Abstract
Background
Mycobacterium chelonae is a species of nontuberculous mycobacteria that typically causes localized cutaneous disease in immunocompetent hosts. There have been few reports of disseminated infections in immunocompetent individuals which have often been associated with invasive medical procedures.
Case Presentation
In this report, we describe a 43-year-old immunocompetent female with an implanted venous access device who presented with skin lesions increasing in size and frequency over the course of five months despite antimicrobial therapy. A diagnosis was not made until mycobacterial culture from a skin biopsy grew M. chelonae.
Conclusion
Disseminated cutaneous M. chelonae infection can be a rare complication of indwelling venous catheterization among immunocompetent patients.
Keywords: Rapidly growing mycobacteria (RGM); Mycobacterium chelonae; Nontuberculous mycobacteria (NTM); Skin infections; Soft Tissue Infections, Central line associated bloodstream infections (CLABSI)
1. Background
Nontuberculous mycobacteria (NTM) are a large group of organisms that encompass all mycobacteria species aside from Mycobacterium tuberculosis complex and Mycobacterium leprae. They are ubiquitous in both natural and constructed environments, and further subdivided into slowly growing mycobacteria (SGM) and rapidly growing mycobacteria (RGM). RGMs grow on solid media within seven days, and include organisms such as Mycobacterium abscessus and Mycobacterium fortuitum. Mycobacterium chelonae is another RGM that has been isolated in soil, dust, water (both treated and untreated), as well as in some fish and amoebae [[1], [2]]. Skin and soft tissue infections are the most common presentation of RGMs, and have been reported to be increasing in incidence in Japan and in the United States [[1], [2], [3], [4]]. M. chelonae is known for causing disseminated cutaneous infection in immunocompromised individuals or localized cutaneous disease in the immunocompetent [[2], [5]]. To the best of the author’s knowledge, this is the first case of disseminated cutaneous M. chelonae infection in an immunocompetent host secondary to an implanted venous access device (IVAD) reported in the literature.
2. Case presentation
A 43-year-old Caucasian woman with history of chronic iron deficiency anemia requiring parenteral iron infusions initially presented to the emergency department with malaise, myalgias and intermittent fevers. Her past medical history included gastric bypass surgery which was reversed due to multiple complications including wound dehiscence, anastomotic leaks, and multiple intra-abdominal infections. It is important to note she had normal liver enzymes and function tests, with no evidence of cirrhosis on multiple abdominal ultrasounds and CT scans. She was admitted for further management and was found to have polymicrobial bacteremia with Stenotrophomonas maltophilia and Enterococcus faecalis thought to be secondary to her peripherally inserted central catheter (PICC). Her PICC was exchanged for an IVAD following treatment of her bacteremia. The IVAD port was placed in the subcutaneous tissue of the patient’s right chest wall under general anesthetic with appropriate sterile technique, and was trimmed to 21 cm with the tip located in the superior vena cava. Shortly after this she developed a small lesion to her right wrist which was biopsied for histopathology, demonstrating chronic lymphocytosis of unclear etiology. Two additional lesions on her right arm developed and progressively increased in size over the next three months before she was admitted again for a central line infection due to S. maltophilia. Her IVAD was not removed and she was discharged home following antimicrobial treatment. Her skin lesions continued to increase in both frequency and size over the following two months, with new lesions appearing in her bilateral calves, followed by her bilateral forearms, eventually spreading up to her bilateral thighs, full arms, breasts, abdomen, shoulders, and neck (Fig. 1A-D). Several lesions would open spontaneously and drain purulent discharge, leaving small ulcerations behind (Fig. 1A). She presented to hospital for evaluation of these lesions in conjunction with ongoing subjective fevers and malaise. She had no history of immunosuppression or risk factors for tuberculosis. Physical examination revealed multiple painful cutaneous erythematous lesions in various stages, including nodules, papules, erosions, and ulcerations. She subsequently underwent biopsy of one of her cutaneous lesions. The biopsy showed necrotizing caseating granulomatous inflammation with acid-fast bacilli highlighted on Ziehl-Neelsen stain (Fig. 2A-C), however mycobacterial culture was not sent. The patient was placed on airborne precautions. At this time laboratory testing revealed a white blood cell count of 5.2 × 109 cells/L with a normal monocyte count, and C-reactive protein level of 36.5 mg/L. Chest radiography and enhanced CT of the abdomen and pelvis were unremarkable, and formal transthoracic echocardiography revealed no vegetations. An ultrasound of her breast was performed which revealed small hypoechoic lesions consistent with abscesses. Three sputum samples were sent for acid-fast bacilli, all of which were negative. Serologic testing for HIV, hepatitis B and C, as well as syphilis were unremarkable. Mycobacterial cultures from blood were negative. Her IVAD was removed with tip/port cultures growing S. maltophilia, achromobacter species, pantoea species, and acremonium species. Unfortunately, the tip was not sent for mycobacterial culture. Mycobacterial culture from a repeat skin biopsy grew M. chelonae after two days of incubation using the automated BACTEC MGIT. Species identification was confirmed with the MALDI Biotyper system (Bruker Daltonics, Billerica, MA, United States) after inactivation and protein extraction using the MBT Mycobacterial Kit (Bruker). The M. chelonae was reported as sensitive to amikacin, tobramycin, clarithromycin, and ciprofloxacin. Tigecycline had a reported minimum inhibitory concentration (MIC) of 0.06. The organism was noted to have intermediate susceptibility to linezolid and imipenem, and was resistant to doxycycline, moxifloxacin, meropenem, trimethoprim-sulfamethoxazole, and cefoxitin. Susceptibility testing was performed using standard Mueller-Hinton broth microdilution method [6]. She completed two months of parenteral amikacin, oral clarithromycin and oral ciprofloxacin with good response before transitioning to oral clarithromycin and ciprofloxacin for an additional four months. Near the end of treatment the patient was found to have new subcutaneous nodules on reassessment. No other sources of infection (e.g. abdominal abscess or vertebral discitis) were suspected, and therefore no additional or repeat imaging were considered. A repeat biopsy of a right forearm lesion was performed, with cultures growing M. chelonae with intermediate susceptibility to ciprofloxacin. The remainder of the susceptibilities were unchanged, however additional testing was performed for clofazimine, revealing an MIC of 0.5. An attempt to increase her ciprofloxacin dose to 750 mg PO BID failed due to intolerance (nausea/vomiting). Amikacin was restarted but was discontinued after two weeks due to nephrotoxicity. Clofazimine was subsequently added to her clarithromycin and ciprofloxacin regimen and continued for an additional nine months. In total, she required approximately 16 months of antimicrobial therapy. She has now been off antibiotics for six months with no signs of recurrence. Additional immunodeficiency testing, including mutations within the interferon gamma (IFN-γ) pathway, were not sought.
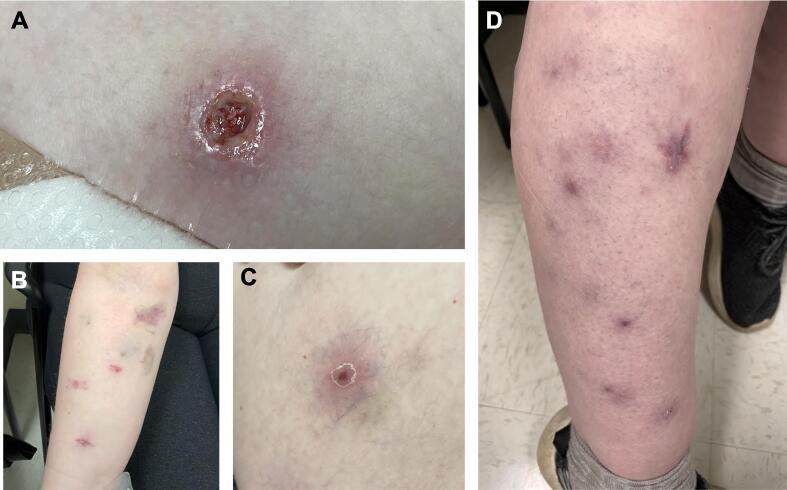
Fig. 1.
Erythematous nodules, papules, erosions, and ulcerations in various stages located on the abdomen (A), right forearm (B), right thigh (C), and the right calf (D). These lesions are all secondary to disseminated cutaneous Mycobacterium chelonae infection.
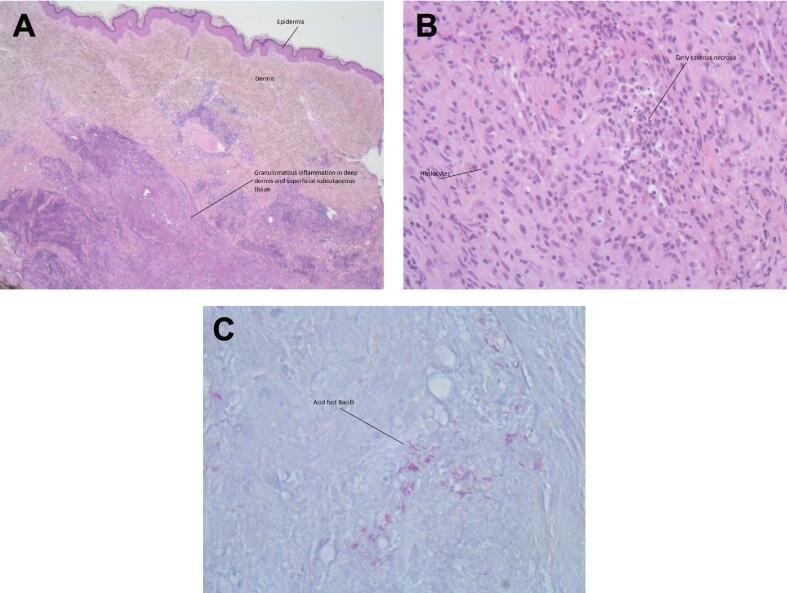
Fig. 2.
(A) Necrotizing granulomatous inflammation involving the deep dermis and superficial subcutaneous tissue (H&E stain, original magnification, ×2.5). (B) Necrotizing granuloma (H&E stain, original magnification, ×20). (C) Acid-fast bacilli consistent with Mycobacterium chelonae (Ziehl Neelsen stain, original magnification, ×40).
3. Discussion
RGMs including M. chelonae typically cause localized cutaneous disease in immunocompetent individuals secondary to puncture wounds, invasive medical or cosmetic procedures (due mainly to inadequate instrument sterilization), or with environmental exposures including contaminated liquids in tattoo studios or nail salons [[2], [5]]. Disseminated infections have largely been associated with immunocompromised patients, however over the last two decades there have been case reports outlining disseminated M. chelonae infection in immunocompetent hosts [[2], [5], [7], [8], [9], [10], [11], [12], [13]]. Two cases of disseminated infection were associated with medical procedures, specifically sclerotherapy of a varicose vein, and pacemaker pulse generator exchange leading to lead endocarditis [[7], [12]]. There have also been cases where a delay in either diagnosis of infection or a delay in seeking treatment has led to dissemination of infection in an immunocompetent host [[8], [9], [10]]. It is also important to note that currently there is no standardized definition as to what constitutes dissemination in these infections. Previous studies have utilized criteria including positive blood or bone marrow cultures, as well as reactive skin disease with anywhere from ≥ 2 to > 5 nodular subcutaneous lesions [[11], [13]]. In the presented case, a likely infected IVAD and a prolonged period of inadequate therapy were the likely culprits leading to disseminated M. chelonae infection. Unfortunately the IVAD was not sent for mycobacterial culture, so it cannot be confirmed with absolute certainty as the origin of her infection. However, given the polymicrobial IVAD cultures and the timeline of the development of her skin lesions, it is the most plausible source. Given the multiple isolated microbes, possible line manipulation was thought a more likely source of infection rather than contamination during IVAD production or implantation.
An important consideration in all of the described cases of disseminated M. chelonae infections in presumably immunocompetent hosts is the potential presence of an undetected immunodeficiency. Specifically, the IFN-γ pathway helps mediate immunity to NTM infections, and mutations in this pathway (such as in genes that encode IL-12Rγ1 or IFNγR1), or the development of anti-IFN-γ autoantibodies could explain disseminated disease in these patients [[14], [15]]. Another consideration could be GATA-2 deficiency, however the patient’s normal monocyte count points away from this as an etiology [16]. Clinicians can consider testing for these immunodeficiencies in patients who present with disseminated or recurrent NTM infections.
RGMs are an uncommon cause of central venous catheter (CVC) associated line infections [[17], [18]]. Mycobacterium mucogenicum and M. fortuitim cause the majority of these infections, however other RGMs including M. chelonae have also been implicated [[17], [18]]. Interestingly, it is rare to see metastatic sites of infection secondary to RGM catheter-related infections, with only three cases being reported in a retrospective study of 116 RGM central line associated bloodstream infections (CLABSI) in patients with cancer [17]. Major risk factors for RGM blood stream infections include immunosuppression, extended duration of catheter in situ, and previous antimicrobial therapy [[17], [18]]. In the presented case, risk factors included her IVAD remaining in situ despite known infection, possible line manipulation/contamination, as well as multiple previous antimicrobial courses. Her presentation is unusual given the rarity of dissemination in immunocompetent hosts and the low complication rate in RGM CLABSIs. Clinical outcomes are generally favourable in non-disseminated cases of RGM CLABSI if the infected catheter is removed and a short course of a combination antimycobacterial regimen is provided [17].
Treatment of disseminated cutaneous M. chelonae infection remains non-standardized. For serious cutaneous M. chelonae infections, the American Thoracic Society/Infectious Diseases Society of America guidelines from 2007 recommend a minimum of four months of a combination drug regimen, as there has been a previous report of clarithromycin resistance following a course of monotherapy [19]. It is generally agreed upon that two to three active agents should be administered with initial parenteral therapy before stepping down to dual oral therapy if the patient is clinically improving [[19], [20], [21], [22]]. Newer consensus guidelines published in 2022 for treatment of M. chelonae pulmonary disease recommend starting treatment with a minimum of two drugs for mild-moderate disease, or three drugs in severe disease, once drug susceptibility has been confirmed [23]. They recommend treating with at least two parenteral drugs for 4–16 weeks, then transitioning to dual oral therapy to complete 12 months total [23]. Tobramycin is the preferred aminoglycoside as it has demonstrated better activity in vitro than amikacin, and moxifloxacin is the preferred fluroquinolone [23].
In the presented case, a prolonged course of antibiotics was required after repeat cultures demonstrated growing ciprofloxacin resistance in M. chelonae despite 6 months of combined antimicrobial therapy. This complication was thought secondary to wavering antimicrobial adherence stemming from persistent nausea/vomiting in the context of a chronic nausea/vomiting disorder. Additionally, the selected treatment regimen was limited by the organism’s resistance to multiple first-line drugs. The use of amikacin over tobramycin was suboptimal given current evidence that tobramycin is more effective for M. chelonae infections [23]. Ciprofloxacin was used given the documented moxifloxacin resistance. The use of clofazimine has been associated with increased mortality in patients with HIV infection and disseminated Mycobacterium avium complex disease, however it remains an option in recent guidelines for treatment of M. chelonae [[23], [24]]. Given this was the only oral drug available to escalate therapy at the time of her disease recurrence, it was determined the benefit of its use outweighed the risk.
4. Conclusion
The present case describes an immunocompetent host with disseminated cutaneous M. chelonae infection secondary to an IVAD. Both dissemination in immunocompetent patients as well as complications from RGM CLABSIs have rarely been reported. Clinicians should be aware disseminated RGM infections in immunocompetent patients can occur in patients with indwelling CVCs and following invasive medical procedures. Early consideration of cutaneous RGM infection in patients with characteristic lesions (Fig. 1A-D) would allow clinicians to order appropriate diagnostic testing. In patients with suspected RGM infection who are clinically stable, empiric therapy should be avoided when possible given the many susceptibility patterns present within this group of organisms and potential for development of antimicrobial resistance as was seen in this case [19]. Instead, tissue biopsy for histopathological examination as well as mycobacterial culture should be sent early in the work-up of these patients to allow for faster diagnosis and improved management of these infections.
Ethics statement
The authors’ institution (University of Alberta) does not require ethical approval for the publication of a single case report. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Nicholas D. Riopel: Conceptualization, Writing – original draft, Visualization. Kimberly Wood: Resources, Writing – review & editing. William Stokes: Conceptualization, Writing – review & editing, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Nicholas D. Riopel, Email: ndriopel@ualberta.ca.
Kimberly Wood, Email: kwood2@ualberta.ca.
William Stokes, Email: wstokes@ualberta.ca.
References
- 1.Misch E.A., Saddler C., Davis J.M. Skin and soft tissue infections due to nontuberculous mycobacteria. Curr Infect Dis Rep. 2018;20(4):6. doi: 10.1007/s11908-018-0611-3. [DOI] [PubMed] [Google Scholar]
- 2.Uslu U., Böhm O., Heppt F., Sticherling M. Skin and soft tissue infections caused by Mycobacterium chelonae: more common than expected? Acta Derm Venereol. 2019;99(10):889–893. doi: 10.2340/00015555-3230. [DOI] [PubMed] [Google Scholar]
- 3.Wentworth A.B., Drage L.A., Wengenack N.L., Wilson J.W., Lohse C.M. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88(1):38–45. doi: 10.1016/j.mayocp.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujishima C., Tahara J., Munemoto S., Hioki C., Sasaki H., Yoshida H., et al. Cutaneous nontuberculous mycobacterial infections in Japan: Review of the Japanese literature. J Dermatol. 2022;49(11):1075–1084. doi: 10.1111/1346-8138.16531. [DOI] [PubMed] [Google Scholar]
- 5.Fowler J., Mahlen S. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138(8):1106–1109. doi: 10.5858/arpa.2012-0203-RS. [DOI] [PubMed] [Google Scholar]
- 6.Brown-Elliott B.A., Woods G.L. Antimycobacterial susceptibility testing of nontuberculous mycobacteria. J Clin Microbiol. 2019;57(10):e00834–e919. doi: 10.1128/JCM.00834-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murback N.D.N., Higa Júnior M.G., Pompílio M.A., Cury E.S.J., Hans Filho G., Takita L.C. Disseminated cutaneous atypical mycobacteriosis by M. chelonae after sclerotherapy of varicose veins in a immunocompetent patient: a case report. An Bras Dermatol. 2015;90(3 Suppl 1):138–142. doi: 10.1590/abd1806-4841.20153504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halpern J., Biswas A., Cadwgan A., Tan B.B. Disseminated cutaneous Mycobacterium chelonae infection in an immunocompetent host. Clin Exp Dermatol. 2009;35(3):269–271. doi: 10.1111/j.1365-2230.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 9.Atikah R., Kahairi A., Asha’ari Z.A. Disseminated cutaneous Mycobacterium fortuitum-chelonae complex infection in a young, immunocompetent patient: a case report. IIUM Medical Journal Malaysia. 2021;20(3):139–142. doi: 10.31436/imjm.v20i3.985. [DOI] [Google Scholar]
- 10.Satta R., Cottoni F., Molicotti P., Lissia A., Cerimele D. Cutaneous Mycobacterium chelonae Infection in a Presumably Immunocompetent Host. Acta Derm Venereol. 2002;82(2):156–157. doi: 10.1080/00015550252948329. [DOI] [PubMed] [Google Scholar]
- 11.Chung W.K., Kim M.S., Kim C.H., Lee M.W., Choi J.H., Moon K.C., et al. Cutaneous Mycobacterium chelonae Infection Presenting as Symmetrical Plaques on Both Shins in an Immunocompetent Patient. Acta Derm Venereol. 2009;89(6):663–664. doi: 10.2340/00015555-0693. [DOI] [PubMed] [Google Scholar]
- 12.Hooda A., Pati P.K., John B., George P.V., Michael J.S. Disseminated Mycobacterium chelonae infection causing pacemaker lead endocarditis in an immunocompetent host. BMJ Case Rep 2014;2014:206042. doi:10.1136/bcr-2014-206042. [DOI] [PMC free article] [PubMed]
- 13.Chou C.H., Chen H.Y., Chen C.Y., Huang C.T., Lai C.C., Hsueh P.R. Clinical features and outcomes of disseminated infections caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 2004–2008. Scand J Infect Dis. 2011;43(1):8–14. doi: 10.3109/00365548.2010.519345. [DOI] [PubMed] [Google Scholar]
- 14.Bhattad S. Mendelian Susceptibility to Mycobacterial Disease: A Clinical and Laboratory Approach. Pediatr Inf Dis. 2019;1(1):34–36. doi: 10.5005/jp-journals-10081-1108. [DOI] [Google Scholar]
- 15.Shih H., Ding J., Yeh C., Chi C., Ku C. Anti-interferon-γ autoantibody-associated immunodeficiency. Curr Opin Immunol. 2021;72:206–214. doi: 10.1016/j.coi.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Hsu A.P., McReynolds L.J., Holland S.M. GATA2 Deficiency. Curr Opin Allergy Clin Immunol. 2015;15(1):104–109. doi: 10.1097/ACI.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Helou G., Hachem R., Viola G.M., El Zakhem A., Chaftari A.-M., Jiang Y., et al. Management of rapidly growing mycobacterial bacteremia in cancer patients. Clin Infect Dis. 2013;56(6):843–846. doi: 10.1093/cid/cis1032. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Coste M.A., Chirca I., Steed L.L., Salgado C.D. Epidemiology of rapidly growing mycobacteria bloodstream infections. Am J Med Sci. 2016;351(3):253–258. doi: 10.1016/j.amjms.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 20.Kasperbauer S.H., De Groote M.A. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36(1):67–78. doi: 10.1016/j.ccm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Santiago T.M., Drage L.A. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33(3):563–577. doi: 10.1016/j.det.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Wallace R.J., Jr., Swenson J.M., Silcox V.A., Bullen M.G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985;152(3):500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- 23.Lange C., Böttger E.C., Cambau E., Griffith D.E., Guglielmetti L., van Ingen J., et al. Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases. Lancet Infect Dis. 2022;22(7):e178–e190. doi: 10.1016/S1473-3099(21)00586-7. [DOI] [PubMed] [Google Scholar]
- 24.Chaisson R.E., Keiser P., Pierce M., Fessel W.J., Ruskin J., Lahart C., et al. Clarithromycin and ethambutol with or without clofazimine for the treatment of bacteremic Mycobacterium avium complex disease in patients with HIV infection. AIDS. 1997;11(3):311–317. doi: 10.1097/00002030-199703110-00008. [DOI] [PubMed] [Google Scholar]