Highlights
-
•
MEG connectivity to emotional faces in children with OCD and controls was investigated.
-
•
Between groups, the OCD group showed increased gamma connectivity to angry faces.
-
•
OCD youth showed greater beta connectivity to happy vs. angry faces.
-
•
Controls engaged a gamma band network, also greater to happy vs. angry faces.
-
•
Increased beta connectivity in OCD suggests the over-use of top-down processing.
Keywords: OCD, Emotional face processing, Functional connectivity, Youth, Magnetoencephalography, Beta/gamma
Abstract
Children and youth with obsessive–compulsive disorder (OCD) demonstrate difficulties with social, emotional and cognitive functions in addition to the core diagnosis of obsessions and compulsions. This is the first magnetoencephalography (MEG) study to examine whole-brain neurophysiological functional connectivity of emotional face processing networks in paediatric OCD. Seventy-two participants (OCD: n = 36; age 8–17 yrs; typically developing controls: n = 36, age 8–17 yrs) completed an implicit emotional face processing task in the MEG. Functional connectivity networks in canonical frequency bands were compared between groups, and within OCD and control groups between emotions (angry vs. happy). Between groups, participants with OCD showed increased functional connectivity in the gamma band to angry faces, suggesting atypical perception of angry faces in OCD. Within groups, the OCD group showed greater engagement of the beta band, suggesting the over-use of top-down processing when perceiving happy versus angry emotions, while controls engaged in bottom-up gamma processing, also greater to happy faces. Over-activation of top-down processing has been linked to difficulties modifying one’s cognitive set. Findings establish altered patterns of neurophysiological connectivity in children with OCD, and are striking in their oscillatory specificity. Our results contribute to a greater understanding of the neurobiology of the disorder, and are foundational for the possibility of alternative targets for intervention.
1. Introduction
Obsessive-compulsive disorder (OCD) is characterized by obsessions, which involve persistent intrusive thoughts/impulses, often accompanied by repetitive behaviours, referred to as compulsions (American Psychiatric Association, 2013, Leckman et al., 1997). In addition to these core diagnostic symptoms, difficulties with social, emotional and cognitive functions are demonstrated but often overlooked in OCD (Kim et al., 2012). These social-cognitive impairments include deficits in emotional face recognition, poor emotional regulation, and difficulties with theory of mind and mentalizing (Aigner et al., 2007, Jansen et al., 2020). A better understanding of the neurobiology of social-cognitive impairments in OCD could lead to better characterizations of the OCD phenotype, and perhaps, lead to an improved ability to identify alternative targets for intervention.
Studies of social-cognitive function in OCD at the behavioural level have focused primarily on emotion recognition, particularly involving disgust (McKay, 2006, Woody and Teachman, 2000), with most studies demonstrating impaired recognition of disgust in adults with the disorder (Corcoran et al., 2008, Daros et al., 2014, Lochner et al., 2012, Sprengelmeyer et al., 1997). Despite some mixed findings, difficulties in emotion recognition (of emotions other than disgust) have been reported (Aigner et al., 2007, Corcoran et al., 2008). For example, in a meta-analysis examining accuracy in emotion recognition across a large sample of OCD patients (n = 221) and controls (n = 224), lower accuracy across emotions (happy, angry, sad, disgust, fear and surprised) was found in OCD compared to controls. The greatest difficulties were seen for negative emotions, particularly disgust and anger (Daros et al., 2014).
Neuroimaging studies in adults with OCD have used functional magnetic resonance imaging (fMRI) to investigate the neural mechanisms underpinning emotion processing. Research has found increased activation of key emotional face processing regions in adults with OCD, including frontal areas (i.e., ventrolateral and dorsolateral prefrontal areas), amygdalae, fusiform gyri and occipital areas (Cardoner et al., 2011, Lawrence et al., 2007, Via et al., 2014, Weidt et al., 2016). For example, using an emotional face-matching task, Cardoner and colleagues (2011) found increased activation in OCD compared to controls during emotional face (i.e., happy and fear) vs. shape matching in the visual cortex, right fusiform gyrus, left thalamus, right dorsolateral prefrontal cortex and right amygdala. Increased functional connectivity in the OCD group was also found between three a priori regions: right amygdala, prefrontal cortex and fusiform gyrus and other core emotion processing areas. The severity of OCD symptoms was positively correlated with both regional dysfunction and functional connectivity.
Paediatric OCD is a common and prevalent neurodevelopmental disorder (NDD) and is just as distressing, stigmatizing and debilitating as in adults; however, there are few studies investigating the younger population (Britton et al., 2010, Vandewouw et al., 2020). Britton et al. (2010) found reduced activation of the amygdala/hippocampal area to emotional faces vs. fixation in OCD compared to typically developing (TD) peers. Further, patterns of hypo-activation in orbital frontal cortex and hyper-activation in VLPFC and anterior cingulate were seen for emotional vs. neutral faces in the OCD group. Another study (Vandewouw et al., 2020) investigated dynamic emotional face processing in children with OCD, compared to other NDDs and controls, and found that the OCD group showed less neural differentiation between dynamic faces and flowers in inferior and middle occipital and fusiform gyri. Reduced neural differentiation in OCD was also reported between angry vs. happy faces in occipital-temporal areas, as well as age-related increases in the recruitment of frontal brain regions to happy faces, while TD peers showed the opposite pattern. The authors suggested less distinctive visual as well as altered salience processing in OCD, and increased difficulty processing happy faces with age.
Taken together, these fMRI data provide a framework to understand the brain regions and neural networks involved in emotion processing in OCD. However, recent studies involving neurophysiological approaches, such as magnetoencephalography (MEG), have demonstrated the importance of exploring the oscillatory dynamics of functional brain networks, as particular cognitive functions have been found toinvolve specific frequency bands. In particular, MEG is an optimal technique as it directly measures neural activity and precisely captures high-resolution spatial–temporal information (Hari and Salmelin, 2012). Given the fast pace of social interactions, understanding the temporal dynamics of social processing is critical and with the functional specificity of the oscillatory domain afforded by MEG, such investigation is possible.
The current MEG study is the first to use whole-brain neurophysiological functional connectivity to investigate the oscillatory neural mechanisms of implicit emotional face processing in youth with OCD compared to age-and sex-matched TD controls. Implicit tasks invoke subconscious, automatic and rapid processing of expressions, for which deficits in perception may not be compensated by learned strategies, experience and greater attention to faces, in those with NDDs (Frith, 2004). Thus, we employed an implicit task to engage real-world social requirements. Based on the larger body of literature in adults with OCD, it was hypothesized that functional connectivity would be increased in OCD compared to TD children, and that the network would involve several key emotional face processing regions (i.e., primary visual areas, fusiform gyri, amygdalae, insulae and prefrontal regions), particularly orbital frontal brain areas. In addition, we hypothesized that significant between-group network functional connectivity strength would be positively associated with the severity of OCD symptoms.
2. Methods and materials
2.1. Participants
Participants were recruited through the Province of Ontario Neurodevelopmental Disorders (POND) network and the cohort included youth 8-to-17 years of age with OCD (n = 42) and age- and sex-matched TD controls (n = 48); all participants were scanned between 2012 and 2020. Eighteen were excluded due to a) < 55% accuracy on the task (n = 3), b) poor head localization (n = 4) or c) < 20 clean happy or angry MEG trials (n = 11). Thus, data from 72 children 8 to 17 years of age with OCD (n = 36) and age- and sex-matched TD controls (n = 36) were included in the analyses. Age did not differ between-groups t(70) = 0.41p = 0.68, nor did the proportion of boys and girls (X2 = 0.06, p = 0.81).
For the OCD participants, a primary diagnosis of OCD was determined by expert clinicians and confirmed with the Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., 1997). For the TD group, children were not included if they were born premature, or had a history of neurodevelopmental, psychiatric or neurological disorders, or learning, language or developmental disabilities. Standard imaging exclusion criteria (e.g., no ferromagnetic implants, no colour blindness, having normal corrected vision) were also applied to both groups.
The Wechsler scales of intelligence (Wechsler, 2012, Wechsler, 2003, Wechsler, 1999, Wechsler, 2014) were used to determine Full-Scale IQ (FSIQ). FSIQ also did not differ between the OCD and TD groups, t(34.40) = -0.15, p = 0.88. The Toronto Obsessive–Compulsive Scale (TOCS) was used to measure paediatric obsessive–compulsive traits, scored on a 7-point Likert scale ranging from −3 “far less than average” to 3 “far more than average” (Lambe et al., 2021). TOCS were collected on 34 OCD and 27 TD participants and a significant between group difference was found, t(59) = -11.38, p = 1.63 × 10-16. Participant demographics are summarized in Table 1. The study protocol was approved by Research Ethics Boards at all participating research sites. Written informed consent was provided by a parent or legal guardian of all child participants; informed verbal assent was given by all child participants.
Table 1.
Participant demographics.
| OCD | TD | ||
|---|---|---|---|
| N | 36 | 36 | |
| Age (years; mean ± std.) | 12.17 ± 2.39 | 12.41 ± 2.52 | |
| Age range | 8.30 – 17.14 | 8.57 – 17.61 | |
| Sex (M:F) | 22:14 | 23:13 | |
| Mean head motion (mm; mean ± std.) | 0.64 ± 0.37 | 0.76 ± 0.63 | |
| Number of non-target trials(mean ± std.) | Happy | 37.36 ± 3.47 | 38.03 ± 2.73 |
| Angry | 37.39 ± 3.11 | 37.06 ± 3.21 | |
| Range of non-target trials | Happy | 28–43 | 31–42 |
| Angry | 28–43 | 30–41 | |
| N | |||
| FSIQ (mean ± std.) | 24 | 34 | |
| 113.17±16.65 | 112.59±9.89 | ||
| N | 34 | 27 | |
| TOCS (mean ± std.) | 21.50 ± 15.83 | -37.93 ± 24.76 | |
Note: FSIQ = Full-scale IQ; TOCS = Toronto Obsessive-Compulsive Scale.
2.2. Emotional face processing task
Faces (26 faces, 12 female) were extracted from the MacBrain Face Stimulus Set (http://www.macbrain.org/resources.htm) (Tottenham et al., 2009). Each face image was sized to 7.4w × 9 h cm with a 2 cm blue or purple border surrounding it. Children attended to the border colour surrounding the images ignoring the emotional content of the faces, thus the task was implicit. Non-target trials were 75% of the total number of trials and required no response. Target trials (25%) ensured task attention; participants responded as quickly as possible on a button box, to faces with a designated border colour (purple or blue). The assigned border colour of the target trials was counter-balanced across subjects; trials were presented randomly. Only correct non-target trials were analysed to avoid motor artefact associated with the button-press for target trials.
Each trial consisted of a happy or angry face presented between 300 and 700 ms, adjusted based on maintenance of error rates (≥95% accuracy for target trials and ≥ 80% accuracy for non-target trials) and subsequent interstimulus interval (fixation cross) presented between 650 and 1300ms, also varied according to error rates (see Supplemental Materials for the detailed weightings for the maintenance of error rates). The task was presented using Presentation® software (www.neurobs.com); face stimuli subtended ∼ 14 × 16 degrees of visual angle, from a viewing distance of 78 cm.
2.3. Data acquisition
MEG data were recorded in a magnetically shielded room while participants lay supine, using a 151-channel CTF system (CTF MEG International Services LP, Coquitlam, BC, Canada). Data were sampled at 600 Hz with an online 0 to 150 Hz anti-aliasing filter, and to attenuate environmental noise a third-order spatial gradient was used. Fiducial coils situated at the left and right pre-auricular points and nasion monitored head location, and were replaced with radio-opaque markers for co-registration with T1-weighted MR images. Individual structural T1-weighted images were obtained on a Siemens 3 T MAGNETOM Trio with a 12-channel head coil (TR/TE = 2300/2.96 ms, FA = 9°, FOV = 240x256mm, # slices = 192, resolution = 1.0 mm isotropic) scanner, or on a PrismaFIT with a 20-channel head and neck coil (TR/TE = 1870/3.14 ms, FA = 9°, FOV = 240x256mm, # slices = 192, resolution = 0.8 mm isotropic) scanner, as a result of a scanner upgrade.
2.4. Preprocessing and source estimation
Preprocessing of the MEG data and source estimation were performed using the FieldTrip toolbox (Oostenveld et al., 2011) in MATLAB (Mathworks) software. A 4th order two-pass Butterworth filter between 1 and 150 Hz was applied to the data, and a discrete Fourier transform notch filter eliminated line noise from the signal at 60 Hz and 120 Hz. The data were segmented into −1000 to 1250 ms trials by emotional face type, relative to face onset. Independent component analysis (ICA) was applied to attenuate motor or ocular artefacts (i.e., eye blinks, saccades), which were then manually removed from the MEG signal. Trials were excluded from analyses if sensor signals surpassed 2000fT, or if the initial median head location shifted greater than 10 mm (Pang, 2011). No significant differences in head motion t(70) = 0.12, p = 0.33 were found between OCD and TD groups. Following artefact rejection, data from participants that included > 20 trials for each emotional face type were analyzed. There were no group differences in the number of trials included in the MEG analyses F(1, 70) = 0.07, p = 0.80, nor in the number of happy or angry trials analyzed F(1, 70) = 1.9, p = 0.17, nor group-by-emotion interaction F(1, 70) = 2.13, p = 0.15 (see Table 1).
For MEG co-registration, we used each individual’s T1-weighted MR image to generate a single-shell head model for each participant (Nolte, 2003). The centre coordinates/centre-of-mass of the first 90 (cortical and subcortical parcels) of the 116 Automated Anatomical Labelling (AAL) atlas (see Supplemental Table 1 for a list of regions) (Tzourio-Mazoyer et al., 2002) were non-linearly transformed from standard template space onto equivalent subject-specific head locations (ICBM 152; (Fonov et al., 2011)). An LCMV beamformer was used to estimate the source activity for each of the source locations (van Veen et al., 1997), with 5% Tikonov regularization. To attenuate the centre-of-head bias the neural activity index was calculated (van Veen et al., 1997).
2.5. Functional connectivity
For each of the source locations the broadband time series data were filtered into canonical frequency bands – theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz) and gamma (30–55 Hz). The Hilbert Transform was computed to obtain the time series of instantaneous phase values at each of the source locations and frequency bands; phase data were then segmented into −400 to 600 ms epochs, relative to happy or angry face onset. Interregional phase synchrony was estimated using the cross-trial phase lag index (PLI) based on Stam et al. (Stam et al., 2007). The PLI is a measure of the consistency of non-zero instantaneous phase lag differences between two source signals; artificial interactions implied by zero or near-zero phase differences are attenuated. For each participant, the PLI was calculated pairwise at each sample across the time series between each of the 90 AAL sources, rendering a 90-by-90 adjacency matrix, for each emotion type and frequency band. For statistical analyses, a latency window of 200 to 400 ms following stimulus onset (i.e., active window) was chosen. The latency window was motivated based on previous research demonstrating between-group differences in functional connectivity during implicit emotion processing in individuals with NDDs compared to typical controls during this latency period (Mennella et al., 2017, Safar et al., 2022, Safar et al., 2021). The relative change from baseline (-200 to 0 ms) was computed for the PLI values at each timepoint within the active window and then averaged.
3. Statistical analyses
Between-group differences in age, head motion, FSIQ and TOCS scores were analysed with independent sample t-tests, with significance held at p < 0.05; differences in the proportion of males and females were analysed with a chi-squared test (p < 0.05). Repeated-measures ANOVAs (within-subject factor: emotion (happy, angry), between-subject factor: group (OCD, TD)) assessed for a significant difference between groups, emotion, or an emotion-by-group interaction in the number of MEG trials included, accuracy and reaction time; significance was held at p < 0.05.
The Network Based Statistic (NBS), a non-parametric method for large network analyses while controlling for the family-wise error rate (FWER (Zalesky et al., 2012, Zalesky et al., 2010) (https://www.nitrc.org/projects/nbs), was used to run a general linear model to identify differences in brain networks between the OCD and TD groups for the emotional faces, with age and sex as covariates, for each frequency range (significance was held at pcorr < 0.05, Bonferroni-corrected for multiple comparisons for emotion). In addition, we identified differences in network connectivity between happy and angry faces within the OCD and TD children. The primary component-forming thresholds were chosen a priori based on the sparsity of the networks, such that the networks comprised 1% of total possible network connections. All NBS analyses were run with 5,000 permutations. The mean connectivity strengths of significant between-group networks identified were correlated with TOCS scores to determine brain-behaviour associations (Bonferroni-corrected for multiple comparisons). Mean connectivity strength was computed by taking the sum of the PLI values at each region to all other brain regions in the significant network, and then computing the mean across these values resulting in the mean connectivity strength for each subject.
4. Results
4.1. Behavioural results
No significant main effects of group F(1, 70) = 2.50, p = 0.12, or emotion F(1, 70) = 0.002, p = 0.97, nor a group-by-emotion interaction F(1, 70) = 0.09, p = 0.76 on accuracy were seen for the non-target trials. Similarly, no significant main effects of group F(1, 70) = 2.35, p = 0.13, or emotion F(1, 70) = 0.46, p = 0.50, nor a group-by-emotion interaction F(1, 70) = 0.95, p = 0.33 on accuracy for target trials were found. Likewise, for reaction time on target trials, we did not find significant effects of group F(1, 70) = 0.59, p = 0.45, or emotion F(1, 70) = 0.19, p = 0.66, nor a group-by-emotion interaction F(1, 70) = 0.72, p = 0.40 (see Table 2). As the task was implicit and non-demanding, measures of accuracy and reaction time were analyzed to ensure task attention. The comparable between-group performance was expected and assured that between-group differences in functional connectivity were not attributed to unequal between-group behavioural performance.
Table 2.
Mean and standard deviation task accuracy and reaction time.
| OCD | TD | |||
|---|---|---|---|---|
| N | 36 | 36 | ||
| Accuracy (%) | Target trials | Happy | 91.15 ± 11.69 | 95.20 ± 7.66 |
| (mean ± std.) | Angry | 92.81 ± 8.52 | 94.90 ± 9.68 | |
| Non-target trials | Happy | 95.52 ± 6.30 | 97.31 ± 3.62 | |
| (mean ± std.) | Angry | 95.40 ± 7.89 | 97.47 ± 2.77 | |
| Reaction time (ms) | Target trials | Happy | 245.57 ± 42.04 | 257.49 ± 51.52 |
| (mean ± std.) | Angry | 250.08 ± 54.84 | 256.07 ± 57.01 | |
4.2. Comparison of MEG between TD and OCD groups
To demonstrate MEG data quality, we performed a time–frequency analysis (Morlet wavelet transformation) to examine the induced task oscillations to happy and angry faces across all participants for well-established face processing areas (see Supplemental Fig. l).
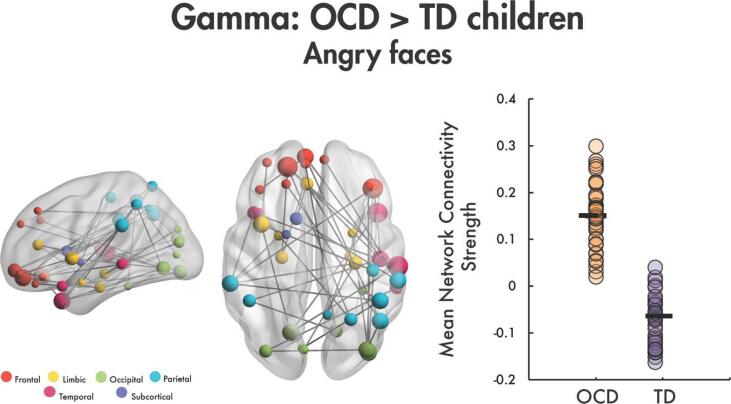
4.3. OCD group showed increased connectivity in gamma to angry faces
In the between-groups comparison, we found a significant network of increased functional connectivity to angry faces in the gamma frequency band in OCD compared to TD youth (t = 2.6, 43 edges, 42 nodes, pcorr < 0.0004; Fig. 1). The network was anchored in right occipital, temporal, and parietal areas including, the right superior and middle temporal gyri, the middle temporal pole, the middle and inferior occipital gyri, the angular gyrus, the inferior parietal and the supramarginal gyri. The network also included several bilateral orbital frontal connections and widespread frontal, limbic, occipital, parietal and temporal brain areas. Additional key face processing areas also identified within the network included the right fusiform, the bilateral amygdalae, the left insula, and the left anterior cingulate gyrus. No significant between-group differences were found for angry faces in the theta, alpha or beta frequency bands, nor for happy faces in theta, alpha, beta or gamma bands. The active window (200 to 400 ms) relative to baseline (-200 to 0 ms) contrast to happy and angry faces was also examined for the gamma band in TD and OCD groups, separately, given the between-group finding. We saw a significant increase from baseline (pcorr < 0.002 for all comparisons in each group; see Supplemental Fig. 2).
Fig. 1.
Increased gamma functional connectivity in OCD compared to TD children to angry faces. A significant gamma band network was identified of increased connectivity to angry faces in OCD vs. TD children. This network is plotted in the glass brains on the left, colour-coded by lobe; node size is scaled by degree (number of connected edges to the node). The mean network connectivity strength by participant is plotted in the dot plot on the right.
To ensure that the between-group effect was not being driven by a whole-brain increase in gamma connectivity, we ran a one-way ANCOVA in SPSS to test for between-group differences across the gamma network while controlling for whole-brain gamma mean connectivity. Findings showed that the effect of group remained significant (p < 0.001), and the whole-brain gamma connectivity covariate was also significant (p = 0.043). Therefore, this network of increased connectivity in OCD to angry faces is still significant even when the global increase in connectivity was controlled for.
4.4. Gamma connectivity strength to angry faces and symptom severity
The mean network connectivity strength of the between-group network (OCD > TD) to angry faces in the gamma band was extracted for each group and correlated with TOCS. Functional connectivity strength was not found to be significantly correlated with TOCS scores in the OCD (r = -0.13, p = 0.46) or TD (r = 0.18, p = 0.36) groups.
4.5. Comparison of MEG to happy vs. angry faces within each group
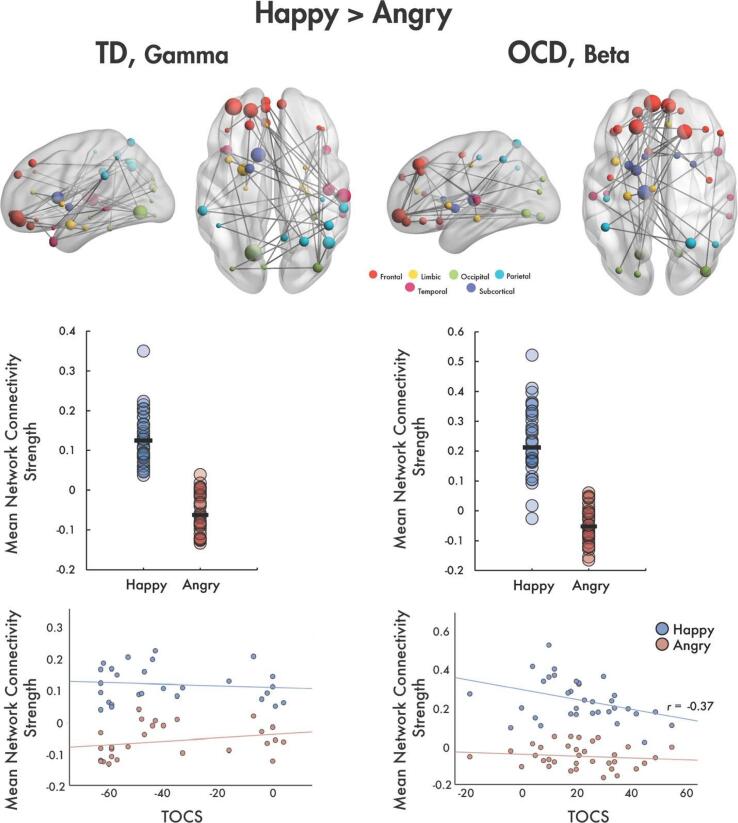
4.5.1. TD children show increased connectivity in gamma band to happy faces
In the TD group, a significant network of increased connectivity was found to happy compared to angry faces in the gamma frequency band (t = 2.53, 40 edges, 39 nodes, pcorr = 0.04; Fig. 2, left). The network was anchored in the right occipital, temporal and parietal areas with the majority of connections with frontal regions, including the orbital part of the left middle frontal gyrus and bilateral superior frontal gyri. The hubs of the network involved the left lingual gyrus, right STG, left amygdala, left caudate, right angular gyrus, and the orbital parts of the left middle frontal gyrus and the superior frontal gyrus; these regions spanned long-range connections across the brain. No significant networks were found for happy and angry contrasts in the theta, alpha or beta bands in the TD group.
Fig. 2.
Left column: (top) A network showing increased functional connectivity to happy vs. angry faces was observed in the gamma band in TD children. The glass brain is shown on top with node size scaled by degree. (middle) Plot of mean network strength for each participant by happy and angry emotion. (bottom) Strength of this network activation did not show significant correlations with TOCS. Right column: (top) For the OCD group, a significant network of increased connectivity to happy vs. angry faces was found in the beta band. Networks are represented in the glass brains with node size scaled by degree. (middle) The mean network connectivity strength for each child is plotted in the dot plots by emotion. (bottom) Mean connectivity strength for happy faces shows a significant negative correlation with TOCS (r = -0.37).
4.5.2. OCD children showed increased connectivity in beta band to happy faces
In the OCD group, a significant network of increased functional connectivity to happy compared to angry faces was found in the beta band (t = 2.62, 40 edges, 38 nodes, pcorr = 0.002; Fig. 2, right). The majority of connections in the network were between the left frontal lobe and left orbital frontal, limbic, occipital, and subcortical brain areas. The most highly connected nodes in the network were the bilateral superior frontal gyri, the left orbital superior frontal gyrus, the bilateral medial orbital superior frontal gyri, the left lingual gyrus, and the right inferior occipital gyrus. In addition, the left insula was connected to bilateral orbital medial superior frontal gyri, and the right anterior cingulate gyrus was linked with the right medial superior frontal gyrus. No significant between-emotion networks were found in the theta, alpha or gamma bands in the OCD group.
4.5.3. Connectivity to happy faces in OCD and symptom severity
In the OCD group, beta functional connectivity strength to happy faces negatively correlated with TOCS (r = -0.37, p = 0.031, Fig. 2, bottom right); this relationship was trending towards significance, pcorr = 0.062 following correction for multiple comparisons across emotion (Bonferroni-corrected). No correlation was observed for angry faces. For TD children, functional connectivity strength to happy and angry faces did not correlate with TOCS (Fig. 2, bottom left).
5. Discussion
In the typically developing brain, implicit emotion processing is a core social-cognitive skill which requires the integration of distributed regions operating at characteristic oscillatory frequencies. In children with NDDs, disruptions in either brain communication or brain oscillatory function can be associated with specific behavioural symptoms. In this study, we used the high-temporal and high-oscillatory resolution of MEG to compare functional connectivity in children with OCD to TD children as they implicitly viewed angry and happy faces. Further, we looked within each group to examine how angry and happy faces were processed. While we had hypothesized that we would find increased functional connectivity in the OCD group, and that atypical functional connectivity strength within key networks would be associated with the severity of OCD symptoms, we had not anticipated the novel findings of the oscillatory specificity of the atypicalities in OCD.
At the group level, two findings stand out. First, a significant network involving well-established face processing areas showed increased functional connectivity to angry faces in the gamma frequency band in OCD; in particular, the orbitofrontal regions were over-recruited. Abnormalities of orbitofrontal areas are consistently reported in OCD compared to controls (Beucke et al., 2013, Evans et al., 2004, Göttlich et al., 2014, Rotge et al., 2008, Saxena et al., 1998), and over-activation and increased connectivity have been seen in tasks involving emotion processing and regulation (Weidt et al., 2016, Rotge et al., 2008). The orbitofrontal cortex plays an important role in regulation of emotional information, and reward processing (Rolls, 2000), which have been suggested to be impacted in OCD (Evans et al., 2004, Figee et al., 2011, Thorsen et al., 2018). Our finding of increased connectivity in children with OCD to angry faces is consistent with this large body of research. Second, the children with OCD also showed greater gamma functional connectivity in right temporal-parietal areas including the superior and middle temporal gyri, temporal pole, and angular and supramarginal gyri, regions involved in both non-verbal memory and face processing. This is consistent with several meta-analyses from neuropsychological (Abramovitch et al., 2013, Shin et al., 2014) and neuroimaging data (Nakao et al., 2014) reporting impairments in visuospatial, non-verbal memory in OCD.
The findings described above involved the gamma band. Gamma synchronization is foundational to sensory and perceptual processing (Simon and Wallace, 2016), implicit emotion processing (Luo et al., 2007) and the integration of facial and emotional information (Uhlhaas et al., 2011). Gamma synchronization has been shown to be both stronger when perceiving salient stimuli (Fries, 2015) and enhanced during the encoding and retrieval of behaviourally important events (Headley and Paré, 2017). Increased connectivity was seen between non-verbal memory and face processing areas, described in the second finding above, and in the classical orbitofrontal-subcortical circuitry, known to be key for social-cognitive functions, described in the first finding. Taken together, the atypical involvement of gamma oscillations in these regions suggest the atypically heightened perception of angry emotional faces in OCD. A meta-analysis has shown that individuals with OCD show reduced accuracy recognizing angry faces, linked to symptomology of the disorder (Daros et al., 2014). The authors suggested that difficulty recognizing angry faces in OCD was related to elevated internal experiences of anger leading to internalization and poorer recognition of anger expressed by others. In addition, or alternatively, perceived anger expressed by others may cause over-arousal in OCD, due to the understanding of the negative impact of their symptoms, leading to recognition deficits (Daros et al., 2014).
Within each group, we examined how angry and happy faces were processed to better understand if children with OCD differed in their processing of these emotions. As expected from the literature on emotion (Lindquist et al., 2016, Murphy et al., 2003) and face (Sabatinelli et al., 2011) processing, TD children showed increased connectivity to happy faces in a network with hubs in left orbito- and dorsolateral-frontal and limbic regions, and right temporo-parietal regions. Again, it is not surprising that this network connectivity was supported by gamma activity as happy faces are highly salient (Fries, 2015) and important (Headley and Paré, 2017) stimuli.
In the OCD group, a similar set of hubs were identified; however, two differences were apparent. First, additional hubs were identified in the insula and anterior cingulate in the OCD group. Previous work with adolescents and young adults with ASD found that insula and anterior cingulate activations were atypical and indexed their difficulties with face processing and understanding social reward and punishment, respectively (Leung et al., 2015, Leung et al., 2018). Our finding of a network involving these hubs aligns with these prior findings of atypical activations and the role that the insula and cingulate play in processing salient stimuli and top-down attention control, respectively, and thus the difficulties the OCD group may have with face and reward processing.
Second, although it is interesting that similar networks were observed in both groups, the OCD group used beta oscillations within this network, in contrast to the gamma oscillatory findings in controls. While beta band was classically associated with motor control (Barratt et al., 2018, Davis et al., 2012, Engel and Fries, 2010), recent studies (for a review see Engel and Fries, 2010) highlight considerable evidence of beta involvement in cognitive tasks, and in particular, a predominance of beta activity in cognitive tasks where endogenous top-down control is exerted, while tasks engaging bottom-up processing are mediated by gamma activity. We observed that those with OCD engaged a network similar to controls, in the beta band, and interestingly, this network strength was correlated with decreased TOCS symptomology in OCD. It is possible that youth with OCD use a beta network because they are overly engaging anticipatory top-down processing when perceiving happy vs. angry faces, while controls engage in bottom-up processing involving a gamma network. Atypical beta oscillations have been implicated in a lack of cognitive-shifting and dysfunction in inhibition (Engel and Fries, 2010), as frequently reported in those with OCD (Berlin and Lee, 2018, Engel and Fries, 2010, Gu et al., 2007, van Velzen et al., 2015). In addition, research has shown deficits in top-down control networks in OCD (Zhang et al., 2011). In the current study, cognitive inflexibility and inhibitory control deficits in the OCD group may reflect engagement of this beta network, and may contribute to greater task difficulty despite comparable behavioural performance. In support of the latter, increased beta phase synchronization has been shown to be associated with task difficulty (Rueda-Delgado et al., 2017). Interestingly, it is the OCD youth with lower TOCS scores who trended towards greater connectivity of this network to happy faces, perhaps supporting recruitment of a compensatory network that is more similar to the controls; it will be important for future work to further explore this relationship in larger samples.
Several limitations of the current study should be noted. First, the between-group mean network connectivity to angry faces in gamma did not significantly correlate with TOCS scores in the OCD group and the correlation between mean network connectivity in OCD in beta and TOCS was only trending towards statistical significance following correction for multiple comparisons. Therefore, it is important to interpret brain-behaviour relations with caution and replicate this study with larger sample sizes. Second, the paradigm was implicit, thus there was no recognition component, and we did not collect measures of recognition accuracy, valence, arousal or dwell time on the faces. Data from these measures would allow for specific relations between functional connectivity and emotion recognition ability to be assessed and should be collected in future research using tasks of explicit emotion recognition. Third, although our sample size of children with OCD is relatively large, it will be important to replicate this research, particularly the brain-behaviour correlates, in larger samples. Fourth, larger trial numbers are optimal for connectivity estimates to attenuate variability and possible biases in increased connectivity; however, data acquisition is challenging in developmental populations, especially in those with neurodevelopmental disorders, limiting the number of non-target trials post-artifact rejection that were included in the present analyses (Pang, 2011, Taylor and Pang, 2014, Puce and Hämäläinen, 2017). We chose a 20 non-target trial per emotion threshold, consistent with previous MEG/EEG studies (Márquez-García et al., 2022, Fogelson et al., 2019, Naumann et al., 2018), to maximize the number of participants included in the analyses and to avoid biasing our population towards those who are older and higher-functioning. Lastly, MEG data from all participants were recorded in the supine position, which minimizes head movement and stabilizes the head in the MEG dewar facilitating data collection in developmental populations. However, caution should be taken when generalizing MEG results from recordings in the supine position to “real-world” conditions, given the unnaturalistic setting and the impact of body posture (i.e., supine vs. sitting) on neural activity in EEG and MEG studies (Spironelli et al., 2016, Thibault et al., 2016). For example, the supine position has been shown to inhibit cortical responses relative to the sitting position during the processing of emotional stimuli in typical adult males (Benvenuti et al., 2013). It will be important for future research to examine how body posture affects task-based functional connectivity in child populations.
The findings in this study demonstrate that while children and adolescents with OCD engage similar brain networks as TD children, they do so by using different oscillatory dynamics. Over-engagement of orbitofrontal networks in the gamma band are consistent with difficulties with emotional information and reward processing, as well as impairments in non-verbal memory and face processing. This was observed as a significant group difference. Further, youth with OCD utilized beta activity suggesting top-down control of emotion processing, in contrast to the bottom-up gamma-mediated processing observed in controls. It has been shown that applying excessive top-down processing results in an inability to modify cognitive sets and therefore an inability to shift attention to new and relevant stimuli. The oscillatory specificity of the findings in this study are theoretically important as they shed light on the underlying neurobiology of OCD, but they are also clinically relevant as they raise the possibility of alternative targets for future interventions.
CRediT authorship contribution statement
Kristina Safar: Methodology, Software, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Elizabeth W. Pang: Writing – original draft, Writing – review & editing. Marlee M. Vandewouw: Methodology, Software, Writing – review & editing. Kathrina de Villa: Investigation, Writing – review & editing. Paul D. Arnold: Resources, Writing – review & editing. Alana Iaboni: Investigation, Writing – review & editing. Muhammed Ayub: Resources. Elizabeth Kelley: Resources, Writing – review & editing. Jason P. Lerch: Resources, Writing – review & editing, Funding acquisition. Evdokia Anagnostou: Conceptualization, Resources, Writing – review & editing, Supervision, Funding acquisition. Margot J. Taylor: Conceptualization, Resources, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
EA has consulted for and received personal fees from Roche, Quadrant Therapeutics, ONO and Impel Pharmaceuticals. EA has also received grants from Roche and Anavex. In addition, EA holds a patent for the device, “Anxiety Meter”, and has received nonfinancial support from AMO Pharma and CRA-Simons Foundation, royalties from APPI and Springer, and editorial honoraria from Wiley. The remaining authors (KS, EWP, MMV, KD, PDA, AI, MA, EK, JPL, and MJT) declare no commercial or financial interests or potential conflicts of interest.
Acknowledgements
Funding was provided for the POND data collection by the Ontario Brain Institute to EA and JPL, and by the Canadian Institutes of Health Research (CIHR) [MOP-119541; MOP-142379] to MJT.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103408.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abramovitch A., Abramowitz J.S., Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin. Psychol. Rev. 2013;33(8):1163–1171. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Aigner M., Sachs G., Bruckmüller E., Winklbaur B., Zitterl W., Kryspin-Exner I., et al. Cognitive and emotion recognition deficits in obsessive-compulsive disorder. Psychiatry Res. 2007;149(1–3):121–128. doi: 10.1016/j.psychres.2005.12.006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Journal of Psychiatry. 10.1176/appi.books.9780890425596.744053.
- Barratt E.L., Francis S.T., Morris P.G., Brookes M.J. Mapping the topological organisation of beta oscillations in motor cortex using MEG. Neuroimage. 2018;181:831–844. doi: 10.1016/j.neuroimage.2018.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuti S.M., Bianchin M., Angrilli A. Posture affects emotional responses: a head down bed rest and ERP study. Brain Cogn. 2013;82(3):313–318. doi: 10.1016/j.bandc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Berlin G.S., Lee H.-J. Response inhibition and error-monitoring processes in individuals with obsessive-compulsive disorder. J. Obs.-Compuls. Relat. Disord. 2018;16:21–27. doi: 10.1016/j.jocrd.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucke J.C., Sepulcre J., Talukdar T., Linnman C., Zschenderlein K., Endrass T., et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiat. 2013;70(6):619. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- Britton J.C., Stewart S.E., Killgore W.D.S., Rosso I.M., Price L.M., Gold A.L., et al. Amygdala activation in response to facial expressions in pediatric obsessive-compulsive disorder. Depress. Anxiety. 2010;27(7):643–651. doi: 10.1002/da.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoner N., Harrison B.J., Pujol J., Soriano-Mas C., Hernández-Ribas R., López-Solá M., et al. Enhanced brain responsiveness during active emotional face processing in obsessive compulsive disorder. World J. Biol. Psychiatry. 2011;12(5):349–363. doi: 10.3109/15622975.2011.559268. [DOI] [PubMed] [Google Scholar]
- Corcoran K.M., Woody S.R., Tolin D.F. Recognition of facial expressions in obsessive–compulsive disorder. J. Anxiety Disord. 2008;22(1):56–66. doi: 10.1016/j.janxdis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Daros A.R., Zakzanis K.K., Rector N.A. A quantitative analysis of facial emotion recognition in obsessive–compulsive disorder. Psychiatry Res. 2014;215(3):514–521. doi: 10.1016/j.psychres.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Davis N.J., Tomlinson S.P., Morgan H.M. January 11): The role of beta-frequency neural oscillations in motor control. J. Neurosci. 2012;32(2):403–404. doi: 10.1523/JNEUROSCI.5106-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations-signalling the status quo? Curr. Opin. Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Evans D.W., Lewis M.D., Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive–compulsive disorder. Brain Cogn. 2004;55(1):220–234. doi: 10.1016/S0278-2626(03)00274-4. [DOI] [PubMed] [Google Scholar]
- Figee M., Vink M., de Geus F., Vulink N., Veltman D.J., Westenberg H., Denys D. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol. Psychiatry. 2011;69(9):867–874. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Fogelson N., Li L., Diaz-Brage P., Amatriain-Fernandez S., Valle-Inclan F. Altered predictive contextual processing of emotional faces versus abstract stimuli in adults with Autism Spectrum Disorder. Clin. Neurophysiol. 2019;130(6):963–975. doi: 10.1016/j.clinph.2019.03.031. [DOI] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L., Group B.D.C. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88(1):220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. Emanuel Miller lecture: Confusions and controversies about Asperger syndrome. J. Child Psychol. Psychiatry. 2004;45 doi: 10.1111/j.1469-7610.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Göttlich M., Krämer U.M., Kordon A., Hohagen F., Zurowski B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum. Brain Mapp. 2014;35(11):5617–5632. doi: 10.1002/hbm.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B.-M., Park J.-Y., Kang D.-H., Lee S.J., Yoo S.Y., Jo H.J., et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2007;131(1):155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Hari, R., Salmelin, R. 2012. Magnetoencephalography: From SQUIDs to neuroscience. Neuroimage 20th Anniversary Special Edition. NeuroImage 61(2): 386-396. [DOI] [PubMed]
- Headley D.B., Paré D. Common oscillatory mechanisms across multiple memory systems. NPJ Sci. Learn. 2017;2(1):1–8. doi: 10.1038/s41539-016-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M., Overgaauw S., de Bruijn E.R.A. Social cognition and obsessive-compulsive disorder: A review of subdomains of social functioning. Front. Psych. 2020;11 doi: 10.3389/fpsyt.2020.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U.M.A., Flynn C., Moreci P., et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim K.L., Reynolds K.C., Alfano C.A. Social impairment in children with obsessive compulsive disorder: Do comorbid problems of inattention and hyperactivity matter? J. Obs.-Compuls. Relat. Disord. 2012;1:228–233. [Google Scholar]
- Lambe L.J., Burton C.L., Anagnostou E., Kelley E., Nicolson R., Georgiades S., et al. Clinical validation of the parent-report Toronto Obsessive-Compulsive Scale (TOCS): A pediatric open-source rating scale. JCPP Advances. 2021;1(4):e12056. doi: 10.1002/jcv2.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N.S., An S.K., Mataix-Cols D., Ruths F., Speckens A., Phillips M.L. Neural responses to facial expressions of disgust but not fear are modulated by washing symptoms in OCD. Biol. Psychiatry. 2007;61(9):1072–1080. doi: 10.1016/j.biopsych.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Leckman J.F., Grice D.E., Boardman J., Zhang H., Vitale A., Bondi C., et al. Symptoms of obsessive-compulsive disorder. Am. J. Psychiatry. 1997;154(7):911–917. doi: 10.1176/ajp.154.7.911. [DOI] [PubMed] [Google Scholar]
- Leung R.C., Pang E.W., Cassel D., Brian J.A., Smith M.L., Taylor M.J. Early neural activation during facial affect processing in adolescents with Autism Spectrum Disorder. Neuroimage Clin. 2015;7:203–212. doi: 10.1016/j.nicl.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung R.C., Pang E.W., Anagnostou E., Taylor M.J. Young adults with autism spectrum disorder show early atypical neural activity during emotional face processing. Front. Hum. Neurosci. 2018;12:57. doi: 10.3389/fnhum.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex. 2016;26(5):1910–1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner C., Simmons C., Kidd M., Chamberlain S.R., Fineberg N.A., van Honk J., et al. Differential effects of escitalopram challenge on disgust processing in obsessive–compulsive disorder. Behav. Brain Res. 2012;226(1):274–280. doi: 10.1016/j.bbr.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Luo Q., Holroyd T., Jones M., Hendler T., Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34(2):839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez-García A.V., Vakorin V.A., Kozhemiako N., Magnuson J.R., Iarocci G., Ribary U., et al. Children with autism spectrum disorder show atypical electroencephalographic response to processing contextual incongruencies. Sci. Rep. 2022;12(1):8948. doi: 10.1038/s41598-022-12475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. Treating disgust reactions in contamination-based obsessive–compulsive disorder. J. Behav. Ther. Exp. Psychiatry. 2006;37(1):53–59. doi: 10.1016/j.jbtep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mennella R., Leung R.C., Taylor M.J., Dunkley B.T. Disconnection from others in autism is more than just a feeling: Whole-brain neural synchrony in adults during implicit processing of emotional faces. Mol. Autism. 2017;8(1):1–12. doi: 10.1186/s13229-017-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.C., Nimmo-Smith I., Lawrence A.D. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nakao T., Okada K., Kanba S. Neurobiological model of obsessive-compulsive disorder: evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin. Neurosci. 2014;68(8):587–605. doi: 10.1111/pcn.12195. [DOI] [PubMed] [Google Scholar]
- Naumann S., Senftleben U., Santhosh M., McPartland J., Webb S.J. Neurophysiological correlates of holistic face processing in adolescents with and without autism spectrum disorder. J. Neurodev. Disord. 2018;10(1):1–13. doi: 10.1186/s11689-018-9244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys. Med. Biol. 2003;48(22):3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:1. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang E.W. Practical aspects of running developmental studies in the MEG. Brain Topogr. 2011;24(3):253–260. doi: 10.1007/s10548-011-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A., Hämäläinen M.S. A review of issues related to data acquisition and analysis in EEG/MEG studies. Brain Sci. 2017;7(6):58. doi: 10.3390/brainsci7060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rotge J.-Y., Guehl D., Dilharreguy B., Cuny E., Tignol J., Bioulac B., et al. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J. Psychiatry Neurosci. 2008;33(5):405–412. [PMC free article] [PubMed] [Google Scholar]
- Rueda-Delgado L.M., Solesio-Jofre E., Mantini D., Dupont P., Daffertshofer A., Swinnen S.P. Coordinative task difficulty and behavioural errors are associated with increased long-range beta band synchronization. Neuroimage. 2017;146 doi: 10.1016/j.neuroimage.2016.10.030. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E.E., Li Q., Siddiqui A., Krafft C., Oliver W.T., et al. Emotional perception: Meta-analyses of face and natural scene processing. Neuroimage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Safar K., Vandewouw M.M., Taylor M.J. Atypical development of emotional face processing networks in autism spectrum disorder from childhood through to adulthood. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar K., Vandewouw M.M., Pang E.W., de Villa K., Crosbie J., Schachar R., et al. Shared and distinct patterns of functional connectivity to emotional faces in Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder children. Front. Psychol. 2022;563 doi: 10.3389/fpsyg.2022.826527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Brody A.L., Schwartz J.M., Baxter L.R. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br. J. Psychiatry. 1998;173(S35):26–37. [PubMed] [Google Scholar]
- Shin N.Y., Lee T.Y., Kim E., Kwon J.S. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol. Med. 2014;44(6):1121–1130. doi: 10.1017/S0033291713001803. [DOI] [PubMed] [Google Scholar]
- Simon D.M., Wallace M.T. Dysfunction of sensory oscillations in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2016;68 doi: 10.1016/j.neubiorev.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spironelli C., Busenello J., Angrilli A. Supine posture inhibits cortical activity: Evidence from delta and alpha EEG bands. Neuropsychologia. 2016;89:125–131. doi: 10.1016/j.neuropsychologia.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer, R., Young, A.W., Pundt, I., Sprengelmeyer, A., Calder, A.J., Berrios, G., et al. 1997. Disgust implicated in obsessive- compulsive disorder. Proc. R. Soc. London Series B: Biol. Sci. 264(1389): 1767–1773. [DOI] [PMC free article] [PubMed]
- Stam C.J., Nolte G., Daffertshofer A. Phase lag index: assessment of functional connectivity from multichannel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007;28(11):1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J., Pang E.W. Magnetoencephalography: From Signals to Dynamic Cortical Networks. 2014. MEG and cognitive developmental studies; pp. 557–577. [Google Scholar]
- The Mathworks Inc. (2018): MATLAB. www.Mathworks.Com/Products/Matlab.
- Thibault R.T., Lifshitz M., Raz A. Body position alters human resting-state: Insights from multi-postural magnetoencephalography. Brain Imaging Behav. 2016;10:772–780. doi: 10.1007/s11682-015-9447-8. [DOI] [PubMed] [Google Scholar]
- Thorsen A.L., Hagland P., Radua J., Mataix-Cols D., Kvale G., Hansen B., van den Heuvel O.A. Emotional processing in obsessive-compulsive disorder: A systematic review and meta-analysis of 25 functional neuroimaging studies. Biol. Psychiatry: Cogn. Neurosci. Neuroimag. 2018;3(6):563–571. doi: 10.1016/j.bpsc.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Pipa G., Neuenschwander S., Wibral M., Singer W. A new look at gamma? High- (>60 Hz) γ-band activity in cortical networks: function, mechanisms and impairment. Prog. Biophys. Mol. Biol. 2011;105(1–2):14–28. doi: 10.1016/j.pbiomolbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- van Veen B., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- van Velzen L.S., de Wit S.J., Ćurĉić-Blake B., Cath D.C., de Vries F.E., Veltman D.J., et al. Altered inhibition-related frontolimbic connectivity in obsessive-compulsive disorder. Hum. Brain Mapp. 2015;36(10):4064–4075. doi: 10.1002/hbm.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewouw M.M., Choi E.J., Hammill C., Arnold P., Schachar R., Lerch J.P., et al. Emotional face processing across neurodevelopmental disorders: a dynamic faces study in children with autism spectrum disorder, attention deficit hyperactivity disorder and obsessive-compulsive disorder. Transl. Psychiatry. 2020;10(1):1–12. doi: 10.1038/s41398-020-01063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via E., Cardoner N., Pujol J., Alonso P., López-Solà M., Real E., et al. Amygdala activation and symptom dimensions in obsessive–compulsive disorder. Br. J. Psychiatry. 2014;204(1):61–68. doi: 10.1192/bjp.bp.112.123364. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Pearson Assessments; Minneapolis, MN: 1999. Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children—4th Edition (WISC-IV®) [Google Scholar]
- Wechsler, D. 2012. Wechsler Preschool and Primary Scale of Intelligence. 4th Ed. San Antonio, TX. Retrieved from http://www.buros.org/copyright-and-permissions.
- Wechsler DJSAPC. 2014. Wechsler intelligence scale for children–5th Edition (WISC-V). Bloomington, MN: Pearson.
- Weidt S., Lutz J., Rufer M., Delsignore A., Jakob N.J., Herwig U., Bruehl A.B. Common and differential alterations of general emotion processing in obsessive-compulsive and social anxiety disorder. Psychol. Med. 2016;46(7):1427–1436. doi: 10.1017/S0033291715002998. [DOI] [PubMed] [Google Scholar]
- Woody S.R., Teachman B.A. Intersection of disgust and fear: Normative and pathological views. Clin. Psychol. Sci. Pract. 2000;7(3):291. [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: Identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Cocchi L., Fornito A., Murray M.M., Bullmore E. Connectivity differences in brain networks. Neuroimage. 2012;60(2):1055–1062. doi: 10.1016/j.neuroimage.2012.01.068. [DOI] [PubMed] [Google Scholar]
- Zhang T., Wang J., Yang Y., Wu Q., Li B., Chen L., et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J. Psychiatry Neurosci. 2011;36(1):23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.