participants, relapse definitions and other aspects of study design. In a recent meta-analysis by Hall et al.11 in 2021 which explored rates of partial cure following discontinuation of oral antivirals in HBeAg-negative patients, rates of VR and CR at 12 months were 63% and 35%, respectively.
Although treatment discontinuation can be considered a therapeutic strategy in its own right with the potential to offer partial and functional cure in some patients, studies of NA discontinuation can also provide unique insights into the virological and immunological conditions required to achieve both partial and functional cure. Several discontinuation studies assessed novel viral markers such as HBcrAg and HBV RNA, and both have been proposed as novel tools to signpost partial and functional cure after NA cessation. Additionally, immune markers, particularly relating to T cell phenotype and function, are differentiated in patient populations who progress to VR and/or CR. Thus, we seek to comprehensively review the data generated to date on novel viral and immune markers in treatment discontinuation studies, aiming to evaluate their potential in providing a roadmap to functional cure.
Materials and methods
Literature search
We performed a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 To retrieve all works of potential relevance, a systematic search of the PubMed/Medline database was performed of all studies through to October 30, 2022. The search used the terms (‘Hepatitis B’ OR ‘Chronic Hepatitis B’) AND (‘Antiviral’ OR ‘Treatment’ OR ‘Therapy’ OR ‘Lamivudine’ OR ‘Adefovir’ OR ‘Entecavir’ OR ‘Telbivudine’ OR ‘Tenofovir’) AND (‘End’ OR ‘Discontinuation’ OR ‘Withdrawal’ OR ‘Cessation’ OR ‘Off-treatment’) which were searched as text words and as exploded medical subject headings where possible, with no language restrictions. The reference lists of relevant articles were also searched for appropriate studies. We requested full texts from authors where we found relevant paper abstracts and conference abstracts. A search for unpublished literature was not performed.
Inclusion criteria
We included randomised or observational studies that met the following inclusion criteria: (1) studies including adult CHB patients who ceased NA only if fulfilling the following standards: HBeAg seroconversion and a minimum mean/median of 6 months of consolidation therapy following virological suppression for initial HBeAg-positive populations, and a minimum median/mean of 12 months of consolidation therapy following virological suppression for initial HBeAg-negative populations, without HBsAg seroclearance; (2) studies providing data in the form of virological and/or clinical relapse rates; (3) studies providing data relating to novel viral and/or immune markers; (4) studies with a minimum follow up of 6 months; (5) studies with a minimum of 10 patients; (6) studies available in English as full papers.
Exclusion criteria
We excluded studies with (1) populations co-infected with HCV or HIV; (2) studies with populations with a history of HCC, liver transplants, or immunosuppressive therapies, (3) studies with populations co-treated with interferon; (4) studies with populations that have exclusively experienced HBsAg seroclearance.
Data extraction
The baseline characteristics of study cohort including age, sex, type of NA, HBeAg status, HBV genotype, and duration of NA were extracted. For each article included, we recorded the author names, year of publication, country of origin, study design, and duration of follow-up. Study outcomes of VR and CR (both regarded as not achieving partial cure) and HBsAg seroclearance (functional cure) as defined in each article were recorded. Regarding novel viral biomarkers, HBcrAg and HBV RNA, the identified cut-off levels, and timing of measurement were presented alongside the associated effect estimates on study outcomes, expressed as either hazard ratios (HRs), odds ratios (ORs), or cumulative rate of study outcomes. Data regarding novel immune markers, namely the phenotype and function of peripheral immune cells, were harvested in the form supplied by the authors.
Quality assessment
For viral markers, the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) assessment tool was used to evaluate study quality, and is available in the Supplementary materials. The judgements within each domain of the tool were carried forward to an overall risk of bias judgement, categorised as low, moderate, serious, or critical. Studies judged to be at critical risk of bias were not included in the analysis. For immune markers, because of the heterogeneous and complex nature of the immunological analyses, no well-established scale could be applied. Two authors (GZ) and (AK) screened the abstracts and selected relevant studies after screening the retrieved full articles. Conflicts of study eligibility or quality assessment were resolved by discussion with a senior author (PTFK).
Results
Characteristics of included studies
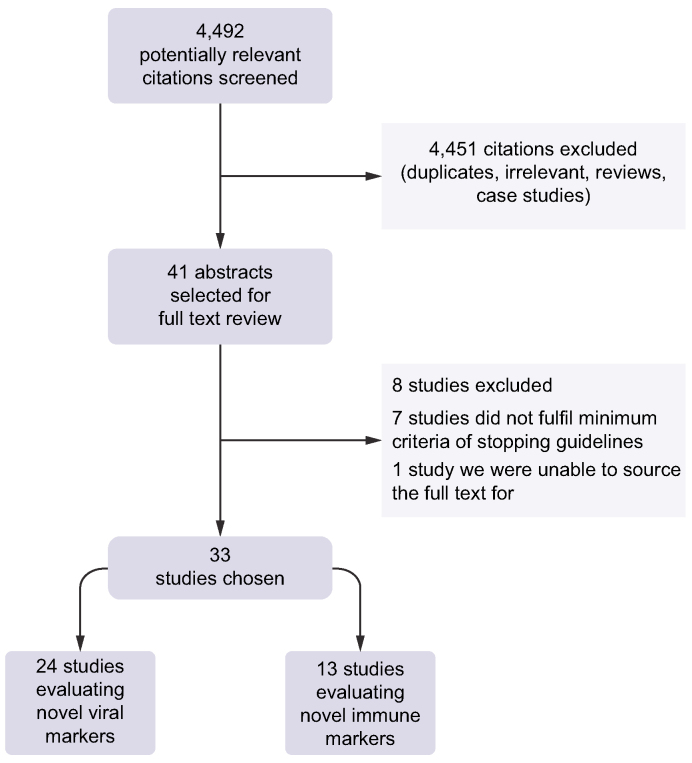
The search identified 4,492 titles and abstracts that were reviewed, with 41 citations being selected for full-text review. Of these, eight studies were excluded after rigorous review. The stopping criteria in seven of these studies did not meet the minimum requirements as per our inclusion criteria and we could not source the full text of another study. Therefore, we evaluated 33 studies,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45] which provided data for a minimum of 2,986 unique patients undergoing treatment cessation. Sonneveld’s 2021 and 2022 studies31,45 extracted data from the CREATE database, which pooled cohorts from previous studies in Asia and Europe that were already included in this meta-analysis.16,17,21,24,25,43,44 In addition, Fan et al. published two included studies18,19 with the same cohort, a different Chinese group published two included studies with likely overlapping cohorts,15,33 and a Taiwanese group published four included studies with likely overlapping cohorts.26,28,29,41 Distinct data on initial e-Antigen-positive populations was provided in five studies, 14 studies provided distinct data on initial e-Antigen-negative populations and 14 studies provided data on combined e-Antigen-positive and e-Antigen-negative populations. Twenty-two studies were conducted in Asian-dominant populations, four studies were conducted in Caucasian-dominant populations, three studies reported on Mediterranean-dominant populations, one study was conducted in a Black African-dominant population and three studies were conducted in heterogenous populations. Fig. 1 displays our study selection process.
Fig. 1.
Study selection process.
The undetectable limit of HBV DNA in the majority of studies was 20 IU/ml (100 copies/ml), but varied from 10 to 100 IU/ml. When specified, the definition of VR was set at HBV DNA >2,000 IU/ml in all but one study,38 which utilised the threshold of HBV DNA >20,000 IU/ml. The definition CR was set as alanine aminotransferase (ALT) >2 × upper limit of normal (ULN) in all studies that specified a threshold, but one study specifically looked at severe hepatitis flares, defined as ALT >10 × ULN.42 The definition of VR and CR in some studies was qualified by multiple time points, for example VR being defined as HBV DNA >2,000 IU/ml verified on two separate occasions 3 months apart. The main study characteristics of these studies are summarised in Tables 1 and 2, and the patient and treatment characteristics of these studies are summarised in Tables 3 and 4.
Table 3.
Patient and treatment characteristics in studies exploring the role of viral markers in prediction of partial and functional cure; stratified by HBeAg status.
| Paper | Total patients | Age | Male | Genotype | Nucleoside analogue | EOT HBsAg (log IU/ml) | TTT (months) | CT (months) | VR | CR | HBsAg loss | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBeAg-positive populations | ||||||||||||
| Fan, R., et al., 2020A18 and Fan, R., et al., 2020B19,∗ | 127 | 30.8 | 94 | B 57, C 73 | LdT ± ADV | 3.1 | 35.7 | 20.4 | 59 | 34 | 1 | 48 |
| Derivation cohort | ||||||||||||
| Evaluation cohort | 59 | 36 | 46 | . | ETV/TDF | 2.6 | 54 | 28.2 | 6 | 66 | ||
| Liao, G., et al., 202130 | 122 | 34 | 95 | B/C 40 | ETV/TDF 71, other 51 | 2.52 | 56.4 | 30 | 44 | 12 | 36 | |
| Xie, Y., et al., 202134 | 139 | 36 | 81 | . | ETV 99, TDF 16, other 24 | 3.2 | 76.8 | 69.6 | 70 | 34 | 13 | 24 |
| Chen, C.H., et al., 202241 |
316 |
ETV 40 TDF 42 |
216 |
B 172, C 144 |
ETV 205, TDF 111 |
ETV 3.0 TDF 2.9 |
ETV 46.0 TDF 46.2 |
ETV 25 TDF 25.8 |
206 |
166 |
15 |
|
| HBeAg-negative populations | ||||||||||||
| Höner Zu Siederdissen, C., et al., 201613 | 15 | 49.1 | 12 | B 3, C 1, D 9 | — | 3.1 | >36 | >36 | 13 | — | 3 | 12 |
| Carey, I., et al., 202017 | 23 | 48 | 14 | A 4, B 3, C 1, D 5, E 10 | TDF 19, ETV 4 | 3.4 | 82.8 | >36 | — | 14 | 0 | 17.9 |
| García-López, M., et al., 202020 | 27 | 56 | 21 | A 3, C 1, D 21, F 2 | TDF 20, ETV 7 | 2.6 | 96 | >36 | 21 | 17 | 8 | 34 |
| Papatheodoridi, M., et al., 202024 | 57 | 60 | 37 | Mainly D | ETV 18, TDF 39 | 2.8 | >96 | 63.6 | 42 | 19 | 14 | 19 |
| Cheng, H.R., et al., 202127 | 54 | 51.3 | 42 | B 54 | ETV 34, TDF 20 | 2.46 | 37.2 | >12 | 39 | . | . | 12 |
| Huang, P.Y., et al., 202128 | 301 | 51.7 | 244 | B 240, C 661 | ETV 301 | 2.43 | 42.2 | 34.6 | 211 | 159 | 41 | 56.3 |
| Kuo, Y.H., et al., 202129 | 185 | 52.2 | 146 | B 139, C 46 | TDF 185 | 2.37 | 39.5 | 31.7 | 128 | 99 | 15 | 35.5 |
| Wübbolding, L.A., et al., 202132 | 43 | 53 | 29 | — | ETV 28, TDF 15 | 3.0 | >48 | >12 | 27 | . | . | 6 |
| Papatheodoridi, M., et al., 202244 |
57 |
60 |
37 |
Mainly D |
ETV 18, TDF 39 |
2.8 |
>96 |
63.6 |
42 |
19 |
14 |
38 |
| Combined HBeAg-positive and HBeAg-negative populations | ||||||||||||
| Hsu, Y.C., et al., 201916 | 135 | 49.5 | 109 | — | ETV 113, TDF 22 | 2.77 | 36.7 | 25.2 | — | 66 | 8 | 25.9 |
| Kaewdech, A., et al., 202021 | 92 | 55 | 59 | — | LMV 51, LMV+TDF 20, ETV 13, LdT 9, TDF 8, LMV+ADV 1 | 2.96 | 78 | >12 | — | — | 2 | 12 |
| Lai, C.L., et al., 202022 | 13 | 56 | — | — | ETV 8, LdT 3, TDF 2 | 2.6 | 160.8 | >12 | 12 | 3 | — | 17.5 |
| Liu, Y., et al., 202023 | 30 | 46 | 21 | — | ETV 17, LMV 8, ADV 2, ADV+LMV 3 | 1.91 | 57.5 | >12 | 11 | 7 | — | 24 |
| Seto, W.K., et al., 202025 | 114 | 58.4 | 75 | — | ETV | 1.74 | 80.4 | 63.6 | 62 | 24 | 8 | 12 |
| Tseng, T.N., et al., 2020a26 | 135 | 52.6 | 104 | B 103, C 32 | ETV 79, TDF 56 | 1.32 | 38.8 | 31.3 | 50 | 38 | 39 | 20.1 |
| Sonneveld, M.J., et al., 202131 | 572 | 52 | 390 | — | ETV 295, TDF 150 | <1.7: 14% 1.7–2: 8% 2–3: 33% >3: 46% |
73.8 | As per APASL and EASL | 267 | 92 | 24 | 12 |
| Xia, M., et al., 202133 | 135 | SR 35 CR 38 |
110 | — | 1st line 74, 2nd line 61 | SR 2.3 CR 2.8 |
. | SR 30.0 CR 28.0 |
— | 50 | 13 | 31.2 |
| Kaewdech, A., et al., 202243 | 92 | 55 | 59 | — | LMV 51, LMV+TDF 20, ETV 13, LdT 9, TDF 8, LMV+ADV 1 | 2.96 | 78 | >12 | — | — | 7 | 35.5 |
| Sonneveld, M.J., et al., 202245 | 1,216 | 50 | 880 | A 19, B 497, C 368, D 81, E 16 | ETV 717, TDF 372 | <1: 5.3% 1–2: 15.8% >2: 78.9% |
41.8 | As per APASL and EASL | — | — | 98 | 25.6 |
ADV, adefovir; ALT, alanine transaminase; APASL, Asian Pacific Association for the Study of the Liver; CR, clinical relapse; CT, consolidation time; eAg+, e-Antigen positive; eAg-, e-Antigen negative; ETV, entecavir; EOT, end of treatment; HBsAg, hepatitis B surface antigen (measured in log IU/ml); HBV DNA, hepatitis B virus deoxyribonucleic acid (measured in log copies/ml); LdT, telbivudine; LMV, lamivudine; SR, sustained response; TDF, tenofovir; TTT, total treatment time (measured in months); ULN, upper limit of normal; VR, viral relapse.
Table 4.
Patient and treatment characteristics in studies exploring the role of immune markers in prediction of partial and functional cure.
| Paper | Total patients | Age (years) | Male | Genotype | NA | EOT HBsAg (log IU/ml) | TTT (months) | CT (months) | VR | CR | HBsAg loss | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Höner Zu Siederdissen, C., et al., 201613,∗ | 15 | 49.1 | 12 | B 3, C 1, D 9 | . | 3.1 | >36 | >36 | 13 | . | 3 | 12 |
| Rinker, F., et al., 201835,∗ | As above | |||||||||||
| Zimmer, C.L., et al., 201836,∗ | As above | |||||||||||
| Rivino, L., et al., 201837 | ||||||||||||
| Cohort 1 | 19 | 46 | A 1, B 4, C 2, D 11, E 1 | TDF + LMV | 3.6 | >18 | >18 | 6 | — | 6 | ||
| Cohort 2 | 27 | 51.7 | B 17, C 7 | ETV/TDF/LdT | 3.1 | >18 | >18 | 11 | — | 8.8 | ||
| Su, T.H., et al., 201814 | 100 | 51 | 72 | B 74 | ETV 66, TDF 34 | ETV 2.64 TDF 2.88 |
37 | 26 | 70 | 45 | — | 35 |
| Chi, H., et al., 201915 | 100 | eAg+ 33 eAg- 41 |
86 | . | 1st line 43, 2nd line 57 | eAg+ 2.8 eAg- 2.5 | eAg+ 46.8 eAg-57.6 |
eAg+ 26.4 eAg- 34.8 | 76 | 39 | 6 | 30 |
| Kranidioti, H., et al., 201938 | 23 | 59 | 15 | . | . | 3.4 | 96 | >36 | . | 13 | 4 | 55.2 |
| Wu, Y., et al., 201939 | 106 | 36 | 84 | . | . | 3.2 | >12 | >12 | . | 36 | . | 22.8 |
| Xie, L., et al., 201940 | 91 | 36 | 75 | . | . | 2.9 | >12 | >12 | 57 | 26 | . | 12 |
| García-López, M., et al., 202020 | 27 | 56 | 21 | A 3, C 1, D 21, F 2 | TDF 20, ETV 7 | 2.6 | 96 | >36 | 21 | 17 | 8 | 34 |
| Papatheodoridi, M., et al., 202024 | 57 | 60 | 37 | Mainly D | ETV 18, TDF 39 | 2.8 | >48 | 63.6 | 42 | 19 | 14 | 19 |
| Wübbolding, L.A., et al., 202132 | 43 | 53 | 29 | . | ETV 28, TDF 15 | 3.0 | >48 | >12 | 27 | — | — | 6 |
| Hall, S.A.L, et al., 202242 | 29 | No flare 54 Flare 60 |
17 | . | ETV 18, TDF 6 | No flare 2.7 Flare 3.1 |
>24 | >18 | — | 17 | 0 | 24 |
ADV, adefovir; ALT, alanine transaminase; CR, clinical relapse; CT, consolidation time; eAg+, e-Antigen-positive; eAg-, e-Antigen-negative; ETV, entecavir; EOT, end of treatment; HBsAg, hepatitis B surface antigen (measured in log IU/ml); HBV DNA, hepatitis B virus deoxyribonucleic acid (measured in log copies/ml); LdT, telbivudine; SR, sustained response; TDF, tenofovir; TTT, total treatment time (measured in months); ULN, upper limit of normal; VR, viral relapse; ‘.’: data not specified.
Höner Zu Siederdissen, C., et al., 2016, Rinker, F., et al., 2018, and Zimmer, C.L., et al., 2018 use the same patient cohort.
Table 1.
Main characteristics of included studies (n = 24) exploring the role of viral markers in prediction of partial cure.
| Paper | Study design | Population | Location (Ethnicity if different) | Sample size | Novel viral markers | VR definition (HBV DNA) | CR definition (ALT) | Retreatment criteria | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Höner Zu Siederdissen, C., et al., 201613 | Prospective | HBeAg- | Germany | 15 | HBcrAg | 2,000 IU/ml | N/A | VR | 12 |
| Hsu, Y.C., et al., 201916 | Prospective | Combined | Taiwan | 135 | HBcrAg | 2,000 IU/ml | ×2 ULN (ULN = 40) | Bili >2 mg/dl, PT >3 s, or ALT >2 × ULN [3 months apart] | 25.9 |
| Carey, I., et al., 202017 | Retrospective | HBeAg- | UK (mostly Black African) | 23 | HBcrAg HBV RNA |
N/A | ×2 ULN (ULN = 19 F, 30 M) [on two occasions] | ‘Clinically significant flare’ | 17.9 |
| Fan, R., et al.,. 2020A18 | Prospective | HBeAg+ | China | 170 | HBV RNA | 2,000 IU/ml [3-4 months apart] | ×2 ULN | CR | 48 |
| Fan, R., et al., 2020B19 | Prospective | HBeAg+ | China | 186 | HBcrAg HBV RNA |
2,000 IU/ml [3–4 months apart] | ×2 ULN | CR | 48 |
| García-López, M., et al., 202020 | Prospective | HBeAg- | Spain | 27 | HBcrAg HBV RNA |
2,000 IU/ml | Not stated | ALT >10 × ULN [on two occasions], ALT >5–10 × ULN and VR [4 wk apart], or ALT >2–5 × ULN and VR [6 months apart] | 34 |
| Kaewdech, A., et al., 202021 | Prospective | Combined | Thailand | 92 | HBcrAg HBV RNA |
2,000 IU/ml | >2 × ULN (ULN = 33) | ALT >10 × ULN, ALT >2–10 × ULN [4 wk apart], Bili >1.5 mg/dl or PT >2 s | 12 |
| Lai, C.L., et al., 202022 | Prospective | Combined | Hong Kong | 13 | HBcrAg HBV RNA |
2,000 IU/ml | — | VR | 17.5 |
| Liu, Y., et al., 202023 | Prospective | Combined | China | 30 | HBV RNA | 2,000 IU/ml [3 months apart] | ×2 ULN | CR | 24 |
| Papatheodoridi, M., et al., 202024 | Prospective | HBeAg- | Greece | 57 | HBcrAg | 2,000 IU/ml | ×2 ULN (ULN = 40) | ALT >10×ULN, ALT >2 × ULN and DNA >100,000 IU/ml or ALT >2 × ULN and DNA >2,000 IU/ml [on three occasions] | 19 |
| Seto, W.K., et al., 202025 | Prospective | Combined | Hong Kong | 114 | HBcrAg HBV RNA |
2,000 IU/ml [1 wk apart] | N/A | VR | 12 |
| Tseng, T.N., et al., 202026 | Not specified | Combined | Taiwan | 135 | HBcrAg | 2,000 IU/ml | >80 | HBeAg+: ALT >2 × ULN and DNA >20,000 IU/ml HBeAg-: ALT >2 × ULN [3 months apart] and DNA >2,000 IU/ml All patients: Bili >2 mg/dl or PT >3 s |
135 |
| Cheng, H.R., et al., 202127 | Prospective | HBeAg- | Taiwan | 54 | HBcrAg | 2,000 IU/ml | — | Not mentioned | 12 |
| Huang, P.Y., et al., 202128 | Not specified | HBeAg- | Taiwan | 301 | HBcrAg | 2,000 IU/ml | ×2 ULN (ULN = 40) | ALT >2× ULN [3 months apart] and DNA >2,000 IU/ml, Bili >2 mg/dl or PT >3 s | 56.3 |
| Kuo, Y.H., et al., 202129 | Retrospective | HBeAg- | Taiwan | 185 | HBcrAg | 2,000 IU/ml | ×2 ULN | ALT >2× ULN [3 months apart] and DNA >2,000 IU/ml, Bili >2 mg/dl or PT >3 s | 35.5 |
| Liao, G., et al., 202130 | Prospective | HBeAg+ | China | 122 | HBcrAg | 2,000 IU/ml | ×2 ULN (ULN = 40) | CR | 36 |
| Sonneveld, M.J., et al., 202131 | Retrospective/prospective | Combined | Multicentre (Asia and Europe) | 572 | HBcrAg | 2,000 IU/ml | ×3 ULN | Not specified | 12 |
| Wübbolding, L.A., et al., 202132 | Prospective | HBeAg- | Asia Pacific | 43 | HBcrAg | 2,000 IU/ml | — | No specified | 6 |
| Xia, M., et al., 202133 | Prospective | Combined | China | 135 | HBV RNA | 2,000 IU/ml | ×2 ULN | CR | 31.2 |
| Xie, Y., et al., 202134 | Prospective | HBeAg+ | China | 139 | HBcrAg HBV RNA |
2,000 IU/ml | ×2 ULN (ULN = 40) | CR | 24 |
| Chen, C.H., et al., 202241 | Prospective | HBeAg+ | Taiwan | 316 | HBcrAg | 2,000 IU/ml | ×2 ULN (ULN = 40) | Not specified | ETV 42 TDF 19 |
| Kaewdech, A., et al., 202243 | Prospective | Combined | Thailand | 92 | HBcrAg HBV RNA |
2,000 IU/ml | >2 × ULN (ULN = 33) | CR and: Bili >1.5 mg/dl, PT >2 s, ALT >10 × ULN, or ALT 2–10 × ULN [4 wk apart] |
35.5 |
| Papatheodoridi, M., et al., 202244 | Prospective | HBeAg- | Greece | 57 | HBcrAg HBV RNA |
2,000 IU/ml | ×2 ULN (ULN = 40) | ALT >10 × ULN, ALT >5 × ULN and Bili >2 mg/dl, ALT >2 × ULN and DNA <100,000 IU/ml, ALT >ULN and DNA >2,000 IU/ml [on three occasions] | 38 |
| Sonneveld, M.J., et al., 202245 | Retrospective/prospective | Combined | Multicentre (Asia and Europe) | 1,216 | HBcrAg | — | — | Not specified | 25.6 |
Anti-HBc, hepatitis B core antibodies; ALT, alanine transaminase; Bili, bilirubin; CR, clinical relapse; ETV, entecavir; F, female; HBcrAg, hepatitis B core-related antigen; HBeAg+, initial e-Antigen-positive population; HBeAg-, initial e-Antigen-negative population; HBV DNA, hepatitis B virus deoxyribonucleic acid; HBV RNA, hepatitis B virus ribonucleic acid; M, male; PT, prothrombin time; ULN, upper limit of normal; VR, virological relapse.
Table 2.
Main characteristics of included studies (n = 13) exploring the role of immune markers in prediction of partial cure.
| Paper | Study design | Population | Location (ethnicity if different) | Sample size | Immune marker explored | VR definition (HBV DNA) | CR definition (ALT) | Retreatment criteria | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Höner Zu Siederdissen, C., et al., 201613 | Prospective | HBeAg- | Germany | 15 | 27 plasma cytokine levels | 2,000 IU/ml | — | VR | 12 |
| Rinker, F., et al., 201835 | HBV-specific T cell activity, phenotype, and function of T cells | ||||||||
| Zimmer, C.L., et al., 201836 | Phenotype and function of NK cells | ||||||||
| Rivino, L., et al., 201837 | Prospective | HBeAg- | Cohort 1 – UK (heterogeneous ethnicity) Cohort 2 – SE Asia |
46 | HBV-specific T cell activity, phenotype, and function of peripheral immune cells, 579 gene expression levels | N/A | ×2 ULN (ULN = 40) | Not specified | Cohort 1–6 Cohort 2–8.8 |
| Su, T.H., et al., 201814 | Prospective | Combined | Taiwan | 100 | SNPs, anti-HBc activity | 2,000 IU/ml | ×2 ULN (ULN = 40) | ALT >2 × ULN [3 months apart] and: DNA >2,000 IU/ml or Bili >2 mg/dl or PT >3 s |
35 |
| Chi, H., et al., 201915 | Prospective | Combined | China | 100 | Anti-HBc | 2,000 IU/ml | ×2 ULN (ULN = 35 F, 40 M) | CR | 30 |
| Kranidioti, H., et al., 201938 | Prospective | HBeAg- | Greece | 23 | 21 key gene expression levels | 20,000 IU/ml | N/A | Not specified | 55.2 |
| Wu, Y., et al., 201939 | Prospective | Combined | China | 106 | SNPs, CXCR5 T cell activity, plasma CXCL13 levels | 2,000 IU/ml | ×2 ULN | Not specified | 23 |
| Xie, L., et al., 201940 | Prospective | Combined | China | 91 | Plasma sST2 levels | 2,000 IU/ml | ×2 ULN | CR | 12 |
| García-López, M., et al., 202020 | Prospective | HBeAg- | Spain | 27 | Global and HBV-specific T cell activity | 2,000 IU/ml | — | ALT >10 × ULN [on two occasions], ALT >5–10 × ULN and VR [4 wk apart], or ALT >2–5 × ULN and VR [6 months apart] | 34 |
| Papatheodoridi, M., et al., 202024, | Prospective | HBeAg- | Greece | 57 | Plasma IP-10 levels | 2,000 IU/ml | ×2 ULN (ULN = 40) | ALT >10 × ULN, ALT >2 × ULN and DNA >100,000 IU/ml or ALT >2 × ULN and DNA >2,000 IU/ml [on three occasions] | 19 |
| Wübbolding, L.A., et al., 202132 | Prospective | HBeAg- | Asia Pacific | 43 | Plasma cytokine, chemokine and growth factor levels | 2,000 IU/ml | — | Not specified | 6 |
| Hall, S.A.H., et al., 202242 | Prospective | HBeAg- | Australia (mostly Asian) | 29 | TLR signalling and TLR/NK receptor expression | — | ×10 ULN (severe flare) | Not specified | 24 |
ALT, alanine transaminase; Bili, bilirubin; CR, clinical relapse; F, female; HBeAg+, initial e-Antigen-positive population; HBeAg-, initial e-Antigen-negative population; M, male; PT, prothrombin time; SNP, single nucleotide polymorphism; ULN, upper limit of normal; VR, virological relapse.
Novel viral markers
HBcrAg and partial cure
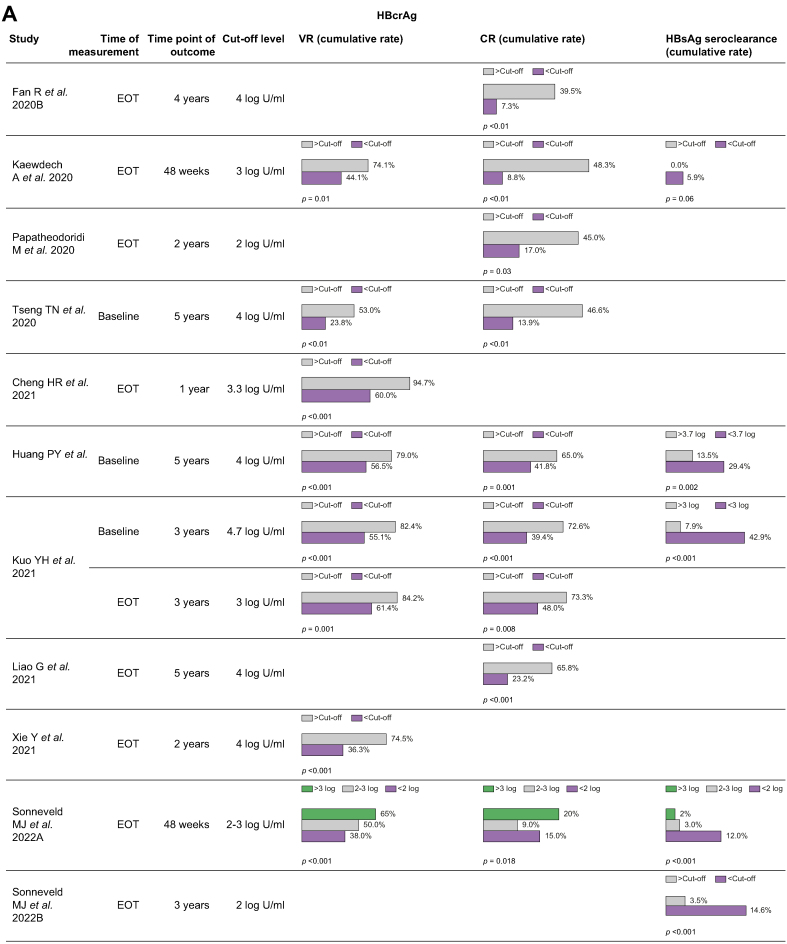
The association between HBcrAg and VR was evaluated in 14 studies (Table 5), four of which produced significant multivariate HRs.30,31,34,41 At the end of treatment (EOT) HBcrAg cut-off of 4 log U/ml in HBeAg-positive populations, Liao et al.30 demonstrated a multivariate HR 1.73 (1.06–2.80, p <0.027) for 5-yr VR, whereas Xie et al.34 demonstrated a multivariate OR of 3.70 (1.61–8.49, p = 0.002) for 2-yr VR. Furthermore, a recent large-scale study pooling European and Asian cohorts31 (including both HBeAg-positive and HBeAg-negative patients) reported that lower EOT HBcrAg levels were significantly associated with higher rates of virological remission/response with multivariate OR 0.73 per log U/ml (0.62–0.86, p <0.001). Seven studies reported the cumulative rates of VR stratified by the level of baseline or EOT HBcrAg, with varying observation periods and cut-off levels of HBcrAg (Fig. 2A). For instance, Tseng et al.26 demonstrated significantly different 5-yr VR rates of 23.8% vs. 53% in patients with baseline HBcrAg <4 log U/ml and >4 log U/ml respectively (p = 0.001), yet returning no significant findings when exploring EOT HBcrAg, in a majority HBeAg-negative population. Huang et al.28 also found that baseline HBcrAg at the cut-off 4 log U/ml was a significant predictor for VR in their HBeAg-negative population, whereas EOT HBcrAg was not.
Table 5.
Summary of novel viral markers.
| Paper | Relation with VR | Relation with CR | Relation with HBsAg clearance |
|---|---|---|---|
|
HBeAg positive populations | |||
| Fan, R., et al., 2020A18 EOT HBV RNA: 26% undetected EOT HBV DNA: 48.5% undetected; 43.8% <20 IU/ml† EOT HBV RNA and EOT HBV DNA: 19.7% undetected |
EOT HBV RNA AUROC 0.775 |
EOT HBV RNA Cumulative incidence of 4-yr CR: p = 0.03∗ RNA <3 log U/ml: 15.3% RNA >3 log U/ml: 37.0% AUROC 0.732 |
N/A |
|
EOT HBV DNA and EOT HBV RNA: Cumulative incidence of 4-yr CR: no p value∗ DNA negative and RNA <3 log U/ml: 8% DNA positive or RNA >3 log U/ml: 37% MV HR (DNA + or RNA >3 log U/ml vs. DNA - and RNA <3 log U/ml): 11.10 (2.69-45.80) p = 0.00 |
EOT HBV DNA and EOT HBV RNA Cumulative incidence of 4-yr CR: p = 0.02∗ DNA negative and RNA <3 log U/ml: 8% (NPV 92%) DNA positive or RNA >3 log U/ml: 31.4% MV HR (DNA + or RNA >3 log U/ml vs. DNA – and RNA <3 log U/ml): 4.54 (1.08-19.00) p = 0.04 |
||
| Fan, R., et al., 2020B19 EOT HBcrAg: 4.3 log U/ml EOT HBV RNA: 3 log copies/ml, 31.5% undetected |
N/A |
EOT HBcrAg Cumulative incidence of 4-yr CR: p = 0.00∗ HBcrAg <4 log U/ml: 7.3% HBcrAg >4 log U/ml: 39.5% MV HR (>4 vs. <4 log U/ml): 5.70 (1.37-23.67) p = 0.02 AUROC 0.621 |
|
|
EOT HBV RNA∗ Cumulative incidence of 4-yr CR: p = 0.00 RNA <3 log U/ml: 12.9% RNA >3 log U/ml: 40.1% MV HR (>3 vs. <3 log U/ml): 3.58 (1.26-10.14) p = 0.02 AUROC 0.635 | |||
|
EOT HBcrAg and EOT HBV RNA∗ Cumulative incidence of 4-yr CR: p = 0.00 RNA <3 log U/ml and HBcrAg <4 log U/ml: 0% RNA >3 log U/ml or HBcrAg >4 log U/ml: 17.3% RNA >3 log U/ml and HBcrAg >4 log U/ml: 46.8% AUROC 0.696 |
EOT HBcrAg and EOT HBV RNA∗ In combination with the validation cohort: Cumulative incidence of 4-yr clearance: p = 0.00 RNA <3 log U/ml and HBcrAg <4 log U/ml: 16.1% RNA >3 log U/ml or HBcrAg >4 log U/ml 1.3% |
||
| Liao, G., et al., 202130 EOT HBcrAg: 3.8 log U/ml |
EOT HBcrAg MV HR (>4 vs. <4 log U/ml): 1.725 (1.063–2.800) p <0.027 |
EOT HBcrAg Cumulative incidence of 5-yr CR: p <0.001∗ HBcrAg < 4 log U/ml: 23.2% HBcrAg > 4 log U/ml: 65.8% MV HR (>4 vs. <4 log U/ml): 2.105 (1.440–3.077) p <0.001 AUROC 0.78 at 1 yr, 0.71 at 3 yr, 0.71 at 5 yr Sensitivity 87.1%, specificity 61.5%, PPV 50%, NPV 92.2% |
EOT HBcrAg Only four of 12 patients achieving HBsAg loss had undetectable HBcrAg |
|
EOT HBsAg and EOT HBcrAg Cumulative incidence of 5-yr CR: p <0.001 HBsAg >2 log IU/ml and HBcrAg <4 log U/ml: 29.4% HBsAg >2 log IU/ml and HBcrAg >4 log U/ml: 78.1% |
|||
|
SCALE-B Score Evaluation Cumulative incidence of 5-yr CR: p <0.001 Low risk: 22.2% Medium risk: 50% High risk: 82.2% AUROC 0.81 at 1 yr, 0.74 at 3 yr, 0.75 at 5 yr |
|||
| Xie, Y., et al., 202134 EOT HBcrAg: 3.8 log U/ml EOT HBV RNA: 0 log copies/ml, 71% undetected |
EOT HBV RNA Cumulative incidence of 2-yr VR: p <0.001∗ RNA negative: 39.4% RNA positive: 77.5% MV OR (RNA – vs. +): 3.453 (1.387–8.597) p = 0.008 AUROC 0.656 |
EOT HBV RNA MV OR (RNA - vs. +): 4.782 (1.968–11.621) p = 0.001 |
|
|
EOT HBcrAg Cumulative incidence of 2-yr VR: p <0.001∗ HBcrAg <4 log U/ml: 36.3% HBcrAg >4 log U/ml: 74.5% MV OR (>4 vs. <4 log U/ml): 3.702 (1.614–8.488) p = 0.002 AUROC 0.616 | |||
|
EOT HBsAg and EOT HBcrAg Cumulative incidence of 2-yr VR: p <0.001 HBsAg <2 log IU/ml and HBcrAg <4 log U/ml: 10.5% HBsAg >2 log IU/ml and/or HBcrAg >4 log U/ml: 56.7% AUROC 0.609 | |||
|
EOT HBsAg and EOT HBV RNA Cumulative incidence of 2-yr VR: p <0.001 HBsAg <2 log IU/ml and HBV RNA negative: 5% HBsAg >2 log IU/ml and/or HBV RNA positive: 58% AUROC 0.698 | |||
|
EOT HBcrAg and EOT HBV RNA∗ Cumulative incidence of 2-yr VR: p <0.001 HBcrAg <4 log U/ml and HBV RNA-negative: 31% HBcrAg >4 log U/ml and/or HBV RNA-positive: 70.6% AUROC 0.631 | |||
|
EOT HBsAg, HBcrAg, and HBV RNA Cumulative incidence of 2-yr VR: HBsAg <2 log IU/ml and HBcrAg <4 log U/ml and HBV RNA-negative: 5.6% AUROC 0.616 | |||
| Chen, C.H., et al., 202241 EOT HBcrAg: 4.4-4.5 log U/ml |
Baseline HBcrAg UV HR (per log U/ml): 1.11 (0.92–1.33) p = 0.265 |
Baseline HBcrAg UV HR (per log U/ml): 1.15 (0.92–1.43) p = 0.220 |
|
|
EOT HBcrAg MV HR (per log U/ml): 1.54 (1.22–1.96) p <0.001 |
EOT HBcrAg MV HR (per log U/ml): 1.63 (1.27–2.09) p <0.001 |
||
|
HBeAg-negative populations | |||
| Höner Zu Siederdissen, C., et al., 201613 | N/A | N/A |
EOT HBcrAg and EOT HBsAg The three out of 15 patients with HBsAg loss demonstrated a >1 log HBsAg reduction over median 33-month (12–50 months) follow-up and had a strong increase in HBV DNA (>4×105IU/ml) and >90-fold increase in HBcrAg at 4–8 wk post-EOT |
| Carey, I., et al., 202017,∗ EOT HBcrAg: 2.0 log U/ml, 83% undetected EOT pgRNA: 0 log U/ml, 87% undetected |
EOT HBsAg No significant change after NA withdrawal Steeper HBsAg decline correlated with lower baseline HBsAg, HBcrAg, RNA, and EOT HBsAg levels |
||
|
EOT HBcrAg and EOT HBV pgRNA Transient resolving elevations after NA cessation CR occurred only in the four patients with HBcrAg >3 log U/ml (100% sensitivity, specificity and PPV) Three of these patients had RNA >1.65 log U/ml (75% sensitivity, 100% specificity, 100% PPV) | |||
| García-López, et al., 202020 EOT HBcrAg: 3.2 log U/ml, 52% undetected EOT HBV RNA: 2.1 log copies/ml, 59% undetected |
EOT HBcrAg and HBV-RNA More frequently undetectable in patients who achieved HBsAg loss than in patients who did not HBcrAg: 75% vs. 42%; p = 0.12 HBV-RNA: 88% vs. 47%; p = 0.053 |
||
| Papatheodoridi, M., et al., 202024 EOT HBcrAg: <2 log U/ml, 62% undetected |
EOT HBcrAg HR: Not significantly associated with VR |
EOT HBcrAg Cumulative incidence of 2-yr retreatment: p = 0.03∗ HBcrAg >2 log U/ml: 45% HBcrAg <2 log U/ml: 17% MV HR per log U/ml: 1.86 (1.11-3.11) p = 0.02 MV HR (>2 vs. <2 log U/ml): 3.64 (1.23-10.75) p = 0.02 |
EOT HBcrAg HR: Not significantly associated with clearance |
| Cheng, H.R., et al., 202127 EOT HBcrAg: 3.6 log U/ml |
EOT HBcrAg Cumulative incidence of 1-yr VR: p <0.001∗ HBcrAg <3.3 log U/ml: 60.0% HBcrAg >3.3 log U/ml: 94.7% HR (>3.3 vs. <3.3 log U/ml): 3.31 (1.72–6.38) p <0.001 AUROC 7.017 |
||
| Huang, P.Y., et al., 202128 Baseline HBcrAg: 4.9 log U/ml EOT HBcrAg: 3.4 log U/ml |
Baseline HBcrAg Cumulative incidence of 5-yr VR: p <0.001∗ HBcrAg <4 log U/ml: 56.5% HBcrAg >4 log U/ml: 79% UV HR (per log U/ml): 1.086 (1.008–1.171) p = 0.031 Not significantly associated in MV analysis |
Baseline HBcrAg Cumulative incidence of 5-yr CR: p = 0.001∗ HBcrAg <4 log U/ml: 41.8% HBcrAg >4 log U/ml: 65% Not significantly associated in UV or MV analysis |
Baseline HBcrAg Cumulative incidence of 5-yr clearance: p = 0.002∗ HBcrAg <3.7 log U/ml: 29.4% HBcrAg >3.7 log U/ml: 13.5% UV HR (per log U/ml): 0.815 (0.692-0.961) p = 0.015 Not significantly associated in MV analysis |
|
EOT HBsAg and baseline HBcrAg Cumulative incidence of 5-yr VR: p = 0.006 HBsAg <150 IU/ml and HBcrAg <4 log U/ml: 27.9% HBsAg <150 IU/ml and HBcrAg >4 log U/ml: 59.1% HBsAg >150 IU/ml and HBcrAg <4 log U/ml: 75.9% HBsAg >150 IU/ml and HBcrAg >4 log U/ml: 84.2% MV HR (HBsAg <150 IU/ml and HBcrAg <4 log U/ml): 0.370 (0.187–0.730) p = 0.004 |
EOT HBsAg and baseline HBcrAg Cumulative incidence of 5-yr CR: p = 0.014 HBsAg <150 IU/ml and HBcrAg <4 log U/ml: 18% HBsAg <150 IU/ml and HBcrAg >4 log U/ml: 48.1% HBsAg >150 IU/ml and HBcrAg <4 log U/ml: 58.8% HBsAg >150 IU/ml and HBcrAg >4 log U/ml: 69.1% MV HR (HBsAg <150 IU/ml and HBcrAg <4 log U/ml): 0.356 (0.156-0.811) p = 0.014 |
||
|
EOT HBcrAg HR: not significantly associated with VR |
EOT HBcrAg HR: not significantly associated with CR |
||
| Kuo, Y.H., et al., 202129 Baseline HBcrAg: 5.3 log U/ml EOT HBcrAg: 3.3 log U/ml |
Baseline HBcrAg Cumulative incidence of 3-yr VR: p <0.001∗ HBcrAg <4.7 log U/ml: 55.1% HBcrAg >4.7 log U/ml: 82.4% UV HR (per log U/ml): 1.201 (1.078–1.338) p = 0.001 Not significantly associated in MV analysis AUROC 0.688 |
Baseline HBcrAg Cumulative incidence of 3-yr CR: p <0.001∗ HBcrAg <4.7 log U/ml: 39.4% HBcrAg >4.7 log U/ml: 72.6% UV HR (per log U/ml): 1.227 (1.083–1.391) p = 0.001 Not significantly associated in MV analysis |
Baseline HBcrAg Cumulative incidence of 3-yr clearance: p <0.001∗ HBcrAg <3 log U/ml: 42.9% HBcrAg >3 log U/ml: 7.9% AUROC 0.688 |
|
EOT HBcrAg Cumulative incidence of 3-yr VR: p = 0.001∗ HBcrAg <3 log U/ml: 61.4% HBcrAg >3 log U/ml: 84.2% UV HR (per log U/ml): 1.489 (1.133–1.955) p = 0.004 Not significantly associated in MV analysis AUROC 0.640 |
EOT HBcrAg Cumulative incidence of 3-yr CR: p = 0.008∗ HBcrAg <3 log U/ml: 48% HBcrAg >3 log U/ml: 73.3% UV HR (per log U/ml): 1.569 (1.157–2.127) p = 0.004 Not significantly associated in MV analysis |
||
|
EOT HBsAg and baseline HBcrAg Cumulative incidence of 3-yr VR: p = 0.003, p = 0.470 HBsAg <2 log IU/ml and HBcrAg <4.7 log U/ml: 20.3% HBsAg <2 log IU/ml and HBcrAg >4.7 log U/ml: 60.4% HBsAg >2 log IU/ml and HBcrAg <4.7 log U/ml: 80.6% HBsAg >2 log IU/ml and HBcrAg >4.7 log U/ml: 87.3% |
EOT HBsAg and baseline HBcrAg Cumulative incidence of 3-yr CR: p <0.001, p = 0.322 HBsAg <2 log IU/ml and HBcrAg <4.7 log U/ml: 10.3% HBsAg <2 log IU/ml and HBcrAg >4.7 log U/ml: 59.5% HBsAg >2 log IU/ml and HBcrAg <4.7 log U/ml: 62.1% HBsAg >2 log IU/ml and HBcrAg >4.7 log U/ml: 75.4% |
||
|
EOT HBsAg and EOT HBcrAg Cumulative incidence of 3-yr VR: p = 0.149 HBsAg <2 log IU/ml and HBcrAg <3 log U/ml: 30.4% HBsAg <2 log IU/ml and HBcrAg >3 log U/ml: 51.8% |
EOT HBsAg and EOT HBcrAg Cumulative incidence of 3-yr CR: p = 0.142 HBsAg <2 log IU/ml and HBcrAg <3 log U/ml: 22.2% HBsAg <2 log IU/ml and HBcrAg >3 log U/ml: 46.4% |
||
| Wübbolding, L. A., et al., 202132 EOT HBcrAg: 3.0 log U/ml |
HBcrAg AUROC 0.56 |
||
| Papatheodoridi, M., et al., 202244 EOT HBcrAg: <2 log U/ml EOT RNA: 93% undetected |
EOT HBV RNA Cumulative incidence of 12-month VR: p = 0.306∗ RNA negative: 68% RNA positive: 100% HR (positive vs. negative); 3.20 (1.10–9.32) p = 0.033 |
EOT HBV RNA Cumulative incidence of 12-month CR: p = 0.01∗ RNA negative: 28% RNA positive: 100% HR (positive vs. negative): 4.73 (1.51–14.86) p = 0.008 |
EOT HBV RNA HR (positive vs. negative): not significantly associated with clearance |
|
EOT HBcrAg and EOT HBV RNA∗ HBcrAg >2 log U/ml and RNA positive: p = 0.042 47% of patients with VR and 18% of patients without VR HR (either positive vs. both negative): not significant |
EOT HBcrAg and EOT HBV RNA∗ HBcrAg >2 log U/ml and RNA positive: p = 0.07 59% of patients with CR and 29% of patients without CR HR (either positive vs. both negative): not significant |
EOT HBcrAg and EOT HBV RNA∗ HBcrAg >2 log U/ml and RNA positive: p = 0.009 0% of patients with HBsAg loss and 46% of patients without HBsAg loss HR (either positive vs. both negative): not significant |
|
|
EOT HBsAg, EOT HBcrAg and EOT HBV RNA HBsAg > 3 log IU/ml and HBcrAg >2 log U/ml and RNA positive: p = 0.209 58% of patients with VR and 35% of patients without VR HR (any positive vs. both negative): not significant |
EOT HBsAg, EOT HBcrAg, and EOT HBV RNA HBsAg > 3 log IU/ml and HBcrAg >2 log U/ml and RNA positive: p = 0.097 71% of patients with CR and 42% of patients without CR HR (any positive vs. both negative): not significant |
EOT HBsAg, EOT HBcrAg, and EOT HBV RNA HBsAg >3 log IU/ml and HBcrAg >2 log U/ml, and RNA positive: p = 0.003 0% of patients with HBsAg loss and 61% of patients without VR HR (any positive vs. both negative): not significant |
|
|
Combined HBeAg-positive and HBeAg-negative populations | |||
| Hsu, Y.C., et al., 201916 EOT HBcrAg: 3.0 log U/ml |
N/A |
EOT HBcrAg MV HR per log U/ml: 1.48 (1.20-1.83) p = 0.00 AUROC 0.61-0.75 |
EOT HBcrAg UV HR per log IU/ml: 0.44 (0.23-0.86) p = 0.02 |
| Kaewdech, A., et al., 202021 EOT HBcrAg: 3.2 log U/ml, 63% undetected (4.0 in HBeAg positive, 3.0 in HBeAg negative) EOT HBV RNA: 2.0 log copies/ml, 49% undetected (2.0 in HBeAg positive, 2.2 in HBeAg negative) |
EOT HBcrAg Cumulative incidence of 48-wk VR: p = 0.01∗ HBcrAg <3 log U/ml: 44.1% HBcrAg >3 log U/ml: 74.1% AUROC 0.686 |
EOT HBcrAg Cumulative incidence of 48-wk CR: p = 0.00∗ HBcrAg <3 log U/ml: 8.8% HBcrAg >3 log U/ml: 48.3% MV HR per log U/ml: 2.21 (1.50–3.24) p = 0.00 AUROC 0.773 |
EOT HBcrAg Cumulative incidence of 48-wk clearance: p = 0.06∗ HBcrAg <3 log U/ml: 5.9% HBcrAg >3 log U/ml: 0% EOT HBV RNA Cumulative incidence of 48-wk clearance: p = 0.5∗ RNA <2 log U/ml: 3.7% RNA >2 log U/ml: 0% |
|
EOT HBV RNA Cumulative incidence of 48-wk VR: p = 0.05∗ HBV RNA <2 log U/ml: 50% HBV RNA >2 log U/ml: 72% AUROC 0.648 |
EOT HBV RNA Cumulative incidence of 48-wk CR: p = 0.04∗ RNA <2 log U/ml: 21.1% RNA >2 log U/ml: 42.6% MV HR per log U/ml: 1.32 (1.02–1.70) p = 0.03 AUROC: 0.657 |
||
|
EOT HBcrAg and EOT HBV RNA AUROC 0.742 |
EOT HBcrAg and EOT HBV RNA Cumulative incidence of 48-wk CR: p = 0.00∗ RNA <2 log U/ml and HBcrAg <3 log U/ml: 0% RNA >2 log U/ml or HBcrAg >3 log U/ml: 22.9% RNA >2 log U/ml and HBcrAg >3 log U/ml: 62.5% AUROC 0.816 |
||
|
EOT HBsAg, EOT HBcrAg, and EOT HBV RNA AUROC 0.746 |
EOT HBsAg, EOT HBcrAg, and EOT HBV RNA AUROC 0.807 |
||
| Lai, C.L., et al., 202022 EOT HBcrAg: 3.4 log U/ml, 31% undetected EOT HBV RNA: 100% undetected |
EOT HBcrAg Median HBcrAg at VR: 3.76 log U/ml Significantly higher than at EOT (p = 0.005) |
||
|
EOT HBV RNA RNA remained undetected in all but one patient after VR | |||
| Liu, Y., et al., 202023 EOT HBV RNA: 55% undetected (59% in HBeAg positive, 46% in HBeAg negative) |
EOT HBV RNA UV HR (negative vs. positive): 0.37 (0.10–1.37) p = 0.14 Not significant |
EOT HBV RNA Cumulative incidence of 2-yr CR: p = 0.04∗ RNA negative: 17.50% RNA positive: 38.27% UV HR (negative vs. positive): 0.17 (0.03–1.09) p = 0.06 |
|
| EOT HBsAg and EOT HBV RNA Cumulative incidence of 2-yr VR: no p value HBsAg <2 log IU/ml and RNA negative 10% HBsAg <3 log IU/ml and RNA negative 23% MV HR (HBsAg <3 log IU/ml and RNA negative): 0.20 (0.05–0.91) p = 0.037 |
EOT HBsAg and EOT HBV RNA MV HR (RNA negative and HBsAg <3 log IU/ml): 0.101 (0.012–0.884) p = 0.04 |
||
| Seto, W.K., et al., 202025 EOT HBcrAg: 3 log U/ml, 19% undetected EOT HBV RNA: 1.65 log U/ml, 36% undetected |
EOT HBcrAg HR: not significantly associated with VR |
EOT HBcrAg HR: not significantly associated with CR |
EOT HBcrAg HR: not significantly associated with clearance |
|
EOT HBV RNA Cumulative incidence of 48-wk VR: p = 0.00∗ RNA undetectable: 36.6% RNA <1.65 log U/ml: 52.1% RNA >1.65 log U/ml: 93.2% MV HR (>1.65 vs. <1.65 U/ml): 2.96 (1.78–4.93) p = 0.00 |
EOT HBV RNA MV HR (>1.65 vs. <1.65 log U/ml): 2.77 (1.21–6.33) p = 0.02 |
EOT HBV RNA HR: not significantly associated with clearance |
|
|
EOT HBsAg and EOT HBV RNA Cumulative incidence of 48-wk VR: p = 0.00 RNA <1.65 log U/ml and HBsAg <10 IU/ml: 9.1% RNA >1.65 log U/ML or HBsAg >10 IU/ml: 63.8% |
|||
| Tseng, T.N., et al., 202026 Baseline HBcrAg: 4.7 log U/ml EOT HBcrAg: 2.9 log U/ml |
Baseline HBcrAg Cumulative incidence of 5-yr VR: p = 0.00∗ HBcrAg <4 log U/ml: 23.8% HBcrAg >4 log U/ml: 53% UV HR per log U/ml: 1.28 (1.11-1.47) p = 0.00 |
Baseline HBcrAg Cumulative incidence of 5-yr CR: p = 0.00∗ HBcrAg <4 log U/ml: 13.9% HBcrAg >4 log U/ml: 46.6% UV HR per log U/ml: 1.33 (1.13–1.56) p = 0.00 |
Baseline HBcrAg HR: not significantly associated with clearance |
|
EOT HBsAg and baseline HBcrAg Cumulative incidence of 5-yr VR: p = 0.00 HBcrAg <4 log U/ml and HBsAg <40 IU/ml: 5.9% HBcrAg >4 log U/ml and HBsAg <40 IU/ml: 27.6% HBcrAg <4 log U/ml and HBsAg >40 IU/ml: 57.3% HBcrAg >4 log U/ml and HBsAg >40 IU/ml: 72.2% MV HR (HBcrAg >4 log U/ml and HBsAg >40 IU/ml): 2.45 (1.82–3.30) p = 0.00 |
EOT HBsAg and baseline HBcrAg Cumulative incidence of 5-yr CR: p = 0.00 HBcrAg <4 log U/ml and HBsAg <40 IU/ml: 2.8% HBcrAg >4 log U/ml and HBsAg <40 IU/ml: 17% HBcrAg <4 log U/ml and HBsAg >40 IU/ml: 34.2% HBcrAg >4 log U/ml and HBsAg >40 IU/ml: 68.3% MV HR (HBcrAg >4 log U/ml and HBsAg >40 IU/ml): 3.02 (2.03–4.50) p = 0.00 |
||
|
EOT HBcrAg HR: not significantly associated with VR |
EOT HBcrAg HR: not significantly associated with CR |
||
| Sonneveld, M.J., et al., 202131 EOT HBcrAg: <2 log U/ml: 22% of patients 2-3 log U/ml: 23% of patients >3 log U/ml: 54% of patients |
EOT HBcrAg Cumulative incidence of 48-wk VR: p <0.001∗ HBcrAg <2 log U/ml: 38% HBcrAg 2–3 log U/ml: 50% HBcrAg >3 log U/ml: 65% MV OR (per log U/ml): 0.73 (0.62–0.86) p <0.001 (MV OR referring to virological remission) |
EOT HBcrAg Cumulative incidence of 48-wk CR: p = 0.018∗ HBcrAg <2 log U/ml: 15% HBcrAg 2–3 log U/ml: 9% HBcrAg >3 log U/ml: 20% MV OR (per log U/ml): 1.29 (1.08–1.54) p = 0.005 |
EOT HBcrAg Cumulative incidence of 48-wk HBsAg loss: p <0.001∗ HBcrAg <2 log U/ml: 12% HBcrAg 2–3 log U/ml: 3% HBcrAg >3 log U/ml: 2% MV OR (per log U/ml): 0.48 (0.33–0.68) p <0.001 |
|
SCALE-B score evaluation Cumulative incidence of 48-wk VR: p <0.001 Low risk: 38% Medium risk: 54% High risk: 65% |
SCALE-B score evaluation Cumulative incidence of 48-wk CR: p <0.001 Low risk: 3% Medium risk: 14% High risk: 31% |
SCALE-B score evaluation Cumulative incidence of 48-wk HBsAg loss: p <0.001 Low risk: 11% Medium risk: 2% High risk: 1% |
|
| Xia, M., et al., 202133 EOT HBV RNA: 23% undetected |
EOT HBV RNA Cumulative incidence of 6-yr CR: p <0.001∗ RNA <3 log copies/ml: 24% RNA 3–4.3 log copies/ml: 61% RNA >4.3 log copies/ml: 100% MV HR per log copies/ml: 1.34 p <0.001 AUROC 0.760 |
EOT HBV RNA Cumulative incidence of 6-yr HBsAg loss: p = 0.007∗ RNA <3 log copies/ml: 30.9% RNA >3 log copies/ml: 1.6% |
|
| Kaewdech, A., et al., 202243 EOT HBcrAg: 3.2 log U/ml EOT HBV RNA: 2.0 log copies/ml |
SCALE-B Cumulative incidence of 2-yr VR: p <0.001 Low risk: 28.6% Medium risk: 61% High risk: 81.5% MV HR (medium vs. low risk): 2.54 (0.88–7.35) p = 0.086 MV HR (high vs. low risk): 5.02 (1.75–14.39) p = 0.003 |
SCALE-B MV HR (medium vs. low risk): 3.01 (0.38–23.87) p = 0.30 MV HR (high vs. low risk): 10.44 (1.38–79.08) p = 0.02 AUROC 0.81 |
SCALE-B Cumulative incidence of 2-yr HBsAg loss: p <0.001 Low risk: 14.3% Medium risk: 2.4% High risk: 0% MV HR (med vs. low risk): 0.09 (0.01–0.52) p = 0.008 MV HR (high vs. low risk): 0.04 (0.00–0.43) p = 0.007 |
|
EOT HBV RNA MV HR (>2 vs. <2 log U/ml): 0.69 (0.41–1.15) p = 0.153 |
EOT HBV RNA MV HR (>2 vs. <2 log U/ml): 0.64 (0.30–1.35) p = 0.24 AUROC 0.66 |
EOT HBV RNA MV HR (>2 vs. <2 log U/ml): 0.20 (0.03–1.16) p = 0.072 |
|
| Sonneveld, M.J., et al., 202245 EOT HBcrAg: <2 log U/ml: 22% of patients 2–3 log U/ml: 19% of patients >3 log U/ml: 58% of patients |
EOT HBcrAg Cumulative incidence of 3-yr HBsAg loss: p <0.001∗ HBcrAg <2 log U/ml: 14.6% HBcrAg >2 log U/ml: 3.5% MV HR (per log U/ml): 0.718 (0.593–0.869) p = 0.001 |
||
|
EOT HBsAg and EOT HBcrAg Among patients with HBsAg <1 log IU/ml, no additive value of HBcrAg (HR = 1.08, p = 0.833) Among patients with HBsAg 10–100 IU/ml: MV HR (<2 vs. >2 log U/ml): 3.397 p = 0.001 Among patients with HBsAg >2 log IU/ml: MV HR (<2 vs. >2 log U/ml): 3.702 p <0.001 | |||
In the first column, mean/median levels of explored biomarkers for each cohort are provided where available.
AUROC, area under region of curve; CR, clinical relapse; EOT, end of treatment; HBcrAg, hepatitis B core-related antigen; HR, hazard ratio; MV, multivariate; OR, odds ratio; UV, univariate; VR: virological relapse.
Cumulative rates of VR, CR, or functional cure stratified by the biomarker(s) of interest were summarised in Fig. 2A–C. †Serum HBV DNA is quantified using the COBAS Taqman HBV test, with a lower limit of detection of 20 IU/ml. This study specifically made a distinction between patients with undetectable HBV DNA (48.5%) and patients with HBV DNA <20 IU/ml (43.8%), which was not performed in other studies.
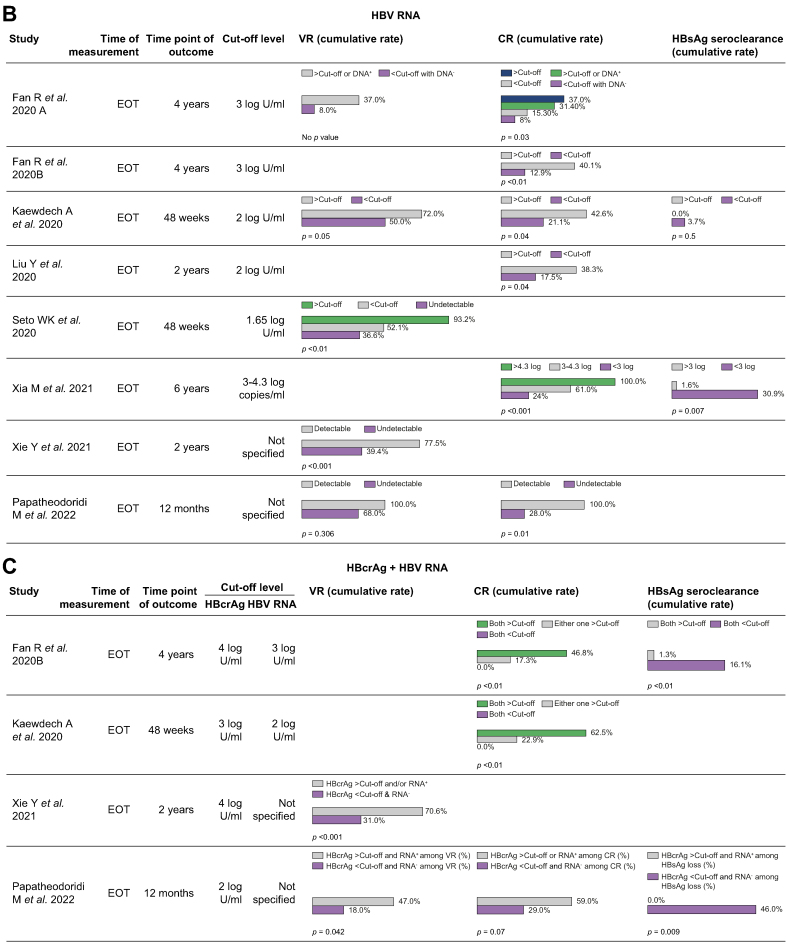
Fig. 2.
Cumulative rates of VR, CR, or functional cure.
(a) Cumulative rates of VR, CR, or functional cure stratified by HBcrAg. Each row represents a single study. The bar charts demonstrate the cumulative rate, shown as %, of the specific outcome (VR, CR, or HBsAg seroclearance) stratified by whether HBcrAg was above the specified cut-off level. For instance, in Fan et al.19, the 4-yr cumulative rate of CR was 39.5% and 7.3% when the end-of-treatment HBcrAg was >4 log and <4 log, respectively (p <0.01). (b) Cumulative rates of VR, CR, or functional cure stratified by HBV RNA. Each row represents a single study. The bar charts demonstrate the cumulative rate, shown as %, of the specific outcome (VR, CR, or HBsAg seroclearance) stratified by whether HBV RNA was above the specified cut-off level. For instance, in Fan et al.,18 the 4-yr cumulative rate of VR was 37.0% and 8.0% when the end-of-treatment HBV RNA was >3 log and <3 log, respectively (no p value). (c) Cumulative rates of VR, CR, or functional cure stratified by HBcrAg + HBV RNA. Each row represents a single study. The bar charts demonstrate the cumulative rate, shown as %, of the specific outcome (VR, CR, or HBsAg seroclearance) stratified by whether HBcrAg and/or HBV RNA was above the specified cut-off level. For instance, in Fan et al.,19 the 4-yr cumulative rate of HBsAg seroclearance was 16.1% when the end-of-treatment HBcrAg was <4 log + HBV RNA <3 log, compared with 1.3% when HBcrAg was >4 log + HBV RNA >3 log (p <0.01). CR, clinical relapse; EOT, end of treatment; VR, virological relapse.
There was a stronger relationship between HBcrAg and CR, as evaluated in 13 studies (Table 5). Fan et al.19 demonstrated a multivariate HR of 5.70 (1.37–23.67; p = 0.017) between patients with EOT HBcrAg >4 log U/ml and <4 log U/ml, with five other studies encompassing HBeAg-positive, HBeAg-negative and combined populations also producing significant multivariate HRs at EOT HBcrAg cut-off levels ranging from 2 to 4 log U/ml.16,19,21,30,31,41 These results were affirmed by the CREATE study group,31 who reported that lower EOT HBcrAg levels were significantly associated with lower rates of CR with multivariate OR 1.29 per log U/ml (1.08–1.54, p = 0.005). Eight studies reported the cumulative rates of CR stratified by various cut-off levels of HBcrAg at baseline or EOT (Fig. 2A). Once again, both Tseng et al.26 and Huang et al.28 found that 5-yr CR rates differed significantly when separating patients at the baseline HBcrAg cut-off of 4 log U/ml. Papatheodoridi et al.24 found that EOT HBcrAg was significantly associated with retreatment (a composite endpoint suggestive of CR) in a HBeAg-negative population, where the 2-yr retreatment rates were 45% and 17% in patients with EOT HBcrAg >2 and <2 log U/ml respectively, alongside a multivariate HR of 3.64 (1.23–10.75; p = 0.019) regarding retreatment with this cut-off level.
Overall, HBeAg-positive populations demonstrate higher mean/median EOT HBcrAg levels, and the EOT cut-off of HBcrAg of 4 log U/ml is a reliable predictor of both VR and CR. HBeAg-negative and combined populations necessitate a lower cut-off level, ranging from 2 to 3.3 log U/ml in the included studies (noting that the validated lower limit of detection is 3 log U/ml). The risk of VR/CR in populations that have a mean/median EOT HBcrAg level at or below 3 log U/ml may be better distinguished by a baseline HBcrAg cut-off of 4 log U/ml.
HBcrAg and functional cure
Fourteen studies evaluated the association between HBcrAg and rates of HBsAg loss, with most studies not returning significant results (Table 5). Five studies reported the cumulative rates of functional cure stratified by baseline or EOT HBcrAg levels (Fig. 2A). Only four of 12 patients in Liao et al.’s study30 achieving HBsAg loss had undetectable EOT HBcrAg. Kaewdech et al.21 found a near-significant difference in 48-wk HBsAg seroclearance rates, 5.9% vs. 0% in patients with EOT HBcrAg <3 log U/ml and >3 log U/ml respectively (p = 0.062). Interestingly, Höner Zu Siederdissen et al.13 demonstrated that HBsAg reduction and seroclearance was associated with the degree of virological relapse. The extent of increase in HBcrAg (in parallel with HBV DNA rebound) at Weeks 4–8 post-treatment cessation correlated with HBsAg decline and were followed by HBsAg loss in three of 15 patients. Carey et al.17 also found that a steeper HBsAg decline post-treatment correlated with lower baseline HBcrAg levels rather than EOT levels, observing transiently resolving elevations of HBcrAg after NA cessation. Recently, a multicentre study45 comprising 1,216 patients demonstrated that EOT HBcrAg was significantly associated with the probability of HBsAg loss (multivariate HR per log U/ml 0.729, 0.603–0.882, p = 0.001).
HBV RNA and partial cure
Eight studies explored the association between HBV RNA levels and rates of VR (Table 5). Five studies reported the cumulative rates of VR at various observation periods stratified by EOT RNA levels (Fig. 2B). For instance, Kaewdech et al.21 initially reported significantly different 48-wk VR rates of 50% and 72% in patients with EOT HBV RNA <2 and >2 log U/ml, respectively (p = 0.048), yet the effect of HBV RNA on both VR and CR was found to be statistically insignificant in their subsequent publication with longer follow-up (median 35.5 months) when adjusted for SCALE-B strata.43 Liu et al.23 did not find a significant association between HBV RNA and VR, but Seto et al.25 demonstrated a multivariate HR of 2.96 (1.78–4.93; p = 0.001) between combined HBeAg-positive and HBeAg-negative patients at RNA cut-off level of 1.65 log U/ml. Papatheodoridi et al.44 found that detectability of EOT HBV RNA was significantly associated with VR in their HBeAg-negative population, quoting a HR of 3.20 (1.10–9.32 p = 0.033), as was detectability of HBV RNA detection at 1 month post-EOT (HR 3.23, 1.57–6.67, p = 0.001). Similarly, Xie et al.34 demonstrated a multivariate OR of 3.453 (1.387–8.597; p = 0.008) between patients with positive vs. negative RNA detection in their HBeAg-positive population. Furthermore, Lai et al.,22 who demonstrated high VR rates in patients with undetectable cccDNA and RNA, found that all but one patient continued to exhibit undetectable HBV RNA levels after relapse.
Eight out of 10 relevant studies affirmed a significant association between HBV RNA and CR (Table 5). Six studies reported the cumulative rates of CR at various observation periods stratified by EOT RNA levels (Fig. 2B). For instance, Fan et al.19 demonstrated a multivariate HR of 3.58 (1.26–10.14; p = 0.017) between HBeAg-positive patients with EOT RNA >3 and <3 log U/ml, alongside significantly different 4-yr CR rates of 12.9% vs. 40.1% according to that cut-off (p = 0.004). Liu et al.23 also reported significantly different 2-yr CR rates of 17.5% vs. 38.3% in patients who were HBV RNA negative and positive, respectively (combined HBeAg-positive and HBeAg-negative population). Papatheodoridi et al.44 also found that detectability of EOT HBV RNA was significantly associated with CR (HR of 4.73, 1.51–14.86, p = 0.008). Carey et al.17 demonstrated transient elevations in HBV RNA after NA cessation and found that three of four patients who demonstrated CR had RNA levels >1.65 log U/ml (75% sensitivity, 100% specificity, 100% PPV).
In conclusion, HBV RNA demonstrates utility in predicting both VR and CR in the majority of publications. There is less of a distinction in mean EOT RNA levels and preferred RNA cut-offs between HBeAg-positive, HBeAg-negative and combined populations when compared with the corresponding HBcrAg findings. This is in part because of a lack of standardisation of RNA assays between different study groups.
HBV RNA and functional cure
The relationship between HBV RNA and HBsAg loss was not reported in most studies. Only two studies reported the cumulative rates of functional cure stratified by EOT RNA levels (Fig. 2B). Kaewdech et al.21 reported a non-significant difference in 48-wk clearance rates between patients with RNA <2 and >2 log U/ml, and Seto et al.25 also reported a non-significant HR associated with seroclearance. However, García-López et al.20 found that EOT HBV RNA was more frequently undetectable in patients who achieved HBsAg loss than in patients who did not (88% vs. 47%, p = 0.053). Xia et al.33 also found that cumulative incidence of 6-yr HBsAg clearance rates was 30.9% vs. 1.6% in patients with EOT HBV RNA <3 vs. >3 log U/ml respectively (p = 0.007).
Combining HBcrAg with qHBsAg
Hsu et al.16 derived the SCALE-B score for CR, consisting of the five predictors: EOT HBsAg, EOT HBcrAg, age, ALT, and use of TDF. Stratifying patient risk, they demonstrated a significant difference in 3-yr CR rates of 86.2%, 55.6%, and 17.2% in the high-, intermediate-, and low-risk subgroups respectively (p = 0.0001). Furthermore, all patients achieving functional cure were drawn from the low-risk subgroup and demonstrated EOT HBsAg levels <2 log IU/ml and EOT HBcrAg levels below 3 log U/ml. Later, Papatheodoridi et al.24 also demonstrated a significant multivariate HR of 0.93 (0.87–0.98; p = 0.012) per 1,000 points increase in the SCALE-B score for HBsAg seroclearance. Lower SCALE-B score was again associated with higher rates of partial and functional cure in Kaewdech et al.’s Thailand study43 and the multicentre CREATE study.31
Combining HBV RNA with qHBsAg
Liu et al.23 found that combining HBV RNA status and EOT HBsAg level was superior to EOT HBsAg level alone in predicting partial cure, with a 2-yr VR rate of 10% in patients with EOT HBsAg <2 log IU/ml and EOT HBV RNA negativity. Seto et al.25 similarly demonstrated that a combination of undetectable EOT HBV RNA level and HBsAg <10 IU/ml was associated with a 1-yr VR rate of 9.1%. Lastly, Xie et al.34 found that the combination of EOT HBsAg <100 IU/ml and EOT HBV RNA undetectability had the highest AUROC for VR or partial cure, with an AUROC of 0.698 that was superior to other singular and combined parameters.
Combining HBcrAg with HBV RNA
The cumulative rates of VR, CR, or functional cure stratified by a combination of EOT HBcrAg and EOT HBV RNA were reported in four studies (Fig. 2C). Xie et al.34 demonstrated that combining EOT HBcrAg and EOT HBV RNA levels was able to strongly predict VR, whereas Fan et al.19 and Kaewdech et al.21 affirmed the same for both rates of CR and functional cure. Kaewdech et al.21 demonstrated that the combination of EOT HBcrAg and EOT HBV RNA was most predictive of subsequent CR with an AUROC of 0.742 (0.64–0.84, p <0.001), indeed superior to qHBsAg alone with an AUROC of 0.609 (0.49–0.73, p = 0.089). In the study by Papatheodorididi et al.,44 although more patients who did not develop VR/CR or achieved HBsAg seroclearance had undetectable HBcrAg and HBV RNA, a combination of detectable HBV RNA and/or HBcrAg at EOT was not significantly associated with partial or functional cure.
Novel immune markers
Non-disease-specific immune markers
Single nucleotide polymorphisms in various genes14,39 have been explored in the context of HBV treatment discontinuation, but the specificity and clinical significance of these findings remain uncertain. For example, Wu et al.39 found that the rs676925 ‘GC’ genotype of the CXCR5 gene was associated with decreased risk of CR, but failed to demonstrate a corresponding difference in percentage of CXCR5-positivity or expression of CXCL13 ligand between genotype groups. With respect to whole genome gene expression analysis, Kranidioti et al.38 found that lower gene expression of CCL20, CCL4, CXCL2, CXCL3, interferon-gamma (IFNγ), IL-8, IL-1A, IL-1B, FASLG and TNFRSF9 in peripheral blood mononuclear cells (PBMCs) predicted off-treatment remission.
Regarding soluble immune markers (SIMs) in the context of treatment discontinuation, Wübbolding et al.32 have proposed a combination of IL-2, CXCL9, CCL5, SCF and TRAIL to be an accurate prognostic marker for VR with an AUROC of 0.89.32 Höner Zu Siederdissen et al.13 found that levels of almost all SIMs increased after treatment cessation, significantly so for TNF, IL-12p70, and IL-10 at Week 4 post-EOT and for TNF and CXCL10/IP10 at Week 8 post-EOT. The increase in SIM levels was associated with VR and HBcrAg rebound, and subsequent decline and loss of HBsAg. Papatheodoridi et al.24 reported that higher EOT IP10 levels at 1 month post-EOT identified patients more likely to achieve HBsAg loss, without mention of whether they underwent transient VR and CR first.
Finally, in terms of innate immunity, Zimmer et al.36 studied changes in the natural killer (NK) cell response in HBV patients following treatment cessation. Stopping NA treatment significantly boosted CD56dim NK cell natural cytotoxicity responses, correlating with increased NK cell functional responses and ALT levels at Weeks 8 and 12 post-EOT. The subgroup of patients who cleared HBsAg experienced higher ALT levels at Week 12 post-EOT and demonstrated higher expression of CD38 on CD56dim NK cells, with increased NK cell functionality. Furthermore, Hall et al.42 reported that severe hepatitis flares were associated with upregulation of the innate immune response, demonstrated by increased activity of TLR2-8 and TLR9 signalling in PBMCs and upregulation of TLR2 and TREM-1 receptor expression on NK cells at peak flare, with no such change from baseline in patients without flares. There was no significant correlation between TLR signalling activity and HBsAg decline or clearance.
HBV-specific immune markers
The association between EOT anti-HBc levels and rates of partial cure after treatment cessation appear unclear (Table 6). Chi et al.15 found that anti-HBc was not significantly associated with VR, but reported a multivariate HR of 0.31 (0.15–0.65; p = 0.002) in predicting 4-yr CR. Patients with anti-HBc >3 log IU/ml demonstrated 4-yr CR rates of 21%, whereas those with anti-HBc <2 log IU/ml demonstrated 4-yr CR rates of 85%. Similar studies did not find a significant association between EOT anti-HBc levels and VR or CR.14
Table 6.
Summary of novel immune markers.
| Paper | Relation with VR | Relation with CR | Relation with HBsAg clearance |
|---|---|---|---|
| Single nucleotide pleomorphisms | |||
| Su, T.H., et al., 201814 |
CTLA (rs231775) non-GG vs. GG genotype: MV HR: 1.74 (1.01–3.00) p = 0.048 |
CTLA4 (rs231775) non-GG vs. GG genotype: MV HR: 2.06 (1.04–4.11) p = 0.039 |
|
| Wu, Y., et al., 201939 |
CXCR5 (rs676925) GC vs. CC genotype: MV OR: 0.25 (0.07–0.95) p = 0.0042 CXCR5 (rs676925) non-CC vs. CC genotype: MV HR: 0.34 (0.12–0.96) p = 0.041 No difference in number or MFI of CXC5-positive cells or plasma CXCL13 levels between genotype groups |
||
| Gene expression levels | |||
| Kranidioti, H., et al., 201938 | Remission associated with lower expression of: CCL20: 14-fold decrease p = 0.03 AUROC 0.929 CCL4: 5.9-fold decrease p = 0.02 UV OR: 27.57 (0.65–1165.96) p = 0.053 CXCL2: 18-fold decrease p = 0.02 CXCL3: 17.6-fold decrease p = 0.01 AUROC 0.857 IFNγ: 5.3-fold decrease p = 0.01 UV OR: 3.46 (1.11-10.79) p = 0.032 AUROC 0.871 IL-8: 5.7-fold decrease p = 0.01 UV OR: 2.97 (1.01-8.73) p = 0.048 AUROC 0.871 IL-1A: 61-fold decrease p = 0.03 IL-1B: 8.6-fold decrease p = 0.05 FASLG: 2-fold decrease p = 0.01 UV OR: 29.78 (1.38-643.08) p = 0.030 AUROC 0.857 TNFRSF9: 2.9-fold decrease p = 0.05 Combination of CCL4, IFNγ, IL-8, and FASLG predicted off-treatment remission with sensitivity 71.4–85.7% and specificity 80–90% |
Patients achieving HBsAg loss had significantly lower expression of: FASLGp = 0.04 IL-8p = 0.02 CCL4p = 0.008 IFNγp = 0.06 |
|
| Serum cytokine/chemokine (immune marker) levels | |||
| Höner Zu Siederdissen, C., et al., 201613 |
SIM levels at EOT vs. Week 4 post-EOT (VR) IL-10: 8.65 → 13.96 pg/ml p = 0.048 IL-12p70: 14.46 → 25.29 pg/ml p = 0.012 CXCL10/IP10: 1,223 → 1,533 pg/ml p = 0.002 TNFα: 18.77 → 57.68 p = 0.027 CXCL10 and TNFα remained significantly elevated at Week 8 |
||
| Xie, L., et al., 201940 |
EOT sST2 levels UV HR per log pg/ml: 2.82 (0.73–10.85) p = 0.132 UV HR (>3.7 vs. <3.7 log pg/ml): 1.72 (0.84–3.51) p = 0.137 12-wk post-EOT sST2 levels MV HR per log pg/ml: 4.40 (2.17–8.93) p <0.001 Patients with CR demonstrated a rising sST2 trend post-EOT and experienced higher sST2 levels at wk 12, 24, and 48 post-EOT compared with patients without CR |
||
| Papatheodoridi, M., et al., 202024 |
EOT IP10 levels HR per 10 pg/ml: 1.03 (0.99–1.07) p = 0.01 1 month post-EOT IP10 levels HR per 10 pg/ml: 1.10 (1.02–1.19) p = 0.01 Compared with EOT, IP10 levels were higher at months 2 (p <0.001) and 3 (p = 0.024), similar at month 6 (p = 0.195) and lower at months 9 and 12 (p <0.004) |
||
| Wübbolding, L.A., et al., 202132 |
EOT SIM levels IL-2: lower in VR p = 0.002 IL-6: lower in VR p = 0.021 MIP-1a/CCL3: lower in VR p = 0.027 RANTES/CCL5: higher in VR p = 0.039 IL-7: lower in VR p = 0.042 All single SIMs had AUROCs <0.67 for VR IL-2, CXCL9, RANTES/CCL5, SCF, TRAIL EOT AUROC: 0.89 (0.5–0.99) 12 wk pre-EOT AUROC: 0.76 (0.34–0.99) 24 wk pre-EOT AUROC: 0.78 (0.1–0.99) |
||
| HBV-specific T cell activity | |||
| Su, T. H., et al., 201814 EOT Anti-HBc: 2.89 log IU/ml in ETV patients, 2.63 log IU/ml in TDF patients |
EOT Anti-HBc MV HR per log IU/ml: 0.92 (0.55–1.56) p = 0.768 Not significant |
EOT Anti-HBc MV HR per log IU/ml: 0.83 (0.45–1.54) p = 0.551 Not significant |
N/A |
| Chi, H., et al., 201915 EOT Anti-HBc: 2.8 log IU/ml in eAg+ patients, 2.5 log IU/ml in eAg- patients |
EOT Anti-HBc UV HR per log IU/ml: 0.69 (0.45–1.06) p = 0.088 Not significant |
EOT Anti-HBc Cumulative incidence of 4-yr CR: p <0.05 Anti-HBc >3 log IU/ml: 21% Anti-HBc 2–3 log IU/ml: 50% Anti-HBc <2 log IU/ml: 85% MV HR per log IU/ml: 0.31 (0.15–0.65) p = 0.002 Patients with CR experienced an anti-HBc increase of 3.6 log IU/ml per yr, while those with SR experienced an anti-HBc increase of 0.5 log IU/ml per year EOT Anti-HBc and EOT HBsAg Cumulative incidence of 4-yr CR: p = 0.009 HBsAg >2 log IU/ml and anti-HBc >3 log IU/ml: 27% HBsAg >2 log IU/ml and anti-HBc <3 log IU/ml: 64% |
N/A |
| Rinker, F., et al., 201835 | Patients achieving HBsAg loss demonstrated a T cell phenotype with lowly expressed PD-1 and KLRG1, and an increase in expression of Ki-67 and CD38 at Week 12 post-EOT Baseline HBsAg was positively correlated with PD-1+ CD8+ T cells, and fold decline of HBsAg at month 12 post-EOT was associated with frequency of Ki-67+ CD38+ T cells at Week 12 post-EOT |
||
| Rivino, L., et al., 201837 | The HBV-specific T cell response, mainly targeting core and polymerase proteins, was at least not superior in patients who flared Patients who did not flare demonstrated increased expression of the most differentially expressed gene, PD-1 (p = 0.009) in CD8+ T cells | ||
| García-López, M., et al., 202020 | Patients remaining off-therapy had functional HBV-specific CD8+ T cell responses against epitopes from multiple HBV proteins, (68% vs. 20%, p = 0.048 for IFNγ production and 77% vs. 40% p = 0.099 for CD107a expression) The percentage of degranulating CD8+ T cells (CD107a) was higher at EOT and Week 12 post-EOT in patients remaining off therapy (p = 0.039 and p = 0.0093) when stimulated with core proteins The percentage of polyfunctional core-specific CD8+ T cells (co-expressing IFNγ and TNFα) was higher among patients remaining off-therapy (p = 0.031) and this increase persisted for more than a year post-EOT (p = 0.01) |
||
| NK cell activity | |||
| Zimmer, C.L., et al., 201836 | Patients achieving HBsAg loss experienced higher ALT levels and higher CD56dim NK cell expression of CD38 at 12 wk post-EOT Patients achieving HBsAg loss experienced elevated responses upon K562 stimulation at 12 wk post-EOT, particularly CD56dim NK cell IFNγ, TNF, and GM-CSF responses |
||
| Hall, S.A.L, et al., 202242 | Hepatitis flares were associated with significant increases in TNF, IL-6 and IL-8 cytokine production after PBMC TLR signalling with stimulation from TLR ligands, whereas patients who did not flare demonstrated no significant changes to baseline Hepatitis flare was associated with increased expression of TREM-1 and TLR2 on NK-bright and NK-T cells, and increased expression of TLR2 alone on NK-dim cells, whereas patients who did not flare demonstrated no significant changes to baseline |
||
AUROC, area under region of curve; CR, clinical relapse; EOT, end of treatment; HBsAg, hepatitis B surface antigen; HR, hazard ratio; IP10, interferon gamma-induced protein 10; MV, multivariate; OR, odds ratio; UV, univariate; VR, virological relapse.
Several studies examined HBV-specific and global T cell populations in patients undergoing treatment cessation. We previously reported that frequencies of in vitro-expanded HBV-specific T cells both during and after discontinuation of therapy were consistently and significantly higher in patients without hepatic flares after treatment-cessation, in particular the responses against core and polymerase proteins.37 Patients who did not develop a biochemical flare upon treatment cessation demonstrated increased gene expression encoding for program celled death protein 1 (PD-1) in CD8+ T cells. García-López et al.20 found that patients who did not require retreatment demonstrated a higher percentage of degranulating CD8+ T cells (CD107a) in addition to polyfunctional CD8+ T cells co-producing IFNγ/tumour necrosis factor-alpha (TNFα). HBV-specific T cell responses did not augment following treatment withdrawal, and were not associated with the development of clinically relevant flares or HBsAg loss. Conversely, Rinker et al.35 found a significant increase in HBV core-specific multifunctional T cell responses at 8 and 12 wk post-EOT, whereas no significant changes were observed following stimulation with polymerase- or envelope-specific peptides. Patients experiencing functional cure demonstrated a less exhausted and more activated T cell phenotype, with increases in Ki-67 and CD38 expression at Week 12 post-EOT. HBV-specific CD4+ and CD8+ T cell responses were also significantly enhanced by PD-L1 blockade at Weeks 4 and 8 post-EOT. The findings from studies of HBV-specific immune markers are summarised in Table 6.
Discussion
Rates of functional cure remain low in CHB patients who remain on antiviral therapy, as evidenced by an 8-yr cumulative incidence of 1.69% in ETV-treated patients and 1.34% in TDF-treated patients in a recent, large multi-ethnic study.46 Treatment cessation in CHB patients has emerged as a possible strategy to achieve functional cure in select patients, but remains a controversial approach given concerns around safety of treatment withdrawal. Overall, the included studies report wide variations in off-therapy outcomes, owing to the heterogeneity of patient populations and stopping criteria. Patient factors, such as ethnicity,47 have been demonstrated to play a role alongside EOT HBsAg levels45 and sustained off-therapy response48,49 in achieving functional cure after treatment discontinuation. However, there is interest in leveraging additional factors to forecast off-therapy HBsAg loss with more certainty. Given the limitations of current treatment strategies in CHB, the aim of this review was to evaluate how the data from treatment discontinuation studies could be applied to the functional cure programme to better predict treatment response and ultimately HBsAg loss.
Reliable biomarkers are essential to identify individuals where NA therapy can be discontinued safely and functional cure achieved. It is well established that the correlation between serum HBsAg level and cccDNA exists only in the HBeAg-positive phase of CHB infection.50,51 Following HBeAg seroconversion, there is continued production of HBsAg, partly from integrated HBV DNA in hepatocytes, and in fact the fraction of integrated HBV DNA as a fraction of total intrahepatic HBV DNA is significantly higher in HBeAg-negative patients compared with HBeAg-positive patients.52 To this end, HBcrAg, which represents the combined antigenic reactivity of e-antigen, core antigen, and defective core-related protein p22cr, has been shown to more strongly correlate with cccDNA quantity in both patients that are treatment naive53 and on NA therapy.54 In situations where serum HBV DNA has become undetectable, the presence of HBcrAg indicates continued secretion of viral-end products. Conversely, serum HBV RNA reflects the amount of virion-like encapsidated particles in which pgRNA was non- or partially reverse transcribed.55 Undetectable HBV RNA despite the persistence of cccDNA in most patients with HBsAg loss after treatment cessation may demonstrate a functional reduction in cccDNA transcriptional activity.20
Our review suggests that EOT HBV RNA and EOT HBcrAg are both strong predictors for sustained partial cure across the included studies, but both markers also have their limitations. The decline in HBcrAg across treatment may result in an EOT level that falls below the accepted lower limit of detection, especially among HBeAg-negative patients, and as a result this assay may not be able to reflect very low but persistent levels of cccDNA. Similarly, Liu et al.23 reported that the lack of highly sensitive methods of detection for HBV RNA may result in a low threshold for undetectable RNA levels. Standardisation of cut-offs of viral markers (especially HBV RNA) in terms of method of detection and quantification is of paramount importance to allow fair comparison between various settings. Although previous studies have failed to find a strong correlation between either of these biomarkers and functional cure, the CREATE study group45 recently pooled multiple large-scale cohorts to conclude that EOT HBcrAg, in isolation or in combination with EOT HBsAg, was significantly associated with HBsAg seroclearance. Various combinations of viral markers have also shown potential in predicting off-therapy responses, but the evidence behind SCALE-B score is the most substantial, having been validated for clinical relapse, retreatment, and HBsAg loss.
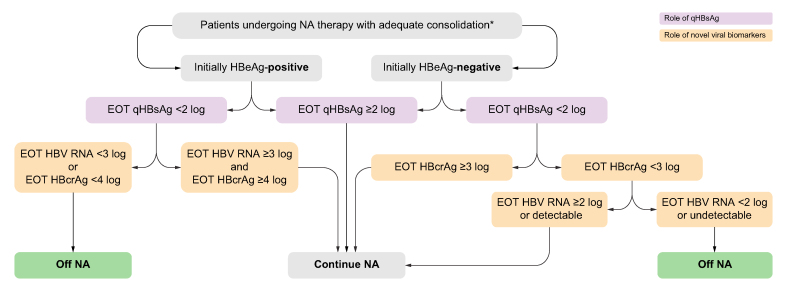
We propose an algorithm, stratified by HBeAg status at NA initiation, based on EOT qHBsAg, in combination with HBcrAg and HBV RNA to decide whether NA should be discontinued in CHB patients (Fig. 3). In general, NA should be continued if EOT qHBsAg is ≥2 log. NA cessation can be considered when the EOT qHBsAg <2 log in combination with HBV RNA <3 log or HBcrAg <4 log for initially HBeAg-positive patients. As the sensitivity of HBcrAg in HBeAg-negative patients is lower, undetectable HBcrAg should not be over interpreted in this scenario; NA cessation could only be considered when HBV RNA is <2 log or undetectable.
Fig. 3.
Proposed algorithm to decide whether NA should be discontinued based on qHBsAg, HBcrAg, and HBV RNA stratified by HBeAg status.
Refer to Table S1 for the guideline-recommended duration of consolidation therapy. EOT, end of treatment; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B envelope antigen; NA, nucleoside analogue; qHBsAg, quantitative hepatitis B surface antigen.
Our understanding of CHB infection is also defined by the patient’s innate and adaptive immune responses.56 The hallmark of CD8+ T cell exhaustion is loss of proliferative capacity, cytotoxicity, and cytokine production, which is enhanced through the upregulation of inhibitory pathways with continued antigen and viral load exposure.57 Regarding innate immunity, NK cells appear to act in inverse correlation to T cells. Their inhibition of CD4+ T cells is likely necessary to limit persistent T cell activation, yet their reversion to a quiescent phenotype is reflective of restoration of HBV-specific T cell function.58 The adaptive humoral response is driven by the role of B cells, which are activated by T-cell dependent and independent pathways to produce disease-specific antibodies. In CHB, HBsAg specific B cells demonstrate defective antibody production and an accumulation of atypical memory B cells with increased expression of inhibitory receptors.59,60 Although all exposed individuals mount an antibody response to HBcAg, higher anti-HBc levels in CHB infection may represent a larger number of activated B cells, which in turn modulate CD4+ and CD8+ T cell activity, and augment naive T-helper cells through their highly potent antigen-presenting function.61
Although the high levels of antigen expression in hepatocytes result in T cell exhaustion and deletion, evidence suggests that long-term antiviral therapy only results in a partial reconstitution of the T cell response. Following in vitro expansion experiments, the HBV-specific polyfunctional T cell response of successfully treated CHB patients (with HBsAg loss) was comparable with patients with spontaneously resolving acute HBV infection. In contrast, in NA-treated CHB patients who were HBV DNA negative but remain HBsAg positive, T cell responses were notably weaker, compounded by the slow decline in HBsAg on long-term NA.62,63 Therefore, there is growing interest in treatment interruption or discontinuation as a strategy to boost the host immune response to facilitate functional cure.
Although studies that explore SIMs in the context of NA cessation demonstrate low replicability potential and lack of disease specificity, it was observed that VR preceded cytokine upregulation and ALT flare, and subsequent HBsAg decline.13,64,65 This suggests that a transient virological rebound, with or without subsequent clinical relapse, may assist in the immune-mediated killing of infected hepatocytes and non-cytolytic degradation of cccDNA. This is in contrast to previous studies that have shown that sustained off-therapy response is associated with higher chance of functional cure,48,49,66 and the role of VR in achieving HBsAg loss remains controversial. We previously demonstrated that increased frequencies of HBV core and polymerase-specific T cells were a promising immunological biomarker for patients who did not experience hepatic flares following treatment cessation, and that hepatic flares were in fact not driven by HBV-specific T-cell responses.37 Recently, a logistic regression model predicting on-treatment presence of functional HBV-specific CD8+ T-cell response has demonstrated a positive correlation with off-treatment HBsAg decline and loss.67 Furthermore, treatment cessation itself triggers a new immunological environment that has been shown to increase frequency and functionality of HBV core-specific T-cell responses in patients achieving functional cure.35
Interpretation of these findings and the path to HBV functional cure
Can NA discontinuation act as an immunomodulator in the functional cure program?
From murine models68 and clinical studies discussed above, a restored immune response against HBV appears to be the prerequisite for a de novo response against HBV during chronic infection. The reappearance of HBV replication after stopping long-term NA treatment could in fact be the necessary trigger for the immune response69 and the effect of viral rebound-induced immune reinvigoration has been shown in a few studies.13,68 A delicate balance exists between the potential immunological benefits of NA discontinuation (i.e. accelerated rates of HBsAg decline/clearance) and the risk of excessive hepatocyte damage and resultant liver failure. To this end, transient VR in absence of a serious clinical flare should be viewed differently from a sustained rise in viraemia levels off-therapy, but this has been poorly delineated in NA discontinuation studies to date, which mostly define VR by a single timepoint.
Patients achieving functional cure following NA discontinuation demonstrate two important events: firstly, a reduction in viral antigen level, and second, evidence of immune recovery. Therefore, any novel approaches intended to enhance functional cure should follow this rule by combining virus-directing agents with immunomodulators. There are no preclinical/clinical studies to date which have evaluated this sequence using novel agents. This combination effect was found to be effective in mice given siRNA (viral antigen knockdown) followed by therapeutic vaccine (composed of recombinant HBV protein), whereby antigen load shift was induced to end the immune tolerance.70 The safety and feasibility of NA discontinuation as a strategy for immune recovery should be guided by EOT antigenic loads. In selected patient groups; characterised by low HBsAg levels, low EOT HBcrAg and/or HBV RNA; NA discontinuation could be utilised in conjunction with virus-directed agents to achieve functional cure without risk of severe CR. However, the safety of this approach could not be over-emphasised, especially after the incidence of a subacute liver failure case necessitating liver transplantation in the REEF-2 study (patient in the placebo arm was continued on TDF for 48 wk which was then stopped).71 Across all included studies, there was a maximum of 15 decompensation events mentioned in six studies, leading to two liver-related deaths (taking into consideration overlapping study cohorts). Although the consensus between studies is that retreatment almost always leads to re-compensation and renewed viral control, there is still a possibility of hepatic decompensation and its sequelae, such as transplantation and death. This was highlighted by a recent meta-analysis which showed that severe hepatitis flares or decompensation would occur in 1.21% and liver transplantation or death was observed in 0.37% following NA discontinuation.72 Regardless of whether NA discontinuation is used as part of a novel combination therapy, close monitoring is essential and standardisation of retreatment criteria will be inevitable to minimise the associated risks.
How can viral markers help to predict response to novel agents?
The results of our review showed that the viral biomarkers that act as a surrogate for transcriptionally active cccDNA are helpful to predict off-therapy partial cure and, to some extent, functional cure. In addition, the timing of assessment has implications on the outcome, and the most commonly used and practical timepoint has been EOT (i.e. end of NA therapy). Recent data suggest that early on-treatment profiles of HBcrAg and HBV RNA can help to identify future responders (HBsAg seroclearance or <2 logs) as early as Week 4 of NA therapy.73 Early biomarker response suggests effective restoration of antiviral immunity, and potentially identifies those likely to achieve HBsAg reduction or seroclearance following treatment. Those with high baseline viral markers or poor viral biomarker response on treatment with novel agents should be continued on NA.
It remains to be determined whether novel agents inducing viral antigen reduction and passive restoration of the immune response will lead to the same sustainable HBsAg seroclearance as observed following long-term NA. Another unanswered question is whether differentiating the HBsAg source (cccDNA vs. integrated DNA) would help to predict risk of severe flare following NA discontinuation/novel treatment strategy. As only hepatocytes containing transcriptionally active cccDNA have the potential to replicate virus and become susceptible to immune attack upon NA discontinuation or immune modulation, those with HBsAg predominantly from integrated DNA have a theoretically lower risk of severe VR or CR if NAs are discontinued or immunomodulators introduced.
Immune assessment – a practical perspective
Ideally, the demonstration of a multi-faceted, poly-cellular immune response together with an assessment of an appropriate panel of inflammatory SIMs would be needed to prove immune restoration. However, as previously discussed, SIMs are heterogenous, non-specific, and so far, inconclusive. Moreover, we lack robust and reproducible assays to predict pro-inflammatory cytokine production with novel therapeutic approaches. To allow a more accessible and reproducible assessment, we propose that HBV-specific T cells should be the immune marker of choice for predicting functional cure on antiviral treatment. The frequency of these T cells, the level of PD-1 expression, and functionality (e.g. CD107a expression, IFNγ production on CD8+ T cells) are relatively specific readouts and efforts are underway to standardise the experimental assays, as well as implement them in all novel clinical trials moving forward.74 For patients receiving immunomodulators, one needs to differentiate responses as being target engagement only or reflecting recovery of the immune response. Ideally, the paired assessment of intra-hepatic HBV-specific T cells in the clinical trial setting would be valuable to inform whether peripheral blood T-cell responses are sufficiently informative.75
Limitations
The limitations regarding the clinical utility of viral markers are the recognised shortfalls in both sensitivity and standardisation. HBcrAg was measured by the Chemiluminscent Enzyme Immunoassay system (Fujirebio, Inc, Tokyo, Japan) in all studies. Although the automated estimation range is quoted as 2–7 log U/ml, the validated lower limit of detection is 3 log U/ml. As a result, readings between 2 and 3 log U/ml are not reliable and many studies state that a large proportion of patients return undetectable readings. In the absence of a unified standard, a range of different HBV RNA assays, platforms, and lower limits of detection have been adopted in the included studies (Table S3). Moreover, most findings from immune marker studies are yet to be comprehensively validated in further independent samples to affirm reproducibility of results. Furthermore, there is also a recognised distinction between in vitro expansion and ex vivo conditions in the generation of immunological data. Unfortunately, we were unable to perform a meta-analysis of the novel markers presented in this review owing to the heterogeneity of the data. There was variation in the cut-offs used for viral markers and the timepoints at which VR, CR, and HBsAg loss were measured. Furthermore, many of these studies had overlapping (but not identical) cohorts, which would have disproportionately skewed the results of a meta-analysis.
Conclusions
Treatment discontinuation has emerged as a valid therapeutic option to maintain partial cure, and has also been associated with higher rates of functional cure compared to patients who remain on long-term NA therapy when trialled in select populations. Nonetheless, treatment discontinuation remains a blunt tool lacking both precision and certainty as to which patients will safely achieve functional cure. The findings of our systematic review demonstrate that HBV RNA and HBcrAg are synergistic to traditional markers, including qHBsAg, in predicting off-therapy VR and CR. Early changes in these parameters with novel therapies should be explored with regard to clinical outcomes. Evidently, the most useful immune markers consist of HBV-specific T cell responses, and these should be assessed in the correct context with accessible and reproducible assays. The achievement of partial cure should be regarded as an important step towards functional cure, which remains the therapeutic goal of novel agents currently under investigation.76 Both virus-targeted and immune modulatory agents (where NA discontinuation can be considered an immunomodulatory strategy) are likely to be required to achieve functional cure, and the best sequence or combination approach needs to be explored further, drawing on the data from NA discontinuation studies.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Conceptualization: PTFK. Data curation: GZ, AK. Drafting of the manuscript: GZ, AK, L-YM. Reviewing and editing of the manuscript: GZ, AK, L-YM, USG, PTFK. Supervision: PTFK.
Data availability statement
All data analysed during this study are available from the corresponding author on reasonable request.
Conflicts of interest
L-YM has served as an advisory board member for Gilead Sciences. PK has served as a speaker, a consultant/advisory board member for Abbott Diagnostics, Aligos, Antios Therapeutics, Assembly Biosciences, Gilead Sciences, Janssen, GlaxoSmithKline, Immunocore and Drug Farm, and has received research funding from Gilead Sciences. GZ, AK and UG have no conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100720.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Trépo C., Chan H.L., Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.Jefferies M., Rauff B., Rashid H., Lam T., Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6:589–599. doi: 10.12998/wjcc.v6.i13.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Hepat B. 2022 https://www.who.int/news-room/fact-sheets/detail/hepatitis-b [Google Scholar]
- 4.Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M., et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 5.Papatheodoridis G.V., Chan H.L., Hansen B.E., Janssen H.L., Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Laras A., Koskinas J., Dimou E., Kostamena A., Hadziyannis S.J. Intrahepatic levels and replicative activity of covalently closed circular hepatitis B virus DNA in chronically infected patients. Hepatology. 2006;44:694–702. doi: 10.1002/hep.21299. [DOI] [PubMed] [Google Scholar]
- 7.Werle-Lapostolle B., Bowden S., Locarnini S., Wursthorn K., Petersen J., Lau G., et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Sarin S., Kumar M., Lau G., Abbas Z., Chan H.L.Y., Chen C.J., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampertico P., Agarwal K., Berg T., Buti M., Janssen H.L., Papatheodoridis G., et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Terrault N.A., Lok A.S., McMahon B.J., Chang K., Hwang J.P., Jonas M.M., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall S.A.L., Vogrin S., Wawryk O., Burns G.S., Visvanathan K., Sundararajan V., et al. Discontinuation of nucleot(s)ide analogue therapy in HBeAg-negative chronic hepatitis B: a meta-analysis. Gut. 2022;71:1629–1641. doi: 10.1136/gutjnl-2020-323979. [DOI] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höner Zu Siederdissen C., Rinker F., Maasoumy B., Wiegand S.B., Filmann N., Falk C.S., et al. Viral and host responses after stopping long-term nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Infect Dis. 2016;214:1492–1497. doi: 10.1093/infdis/jiw412. [DOI] [PubMed] [Google Scholar]
- 14.Su T.H., Yang H.C., Tseng T.C., Liou J.M., Liu C.H., Chen C.L., et al. Distinct relapse rates and risk predictors after discontinuing tenofovir and entecavir therapy. J Infect Dis. 2018;217:1193–1201. doi: 10.1093/infdis/jix690. [DOI] [PubMed] [Google Scholar]
- 15.Chi H., Li Z., Hansen B.E., Yu T., Zhang X., Sun J., et al. Serum level of antibodies against hepatitis B core protein is associated with clinical relapse after discontinuation of nucleos(t)ide analogue therapy. Clin Gastroenterol Hepatol. 2019;17:182–191.e1. doi: 10.1016/j.cgh.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Hsu Y.C., Nguyen M.H., Mo L.R., Wu M.S., Yang T.H., Chen C.C., et al. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment Pharmacol Ther. 2019;49:107–115. doi: 10.1111/apt.15058. [DOI] [PubMed] [Google Scholar]
- 17.Carey I., Gersch J., Wang B., Moigboi C., Kuhns M., Cloherty G., et al. Pregenomic HBV RNA and hepatitis B core-related antigen predict outcomes in hepatitis B e antigen-negative chronic hepatitis B patients suppressed on nucleos(t)ide analogue therapy. Hepatology. 2020;72:42–57. doi: 10.1002/hep.31026. [DOI] [PubMed] [Google Scholar]
- 18.Fan R., Zhou B., Xu M., Tan D., Niu J., Wang H., et al. Association between negative results from tests for HBV DNA and RNA and durability of response after discontinuation of nucles(t)ide analogue therapy. Clin Gastroenterol Hepatol. 2020;18:719–727.e7. doi: 10.1016/j.cgh.2019.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Fan R., Peng J., Xie Q., Tan D., Xu M., Niu J., et al. Combining hepatitis B virus RNA and hepatitis B core-related antigen: guidance for safely stopping nucleos(t)ide analogues in hepatitis B e antigen-positive patients with chronic hepatitis B. J Infect Dis. 2020;222:611–618. doi: 10.1093/infdis/jiaa136. [DOI] [PubMed] [Google Scholar]
- 20.García-López M., Lens S., Pallett L.J., Testoni B., Rodriguez-Tajes S., Marino Z., et al. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol. 2021;74:1064–1074. doi: 10.1016/j.jhep.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaewdech A., Tangkijvanich P., Sripongpun P., Witeerungrot T., Jandee S., Tanaka Y., et al. Hepatitis B surface antigen, core-related antigen and HBV RNA: predicting clinical relapse after NA therapy discontinuation. Liver Int. 2020;40:2961–2971. doi: 10.1111/liv.14606. [DOI] [PubMed] [Google Scholar]
- 22.Lai C.L., Wong D.K., Wong G.T., Seto W.K., Fung J., Yuen M.F. Rebound of HBV DNA after cessation of nucleos/tide analogues in chronic hepatitis B patients with undetectable covalently closed. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Xue J., Liao W., Yan H., Liang X. Serum HBV RNA dynamic and drug withdrawal predictor value in patients with chronic HBV infection on long-term nucleos(t)ide analogue (NA) therapy. J Clin Gastroenterol. 2020;54:e73–e82. doi: 10.1097/MCG.0000000000001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papatheodoridi M., Hadziyannis E., Berby F., Zachou K., Testoni B., Rigopoulou E., et al. Predictors of hepatitis B surface antigen loss, relapse and retreatment after discontinuation of effective oral antiviral therapy in noncirrhotic HBeAg-negative chronic hepatitis B. J Viral Hepat. 2020;27:118–126. doi: 10.1111/jvh.13211. [DOI] [PubMed] [Google Scholar]
- 25.Seto W.K., Liu K.S., Mak L.Y., Cloherty G., Wong D.K., Gersch J., et al. Role of serum HBV RNA and hepatitis B surface antigen levels in identifying Asian patients with chronic hepatitis B suitable for entecavir cessation. Gut. 2020;70:775–783. doi: 10.1136/gutjnl-2020-321116. [DOI] [PubMed] [Google Scholar]
- 26.Tseng T.N., Hu T.H., Wang J.H., Kuo Y.H., Hung C.H., Lu S.N., et al. Incidence and factors associated with HBV relapse after cessation of entecavir or tenofovir in patients with HBsAg below 100 IU/mL. Clin Gastroenterol Hepatol. 2020;18:2803–2812. doi: 10.1016/j.cgh.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H.R., Yang H.C., Lin S.R., Yang T.Y., Lin Y.Y., Su T.H., et al. Combined viral quasispecies diversity and hepatitis B core-related antigen predict off-nucleos(t)ide analog durability in HBeAg-negative patients. Hepatol Int. 2021;15:582–592. doi: 10.1007/s12072-021-10186-7. [DOI] [PubMed] [Google Scholar]
- 28.Huang P.Y., Wang J.H., Hung C.H., Lu S.N., Hu T.H., Chen C.H. The role of hepatitis B virus core-related antigen in predicting hepatitis B virus relapse after cessation of entecavir in hepatitis B e antigen-negative patients. J Viral Hepat. 2021;28:1141–1149. doi: 10.1111/jvh.13528. [DOI] [PubMed] [Google Scholar]
- 29.Kuo Y.H., Wang J.H., Hung C.H., Lu S.N., Hu T.H., Chen C.H. Combining end-of-treatment HBsAg and baseline hepatitis B core-related antigen reduce HBV relapse rate after tenofovir cessation. Hepatol Int. 2021;15:301–309. doi: 10.1007/s12072-021-10159-w. [DOI] [PubMed] [Google Scholar]
- 30.Liao G., Ding X., Xia M., Wu Y., Chen H., Fan R., et al. Hepatitis B core-related antigen is a biomarker for off-treatment relapse after long-term nucleos(t)ide analog therapy in patients with chronic hepatitis B. Int J Gen Med. 2021;14:4967–4976. doi: 10.2147/IJGM.S321253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonneveld M.J., Park J.Y., Kaewdech A., Seto W.K., Tanaka Y., Carey I., et al. Prediction of sustained response after nucleo(s)tide analogue cessation using HBsAg and HBcrAg levels: a multicenter study (CREATE) Clin Gastroenterol Hepatol. 2022;20:e784–e793. doi: 10.1016/j.cgh.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Wübbolding M., Lopez Alfonso J.C., Lin C.Y., Binder S., Falk C., Debarry J., et al. Pilot study using machine learning to identify immune profiles for the prediction of early virological relapse after stopping nucleos(t)ide analogues in HBeAg-negative CHB. Hepatol Commun. 2021;5:97–111. doi: 10.1002/hep4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia M., Chi H., Wu Y., Hansen B.E., Li Z., Liu S., et al. Serum hepatitis B virus RNA level is associated with biochemical relapse in patients with chronic hepatitis B infection who discontinue nucleos(t)ide analogue treatment. Aliment Pharmacol Ther. 2021;54:709–714. doi: 10.1111/apt.16538. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y., Li M., Ou X., Zheng S., Gao Y., Xu X., et al. HBeAg-positive patients with HBsAg < 100 IU/mL and negative HBV RNA have lower risk of virological relapse after nucleos(t)ide analogues cessation. J Gastroenterol. 2021;56:856–867. doi: 10.1007/s00535-021-01812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinker F., Zimmer C.L., Höner Zu Siederdissen C., Manns M.P., Kraft A.R.M., Wedemeyer H., et al. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69:584–593. doi: 10.1016/j.jhep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Zimmer C.L., Rinker F., Höner Zu Siederdissen C., Manns M.P., Wedemeyer H., Cornberg M., et al. Increased NK cell function after cessation of long-term nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss. J Infect Dis. 2018;217:1656–1666. doi: 10.1093/infdis/jiy097. [DOI] [PubMed] [Google Scholar]
- 37.Rivino L., Le Bert N., Gill U.S., Kunasegaran K., Cheng Y., Tan D.Z., et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668–681. doi: 10.1172/JCI92812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kranidioti H., Manolakopoulos S., Kontos G., Breen M.S., Kourikou A., Deutsch M., et al. Immunological biomarkers as indicators for outcome after discontinuation of nucleos(t)ide analogue therapy in patients with HBeAg-negative chronic hepatitis B. J Viral Hepat. 2019;26:697–709. doi: 10.1111/jvh.13068. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y., Fan J., Liao G., Xia M., Jiang D., Peng J., et al. Genetic variations in the CXCR5 gene decrease the risk of clinical relapse after discontinuation of nucleos(t)ide analogue therapy in patients with chronic hepatitis B. Infect Genet Evol. 2020;78 doi: 10.1016/j.meegid.2019.104124. [DOI] [PubMed] [Google Scholar]
- 40.Xie L., Liao G., Chen H., Xia M., Huang X., Fong R., et al. Elevated expression of serum soluble ST2 in clinical relapse after stopping long-term nucleos(t)ide analogue therapy for chronic hepatitis B. BMC Infect Dis. 2019;19:640. doi: 10.1186/s12879-019-4261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C.H., Peng C.Y., Kuo Y.H., Hu T.H., Hung C.H., Wang J.H., et al. Earlier and higher rate of hepatitis B virus relapse after discontinuing tenofovir versus entecavir in hepatitis B e Antigen-positive patients. J Infect Dis. 2022;225:1974–1981. doi: 10.1093/infdis/jiab596. [DOI] [PubMed] [Google Scholar]
- 42.Hall S.A., Burns G.S., Mooney B.J., Millen R., Morris R., Vogrin S., et al. Hepatitis B virus flares following nucleot(s)ide analogue cessation are associated with activation of TLR signalling pathways. J Infect Dis. 2022;227:123–132. doi: 10.1093/infdis/jiac375. [DOI] [PubMed] [Google Scholar]
- 43.Kaewdech A., Assawasuwannakit S., Sripongpun P., Chamroonkul N., Tangkijvanich P., Piratvisuth T. Clinical utility of SCALE-B to predict hepatitis B virus relapse, hepatitis B surface antigen loss after antiviral cessation in Asian patients after 2-year follow-up. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.859430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papatheodoridi M., Papachristou E., Moschidis Z., Hadziyannis E., Rigopoulou E., Zachou K., et al. Significance of serum HBV RNA in non-cirrhotic HBeAg-negative chronic hepatitis B patients who discontinue effective antiviral therapy. J Viral Hepat. 2022;29:948–957. doi: 10.1111/jvh.13729. [DOI] [PubMed] [Google Scholar]
- 45.Sonneveld M.J., Chiu S.M., Park J.Y., Brakenhoff S.M., Kaewdech A., Seto W.K., et al. Probability of HBsAg loss after nucleo(s)tide analogue withdrawal depends on HBV genotype and viral antigen levels. J Hepatol. 2022;76:1042–1050. doi: 10.1016/j.jhep.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Hsu Y.-C., Jun D.W., Peng C.-Y., Yeh M.-L., Trinh H., Wong G.L.-H., et al. Effectiveness of entecavir vs tenofovir disoproxil fumarate for functional cure of chronic hepatitis B in an international cohort. Hepatol Int. 2022;16:1297–1307. doi: 10.1007/s12072-022-10411-x. [DOI] [PubMed] [Google Scholar]
- 47.Hirode G., Choi H.S.J., Chen C.H., Su T.H., Seto W.K., Van Hees S., et al. Off-therapy response after nucleos(t)ide analogue withdrawal in patients with chronic hepatitis B: an international, multicenter, multiethnic cohort (RETRACT-B Study) Gastroenterology. 2022;162:757–771.e4. doi: 10.1053/j.gastro.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Jeng W.J., Chen Y.C., Chien R.N., Sheen I.S., Liaw Y.F. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68:425–434. doi: 10.1002/hep.29640. [DOI] [PubMed] [Google Scholar]
- 49.Chen C.H., Hung C.H., Wang J.H., Lu S.N., Lai H.C., Hu T.H., et al. The incidence of hepatitis B surface antigen loss between hepatitis B E antigen-negative noncirrhotic patients who discontinued or continued entecavir therapy. J Infect Dis. 2019;219:1624–1633. doi: 10.1093/infdis/jiy697. [DOI] [PubMed] [Google Scholar]
- 50.Liang L.B., Zhu X., Yan L.B., Du L.Y., Liu C., Liao J., et al. Quantitative intrahepatic HBV cccDNA correlates with histological liver inflammation in chronic hepatitis B virus infection. Int J Infect Dis. 2016;52:77–82. doi: 10.1016/j.ijid.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Thompson A.J., Nguyen T., Iser D., Ayres A., Jackson K., Littlejohn M., et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 52.Rydell G.E., Larsson S.B., Prakash K., Andersson M., Norder H., Hellstrand K., et al. Abundance of noncircular intrahepatic hepatitis B virus DNA may reflect frequent integration into human DNA in chronically infected patients. J Infect Dis. 2022;225:1982–1990. doi: 10.1093/infdis/jiaa572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svicher V., Salpini R., Piermatteo L., Carioti L., Battisti A., Colagrossi L., et al. Whole exome HBV DNA integration is independent of the intrahepatic HBV reservoir in HBeAg-negative chronic hepatitis B. Gut. 2021;70:2337. doi: 10.1136/gutjnl-2020-323300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong D.K., Tanaka Y., Lai C.L., Mizokami M., Fung J., Yuen M.F. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J Clin Microbiol. 2007;45:3942–3947. doi: 10.1128/JCM.00366-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prakash K., Rydell G.E., Larsson S.B., Andersson M., Norkrans G., Norder H., et al. High serum levels of pregenomic RNA reflect frequently failing reverse transcription in hepatitis B virus particles. Virol J. 2018;15:86. doi: 10.1186/s12985-018-0994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertoletti A., Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754–1764. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 57.Ye B., Liu X., Li X., Kong H., Tian L., Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boni C., Lampertico P., Talamona L., Giuberti T., Invernizzi F., Barili V., et al. Natural killer cell phenotype modulation and natural killer/T-cell interplay in nucleos(t)ide analogue-treated hepatitis e antigen-negative patients with chronic hepatitis B. Hepatology. 2015;62:1697–1709. doi: 10.1002/hep.28155. [DOI] [PubMed] [Google Scholar]
- 59.Burton A.R., Pallett L.J., McCoy L.E., Suveizdyte K., Amin O.E., Swadling L., et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest. 2018;128:4588–4603. doi: 10.1172/JCI121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salimzadeh L., Le Bert N., Dutertre C.A., Gill U.S., Newell E.W., Frey C., et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J Clin Invest. 2018;128:4573–4587. doi: 10.1172/JCI121957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milich D.R., Chen M., Schödel F., Peterson D.L., Jones J.E., Hughes J.L. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci U S A. 1997;94:14648–14653. doi: 10.1073/pnas.94.26.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boni C., Laccabue D., Lampertico P., Giuberti T., Vigrano M., Schivazappa S., et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–973.e9. doi: 10.1053/j.gastro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Lam Y.F., Seto W.K., Wong D., Cheung K.S., Fung J., Mak L.Y., et al. Seven-year treatment outcome of entecavir in a real-world cohort: effects on clinical parameters, HBsAg and HBcrAg levels. Clin Transl Gastroenterol. 2017;8:e125. doi: 10.1038/ctg.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaroszewicz J., Ho H., Markova A., Deterding K., Wursthorn K., Schulz S., et al. Hepatitis B surface antigen (HBsAg) decrease and serum interferon-inducible protein-10 levels as predictive markers for HBsAg loss during treatment with nucleoside/nucleotide analogues. Antivir Ther. 2011;16:915–924. doi: 10.3851/IMP1866. [DOI] [PubMed] [Google Scholar]
- 65.Schurich A., Pallett L.J., Lubowiecki M., Singh H.D., Gill U.S., Kennedy P.T.F., et al. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C.H., Hu T.H., Wang J.H., Lai H.C., Hung C.H., Lu S.N., et al. Comparison of HBsAg changes between HBeAg-negative patients who discontinued or maintained entecavir therapy. Hepatol Int. 2020;14:317–325. doi: 10.1007/s12072-019-09991-y. [DOI] [PubMed] [Google Scholar]
- 67.Peña-Asensio J., Calvo H., Miquel J., Sanz-de-Villalobos E., Gonzalez-Praetorius A., Torralba M., et al. Model to predict on-treatment restoration of functional HBV-specific CD8(+) cell response foresees off-treatment HBV control in eAg-negative chronic hepatitis B. Aliment Pharmacol Ther. 2022;55:1545–1559. doi: 10.1111/apt.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J., Zhang E., Ma Z., Wu W., Kosinska, Zhang X., et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Bommel F., Berg T. Risks and benefits of discontinuation of nucleos(t)ide analogue treatment: a treatment concept for patients with HBeAg-negative chronic hepatitis B. Hepatol Commun. 2021;5:1632–1648. doi: 10.1002/hep4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michler T., Kosinska A.D., Festag J., Bunse T., Su J., Ringelhan M., et al. Knockdown of virus antigen expression increases therapeutic vaccine efficacy in high-titer hepatitis B virus carrier mice. Gastroenterology. 2020;158:1762–1775.e9. doi: 10.1053/j.gastro.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 71.Agarwal K., Lok J., Carey I., Shivkar Y., Biermer M., Berg T., et al. A case of HBV-induced liver failure in the REEF-2 phase II trial: implications for finite treatment strategies in HBV 'cure. J Hepatol. 2022;77:245–248. doi: 10.1016/j.jhep.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Tseng C.H., Chen T.H., Wu J.L., Lee T.Y., Borghi J.A., Lin J.T., et al. Serious adverse events after cessation of nucleos(t)ide analogues in individuals with chronic hepatitis B: a systematic review and meta-analysis. JHEP Rep. 2023;5 doi: 10.1016/j.jhepr.2022.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mak L.Y., Wong D., Kuchta A., Hilfiker M., Hamilton A., Chow N., et al. Hepatitis B virus pre-genomic RNA and hepatitis B core-related antigen reductions at week 4 predict favourable hepatitis B surface antigen response upon long-term nucleos(t)ide analogue in chronic hepatitis B. Clin Mol Hepatol. 2023;29:146–162. doi: 10.3350/cmh.2022.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gehring A.J., Mendez P., Richter K., Ertl H., Donaldson E.F., Mishra P., et al. Immunological biomarker discovery in cure regimens for chronic hepatitis B virus infection. J Hepatol. 2022;77:525–538. doi: 10.1016/j.jhep.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 75.Gill U.S., Pallett L.J., Thomas N., Burton A.R., Patel A.A., Yona S., et al. Fine needle aspirates comprehensively sample intrahepatic immunity. Gut. 2019;68:1493–1503. doi: 10.1136/gutjnl-2018-317071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cornberg M., Lok A.S.-F., Terrault N.A., Zoulim F., Berg T., Brunetto M.R., et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B – Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. J Hepatol. 2020;72:539–557. doi: 10.1016/j.jhep.2019.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are available from the corresponding author on reasonable request.