Summary
Background
This analysis evaluated the immune response to the two-dose, heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola virus vaccine regimen, administered 56-days apart, from multiple African sites based on results from one analytic laboratory.
Methods
Immunogenicity across three trials (EBL2002, EBL2004/PREVAC, EBL3001) conducted in East and West Africa is summarised. Vaccine-induced Ebola glycoprotein-binding antibody concentrations were analysed by Q2 Solutions laboratory at baseline, 21 days (EBL2002 and EBL3001) or 28 days (EBL2004) post-dose 2 (regimen completion), and 12 months post-dose 1 using the validated Filovirus Animal Nonclinical Group Ebola glycoprotein enzyme-linked immunosorbent assay (ELISA). Responders were defined as those with a >2.5-fold increase from baseline or the lower limit of quantification (LLOQ) if <LLOQ at baseline.
Findings
At 21 or 28 (21/28) days post-dose 2, the geometric mean concentration (GMC) range was 3810–7518 ELISA units (EU)/mL (percent responders: ≥98%) in adults, 9929–13532 EU/mL (≥98%) in adolescents aged 12–17 years, 10,212–17388 EU/mL (≥99%) in older children, and 22,568–25111 EU/mL (≥98%) in younger children. When stratified by country, GMCs at 21/28 days post-dose 2 were generally similar among adults and within paediatric cohorts (percent responders: 95%–100%). At month 12, GMC range was 259–437 EU/mL (percent responders: 49%–88%) in adults and 386–1139 EU/mL (70%–100%) in paediatric
participants.
Interpretation
Based on data from a single laboratory using a single validated assay, Ad26.ZEBOV, MVA-BN-Filo induced a strong humoral immune response, with ≥95% of participants across countries classified as responders at 21/28 days post-dose 2 (regimen completion), regardless of age.
Funding
Janssen Vaccines & Prevention BV; Innovative Medicines Initiative.
Keywords: Ad26.ZEBOV, MVA-BN-Filo, Africa, Ebola, Vaccine
Research in context.
Evidence before this study
Outbreaks of Ebola virus disease present a global public health concern and, therefore, development of effective vaccines is crucial. In clinical trials, specific biomarkers can act as surrogates for clinical outcomes, especially when data for the latter may take many years to gather or the population to be studied is very large. For infectious diseases in particular, elicitation of an immune response and suppression of pathogen replication according to predefined thresholds have been used as surrogate endpoints for the regulatory approval of antiviral therapies and prophylactic vaccines, rather than progression of disease or development of organ damage. However, a consistent antibody assay to determine the response to a vaccine across different populations is required to accurately assess outcomes.
We searched PubMed on December 13, 2021, using the following search terms “ebola OR hepatitis B OR yellow fever OR pneumococcus OR measles” AND “vaccine response heterogeneity OR vaccine response variability OR vaccine immunogenicity heterogeneity OR vaccine immunogenicity response variability OR vaccine response geographical variation”. We searched for clinical and epidemiological research, published with no time limits, up to December 13, 2021, with no language restrictions. This search yielded two meta-analyses and three studies that directly compared geographic variations in vaccine response rates and identified differences in response rates between studies and countries. One meta-analysis found that antibody responses to pneumococcal immunisation after two or three doses (most studies had an interval of 1 or 2 months between the first two doses) were generally higher in the Western Pacific region, Southeast Asia, and Africa relative to Europe and North America; similarly, the other meta-analysis reported that antibody response to yellow fever vaccination was higher in non-endemic versus endemic regions. With respect to Dengue fever vaccination, one study described a greater response 28 days after each dose (given at months 0, 6, and 12) in Latin America compared with Southeast Asia. The second study observed differences in antibody response assessed at various timepoints (range, 4–67 weeks) after hepatitis B vaccination across regions within the Gambia. The third study evaluated results from three phase 1 clinical trials of vaccine regimens containing Ad26.ZEBOV and MVA-BN-Filo vaccines for Ebola virus disease and reported significantly higher antibody responses 1 year after the first vaccination dose among European participants compared with participants from East Africa. Lower immune responses with an Ad26.ZEBOV, MVA-BN-Filo vaccination 21 days after the second dose have also been observed in participants from Sierra Leone compared to those in participants from other African countries, Europe, and the United States.
Added value of this study
In the current analysis of data from multiple African sites in three phase 2 studies, the primary outcome was the vaccine-elicited Ebola virus glycoprotein-binding antibody response to the two-dose, heterologous Ad26.ZEBOV, MVA-BN-Filo vaccine regimen administered in a 56-day interval. Immunogenicity was measured at a single analytic laboratory using the validated Filovirus Animal Nonclinical Group enzyme-linked immunosorbent assay.
Ebola virus glycoprotein-binding antibody concentrations were measured at 21 or 28 days, depending on the study, after the second dose and at month 12 after the first dose in adults and paediatric participants. While there were differences in antibody geometric mean concentrations (GMCs) across countries (Sierra Leone, Mali, Liberia, Guinea, Burkina Faso, Côte d’Ivoire, Uganda, and Kenya), the overall GMCs were generally similar in adults and within paediatric age groups at each timepoint.
Implications of all the available evidence
According to data analysed at a single laboratory using a validated assay, the two-dose, heterologous Ad26.ZEBOV, MVA-BN-Filo vaccine regimen elicited robust Ebola virus glycoprotein antibody responses, in participants from eight African countries, that persisted up to approximately 12 months after the first vaccine dose.
Introduction
Recent large outbreaks of Ebola virus (EBOV) disease (EVD) resulted in nearly 2300 deaths in the Democratic Republic of the Congo (DRC) and Uganda (2018–2020) and more than 11,300 deaths in Guinea, Liberia, and Sierra Leone (2014–2016).1 There were three reported EVD outbreaks in 2021 (one in Guinea, two in the DRC), two outbreaks in 2022 in the DRC, and one outbreak spanning 2022 to 2023 in Uganda.2, 3, 4, 5
Two EVD vaccines, rVSV-ZEBOV manufactured by Merck (Whitehouse Station, NJ, USA) and the Ad26.ZEBOV, MVA-BN-Filo regimen manufactured by Janssen (Beerse, Belgium), are currently pre-qualified by the World Health Organization. The heterologous, two-dose Ad26.ZEBOV, MVA-BN-Filo vaccine regimen comprises one dose of the monovalent, replication-incompetent, adenovirus type 26 vector–based vaccine encoding the EBOV glycoprotein (GP; Mayinga variant, Ad26.ZEBOV), followed 56 days later by a dose of the recombinant, non-replicating, modified vaccinia Ankara-vectored vaccine encoding GPs from the EBOV (Mayinga variant), Sudan virus (Gulu variant), and Marburg virus (Musoke variant), plus the Tai Forest virus nucleoprotein (MVA-BN-Filo).
The current analysis evaluated the immune response to the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen, administered in a 56-day interval, from multiple African sites based on results from a single analytic laboratory. Regulatory agencies recognise the need for validated surrogate endpoints6,7 and prefer the use of a fully validated assay, with testing conducted at a single (central) accredited laboratory, with the same laboratory used throughout the clinical development programme to eliminate inter-laboratory variability that may impact results and possibly confound conclusions.6,7 The use of a single laboratory can also facilitate comparison of responses across populations and lend credence to a vaccine's ‘true’ response.
EBOV GP-binding antibodies were previously identified as an immune parameter highly correlated with survival after EBOV challenge in non-human primates vaccinated with Ad26.ZEBOV, MVA-BN-Filo.8 A single assay from a single laboratory was validated and subsequently accepted by regulatory agencies to measure the vaccine-elicited binding antibody responses in both non-human primates and humans.9 Therefore, the primary immunogenicity outcome measure for the Ad26.ZEBOV, MVA-BN-Filo regimen is binding antibody concentrations against EBOV GP, as measured by the Filovirus Animal Nonclinical Group (FANG) enzyme-linked immunosorbent assay (ELISA) at Q2 Solutions Vaccine Testing Laboratory. The aim of this study was to assess EBOV GP-binding antibody concentrations measured using a one-assay, one-lab strategy (ie, FANG ELISA at Q2 Solutions) in different age groups across multiple African countries using samples from three clinical trials of the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen.
Methods
Three late-development studies were selected for inclusion in this report: EBL2002, Partnership for Research on Ebola VACcination (PREVAC; hereafter referred to as EBL2004), and EBL3001.10, 11, 12, 13, 14 This analysis included only studies that analysed samples using the FANG ELISA assay at Q2 Solutions laboratory, were conducted in sub-Saharan African countries, and evaluated the safety and immunogenicity of the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen with dosing on days 1 and 57, respectively, in individuals aged ≥1 year. This allowed immune responses to be examined more accurately in different age groups and across different countries within sub-Saharan Africa. A formal literature review was not performed.
Studies EBL2002 and EBL3001 assessed the EBOV GP-binding antibody response at various timepoints after vaccination via the validated FANG ELISA assay undertaken at a single analytic laboratory (Q2 Solutions, San Juan Capistrano, CA, USA). Study EBL2004 assessed EBOV GP-binding antibody concentrations at different timepoints after vaccination via a FANG ELISA, but at two different laboratories (Liberian Institute for Biomedical Research [Charlesville, Liberia] and the National Institute of Allergy and Infectious Diseases Integrated Research Facility [Frederick, Maryland, USA]). For the current analysis, a subset of samples from EBL2004 was analysed by Q2 Solutions laboratory using the validated FANG ELISA assay.
Completed phase 1 studies of vaccination with Ad26.ZEBOV, MVA-BN-Filo, even if conducted in African countries, were not included in this analysis because the FANG ELISAs were run at Battelle Biomedical Research Center (BBRC; Columbus, OH, USA). The FANG ELISA at BBRC is a validated assay and BBRC is an accredited laboratory; however, comparing these results with FANG ELISA results from Q2 Solutions would not align with the one-assay, one-lab strategy. Other completed studies were not included because they either were not conducted in African countries (EBL2001, EBL3002, EBL3003), did not enrol participants aged ≥1 year (EBL2005), or did not include assessment of the licensed regimen (EBL2003, EBL2010, EBL2011).
Results of the current analysis are presented by study and stratified by geographic location and, for paediatric participants, further stratified by age group. In EBL2002 and EBL3001 stage 2,11,14 the EBOV GP-binding antibody response was assessed in adolescents aged 12–17 years, older children aged 4–11 years, and, in EBL3001 stage 2 only, younger children aged 1–3 years. EBL2004 assessed EBOV GP-binding antibody response in adolescents aged 12–17 years, older children aged 5–11 years, and younger children aged 1–4 years.12
Trial designs and participants
The phase 2, randomised, placebo-controlled EBL2002 study (ClinicalTrials.gov identifier: NCT02564523) evaluated the safety and immunogenicity of Ad26.ZEBOV followed by MVA-BN-Filo 28, 56, and 84 days later in healthy adults, human immunodeficiency virus (HIV)-infected adults, and children aged 4–17 years at seven sites in four African countries (Uganda, Kenya, Burkina Faso, Côte d’Ivoire).10,11 Enrolment began on 9 November 2015 for adults and 26 February 2016 for paediatric participants; the last visits for these groups for immunogenicity occurred on 12 February 2019 and 28 November 2018, respectively. All samples were analysed by Q2 Solutions between July 2017 and March 2019.
EBL2004/PREVAC was a randomised, double-blind, placebo-controlled, phase 2 trial (NCT02876328) conducted at six sites in Guinea, Liberia, Mali, and Sierra Leone.12,15 Healthy individuals aged ≥1 year were eligible to participate and were randomised to one of five study arms. Enrolment began on 16 April 2018 and the last visits for immunogenicity occurred on 25 November 2019 for adults and 12 December 2019 for paediatric participants. Additional details of the EBL2004 study design are provided in the Supplementary methods.
Serum aliquots from a subset of participants in EBL2004, at selected timepoints, were sent to Q2 Solutions laboratory for FANG ELISA analysis. Data presented here from the Ad26.ZEBOV, MVA-BN-Filo (56-day interval) and matching placebo arms are based on this subset of samples analysed by Q2 Solutions. All samples were analysed by Q2 Solutions between July 2020 and September 2021.
EBL3001 (NCT02509494) evaluated the safety and immunogenicity of Ad26.ZEBOV followed by MVA-BN-Filo 56 days later in individuals ≥1 year of age in Sierra Leone.13,14 Stage 1 was an open-label study conducted in adults and stage 2 was a randomised, active-controlled (meningococcal quadrivalent conjugate vaccine [MenACWY] followed by placebo) trial in adults and paediatric participants. Enrolment began on 30 September 2015 for adults and 04 April 2017 for paediatric participants; the last visits for immunogenicity occurred on 24 October 2018 and 28 June 2019, respectively. All samples were analysed by Q2 Solutions between May 2017 and August 2019.
In all three studies, male and female adults and children were enroled, with sex self-reported by participants or by observation, and previous history of EVD was an exclusion criterion. In all three studies, Ad26.ZEBOV (5 × 1010 viral particles) and MVA-BN-Filo (1 × 108 infectious units) were administered by intramuscular injection.
Outcomes
The principal immunogenicity readout for EBL2002 and EBL3001 was the EBOV GP-binding antibody concentration at day 21 post–MVA-BN-Filo vaccination (post-dose 2 [regimen completion]).10,11,13,14 Secondary outcomes included EBOV GP-binding antibody concentrations measured 12 months post-Ad26.ZEBOV vaccination (post-dose 1). The primary endpoints for the current analysis of EBL2004 were EBOV GP-binding antibody response at day 28 post-dose 2 (regimen completion) and month 12 post-dose 1. Samples from all studies were analysed according to Q2 Solution's FANG ELISA standard operating procedure (SOP) and a single reportable value for each participant sample at each timepoint was uploaded for statistical analysis.
For all studies, a response was defined as a >2.5-fold increase from baseline in EBOV GP-binding antibody concentration or a post-baseline value >2.5 times the lower limit of quantification (LLOQ) of 36.11 ELISA units (EU)/mL, if the sample was negative at baseline.
Ethics
All study protocols were approved by local and national independent ethics committees and institutional review boards (IRBs), and the studies were conducted according to the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice Guidelines.10, 11, 12, 13, 14
The EBL2002 protocol and amendments were reviewed and approved in Burkina Faso by the Burkina Faso Central Ethics Committee (approval number: 2017-02-023); in Côte d’Ivoire by the Comite National D'Ethique de La Recherche (IRB00009111, reference number: 063-18/MSHP/CNER-km); in Kenya by the Kenyatta National Hospital/University of Nairobi Ethical Review Committee (IRB00002139, reference number: KNH-ERC/Mod&SAE/290); and in Uganda by the Uganda Virus Research Institute Research Ethics Committee (IRB00001693, reference number: GC/127/17/10/517) and the Makerere University School of Public Health Research and Ethics Committee (IRB00005876, reference number: MUSPH#326).10,11
The EBL2004 study protocol and the informed consent and assent forms, including participants' information materials, were approved by ethics committees of the sponsors (Comité d’Evaluation Ethique de l’Inserm [IRB00003888] and the London School of Hygiene & Tropical Medicine Ethics Committee [IRB00008403]) and the implementing countries (Guinea: Comité National d’Ethique pour la Recherche en Santé [IRB00009682]; Liberia: National Health Science Research Ethics Committee [IRB00010040]; Mali: U Mali Faculty Med Pharmacy & Dentistry [IRB00001983]; and Sierra Leone: Sierra Leone Ethics and Scientific Review Committee [IRB00007981]) before each version of the protocol was implemented.12 The National Institutes of Health established an institutional reliance agreement with Inserm to rely upon the Inserm ethics committee.
The EBL3001 protocol and amendments were reviewed and approved by the Sierra Leone Ethics and Scientific Review Committee (IRB00007981) and the London School of Hygiene & Tropical Medicine Ethics Committee (reference number: 9726).13,14
All adult participants supplied written informed consent before enrolment. For paediatric participants, parents or guardians provided written informed consent for their child to join the trial. Older children (aged 6 or 12 years and older, depending on country, in EBL2002, and aged 7 years and older in EBL2004 and EBL3001) also gave written assent.
Statistics
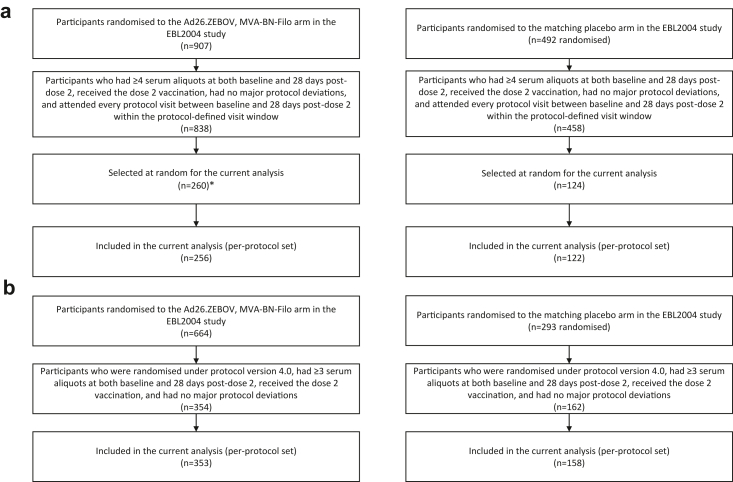
Immunogenicity results from EBL2002, a subset of EBL2004 participants randomised to the Ad26.ZEBOV, MVA-BN-Filo regimen and matching placebo group, and EBL3001 are reported here. Baseline demographic characteristics are also reported. For EBL2004, a subset of 373 (27%) of 1399 participants aged ≥18 years, stratified by country, were selected at random in a 2:1 ratio of Ad26.ZEBOV, MVA-BN-Filo regimen to matching placebo to ensure that the distribution of the selected participants was similar to the overall participant distribution across groups (Fig. 1a). Random selection was done before sample analysis. Additionally, 11 adult participants were selected from the Ad26.ZEBOV, MVA-BN-Filo group after unblinding due to unavailability of month 12 samples for some selected participants. For paediatric participants aged 1–17 years, available samples of all participants without protocol deviations that could influence immune responses were analysed (Fig. 1b). For all studies, outcomes were assessed separately for adults and paediatric participants.
Fig. 1.
Flow diagram for (a) adult and (b) paediatric participants in the EBL2004 study included in the analysis. Flow diagrams showing the derivation of samples from participants in the EBL2004 study included in the current analysis with immunogenicity assessments performed by Q2 Solutions. ∗This number includes an additional 11 participants who were selected for the Ad26.ZEBOV, MVA-BN-Filo regimen after unblinding due to missing month 12 samples for some randomly selected participants.
The analysis set for immunogenicity (per-protocol) included all vaccinated participants who received both vaccinations within the protocol-defined time window, had ≥1 evaluable post-vaccination immunogenicity sample, and had no major protocol deviations that could have influenced the immune response.
Antibody concentrations for the Ad26.ZEBOV, MVA-BN-Filo and matched control groups were summarised as geometric mean concentrations (GMCs) with 95% confidence intervals (CIs) at each timepoint. As the minimum clinically meaningful difference in terms of binding antibody levels is not known, non-overlapping CIs served to highlight large differences between subgroups. Responder rates were reported for each post-baseline timepoint. Subgroup analyses for the proportion of responders at 21 or 28 days post-dose 2 and 12 months post-dose 1 were conducted according to age at baseline for paediatric participants, and by country.
Mixed effects models were used to estimate an overall GMC for adult and paediatric participants that accounted for between-study variation. To estimate an overall GMC across studies for the adult participants, the unconditional random intercept model with no predictors was used to assess between-study variation in binding antibody concentrations (EU/mL). This model was fitted on log10-transformed binding antibody concentrations (EU/mL) and investigated the variation in binding antibody concentrations attributable to the studies. Using the covariance parameter estimates from the model, the intraclass correlation coefficient (ICC) that indicated how much of the total variation in immune response was attributable to the studies was computed. The general equation for the ICC is given by:
where denotes the covariance estimate for the intercept and denotes the covariance estimate for the residual. Accounting for the between-study variation, the GMCs were obtained by back-transformation of the estimated mean values and corresponding 95% CI.
For the paediatric participants, the ICC based on the covariance parameter estimates from a random intercept model with age category as a predictor in the model was similarly calculated. Afterwards, the overall GMC for each age category across studies was estimated by fitting a random intercept and random slope model with age category as a predictor in the model. These models were also fitted on log10-transformed binding antibody concentrations (EU/mL) and the GMCs were obtained by back-transformation. Before fitting the models, EBL2004 paediatric participants were reclassified into the following age groups: 1–3 years, 4–11 years, and 12–17 years, in alignment with the age groups in EBL2002 and EBL3001. The proportion of variance explained when controlling for baseline characteristics was also computed and the results are presented in Supplementary Tables S1 and S2 for adults and paediatric participants, respectively.
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc.; Cary, NC, USA).
Role of the funding source
The sponsors of each study were responsible for the study design; data collection, analysis, and interpretation; and writing the study reports. Within study EBL2004/PREVAC, Janssen was responsible for the collection, analysis, and interpretation of the FANG ELISA data presented in this article. The decision to submit the manuscript for publication was initiated by Janssen, with approval from the PREVAC Trial Steering Committee.
Results
Study participants
Baseline characteristics of participants within each of the three studies in the Ebola vaccine and control groups and across countries are shown in Tables 1 and 2.10,11,13,14 In the adult subgroups of the Ebola vaccine group, the median age ranged from 23.0 to 39.0 years, the percentage of females ranged from 2% (1 of 43 participants in EBL3001 stage 1) to 71% (42 of 59 HIV-infected adults in EBL2002), and the median body-mass index ranged from 21.6 to 23.7 kg/m2 across studies. In the adolescent subgroups of the Ebola vaccine group, the median age was 14.0 years in all studies and the percentage of females ranged from 39% (52 of 132 participants in EBL2004) to 48% (68 of 142 participants in EBL3001 stage 2). In the older child subgroups of the Ebola vaccine group, the median age was 8.0 years in all studies and the percentage of females ranged from 48% (54 of 112 participants in EBL2004) to 52% (28 of 54 and 70 of 134 participants in EBL2002 and EBL3001 stage 2, respectively). In the younger child subgroups of the Ebola vaccine group, the median age was 3.0 years in EBL2004 and 2.0 years in EBL3001 stage 2 and the percentage of females was 55% (60 of 110 participants) and 43% (54 of 126 participants), respectively.
Table 1.
Baseline demographic characteristics for adults in EBL2002, EBL2004,a and EBL3001 (per-protocol analysis set).
| Study | N | Age, years |
Sex, n (%)b |
Body-mass index, kg/m2 |
|||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Male | Female | Median (IQR) | ||||
| EBL2002 | Cohort 1 (healthy adults and elderly) | Vaccine | 137 | 31.0 (24.0; 42.0) | 89 (65) | 48 (35) | 22.6 (20.5; 25.4) |
| Burkina Faso | 40 | 29.0 (22.5; 38.5) | 27 (68) | 13 (33) | 21.4 (20.1; 24.9) | ||
| Côte d’Ivoire | 18 | 37.0 (33.0; 40.0) | 17 (94) | 1 (6) | 23.5 (22.1; 25.8) | ||
| Kenya | 20 | 29.0 (22.5; 48.0) | 9 (45) | 11 (55) | 22.5 (20.3; 27.6) | ||
| Uganda | 59 | 29.0 (23.0; 44.0) | 36 (61) | 23 (39) | 23.1 (21.0; 26.0) | ||
| Control | 24 | 30.0 (24.5; 34.0) | 15 (63) | 9 (38) | 21.1 (20.0; 24.5) | ||
| Burkina Faso | 6 | 30.5 (30.0; 31.0) | 4 (67) | 2 (33) | 20.7 (19.6; 22.0) | ||
| Côte d’Ivoire | 3 | 23.0 (23.0; 47.0) | 2 (67) | 1 (33) | 23.4 (19.3; 25.3) | ||
| Kenya | 5 | 22.0 (21.0; 31.0) | 3 (60) | 2 (40) | 20.3 (19.0; 20.7) | ||
| Uganda | 10 | 29.5 (27.0; 37.0) | 6 (60) | 4 (40) | 22.5 (20.8; 25.1) | ||
| EBL2002 | Cohort 2a (HIV-infected) | Vaccine | 59 | 39.0 (36.0; 44.0) | 17 (29) | 42 (71) | 23.7 (20.1; 27.5) |
| Control | 12 | 41.5 (38.0; 46.5) | 2 (17) | 10 (83) | 23.1 (21.8; 27.4) | ||
| EBL2004 | Vaccine | 256 | 28.0 (22; 41) | 136 (53) | 120 (47) | 22.6 (20.6; 26.5) | |
| Guinea | 87 | 30.0 (23.0; 44.0) | 35 (40) | 52 (60) | 23.7 (21.2; 28.5) | ||
| Liberia | 67 | 28.0 (21.0; 40.0) | 41 (61) | 26 (39) | 22.4 (20.6; 28.2) | ||
| Mali | 49 | 28.0 (22.0; 40.0) | 28 (57) | 21 (43) | 22.8 (20.3; 28.8) | ||
| Sierra Leone | 53 | 26.0 (19.0; 46.0) | 32 (60) | 21 (40) | 21.5 (20.3; 23.5) | ||
| Control | 122 | 29.0 (22; 40) | 64 (53) | 58 (48) | 22.6 (20.6; 25.6) | ||
| Guinea | 44 | 29.5 (23.0; 40.0) | 23 (52) | 21 (48) | 22.5 (20.5; 26.1) | ||
| Liberia | 33 | 24.0 (22.0; 34.0) | 16 (49) | 17 (52) | 23.3 (21.8; 24.6) | ||
| Mali | 25 | 31.0 (20.0; 40.0) | 11 (44) | 14 (56) | 22.9 (20.4; 30.3) | ||
| Sierra Leone | 20 | 30.5 (22.5; 45.5) | 14 (70) | 6 (30) | 21.7 (20.2; 24.1) | ||
| EBL3001 | Stage 1 | Vaccine | 43 | 23.0 (20.0; 27.0) | 42 (98) | 1 (2) | 21.9 (20.6; 22.9) |
| Stage 2 | Vaccine | 191 | 23.0 (21.0; 30.0) | 160 (84) | 31 (16) | 21.6 (19.8; 22.9) | |
| Control | 68 | 24.0 (20.5; 35.0) | 56 (82) | 12 (18) | 21.3 (20.1; 22.8) | ||
HIV; Human immunodeficiency virus, IQR; Interquartile range.
One participant in the per-protocol set is excluded from this table as the participant had data at month 1 but not at month 3 and month 12.
Percentages may not add up to 100 due to rounding.
Table 2.
Baseline demographic characteristics for children in EBL2002, EBL2004, and EBL3001 stage 2 (per-protocol analysis set).a
| Study | Adolescentsb |
Older childrenc |
Younger childrend |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age, years |
Sex, n (%)e |
N | Age, years |
Sex, n (%)e |
N | Age, years |
Sex, n (%)e |
||||
| Median (IQR) | Male | Female | Median (IQR) | Male | Female | Median (IQR) | Male | Female | ||||
| EBL2002 | ||||||||||||
| Vaccine | 53 | 14.0 (13.0; 15.0) | 29 (55) | 24 (45) | 54 | 8.0 (6.0; 10.0) | 26 (48) | 28 (52) | NA | NA | NA | NA |
| Burkina Faso | 29 | 14.0 (13.0; 16.0) | 19 (66) | 10 (34) | 23 | 8.0 (6.0; 9.0) | 11 (48) | 12 (52) | NA | NA | NA | NA |
| Côte d’Ivoire | 0 | – | – | – | 12 | 6.5 (4.0; 8.0) | 7 (58) | 5 (42) | NA | NA | NA | NA |
| Kenya | 12 | 13.5 (13.0; 15.0) | 5 (42) | 7 (58) | 5 | 8.0 (7.0; 10.0) | 2 (40) | 3 (60) | NA | NA | NA | NA |
| Uganda | 12 | 13.5 (12.5; 14.5) | 5 (42) | 7 (58) | 14 | 10.0 (6.0; 10.0) | 6 (43) | 8 (57) | NA | NA | NA | NA |
| Control | 10 | 14.0 (12.0; 16.0) | 6 (60) | 4 (40) | 11 | 7.0 (6.0; 9.0) | 6 (55) | 5 (45) | NA | NA | NA | NA |
| Burkina Faso | 4 | 16.0 (15.0; 16.5) | 3 (75) | 1 (25) | 4 | 7.5 (7.0; 8.0) | 2 (50) | 2 (50) | NA | NA | NA | NA |
| Côte d’Ivoire | 0 | – | – | – | 2 | 6.0 (5.0; 7.0) | 1 (50) | 1 (50) | NA | NA | NA | NA |
| Kenya | 2 | 12.0 (12.0; 12.0) | 0 | 2 (100) | 1 | 9.0 (9.0; 9.0) | 1 (100) | 0 | NA | NA | NA | NA |
| Uganda | 4 | 14.0 (13.0; 14.5) | 3 (75) | 1 (25) | 4 | 8.0 (5.5; 10.5) | 2 (50) | 2 (50) | NA | NA | NA | NA |
| EBL2004 | ||||||||||||
| Vaccine | 132 | 14.0 (13; 16) | 80 (61) | 52 (39) | 112 | 8.0 (6; 9) | 58 (52) | 54 (48) | 110 | 3.0 (2; 4) | 50 (46) | 60 (55) |
| Guinea | 42 | 14.5 (13.0; 16.0) | 25 (60) | 17 (41) | 53 | 7.0 (6.0; 9.0) | 27 (51) | 26 (49) | 57 | 3.0 (2.0; 3.0) | 26 (46) | 31 (54) |
| Liberia | 24 | 13.5 (12.0; 15.0) | 14 (58) | 10 (42) | 14 | 7.5 (5.0; 8.0) | 6 (43) | 8 (57) | 5 | 1.0 (1.0; 3.0) | 1 (20) | 4 (80) |
| Mali | 22 | 15.0 (13.0; 16.0) | 11 (50) | 11 (50) | 20 | 8.0 (6.5; 10.0) | 14 (70) | 6 (30) | 38 | 3.0 (2.0; 4.0) | 19 (50) | 19 (50) |
| Sierra Leone | 44 | 14.0 (13.0; 16.0) | 30 (68) | 14 (32) | 25 | 9.0 (7.0; 10.0) | 11 (44) | 14 (56) | 10 | 3.0 (2.0; 4.0) | 4 (40) | 6 (60) |
| Control | 55 | 15.0 (13; 16) | 30 (55) | 25 (46) | 54 | 8.0 (6; 9) | 30 (56) | 24 (44) | 49 | 3.0 (2; 4) | 23 (47) | 26 (53) |
| Guinea | 24 | 15.0 (13.0; 15.0) | 12 (50) | 12 (50) | 20 | 7.0 (6.0; 9.0) | 11 (55) | 9 (45) | 24 | 2.0 (1.5; 4.0) | 12 (50) | 12 (50) |
| Liberia | 8 | 15.0 (13.5; 15.5) | 6 (75) | 2 (25) | 9 | 8.0 (5.0; 9.0) | 6 (67) | 3 (33) | 1 | 2.0 (2.0; 2.0) | 0 | 1 (100) |
| Mali | 7 | 13.0 (12.0; 15.0) | 2 (29) | 5 (71) | 9 | 9.0 (8.0; 10.0) | 3 (33) | 6 (67) | 18 | 3.0 (2.0; 3.0) | 7 (39) | 11 (61) |
| Sierra Leone | 16 | 15.5 (13.0; 16.5) | 10 (63) | 6 (38) | 16 | 7.0 (6.0; 9.0) | 10 (63) | 6 (38) | 6 | 2.5 (2.0; 3.0) | 4 (67) | 2 (33) |
| EBL3001 stage 2 | ||||||||||||
| Vaccine | 142 | 14.0 (13.0; 16.0) | 74 (52) | 68 (48) | 134 | 8.0 (7.0; 9.0) | 64 (48) | 70 (52) | 126 | 2.0 (1.0; 3.0) | 72 (57) | 54 (43) |
| Control | 46 | 13.5 (13.0; 15.0) | 25 (54) | 21 (46) | 45 | 8.0 (7.0; 9.0) | 20 (44) | 25 (56) | 41 | 2.0 (1.0; 3.0) | 24 (59) | 17 (41) |
IQR; Interquartile range, NA; Not applicable.
Body-mass index was not included as it is not a reliable reflection of growth index in children below 12 years of age.
Participants aged 12–17 years in EBL2002, EBL2004, and EBL3001 stage 2.
Participants aged 4–11 years in EBL2002 and EBL3001 stage 2 and 5–11 years in EBL2004.
Participants aged 1–4 years in EBL2004 and 1–3 years in EBL3001 stage 2.
Percentages may not add up to 100 due to rounding.
Immunogenicity
The validated FANG ELISA, analysed by Q2 Solutions, was used to assess the EBOV GP-binding antibody response to Ad26.ZEBOV, MVA-BN-Filo for all three studies. Results presented in the sections that follow focus on the 56-day interval regimen (ie, dose 1 and dose 2 administered 56 days apart). Results for the other interval regimens in EBL2002 are presented in the original publications.10,11 In all studies, the highest immune responses were detected with the FANG ELISA a few weeks after administration of dose 2 (sampling at 21 days post-dose 2 in EBL2002 and EBL3001 and at 28 days post-dose 2 in EBL2004).
Results for control group GMCs10,13 and country-specific GMCs from EBL200210 and EBL2004 in adults are provided in Supplementary Table S3. Results for control group GMCs11,14 and country- and age group-specific GMCs in paediatric participants are presented in Supplementary Table S4.
EBOV GP-binding antibody response in adults over time
EBOV GP-binding antibody GMCs at baseline in adults
EBOV GP-binding antibody GMCs at baseline across all studies and countries were low to undetectable (supplementary results, Supplementary Table S3).
EBOV GP-binding antibody GMCs at 21 or 28 days post-dose 2 in adults
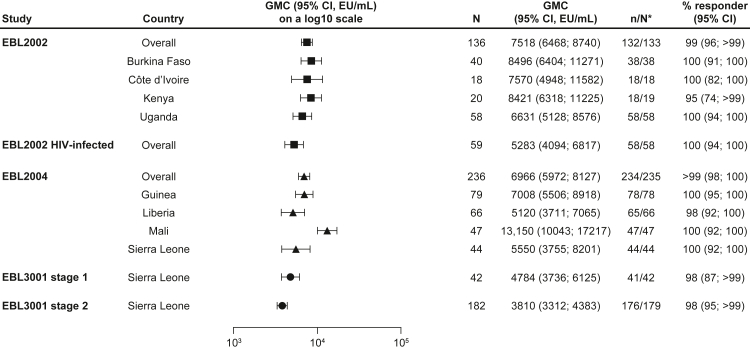
The overall GMC for the 56-day regimen at 21 days post-dose 2 in EBL2002 was 7518 EU/mL (95% CI: 6468–8740; percent responders: 99%) in healthy adults and 5283 EU/mL (4094–6817; 100%) in HIV-infected adults (Fig. 2, Supplementary Figure S1, Supplementary Table S3).10 The overall GMC at 28 days post-dose 2 in EBL2004 was 6966 EU/mL (5972–8127; >99%). For EBL3001, the overall GMCs at 21 days post-dose 2 were 4784 EU/mL (3736–6125; 98%) and 3810 EU/mL (3312–4383; 98%) in adults in stages 1 and 2, respectively.13
Fig. 2.
EBOV GP-binding antibody GMCs in adult participants at 21 or 28 days post-dose 2, stratified by country. EBOV GP-binding antibody GMCs at 21 or 28 days post-dose 2 are presented overall for adults who received the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen in EBL2002 (n = 136), HIV-infected adults in EBL2002 (n = 59), adults in EBL2004 (n = 236), and adults in EBL 3001 stage 1 (n = 42) and stage 2 (n = 182), as well as by country in each study. Samples were analysed according to Q2 Solution's FANG ELISA standard operating procedure, and a single reportable value for each participant sample at each timepoint was uploaded for statistical analysis. Error bars represent 95% CIs. CI; Confidence interval. EBOV GP; Ebola virus glycoprotein. ELISA; Enzyme-linked immunosorbent assay. EU; ELISA unit. FANG; Filovirus Animal Nonclinical Group. GMC; Geometric mean concentration. ∗N is the number of participants with data at baseline and at 21 days (EBL2002/EBL3001) or 28 days (EBL2004) post-dose 2.
When results were stratified by country across studies, the 95% CI of participants from Mali was completely above the 95% CIs of participants from Guinea, Liberia, Sierra Leone (in studies EBL2004 and EBL3001 stages 1 and 2), and Uganda (Supplementary Table S3, Fig. 2).
EBOV GP-binding antibody GMCs at month 12 in adults
In EBL2002 at month 12, the overall GMC for the 56-day regimen was 342 EU/mL (95% CI: 291–401; percent responders: 78%) in healthy adults and 338 EU/mL (253–450; 88%) in HIV-infected adults (Supplementary Figure S2, Supplementary Table S3).10 At month 12 in EBL2004, the overall GMC was 437 EU/mL (352–542; 80%). For EBL3001, the 12-month overall GMC was 325 EU/mL (238–445; 77%) and 259 EU/mL (223–301; 49%) for stages 1 and 2, respectively.13
When assessing non-overlapping 95% CIs across studies, the overall response at month 12 in EBL2004 was higher than the response in EBL3001 stage 2, but not stage 1. When results were stratified by country at month 12, the 95% CI in participants from Mali was above the 95% CIs in participants from Sierra Leone in EBL3001 stage 2 and Uganda (Supplementary Figure S2, Supplementary Table S3).
EBOV GP-binding antibody response in paediatric participants over time
EBOV GP-binding antibody GMCs at baseline in paediatric participants
EBOV GP-binding antibody GMCs at baseline across all studies, age groups, and countries were low to undetectable (supplementary results, Supplementary Table S4).
EBOV GP-binding antibody GMCs at 21 or 28 days post-dose 2 in paediatric participants
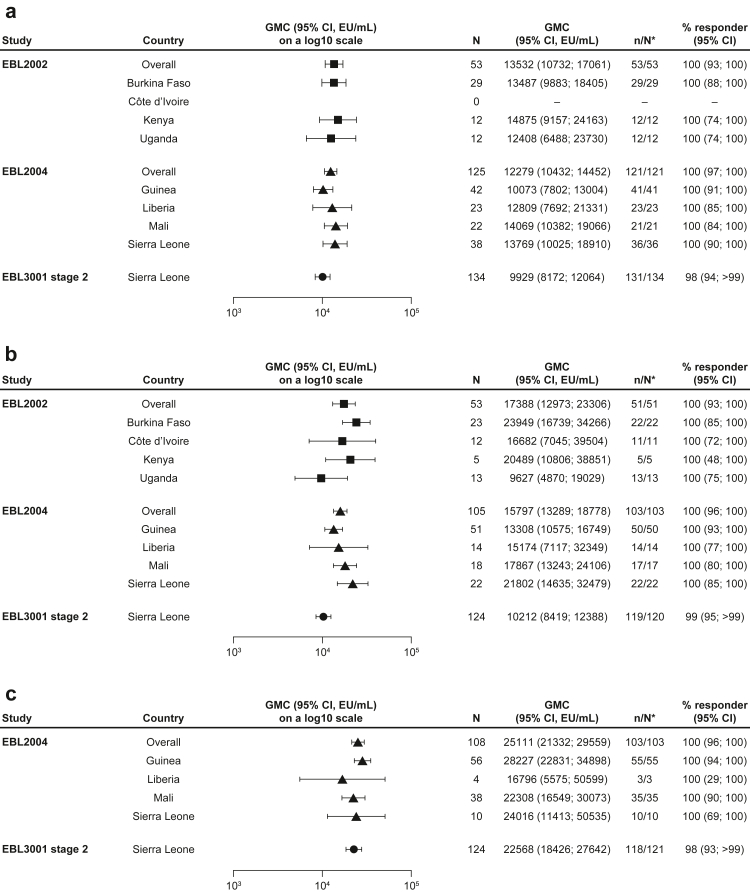
In EBL2002, the overall GMC at 21 days post-dose 2 for the 56-day regimen was 13,532 EU/mL (95% CI: 10,732–17061; percent responders: 100%) in adolescents and 17,388 EU/mL (12,973–23306; 100%) in older children (Fig. 3a and b, Supplementary Figure S3a and b, Supplementary Table S4).11
Fig. 3.
EBOV-GP binding antibody GMCs in (a) adolescents, (b) older children, and (c) younger children at 21 or 28 days post-dose 2, stratified by country. EBOV GP-binding antibody GMCs at 21 or 28 days post-dose 2 are presented overall for paediatric participants who received the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen, including adolescents aged 12–17 years in EBL2002 (n = 53), EBL2004 (n = 125), and EBL3001 stage 2 (n = 134); older children aged 4–11 years in EBL2002 (n = 53) and EBL3001 stage 2 (n = 124) and 5–11 years in EBL2004 (n = 105); and younger children aged 1–4 years EBL2004 (n = 108) and 1–3 years in EBL3001 stage 2 (n = 124). GMCs are also shown by country for each study. Samples were analysed according to Q2 Solution's FANG ELISA standard operating procedure, and a single reportable value for each participant sample at each timepoint was uploaded for statistical analysis. Error bars represent 95% CIs. CI; Confidence interval. EBOV GP; Ebola virus glycoprotein. ELISA; Enzyme-linked immunosorbent assay. EU; ELISA unit. FANG; Filovirus Animal Nonclinical Group. GMC; Geometric mean concentration. ∗N is the number of participants with data at baseline and at 21 days (EBL2002/EBL3001) or 28 days (EBL2004) post-dose 2.
The overall GMC at 28 days post-dose 2 in EBL2004 was 12,279 EU/mL (95% CI: 10,432–14452; percent responders: 100%) in adolescents, 15,797 EU/mL (13,289–18778; 100%) in older children, and 25,111 EU/mL (21,332–29559; 100%) in younger children (Fig. 3a–c, Supplementary Figure S3a–c, Supplementary Table S4).
In EBL3001 stage 2, the overall GMC at 21 days post-dose 2 was 9929 EU/mL (95% CI: 8172–12064; percent responders: 98%) in adolescents, 10,212 EU/mL (8419–12388; 99%) in older children, and 22,568 EU/mL (18,426–27642; 98%) in younger children.14
No notable differences in binding antibody GMCs at 21 or 28 days post-dose 2 were observed between the three age groups across studies (Fig. 3a–c, Supplementary Figure S3a–c, Supplementary Table S4). When results from the three studies were stratified by both age group and country, responses in older children in Burkina Faso and older children in Sierra Leone in EBL2004 were higher than the response in older children in Sierra Leone in EBL3001 stage 2, based on non-overlapping 95% CIs (Fig. 3, Supplementary Table S4). Of note, the number of participants per group was often <10 when paediatric participants were stratified by both age group and country at all timepoints.
EBOV GP-binding antibody GMCs at month 12 in paediatric participants
The overall binding antibody GMC at 12 months post-dose 1 in EBL2002 was 541 EU/mL (95% CI: 433–678; percent responders: 90%) for adolescents and 637 EU/mL (529–767; 98%) for older children (Supplementary Figure S4a and b, Supplementary Table S4).11
At month 12, the overall GMC in EBL2004 was 731 EU/mL (95% CI: 589–907; percent responders: 77%) in adolescents, 739 EU/mL (585–933; 94%) in older children, and 1139 EU/mL (905–1432; 100%) in younger children (Supplementary Figure S4a–c, Supplementary Table S4). Data were not available at this timepoint from Liberia.
In EBL3001 stage 2, the overall binding antibody GMC at month 12 was 386 EU/mL (95% CI: 326–457; percent responders: 70%) in adolescents, 436 EU/mL (375–506; 71%) in older children, and 750 EU/mL (629–894; 96%) in younger children.14
When the paediatric month 12 GMCs observed across studies were stratified by age group, the 95% CI of older children in EBL2002 was higher than the 95% CI of older children in EBL3001 stage 2 (Supplementary Figure S4b, Supplementary Table S4). Additionally, responses in EBL2004 were higher than responses in EBL3001 stage 2 in all three age groups, based on non-overlapping 95% CIs (Supplementary Figure S4a–c, Supplementary Table S4).
When results from the three studies were stratified by both age group and country, the 95% CIs of adolescents in Guinea and Sierra Leone in EBL2004 were higher than the 95% CI of adolescents in Sierra Leone in EBL3001 stage 2 (Supplementary Figure S4a, Supplementary Table S4). For older children, the 95% CIs in Burkina Faso, Kenya, Guinea, and Sierra Leone in EBL2004 were higher than the 95% CI in Sierra Leone in EBL3001 stage 2 (Supplementary Figure S4b, Supplementary Table S4). In younger children, the 95% CI in Mali was higher than the 95% CI in Sierra Leone in EBL3001 stage 2 (Supplementary Figure S4c, Supplementary Table S4).
EBOV GP-binding antibody GMCs after accounting for between-study variation
To estimate how much of the total variation in immune response was attributable to the studies, the ICC for adult and paediatric participants at 21 or 28 days post-dose 2 was calculated. The ICCs were 10% and 3% for adult and paediatric participants, respectively (Supplementary Tables S5 and S6). This indicates that 10% of the variability in the observed EBOV GP-binding antibodies of adult participants was accounted for by the studies and 90% of the variability was accounted for by the individual participants. Similarly, 3% of the variability in the paediatric cohorts was accounted for by the studies. The models adequately fit the data (Supplementary Figures S5 and S6). After accounting for between-study variation, the overall GMC (95% CI) was 5914 EU/mL (2503–13976) for adults (Table 3), and 11,760 EU/mL (7827–17668), 14,557 EU/mL (9688–21872), and 25,767 EU/mL (16,381–40528) for adolescents, older children, and younger children, respectively (Table 4). Controlling for baseline characteristics (adults: age and sex; adolescents and children: sex) reduced the variability attributable to individual participants by at most 6% (supplementary results, Supplementary Tables S1 and S2).
Table 3.
EBOV GP-binding antibody GMCs in adult participants at 21 or 28 days post-dose 2, accounting for between-study variations (random intercept model).
| Study | GMC, EU/mL | 95% CI |
|---|---|---|
| Overall | 5914 | 2503; 13,976 |
| EBL2002 | 7434 | 6410; 8621 |
| EBL2004 | 6910 | 5939; 8041 |
| EBL3001 stages 1 and 2 | 4027 | 3567; 4516 |
CI; Confidence interval, EBOV GP; Ebola virus glycoprotein, EU; Enzyme-linked immunosorbent assay unit, GMC; Geometric mean concentration.
Table 4.
EBOV GP-binding antibody GMCs in paediatric participants at 21 or 28 days post-dose 2, accounting for between-study variations (random intercept and random slope model).
| Age category | Study | GMC, EU/mL | 95% CI |
|---|---|---|---|
| Adolescentsa | Overall | 11,760 | 7827; 17,668 |
| EBL2002 | 13,307 | 10,824; 16,360 | |
| EBL2004 | 12,456 | 10,825; 14,332 | |
| EBL3001 stage 2 | 9812 | 8346; 11,535 | |
| Older childrenb | Overall | 14,557 | 9688; 21,872 |
| EBL2002 | 16,626 | 13,523; 20,440 | |
| EBL2004 | 16,331 | 14,251; 18,714 | |
| EBL3001 stage 2 | 11,361 | 9623; 13,412 | |
| Younger childrenc | Overall | 25,767 | 16,381; 40,528 |
| EBL2004 | 27,093 | 22,772; 32,234 | |
| EBL3001 stage 2 | 21,776 | 18,313; 25,892 |
CI; Confidence interval, EBOV GP; Ebola virus glycoprotein, EU; Enzyme-linked immunosorbent assay unit, GMC; Geometric mean concentration.
Participants aged 12–17 years in EBL2002, EBL2004, and EBL3001 stage 2.
Participants aged 4–11 years in EBL2002 and EBL3001 stage 2 and 5–11 years in EBL2004.
Participants aged 1–4 years in EBL2004 and 1–3 years in EBL3001 stage 2.
Discussion
In this analysis, data from three clinical studies, conducted in eight sub-Saharan African countries, were included to examine immunogenicity of the heterologous two-dose Ad26.ZEBOV, MVA-BN-Filo regimen administered in a 56-day interval to adults and children (≥1 year old), stratified by country. Overall, the results suggest that the immune response was greater in younger children than in older age groups and that the immune response decreased by 12 months. In addition, participants from Mali had the highest responses compared with participants from other countries, while participants from Sierra Leone in EBL3001 had the lowest responses. Safety and reactogenicity of the Ad26.ZEBOV, MVA-BN-Filo regimen were reported previously10, 11, 12, 13, 14 and these studies confirm that the regimen induces robust humoral immune responses and is well tolerated in both adults and children in this population.
At 21 or 28 days post-dose 2, GMCs in adults were comparable overall, although responses were higher in participants in Mali in EBL2004 than those in Liberia in EBL2004, Sierra Leone in both EBL2004 and EBL3001, and Uganda in EBL2002. Responder rates at this timepoint did not differ across African countries (percent responder range: 95%–100%). Binding antibody GMCs in HIV-infected adults were similar to the overall study population at 21 days post-dose 2 and the responder rate was 100%, confirming that the vaccine regimen is immunogenic in this population.
Similar results were observed in adults at month 12; binding antibody GMCs in EBL2002, EBL2004, and EBL3001 were generally comparable, with higher responses in participants in Mali in EBL2004 than in Sierra Leone in EBL3001 and Uganda in EBL2002. Responder rates across countries in adults at month 12 ranged from 49% to 88%. Similar results were also observed in HIV-infected adults at month 12, with binding antibody GMCs similar to the overall study population.
Paediatric responses tended to be numerically higher than adult responses, consistent with previous reports.11,14 GMCs at 21 or 28 days post-dose 2 in children were generally comparable across countries, although responses in older children in Burkina Faso in EBL2002 and Sierra Leone in EBL2004 were higher than those in older children in Sierra Leone in EBL3001 stage 2. However, these results should be interpreted with caution given the small sample sizes (often <10) when paediatric participants are stratified by both country and age group.
Differences in paediatric GMCs at month 12 were more obvious than at 21 or 28 days post-dose 2. The 95% CIs in adolescents from Guinea and Sierra Leone in EBL2004; older children from Burkina Faso and Kenya in EBL2002 and Guinea, and Sierra Leone in EBL2004; and younger children in Mali in EBL2004 did not overlap those in the corresponding age groups from Sierra Leone in EBL3001 stage 2. However, these results should also be interpreted with caution given the small sample sizes.
Immune response to the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen did wane after 12 months in both adult and paediatric participants, but this does not necessarily mean that a booster dose is needed. Studies EBL2002 and EBL3001 administered an Ad26.ZEBOV booster dose at one and two years post-dose 1, respectively, in adults and a rapid and robust anamnestic response was observed in both studies.10,13 Likewise, results from a study in children aged 4–15 years who had received the Ad26.ZEBOV, MVA-BN-Filo regimen >3 years earlier16 showed that an Ad26.ZEBOV booster vaccination elicits a rapid and robust anamnestic response. An Ad26.ZEBOV booster may be an effective strategy in preventing EVD in previously vaccinated individuals at imminent risk of infection.16
The strength of the current analysis is that it included data from several sub-Saharan African countries and provided a detailed overview of immune responses across the region. Furthermore, the variation in GMC responses in adult and paediatric participants was shown to be largely due to participant characteristics rather than between-study variance. A limitation of the analysis is that no concrete conclusions can be made on whether there were differences in response by country or study. This is due to the small sample size of the subgroups when participants were stratified by study, country, and age group. In addition, the study timeframes and analysis of samples occurred in different years. Although samples were analysed by the same laboratory in all studies, and the laboratory performed stability monitoring of the FANG ELISA assay, it is possible that laboratory drift may have occurred over time and responses could vary over time.
Another limitation is that many factors (eg, socioeconomic status, pathogen exposure) that could be associated with the observed differences in responses among countries were not available to aid further evaluation. Other intramuscularly administered viral vaccines have shown varying immune responses across sub-Saharan African countries. Lower vaccine immunogenicity has been described for a measles vaccine in Kenya and Mozambique compared with more developed countries, while lymph node inflammation and fibrosis resulting in T follicular helper cell loss was associated with blunted vaccine-induced antibody responses to a yellow fever vaccine in Uganda.17, 18, 19, 20
Factors that could potentially be involved in geographic differences in binding antibody response across African countries are numerous. Participants from rural settings are more likely to have a lower overall health status,21 as demonstrated by low body-mass index, poor nutritional status, low life expectancy, and high under-5-year mortality rates, which can impact immune response to vaccination.22,23 Concomitant disease burdens, such as acute respiratory diseases, helminth infections, and malaria, vary with geographic location and can modulate the immune system towards a type 2 helper T cell and regulatory T cell immune response,24,25 resulting in lower response to vaccine antigens.24,26 As concomitant parasitic disease burdens are generally lower in younger children, likely because they have had less time to be exposed, this may have partly contributed to the higher EBOV GP-binding antibody responses observed in younger participants in this analysis across the African countries. Other factors known to affect vaccine immunogenicity, such as micronutrient deficiencies (particularly vitamin A and zinc) and genetic variability, may also play a role in geographic differences in immune response to vaccination.27, 28, 29
Immune responses to the Ad26.ZEBOV, MVA-BN-Filo regimen were lower in participants from Sierra Leone in EBL3001 compared with participants from other studies which may be due, in part, to regional differences in factors that can affect the immune system. Sierra Leone has a high concomitant parasitic disease burden and is affected by high rates of chronic malnutrition and maternal and infant mortality, which are indicative of a poor overall health status among this population that may impact immune responses.30,31 Furthermore, the EBL3001 study sites were located in Kambia town and Rokupr village, both in the rural Kambia District of Sierra Leone. In contrast, the study sites in Mali, where immune responses in adults were the highest, were located in Bamako, Mali's capital and largest city. The study sites in Burkina Faso and Kenya, which had the second and third highest immune responses at 21 days post-dose 2, were also located in large cities. Interestingly, the immune response observed in participants from Mambolo, also located in the rural Kambia District of Sierra Leone, in EBL2004 was generally higher than that observed in participants from Kambia town and Rokupr in EBL3001. It is also possible that genetic polymorphisms in these populations could result in lower or higher post-vaccination immune responses. However, these potential reasons have not been formally studied, and further research is needed to determine whether any of these factors contributed to the differences in the immune response across countries.
Because of the myriad factors with a potential impact on immunogenicity, use of a single validated assay, run in a single accredited laboratory, is of utmost importance in minimising additional variables that could influence results. The assay validation process includes assessment of various assay performance parameters, including dilutional linearity, intermediate precision and repeatability, limit of detection, limit of quantification, selectivity/matrix effects, and interference of serum proteins. After validation, the stability of assay performance is monitored to ensure that FANG ELISA results from a single laboratory are stable and comparable over time. However, validation in a single laboratory does not ensure the results from the same assay will be comparable when performed in different laboratories. Indeed, experience indicates that even if stable and reproducible within one laboratory, FANG ELISA results from different laboratories may not be comparable.12 Even results between two accredited laboratories cannot be directly compared until an inter-lab comparability assessment has been performed. An inter-lab comparability assessment, performed to determine whether FANG ELISA results produced at the BBRC and Q2 Solutions accredited laboratories were comparable, indicated that results from Q2 Solutions trended lower than those from BBRC (unpublished). Therefore, the only way to eliminate inter-lab variability and to maximise comparability of FANG ELISA results is to employ a one-assay, one-lab strategy.
In conclusion, while vaccine-induced EBOV GP-binding antibody responses showed variation across geographic locations assessed in studies EBL2002, EBL2004, and EBL3001, the two-dose heterologous Ad26.ZEBOV, MVA-BN-Filo vaccine regimen induced a strong humoral response, with ≥95% of participants from all eight African countries classified as responders at 21 or 28 days post-dose 2 (regimen completion), regardless of age group. Binding antibodies persisted up to at least 12 months after administration of dose 1. These results demonstrate that the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen induces immune responses against EBOV in adults and children that persist for at least one year.
Contributors
CM contributed to data collection (EBL2004), conception and design of the pooled analysis, data interpretation and presentation, and the manuscript first draft. HB contributed to the conduct and data collection of the EBL2002 study, had full access to the data, and reviewed the data and first and final versions of the manuscript. M Kieh and SBK contributed to the development of the EBL2004/PREVAC study protocol, scientifically and administratively managed the implementation of the EBL2004/PREVAC study at one of the clinical trial sites, participated in the conceptualisation of the manuscript and review of the data, provided input on the various versions of the draft manuscript, and reviewed and approved the final manuscript. ZA contributed to data collection (EBL2002); had full access to, reviewed, and interpreted the data; and reviewed and approved the final version of the manuscript. BTR contributed to the data collection of the Sierra Leone EBOVAC vaccine studies, had access to the data, and reviewed the final versions of both adult and paediatric safety and immunogenicity studies. SD contributed to the design and conduct of the studies in Mali and reviewed the data and the draft manuscript. SBS contributed to the design and conduct of the Burkina Faso studies, which contributed to this analysis; reviewed the data and had full access to the data; and reviewed the final version of the manuscript. AS-B contributed to the conduct and data collection of the Sierra Leonean studies. AHB, MS, and SS contributed to the design, conduct, and data collection for the EBL2002, EBL3001, and EBL2004 studies. AG contributed to the conception and design of the statistical analysis, data interpretation and presentation, and the manuscript first draft. M Katwere contributed to data collection (EBL2002), data interpretation and presentation, and manuscript drafting and review. JH contributed to the data collection, interpretation, and presentation, and reviewed and approved the final version of the manuscript. BK contributed to the conduct and data analysis of EBL2004 and EBL3001, conception and design of the pooled analysis, data interpretation and presentation, manuscript first draft, and manuscript review. SE contributed to the design and conduct of the Ivorian studies, which contributed to this analysis; had full access to the data; and reviewed the final version of the manuscript. HK contributed to the conduct of EBL2002, which contributed to the analysis; had full access to the data; and reviewed the final version of the manuscript. OA contributed to the collection of primary data and reviewed the manuscript prior to submission. EDO contributed to the design, conduct, and data collection for the EBL2004 study, contributed to the manuscript first draft, and reviewed the final version of the manuscript. BL was the site principal investigator of the EBL3001 study and reviewed the data and the final version of the manuscript. RT contributed to design and conduct of EBL2002 as EBOVAC2 coordinator, data interpretation, and the manuscript. BG and DW-J contributed to the design and conduct of the Sierra Leonean studies and to the EBL2004/PREVAC study which contributed to this analysis; reviewed the data; had full access to the data; and reviewed the final version of the manuscript. MD and KL contributed to the design and conduct of the EBL2002, EBL3001, and EBL2004 studies; and data interpretation and presentation. CR contributed to the individual study conception and conduct, data analysis and interpretation, and manuscript preparation. All authors had full access to the data and approved the final draft for publication. CM, HB, ZA, AG, and DW-J verified the underlying data.
Data sharing statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Declaration of interests
CM, AG, JH, BK, MD, KL, and CR were full-time employees of Janssen Pharmaceuticals at the time of the study and report stock or stock options in Janssen Pharmaceuticals. HB's institution has received funding from the Innovative Medicines Initiative to conduct the EBL2002 study. M Katwere was a full-time employee of Janssen Pharmaceuticals at the time of the study. SE's institution has received funding from Inserm France to conduct the EBL2002 study. BL's institution has received funding from the Innovative Medicines Initiative to conduct the EBL3005 study. DW-J is a consortia partner with Janssen Pharmaceuticals on EBOVAC1 and EBOVAC3 projects, has received Ad26.MVA Ebola vaccines donated by Janssen Pharmaceuticals for clinical trials under EBOVAC1 and EBOVAC3 projects, and her institution has received funding from the Innovative Medicines Initiative to conduct the EBL3005 study. M Kieh, ZA, BTR, SD, SBS, AS-B, AHB, HK, SBK, OA, MS, EDO, SS, RT, and BG declare no competing interests.
Acknowledgements
The authors thank the EBL2002, EBL2004/PREVAC, and EBL3001 study teams and the participants who consented to the trials. We are grateful to the Ministries of Health of Burkina Faso, Côte d’Ivoire, Guinea, Kenya, Liberia, Mali, Sierra Leone, and Uganda who permitted the conduct of the trials, and we furthermore thank The Alliance for International Medical Action (ALIMA) and all site collaborators for their contribution in the implementation of this EBL2004/PREVAC trial. Medical writing and editorial support were provided by Kimberly Fuller, PhD, and Courtney St. Amour, PhD, of Lumanity Communications Inc., and were funded by Janssen Vaccines & Prevention BV. Publication coordination was provided by Sónia Silva and Valérie Oriol Mathieu (Janssen Vaccines & Prevention, Leiden, The Netherlands).
The EBL2002 and EBL3001 studies received funding from Janssen Vaccines & Prevention BV and the Innovative Medicines Initiative 2 (www.imi.europa.eu) Joint Undertaking (IMI2JU) under grant agreements No 115854 (EBOVAC1) and No 115861 (EBOVAC2). These Joint Undertakings receive support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Association (EFPIA). The dissemination represents only the authors' views and IMI2JU is not responsible for any use of the information contained in the dissemination. Janssen Vaccines & Prevention BV provided the vaccines for the conduct of EBL2002 and EBL3001.
The EBL2004/PREVAC research was supported in part by the National Institutes of Health (NIH), Institut national de la santé et de la recherche médicale (Inserm), and by the London School of Hygiene and Tropical Medicine (LSHTM) through funding from the IMI2JU under grant agreement No 115854 (EBOVAC1). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. The dissemination of this project represents only the authors' views and IMI2JU is not responsible for any use of the information contained in the dissemination. The clinical trial was conducted with the support of Janssen, Bavarian Nordic, and Merck Sharp & Dohme Corp., which provided the vaccines according to EBOVAC1 grant agreement. Funding was also provided in part by the National Cancer Institute (NCI) contract HHSN261201500003I through the Frederick National Laboratory for Cancer Research. The project has been funded by a dedicated Inserm allocation on behalf of the Minister of Higher Education, Research, and Innovation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104562.
Appendix A. Supplementary data
References
- 1.Centers for Disease Control and Prevention . 1976. Ebola virus disease distribution map: cases of Ebola virus disease in Africa since.https://www.cdc.gov/vhf/ebola/history/distribution-map.html [Google Scholar]
- 2.World Health Organization Ebola virus disease. https://www.who.int/health-topics/ebola#tab=tab_1
- 3.World Health Organization Ebola virus disease - democratic republic of the Congo. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON398
- 4.World Health Organization Ebola virus disease – democratic republic of the Congo. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON404
- 5.World Health Organization Ebola disease caused by Sudan ebolavirus – Uganda. 2023. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON433
- 6.US Food and Drug Administration Surrogate endpoint resources for drug and biologic development. 2018. https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development
- 7.European Medicines Agency Guideline clinical evaluation of vaccines. 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-clinical-evaluation-vaccines-revision-1_en.pdf
- 8.Roozendaal R., Hendriks J., van Effelterre T., et al. Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate. NPJ Vaccines. 2020;5:112. doi: 10.1038/s41541-020-00261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudge T.L., Jr., Sankovich K.A., Niemuth N.A., et al. Development, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry H., Mutua G., Kibuuka H., et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in healthy and HIV-infected adults: a randomised, placebo-controlled phase II clinical trial in Africa. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anywaine Z., Barry H., Anzal O., et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in children and adolescents in Africa: a randomised, placebo-controlled, multicentre phase II clinical trial. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PREVAC Study Team. Kieh M., Richert L., et al. Randomized trial of vaccines for Zaire Ebola virus disease. N Engl J Med. 2022;387:2411–2424. doi: 10.1056/NEJMoa2200072. https://www.nejm.org/doi/full/10.1056/NEJMoa2200072 [DOI] [PubMed] [Google Scholar]
- 13.Ishola D., Manno D., Afolabi M.O., et al. Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial. Lancet Infect Dis. 2022;22:97–109. doi: 10.1016/S1473-3099(21)00125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afolabi M.O., Ishola D., Manno D., et al. Safety and immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in children in Sierra Leone: a randomised, double-blind, controlled trial. Lancet Infect Dis. 2022;22:110–122. doi: 10.1016/S1473-3099(21)00128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badio M., Lhomme E., Kieh M., et al. Partnership for Research on Ebola VACcination (PREVAC): protocol of a randomized, double-blind, placebo-controlled phase 2 clinical trial evaluating three vaccine strategies against Ebola in healthy volunteers in four West African countries. Trials. 2021;22:86. doi: 10.1186/s13063-021-05035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manno D., Bangura A., Baiden F., et al. Safety and immunogenicity of an Ad26.ZEBOV booster dose in children previously vaccinated with the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen: an open-label, non-randomised, phase 2 trial. Lancet Infect Dis. 2023;23:352–360. doi: 10.1016/S1473-3099(22)00594-1. [DOI] [PubMed] [Google Scholar]
- 17.Clifford H.D., Hayden C.M., Khoo S.K., et al. Genetic variants in the IL-4/IL-13 pathway influence measles vaccine responses and vaccine failure in children from Mozambique. Viral Immunol. 2017;30:472–478. doi: 10.1089/vim.2017.0014. [DOI] [PubMed] [Google Scholar]
- 18.Kaan J.A., van Vlokhoven P.C., Schneeberger P.M., Nijhof W. Immunogenicity of measles vaccine from a hospital based and outreach programme in rural Kenya. Trop Doct. 1992;22:30–32. doi: 10.1177/004947559202200110. [DOI] [PubMed] [Google Scholar]
- 19.Kityo C., Makamdop K.N., Rothenberger M., et al. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J Clin Invest. 2018;128:2763–2773. doi: 10.1172/JCI97377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muyanja E., Ssemaganda A., Ngauv P., et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest. 2014;124:3147–3158. doi: 10.1172/JCI75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strasser R., Kam S.M., Regalado S.M. Rural health care access and policy in developing countries. Annu Rev Public Health. 2016;37:395–412. doi: 10.1146/annurev-publhealth-032315-021507. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization Regional Office for Africa The state of health in the WHO African Region: an analysis of the status of health, health services and health systems in the context of the Sustainable Development Goals. 2018. https://apps.who.int/iris/bitstream/handle/10665/275292/9789290234098-eng.pdf?sequence=1&isAllowed=y
- 23.World Health Organization Regional Office for Africa Nutrition in the WHO African region. 2017. https://www.afro.who.int/publications/nutrition-who-african-region
- 24.Salgame P., Yap G.S., Gause W.C. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishola D. EBOVAC-Salone Malaria Infection (MALI) Sub-Study Team. Asymptomatic malaria infection and the immune response to the two-dose Ad26.ZEBOV, MVA-BN-Filo Ebola vaccine regimen in adults and children. Clin Infect Dis. 2022;75:1585–1593. doi: 10.1093/cid/ciac209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labeaud A.D., Malhotra I., King M.J., King C.L., King C.H. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mentzer A.J., O'Connor D., Pollard A.J., Hill A.V. Searching for the human genetic factors standing in the way of universally effective vaccines. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdez Y., Brown E.M., Finlay B.B. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35:526–537. doi: 10.1016/j.it.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Jackson B.A., Wilson J.L., Kirbah S., et al. Mitochondrial DNA genetic diversity among four ethnic groups in Sierra Leone. Am J Phys Anthropol. 2005;128:156–163. doi: 10.1002/ajpa.20040. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization WHO country cooperation strategy 2017-2021: Sierra Leone. 2017. https://www.who.int/publications/i/item/9789290233572
- 31.World Food Programme Sierra Leone. https://www.wfp.org/countries/sierra-leone
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.