Abstract
Background & Aims
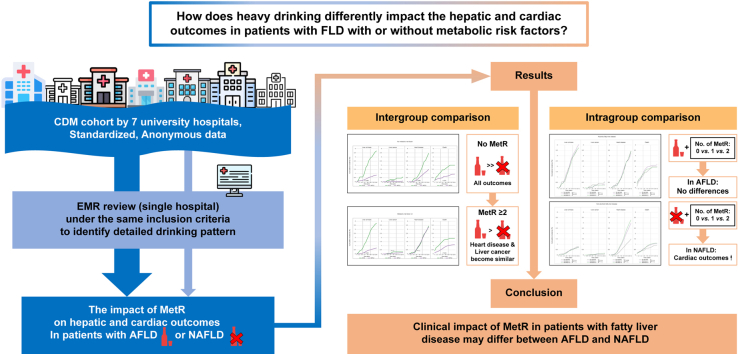
Metabolic risk factors (MetRs) are associated with hepatic and cardiac outcomes in patients with fatty liver disease (FLD). We evaluated whether MetRs have different effects on alcoholic FLD (AFLD) and non-alcoholic FLD (NAFLD).
Methods
We used a standardised common data model to analyse data from seven university hospital databases between 2006 and 2015. MetRs included diabetes mellitus, hypertension, dyslipidaemia, and obesity. Follow-up data were analysed for the incidence of hepatic outcomes, cardiac outcomes, and death in patients with AFLD or NAFLD and based on MetRs within AFLD and NAFLD.
Results
Out of 3,069 and 17,067 patients with AFLD and NAFLD, respectively, 2,323 (75.7%) and 13,121 (76.9%) had one or more MetR, respectively. Patients with AFLD were at a higher risk of hepatic outcomes (adjusted risk ratio [aRR], 5.81) compared with those with NAFLD irrespective of MetR. The risk of cardiac outcomes in AFLD and NAFLD became similar with the increasing number of MetRs. Patients with NAFLD without MetRs demonstrated a lower risk of cardiac outcomes, but not hepatic outcomes, compared with those with MetRs (aRR, 0.66 and 0.61 for MetR ≥1 and MetR ≥2, respectively; p <0.05). In patients with AFLD, hepatic and cardiac outcomes were not associated with MetRs.
Conclusions
The clinical impact of MetRs in patients with FLD may differ between patients with AFLD and those with NAFLD.
Impact and Implications
With the increasing prevalence of fatty liver disease (FLD) and metabolic syndrome, the increase in associated complications, such as liver and heart diseases, has become an important social issue. Particularly in patients with FLD with excessive alcohol consumption, the incidence of liver and heart disease is pronounced because of the dominant effect of alcohol over the effects of other factors. Thus, appropriate screening and management of alcohol consumption in patients with FLD are vital.
Keywords: Alcoholic fatty liver disease, Non-alcoholic fatty liver disease, Metabolic risk factor, Outcome, Common data model
Graphical abstract
Highlights
-
•
In AFLD, cardiac and hepatic outcomes were higher than in NAFLD regardless of MetRs.
-
•
The incidence of cardiac outcomes in patients with NAFLD matched that in patients with AFLD as the number of MetRs increased.
-
•
In NAFLD, the incidence of cardiac outcomes increased as the number of MetR increased, but there was no prominent difference in hepatic outcomes.
-
•
In AFLD, the number of MetRs did not correlate with cardiac and hepatic outcomes.
Introduction
Fatty liver disease (FLD) is the most common cause of chronic liver disease, which is responsible for a majority of morbidity and mortality in patients with chronic liver disease worldwide.1 Traditionally, FLD can be divided into alcoholic fatty liver disease (AFLD) and non-alcoholic fatty liver disease (NAFLD), which, between them, account for almost every patient with FLD.[2], [3], [4] Progressive forms of FLD eventually result in hepatic fibrosis and primary liver cancer, which are well-known prognostic factors in chronic liver disease.5,6 Additionally, various studies have reported the inseparable relationship between FLD and cardiovascular disease in terms of life-threatening medical events.[7], [8], [9] Given the concern that hepatic and cardiac outcomes are important during the clinical course of FLD, recent studies have reported that FLD-related outcomes could be accelerated under the influence of metabolic risk factors (MetRs), such as diabetes mellitus, hypertension, dyslipidaemia, and obesity.[9], [10], [11]
With the increasing number of patients with FLD worldwide, especially NAFLD, identifying high-risk patients is an important issue. In this regard, a panel of international experts has proposed a new classification, called ‘metabolic-associated fatty liver disease’ (MAFLD).12 MAFLD is defined as FLD in patients who are overweight/obesity and/or who have type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation irrespective of any other etiology of liver disease. Although the concept of MAFLD has some advantages in identifying high-risk patients and predicting outcomes, several issues with this proposal have been raised.[13], [14], [15]
Most patients with MAFLD include those with NAFLD and MetRs who are considered to have a relatively benign course compared with those with AFLD in terms of hepatic and non-hepatic outcomes.10,[16], [17], [18] One study suggested that almost all patients (99.1%) who have MAFLD but not NAFLD also had AFLD.13 In other words, when analysing the outcomes of FLD under the concept of MAFLD, the results vary based on the proportion of AFLD.7 Nevertheless, there have been few attempts to assess the outcomes of AFLD and NAFLD separately, and no study has analysed FLD-related outcomes according to the presence of MetRs.8,19,20
Another crucial issue is the effect of concomitant comorbidities (except for MetRs) on AFLD and NAFLD. In fact, patients with AFLD and NAFLD often have varying numbers of comorbidities, and it is possible that the effects of comorbidities on AFLD and NAFLD outcomes can vary.21,22 Therefore, the effects of MetRs on FLD outcomes should be analysed separately from the influence of comorbidities; however, no previous studies have used this approach.
Therefore, we investigated the effects of MetRs on the incidence of hepatic and cardiac outcomes using a common data model, including data from seven university hospital databases, to identify clinical information for risk stratification in patients with FLD. To compare the outcomes between the AFLD and NAFLD groups of patients, we analysed: (i) intergroup differences between patients with AFLD vs. those with NAFLD; and (ii) intragroup differences according to MetRs within groups of patients with AFLD vs. those with NAFLD.
Patients and methods
Data source
This multicentre observational study used data from seven university hospitals in six provinces of Republic of Korea. It is difficult to combine and analyse data from different hospitals because different data sets are built using different data models and often local terminologies. Furthermore, integrating data from multiple hospitals entails not only problems of data standardisation, but also pseudonymisation issues.23 To overcome these limitations and secure a basis for integrated data analysis, we used data previously converted to the standardised Observational Medical Outcome Partnership (OMOP)-Common Data Model (CDM) v5.3.24,25 Since 2018, OMOP-CDM data from various hospitals have been converted and used through the platform called FEEDER-NET (https://feedernet.com), a health Big-Data platform supported by National CDM projects in Korea. The OMOP-CDM version of hospital data has been validated in several observational health data science and informatics (OHDSI) studies.[26], [27], [28] Non-standardised medical records and terms were mapped to the Systemized Nomenclature for Medicine-Clinical Terms (SNOMED-CT) code, and Logical Observation Identifiers, Names, and Codes (LOINC) were also used for numeric data extraction.29,30
The analysis in this study included data from seven hospitals (Kyung Hee University Hospital at Gangdong, Hallym University Kangdong Sacred Heart Hospital, Kangwon National University Hospital, Daegu Catholic University Medical Center, Pusan National University Hospital, Wonkwang University Hospital, and Ajou University Hospital) corresponding to the Research Border-free Zone (RFZ), a research-free zone for multi-institution-distributed Big Data research. Institutions affiliated with the RFZ provide researchers from other institutions with the same level of CDM research rights as those permitted to in-house researchers.31 All data were obtained from the hospital-based CDM databases. This study protocol was approved by the Institutional Review Board (IRB) of Kyung Hee University, Republic of Korea, and all study methods were performed in accordance with the appropriate guidelines and regulations. The study protocol also conforms to the ethical guidelines of the Declaration of Helsinki, as reflected in prior approval by the Institution’s IRB (KHNMC IRB 2020-08-003).
Study subjects and definitions
In this retrospective, multicentre, observational, comparative cohort study, we included patients aged over 20 years and cohort entries from July 2006 to December 2015, with the end of the observational period being December 2019. All study variables were defined by standard concept identification and the codes for the concept sets, as summarised in Table S1. The medical histories of 23,884 patients found to have FLD for the first time during the study period were initially extracted from databases from each of the seven hospitals. The patients included were only those with confirmed AFLD or NAFLD according to the standard code. If available, CDM data regarding drinking were used to confirm the diagnostic accuracy of AFLD and NAFLD. Patients with AFLD were defined by diagnostic codes (50325005) with or without a current drinker code (228279004, 86933000, and 219006) confirmed within 1 year of diagnosis. Patients with NAFLD were defined by the presence of both diagnostic codes (197321007) with or without a non-drinker code (105542008) confirmed within 1 year of diagnosis. Patients with both AFLD and NAFLD codes were excluded.
For the study analysis, we also excluded patients with a medical history of cirrhosis or primary liver cancer diagnosed any time before or up to 1 year after their AFLD or NAFLD diagnosis (n = 617). We also excluded patients with chronic viral hepatitis (hepatitis B and C virus infection, n = 1,316) and minor etiology of chronic liver disease, including primary biliary cholangitis, primary sclerosing cholangitis, Wilson’s disease, hemochromatosis, and autoimmune hepatitis, diagnosed at any time point during the observational period (n = 64). Additionally, to ensure that the study focused on only the effect of metabolic risk factors on AFLD and NAFLD, patients with a medical history of severe comorbidities (heart failure, ischaemic heart disease, atrial fibrillation, chronic obstructive lung disease, cerebral infarction, and chronic kidney disease; n = 1,751) diagnosed any time before and up to 1 year after their AFLD or NAFLD diagnosis were also excluded (Fig. S1).21,22,[32], [33], [34], [35], [36], [37]
MetRs were defined using standardised diagnosis codes and/or numeric data and were recorded if they occurred any time before and up to 1 year after the AFLD or NAFLD diagnosis or nearest to the index date, as follows11,28,38: (i) diabetes mellitus, defined as fasting serum glucose level ≥126 mg/dL or glycated hemoglobin (HbA1c) ≥6.5 mg/dL or a diagnosis of diabetes mellitus; (ii) hypertension, defined as systolic/diastolic blood pressure ≥140/90 mmHg or a diagnosis of hypertension; (iii) dyslipidaemia, defined as a serum total cholesterol level ≥200 mg/dL, low-density lipoprotein (LDL) cholesterol level ≥100 mg/dL, triglyceride level ≥150 mg/dL, high-density lipoprotein (HDL)-cholesterol <40 mg/dL, or a diagnosis of dyslipidaemia; and (iv) obesity, defined as a body mass index (BMI) ≥25 kg/m2.
Direct data extraction from electronic medical records for detailed information on alcohol consumption
The CDM code for the amount of drinking varied from 12.8% to 52.6% according to the conversion rate of each hospital database. To evaluate whether the standard code-based classification sufficiently classified actual patients with AFLD and NAFLD, we conducted a manual review of electronic medical records from accessible data using the same inclusion criteria as the CDM code-based extraction. A total of 3,253 patients (∼16.2% of patients in the entire study group) were extracted from the database of Kyung Hee University Hospital at Gangdong using the same inclusion criteria. Data related to alcohol consumption were manually reviewed except for cases where the patient refused to disclose their level of alcohol consumption or when it was difficult to obtain complete information because of loss of data (Table S2). The results of the additional analysis showed that the agreement between the CDM code and clinical diagnosis was 97.9% for AFLD (heavy or very heavy drinker with FLD) and 98.8% for NAFLD (equal to, or less than, moderate drinking with FLD). These results suggest that AFLD and NAFLD defined by standard codes could be useful in distinguishing FLD based on whether there was a history of heavy drinking.
Study outcomes
This study was conducted to address the effects of MetRs on the incidence of cirrhosis, primary liver cancer, cardiac outcomes, and death in patients with AFLD or NAFLD. The hepatic outcomes included the incidence of cirrhosis and primary liver cancer. The cardiac outcomes included the incidence of ischaemic heart disease, heart failure, and atrial fibrillation.8,11,33,34 Subsequently, intergroup comparisons (AFLD vs. NAFLD, depending on the presence of the same level of MetR [all patients, patients without MetRs, patients with one or more MetR, and patients with two or more MetRs]), and intragroup comparisons (AFLD without MetRs vs. AFLD with one [or two] or more MetRs; NAFLD without MetR vs. NAFLD with one [or two] or more MetRs) were performed.
Statistical analyses
OHDSI analysis tools are embedded in the interactive analysis platform, ATLAS. We analysed ATLAS version 2.7.6 on FEEDER-NET, a health Big-Data platform based on OHDSI-CDM and supported by the Korean National Project.26,31 In this study, OHDSI analysis tools were used for primary data extraction, annual and cumulative incidence analysis, and regression analysis for each hospital. The data extraction of the study population and the incidence-based analysis were performed using the overall data; normally distributed continuous variables are presented as mean ± SD, and data for categorical variables are presented as numbers (percentages). The Chi-square test was used to examine the relationships between categorical variables, and the Student’s t test was used to compare the mean values of continuous variables across the groups. The Kaplan-Meier method with a log-rank test was used to compare the cumulative incidence of the outcome in terms of inter- and intragroup analyses. The hazard ratios (HRs) and 95% CIs for the incidence (event per person-year) of outcomes based on Cox proportional hazard models (COX-PH) were used only when the analysis was possible in each hospital (i.e. all the target and comparator groups had data available for at least six of the seven hospitals). COX-PH analysis was performed for hepatic and cardiac outcomes in terms of inter- and intragroup comparisons using unadjusted and age-sex adjusted regression analysis. Once the data were extracted from each hospital database, the risk of outcome data was combined and analysed using meta-analyses, which were performed to estimate the incidence (by person-years) using a random-effects model. Statistical analyses for pooled data were performed using R v4.1.3 (R Foundation Inc.; http://cran.r-project.org), and meta-analysis was performed using Review Manager (RevMan, version 5.4.1; Cochrane Collaboration). A p value <0.05 was considered significant.
Results
Demographic characteristics and trends of outcomes in AFLD and NAFLD
Table 1 summarises the baseline characteristics of the patients with FLD. The study population included 3,069 patients with AFLD and 17,067 patients with NAFLD; those with AFLD included a higher proportion of males (91.2% in AFLD and 63.2% in NAFLD) and older patients (mean age, 49.12 years in AFLD and 47.33 years in NAFLD) compared with those with NAFLD. Those without MetRs included 746 (24.3%) patients with AFLD and 3,946 (23.1%) patients with NAFLD. In patients without MetRs, a similar trend was observed; AFLD included a higher proportion of males (88.5% in AFLD and 61.8% in NAFLD) and older age (mean age, 48.20 in AFLD and 43.84 years in NAFLD) compared with those with NAFLD. According to the presence of each MetR, males were more likely to have AFLD than NAFLD (range, 91.5–92.8% in AFLD and 59.8–66.5% in NAFLD). As for age, there were no statistically significant differences between the AFLD and NAFLD groups in terms of diabetes mellitus, hypertension, and dyslipidaemia, whereas those with obesity and AFLD were older (mean age, 48.68 years in AFLD and 46.098 years in NAFLD) compared with those with obesity and NAFLD. The AFLD group had a higher proportion of males (MetR ≥1, 92.0% in AFLD and 63.5% in NAFLD; MetR ≥2, 92.4% in AFLD and 64.1% in NAFLD) and older age (mean age: MetR ≥1, 49.99 years in AFLD and 48.81 years in NAFLD; MetR ≥2, 50.01 years in AFLD and 48.83 years in NAFLD) in patients with both one or more MetR and two or more MetRs. Generally, the presence of MetRs tended to increase with age in patients with FLD. Based on the data extracted from each hospital database, there were differences in the absolute values or ratios according to region or hospital; however, the trend was maintained overall in terms of the proportion of males and the mean age in each group (Table S3).
Table 1.
Baseline characteristics of patients with FLD.
| Group | AFLD (n = 3,069) | NAFLD (n = 17,067) | p value |
|---|---|---|---|
| Male, n (%) | 2,798 (91.2) | 10,792 (63.2) | <0.001 |
| Age, mean (SD) | 49.12 (10.62) | 47.33 (13.13) | <0.001 |
| Age group (years), n (%) | |||
| 20–24 | 28 (1.0) | 734 (4.3) | — |
| 25–29 | 70 (2.3) | 970 (5.7) | — |
| 30–34 | 193 (6.3) | 1,535 (9.0) | — |
| 35–39 | 290 (9.4) | 1,841 (10.8) | — |
| 40–44 | 453 (14.8) | 1,976 (11.6) | — |
| 45–49 | 533 (17.4) | 2,217 (13.0) | — |
| 50–54 | 550 (17.9) | 2,606 (15.3) | — |
| 55–59 | 441 (14.4) | 2,103 (12.3) | — |
| 60–64 | 251 (8.2) | 1,436 (8.4) | — |
| 65–69 | 152 (5.0) | 868 (5.1) | — |
| ≥70 |
108 (3.5) |
781 (4.6) |
— |
| Absence of MetR | AFLD (n = 746; 24.3% of total AFLD) | NAFLD (n = 3,946; 23.1% of total NAFLD) | – |
| Male, n (%) | 660 (88.5) | 2,437 (61.8) | <0.001 |
| Age, mean (SD) |
48.20 (12.31) |
43.84 (13.86) |
<0.001 |
| Presence of MetR | |||
| Diabetes mellitus | 555 | 2,997 | — |
| Male, n (%) | 508 (91.5) | 1,791 (59.8) | <0.001 |
| Age, mean (SD) | 52.00 (10.00) | 51.74 (12.27) | 0.78 |
| Hypertension | 778 | 4,259 | — |
| Male, n (%) | 716 (92.0) | 2,658 (62.4) | <0.001 |
| Age, mean (SD) | 50.71 (10.31) | 51.08 (13.18) | 0.63 |
| Dyslipidaemia | 1,932 | 11,193 | — |
| Male, n (%) | 1,780 (92.1) | 7,112 (63.5) | <0.001 |
| Age, mean (SD) | 48.79 (10.15) | 48.17 (12.46) | 0.11 |
| Obesity∗ | 306 | 2,245 | — |
| Male, n (%) | 284 (92.8) | 1,493 (66.5) | <0.001 |
| Age, mean (SD) |
48.68 (9.98) |
46.09 (12.75) |
0.002 |
| Presence of ≥1 MetRs | 2,323 | 13,121 | |
| Male, n (%) | 2,137 (92.0) | 8,329 (63.5) | <0.001 |
| Age, mean (SD) |
49.99 (10.10) |
48.41 (12.72) |
<0.001 |
| Presence of ≥2 MetRs | 1,556 | 9,288 | |
| Male, n (%) | 1,437 (92.4) | 5,956 (64.1) | <0.001 |
| Age, mean (SD) | 50.01 (10.12) | 48.83 (12.61) | 0.005 |
The Chi-square test was used to examine the relationships between categorical variables, and Student’s t test was used to compare the mean values of continuous variables across the groups.
Differences significant at p <0.05.
AFLD, alcoholic fatty liver disease; FLD, fatty liver disease; MetR, metabolic risk factor; NAFLD, non-alcoholic fatty liver disease.
Obesity data from three centres (Kangwon National University Hospital, Daegu Catholic University Medical Center, and Pusan National University Hospital) were not available.
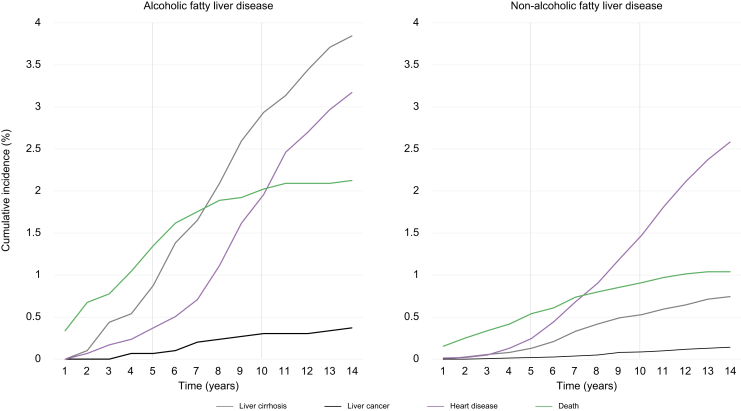
The incidences of four outcomes (cirrhosis, liver cancer, heart disease, and death) were estimated in the entire patient group (Fig. 1). The mean follow-up duration was 5.52 years in the AFLD group and 5.56 years in the NAFLD group. In all patients, the 5-year and 10-year cumulative incidence of cirrhosis was 0.88% and 2.93% in the AFLD and 0.13% and 0.53% in NAFLD groups, respectively. The 5-year and 10-year liver cancer rate was 0.07% and 0.30% in the AFLD group, respectively, and 0.02% and 0.09% in the NAFLD group, respectively. For heart disease, the 5-year and 10-year incidence was 0.37% and 1.96% in the AFLD group and 0.25% and 1.48% in the NAFLD group, respectively. The 5-year and 10-year cumulative mortality rate was 1.35% and 2.02% in the AFLD group and 0.54% and 0.91% in the NAFLD group, respectively. Overall, the incidence of cirrhosis, liver cancer, and death was higher in the AFLD group than in the NAFLD group. By contrast, the proportional difference in heart disease was less prominent than that of other outcomes. The risk of incidence of hepatic and cardiac outcomes between the AFLD and NAFLD groups (Table S4) and the adjusted risk ratio (aRR) revealed that hepatic outcomes were more common in the AFLD group than in the NAFLD group (aRR: 95% CI 5.81 [4.14–8.15], p <0.001) but not cardiac outcomes (aRR: 95% CI 1.19 [0.89–1.58], p >0.05).
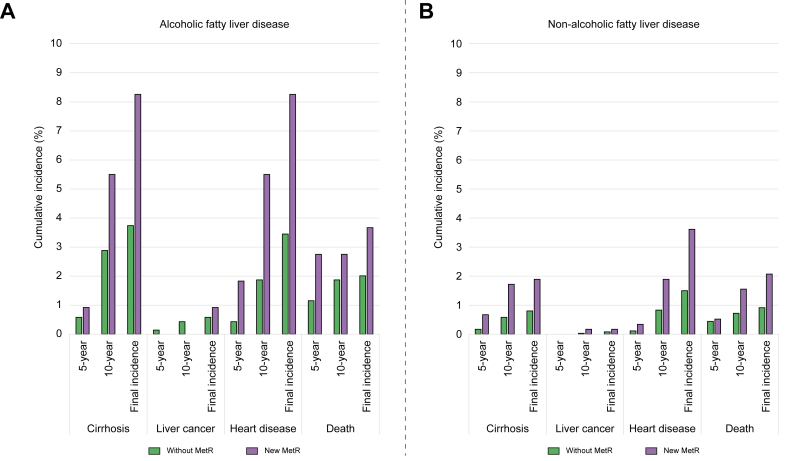
Fig. 1.
Cumulative incidence of cirrhosis, liver cancer, heart disease, and death in patients with (A) alcoholic fatty liver disease and (B) non-alcoholic fatty liver disease.
Comparisons of intergroup and intragroup outcomes according to MetR
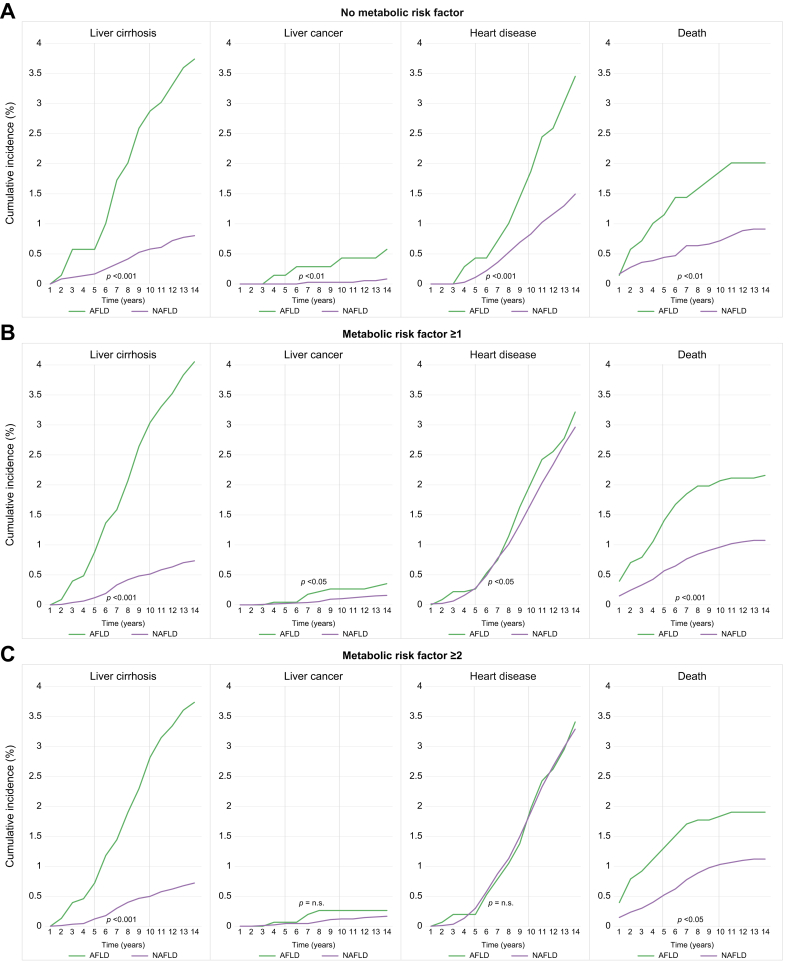
Intergroup analysis was performed in patients without MetRs, MetR ≥1, and MetR ≥2 for the incidence of cirrhosis, liver cancer, heart disease, and death (Fig. 2). In patients without MetRs, AFLD had a higher cumulative 5-year and 10-year incidence of all four outcomes compared with NAFLD (cirrhosis, 0.58% and 2.88% in the AFLD group and 0.17% and 0.58% in the NAFLD group, respectively; liver cancer, 0.14% and 0.43% in the AFLD group and 0% and 0.03% in the NAFLD group, respectively; heart disease, 0.43% and 1.87% in the AFLD group and 0.11% and 0.83% in the NAFLD group, respectively; death, 1.15% and 1.87% in the AFLD group and 0.44% and 0.72% in the NAFLD group, respectively; all p <0.05, log-rank test).
Fig. 2.
Intergroup comparison of cumulative incidence of cirrhosis, liver cancer, heart disease, and death in patients (A) without MetRs (B) MetR ≥1, and (C) MetR ≥2.
Log-rank tests used to examine the differences in cumulative risks; differences significant at p <0.05. AFLD, alcoholic fatty liver disease; MetR, metabolic risk factor; NAFLD, non-alcoholic fatty liver disease.
In patients with one or more MetR, there was no statistically significant difference in AFLD and NAFLD in the incidence of heart disease (5- and 10-year incidences, 0.26% and 2.03% in the AFLD group and 0.28% and 1.68% in the NAFLD group, respectively; all p >0.05). There was no statistically significant difference in the incidence of liver cancer and heart disease between the AFLD and NAFLD groups in patients with two or more MetRs (5- and 10-year incidence: liver cancer, 0.07% and 0.26% in the AFLD group and 0.04% and 0.12% in the NAFLD group, respectively; heart disease, 0.20% and 1.97% in the AFLD group and 0.30% and 1.90% in the NAFLD group, respectively; all p >0.05). By contrast, the incidence of cirrhosis and death was higher in the AFLD group than in the NAFLD group irrespective of MetR (all p <0.05).
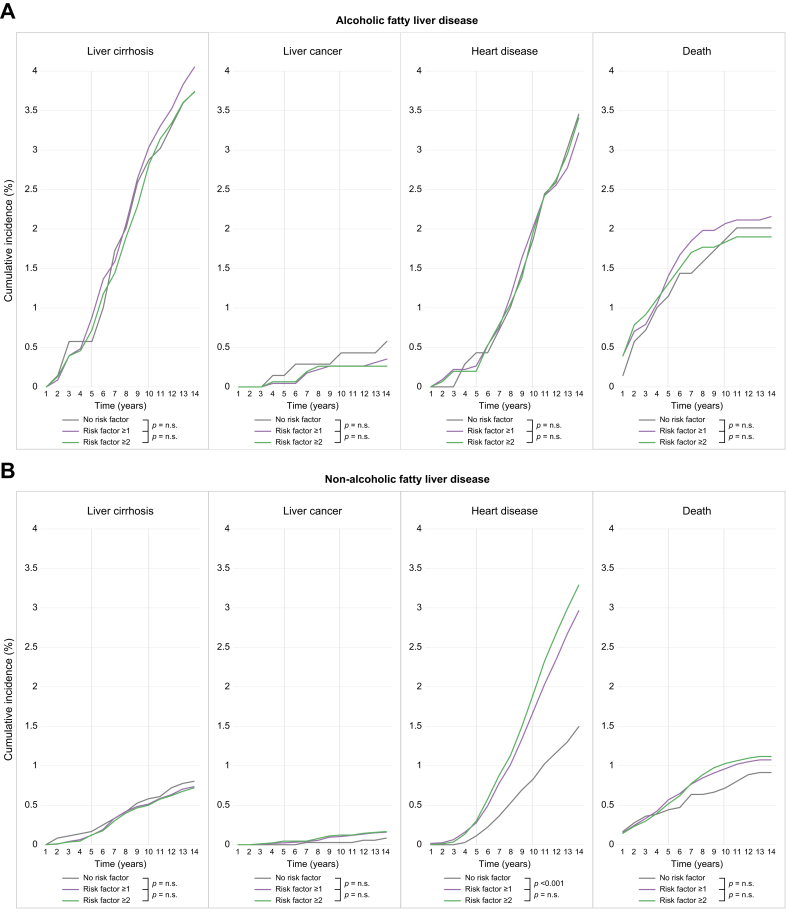
In terms of intragroup comparative analyses (Fig. 3), there was no statistically significant difference in the incidence rates of cirrhosis, liver cancer, heart disease, and death among the patients without MetR, MetR ≥1, and MetR ≥2 in the AFLD group (5- and 10-year incidence: cirrhosis, 0.58% and 2.88%, 0.88% and 3.04%, 0.72% and 2.82%, respectively; liver cancer, 0.14% and 0.43%, 0.04% and 0.26%, 0.07 and 0.26%, respectively; heart disease, 0.43% and 1.87%, 0.26% and 2.03%, 0.20%, and 1.97%, respectively; and death, 1.15% and 1.87%, 1.41% and 2.07%, 1.31%, and 1.83%, respectively; all p >0.05). In the NAFLD group, there was no statistically significant difference between the incidence of cirrhosis and liver cancer among patients without MetR, MetR ≥1, and MetR ≥2 (5- and 10-year incidence: cirrhosis and liver cancer, 0% and 0.03%, 0.02% and 0.10%, 0.04% and 0.12%, respectively; all p >0.05). By contrast, the incidence of heart disease was significantly higher in patients with MetR ≥1 and MetR ≥2 than in those without MetR (5- and 10-year incidence: no MetR vs. MetR ≥1, 0.11% and 0.83% vs. 0.28% and 1.68%, respectively; no MetR vs. MetR ≥2, 0.11% and 0.83% vs. 0.30% and 1.90%, respectively; all p <0.05). The incidence of mortality was higher in those with MetR ≥1 and MetR ≥2 than in those without MetR; however, there was no statistically significant difference between the groups (5- and 10-year incidence: no MetR vs. MetR ≥1, 0.44% and 0.72% vs. 0.57% and 0.96%, respectively; no MetR vs. MetR ≥2, 0.44% and 0.72% vs. 0.52% and 1.03%, respectively; all p >0.05). The annualised incidence and number of patients at risk are summarised in Table S5.
Fig. 3.
Intragroup comparison of cumulative incidence of cirrhosis, liver cancer, heart disease, and death according to MetRs in patients with (A) AFLD and (B) NAFLD.
Log-rank tests used to examine the differences in cumulative risks; differences significant at p <0.05. AFLD, alcoholic fatty liver disease; MetR, metabolic risk factor; NAFLD, non-alcoholic fatty liver disease; n.s., non-significant.
Intergroup and intragroup risk of hepatic and cardiac outcomes according to MetRs
Intergroup comparisons (AFLD vs. NAFLD) were performed in patients without MetR, MetR ≥1, and MetR ≥2 to compare the risk of incidence of hepatic and cardiac outcomes (Table 2). The aRR in patients without MetR, MetR ≥1, and MetR ≥2 in terms of cardiac outcomes were not statistically significantly different across the groups (aRR: 95% CI 1.46 [0.77–2.75] in patients without MetR; 1.16 [0.84–1.61] in patients with MetR ≥1; 0.94 [0.63–1.39] in patients with MetR ≥2; all p >0.05). In contrast to cardiac outcomes, the hepatic outcomes consistently favoured the NAFLD group in patients without MetR, MetR ≥1, and MetR ≥2 (aRR: 95% CI: 2.94 [1.47–5.87] in patients without MetR; 6.74 [3.69–12.29] in patients with MetR ≥1; 5.56 [2.68–11.51] in patients with MetR ≥2, all p <0.05).
Table 2.
Risk of adverse cardiac and hepatic outcomes on intergroup comparison between those with AFLD and those with NAFLD according to MetR.
| Presence of MetR | Outcome incidence | Outcome incidence (events/follow-up time [person-year]) | Adjusted risk ratio∗ | 95% CI | p value† |
|---|---|---|---|---|---|
| AFLD vs. NAFLD | |||||
| Without MetR‡ | Cardiac outcomes | 15/2,459 vs. 27/6,344 | 1.46 | 0.77–2.75 | 0.25 |
| Hepatic outcomes | 21/2,423 vs. 15/6,404 | 2.94 | 1.47–5.87 | 0.002 | |
| MetR ≥1‡ | Cardiac outcomes | 50/8,388 vs. 139/27,451 | 1.16 | 0.84–1.61 | 0.36 |
| Hepatic outcomes | 76/8,396 vs. 33/27,837 | 6.74 | 3.69–12.29 | <0.001 | |
| MetR ≥2 | Cardiac outcomes | 36/6,564 vs. 141/22,712 | 0.94 | 0.63–1.39 | 0.75 |
| Hepatic outcomes | 49/6,543 vs. 30/23,128 | 5.56 | 2.68–11.51 | <0.001 | |
†Differences significant at p <0.05.
AFLD, alcoholic fatty liver disease; MetR, metabolic risk factor; NAFLD, non-alcoholic fatty liver disease.
Model adjusted for age and sex in each hospital data included, and meta-analyses were undertaken to obtain cumulative incidence estimates (by person-year) using a random-effects model.
Data of hazard ratios and 95% CIs for the incidence (event per person-year) of outcomes by Cox proportional hazard models were used in six out of seven hospitals (not Wonkwang University Hospital).
Intragroup comparisons between patients with AFLD and those with NAFLD are summarised in Table 3. In patients with AFLD, there was no statistically significant difference between patients without MetR vs. MetR ≥1 and MetR ≥2 in terms of cardiac outcomes (no MetR vs. MetR ≥1: 95% CI 0.98 [0.56–1.71]; no MetR vs. MetR ≥2: 95% CI 1.04 [0.58–1.85], all p >0.05) and hepatic outcomes (no MetR vs. MetR ≥1: 95% CI 1.09 [0.37–3.19]; no MetR vs. MetR ≥2: 95% CI 1.08 [0.33–3.52], all p >0.05). However, in patients with NAFLD, cardiac outcomes were significantly more favourable in patients without MetR than in patients with MetR ≥1 and MetR ≥2 (0.66 [0.46–0.94] and 0.61 [0.43–0.87], respectively; all p <0.05). There was no significant risk difference in hepatic outcomes in patients with NAFLD with any MetR than those without MetR (95% CI 1.35 [0.52–3.52] and 1.59 [0.65–3.92], respectively, all p >0.05).
Table 3.
Risk of adverse cardiac and hepatic outcomes on intragroup comparison in those with AFLD and those with NAFLD according to MetR.
| Presence of MetR | Outcome incidence | Outcome incidence (events/follow-up time [person-year]) | Adjusted risk ratio∗ | 95% CI | p value† |
|---|---|---|---|---|---|
| AFLD | |||||
| No MetR vs. MetR ≥1‡ | Cardiac outcomes | 18/2,531 vs. 40/5,395 | 0.98 | 0.56–1.71 | 0.93 |
| Hepatic outcomes | 21/2,569 vs. 47/5,556 | 1.09 | 0.37–3.19 | 0.88 | |
| No MetR vs. MetR ≥2‡ | Cardiac outcomes | 18/2,606 vs. 32/4,771 | 1.04 | 0.58–1.85 | 0.90 |
| Hepatic outcomes |
21/2,567 vs. 39/4,786 | 1.08 |
0.33–3.52 |
0.89 |
|
| NAFLD | |||||
| No MetR vs. MetR ≥1‡ | Cardiac outcomes | 40/13,915 vs. 126/28,676 | 0.66 | 0.46–0.94 | 0.02 |
| Hepatic outcomes | 25/14,060 vs. 36/27,973 | 1.35 | 0.52–3.52 | 0.54 | |
| No MetR vs. MetR ≥2‡ | Cardiac outcomes | 40/13,174 vs. 126/24,696 | 0.61 | 0.43–0.87 | 0.006 |
| Hepatic outcomes | 25/13,298 vs. 30/25,152 | 1.59 | 0.65–3.92 | 0.31 | |
†Differences significant at p <0.05.
AFLD, alcoholic fatty liver disease; MetR, metabolic risk factor; NAFLD, non-alcoholic fatty liver disease.
Model adjusted for age and sex in each hospital data included, and meta-analyses were undertaken to obtain cumulative incidence estimates (by person-year) using a random-effects model.
Data of hazard ratios and 95% CIs for the incidence (event per person-year) of outcomes by Cox proportional hazard models were used in six hospitals, except Wonkwang University Hospital.
Different effects of newly developed MetRs on outcomes in AFLD and NAFLD
Subgroup analyses were performed to identify the incidence of outcomes of one or more newly developed MetRs (nMetRs) within 5 years in patients with no MetR at the time of AFLD or NAFLD diagnosis (Fig. 4). Although the absolute increment in the incidence of outcomes was consistently higher in the AFLD group than in the NAFLD group, the rate of increase was different across the four outcomes. In patients with AFLD, 5- and 10-year cumulative outcome incidences were higher in patients with nMetRs than in patients without nMetRs, except for the incidence of liver cancer (cirrhosis, 0.58% and 2.88% vs. 0.92% and 5.50%; liver cancer, 0.14% and 0% vs. 0% and 0%; heart disease, 0.43% and 1.87% vs. 1.87% and 5.50%; and death, 1.15% and 1.87% vs. 2.75% and 2.75%, respectively). In patients with NAFLD, the 5- and 10-year cumulative outcome incidences of nMetR patients compared with those in patients without MetR were higher in terms of the incidence of cirrhosis (0.17% and 0.58% vs. 0.67% and 1.72%, respectively), heart disease (0.11% and 0.83% vs. 0.34% and 1.89%, respectively), and death (0.44% and 0.72% vs. 0.52% and 1.55%, respectively). However, there was no prominent difference in the incidence of liver cancer (0% and 0.03% vs. 0% and 0.17%, respectively). Patients from either the AFLD or NAFLD groups in whom MetRs were recorded within 5 years of their AFLD or NAFLD diagnosis demonstrated a more prominent incidence of outcomes compared to patients without MetR from baseline to 5 years in terms of cirrhosis, heart disease, and death (Fig. S2).
Fig. 4.
Comparison of the incidence of cirrhosis, liver cancer, heart disease, and death between patients without MetRs and those with newly developed MetRs within 5 years of cohort entry in patients with (A) AFLD and (B) NAFLD.
AFLD, alcoholic fatty liver disease; MetR, metabolic risk factor; NAFLD, non-alcoholic fatty liver disease.
Discussion
Recent studies on FLD within the concept of MAFLD have attempted to analyse whether there are differences in morbidity or mortality between patients with MAFLD and those with NAFLD.13,14 However, the integrated approach of FLD with MetRs has some problems in that it is difficult to clearly evaluate the effects of MetR on FLD of different etiologies. Given that most patients with FLD also have either NAFLD or AFLD, it is important to separate the analyses to identify the effects of MetRs on the incidence of hepatic and cardiac outcomes.7,39 Furthermore, it is important to exclude well-known clinical factors, such as chronic viral hepatitis and severe underlying comorbidities, related to the outcomes21,22,[32], [33], [34], [35], [36], [37],40 to analyse the effects of MetR on FLD per se. Nevertheless, no study has yet evaluated the effects of MetRs on the incidence of outcomes in two different categories of patients with FLD after excluding confounding factors.
The current study demonstrated that both hepatic and cardiac outcomes were more frequent in patients with AFLD than in those with NAFLD. However, when the incidence analysis was limited to patients with AFLD, the incidence of outcomes did not depend on the severity of MetRs. By contrast, the incidence of heart disease in patients with NAFLD increased by MetR, becoming similar to those of patients with AFLD. In patients with NAFLD, the incidence of liver cancer and mortality increased but there was no statistically significant difference between the cumulative incidence and adjusted regression analysis. These results suggest that the presence of MetR affects the incidence of hepatic and cardiac outcomes differently in patients with AFLD vs. those with NAFLD, thus indicating that careful risk stratification is required under the concept of MAFLD.
No previous studies have directly compared AFLD and NAFLD groups of patients according to the presence of MetRs. One hospital cohort study evaluated the incidence of liver and cardiovascular disease in patients with FLD. The risk of hepatic outcomes in the AFLD group compared with the NAFLD group was similar to that in our study in the age- and sex-adjusted regression analysis. Furthermore, our intergroup analysis revealed that the incidence of hepatic outcomes was at least three to six times higher in the AFLD group than in the NAFLD group and was dependent on MetRs. Although a gradual increase in liver cancer was observed, most of the hepatic outcomes were cirrhosis, which demonstrated a consistently higher incidence in the AFLD group compared with the NAFLD group. Given that the incidence of liver cancer before advanced fibrosis or cirrhosis is very low,41 we considered cirrhosis to be the main hepatic outcome in our study. Interestingly, the results of previous studies did not demonstrate a statistically significant difference in the cardiovascular risk between patients with AFLD and NAFLD even after adjusting the regression model for MetRs, such as diabetes, hypertension, and dyslipidaemia. Our intergroup analysis revealed that the difference in the incidence of heart disease between the AFLD and NAFLD groups disappeared with an increase in the number of MetRs. Overall, the AFLD group had consistently more frequent hepatic and cardiac outcomes compared with the NAFLD group irrespective of the number of MetRs. By contrast, in NAFLD, there was a pattern of catching up with cardiac outcomes as the severity of MetR increased, as also seen in AFLD.
In the intragroup analysis, the AFLD group did not demonstrate clinical effects according to an increase in MetRs for all outcomes. However, both cardiac outcomes and mortality increased in the NAFLD group. In terms of AFLD, the effects of MetR may not be clinically significant in terms of the incidence of hepatic outcomes, such as cirrhosis or liver cancer. In NAFLD, increases in MetRs affected the cardiac outcomes and mortality, as reported previously.9,42 Thee current results suggested that AFLD and NAFLD are differently affected depending on the severity of MetRs.
There are two possible explanations for the results from patients with AFLD: first, alcohol consumption itself could have a dominant effect on outcomes in the AFLD group irrespective of MetRs. Previous studies targeting FLD and alcohol consumption have reported MetRs as independent risk factors for overall survival and the incidence of severe forms of liver disease.10,14,43 By contrast, one meta-analysis reported that the consumption of alcohol increased hepatic outcomes under conditions accompanied by MetRs, such as BMI.44 The results of previous studies and those of our study on the distribution of alcohol consumption suggest that AFLD has a dominant effect on the incidence of hepatic outcomes over cardiac outcomes.8 Furthermore, it is possible that alcohol consumption itself had a greater influence than MetRs on the occurrence of clinically significant outcomes. Second, it is possible that not only the number of MetRs at cohort entry, but also nMetRs have different effects on the outcomes in the AFLD and NAFLD groups. To identify the difference in the outcomes according to the number of MetRs, the outcome incidence was compared between the groups in which MetR occurred within 5 years of diagnosis (nMetR) and the group without MetR at cohort entry. As a result, the cumulative incidence of cirrhosis and heart disease more than doubled in the nMetR group for both the AFLD and NAFLD groups, being far more prominent in the AFLD group. In particular, the group in which nMetR developed demonstrated a higher incidence of outcomes compared with the group that already had MetR at cohort entry. This result suggests that more careful monitoring and management are required in patients with nMetRs, particularly those with AFLD. Therefore, further studies need to consider the following: (i) when screening risk groups according to MetRs in patients with FLD, different risk stratification is needed between AFLD and NAFLD groups; and (ii) it is necessary to investigate in patients with FLD and nMetR whether we can effectively predict those with a high risk of hepatic and cardiac outcomes.
Previous studies on FLD evaluated the incidence of cardiovascular events with the aim of assessing complications of vascular origin.9,45 These studies reflect the underlying mechanisms mainly associated with NAFLD. However, when atrial fibrillation occurs in patients with excessive alcohol consumption, ischaemic stroke has been reported to increase following atrial fibrillation.46,47 That is, many stroke events may be caused by embolic events that occur after atrial fibrillation in patients with AFLD (the effect of non-vascular heart disease). This pattern is different from that of NAFLD. Although the association between NAFLD and atrial fibrillation has been frequently reported,34,[48], [49], [50] a recent Mendelian randomised study showed that NAFLD has a high correlation with small/large vessels and stroke; however, there is a possibility that this correlation is not related to embolic events.51 Interestingly, based on the additional CDM-based analysis, the incidence of stroke revealed hepatic outcome patterns in the intergroup analysis and cardiac outcome patterns in the intragroup analysis (Fig. S3–S6;Tables S6 and S7). Thus, future research is needed to evaluate the different incidence patterns of heart disease and stroke in patients with AFLD and NAFLD according to MetRs.
This study has several advantages. With the recent increase in the prevalence of FLD, it is necessary to separately analyse the effects of MetRs on FLD to facilitate clinical risk stratification. Our study provides basic information for future research by excluding confounders, such as chronic viral hepatitis and severe comorbidities. Additionally, this multicentre observational study, using standardised data extraction and CDM-based analysis, provides a methodological example of how to conduct research that requires long-term observational studies across multiple regions.
However, there are problems that could not be addressed by this study. First, because of the limitations of data acquisition, MetRs included only four representative diseases: diabetes mellitus, hypertension, dyslipidaemia, and obesity. Therefore, compared with the MAFLD concept, it was impossible to completely separate patients with MetRs corresponding to the grey zone (i.e., milder metabolic risk abnormalities with lean/normal weight) according to the differences in the definition.12 It was also not possible to analyse how different components of each MetR could affect the AFLD and NAFLD groups differently. In addition, the difference in baseline comorbidities or the risk of outcome changes according to treatment history could not be analysed separately. The degree of alcohol consumption also could not analysed separately. However, in our results from a single-centre EMR review, the CDM code for AFLD and NAFLD could be used to divide patients with FLD based on approximate alcohol consumption amounts (i.e. heavy drinking). Although the incidence of outcomes was likely to vary depending on the regional drinking patterns and the possibility of abstinence in the AFLD group, the rate of alcohol cessation is very low in Korea, it is believed that the study cohort reflects the general patient population.28,52 Finally, it was not possible to analyse the degree of steatosis, inflammation, and fibrosis because the data in this study were analysed based on clinical diagnosis and indicators and not on biopsy results, which is the gold standard test for the diagnosis of FLD.
In conclusion, compared with the NAFLD group, the AFLD group showed more hepatic and cardiac outcomes irrespective of the number of MetRs. In the NAFLD group, the proportion of cardiac outcomes increased with the increase in the number of MetRs, eventually matching that of cardiac outcomes in the AFLD group. By contrast, in the AFLD group, both hepatic and cardiac outcomes were not associated with the number of MetRs. Overall, the clinical impact of MetRs in patients with FLD may differ between those with AFLD vs. those with NAFLD.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
JL, HS, HIK: study concept and design, data analysis, interpretation, drafting, and critical revision, of manuscript.
HIK: data acquisition and study supervision.
Data availability statement
CDM data are designed to support a distributed research network. Thus, access to the data is restricted to internal private networks. Therefore, data are not publicly available.
Conflict of interest
The authors have no conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
Data extraction and statistical analyses of each of the seven medical centres were performed using FEEDER-NET (EvidNet Inc., https://feedernet.com), a healthcare Big-Data platform.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100721.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Younossi Z., Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778–1785. doi: 10.1053/j.gastro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Park S.H., Plank L.D., Suk K.T., Park Y.E., Lee J., Choi J.H., et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998-2017. Clin Mol Hepatol. 2020;26:209–215. doi: 10.3350/cmh.2019.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griswold M.G., Fullman N., Hawley C., Arian N., Zimsen S.R.M., Tymeson H.D., et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Parker R., Aithal G.P., Becker U., Gleeson D., Masson S., Wyatt J.I., et al. Natural history of histologically proven alcohol-related liver disease: a systematic review. J Hepatol. 2019;71:586–593. doi: 10.1016/j.jhep.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Idalsoaga F., Kulkarni A.V., Mousa O.Y., Arrese M., Arab J.P. Non-alcoholic fatty liver disease and alcohol-related liver disease: two intertwined entities. Front Med (Lausanne) 2020;7:448. doi: 10.3389/fmed.2020.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y., Cho J., Cho Y.K., Cho A., Hong Y.S., Zhao D., et al. Alcoholic and nonalcoholic fatty liver disease and incident hospitalization for liver and cardiovascular diseases. Clin Gastroenterol Hepatol. 2020;18:205–215. doi: 10.1016/j.cgh.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Simon T.G., Roelstraete B., Hagstrom H., Sundstrom J., Ludvigsson J.F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut. 2022;71:1867–1875. doi: 10.1136/gutjnl-2021-325724. [DOI] [PubMed] [Google Scholar]
- 10.Aberg F., Helenius-Hietala J., Puukka P., Farkkila M., Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67:2141–2149. doi: 10.1002/hep.29631. [DOI] [PubMed] [Google Scholar]
- 11.Kanwal F., Kramer J.R., Li L., Dai J., Natarajan Y., Yu X., et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71:808–819. doi: 10.1002/hep.31014. [DOI] [PubMed] [Google Scholar]
- 12.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z.M., Paik J.M., Al Shabeeb R., Golabi P., Younossi I., Henry L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology. 2022;76:1423–1437. doi: 10.1002/hep.32499. [DOI] [PubMed] [Google Scholar]
- 14.van Kleef L.A., de Knegt R.J., Brouwer W.P. MAFLD and excessive alcohol consumption are both independent risk factors for mortality. Hepatology. 2022;76:1423–1437. doi: 10.1002/hep.32642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H, Yoon EL, Kim M, Cho S, Nah EH, Jun DW. Nomenclature dilemma of metabolic associated fatty liver disease (MAFLD): considerable proportions of MAFLD are metabolic healthy. Clin Gastroenterol Hepatol. 2023;21:1041–1049. doi: 10.1016/j.cgh.2022.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Toshikuni N., Tsutsumi M., Arisawa T. Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:8393–8406. doi: 10.3748/wjg.v20.i26.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P., Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: spectrum and diagnosis. Transl Gastroenterol Hepatol. 2020;5:19. doi: 10.21037/tgh.2019.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dam-Larsen S., Franzmann M., Andersen I.B., Christoffersen P., Jensen L.B., Sorensen T.I., et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haflidadottir S., Jonasson J.G., Norland H., Einarsdottir S.O., Kleiner D.E., Lund S.H., et al. Long term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014;14:166. doi: 10.1186/1471-230X-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dam-Larsen S., Becker U., Franzmann M.B., Larsen K., Christoffersen P., Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 21.Glass L.M., Hunt C.M., Fuchs M., Su G.L. Comorbidities and nonalcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36:64–71. [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T.W., Wang C.C., Tsai M.C., Wang Y.T., Tseng M.H., Lin C.C. Comorbidities and outcome of alcoholic and non-alcoholic liver cirrhosis in Taiwan: a population-based study. Int J Environ Res Public Health. 2020;17:2825. doi: 10.3390/ijerph17082825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yap C., Bedding A., de Bono J., Dimairo M., Espinasse A., Evans J., et al. The need for reporting guidelines for early phase dose-finding trials: dose-finding CONSORT extension. Nat Med. 2022;28:6–7. doi: 10.1038/s41591-021-01594-1. [DOI] [PubMed] [Google Scholar]
- 24.Overhage J.M., Ryan P.B., Reich C.G., Hartzema A.G., Stang P.E. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc. 2012;19:54–60. doi: 10.1136/amiajnl-2011-000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hripcsak G., Duke J.D., Shah N.H., Reich C.G., Huser V., Schuemie M.J., et al. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon D., Ahn E.K., Park M.Y., Cho S.Y., Ryan P., Schuemie M.J., et al. Conversion and data quality assessment of electronic health record data at a Korean tertiary teaching hospital to a common data model for distributed network research. Healthc Inform Res. 2016;22:54–58. doi: 10.4258/hir.2016.22.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo S.I., Park C.H., You S.C., Kim J.Y., Lee K.J., Kim J., et al. Association between proton pump inhibitor use and gastric cancer: a population-based cohort study using two different types of nationwide databases in Korea. Gut. 2021;70:2066–2075. doi: 10.1136/gutjnl-2020-323845. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.I., Park S.Y., Shin H.P. Incidence and management patterns of alcohol-related liver disease in Korea: a nationwide standard cohort study. Sci Rep. 2021;11:6648. doi: 10.1038/s41598-021-86197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald C.J., Huff S.M., Suico J.G., Hill G., Leavelle D., Aller R., et al. LOINC, a universal standard for identifying laboratory observations: a 5-year update. Clin Chem. 2003;49:624–633. doi: 10.1373/49.4.624. [DOI] [PubMed] [Google Scholar]
- 30.Bodenreider O., Cornet R., Vreeman D.J. Recent developments in clinical terminologies - SNOMED CT, LOINC, and RxNorm. Yearb Med Inform. 2018;27:129–139. doi: 10.1055/s-0038-1667077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byun J., Lee D.Y., Jeong C.W., Kim Y., Rhee H.Y., Moon K.W., et al. Analysis of treatment pattern of anti-dementia medications in newly diagnosed Alzheimer’s dementia using OMOP CDM. Sci Rep. 2022;12:4451. doi: 10.1038/s41598-022-08595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J., Dai L., Zhang Y., Wang A., Li H., Wang Y., et al. Severity of nonalcoholic fatty liver disease and risk of future ischemic stroke events. Stroke. 2021;52:103–110. doi: 10.1161/STROKEAHA.120.030433. [DOI] [PubMed] [Google Scholar]
- 33.Fudim M., Zhong L., Patel K.V., Khera R., Abdelmalek M.F., Diehl A.M., et al. Nonalcoholic fatty liver disease and risk of heart failure among medicare beneficiaries. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai X., Zheng S., Liu Y., Zhang Y., Lu J., Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. 2020;40:1594–1600. doi: 10.1111/liv.14461. [DOI] [PubMed] [Google Scholar]
- 35.Viglino D., Jullian-Desayes I., Minoves M., Aron-Wisnewsky J., Leroy V., Zarski J.P., et al. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur Respir J. 2017;49 doi: 10.1183/13993003.01923-2016. [DOI] [PubMed] [Google Scholar]
- 36.Wong V.W., Wong G.L., Yip G.W., Lo A.O., Limquiaco J., Chu W.C., et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–1727. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 37.Targher G., Chonchol M., Zoppini G., Abaterusso C., Bonora E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: is there a link? J Hepatol. 2011;54:1020–1029. doi: 10.1016/j.jhep.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y.B., Moon H., Lee J.H., Cho E.J., Yu S.J., Kim Y.J., et al. Association of metabolic risk factors with risks of cancer and all-cause mortality in patients with chronic hepatitis B. Hepatology. 2021;73:2266–2277. doi: 10.1002/hep.31612. [DOI] [PubMed] [Google Scholar]
- 39.Ventura-Cots M., Ballester-Ferré M.P., Ravi S., Bataller R. Public health policies and alcohol-related liver disease. JHEP Rep. 2019;1:403–413. doi: 10.1016/j.jhepr.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheemerla S., Balakrishnan M. Global epidemiology of chronic liver disease. Clin Liver Dis. 2021;17:365–370. doi: 10.1002/cld.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim J.H. Should you advocate for hepatocellular carcinoma surveillance in patients with alcohol-related liver disease or non-alcoholic fatty liver disease? Clin Mol Hepatol. 2020;26:183–184. doi: 10.3350/cmh.2020.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H., Lee Y.H., Kim S.U., Kim H.C. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19:2138–2147. doi: 10.1016/j.cgh.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Younossi Z.M., Stepanova M., Ong J., Yilmaz Y., Duseja A., Eguchi Y., et al. Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol-related fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:1625–1633. doi: 10.1016/j.cgh.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 44.Glyn-Owen K., Bohning D., Parkes J., Roderick P., Buchanan R. The combined effect of alcohol and body mass index on risk of chronic liver disease: a systematic review and meta-analysis of cohort studies. Liver Int. 2021;41:1216–1226. doi: 10.1111/liv.14754. [DOI] [PubMed] [Google Scholar]
- 45.Polyzos S.A., Kechagias S., Tsochatzis E.A. Review article: non-alcoholic fatty liver disease and cardiovascular diseases: associations and treatment considerations. Aliment Pharmacol Ther. 2021;54:1013–1025. doi: 10.1111/apt.16575. [DOI] [PubMed] [Google Scholar]
- 46.Lee S.R., Choi E.K., Jung J.H., Han K.D., Oh S., Lip G.Y.H. Lower risk of stroke after alcohol abstinence in patients with incident atrial fibrillation: a nationwide population-based cohort study. Eur Heart J. 2021;42:4759–4768. doi: 10.1093/eurheartj/ehab315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voskoboinik A., Prabhu S., Ling L.H., Kalman J.M., Kistler P.M. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. 2016;68:2567–2576. doi: 10.1016/j.jacc.2016.08.074. [DOI] [PubMed] [Google Scholar]
- 48.Jin Q., Yang R.X., Fan J.G. Does nonalcoholic fatty liver disease predispose patients to carotid arteriosclerosis and ischemic stroke? Clin Mol Hepatol. 2022;28:473–477. doi: 10.3350/cmh.2022.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haghbin H., Gangwani M.K., Ravi S.J.K., Perisetti A., Aziz M., Goyal H., et al. Nonalcoholic fatty liver disease and atrial fibrillation: possible pathophysiological links and therapeutic interventions. Ann Gastroenterol. 2020;33:603–614. doi: 10.20524/aog.2020.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baik M., Kim S.U., Nam H.S., Heo J.H., Kim Y.D. The paradoxical protective effect of liver steatosis on severity and functional outcome of ischemic stroke. Front Neurol. 2019;10:375. doi: 10.3389/fneur.2019.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu M., Zha M., Lv Q., Xie Y., Yuan K., Zhang X., et al. Non-alcoholic fatty liver disease and stroke: a Mendelian randomization study. Eur J Neurol. 2022;29:1534–1537. doi: 10.1111/ene.15277. [DOI] [PubMed] [Google Scholar]
- 52.Jang J.Y., Kim D.J. Epidemiology of alcoholic liver disease in Korea. Clin Mol Hepatol. 2018;24:93–99. doi: 10.3350/cmh.2017.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CDM data are designed to support a distributed research network. Thus, access to the data is restricted to internal private networks. Therefore, data are not publicly available.