Abstract
Introduction
This study assessed the levels of compliance to topical minoxidil (TM) among male and female patients with androgenetic alopecia (AGA) and analyzed the factors associated with minoxidil discontinuation.
Method
A retrospective study was conducted among 400 consecutive patients with AGA who presented to a dermatology clinic and who were prescribed minoxidil 2% or 5% in the past 5 years. Demographic factors, other previous treatments, and minoxidil parameters including the dose (2% or 5%), total duration of use, treatment results, and side effects were collected.
Result
The mean age of the patients was 32.41 years [standard deviation (SD) 8.18], and 66.5% were female. The majority of patients (82.5%) did not receive any previous treatment for AGA. Of the total patients, 345 (86.3%) have discontinued minoxidil. Discontinuation rate showed no association with sex (p = 0.271), age category (p = 0.069), or previous treatment (p = 0.530). Furthermore, the likelihood of minoxidil discontinuation decreased with the increase in treatment duration (p < 0.001) and was significantly lower among patients who reported improvement (69.3%) or stabilization of hair shedding (64.1%) compared with those who reported baby hair (88.9%) or no efficacy (95.3%) (p < 0.001). Furthermore, having experienced an adverse effect of minoxidil was associated with 93.6% discontinuation rate compared with 75.8% in the case of no side effects (p < 0.001). Adjusted analysis showed that minoxidil discontinuation was independently association with longer duration of use [> 1 year; odds ratio (OR) 0.22; p < 0.001], perceived improvement (OR 0.17; p < 0.001) or stabilization (OR 0.14; p < 0.001), and the occurrence of side effects (OR 3.06; p = 0.002).
Conclusions
The clinical use of TM in AGA is limited by a substantially low compliance even in absence of adverse effects. We emphasize the importance of educating patients regarding the treatment’s side effects and the need to use minoxidil for a minimum of 12 months to assess treatment efficacy.
Keywords: Androgenetic alopecia, Compliance, Minoxidil, Pattern hair loss, Side effects
Key Summary Points
| We assessed the determinants of compliance to topical minoxidil (TM) among patients with androgenetic alopecia. |
| The observed discontinuation rate of TM was 86.3%. The occurrence of side effects was reported in 46.5% of the patients and was associated with a higher TM discontinuation rate. |
| Adjusted analysis showed that the likelihood of TM discontinuation independently decreased with a longer duration of use of more than 1 year and with perceived improvement or stabilization. We also observed a positive relationship between the duration of TM use and patient-reported efficacy. |
| On the basis of our results, patients should be encouraged to use TM for a minimum of 1 year to assess its efficacy and be educated about possible side effects and how to deal with them. |
Introduction
Androgenetic alopecia (AGA) is by far the most common cause of hair loss. It affects approximately 50% of men by the age of 50 and 20–53% of women by the age of 50, and its prevalence increases with advancing age [1, 2]. Topical minoxidil (TM) is the only treatment approved by the Food and Drug Administration (FDA) for female patients with patterned hair loss, while for males, TM and oral finasteride are the only FDA-approved treatments for AGA. Nevertheless, the efficacy of minoxidil is limited. Studies suggest that only one-third of patients (32%) experience a positive cosmetic effect and terminal hair regrowth after 1 year of use [3–6]. Additionally, the use of TM in AGA requires a daily long-term commitment, most likely for the duration of an individual’s life, which constitutes a major limitation for compliance [5]. Unfortunately, months after stopping minoxidil, all the newly grown hairs will fall out [3].
Finally, minoxidil use is associated with several side effects, of which itching of the scalp, increased dandruff, and erythema are commonly reported. The most common causes underlying these symptoms include irritant contact dermatitis, allergic contact dermatitis, or an exacerbation of seborrheic dermatitis [7]. The development of hypertrichosis on the face and hands is another common side effect observed with TM. Studies have reported undesired hair growth in up to 51% of female participants [8, 9]. Moreover, headache was reported in 7% of individuals using minoxidil [10]. These observations indicate that minoxidil users are exposed to a high risk of nonadherence and treatment interruption for multiple reasons that need to be explored to improve the care offered to these patients.
This study aimed to assess the levels of compliance to TM among male and female patients with AGA, and to analyze the occurrence of side effects and other factors associated with minoxidil discontinuation.
Methods
Design and Setting
This was a retrospective study conducted at the author’s private dermatology clinic located in Jeddah, Saudi Arabia, between February and September 2022. The study protocol and tools were reviewed and ethically approved by the institutional review board of Jeddah University (Registration number: HAP-O2-J-094).
Population
The study included consecutive patients with AGA, who presented to the clinic for a hair consultation during the study period, and who were prescribed and used minoxidil 2% or 5% for less than 5 years and more than 3 months before the visit.
Sampling
A convenience, non-random sampling was used to include all patients who provided consent. A minimum sample size of 377 was targeted to detect an unknown percentage of minoxidil discontinuation (p = 50%) with 80% statistical power and 5% margin of error.
Data Collection
An Excel sheet was designed to collect the following data: (1) demographic data; (2) other treatments used for AGA; (3) minoxidil dose (2% or 5%); (4) total duration of use; (5) treatment results including improvement with hair regrowth, stabilization of hair shedding and/or development of baby hair, or no efficacy and/or worsening; (6) side effects; and (7) current minoxidil use (continued versus discontinued). If minoxidil was discontinued, the questionnaire also included the reasons for discontinuation.
Ethical Clearance
All participants signed an informed consent stating that their data will be used for research purposes and that the results will be published in a peer-reviewed specialized journal.
Statistical Methods
Data were coded and analyzed using the Statistical Package for Social Sciences version 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to present the study data as frequencies and percentages for categorical variables and means [standard deviation (SD)] for continuous variables. Chi-squared test was used to analyze factors associated with minoxidil discontinuation. An unadjusted and adjusted logistic regression model was used to analyze independent factors of minoxidil discontinuation; results were presented as odds ratio (OR) with 95% confidence interval (95% CI). In the adjusted model, a dummy variable was created for long duration of use (> 12 months) as it was the only category showing significance in the unadjusted model. A p-value < 0.05 was considered to reject the null hypothesis.
Results
A total of 400 consecutive patients with AGA were included, with a mean age of 32.41 years (SD 8.18) and 66.5% female. The majority of patients (82.5%) did not receive any other treatment for AGA, while the remaining patients received one (14.5%) or two (3.0%). The most frequently used previous treatments included platelet-rich plasma (PRP) (8.5%), hair transplantation (4.0%), and autologous cellular micrografts (ACM) (3.8%) (Table 1).
Table 1.
Participants’ characteristics (N = 400)
| Parameter | Level | Mean | SD |
|---|---|---|---|
| Age | (Years) | 32.41 | 8.18 |
| Parameter | Level | Frequency | Percentage |
| Sex | Male | 134 | 33.5 |
| Female | 266 | 66.5 | |
| Other treatments for androgenetic alopeciaa | Platelet-rich plasma | 34 | 8.5 |
| Hair transplant | 16 | 4.0 | |
| Autologous cellular micrografs | 15 | 3.8 | |
| Finastaride tablets | 10 | 2.5 | |
| Mesotherapy | 5 | 1.3 | |
| Spinorolactone | 2 | 0.5 | |
| Adipose-derived stem cells | 1 | 0.3 | |
| Other | 4 | 1.0 | |
| Number of other treatments | 0 | 165 | 82.5 |
| 1 | 29 | 14.5 | |
| 2 | 6 | 3.0 |
aA patient may have used more than one treatment
The majority of patients took minoxidil for a short period of time, such as < 1 month (20.8%), 2–3 months (24.8%), and 4–6 months (21.0%), while very few patients took it for > 1 year (12%). The most frequently used dosage form was 5% (91.0%). Regarding treatment outcomes, 22.0% of the patients reported improvement and hair regrowth, 9.8% reported stabilization of hair loss, and 4.5% reported having baby hair, while 63.8% reported no efficacy of the treatment or worsening. At least one side effect was reported in 46.5% of the patients, and two or more side effects were reported in 12.8% of the patients (Table 2).
Table 2.
Minoxidil-related parameters (N = 400)
| Parameter | Level | Frequency | Percentage |
|---|---|---|---|
| Duration of use | ≤ 1 month | 83 | 20.8 |
| 2–3 months | 99 | 24.8 | |
| 4–6 months | 84 | 21.0 | |
| 6–12 months | 62 | 15.5 | |
| > 1 year | 48 | 12.0 | |
| Not documented | 24 | 6.0 | |
| Dose | 2% | 36 | 9.0 |
| 5% | 364 | 91.0 | |
| Patient-reported outcomes | Improved | 88 | 22.0 |
| Stabilized | 39 | 9.8 | |
| Baby hair | 18 | 4.5 | |
| No efficacy | 255 | 63.8 | |
| Side effects | No | 214 | 53.5 |
| Yes | 186 | 46.5 | |
| Number of complications | None | 214 | 53.5 |
| 1 | 135 | 33.8 | |
| 2 | 42 | 10.5 | |
| 3+ | 9 | 2.3 | |
| Discontinuation | No | 55 | 13.8 |
| Yes | 345 | 86.3 |
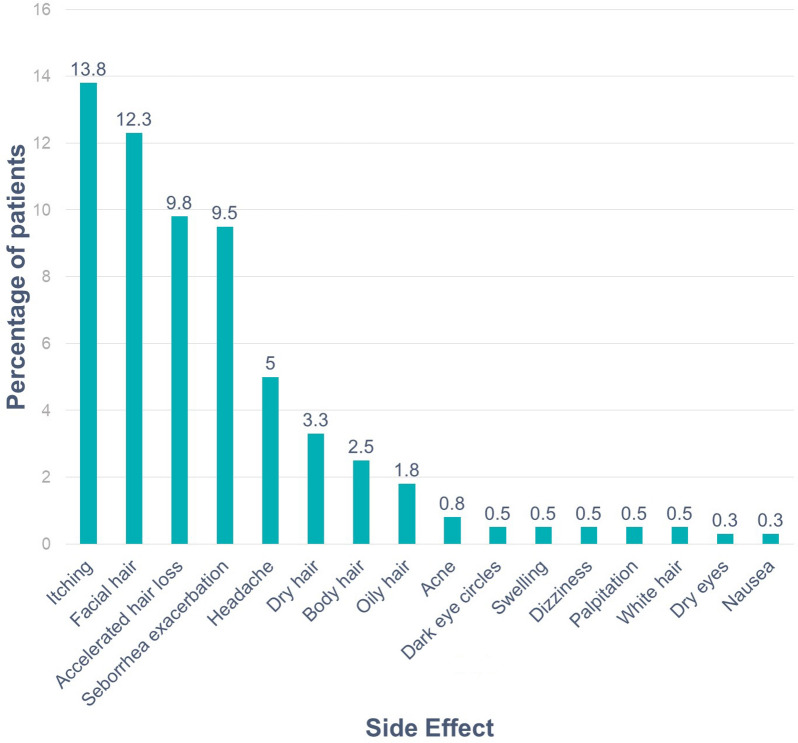
The five most frequently reported side effects were scalp itching (13.8%), facial hair (12.3%), increased hair loss (9.8%), seborrhea exacerbation (9.5%), and headache (5.0%) (Fig. 1).
Fig. 1.
Side effects of minoxidil. Bars represent the percentage of patients who reported the given side effect
Of the total patients, 345 (86.3%) discontinued minoxidil. Discontinuation rate showed no association with sex (p = 0.271), age category (p = 0.069), or previous treatment (p = 0.530). Furthermore, the likelihood of minoxidil discontinuation decreased with increased treatment duration (p < 0.001) and was significantly lower among patients who reported improvement (69.3%) or stabilization of hair shedding (64.1%), compared with those who reported baby hair (88.9%) or no efficacy (95.3%) (p < 0.001). Having experienced an adverse effect of minoxidil was associated with 93.6% discontinuation rate compared with 75.8% in the case of no side effects (p < 0.001) (Table 3).
Table 3.
Factors associated with minoxidil discontinuation
| Factor | Treatment discontinuation | p-Value | |||
|---|---|---|---|---|---|
| No (N = 51) | Yes (N = 345) | ||||
| n | % | N | % | ||
| Sex | |||||
| Male | 22 | 16.4 | 112 | 83.6 | |
| Female | 33 | 12.4 | 233 | 87.6 | 0.271 |
| Age | |||||
| ≤ 32 years | 26 | 11.1 | 208 | 88.9 | |
| > 32 years | 29 | 17.5 | 137 | 82.5 | 0.069 |
| Other treatments for androgenetic alopecia | |||||
| No | 43 | 13.2 | 282 | 86.8 | |
| Yes | 12 | 16.0 | 63 | 84.0 | 0.530 |
| Minoxidil duration | |||||
| ≤ 1 month | 6 | 7.2 | 77 | 92.8 | |
| 2–3 months | 3 | 3.0 | 96 | 97.0 | |
| 4–6 months | 11 | 13.1 | 73 | 86.9 | |
| 6–12 months | 9 | 14.5 | 53 | 85.5 | |
| > 1 year | 22 | 45.8 | 26 | 54.2 | |
| Not documented | 4 | 16.7 | 20 | 83.3 | < 0.001* |
| Self-reported outcomes | |||||
| Improvement | 27 | 30.7 | 61 | 69.3 | |
| Stabilization | 14 | 35.9 | 25 | 64.1 | |
| Baby hair | 2 | 11.1 | 16 | 88.9 | |
| No improvement | 12 | 4.7 | 243 | 95.3 | < 0.001* |
| Complications | |||||
| No | 40 | 24.2 | 125 | 75.8 | |
| Yes | 15 | 6.4 | 220 | 93.6 | < 0.001* |
| No. of complications | |||||
| 0 | 40 | 24.2 | 125 | 75.8 | |
| 1 | 11 | 6.6 | 155 | 93.4 | |
| 2+ | 4 | 5.8 | 65 | 94.2 | < 0.001* |
*Statistically significant result (p < 0.05)
In the adjusted regression model, minoxidil discontinuation was independently association with longer duration of use (> 1 year; OR 0.22; p < 0.001), perceived improvement (OR 0.17; p < 0.001) or stabilization (OR 0.14; p < 0.001), and the occurrence of side effects (OR 3.06; p = 0.002) (Table 4).
Table 4.
Independent factors associated with minoxidil discontinuation
| Predictor/level | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Minoxidil duration | ||||||||
| ≤ 1 month | Ref | < 0.001* | – | – | – | |||
| 2–3 months | 2.48 | 0.60 | 10.29 | 0.207 | – | – | – | |
| 4–6 months | 0.52 | 0.18 | 1.47 | 0.216 | – | – | – | |
| 6–12 months | 0.46 | 0.15 | 1.37 | 0.162 | – | – | – | |
| > 1 year§ | 0.09 | 0.03 | 0.25 | < 0.001* | 0.22 | 0.10 | 0.46 | < 0.001* |
| Not documented | 0.39 | 0.10 | 1.51 | 0.174 | – | – | – | |
| Self-reported outcomes | ||||||||
| Improvement | 0.11 | 0.05 | 0.23 | < 0.001* | 0.17 | 0.08 | 0.37 | < 0.001* |
| Stabilization | 0.09 | 0.04 | 0.21 | < 0.001* | 0.14 | 0.06 | 0.37 | < 0.001* |
| Baby hair | 0.40 | 0.08 | 1.92 | 0.249 | 0.48 | 0.09 | 2.49 | 0.383 |
| No improvement | Ref | < 0.001* | Ref | |||||
| Complications | ||||||||
| No | Ref | Ref | ||||||
| Yes | 4.64 | 2.46 | 8.73 | < 0.001* | 3.06 | 1.53 | 6.10 | 0.002* |
| No. of complications | ||||||||
| 0 | Ref | – | – | – | ||||
| 1 | 4.51 | 2.22 | 9.15 | < 0.001* | – | – | – | |
| 2+ | 5.20 | 1.78 | 15.17 | 0.003* | – | – | – | |
OD odds ratio, CI confidence interval
Ref: category used as reference in calculation of OR
*Statistically significant result (p < 0.05)
§Used as dummy variable in adjusted model
Of the 345 patients who discontinued minoxidil, 150 provided the reason for discontinuation as a response to the questionnaire. The most frequently mentioned reason was lack of compliance (20.0%), followed by unsatisfactory results (11.3%). In addition, one patient elicited cost of the treatment as a reason for discontinuation (results not presented in tables).
Further analysis of the association between duration of minoxidil use and treatment outcomes, including improvement, was carried out in the total population and among the patients who discontinued separately (Table 5). Results showed that longer treatment duration was associated with a gradual increase in the percentage of patient-reported improvement (p < 0.001) in both populations.
Table 5.
Association of duration of minoxidil use with patient-reported improvement among all participants and discontinuers (N = 400)
| Duration of use | All participants (N = 400) | Discontinuers (N = 346) | ||
|---|---|---|---|---|
| n | Improvement (%) | n | Improvement (%) | |
| ≤ 1 month | 83 | 1.2 | 77 | 0.0 |
| 2–3 months | 99 | 19.2 | 96 | 17.7 |
| 4–6 months | 84 | 29.8 | 73 | 24.7 |
| 6–12 months | 62 | 25.8 | 53 | 20.8 |
| > 12 months | 48 | 43.8 | 26 | 42.6 |
| Not documented | 24 | 25.0 | 20 | 20.0 |
| p-Value | < 0.001* | < 0.001* | ||
*Statistically significant result (p < 0.05)
Discussion
Summary of the Findings
The purpose of this research is to evaluate the level of treatment compliance in patients treated with TM for AGA. This subsequently enables us to identify the factors associated with discontinuation of minoxidil. The observed discontinuation rate of TM (including both 2% and 5% forms) was 86.3%. Only 15.5% and 12.0% of the total patients achieved a long-tern treatment duration of 6–12 months and ≥ 12 months, respectively. The occurrence of side effects was reported in 46.5% of the patients and was associated with a higher minoxidil discontinuation rate (93.6%); however, the discontinuation rate remained very high (75.8%) even in the absence of complications. The most common complications were irritation (13.8%), facial hair (12.3%), increased hair loss (9.8%), and seborrhea exacerbation (9.5%). The discontinuation rate increased with the increase in the number of adverse events. Otherwise, there was no difference across sex or age groups. Adjusted analysis showed that the likelihood of minoxidil discontinuation independently decreased by 78% (OR 0.22) with a longer duration of use (> 1 year), and by 83% and 86%, respectively, in the case of perceived improvement or stabilization. Furthermore, the likelihood of discontinuation increased by threefold in the case of side effects (OR 3.06), and we observed a positive relationship between the duration of minoxidil use and patient-reported efficacy.
Irritant and Allergic Contact Dermatitis
The use of TM is frequently associated with scalp irritation or allergic contact dermatitis, which induces debilitating symptoms, such as pruritus and erythema, ultimately leading to treatment cessation [9]. In most cases, contact dermatitis is caused by irritation or sometimes contact allergy from propylene glycol, a nonactive ingredient in commercial formulations, rather than the active principle [7]. Less commonly, there is a true allergic contact dermatitis from minoxidil itself. Patch testing may be useful to determine the source of sensitization.
Seborrhea Exacerbation
Exacerbation of seborrheic dermatitis is another concern that was reported with minoxidil [7]. This reaction may mimic irritant or allergic contact dermatitis; therefore, it is necessary to differentiate the two entities to provide adequate management and enable treatment continuation since seborrhea can be controlled without interruption of minoxidil [7]. The mechanism behind minoxidil-induced seborrhea is not elucidated; however, minoxidil is still believed to suppress the androgen receptor activity, which would decrease androgen-induced sebum production [11, 12]. Minoxidil may possibly exhibit a paradoxical action on sebocytes, stimulating their androgen-receptor-related pathways instead of inhibiting them.
Hypertrichosis
Minoxidil is a potent arteriolar vasodilator that opens potassium channels of smooth muscle cells leading to local hyperpolarization. This mechanism results in better perfusion to hair follicles, which may explain the reversal of hair loss [9]. Minoxidil also stimulates the vascular endothelial growth factor (VEGF)-mediated perifollicular angiogenesis in human dermal papilla cells, leading to acceleration of hair growth in a dose-dependent fashion as shown by Lachgar et al. [13]. However, these effects are not specific to scalp follicles, which may cause hair growth in other non-desired areas, such as the temples, forehead, cheeks, and other more remote areas of the skin [9]. In a previously cited study by Blume-Peytavi et al., the prevalence of hypertrichosis in sideburns and temples was 26% and 25% in patients treated with 2% minoxidil solution, versus 11% and 22% in those treated with 5% minoxidil foam, respectively [10]. Fortunately, drug-induced hypertrichosis is a reversible condition in most cases and resolves spontaneously after treatment cessation [14].
Increased Hair Loss
Another anti-alopecic mechanism of minoxidil with the initiation of minoxidil treatment is the shortening of the telogen phase, which induces the shedding of the club telogen hairs and subsequent shift to reenter anagen phase with the regrowth of healthy and thick anagen hairs (immediate telogen release) [9]. This results in the development of increased hair loss/shedding as an adverse event [9], as reported in our study.
Lesser Likelihood of Discontinuation with Prolonged Use
We observed a tendency for better adherence to TM after long-term use (6 months and beyond). This may be explained by early discontinuation being motivated by side effects that occur during the first weeks or months of treatment. Beyond this period, the likelihood of adverse effects may decrease. More notably, we observed that duration of use for > 1 year was independently associated with 78% decrease of the discontinuation rate, which may constitute a threshold for enhanced compliance. Another possible explanation is that long-term adherence may be motivated by perceived improvement in the initial period of treatment. This is in agreement with the observed gradual increase in patient-reported improvement with the duration of use, reaching up to 43.8% after > 1 year of use, even among the patients who discontinued TM. This emphasizes the importance of achieving a long-term compliance to minoxidil, in the absence of serious side effects, to induce a virtuous cycle of improvement—satisfaction—adherence. Beyond these factors, several patient- and physician-related factors may contribute to minoxidil adherence [15].
High Rate of Discontinuation Even in the Absence of Side Effects
Our findings revealed that the use of TM had led to clinical improvement in a modest proportion (22.0%) of patients. This is associated with early interruption of treatment occurring even in the absence of adverse events in several patients, probably because of low perceived clinical improvement. A recent survey of 93 patients with AGA found that 68% of previous TM users had stopped because of lack of effectiveness [16]. While safety outcomes were not mentioned as a real complaint, a substantial percentage of patients reported significant concerns about cost (47%) and life-long use (32%). In the present study, only one patient was concerned with minoxidil cost and another one was concerned with life-long use; both were reported as reasons for discontinuation.
Other Observations
In the present study, the most frequently used previous treatment was platelet-rich plasma (8.5%), followed by hair transplantation (4.0%) and ACM (3.8%). Furthermore, only 2.5% of the patients reported having used finasteride previously. A meta-analysis of ten studies (N = 165 participants) examining PRP treatment in patients with AGA showed a statistically significant overall standardized mean difference in hair density of 0.58 compared with baseline. Authors concluded that PRP is beneficial in the treatment of AGA [17]. Hair transplantation is an established effective treatment for AGA in male patients, especially in advanced stages, with graft survival being greater than 90% [18], but is limited by a weak occipital donor area in many female patients with AGA [19, 20]. ACM is an autologous cellular suspension obtained from three scalp tissue specimens disaggregated by Rigeneracons medical device (Human Brain Wave, Turin, Italy). The author, in a retrospective cohort study of 140 patients (80.7% of them female), concluded that ACM is a promising treatment in early AGA with a short-term favorable response observed in up to approximately two-thirds of patients [21]. Ruiz et al. studied the efficacy of ACM among 100 cases of AGA and showed significant increase in hair density and thick hair percentage at 2 months [22].
Although finasteride has proven efficacy and is FDA approved for treating male patients with AGA, it was previously used by a limited number of patients in the present study. In clinical trials, patients taking oral finasteride predominantly reported sexual dysfunction as a side effect (erectile dysfunction, loss of libido, ejaculation disorder, and gynecomastia) in 4.5% of cases [23]. Post-finasteride syndrome (PFS), which includes sexual dysfunction, is believed to persist after cessation of finasteride therapy [24]. The probable reason for the low percentage of finasteride usage in this study is the fear of potential sexual dysfunction and PFS, which may also be influenced by the culture in which the study was conducted.
Furthermore, the low male ratio observed in the present study, approximately half the number of female patients, reflects the actual percentage of patients presenting to the author’s clinic, as female patients are more concerned about their hair loss than males, especially in the local culture. Psychological stress in women is usually more severe, as hair is one of the most important components of women’s physical appearance [25–27].
Strategies to Reduce Discontinuation
Patient education is of paramount importance for optimizing compliance to TM. Before prescribing the drug, the expected level and time of response must be thoroughly discussed with patients to avoid any feelings of disappointment. Patients should be informed about the necessity of long-term application of minoxidil to achieve the desired cosmetic outcome and psychosocial well-being, since the clinical response can only be significantly noticed after an average of 4–6 months of treatment [3, 9]. Furthermore, patients must be informed about the possible side effects, as full disclosure of side effects was shown to enhance primary adherence [28]. Nonetheless, patients should be reassured about the reversibility and normality of minoxidil-related esthetic complications (i.e., increased hair shedding and hypertrichosis), as these effects are linked to its mechanism of action. Thus, increased hair loss and hypertrichosis usually disappear shortly once the treatment progresses and ends, respectively.
In patients with contact dermatitis, a patch test should be performed to identify the allergen. In the case of irritation or allergy from propylene glycol, a propylene-glycol-free formulation can be prescribed to reverse the sensitization process and enhance drug acceptability and tolerability [9, 29, 30]. In such cases, the foam form is preferred over the liquid form, as it does not contain any propylene glycol, usually required for the liquid formulation to serve as a vehicle that dissolves hydrophobic minoxidil in water [31]. Minoxidil-induced seborrhea and itching can also be treated by alternating between ketoconazole or zinc pyrithione or selenium-sulfide-based shampoos. Corticosteroid shampoos can also be used once weekly in some patients [32]. The off-label use of low-dose oral minoxidil can be also used in selected patients after discussing its side effects, which include hypertrichosis (15.1%), lightheadedness (1.7%), fluid retention (1.3%), tachycardia (0.9%), headache (0.4%), and periorbital edema (0.3%) [33]. Therianou and colleagues recently reported nine patients who demonstrated true patch test proven contact allergy to TM but tolerated oral minoxidil without any notable complications. The patients used oral minoxidil at the low dose of 0.25 mg twice daily, and follow-up for these patients ranged from 7–33 months. None of the nine patients reported side effects and all were satisfied with their treatment results [34].
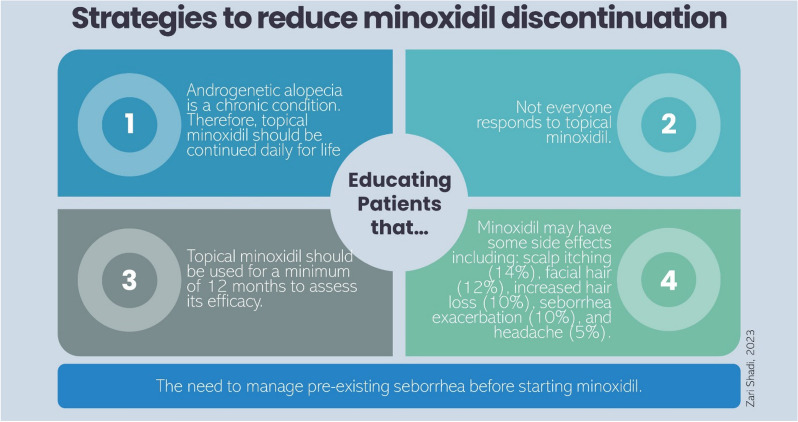
Additionally, selecting the most suitable candidates to long-term TM therapy can prevent treatment discontinuation. Preexisting seborrhea should be managed prior to intuition of TM. Goren et al. demonstrated that minoxidil efficacy in patients with AGA can be predicted by studying the level of enzymatic activity of hair follicle sulfotransferase (SULT1A1), the enzyme that converts minoxidil to its active form minoxidil sulfate, with 95% sensitivity and 73% specificity [35]. Consistently, Roberts et al. supported the same hypothesis and found a sensitivity of 93% and a specificity of 83% [36]; however, the clinical validity of this biomarker requires further assessment [37]. The availability of such prognostic tools would provide more accurate predictions of the treatment benefit–risk ratio, which can be shared with patients to involve them in therapeutic decision-making. The most important strategies to prevent minoxidil discontinuation are summarized in Fig. 2.
Fig. 2.
Strategies to reduce minoxidil discontinuation
Limitations
One essential limitation of this study is the inability to address other barriers that may compromise patients’ adherence to treatment, such as personal factors, socioeconomic status, comorbidities, level of education, stressful events, previous experiences with medications, difficulties of treatment application, etc. Moreover, the study failed to assess the levels of patients’ knowledge about minoxidil and their levels of expectations regarding its efficacy, as well as their actual timing and method of application.
Conclusions
The clinical use of TM in AGA is limited by a substantially low compliance even in the absence of adverse effects. A majority of the discontinuation events occurred during the first few months of treatment. The occurrence of side effects further increased the likelihood of minoxidil discontinuation. We emphasize the importance of educating patients that TM should be used for a minimum of 12 months to assess its efficacy, and to educate them about possible side effects to engage them in the decision-making process. Furthermore, patients should be closely monitored for adverse effects related to minoxidil, and adequate interventions should be implemented in a timely manner to reverse or reduce the severity of these adverse effects and improve patient compliance.
Acknowledgements
The author thanks Dr. Mohamed Amine HAIRECHE for his valuable assistance in analyzing the data of the present study. The author also thanks the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
S. Zari conceived the research idea, designed the study, supervised the data collection and analysis, interpreted the results, redacted the scientific content of the manuscript, and reviewed and validated the final version.
Ethics and Compliance guidelines
The present study was conducted in compliance with the international ethical standards, with respect of the principles of autonomy, privacy, and nonmaleficence. It received ethical approval from the institutional review board of Jeddah University (Registration number: HAP-O2-J-094). All participants have signed an informed consent stating that their data will be used for research purpose and that the results will be published in a peer-reviewed specialized journal.
Disclosures
S. Zari has nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Bolduc C, Shapiro J. Management of androgenetic alopecia. Am J Clin Dermatol. 2000;1:151–158. doi: 10.2165/00128071-200001030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Price VH. Treatment of hair loss. New Engl J Med. 1999;341:964–973. doi: 10.1056/NEJM199909233411307. [DOI] [PubMed] [Google Scholar]
- 3.de Villez RL. Topical minoxidil therapy in hereditary androgenetic alopecia. Arch Dermatol. 1985;121:197–202. doi: 10.1001/archderm.1985.01660020055017. [DOI] [PubMed] [Google Scholar]
- 4.Olsen EA, DeLong ER, Weiner MS. Dose–response study of topical minoxidil in male pattern baldness. J Am Acad Dermatol. 1986;15:30–37. doi: 10.1016/S0190-9622(86)70138-2. [DOI] [PubMed] [Google Scholar]
- 5.Katz HI. Topical minoxidil: review of efficacy. Clin Dermatol. 1988;6:195–199. doi: 10.1016/0738-081X(88)90087-9. [DOI] [PubMed] [Google Scholar]
- 6.Olsen EA, Whiting D, Bergfeld W, Miller J, Hordinsky M, Wanser R, et al. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2007;57:767–774. doi: 10.1016/j.jaad.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Friedman ES, Friedman PM, Cohen DE, Washenik K. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309–312. doi: 10.1067/mjd.2002.119104. [DOI] [PubMed] [Google Scholar]
- 8.Tsuboi R, Tanaka T, Nishikawa T, Ueki R, Yamada H, Katsuoka K, et al. A randomized, placebo-controlled trial of 1% topical minoxidil solution in the treatment of androgenetic alopecia in Japanese women. Eur J Dermatol. 2007;17:37–44. doi: 10.1684/ejd.2007.0187. [DOI] [PubMed] [Google Scholar]
- 9.Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 10.Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia BN. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65:1126–1134.e2. doi: 10.1016/j.jaad.2010.09.724. [DOI] [PubMed] [Google Scholar]
- 11.Hsu C-L, Liu J-S, Lin A-C, Yang C-H, Chung W-H, Wu W-G. Minoxidil may suppress androgen receptor-related functions. Oncotarget. 2014;5:2187–2197. doi: 10.18632/oncotarget.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceruti JM, Leirós GJ, Balañá ME. Androgens and androgen receptor action in skin and hair follicles. Mol Cell Endocrinol. 2018;465:122–133. doi: 10.1016/j.mce.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Lachgar S, Charveron M, Gall Y, Bonafe JL. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol. 1998;138:407–411. doi: 10.1046/j.1365-2133.1998.02115.x. [DOI] [PubMed] [Google Scholar]
- 14.Saleh D, Yarrarapu SN, Cook C. Hypertrichosis. Island: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 15.Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26:155–159. doi: 10.5001/omj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nussbaum D, Murphy E, Nelson K, Gonzalez-Lopez A, Qureshi A, Schwartz J, Friedman A. A survey of patient attitudes towards topical minoxidil in the treatment of hair loss. J Drugs Dermatol JDD. 2022;21(10):1140–1142. [PubMed] [Google Scholar]
- 17.Gupta AK, Cole J, Deutsch DP, Everts PA, Niedbalski RP, Panchaprateep R, et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatol Surg. 2019;45:1262–1273. doi: 10.1097/DSS.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 18.Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010;11:1295–1304. doi: 10.1517/14656561003752730. [DOI] [PubMed] [Google Scholar]
- 19.RezanEkmekci T, Koslu A. Phototrichogram findings in women with androgenetic alopecia. Skin Res Technol. 2006;12:309–312. doi: 10.1111/j.0909-752X.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Ekmekci T, Sakiz D, Koslu A. Occipital involvement in female pattern hair loss: histopathological evidences. J Eur Acad Dermatol Venereol. 2010;24:299–301. doi: 10.1111/j.1468-3083.2009.03411.x. [DOI] [PubMed] [Google Scholar]
- 21.Zari S. Short-term efficacy of autologous cellular micrografts in male and female androgenetic alopecia: a retrospective cohort study. Clin Cosmet Investig Dermatol. 2021;14:1725–1736. doi: 10.2147/CCID.S334807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz RG, Rosell JMC, Ceccarelli G, de Sio C, de Angelis GC, Pinto H, et al. Progenitor-cell-enriched micrografts as a novel option for the management of androgenetic alopecia. J Cell Physiol. 2020;235:4587–4593. doi: 10.1002/jcp.29335. [DOI] [PubMed] [Google Scholar]
- 23.Gupta AK, Talukder M, Williams G. Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J Dermatol Treat. 2022;33:2946–2962. doi: 10.1080/09546634.2022.2109567. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AK, Venkataraman M, Talukder M, Bamimore MA. Finasteride for hair loss: a review. J Dermatol Treat. 2022;33:1938–1946. doi: 10.1080/09546634.2021.1959506. [DOI] [PubMed] [Google Scholar]
- 25.Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189–199. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuang X-S, Zheng Y-Y, Xu J-J, Fan W-X. Quality of life in women with female pattern hair loss and the impact of topical minoxidil treatment on quality of life in these patients. Exp Ther Med. 2013;6:542–546. doi: 10.3892/etm.2013.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29:568–575. doi: 10.1016/0190-9622(93)70223-G. [DOI] [PubMed] [Google Scholar]
- 28.Polinski JM, Kesselheim AS, Frolkis JP, Wescott P, Allen-Coleman C, Fischer MA. A matter of trust: patient barriers to primary medication adherence. Health Educ Res. 2014;29:755–763. doi: 10.1093/her/cyu023. [DOI] [PubMed] [Google Scholar]
- 29.Lopedota A, Cutrignelli A, Denora N, Laquintana V, Lopalco A, Selva S, et al. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-β-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev Ind Pharm. 2015;41:728–736. doi: 10.3109/03639045.2014.900078. [DOI] [PubMed] [Google Scholar]
- 30.Barbareschi M, Vescovi V, Starace M, Piraccini BM, Milani M. Propylene glycol free 5% minoxidil lotion formulation: cosmetic acceptability, local tolerability, clinical efficacy and in-vitro skin absorption evaluations. G Ital Dermatol Venereol. 2020;155:341–345. doi: 10.23736/S0392-0488.20.06554-2. [DOI] [PubMed] [Google Scholar]
- 31.Purnak T, Senel E, Sahin C. Liquid formulation of minoxidil versus its foam formulation. Indian J Dermatol. 2011;56:462. doi: 10.4103/0019-5154.84714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44–49. [PMC free article] [PubMed] [Google Scholar]
- 33.Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, Moreno-Arrones ÓM, Saceda-Corralo D, Rodrigues-Barata R, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644–1651. doi: 10.1016/j.jaad.2021.02.054. [DOI] [PubMed] [Google Scholar]
- 34.Therianou A, Vincenzi C, Tosti A. How safe is prescribing oral minoxidil in patients allergic to topical minoxidil? J Am Acad Dermatol. 2022;86:429–431. doi: 10.1016/j.jaad.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Goren A, Castano JA, McCoy J, Bermudez F, Lotti T. Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther. 2014;27:171–173. doi: 10.1111/dth.12111. [DOI] [PubMed] [Google Scholar]
- 36.Roberts J, Desai N, McCoy J, Goren A. Sulfotransferase activity in plucked hair follicles predicts response to topical minoxidil in the treatment of female androgenetic alopecia. Dermatol Ther. 2014;27:252–254. doi: 10.1111/dth.12130. [DOI] [PubMed] [Google Scholar]
- 37.Pietrauszka K, Bergler-Czop B. Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: a review. Postepy Dermatol Alergol. 2022;39:472–478. doi: 10.5114/ada.2020.99947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.